Abstract
Background
Optimization of combination antiretroviral therapy (cART) can impact the human immunodeficiency virus (HIV) reservoir. We evaluated the effect on the HIV reservoir in peripheral blood and ileum biopsies in patients switching from boosted protease inhibitor (PI/r)–based therapy to dolutegravir (DTG)–based therapy.
Methods
Impact of Integrase-inhibitor DOlutegravir On the viral Reservoir (INDOOR) is a phase 4 open-label clinical trial that randomly included 42 HIV type 1–infected individuals on effective cART: 20 who switched from PI/r-based to DTG-based cART (switch group), and 22 who remained in PI/r-based regimens (control group). We analyzed blood and ileum biopsies to quantify episomal, total, and integrated HIV DNA, cell-associated HIV RNA, residual plasma viremia, T-cell subsets, cell activation, and inflammation markers.
Results
There were no related adverse events or treatment discontinuations due to drug intolerance. The HIV reservoir was consistently larger in ileal than in peripheral CD4+ T cells in both groups (P < .01). Residual viremia in plasma decreased in the switch group (P = .03). However, we did not observe significant longitudinal changes in low-level viral replication, total and integrated HIV reservoir, HIV transcription, T-cell maturation subsets, immunoactivation markers, inflammatory soluble proteins, or cellular markers of latently infected cells.
Conclusions
The INDOOR study is the first evaluation of changes in HIV reservoir size in ileum biopsies and in peripheral blood in individuals switched from PI/r- to DTG-based cART. Although this switch was safe and well tolerated, it had no impact on a large array of immunological and inflammatory markers or on HIV reservoir markers in peripheral or in ileal CD4+ T cells.
Clinical Trials Registration
EudraCT 2014-004331-39.
Keywords: switching, dolutegravir, HIV reservoir, ileum biopsies, immunological and inflammation markers
INDOOR evaluated the effect of switching from boosted protease inhibitor– to dolutegravir-based antiretroviral therapy on the HIV reservoir in peripheral blood and ileum. Residual plasma viremia and soluble CD14 levels decreased, but low-level HIV replication, the HIV reservoir, and immune activation remained unaffected.
Effective combination antiretroviral therapy (cART) suppresses viral replication to undetectable levels as assessed using standard clinical assays. It also reduces morbidity and mortality and improves the quality of life of human immunodeficiency virus (HIV)–infected individuals [1, 2]. Around 30% of infected individuals are currently treated using cART regimens based on boosted protease inhibitors (PI/r). However, PIs have been associated with adverse events such as long-term toxicity, interactions with other drugs, and an increased risk of cardiovascular disease [3]. Therefore, in clinical practice, switching to an integrase strand transfer inhibitor (INSTI) has become a common strategy to prevent long-term metabolic disorders and to manage drug–drug interactions [4, 5]. Furthermore, some studies report a decrease in the viral reservoir and host immune activation after switching the third drug to an INSTI [6, 7]. Several mechanisms contribute to the maintenance of the viral reservoir, including homeostatic dynamics within the CD4+ T-cell pool [8, 9], anatomical sanctuary sites [10, 11], and low-level viral replication [12–14], which leads to the detection of residual plasma viremia in antiretroviral therapy (ART)–suppressed individuals. However, the origin of residual virus production during ART remains unknown.
Raltegravir (RAL) is the only INSTI that has been investigated in cART-switching clinical trials evaluating the effects of therapy on the HIV reservoir [6, 15, 16]. However, the effect of switching to RAL-based regimens remains unclear. Two studies have shown no effect on total or episomal HIV DNA [15, 16], while another showed a decrease in the HIV reservoir size after 48 weeks of switching to a RAL-based regimen [6]. Furthermore, the effect of switching to integrase inhibitors on the HIV reservoir has been evaluated in peripheral blood [6, 15, 16], but not in lymphoid tissue. Gut tissue is a key site for the HIV reservoir [17], although sampling remains difficult [18].
Dolutegravir (DTG) was superior to RAL with respect to virological suppression (<50 copies/mL) in treatment-experienced individuals who were INSTI-naive [19]. A recent study showed that individuals receiving RAL or DTG have comparable HIV DNA in gut-associated lymphoid tissue (GALT) [20]. However, whether its role in the dynamics of the HIV type 1 (HIV-1) reservoir is similar to that of RAL is unknown. Therefore, we compared the effect of switching to DTG-based cART or maintaining PI/r-based cART on the HIV reservoir, in both peripheral blood, and ileum biopsies from HIV-infected, ART-suppressed individuals.
MATERIALS AND METHODS
Study Design
Impact of Integrase-inhibitor DOlutegravir On the viral Reservoir (INDOOR) (EudraCT identifier 2014-004331-39) was a phase 4, randomized, open-label clinical trial, conducted from June 2015 to November 2016 at Vall d’Hebron University Hospital (Barcelona, Spain). We enrolled HIV-1–infected adults with HIV RNA <50 copies/mL for at least 1 year on a stable regimen containing a PI/r plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) for at least 3 months and CD4 T-cell count >200 cells/μL.
Selected participants were randomly assigned (1:1) to switch to DTG plus 2 NRTIs or to continue the same regimen containing a PI/r plus 2 NRTIs (Figure 1). A blocked randomization method was used by creating random block sizes and blinding the investigator to the size of each block.
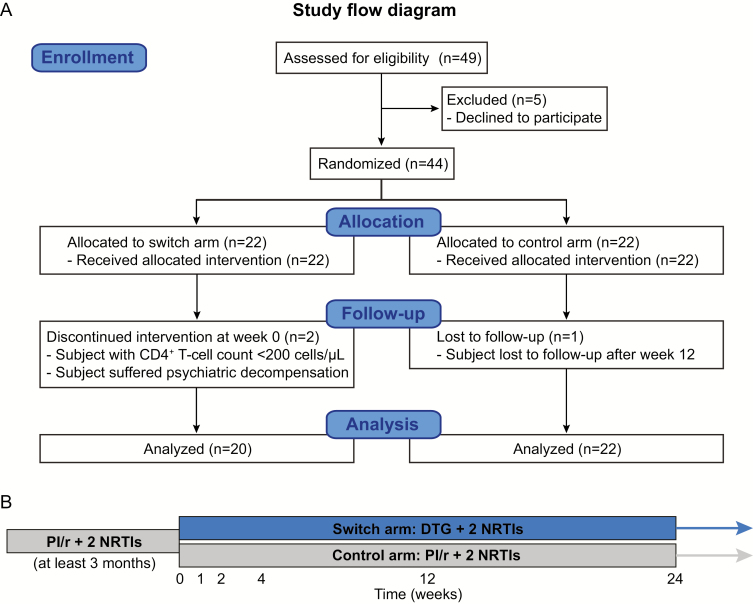
Figure 1.
Trial design. A, Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial showing the enrollment of 44 subjects who were randomized to the switch or control groups. According to our experience, in order to assess the impact on cryptic human immunodeficiency virus (HIV) replication, it is important to evaluate changes affecting 2-LTR, which, owing to their ephemeral nature, can only be detected in recently infected cells, and changes affecting HIV RNA expression during the first 4 weeks following switching from boosted protease inhibitor to dolutegravir (DTG). Consequently, sample size was estimated on the assumption that switching to DTG is associated with an increase of >30% in the proportion of patients with detectable episomal HIV DNA (2-LTR) in peripheral blood mononuclear cells at study week 4 [13]. Mathematical modeling of data obtained from this study suggests that the transient increase in measured 2-LTR concentration is consistent with the blocking effect of the integrase inhibitor on de novo infection [27]. Taking into account all of the above, a total of 40 individuals was needed, 20 in each group. We recruited 44 subjects to accommodate for dropouts and a 10% loss to follow-up. B, Study design. Abbreviations: NRTI, nucleoside reverse transcriptase inhibitor; PI/r, boosted protease inhibitor.
All of the individuals included in the study were asked to participate in a substudy to obtain endoscopic ileum biopsies.
The local ethics committee approved the protocol, and the study was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent before inclusion.
Procedures
We collected blood samples at weeks 0, 1, 2, 4, 12, and 24 from all participants, and ileum samples at weeks 0 and 24 from those included in the GALT biopsy substudy. Procedures are described in Supplementary Materials and in Supplementary Figure 1.
Statistical Analyses
Statistical significance was set at 5% for all the tests, and significant P values were adjusted using the false discovery rate method for multiple comparisons. The analyses were performed using R (version 3.0.2) and GraphPad (version 5.01) software, and are described in Supplementary Materials.
RESULTS
Cohort Characteristics and Clinical Outcome
A total of 49 participants were screened; of these, 44 were randomized to switch to DTG (n = 22, switch arm) or to continue the same PI/r-based cART regimen (n = 22, control arm). Two participants discontinued the study protocol: 1 had a previous psychiatric history and experienced a psychotic decompensation; the other had a baseline CD4+ count <200 cells/μL at the first visit. One subject in the control group with suppressed viremia at week 12 did not attend at week 24. The remaining randomized individuals completed the study protocol (Figure 1).
The baseline characteristics are summarized in Table 1. The number of participants with heterosexual HIV infection was significantly higher in the switching arm (P = .02). Otherwise, treatment arms were well balanced for demographic and clinical data.
Table 1.
Baseline Characteristics
| INDOOR Trial | ||||
|---|---|---|---|---|
| Characteristic | All (N = 42) | Switch Arm (n = 20) | Control Arm (n = 22) | P Value |
| Age, y, median (IQR) | 49 (46–54) | 49 (45–53) | 53 (46–55) | .21 |
| Sex | ||||
| Female | 12 (29) | 8 (40) | 4 (18) | .17 |
| Male | 30 (71) | 12 (60) | 18 (82) | |
| HIV infection route | ||||
| IDU | 23 (55) | 10 (50) | 13 (58) | .02 |
| MSM | 3 (7) | 0 | 3 (14) | |
| Heterosexual | 13 (31) | 10 (50) | 3 (14) | |
| Unknown | 3 (7) | 0 | 3 (14) | |
| Time since diagnosis of HIV, y, median (IQR) | 17.5 (11.8–25) | 16 (11–23.8) | 20 (13.8–25.3) | .19 |
| Time since initiation of cART, y, median (IQR) | 12 (6–18.3) | 12 (5.3–18) | 12 (7.5–19) | .65 |
| Time on current PI-based cART regimen, y, median (IQR) | 4 (3–7) | 4.5 (3–7) | 4 (2.8–8.5) | .96 |
| Time with suppressed VL, median (IQR) | 4 (3–8.3) | 3 (2.3–7.3) | 5.5 (3–9) | .23 |
| Nadir CD4+ count, cells/μL, median (IQR) | 199 (83–297) | 204 (95–259) | 197 (89–289) | .85 |
| Absolute CD4+ count, cells/μL, median (IQR) | 620 (495–920) | 610 (470–760) | 625 (503–1235) | .11 |
| % CD4+ T cells, median (IQR) | 33.2 (28.4–39.2) | 31.8 (28.3–35.2) | 35.6 (29.5–42.1) | .16 |
| Absolute CD8+ count, cells/μL, median (IQR) | 810 (605–1050) | 670 (605–890) | 905 (680–1215) | .19 |
| % CD8+ T cells, median (IQR) | 40.9 (34.8–47.8) | 40.4 (32.2–47.9) | 41.7 (35.8–47.7) | .51 |
| CD4:CD8 ratio, median (IQR) | 0.8 (0.6–1.1) | 0.8 (0.6–1.0) | 0.9 (0.7–1.1) | .68 |
| HIV-1 RNA copies/mL plasma, median (IQR) | <50 | <50 | <50 | .50 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; INDOOR, impact of Integrase-inhibitor DOlutegravir On the viral Reservoir; MSM, men who have sex with men; PI, protease inhibitor; VL, viral load.
Ninety percent (18/20) of participants in the DTG arm and 76.2% (16/21) in the control group maintained viral suppression at week 24. At the safety visit, 4 weeks after completing the 24-week study duration, 100% (20/20) of subjects in the switch group had viral load <50 copies/mL, whereas 3 individuals (14.3%) receiving a PI-based regimen had detectable viremia of 50–200 copies/mL. Self-reported adherence was >95%. No drug-related adverse events during follow-up or discontinuations due to drug intolerance were reported. Individuals switching to DTG showed an improved lipid profile. Total cholesterol, low-density lipoprotein cholesterol, triglycerides, and the cholesterol/high-density lipoprotein cholesterol ratio changed a median of –27 (interquartile range [IQR], –39 to –13.5) mg/dL, –21.3 (IQR, –28.3 to 4.3) mg/dL, –37.5 (IQR, –84.8 to 7) mg/dL, and –0.32 (IQR, –0.57 to 0.05), respectively (Supplementary Figure 2).
Colonoscopy Findings
Thirty-three participants participated in the ileum biopsy substudy: 13 from the switch arm and 20 from the control group (Supplementary Table 1). Of these, 11 and 19, respectively, had paired biopsies. Individuals in the switch arm of the substudy were younger (48 vs 54 years; P = .02), and time since HIV diagnosis was shorter than in the control group (15 [IQR, 10.5–21] years vs 20.5 [IQR, 15.25–26.5] years; P = .04). Colonoscopy was abnormal in 9 (27.3%) subjects. The main findings were tubular adenoma with low-grade dysplasia (n = 5), tubular adenoma with high-grade dysplasia (n = 1), hyperplastic polyps (n = 2), and squamous cell carcinoma (n = 1). No participant had gastrointestinal complaints before colonoscopy.
2-LTR Dynamics in Peripheral CD4+ T Cells
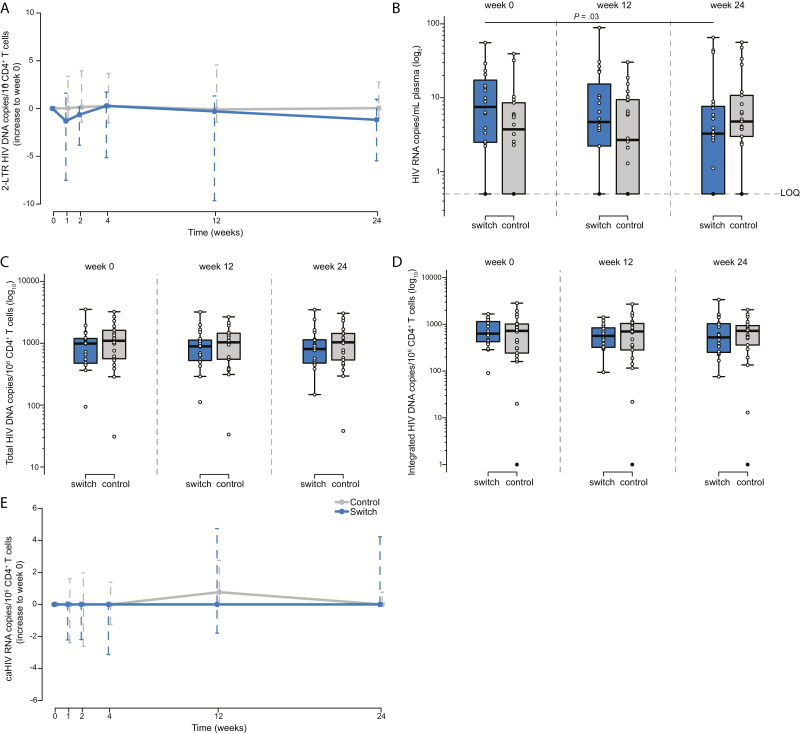
To determine the effect of switching, we analyzed changes in low-level viral replication by quantification of 2-LTR circles in peripheral CD4+ T cells. At baseline, we detected episomal HIV DNA at all the time points in 14 participants (33%) and at ≥1 time point in 41 participants (98%), with a median level of 6.20 (IQR, 0.00–17.35) 2-LTR copies per million CD4+ T cells in the switch group and 2.35 (IQR, 0.00–7.65) in the control group. We observed no statistically significant longitudinal changes in either of the groups or between the groups during the study (Figure 2A). These results confirm the persistence of low-level viral replication during PI/r-based regimens that is not affected by switching to DTG-based ART.
Figure 2.
Analysis of human immunodeficiency virus (HIV) reservoir dynamics in peripheral CD4+ T cells. A, 2-LTR circles. B, Ultrasensitive viral load in plasma. C, Total HIV DNA. D, Integrated HIV DNA. E, Cell-associated unspliced HIV RNA. The switch group is shown in blue and the control group in gray. Solid black dots represent determinations below the limit of quantification. Abbreviations: caHIV, cell-associated HIV; LOQ, limit of quantification.
Residual Viremia in Plasma
To determine the effect of switching on residual plasma viremia, we quantified the HIV RNA copies/mL of plasma by detecting ultrasensitive viral load. Residual plasma viremia was detected in 95% of the participants with a median level of 7.7 (IQR, 2.5–19.2) HIV RNA copies/mL plasma in the switch group and 3.8 (IQR, 1.0–8.6) in the control group. Interestingly, we observed a decrease in residual viremia in the switch group at week 12. This became significant at week 24 (P = .03; Figure 2B); however, the significance was lost after correction for multiple comparisons. Although we observed no significant differences between groups over time, there was a trend between groups at baseline, namely, a slightly higher residual viral load in the switch arm (P = .07). Nonetheless, this trend was not related to time to suppression or any other clinical parameter evaluated in the study. These findings suggest that switching may decrease residual plasma viremia in individuals who have a relatively higher residual viral load.
Reservoir Quantification in Ileal and Peripheral CD4+ T Cells
To provide further insights into the effect of switching on the size of the HIV reservoir, we quantified total HIV DNA in ileal CD4+ T cells and total and integrated HIV DNA and unspliced cell-associated HIV RNA in peripheral CD4+ T cells.
At baseline, using cell lysates, we detected total HIV DNA in 100% of samples, with a median of 989 (IQR, 472–1210) total HIV DNA copies/million peripheral CD4+ T cells in the switch group, and 1099 (IQR, 554–1624) in the control group (Figure 2C). Using DNA extraction, we detected total HIV DNA in 41 participants (98%) and observed a strong correlation between quantification using lysates and extracted DNA at week 0 (n = 41; P < .0001; ρ = 0.89; Supplementary Figure 3A). We detected integrated HIV DNA levels in peripheral CD4+ T cells in 41 participants (98%), with a median level of 634 (IQR, 415–1199) HIV DNA integrated copies/million CD4+ T cells in the switch group, and 725 (IQR, 235–1023) in the control group (Figure 2D). Moreover, we detected unspliced HIV RNA in 34 participants (81%), with a median level of 2.5 (IQR, 0.0–4.8) unspliced HIV RNA/million CD4+ T cells in the switch group and 2.3 (IQR, 0.0–4.3) in the control group (Figure 2E). We also observed strong correlations between total and integrated HIV DNA (P < .0001; ρ = 0.88; Supplementary Figure 3B) and total or integrated HIV DNA and unspliced HIV RNA (P = .004, ρ = 0.58; P = .007, ρ = 0.55, respectively; Supplementary Figure 3C and 3D). However, we observed no statistically significant longitudinal changes in any of these reservoir markers within or between the groups.
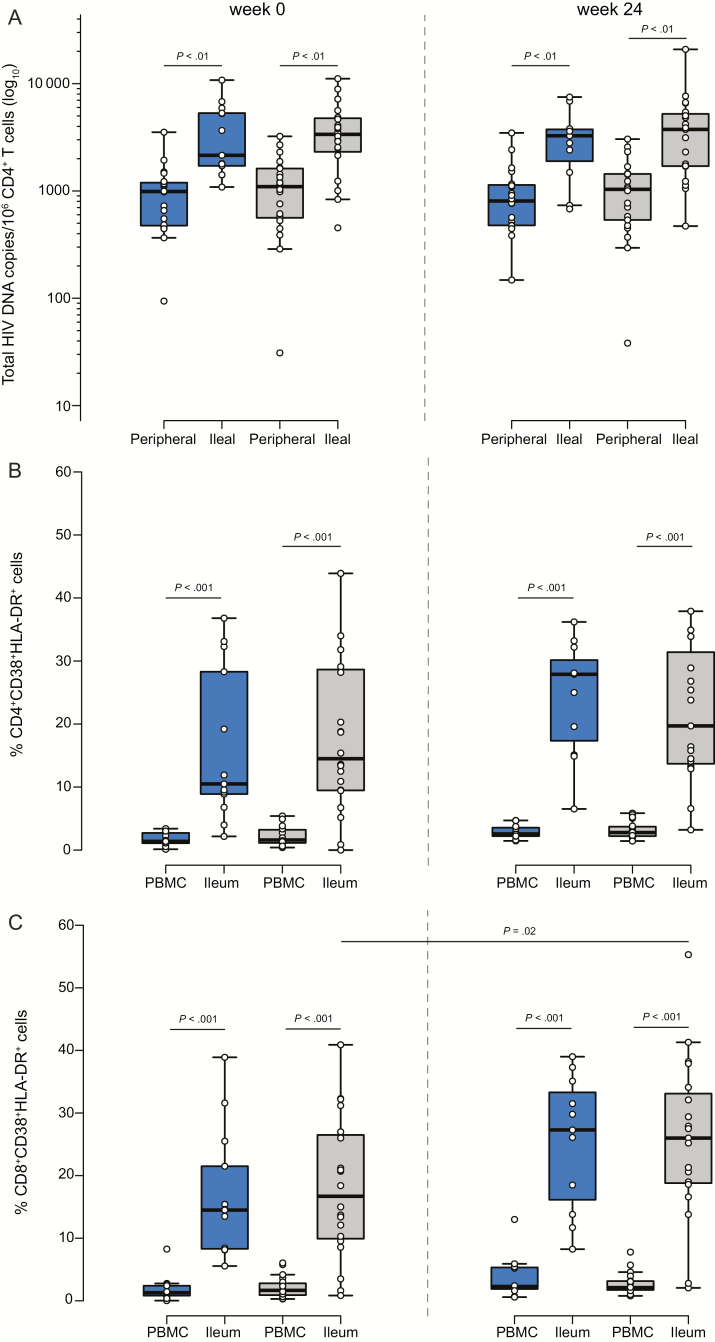
We detected total HIV DNA in 100% of the ileum biopsies. We found 2151 (IQR, 1564–5615) total HIV DNA copies/million CD4+ T cells in the switch group and 3372 (IQR, 2234–4806) in the control group at week 0 (Figure 3A), showing higher levels of total HIV DNA in ileal than in peripheral CD4+ T cells in both the switch and control groups (P < .001). We also observed a direct trend between the size of the HIV reservoir in CD4+ T cells from the ileum and from peripheral blood at week 0 (n = 33; P = .07; ρ = 0.32; Supplementary Figure 3E). As in peripheral blood, we did not detect longitudinal changes in the size of the HIV reservoir in ileum samples.
Figure 3.
Human immunodeficiency virus (HIV) reservoir dynamics and T-cell activation in ileum biopsies. A, Total HIV DNA in ileal vs peripheral CD4+ T cells. B and C, Comparison of CD4+ and CD8+ CD38+HLA-DR+ T cells in ileum biopsies and in peripheral blood mononuclear cells quantified by FACSAria II and longitudinal follow-up of CD4+ and CD8+ CD38+HLA-DR+ T cells in the switch and the control groups. The switch group is shown in blue and the control group in gray. Abbreviation: PBMC, peripheral blood mononuclear cell.
These results indicate that the HIV reservoir is larger in the ileum than in peripheral blood and that there is a strong positive correlation between total and integrated HIV DNA and between HIV DNA and unspliced HIV RNA.
Activation Markers in Ileum Biopsies and in Peripheral Blood
To determine whether switching to DTG-based therapy decreases cell activation, we compared the percentage of CD38+HLA-DR+ in CD4+ and in CD8+ T cells in both groups in ileum biopsies and in peripheral blood. We observed a higher percentage of activated CD4+ (14.5%) and CD8+ T cells (16.7%) from ileum biopsies than in cells from peripheral blood (1.6% and 1.7%, respectively; P < .001; Figure 3B and 3C). However, we only observed an increase in the percentage of CD8+CD38+HLA-DR+ cells in the control group over time (P = .02; Figure 3C). We did not observe changes between groups. These results confirmed that there are more activated cells in tissue than in peripheral blood and that switching did not reduce this activation.
Dynamics of Cellular Subsets, Activation, and Reservoir Surrogate Markers in Peripheral Blood
No significant differences were observed for frequency of CD4+ and CD8+ T cells and the CD4/CD8 ratio between the groups at any time point. Furthermore, no significant longitudinal differences were observed in either group (Supplementary Table 2).
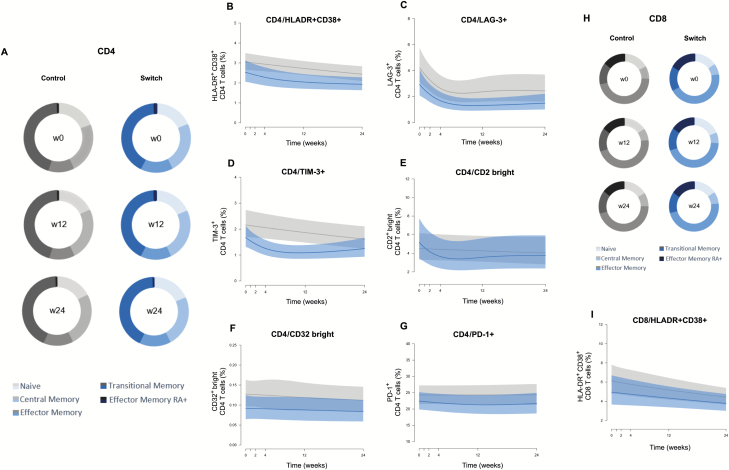
In CD4+ T cells, we observed a decrease in naive and terminal effector CD45RA+ cells during week 0–24 in the control group, an increase in effector cells during weeks 0–12 in the control group, and an increase in the switch group during weeks 0–24. However, no differences between groups were observed for any maturation subset at any time point (Figure 4A). Activation levels decreased over time in both groups (weeks 0–12 and weeks 12–24) without intergroup differences (Figure 4B and Supplementary Table 2). We found the same behavior in both groups when we analyzed LAG-3 and TIM-3 expression in CD4+ T cells (Figure 4C and 4D). CD2bright showed a significant decrease (weeks 0–24) in the switch group, although no significant differences between groups were observed (Figure 4E). Finally, the expression of CD32 and PD-1 in CD4+ T cells was stable over time and similar between groups (Figure 4F and 4G and Supplementary Table 2).
Figure 4.
Analysis of CD4+ and CD8+ T-cell subsets and activation markers. A, Median CD4+ T-cell values for the frequency of the maturation subsets (naive, central memory, effector memory, transitional memory, and effector memory RA+) in the control and switch groups at 3 different time points (weeks 0, 12, and 24). Maturation stages were defined based on the combination of CD45RA, CD197 (CCR7), and CD27, as described in “Materials and Methods.” B–G, Dynamics of CD4+ T-cell activation (percentage CD4+HLA-DR+CD38+ cells), exhaustion (percentage CD4+PD-1+), and potential HIV reservoir markers (percentage of CD4+LAG-3+, CD4+TIM-3+, CD4+CD2bright, and CD4+CD32bright) in both groups. H, Median CD8+ T-cell values for the frequency of the maturation subsets (naive, central memory, effector memory, transitional memory, and effector memory RA+) in the control and switch groups at 3 different time points (weeks 0, 12, and 24). I, Dynamics of CD8+ T-cell activation (percentage CD8+HLA-DR+CD38+ cells) in both groups. The switch group is shown in blue and the control group in gray.
Maturation in CD8+ T cells showed significant increases in central memory cells (weeks 12–24), naive cells (weeks 0–12), and effector cells (weeks 0–12) in the control group, while only the latter cell subset increased in the switch group (weeks 0–24). Again, no differences between groups were observed for any maturation subset at any time point (Figure 4H). The percentage of activated CD8 cells decreased similarly in both groups (Figure 4I). No major changes were noticed for other markers in CD8+ T cells between the groups (Supplementary Table 2).
Inflammatory Markers in Plasma
We did not observe any differences in interleukin 6, C-reactive protein, TNF-related apoptosis-inducing ligand (TRAIL), or interferon gamma-induced protein 10 (IP-10) levels over time or between groups. However, we observed a significant decrease in soluble CD14 (sCD14) levels (weeks 0–12 and weeks 0–24) in the switch group that was not significant between groups (Supplementary Table 3).
DISCUSSION
This is the first study to compare the dynamics of HIV-1 reservoirs after the switch from PI/r- to DTG-based regimens in peripheral blood and in ileum biopsies from cART-suppressed individuals. Our results showed that switching to DTG-based therapy is not associated with changes in low-level viral replication, as no fluctuation in the level of episomal HIV DNA (2-LTR) was observed. In addition, there was no decay in other HIV reservoir markers, such as total and integrated HIV DNA, and unspliced HIV RNA, either in peripheral or in ileal CD4+ T cells after 24 weeks. Interestingly, we detected a significant decrease in residual plasma viremia and sCD14 after 24 weeks of switching to DTG-based therapy, although significance is lost after correction for multiple comparisons. Furthermore, we did not detect significant changes in T-cell activation or other plasma inflammation markers. Therefore, switching to DTG in individuals on suppressive PI/r-based regimens may reduce residual plasma viremia and sCD14, although it did not significantly alter the size of the viral reservoir, low-level viral replication, or immune activation.
These findings are consistent with those of previous studies comparing switching to RAL [15, 16] or intensification of treatment with DTG [21], in which there were no significant changes in HIV reservoir size or in low-level viral replication associated with the intervention. However, our results contrast with those of the RAltegravir Switch for Toxicity or Adverse Events (RASTA) study [6], which showed a significant decrease in HIV reservoir size, measured by total HIV DNA in peripheral blood after 48 weeks of switching successful therapy to RAL-based regimens. HIV reservoir size can decrease during the first 3 years after initiation of cART [22]. Nonetheless, in the RASTA study, the switch group comprised individuals who had been ART suppressed for <3 years, whereas subjects from the control group had been suppressed for >5 years (P = .02), and effective viral suppression was considered a viral load <200 copies/mL. In contrast, in our study, we considered effective suppression a viral load <50 copies/mL, and the INDOOR participants were suppressed for >3 years in both groups. Moreover, we followed participants until week 24 after switching to DTG, while RASTA follow-up was until week 48, with no significant decreases in the HIV reservoir at week 24. Beyond cohort differences, the discrepancy between the 2 studies may also be explained by differences between RAL and DTG pharmacokinetics and tissue penetration. Previous studies estimated concentrations of DTG in rectal tissue to be 17% of those in plasma [23], as compared with RAL, where concentrations in the gastrointestinal tract can be >600 times higher than in plasma [24]. Moreover, Weber et al recently showed that individuals receiving RAL or DTG have comparable HIV DNA in GALT [20]. Nevertheless, the lack of effect of DTG on episomal markers of viral replication in our switch and in a recent intensification study [21] contrasts with previous reports on intensification of RAL [13, 14]. It is debatable whether transiently produced 2-LTR circles are the result of ongoing cycles of viral replication or merely persistent viral release from productively infected cells. However, mathematical modeling of the data suggests that the transient dynamics of 2-LTR after intensification with integrase inhibitors would be more compatible with the former hypothesis [25–27]. Consequently, we might suspect a differential effect of DTG on the dynamics of 2-LTR circles, although we verified the absence of such differences in vitro (Supplementary Figure 4). Alternatively, lower levels of residual viral replication in recent studies might be explained by the inclusion of individuals with a more favorable profile, including a higher CD4+ T-cell nadir, probably due to earlier initiation of treatment. Therefore, further investigation comparing switching to RAL and to DTG in samples from peripheral blood and different tissues and measurement of drug concentrations and residual viral replication in tissues would be needed to better understand differences in the pharmacological effect of RAL and DTG switching interventions in vivo.
Nevertheless, as previously reported, our results revealed a larger HIV reservoir and greater activation in CD4+ T cells from ileum biopsies than in those from peripheral blood [28]. We also found a strong correlation between markers of persistence of HIV [29].
Furthermore, the lack of change in cell activation and plasma inflammation markers is consistent with the lack of effect on HIV reservoir size in both the ileum and blood and with the absence of change in low-level viral replication in blood. Thus, our data suggest that switching to a DTG-based regimen from a PI/r-based regimen does not reduce immune activation or inflammation markers in blood and does not improve standard cART beyond avoiding long-term metabolic disorders and managing drug–drug interactions [4]. In line with previous studies, switching to DTG in the INDOOR study did not jeopardize virological efficacy [30–32], but improved triglycerides and the remaining proatherogenic lipid fractions [30–32].
The strengths of this study lie in its prospective design with randomization, switch-control (1:1), predefined frequent analyses of 2-LTR changes within the first 4 weeks, and the analysis of ileum biopsies. Our study is also subject to limitations. First, it is difficult to exclude a small effect on 2-LTR circles owing to the number of undetectable determinations. Second, we observed baseline differences between groups in the infection route and a trend toward higher residual plasma viremia in the switch group. However, we corrected for baseline differences in the statistical analysis and did not observe any association between these variables and the overall results. Finally, sampling limitations prevented us from measuring 2-LTR in the ileum biopsies. Consequently, an effect on low-level viral replication in lymphoid tissue cannot be excluded.
In conclusion, we found that switching from PI/r- to DTG-based therapy maintains viral suppression, improves lipid profile, and may reduce HIV residual plasma viremia. However, we observed no evidence of an increase in the level of 2-LTR circles in peripheral blood CD4+ T cells or a decrease in total HIV reservoir size in either ileal or peripheral CD4+ T cells after 24 weeks of treatment in HIV-infected, ART-suppressed individuals. Furthermore, switching to DTG did not affect T-cell activation or inflammation markers.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. M.-L., M. J., J. N., and S. R. acquired, analyzed, and interpreted the data and wrote the manuscript. M. C. P., C. G., and M. S. acquired data and revised the manuscript. V. U. performed the statistical analysis and revised the manuscript. J. N. designed and performed the clinical study, analyzed and interpreted the data, and wrote the manuscript. A. T., B. P. R., M. C., J. B., and L. V. designed and performed the clinical study and revised the manuscript. J. M.-P. participated in study design and data interpretation and wrote the manuscript.
Acknowledgments. The authors thank the Institute Germans Trias i Pujol Cytometry Core Facility and staff (M. A. Fernandez and G. Requena) and the Microbiology Service of Hospital Universitari Germans Trias i Pujol (L. Matas and A. Hernández-Rodríguez) for their contributions to this publication.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or drafting of the manuscript.
Financial support. This study was partially funded by an unrestricted grant from ViiV Healthcare. IrsiCaixa was supported by the Centres de Recerca de Catalunya program from Generalitat de Catalunya. M. S. was supported by a postdoctoral training scholarship (Juan de la Cierva) from the Ministerio de Economía y Competitividad de España (FPDI-2013-17134). S. M.-L. holds a fellowship contract from Agencia de Gestión de Ayudas Universitarias y de Investigación (2013FI_B00275). C. G. was partially supported by a predoctoral fellowship contract from the Ministerio de Educación, Cultura y Deporte de España (FPU15/03698). L. V. is funded by the Fonds Wetenschappelijk Onderzoek/International (FWO, 1.8.020.09.N.00). S. R. received a strategic basic research grant from the Fonds Wetenschappelijk Onderzoek/International (FWO, 1S32916N). This study was partially supported by the SPANISH AIDS Research Network RD16/0025/0007 project as part of the Plan Nacional R+D+I and by Instituto de Salud Carlos III-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional.
Potential conflicts of interest. J. N. has received personal fees from AbbVie, Merck Sharp & Dohme, ViiV Healthcare, Gilead, and Janssen and nonfinancial support from AbbVie, Gilead, and Janssen. J. B. has received personal fees from AlbaJuna Therapeutics. J. M.-P. has received grants from ViiV Healthcare, Merck, Gilead, and AbiVax, and personal fees from ViiV Healthcare, Merck, Gilead, Janssen, and AbiVax. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johnson VA. Combination therapy: more effective control of HIV type 1? AIDS Res Hum Retroviruses 1994; 10:907–12. [DOI] [PubMed] [Google Scholar]
- 2. Palella FJ Jr, Armon C, Buchacz K, et al. . HOPS Investigators Factors associated with mortality among persistently viraemic triple-antiretroviral-class-experienced patients receiving antiretroviral therapy in the HIV Outpatient Study (HOPS). J Antimicrob Chemother 2014; 69:2826–34. [DOI] [PubMed] [Google Scholar]
- 3. Lang S, Mary-Krause M, Cotte L, et al. . Clinical Epidemiology Group of the French Hospital Database on HIV Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010; 170:1228–38. [DOI] [PubMed] [Google Scholar]
- 4. Potard V, Simon A, Lacombe J-M, Parienti J-J, Costagliola D; French Hospital Database on HIV (FHDH-ANRS CO4) Switching to raltegravir from a virologically effective boosted protease inhibitor regimen: a comparative effectiveness analysis from the French Hospital Database on HIV (FHDH-ANRS CO4). Clin Infect Dis 2016; 63:1254–61. [DOI] [PubMed] [Google Scholar]
- 5. Negredo E, Estrada V, Domingo P, et al. . Switching from a ritonavir-boosted PI to dolutegravir as an alternative strategy in virologically suppressed HIV-infected individuals. J Antimicrob Chemother 2016; 72:844–9. [DOI] [PubMed] [Google Scholar]
- 6. Rossetti B, Meini G, Bianco C, et al. . Total cellular HIV-1 DNA decreases after switching to raltegravir-based regimens in patients with suppressed HIV-1 RNA. J Clin Virol 2017; 91:18–24. [DOI] [PubMed] [Google Scholar]
- 7. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017; 14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1:1284–90. [DOI] [PubMed] [Google Scholar]
- 9. Simonetti FR, Sobolewski MD, Fyne E, et al. . Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci 2016; 113:1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rothenberger MK, Keele BF, Wietgrefe SW, et al. . Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA 2015; 112:E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukazawa Y, Lum R, Okoye AA, et al. . B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. . Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buzón MJ, Massanella M, Llibre JM, et al. . HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 2010; 16:460–5. [DOI] [PubMed] [Google Scholar]
- 14. Hatano H, Strain MC, Scherzer R, et al. . Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis 2013; 208:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delaugerre C, Charreau I, Braun J, et al. . ANRS 138 Study Group Time course of total HIV-1 DNA and 2-long-terminal repeat circles in patients with controlled plasma viremia switching to a raltegravir-containing regimen. AIDS 2010; 24:2391–5. [DOI] [PubMed] [Google Scholar]
- 16. Lam YM, McBride KL, Amin J, et al. . Switching virally suppressed, treatment-experienced patients to a raltegravir-containing regimen does not alter levels of HIV-1 DNA. PLoS One 2012; 7:e31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chun TW, Nickle DC, Justement JS, et al. . Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis 2008; 197:714–20. [DOI] [PubMed] [Google Scholar]
- 18. Mehraj V, Ghali P, Ramendra R, et al. . The evaluation of risk-benefit ratio for gut tissue sampling in HIV cure research. J Virus Erad 2017; 3:212–7. [PMC free article] [PubMed] [Google Scholar]
- 19. Cahn P, Pozniak AL, Mingrone H, et al. . Extended SAILING Study Team Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 20. Weber MD, Andrews E, Prince HA, et al. . Virological and immunological responses to raltegravir and dolutegravir in the gut-associated lymphoid tissue of HIV-infected men and women. Antivir Ther 2018; 23:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen TA, McMahon JH, Chang JJ, et al. . The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: a randomised, placebo-controlled, double-blind trial. Lancet HIV 2018; 5:e221–30. [DOI] [PubMed] [Google Scholar]
- 22. Besson GJ, Lalama CM, Bosch RJ, et al. . HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greener BN, Patterson KB, Prince HM, et al. . Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr 2013; 64:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patterson KB, Prince HA, Stevens T, et al. . Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS 2013; 27:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS 2016; 11:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez-Picado J, Zurakowski R, Buzón MJ, Stevenson M. Episomal HIV-1 DNA and its relationship to other markers of HIV-1 persistence. Retrovirology 2018; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo R, Cardozo EF, Piovoso MJ, et al. . Modelling HIV-1 2-LTR dynamics following raltegravir intensification. J R Soc Interface 2013; 10:20130186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yukl SA, Gianella S, Sinclair E, et al. . Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 2010; 202:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eriksson S, Graf EH, Dahl V, et al. . Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gatell JM, Assoumou L, Moyle G, et al. . NEAT022 Study Group Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31:2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trottier B, Lake JE, Logue K, et al. . Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther 2017; 22:295–305. [DOI] [PubMed] [Google Scholar]
- 32. Gatell JM, Assoumou L, Moyle G, et al. . Immediate versus deferred switching from a boosted protease inhibitor–based regimen to a dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age ≥50 years: final 96-week results of the NEAT022 study [manuscript published online ahead of print 14 June 2018]. Clin Infect Dis 2018. doi:10.1093/cid/ciy505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.