Abstract
Background
Oropharyngeal cancer incidence is rapidly rising due to human papillomavirus (HPV) type 16 infection. The dearth of data on effectiveness of national female-only vaccination programs in preventing oral HPV infection and potential herd immunity in unvaccinated males has resulted in considerable controversy regarding the need to vaccinate males, especially in countries with high female vaccination coverage.
Methods
Subjects aged 0–65 years undergoing tonsillectomy for nonmalignant indications were recruited in 6 hospitals in the United Kingdom. Oral samples were collected as follows: oral rinse, tongue base, and pharyngeal wall brushes, then tonsil tissue (tonsillectomy). Vaccination data were obtained from regional health authorities. All samples were centrally tested for HPV DNA by polymerase chain reaction.
Results
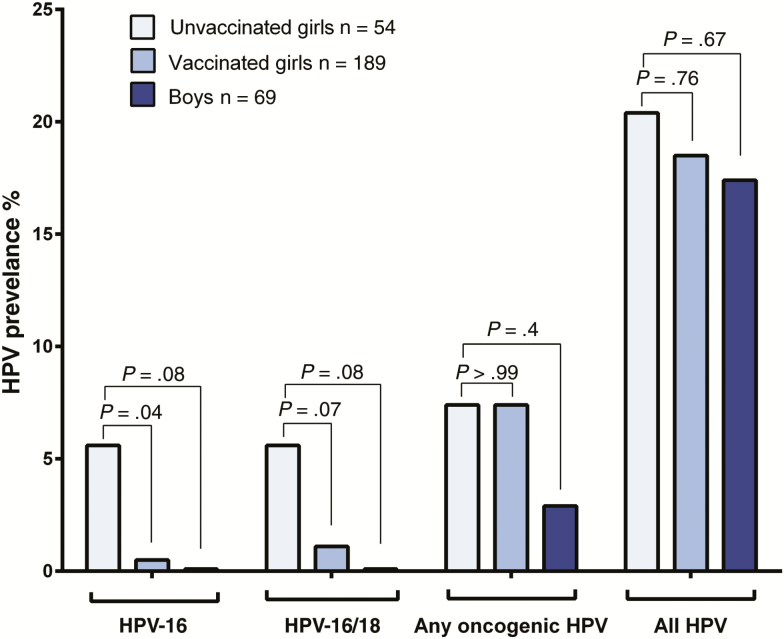
Of 940 subjects, 243 females and 69 males were aged 12–24 years (median age, 18.6 years), with 189 (78%) females and no males vaccinated against HPV. Overall, oropharyngeal HPV-16 prevalence was significantly lower in vaccinated versus unvaccinated females (0.5% vs 5.6%, P = .04). In contrast, prevalence of any oropharyngeal HPV type was similar in vaccinated and unvaccinated females (19% vs 20%, P = .76). Oropharyngeal HPV-16 prevalence in unvaccinated males was similar to vaccinated females (0% vs 0.5%, P > .99), and lower than unvaccinated females (0% vs 5.6%, P = .08).
Conclusions
Our findings indicate that the UK female-only vaccination program is associated with significant reductions in oropharyngeal HPV-16 infections. These are also the first data to suggest potential herd immunity from female-only vaccination against oropharyngeal HPV infection in contemporaneously aged males.
Keywords: head and neck cancer, vaccination, oropharyngeal cancer, cancer prevention, clinical trial
Human papillomavirus (HPV)–related oropharyngeal cancers are rapidly increasing. This study shows that vaccinating girls and young women in a national program against HPV reduces oropharyngeal oncogenic HPV-16 infection. The data also show low oral HPV-16 prevalence in unvaccinated males, suggesting potential herd immunity.
Infection with human papillomavirus (HPV) can cause oropharyngeal cancers, as well as cervical, anal, penile, and vulvovaginal cancers, and genital warts. HPV is the main cause for the increasing incidence of oropharyngeal cancers in the United States and many Western European countries [1–5], and affects 3 times as many men than women. HPV-16 has been identified as the primary type causing these cancers [4, 5]. Three HPV vaccines are now licensed in many countries worldwide: the HPV-16/18 AS04-adjuvanted vaccine (AS04-HPV-16/18v; Cervarix, GSK) and the 4-valent (4vHPVv) and 9-valent (9vHPVv) aluminum hydroxyphosphate sulfate adjuvanted vaccines (Gardasil, Merck). These vaccines have been shown to prevent anogenital HPV-16/18 infection and high-grade cervical and anogenital lesions [6–11]. The AS04-HPV-16/18 vaccine targets 2 types of HPV that together cause >70% of cervical cancers (HPV-16 and -18) and has also shown cross-protection against HPV-31, -33, and -45, the next most common HPV types in cervical cancer [12–15]. In addition to HPV-16 and -18, the 4vHPV vaccine targets HPV-6 and HPV-11, which cause >86% of genital warts [16]. The 9vHPV vaccine (against HPV types 6/11/16/18/31/33/45/52/58) has also been recently approved in many countries [17].
HPV vaccination was first introduced in the United Kingdom in September 2008, with AS04-HPV-16/18v offered to all girls aged 12–13 years (UK year 8) as well as all girls aged 14–17 as part of a time-limited catch-up program, with a switch to 4vHPV vaccine in September 2012. HPV vaccination in UK girls has had high uptake with 77% of 12- to 13-year-olds and 49% of 14- to 17-year-olds in the “catch-up” cohort having received all 3 doses [18].
In addition to trial data demonstrating that HPV vaccination effectively reduces cervical HPV infection and precancerous lesions, there have now been several studies showing population effects of the national vaccination program. A systematic review and meta-analysis and several studies of the impact of the national immunization program have shown considerable reductions in the risk of cervical HPV-16/18 and HPV-31/33/45 infections, anogenital warts, and cervical abnormalities (including invasive HPV-associated cancers) among females vaccinated before 20 years of age [15, 19–24].
To date, the effect of vaccination on oral HPV infection has not been well explored. Secondary analysis of a randomized controlled trial assessing AS04-HPV-16/18 vaccine efficacy on cervical HPV in Costa Rica [25] demonstrated that vaccination was associated with a 93% (95% confidence interval, 63%–100%) decrease in the prevalence of oral HPV-16/18 in adult women 4 years after vaccination. More recently, evidence has been reported supporting reduced HPV-6/11/16/18 oral prevalence rates in vaccinated compared to unvaccinated subjects aged 18–33 years in the United States (0.11% vs 1.61%, P = .08) [26]. Importantly, all studies have been carried out using oral rinse, and there have been no studies examining HPV prevalence using oral rinse and tonsil tissue together, or the effect of the vaccine on HPV prevalence in tonsil tissue (the primary site of oropharyngeal cancer). In addition, there have been no studies evaluating the efficacy of vaccination programs on oral HPV prevalence in children, or studying protection of males from oral HPV infection by the potential herd effect from a national female-only vaccination program.
To address that, this study aimed to assess the effect of HPV vaccination on HPV prevalence in tonsillar tissue and oral exfoliated cells among girls and young adult women in the United Kingdom undergoing voluntary tonsillectomy for nonmalignant indications, and to compare levels of infection to those of unvaccinated, contemporaneous young males of the same age.
METHODS
Study Design
This article uses data collected in the Oromouth study (NCT01330147), a cohort of 940 patients (340 males, 600 females) aged 0–65 years undergoing tonsillectomy for nonmalignant indications. Subjects were enrolled across 6 hospitals in the UK from 2013 to 2015. To assess vaccine effectiveness, we concentrated the analysis on female subjects aged 12–24 years at enrollment who could have been vaccinated under the national UK HPV vaccination program, and on contemporaneous males of the same age. The West Midlands–Solihull National Health Service Research Ethics Committee approved this study (approval number 11/WM/0283), and all patients or parent(s)/legal guardian(s) gave written informed consent.
Data Collection
Oral samples were collected in the following order: oral mucosal transudate (using Oracol S10 devices, Malvern Medical Developments) followed by a 60-second, sterile-saline oral rinse and gargle; an oropharyngeal brush of the base of the tongue (using Orcellex brushes, Rovers, The Netherlands); an oropharyngeal brush of the posterior pharyngeal wall; and finally, all left and right tonsil tissue by tonsillectomy. Further details on collection and processing of all samples are provided in the Supplementary Methods and Supplementary Figure 1. Urine, blood, and nail brush samples were also collected preoperatively (results not reported here). Samples were collected using predefined protocols by research nurses and surgeons who were trained before embarking on the study.
A standardized survey was completed by participants (sample shown in Supplementary Figure 2). The survey included detailed demographic information; vaccination and clinical history; and, for subjects aged ≥16 years, sexual, smoking, and drinking behaviors. To avoid feelings of embarrassment and underreporting by patients, surveys forms had unique identifiers only, with no names, and were submitted in closed envelopes deposited in locked ballot-type boxes, only to be opened by researchers who were independent and did not know the clinical teams.
Data on vaccination were obtained from the regional health authorities that provided information on which patients received vaccination through the school program and the catch-up program, and how many doses they received.
A study log was maintained to record those approached to be part of the Oromouth study and to record reasons for lack of consent. A total of 1356 individuals were approached, of whom 71.6% consented. The main reasons for not gaining consent were patients refusing (38.9%) and parents declining (21.5%). Of this cohort, 30 patients were part of a pilot study and were therefore not included in the analysis for the main study.
Processing and HPV Testing of Samples
All samples were tested centrally for the presence of HPV DNA by polymerase chain reaction (PCR) amplification using the 10-primer HPV short PCR fragment (SPF10) PCR DNA enzyme immunoassay (DEIA)–line probe assay (LiPA25) version 1 (Laboratory Biomedical Products, Rijswijk, The Netherlands). In brief, this broad-spectrum PCR-based HPV DNA testing system uses SPF10 primers to amplify and a DEIA to detect at least 57 HPV genotypes and the LiPA25 to genotype 25 carcinogenic and noncarcinogenic HPVs in all samples (HPV types 6, 11, 16, 18, 31, 33–35, 39, 40, 42–45, 51–54, 56, 58, 59, 66, 68, 70, and 74) [27, 28]. To increase the specificity of type-specific detection of HPV using the SPF10 DEIA system, all specimens that were SPF10 PCR/DEIA-positive were tested with the E6-based multiplex type-specific system (MPTS123) that uses xMAP technology (Luminex, Austin, Texas) [29]. The HPV types detected by the MPTS123 assay are HPV types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. See the Supplementary Materials for details.
Oropharyngeal HPV positivity was defined as HPV DNA detection in any of the collected oral samples (oral rinse, either of the oral brushes, or the tonsillar tissue samples) regardless of type. On the basis of previous work [30], oncogenic (high-risk) HPV was defined as type 16, 18, 31, 33, 35, 39, 45, 51, 52, 58, or 59.
Risk of Bias Mitigation
Consecutive patients were recruited to avoid bias. Samples were analyzed at laboratories in a blinded fashion, with no knowledge of patient characteristics or behaviors. Questionnaires were collected and analyzed in a pseudo-anonymized manner, as described above.
Statistical Analysis
In this prespecified analysis of secondary outcome measures, demographic characteristics, risk factors, and sample-specific HPV prevalence for females and males aged 12–24 years were compared by vaccination status and tested for differences using Pearson χ2 tests or Fisher exact test. The following HPV type-specific outcomes for prevalence were compared between differences by vaccinated and unvaccinated subjects and by sample type: HPV-16, HPV-16/18, HPV-31/33/45, any oncogenic HPV, and any HPV. To explore previously found cross-protective effects of Cervarix (AS04-HPV-16/18v) vaccination [12–14] with HPV types 31, 33, and/or 45, positivity to these types was considered as a separate outcome. Logistic regressions were performed for each of the outcomes to test the association between vaccination and prevalence of HPV after controlling for age. Because behavioral factors were collected for subjects aged ≥16 years, there were insufficient vaccinated patient numbers to undertake multiple logistic regressions to adjust for behavioral factors.
RESULTS
Of the 940 subjects in the study, there were 243 females and 69 males aged 12–24 years, with a median age of 18.6 years (interquartile range [IQR], 16.3–20.7 years) and 19.1 years (IQR, 15.0–21.0 years), respectively. Of the females, 189 (78%) received HPV vaccination. None of the males were vaccinated. Females who were vaccinated were more likely than unvaccinated females to be white (90% vs 76%, P = .03) and <20 years old at enrollment (70% vs 54%, P = .01), but were similar in terms of enrollment center, year enrolled, and sexual behavior. Eighty-nine percent of those vaccinated received the AS04-HPV-16/18 vaccine (Table 1).
Table 1.
Description of Males and Females Aged 12–24 Years in the Study Population, With Data on Females by Human Papillomavirus Vaccination History
| Females | Males | ||||
|---|---|---|---|---|---|
| Received HPV Vaccine | |||||
| Participant characteristic | No (n = 54) |
Yes (n = 189) |
P Value for Unvaccinated vs Vaccinated Females | Unvaccinated Males (n = 69) | P Value for Males vs Vaccinated Females |
| Age, y | .01 | .02 | |||
| 12–15 | 16 (29.6) | 41 (21.7) | 21 (30.4) | ||
| 16–19 | 13 (24.1) | 92 (48.7) | 20 (29.0) | ||
| 20–24 | 25 (46.3) | 56 (29.6) | 28 (40.6) | ||
| Race/ethnicity | .03 | .38 | |||
| White | 41 (75.9) | 171 (90.5) | 59 (85.5) | ||
| Black or black British mixed | 2 (3.7) | 4 (2.1) | 5 (7.3) | ||
| Asian or British Asian | 5 (9.3) | 5 (2.7) | 2 (2.9) | ||
| Mixed or other ethnic group | 6 (11.1) | 9 (4.8) | 3 (4.4) | ||
| Center enrolled | .35 | .78 | |||
| Worcester Royal Hospital | 1 (1.9) | 6 (3.2) | 2 (2.9) | ||
| University Hospital Coventry and Warwickshire | 27 (50.0) | 66 (34.9) | 31 (44.9) | ||
| University Hospital Birmingham | 13 (24.1) | 63 (33.3) | 20 (29.0) | ||
| New Cross Hospital Wolverhampton | 2 (3.7) | 4 (2.1) | 1 (1.5) | ||
| Kidderminister General Hospital | 1 (1.8) | 10 (5.3) | 4 (5.8) | ||
| Birmingham Heartlands Hospital | 10 (18.5) | 40 (21.2) | 11 (15.9) | ||
| Year enrolled | .60 | .16 | |||
| 2013 | 17 (31.5) | 66 (34.9) | 23 (33.3) | ||
| 2014 | 23 (42.6) | 86 (45.5) | 25 (36.2) | ||
| 2015 | 14 (25.9) | 37 (19.6) | 21 (30.4) | ||
| Survey among those aged ≥16 y only | |||||
| Age at first sex, y, mean (SD) | 16.2 (1.7) | 15.9 (1.5) | .24 | 16.2 (1.3) | .12 |
| Ever had sex | .31 | .57 | |||
| No | 1 (2.9) | 14 (10.3) | 3 (6.5) | ||
| Yes | 34 (97.1) | 122 (89.7) | 43 (93.5) | ||
| Ever had oral sex | .08 | .09 | |||
| No | 2 (6.5) | 25 (19.7) | 3 (7.1) | ||
| Yes | 29 (93.5) | 102 (80.3) | 39 (92.9) | ||
| No. of lifetime oral sex partners | .09 | .02 | |||
| 0 | 3 (10.7) | 26 (21.1) | 7 (46.7) | ||
| 1 | 8 (28.6) | 24 (19.5) | 1 (6.7) | ||
| 2–5 | 16 (57.1) | 51 (41.5) | 2 (13.3) | ||
| ≥6 | 1 (3.6) | 22 (17.9) | 5 (33.3) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HPV, human papillomavirus; SD, standard deviation.
Effect of Vaccination on HPV Prevalence
HPV prevalence was compared in vaccinated and unvaccinated females, by HPV type and by sample type (Figure 1; Table 2). Overall oropharyngeal HPV-16 prevalence was significantly lower in vaccinated than unvaccinated females (0.5% vs 5.6%, P = .04). Prevalence of oropharyngeal HPV-16 appeared to be lower among vaccinated than unvaccinated females in both the routine and catch-up vaccination cohorts (Supplementary Table 1). Prevalence of oropharyngeal HPV-16 and/or HPV-18 together (1.1% vs 5.6%, P = .07) also appeared to be reduced (Figure 1). All 4 participants who had oropharyngeal HPV-16 infections had HPV-16 detected in tonsillar tissue. Only 1 of these participants with tonsillar HPV-16 had HPV-16 detected in an oral rinse sample. Of the 4 participants with oropharyngeal HPV-16 infections, 3 were unvaccinated and 1 was vaccinated. The vaccinated participant was a young woman who was 20 years old when she enrolled in the study in 2015, reported receiving 3 doses of AS04-HPV-16/18v, had 8 oral sex partners, and was a current smoker. One (vaccinated) participant had an oropharyngeal HPV-18 infection detected in an oral brush sample.
Figure 1.
Oropharyngeal human papillomavirus (HPV) prevalence in unvaccinated females, vaccinated females, and unvaccinated males aged 12–14 years, by vaccination status and HPV type. P values represent comparisons to unvaccinated females using Pearson χ2 test or Fisher exact test. Abbreviation: HPV, human papillomavirus.
Table 2.
Difference in Human Papillomavirus (HPV) Prevalence Among 69 Unvaccinated Males, 189 Females Vaccinated With Any HPV Vaccine, and 54 Unvaccinated Females Aged 12–24 Years at Enrollment, by Sample Type and Among Select HPV Types
| Females | Unvaccinated vs Vaccinated Females | Males (All Unvaccinated) | Males vs Vaccinated Females | Males vs Unvaccinated Females | ||
|---|---|---|---|---|---|---|
| HPV type and sample type | Not Vaccinated (n = 54) |
Vaccinateda (n = 189b) |
P Value | (n = 69) | P Value | P Value |
| HPV-16 | ||||||
| Oropharyngeal (overall) | 3 (5.6) | 1 (0.5) | .04 | 0 (0) | >.99 | .08 |
| Oral rinse | 1 (1.9) | 0 (0.0) | .22 | 0 (0) | … | .44 |
| Oral brush (either sample) | 0 (0.0) | 0 (0.0) | … | 0 (0) | … | … |
| Tonsil | 3 (5.6) | 1 (0.5) | .04 | 0 (0) | >.99 | .08 |
| HPV-16 or -18 | ||||||
| Oropharyngeal (overall) | 3 (5.6) | 2 (1.1) | .07 | 0 (0) | > .99 | .08 |
| Oral rinse | 1 (1.9) | 0 (0.0) | .22 | 0 (0) | … | .44 |
| Oral brush (either sample) | 0 (0.0) | 1 (0.5) | >.99 | 0 (0) | >.99 | … |
| Tonsil | 3 (5.6) | 1 (0.5) | .04 | 0 (0) | >.99 | .08 |
| HPV-31, -33, or -45 | ||||||
| Oropharyngeal (overall) | 1 (1.9) | 0 (0.0) | .22 | 1 (1.5) | .27 | >.99 |
| Oral rinse | 1 (1.9) | 0 (0.0) | .22 | 0 (0) | … | .44 |
| Oral brush (either sample) | 0 (0.0) | 0 (0.0) | … | 1 (1.5) | .27 | >.99 |
| Tonsil | 0 (0.0) | 0 (0.0) | … | 0 (0) | … | … |
| Any oncogenic HPV type | ||||||
| Oropharyngeal (overall) | 4 (7.4) | 14 (7.4) | >.99 | 2 (2.9) | .25 | .40 |
| Oral rinse | 2 (3.7) | 12 (6.4) | .74 | 1 (1.5) | .20 | .58 |
| Oral brush (either sample) | 0 (0.0) | 2 (1.1) | >.99 | 1 (1.5) | >.99 | >.99 |
| Tonsil | 3 (5.6) | 1 (0.5) | .04 | 0 (0) | >.99 | .08 |
| Any type of HPV | ||||||
| Oropharyngeal (overall) | 11 (20.4) | 35 (18.5) | .76 | 12 (17.4) | .84 | .67 |
| Oral rinse | 8 (14.8) | 28 (14.8) | >.99 | 9 (13.2) | .72 | .77 |
| Oral brush (either sample) | 1 (1.9) | 8 (4.2) | .69 | 3 (4.4) | > .99 | .63 |
| Tonsil | 3 (5.6) | 2 (1.1) | .07 | 1 (1.5) | >.99 | .32 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviation: HPV, human papillomavirus.
aHPV-16 was detected in the tonsil sample of 1 person who was vaccinated with HPV-16/18 AS04-adjuvanted vaccine (AS04-HPV-16/18v) (with 3 doses), reported having 8 lifetime oral sex partners, was a current smoker, and was enrolled in 2015 when she was 20 years old. Only 1 HPV-18 infection was detected in any oral sample: It was in a participant who received all 3 doses of AS04-HPV-16/18v, reported never performing oral sex or any other sexual activity, was a never smoker, and was enrolled in 2013 at age 17 years.
bTwo vaccinated subjects did not have tonsil samples (tonsillar data for vaccinated subjects shown are among 187 subjects). Three vaccinated subjects and 1 unvaccinated subject did not have oral rinse samples (oral rinse data for vaccinated and unvaccinated subjects shown are 186 and 53, respectively).
Oropharyngeal prevalence of HPV-31, -33, and/or -45 was 0 in vaccinated females compared with 1.9% (1 case) in unvaccinated females (P = .22). Prevalence of any type of oropharyngeal HPV (19% vs 20%, P = .76) or any oncogenic HPV type (7.4% vs 7.4%, P > .99) was similar in vaccinated and unvaccinated females. Adjustment for age did not change the results materially (Supplementary Table 2).
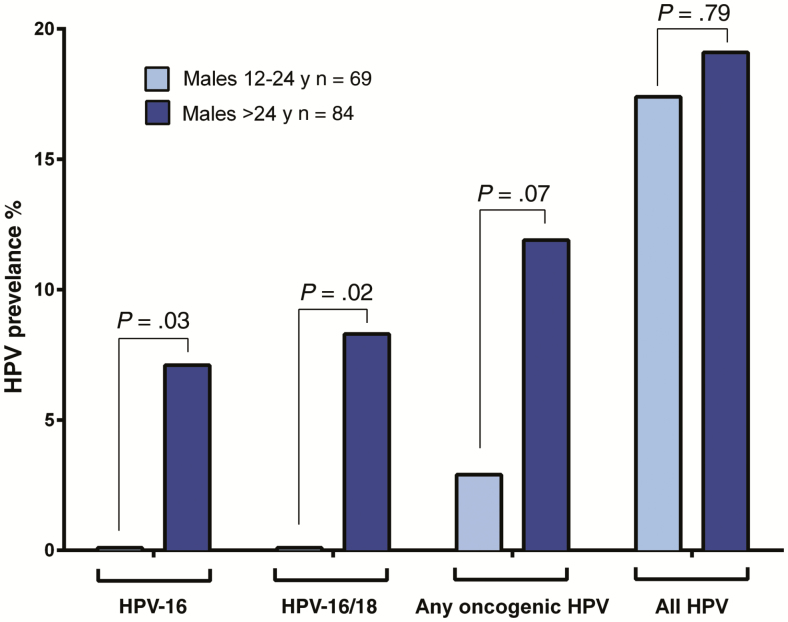
Next, HPV prevalence among unvaccinated males 12–24 years of age was compared to that among unvaccinated and vaccinated females of the same ages. There were no oropharyngeal HPV-16 or HPV-18 infections detected among males. Indeed, oropharyngeal HPV-16 prevalence in males appears to be similar to that of vaccinated females (0 vs 0.5%, P > .99), and lower than unvaccinated females (0 vs 5.6%, P = .08) (Figure 1; Table 2). Among 84 older men in the study, aged 25–56 years, prevalence of oropharyngeal HPV-16 (7.1%, P = .03), and of combined oropharyngeal HPV-16 and/or HPV-18 infections (8.3%, P = .02), was significantly higher than that observed among the 12- to 24-year-old males (Figure 2; Supplementary Table 3).
Figure 2.
Oropharyngeal human papillomavirus (HPV) prevalence in males 12–24 years of age and men >24 years of age, by HPV type. P values represent comparisons to males 12–24 years old using Pearson χ2 test or Fisher exact test.
Effect of Vaccination by Sample Type
When considering each sample type separately, HPV-16 prevalence in tonsillar tissue samples was significantly lower in vaccinated than unvaccinated females aged 12–24 years (HPV-16: 0.5% vs 5.6%, P = .04). Only 1 non–HPV-16 type was detected in tonsillar samples in this age group, an HPV-6 infection in a participant aged 17 years who received 3 doses of AS04-HPV-16/18v. When considering HPV-16 in oral rinse samples alone, smaller differences were seen between vaccinated females aged 12–24 years, compared to unvaccinated ones (0 vs 1.9%, P = .44) (Table 2). HPV detection in oropharyngeal brushes was low, with no HPV-16 being detected.
DISCUSSION
Our findings are the first to indicate that routine vaccination against HPV, as part of a national program, is associated with reductions in oropharyngeal HPV-16 infections (the primary HPV type linked to oropharyngeal cancers) in children and young adults. Specifically, vaccination reduces the prevalence of tonsillar HPV infections, which is the commonest site of oropharyngeal cancer and for which data have hitherto been lacking. These data are consistent with data in adults from post hoc analyses of the GlaxoSmithKline HPV-040 study [31], with data from a randomized controlled trial in Costa Rica [25], and with recent data from the United States [32]. The differences in oropharyngeal HPV-16 infection shown within this relatively small study population suggest that the population impact of the UK vaccination program on oropharyngeal HPV is likely to be substantial.
Importantly, our data also demonstrate low HPV-16 prevalence among unvaccinated males aged 12–24 years. Males’ prevalence rates were similar to rates in vaccinated females, and considerably lower than in unvaccinated females and men aged ≥25 years, despite males reporting significantly more sexual activity (ever had sex) and more sexual partners than vaccinated females. This effect was also demonstrated despite a likely reduction in prevalence rates in unvaccinated females due to the potential herd effect from vaccinated females, as demonstrated for cervical infections in Scotland, England, and the Netherlands [15, 21, 23, 24, 33]. Previously, the only evidence of any potential herd immunity in males from the UK female vaccination program was a reported 62% reduction in genital warts in heterosexual boys and young men in England since 2009 [34]. Our data may be one of the first indications of a potential herd immunity effect from the female-only vaccination program on oropharyngeal HPV infection in contemporaneously aged males. If confirmed in larger population-based studies, these new findings could carry important implications for the decision to extend national HPV vaccination programs to include males, where there is high coverage of females.
No previous study has had the opportunity to prospectively test tonsillar tissue for HPV in vaccinated and unvaccinated individuals. The few studies available were undertaken retrospectively on formalin-fixed tissue samples from historic cohorts and have reported rates of 0–1% [35–37]. By including tonsillar samples in our combined oropharyngeal HPV outcome, we were able to detect HPV in participants with greater sensitivity than by oral rinse alone. We were therefore able to find HPV in considerably more subjects, enabling us to detect a compelling difference in HPV-16 prevalence between the vaccinated and unvaccinated groups in the tissue expected to be most relevant for disease. These results suggest that current estimates of oral HPV-16 prevalence rates, based predominantly on oral rinse samples, may be an underestimate of the true prevalence. It should be noted that more HPV-16 was identified in tonsils than oral rinse samples, whereas HPV subtypes overall were identified much more commonly in oral rinse than tonsil samples. This may reflect a predilection of HPV-16 to tonsils, compared to other HPV subtypes.
Our study had limitations in that there were a small number of people with infection, especially for non–HPV-16 oncogenic types, which limited the analyses and adjustments that could be undertaken. There was only 1 HPV-18 case (in a vaccinated female) and only 1 HPV-31/33/45 infection detected in our study (in an unvaccinated female), so we could not make reliable conclusions for non–HPV-16 oncogenic infections or adequately evaluate the cross-protective effects that have been found in previous studies [12–14]. However, these are rare causes of HPV-related oropharyngeal cancer. Furthermore, only participants aged ≥16 years at enrollment completed the risk behavior survey, and we therefore could not adjust for these factors in our overall analysis without severely truncating our dataset. This means that residual confounding could remain in the estimates from the logistic regression. However, when restricting analyses to those who completed the survey and adjusting for behavioral risk factors, the results were of a similar magnitude to those displayed by the whole sample (Supplementary Table 2). Furthermore, we undertook multiple analysis of secondary outcomes, with no control for multiplicity of inferences, which should be kept in mind when interpreting these results. Despite these limitations above, our results demonstrated convincing differences. Finally, more females aged 12–24 years were recruited compared to males. This reflects a lower willingness of males to agree to participate in the study. This may introduce biases, although the prevalence of overall HPV and, importantly, all (sexually transmitted) high-risk HPV infections was the same in females and males of the same age (data not shown), suggesting that the differences seen in HPV-16 prevalence were not due to recruitment bias.
While the UK vaccination program was designed to prevent cervical cancers in women, the secondary effects of preventing oropharyngeal HPV infection are important to consider. With a rising public health focus on preventing HPV-positive oropharyngeal cancers due to their increasing incidence [38], the effective reduction in oropharyngeal HPV-16 prevalence in vaccinated adolescents and young adults seen in our study means that national vaccination programs could considerably reduce the incidence of oropharyngeal HPV cancers. Our study also demonstrated reduced oropharyngeal HPV-16 prevalence in the vaccinated groups of both the routine and catch-up vaccine programs. As with cervical cancer, however, longitudinal data are necessary to fully establish the effectiveness of vaccination for preventing oropharyngeal cancers.
In summary, our results are one of the first to show that a female-only vaccination program protects against oncogenic oropharyngeal HPV-16 infection in young females, and may also confer protection on contemporaneously aged unvaccinated males through potential herd immunity. This suggests that oropharyngeal HPV prevalence may be reduced by female-only national HPV vaccination programs with high coverage.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. H. M. conceived of, designed, conducted, and interpreted the study and wrote the manuscript. J. L. B., R. J. S., N. B., O. O., J. B., and J. J. conducted the study, interpreted results, and wrote the manuscript. S. T. and D. R. participated in the study design, analysis and interpretation of the data, and writing the manuscript. G. D. and T. S. B. analyzed the data and wrote the manuscript. A. M., L. S., and A. V. participated in the design of the sampling procedures, laboratory testing, and interpretation of the results and writing of the manuscript.
Acknowledgments. The authors thank all study participants and their families and all clinical study site personnel who contributed to the conduct of this trial. The authors also thank Dimitrie Grégoire, Dominique Gilson, Stéphanie Maerlan, Nathalie Houard, Jean-Marc Delroisse, Serge Durviaux, and Thierry Pascal for their help in sample testing; Pam Kalodimos, Corinne Willame, Monique Dodet, and Edwin Kolp for their help in study coordination; Sylviane Poncelet and Martin Ryser for manuscript review; Sarah Welby for her review and input to the study and manuscript; and Gemma Jones for manuscript preparation.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA (GSK) via an unrestricted research grant. A. M. received support for laboratory testing and travel to meetings from GSK for the present study.
Potential conflicts of interest. S. T. and D. R. are employees of and hold shares in the GSK group of companies. H. M. has received research grants and advisory consultancy fees from AstraZeneca and Merck, Sharpe & Dohme (MSD), and has received previous grants from the GSK group of companies, MSD, Sanofi Pasteur, Silence Therapeutics, and AstraZeneca. A. V.’s institution, the University of Antwerp, received support from GSK for DNA extraction of the urine samples. A. V. has an issued patent for a liquid collection device and is co founder and board member of the University of Antwerp spin-off Novosanis NV. This company distributes a urine collection device; this device was not used in this study, and the manuscript does not report on the urine data. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. D’Souza G, Kreimer AR, Viscidi R, et al. . Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944–56. [DOI] [PubMed] [Google Scholar]
- 2. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14:467–75. [DOI] [PubMed] [Google Scholar]
- 3. Saraiya M, Unger ER, Thompson TD, et al. . US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehanna H, Beech T, Nicholson T, et al. . Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck 2013; 35:747–55. [DOI] [PubMed] [Google Scholar]
- 5. Mehanna H, Franklin N, Compton N, et al. . Geographic variation in human papillomavirus-related oropharyngeal cancer: data from 4 multinational randomized trials. Head Neck 2016; 38(Suppl 1):E1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paavonen J, Naud P, Salmerón J, et al. . HPV PATRICIA Study Group . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–14. [DOI] [PubMed] [Google Scholar]
- 7. FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–27. [DOI] [PubMed] [Google Scholar]
- 8. Garland SM, Hernandez-Avila M, Wheeler CM, et al. . Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators . Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 9. Palefsky JM, Giuliano AR, Goldstone S, et al. . HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–85. [DOI] [PubMed] [Google Scholar]
- 10. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis 2012; 54:891–8. [DOI] [PubMed] [Google Scholar]
- 11. Harper DM. Impact of vaccination with Cervarix (trade mark) on subsequent HPV-16/18 infection and cervical disease in women 15-25 years of age. Gynecol Oncol 2008; 110:S11–7. [DOI] [PubMed] [Google Scholar]
- 12. Kavanagh K, Pollock KG, Potts A, et al. . Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 2014; 110:2804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wheeler CM, Castellsagué X, Garland SM, et al. ; HPV PATRICIA Study Group . Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100–10. [DOI] [PubMed] [Google Scholar]
- 14. Einstein MH, Baron M, Levin MJ, et al. ; HPV-010 Study Group . Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin 2011; 7:1359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kavanagh K, Pollock KG, Cuschieri K, et al. . Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 16. Garland SM, Steben M, Sings HL, et al. . Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 17. European Medicines Agency. Gardasil 9: EPAR summary for the public. EMA, 2015:1–4. Available at: https://www.ema.europa.eu/documents/overview/gardasil-9-epar-summary-public_en.pdf
- 18. Public Health England. Human papillomavirus (HPV) vaccine coverage in England, 2008/09 to 2013/14: a review of the full six years of the three-dose schedule. London: Public Health England,2015. [Google Scholar]
- 19. Drolet M, Bénard É, Boily MC, et al. . Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open 2016; 6:e009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mesher D, Panwar K, Thomas SL, et al. . The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type-specific HPV in young females, 2010–2016. J Infect Dis 2018; 218:911–21. [DOI] [PubMed] [Google Scholar]
- 22. Pollock KG, Kavanagh K, Potts A, et al. . Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111:1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donken R, King AJ, Bogaards JA, Woestenberg PJ, Meijer CJLM, de Melker HE. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018; 217:1579–89. [DOI] [PubMed] [Google Scholar]
- 24. Luostarinen T, Apter D, Dillner J, et al. . Vaccination protects against invasive HPV-associated cancers. Int J Cancer 2018; 142:2186–7. [DOI] [PubMed] [Google Scholar]
- 25. Herrero R, Quint W, Hildesheim A, et al. ; CVT Vaccine Group . Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013; 8:e68329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaturvedi AK, Graubard BI, Broutian T, et al. . Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol 2018; 36:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleter B, van Doorn LJ, Schrauwen L, et al. . Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999; 37:2508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kleter B, van Doorn LJ, ter Schegget J, et al. . Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 1998; 153:1731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Alewijk D, Kleter B, Vent M, et al. . A human papilloma virus testing algorithm comprising a combination of the L1 broad-spectrum SPF10 PCR assay and a novel E6 high-risk multiplex type-specific genotyping PCR assay. J Clin Microbiol 2013; 51:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castle PE. The evolving definition of carcinogenic human papillomavirus. Infect Agent Cancer 2009; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehtinen M, Eriksson T, Natunen K, Damaso S, Bi D, Struyf F.. HN03-03 efficacy of AS04-adjuvanted HPV-16/18 vaccine in reducing oropharyngeal HPV infections in adolescent girls—results from a community-randomized trial. EUropean Research Organisation on Genital Infection and Neoplasia (EUROGIN):Amsterdam, the Netherlands, 2017. [Google Scholar]
- 32. Sonawane K, Suk R, Chiao EY, et al. . Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med 2017; 167:714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cameron RL, Kavanagh K, Pan J, et al. . Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 2016; 22:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Public Health England. Sexually transmitted infections and chlamydia screening in England, 2016. London: Public Health England, 2017.
- 35. Ernster JA, Sciotto CG, O’Brien MM, Robinson LJ, Willson T. Prevalence of oncogenic human papillomavirus 16 and 18 in the palatine tonsils of the general adult population. Arch Otolaryngol Head Neck Surg 2009; 135:554–7. [DOI] [PubMed] [Google Scholar]
- 36. Klingenberg B, Hafkamp HC, Haesevoets A, et al. . p16 INK4A overexpression is frequently detected in tumour-free tonsil tissue without association with HPV. Histopathology 2010; 56:957–67. [DOI] [PubMed] [Google Scholar]
- 37. Bekker JB, Evans MF, Threlkeld KJ, Rajendran V, Adamson CS, Cooper K. Screening for HPV in clinically benign tonsillectomy specimens. Modern Pathol 2012; 25:305. [Google Scholar]
- 38. Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus-related oropharyngeal cancer. Ann Oncol 2017; 28:2386–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.