Abstract
Background
GMZ2 is a recombinant malaria vaccine inducing immune responses against Plasmodium falciparum (Pf) merozoite surface protein-3 and glutamate-rich protein. We used standardized controlled human malaria infection (CHMI) to assess the efficacy of this asexual blood-stage vaccine.
Methods
We vaccinated 50 healthy, adult volunteers with lifelong exposure to Pf 3 times, at 4-week intervals, with 30 or 100 µg GMZ2 formulated in CAF01, a liposome-based adjuvant; 100 µg GMZ2, formulated in Alhydrogel; or a control vaccine (Verorab). Approximately 13 weeks after the last vaccination, 35/50 volunteers underwent CHMI by direct venous inoculation of 3200 Pf sporozoites (Sanaria® PfSPZ Challenge).
Results
Adverse events were similarly distributed between GMZ2 and control vaccinees. Baseline-corrected anti-GMZ2 antibody concentrations 4 weeks after the last vaccination were higher in all 3 GMZ2-vaccinated arms, compared to the control group. All GMZ2 formulations induced similar antibody levels. CHMI resulted in 29/34 (85%) volunteers with Pf parasitemia and 15/34 (44%) with malaria (parasitemia and symptoms). The proportion of participants with malaria (2/5 control, 6/10 GMZ2-Alhydrogel, 2/8 30 µg GMZ2-CAF01, and 5/11 100 µg GMZ2-CAF01) and the time it took them to develop malaria were similar in all groups. Baseline, vaccine-specific antibody concentrations were associated with protection against malaria.
Conclusions
GMZ2 is well tolerated and immunogenic in lifelong–Pf-exposed adults from Gabon, with similar antibody responses regardless of formulation. CHMI showed no protective effect of prior vaccination with GMZ2, although baseline, vaccine-specific antibody concentrations were associated with protection. CHMI with the PfSPZ Challenge is a potent new tool to validate asexual, blood-stage malaria vaccines in Africa.
Clinical Trials Registration
Pan-African Clinical Trials: PACTR201503001038304
Keywords: clinical trial, Plasmodium falciparum, malaria vaccine, controlled human malaria infection
GMZ2, formulated with Alhydrogel or CAF01, was well tolerated and immunogenic in healthy adults from Gabon. Controlled human malaria infection to assess naturally acquired and vaccine-induced immunities showed no protection by vaccination, but an association with preexisting vaccine-specific antibodies.
Malaria vaccines have the potential to transform malaria control. RTS,S (Mosquirix) is the first vaccine to complete clinical development [1], but large-scale implementation studies have been requested before market authorization [2]. Immunization with Plasmodium falciparum sporozoites (PfSPZ), either attenuated by irradiation (PfSPZ Vaccine) or chemoprophylaxis (PfSPZ-CVac), is a promising alternative, with high-level protection in controlled human malaria infection (CHMI) [3–7] and under natural exposure [8]. Together with a viral-vectored vaccine candidate that showed some protection in 1 of 2 trials [9, 10], these all represent preerythrocytic vaccine candidates. An immunity to the preerythrocytic stages of malaria parasites does not prevent asexual blood stage replication [11]: that is, when a fraction of parasites escapes the immune response and invades erythrocytes. Therefore, an immunity that limits the blood-stage parasite multiplication would ideally complement preerythrocytic vaccines. Blood-stage immunity can be naturally acquired and prevents complications in lifelong–malaria-exposed adults. Vaccine candidates based on apical membrane antigen-1 (AMA-1) [12], merozoite surface protein-2 (MSP2) [13], merozoite surface protein-3 (MSP3) [14], and serine repeat antigen-5 (SERA5) [15] showed some efficacy—at least strain-specific efficacy—in exploratory post hoc analyses of clinical trials performed under natural malaria exposure. GMZ2 was the first asexual blood-stage candidate that showed significant, although modest, efficacy in a Phase II trial [16].
GMZ2 is a fusion protein of fragments of P. falciparum glutamate-rich protein (GLURP) and MSP3. The antigens were selected based on epidemiological and in vitro studies. Better efficacy of GMZ2 in children with high post-immunization titers [16] motivated the search for more immunogenic formulations.
CAF01 is a liposome-based adjuvant with potent, immune-enhancing properties on humoral and cellular immune responses [17], and has been tested successfully with tuberculosis [18] and human immunodeficiency virus [19, 20] protein and peptide vaccines.
Here, we report the first human trial of GMZ2-CAF01 in healthy, adult volunteers, using standardized CHMI with the PfSPZ Challenge [21] to assess efficacy. It is the first use of standardized CHMI with the PfSPZ Challenge to detect an efficacy signal in the development of an asexual blood-stage candidate.
METHODS
Study Design
The study was a randomized, controlled, double-blind, single-center, Phase I trial, conducted in Lambaréné, Gabon [22]. Study participants were healthy, adult volunteers with a history of at least 10 years residence in areas with high malaria endemicity. Inclusion and exclusion criteria were chosen to minimize risks for the volunteers (Supplementary Material 1). In particular, immunosuppression, inflammation, chronic disease, cardiovascular disease, and neurological as well psychiatric risk factors were assessed. Participants were allocated to 1 of 5 groups: (1) Group A (control vaccine; n = 8), (2) Group B (100 µg GMZ2-Alhydrogel; n = 12), (3) Group C (30 µg GMZ2-CAF01; n = 8), (4) Group D (100 µg GMZ2-CAF01; n = 12), and (5) Group E (100 µg GMZ2-CAF01, without subsequent CHMI; n = 10). All injections were administered intramuscularly in the deltoid muscle on study days (D) 0, 28, and 56, in alternating sides. After completion of the immunization, volunteers of Groups A–D underwent CHMI by direct venous inoculation (DVI) of 3200 aseptic, purified, cryopreserved P. falciparum sporozoites (Sanaria® PfSPZ Challenge), strain NF54, to assess vaccine efficacy. Group E volunteers were followed for 6 months post–immunization without CHMI.
Vaccines
GMZ2 is a recombinant fusion protein of fragments of GLURP (GLURP27-500) and MSP3 (MSP3212-380), produced in Lactococcus lactis. The vaccine was reconstituted with either Alhydrogel or CAF01 adjuvant. Alhydrogel (aluminum hydroxide suspension) was used at a concentration of 0.85 mg aluminum (Al3+) per dose. CAF01 is a liposome-based adjuvant consisting of the immune-stimulating, synthetic glycolipid trehalose-dibehenate, incorporated into cationic di-methyldioctadecylammonium bromide liposomes [17]. Per dose, 625 µg di-methyldioctadecylammonium bromide and 125 µg trehalose-dibehenate were used. Volunteers allocated to the control group (Group A) were vaccinated with a rabies vaccine (Verorab, Sanofi Pasteur).
Safety
Vaccinations were done at a clinical trial facility. Volunteers remained at the clinic for at least 30 minutes and had scheduled visits at 1, 7, and 14 days following each vaccination, as well as 4 weeks after the last vaccination (D84). In addition, participants were visited at home 2, 4, and 6 days following each vaccination, by field workers. During visits, local and systemic symptoms were solicited and open questions were asked to assess vaccine tolerability. Adverse event (AE) severity was assessed using the US Food and Drug Administration grading scale [23], and causality was attributed to each AE by a physician. Biochemistry and hematologic laboratory parameters were measured before and at 7 and 14 days following each vaccination, as well as on D84. During CHMI, volunteers remained in the vicinity of the clinical trial facility, which is an area with low malaria transmission. Group E was recruited to detect potential, late AEs following immunization with 100 µg GMZ2-CAF01 and not associated with CHMI.
Vaccine Efficacy Assessment
Out of the 50 participants enrolled in the study, 40 (Groups A–D) were allocated to receive CHMI to assess vaccine efficacy. Before CHMI, all participants of Groups A–D were treated with 300 mg of clindamycin every 12 hours for 5 days, to clear P. falciparum infections. CHMI was done approximately 13 weeks after the last vaccine injection and at least 48 hours after the last clindamycin dose, by DVI, of 3200 PfSPZ of PfSPZ Challenge, as described [21]. The PfSPZ Challenge was produced from P. falciparum strain NF54 by Sanaria Inc. and shipped in liquid nitrogen vapor phase to Gabon. Following DVI, participants were observed for at least 30 minutes and were examined the next day. All subjects were seen daily from 6 to 35 days following DVI. Thick blood smears (TBS) and 1 ml blood samples were collected daily to assess parasitemia by quantitative, real-time polymerase chain reaction (qPCR). The primary efficacy endpoint was malaria, defined as a positive TBS and at least 1 malaria-related symptom. The first-line treatment of P. falciparum malaria was artemether-lumefantrine. A cure was defined as 2 consecutive, negative TBSs, 1 day apart. All participants who did not develop malaria during follow-up received a full treatment course with artemether-lumefantrine at 35 days following DVI. Volunteers were followed for at least 8 weeks post-CHMI.
Immunogenicity
Immunoglobulin G (IgG) against GMZ2, as well as the GLURP27-500 and MSP3212-380, was measured by an enzyme-linked immunosorbent assay (ELISA) in plasma collected at baseline and on D84. GLURP and MSP3 fragments contained in GMZ2 were expressed in Escherichia coli. IgG concentrations were derived from fitting the optical density values of serial plasma dilutions. ELISA and data analysis were done as previously described [24–26], with minor modifications: plasma was diluted in phosphate-buffered saline, 3% non-fat milk, and 0.1% Tween 20, and a peroxidase-conjugated, goat, anti-human IgG (Invitrogen) was used at a 1:65 000 dilution. Pooled sera from lifelong–malaria- exposed adults were used as a positive control, and a pool of malaria-naive European adults served as a negative control.
Molecular Analyses
All molecular assays were performed blinded, a posteriori. Nucleic acids were extracted from 0.5 mL blood. Extraction was automated on the QIAsymphony sample prep module (SP) using QIAsymphony diagnostic sample preparation (DSP) DNA kit (Qiagen), followed by qPCR, as described previously [5], with a lower limit of detection of 5 parasites/mL. Since the study took place in a malaria-endemic setting, naturally acquired infections can occur. We used a 3-step approach to discriminate between strain NF54 and naturally acquired infections: (1) species differentiation by qPCR, (2) genotyping of the marker of chloroquine resistance, the CRT (PF3D7_0709000) gene (strain NF54 is wild-type, and >90% of circulating strains in the area are chloroquine resistant [27]), followed by (3) MSP1 (PF3D7_0930300) genotyping (Supplementary Material 2). The qPCR assay for Plasmodium spp. identification was performed as previously described [28]. The CRT genotyping was conducted using a highly sensitive triplex qPCR assay, with hydrolysis probes specific to the wild-type genotype and 2 common, resistance-associated genotypes at codons 72–76. The result was further confirmed by MSP1 genotyping, analyzed by a QIAxcel capillary electrophoresis system (Qiagen). The single-nucleotide polymorphisms responsible for sickle cell disease (rs334) in the human β-globin gene were determined by nested-PCR amplification of the exon 1, followed by bidirectional sequencing of the amplicon.
Statistics
The sample size was calculated to detect frequent safety and tolerability signals in volunteers vaccinated with 100 µg GMZ2-CAF01 (72% power to detect 1 occurrence of an event). With 12 vaccinees and 8 control-vaccine recipients, a change from 25% to 92% protection against malaria following CHMI could be detected with 90% power. The number of participants with malaria, defined as parasitemia and at least 1 symptom, was used to calculate vaccine efficacy. For safety analyses, all volunteers who received at least 1 vaccination were included in the analysis; for efficacy, all volunteers who completed CHMI were included in the analysis. Cox proportional hazard models were used for exploratory analyses of the influence of co-variates on vaccine efficacy. All immunological assays were considered exploratory. The level of significance was set at a 2-tailed P value <5%. Further details are given in the Clinical Trial Protocol (Supplementary Material 1).
Ethics
The study was approved by the National Ethics Committee of Gabon and authorized by the Gabonese Ministry of Health. Subject safety was monitored by a local safety monitor and a scientific monitoring committee. The trial was performed according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines and the Declaration of Helsinki, and is registered with the Pan-African Clinical Trials Registry (trial number PACTR201503001038304).
RESULTS
Study Flow and Characteristics of the Study Population
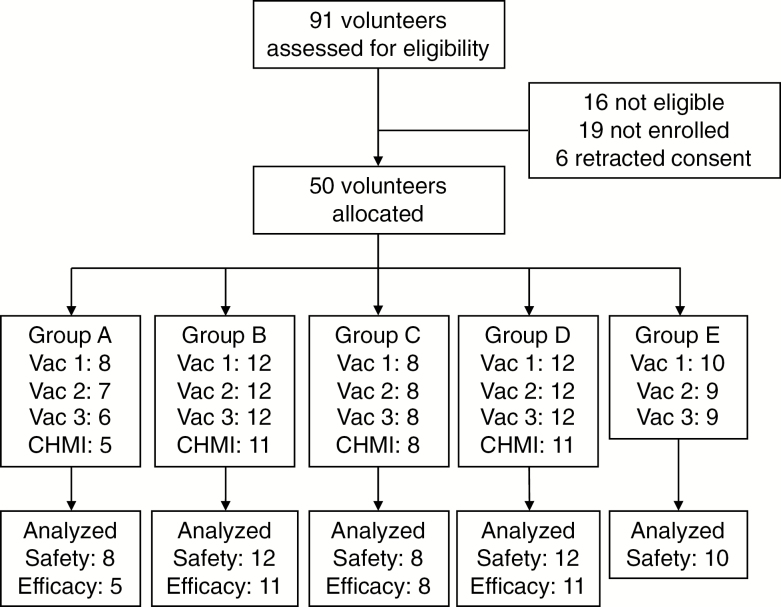
We enrolled 50 healthy, male subjects in the trial. They were immunized between 20 April 2015 and 18 June 2015 (Figure 1). Baseline demographic characteristics were similar between groups (Table 1). All 50 volunteers received the first vaccination, 48 received the second, and 47 received the full course of immunization. The time windows between vaccinations were 28 ± 1 days for those who completed the immunization.
Figure 1.
Study flow diagram. Abbreviation: CHMI, controlled human malaria infection; Vac, vaccination.
Table 1.
Baseline Demographics
| Group A | Group B | Group C | Group D | Group E | |
|---|---|---|---|---|---|
| Vaccine, n | 8 | 12 | 8 | 12 | 10 |
| CHMI, n | 5 | 11 | 8 | 11 | NA |
| Agea, in years | 23.8 (21.8; 35.5) |
24.4 (19.2; 32.2) |
22.5 (20.3; 35.1) |
21.8 (18.1; 34.0) |
21.5 (18.2; 37.4) |
| BMIa, in kg/m2 | 23.3 (16.7; 25.3) |
22.1 (18.8; 29.7) |
21.6 (19.1; 23.1) |
21.4 (18.8; 24.1) |
21.6 (18.8; 25.6) |
| HbAS-to-HbAA ratio | 0.25 | 0.1 | 0.33 | 0.1 | NA |
Abbreviations: BMI, body mass index; CHMI, controlled human malaria infection; HbAA, hemoglobin AA; HbAS, hemoglobin AS; NA, not available.
aMedian (minimum; maximum)
DVI was performed between 18 and 21 September 2015 in a subgroup of 35 volunteers. All 3 volunteers (2 allocated to Group A and 1 to Group E) who did not complete the vaccination schedule relocated out of the study area. Finally, 5 fewer volunteers than originally planned (3 allocated to Group A, 1 to Group B, and 1 to Group D) underwent CHMI. DVI was done 93–97 days following the last vaccination. There was 1 volunteer (Group B) who relocated to another country during the CHMI period. Therefore, his follow-up was prematurely stopped and he received presumptive antimalarial treatment with artemether-lumefantrine beginning at 14 days post-DVI. The volunteer was contacted regularly until the end of the trial and reported no health problems.
Vaccine and CHMI Safety
No serious adverse event occurred during the study, and no participant was withdrawn for safety reasons. There were 496 AEs recorded: 454 were Grade 1, 42 were Grade 2, and none were Grade 3. Every volunteer had at least 1 AE (range 1–29). There were 170 AEs that were considered to be at least possibly related to the study (151 were Grade 1 and 19 were Grade 2).
From the day of first vaccination to D84, 221 AEs occurred (196 were Grade 1 and 25 were Grade 2), of which 130 were judged to be at least possibly related to the study (115 were Grade 1 and 15 were Grade 2). There were 2 volunteers who had no AE during the immunization period (1 in Group A and 1 in Group B). At least possibly related Grade 2 AEs during immunization occurred in 13 volunteers, consisting of 14 instances of injection site pain and 1 of myalgia; all of these were in GMZ2-immunized volunteers (4 in Group B, 5 in Group C, 3 in Group D, and 3 in Group E). The AE pattern was similar between the groups (Table 2).
Table 2.
Adverse Event (AE) Pattern, of At Least Possibly Related AE During Vaccination
| MedDRA Preferred Term | Severity | Group A (n = 8) |
Group B (n = 12) |
Group C (n = 8) |
Group D (n = 12) |
Group E (n = 10) |
|---|---|---|---|---|---|---|
| Asthenia | 1 | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 1 | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Diarrhea | 1 | 2 (2) | 3 (2) | 1 (1) | 0 (0) | 1 (1) |
| Fatigue | 1 | 2 (2) | 3 (2) | 2 (2) | 2 (2) | 1 (1) |
| Headache | 1 | 1 (1) | 2 (2) | 3 (3) | 2 (2) | 2 (1) |
| Injection site pain | 1 | 8 (5) | 15 (9) | 17 (8) | 18 (10) | 13 (9) |
| Injection site pain | 2 | 0 (0) | 4 (4) | 5 (4) | 2 (2) | 3 (3) |
| Injection site pruritus | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Injection site swelling | 1 | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Myalgia | 2 | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Nausea | 1 | 0 (0) | 4 (4) | 1 (1) | 2 (2) | 1 (1) |
| Pruritus | 1 | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
| Pyrexia | 1 | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Pyuria | 1 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Data were coded according to MedDRA. Data are shown as number of adverse events (number of volunteers).
Abbreviation: MedDRA, the Medical Dictionary for Regulatory Activities.
CHMI was generally well tolerated. Between the day of DVI and the end of follow-up, 174 AEs occurred (161 were Grade 1 and 13 were Grade 2). There were 3 volunteers who experienced no AE (2 in Group C and 1 in Group D). There were 35 AEs that were considered to be at least possibly related to the study (31 were Grade 1 and 4 were Grade 2). As expected, parasitemic and protected volunteers had different AE patterns (Table 3).
Table 3.
Adverse Event (AE) Pattern, of At Least Possibly Related AE During Controlled Human Malaria Infection
| MedDRA Preferred Term | Severity | Controla (n = 20) |
Malaria (n = 15) |
|---|---|---|---|
| Arthralgia | 1 | 0 (0) | 3 (3) |
| Chills | 1 | 0 (0) | 1 (1) |
| Diarrhea | 1 | 1 (1) | 3 (2) |
| Fatigue | 1 | 1 (1) | 4 (4) |
| Fatigue | 2 | 0 (0) | 1 (1) |
| Feeling cold | 1 | 0 (0) | 2 (2) |
| Headache | 1 | 0 (0) | 7 (6) |
| Headache | 2 | 0 (0) | 1 (1) |
| Injection site pain | 1 | 1 (1) | 0 (0) |
| Myalgia | 1 | 0 (0) | 1 (1) |
| Myalgia | 2 | 0 (0) | 1 (1) |
| Nausea | 1 | 0 (0) | 5 (5) |
| Pyrexia | 1 | 0 (0) | 2 (2) |
| Pyrexia | 2 | 0 (0) | 1 (1) |
Data were coded according to MedDRA. Data are shown as number of adverse events (number of volunteers).
Abbreviation: MedDRA, the Medical Dictionary for Regulatory Activities.
aNo fully protected volunteer experienced a related AE.
In Group E, no possibly related AE occurred later than 28 days following the last immunization.
Vaccine Efficacy
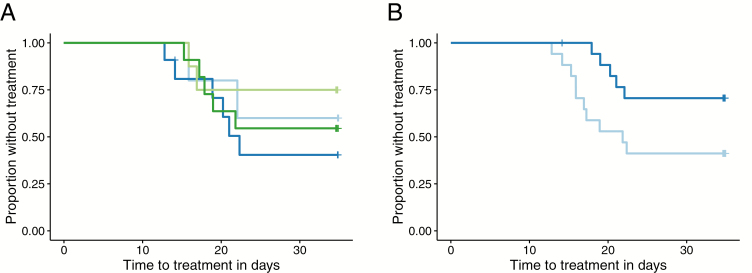
Of the 35 subjects, 34 completed the follow-up until Day 35. Of these, 15 volunteers were treated because of malaria: 2/5 (40%) in Group A, 6/10 (60%) in Group B, 2/8 (25%) in Group C, and 5/11 (45%) in Group D. The time to malaria was similar between the interventions (log rank test P = .6; Figure 2A). Of the 35 volunteers, 5 (14%) had sickle cell trait. Including sickle cell trait in the model did not change the interpretation of the results.
Figure 2.
Kaplan-Meier plot. A, The time to malaria treatment in control is shown in light blue, GMZ2-Alhydrogel in dark blue, 30 µg GMZ2-CAF01 in light green, and 100 µg GMZ2-CAF01 in dark green for vaccinated volunteers. B, The time to malaria treatment with anti-GMZ2 immunoglobin G concentration at baseline above the median is shown in dark blue, and below the median is in light blue.
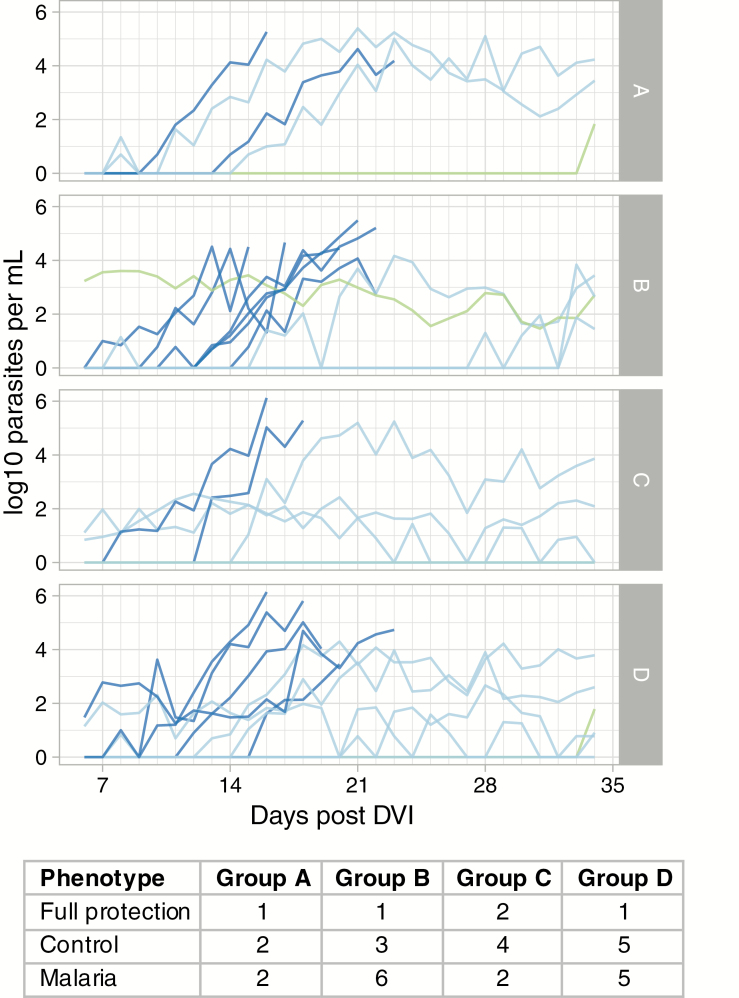
The time to the first positive TBS (log rank test P = .6), parasite growth rates, and patterns were similar between the intervention groups (Figure 3), and immunization with GMZ2-CAF01 did not explain the differences in the pattern distributions of the 3 CHMI phenotypes in adults from malaria-endemic areas [29] (Fisher’s Exact Test; P = .86): (1) full protection (no parasitemia, no symptoms), (2) control (low oscillating parasitemia, no symptoms), and (3) malaria (monotone increase of parasitemia, symptoms). There were 2 volunteers with delayed NF54 parasitemia (≥3 weeks following DVI). There was 1 volunteer (Group B) who had continuous, submicroscopic, non-NF54 parasitemia without symptoms over the whole CHMI period. There were 4 volunteers who had mixed infections: 1 with P. malariae followed by NF54 and 3 with NF54 and a naturally acquired strain. The volunteers with mixed P. falciparum remained asymptomatic and TBS negative (control phenotype). The other volunteer showed decreasing P. malariae parasitemia, and subsequently developed NF54 parasitemia and symptoms.
Figure 3.
Parasitemia over time. Parasitemia was measured by quantitative, polymerase chain reaction. Data until treatment with an antimalarial (either due to symptoms or at the end of the 35-day follow-up period) are shown. Volunteers who developed malaria are shown in dark blue, those with controlled parasitemia at low levels without symptoms are in light blue, and those who were fully protected are in green. There was 1 volunteer of Group B who had non-NF54 submicroscopic parasitemia over the complete CHMI period and was considered protected (green). Abbreviations: CHMI, controlled human malaria infection; DVI, direct venous inoculation.
Immunogenicity
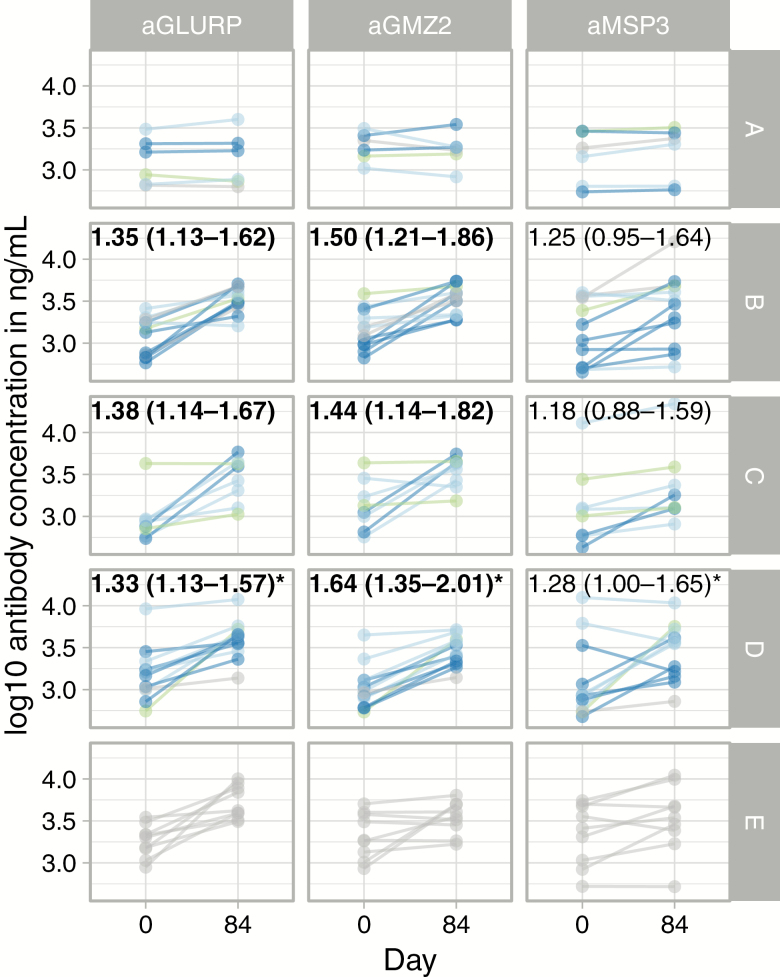
GMZ2-, GLURP-, and MSP3-specific IgG were detected at baseline in all participants, with similar levels between groups (Figure 4). By 4 weeks after the last vaccination, the plasma concentrations of anti-GMZ2 and anti-GLURP IgG were significantly increased from baseline in those subjects vaccinated with 30 μg GMZ2-CAF01, 100 µg of GMZ2-CAF01, or 100 µg GMZ2-Alhydrogel. The MSP3-specific IgG changed only little following immunization; the strongest increase was observed in those volunteers vaccinated with 100 µg of GMZ2-CAF01 (Figure 4). Vaccination with the control vaccine did not increase IgG reactivity against GMZ2, GLURP, or MSP3. The variance of vaccine-specific IgGs within GMZ2-immunized groups decreased over time (P = .026), since the effect of boosting was more pronounced in those with a low baseline value (Figure 4).
Figure 4.
Vaccine-specific IgG over time. The increase of IgG from baseline to 4 weeks following the last vaccination (Day 84) is shown. The numbers indicate baseline-corrected fold-increase over control vaccine on Day 84 (95% confidence interval). Note the large range of IgG at baseline. Volunteers who developed malaria are shown in dark blue, those with controlled parasitemia at low levels without symptoms are in light blue, those who were fully protected are in green, and those who did not undergo CHMI are in gray. Abbreviations: aGLURP, anti-glutamate rich protein IgG; aGMZ2, anti-GMZ2 IgG; aMSP3, anti-merozoite protein-3 IgG; CHMI, controlled human malaria infection; IgG, immunoglobin G. *Indicates pooled Group D and E estimates. Bolded values: statistically significant change.
Including participants’ baseline, vaccine-specific IgG (as a measure of the previous exposure to malaria) and treatment allocation in a Cox model showed that the time to their development of malaria depended on their baseline, GMZ2-specific (hazard ratio 0.10, 95% confidence interval 0.01–0.85) and MSP3-specific IgG concentrations (hazard ratio 0.13, 95% confidence interval 0.02–0.82), irrespective of treatment allocation. Splitting the IgG ELISA data on the median revealed the same trend (Figure 2B). Post-immunization, only MSP3-specific IgG were associated with a longer time to the development of malaria.
DISCUSSION
GMZ2 is modestly efficacious against naturally acquired malaria [16]. It has an excellent safety and tolerability profile, and its efficacy increases with higher vaccine-specific antibody titers. Hence, increasing immunogenicity may result in improved efficacy. A straightforward approach is to use a more potent adjuvant. CAF01 is a liposome-based adjuvant, developed to induce potent and long-lived humoral and cellular immune responses [17]. In preclinical studies, the CAF01-based formulation of GMZ2 was superior, compared to Alhydrogel. Several clinical trials with tuberculosis and human immunodeficiency virus vaccines corroborated its immune-enhancing properties, tolerability, and safety in humans [18–20]. We confirmed that CAF01 is well tolerated and safe.
To assess vaccine efficacy, a subgroup of volunteers underwent CHMI. It is the first time that standardized CHMI with the PfSPZ Challenge was used to measure the efficacy of a blood-stage vaccine candidate. The development of GMZ2-Alhydrogel took 1 decade and almost 2000 study participants from the first trial in a human until the completion of a Phase II trial [16, 25, 26, 30]. With CHMI, the development process becomes much more effective. In malaria-naive adults, only 3 replication cycles (of 48 hours each) can be observed with acceptable tolerability [31]. To assess activity against asexual, blood-stage parasites, CHMI over longer time periods and with higher parasite thresholds would be advantageous. The number of replication cycles can be increased by (1) inoculation of asexual, blood-stage parasites [32] or (2) CHMI in malaria-exposed volunteers, who tolerate higher parasitemias and can be followed for longer time periods [29]. Since small, asexual, blood-stage inocula may result in biased immune responses and do not allow higher parasitemias [33], we used CHMI in malaria-exposed, healthy adults to assess vaccine efficacy, and observed up to 14 asexual replication cycles.
Vaccination with GMZ2 did not result in protection, despite a robust, vaccine-specific immune response. Unexpectedly, immunogenicity of the GMZ2-CAF01 formulation was not superior to GMZ2-Alhydrogel. Since we only measured antibody concentration by ELISA, it is possible that other (ie, cellular) responses are differentially affected. Since the development of functional antibodies to some plasmodial antigens is different from the prototypic affinity maturation [34] and involves homotypic interactions [35] and individual response patterns [5], further analyses will improve our understanding of the critical parameters of a highly efficacious malaria vaccine. Nevertheless, it is the first head-to-head comparison of CAF01 with another adjuvant, and our immunological aim—increasing vaccine-induced IgG—could not be achieved. Further studies on cellular responses and antibody functions, specificity, and avidity are ongoing.
Interestingly, baseline, vaccine-specific IgG levels predicted the time to the development of malaria. Possible interpretations of this finding are: (1) vaccine-specific IgG is correlated with previous exposure and protective immune mediators, but is not causally linked to protection, (2) vaccine-induced antibodies are not functional (eg, have a different affinity), whereas naturally acquired GMZ2-specific antibodies are anti-parasitic, or (3) the response was short-lived, and vaccine-specific IgG levels were not maintained until CHMI. In vitro, GMZ2-induced antibodies are functional [36], and an animal model showed partial protection [37]. Hence, the first hypothesis is more likely than the second, and suggests that CHMI in lifelong–malaria-exposed adults is an efficient way to down-select vaccine candidates derived from association studies.
In conclusion, we showed that CHMI in lifelong–malaria-exposed volunteers can be used to assess asexual, blood-stage immunity to develop new interventions. GMZ2 was well tolerated, safe, and immunogenic, but did not complement naturally acquired immunity to increase protection following CHMI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the volunteers and the teams at Centre de Recherches Médicales de Lambaréné, Statens Serum Institut, Sanaria, and the University of Tübingen: in particular, Annette Knoblich, Cigdem Uyanik, Eric R. James, Peter F. Billingsley, Markus Gmeiner, and Patricia Granados. They also thank Ingrid Kromann and Lars Vibe Andreasen for providing CAF01 and developing its formulation with GMZ2.
Financial support. This work was supported by the German Center for Infection Research (grant numbers TTU 03.801, TTU 03.702, and TTU 03.703) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers 5R44AI058375 and 5R44AI055229).
Potential conflicts of interest. M. E. has received grants from GlaxoSmithKline, Takeda, International Vaccine Institute, Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung (BMBF), and European and Developing Countries Clinical Trials Partnership (EDCTP), outside the submitted work. P. G. K. has received grants from EDCTP and Deutsche Zentrum für Infektionsforschung (BMBF). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386: 31–45. Available at:http://apps.who.int/iris/bitstream/handle/10665/259874/WER9303.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Weekly Epidemiological Record 2018; 93:17–9.29350500 [Google Scholar]
- 3. Ishizuka AS, Lyke KE, DeZure A, et al. ; VRC 312 and VRC 314 Study Teams. protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA 2017; 114:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mordmüller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seder RA, Chang LJ, Enama ME, et al. ; VRC 312 Study Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 7. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 2017; 17:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogwang C, Kimani D, Edwards NJ, et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci Transl Med 2015; 7:286re5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mensah VA, Gueye A, Ndiaye M, et al. ; MVVC group Safety, immunogenicity and efficacy of prime-boost vaccination with ChAd63 and MVA encoding ME-TRAP against Plasmodium falciparum infection in adults in Senegal. PLOS One 2016; 11:e0167951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bijker EM, Bastiaens GJ, Teirlinck AC, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci USA 2013; 110:7862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011; 365:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis 2002; 185:820–7. [DOI] [PubMed] [Google Scholar]
- 14. Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. N Engl J Med 2011; 365:1062–4. [DOI] [PubMed] [Google Scholar]
- 15. Palacpac NM, Ntege E, Yeka A, et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLOS One 2013; 8:e64073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sirima SB, Mordmüller B, Milligan P, et al. ; GMZ2 Trial Study Group A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 2016; 34:4536–42. [DOI] [PubMed] [Google Scholar]
- 17. Davidsen J, Rosenkrands I, Christensen D, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6’-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta 2005; 1718:22–31. [DOI] [PubMed] [Google Scholar]
- 18. van Dissel JT, Joosten SA, Hoff ST, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014; 32:7098–107. [DOI] [PubMed] [Google Scholar]
- 19. Karlsson I, Brandt L, Vinner L, et al. Adjuvanted HLA-supertype restricted subdominant peptides induce new T-cell immunity during untreated HIV-1-infection. Clin Immunol 2013; 146:120–30. [DOI] [PubMed] [Google Scholar]
- 20. Román VR, Jensen KJ, Jensen SS, et al. Therapeutic vaccination using cationic liposome-adjuvanted HIV type 1 peptides representing HLA-supertype-restricted subdominant T cell epitopes: safety, immunogenicity, and feasibility in Guinea-Bissau. AIDS Res Hum Retroviruses 2013; 29:1504–12. [DOI] [PubMed] [Google Scholar]
- 21. Mordmüller B, Supan C, Sim KL, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J 2015; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wildling E, Winkler S, Kremsner PG, Brandts C, Jenne L, Wernsdorfer WH. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop Med Parasitol 1995; 46:77–82. [PubMed] [Google Scholar]
- 23. US Food and Drug Administration. Guidance for industry – toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM091977.pdf. Accessed 15 February 2018. [Google Scholar]
- 24. Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun 2000; 68:2617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esen M, Kremsner PG, Schleucher R, et al. Safety and immunogenicity of GMZ2 - a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine 2009; 27:6862–8. [DOI] [PubMed] [Google Scholar]
- 26. Mordmüller B, Szywon K, Greutelaers B, et al. Safety and immunogenicity of the malaria vaccine candidate GMZ2 in malaria-exposed, adult individuals from Lambaréné, Gabon. Vaccine 2010; 28:6698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frank M, Lehners N, Mayengue PI, et al. A thirteen-year analysis of Plasmodium falciparum populations reveals high conservation of the mutant pfcrt haplotype despite the withdrawal of chloroquine from national treatment guidelines in Gabon. Malar J 2011; 10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Groger M, Veletzky L, Lalremruata A, et al. Prospective clinical trial assessing species-specific efficacy of artemether-lumefantrine for the treatment of Plasmodium malariae, Plasmodium ovale, and mixed Plasmodium malaria in Gabon. Antimicrob Agents Chemother 2018; 62:e01758–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lell B, Mordmüller B, Dejon Agobe JC, et al. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg 2018; 98:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bélard S, Issifou S, Hounkpatin AB, et al. A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PLOS One 2011; 6:e22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walk J, Schats R, Langenberg MC, et al. Diagnosis and treatment based on quantitative PCR after controlled human malaria infection. Malar J 2016; 15:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Payne RO, Milne KH, Elias SC, et al. Demonstration of the blood-stage Plasmodium falciparum controlled human malaria infection model to assess efficacy of the P. falciparum apical membrane antigen 1 vaccine, FMP2.1/AS01. J Infect Dis 2016; 213:1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pombo DJ, Lawrence G, Hirunpetcharat C, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 2002; 360:610–7. [DOI] [PubMed] [Google Scholar]
- 34. Triller G, Scally SW, Costa G, et al. Natural parasite exposure induces protective human anti-malarial antibodies. Immunity 2017; 47:1197–209.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Imkeller K, Scally SW, Bosch A, et al. Antihomotypic affinity maturation improves human B cell responses against a repetitive epitope. Science 2018; 360:1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jepsen MP, Jogdand PS, Singh SK, et al. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J Infect Dis 2013; 208:479–88. [DOI] [PubMed] [Google Scholar]
- 37. Carvalho LJ, Alves FA, Bianco C Jr, et al. Immunization of Saimiri sciureus monkeys with a recombinant hybrid protein derived from the Plasmodium falciparum antigen glutamate-rich protein and merozoite surface protein 3 can induce partial protection with Freund and Montanide ISA720 adjuvants. Clin Diagn Lab Immunol 2005; 12:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.