Abstract
Background
This study assessed the penetration and efficacy of tenofovir alafenamide (TAF) in the male genital tract (MGT) and the semen quality of individuals infected with human immunodeficiency virus (HIV)-1 who were treated with a TAF-containing regimen.
Methods
This was a prospective, open-label, single-arm study of 14 virologically-suppressed, HIV-1–infected men on stable antiretroviral therapy with elvitegravir, cobicistat, emtricitabine (E/C/F) and tenofovir disoproxil fumarate (TDF) who switched to E/C/F and TAF. At baseline (pre-switch) and at 12 weeks post-switch, we measured HIV-1 RNA in seminal plasma (SP) and blood plasma (BP), tenofovir (TFV) in SP and BP, and TFV-diphosphate (dp) in peripheral blood mononuclear cells (PBMCs) and seminal mononuclear cells (SMCs) at the end of the dosing interval (C24h). Semen quality was assessed before switching and after 12 weeks on TAF.
Results
With TAF, TFV C24 was 11.9-fold higher in SP than in BP. This concentration was significantly lower than TFV C24 in SP with TDF, but 9.6-fold higher than the 50% inhibitory concentration (IC50) (11.5 ng/mL). By contrast, the median TFV-dp concentration achieved with TAF in SMCs was 6% that of TFV-dp in PBMCs. The TFV-dp SMC:PBMC ratio was also significantly lower with TAF. Nonetheless, TFV-dp C24 in SMC was comparable with TAF and TDF. All the patients had HIV-1 RNA <40 copies/mL in BP and SP at baseline and at 12 weeks post-switch. No significant differences were observed in semen quality between TAF and TDF.
Conclusions
Extracellular and intracellular seminal TFV distribution differs between TAF and TDF. Nevertheless, both formulations, combined with elvitegravir/cobicistat/emtricitabine, maintained HIV-1 RNA suppression in semen. Differences in MGT distribution were not associated with differences in semen quality.
Clinical Trials Registration
EudraCT: 2016-001371-69
Keywords: antiretroviral therapy, tenofovir alafenamide, semen, male genital tract, HIV reservoirs
Both tenofovir alafenamide and tenofovir disoproxil fumarate maintained human immunodeficiency virus–1 RNA suppression in semen, and no clinically-relevant differences were observed in the extracellular and intracellular distribution of tenofovir in semen.
The persistence of human immunodeficiency virus (HIV) in reservoirs is a barrier for HIV eradication [1]. The male genital tract (MGT) is such a reservoir [2]. The ability of antiretroviral (ARV) drugs to penetrate in the MGT is a key factor for achieving HIV suppression in this reservoir and for preventing sexual transmission of the virus [3, 4].
Tenofovir alafenamide (TAF) is a novel prodrug of tenofovir (TFV). Used in combination with other ARVs, TAF has demonstrated non-inferior efficacy, with an improved renal and bone safety profile, when compared with tenofovir disoproxil fumarate (TDF) in Phase III, randomized, clinical trials in treatment-naive and virologically suppressed HIV patients previously treated with other regimens [5–8]. TAF is thus recommended in treatment guidelines as a component of preferred first-line ARV therapy (ART) regimens [9–11] and as a switching option to manage or prevent drug toxicity [9–11]. Moreover, TAF is available in combination with other drugs in single-tablet regimens, which are currently an attractive option for ART simplification [7, 12–14].
High concentrations of TFV in seminal plasma (SP) and TFV-diphosphate (TFV-dp) in seminal mononuclear cells (SMCs) have been observed in HIV type 1 (HIV-1)-infected men receiving TDF [15]. The same study also detected a potent and rapid reduction in seminal HIV-1 RNA concentrations. Compared with TDF, TAF achieves higher concentrations of TFV-dp (the pharmacologically-active drug) in target lymphocytic cells, shows potent antiviral activity at oral doses 10 times lower than those used in TDF, and is associated with a reduction in plasma TFV exposure of around 90% [16]. However, there is little information about the extracellular and intracellular distribution of TAF in the MGT [17], and no studies to date have described the efficacy of this drug in this viral reservoir. Additionally, although it is known that seminal drug concentrations can influence semen quality [18], there is no information about semen quality in patients being treated with TAF.
The aim of this study was to evaluate the extracellular and intracellular concentrations of TFV in semen; seminal HIV-1 RNA suppression; and changes in semen quality parameters after switching from TDF to TAF, when both are in combination with elvitegravir, cobicistat, and emtricitabine (E/C/F).
METHODS
Study Design and Population
This was a prospective, single-arm, open-label study. Initially, 15 individuals were included, but 1 was lost to follow-up after the baseline visit. The analysis is thus restricted to the 14 individuals who completed all study procedures. The study was carried out at the HIV outpatient clinic at Bellvitge University Hospital in Barcelona, Spain, between January and August 2017. Eligible participants were male adults (≥18 years old) with chronic HIV-1 infection, on stable ART with E/C/F/TDF (≥3 months), and with blood plasma (BP) HIV-1 RNA suppression below 40 copies/mL (≥6 months). The exclusion criteria were evidence of a primary viral resistance to the study drugs, a concomitant sexually transmitted infection, a hepatitis C virus infection that might need treatment during the study period, severe hepatic impairment, an estimated glomerular filtration rate <50 mL/min, a currently-active opportunistic infection, and any active malignancy.
At baseline, ART was switched to TAF at 10 mg, emtricitabine at 200 mg, elvitegravir at 150 mg, and cobicistat at 150 mg, co-formulated as a fixed-dose combination in a single tablet to be given once daily (Genvoya, Gilead Science).
The primary objective was to evaluate TFV concentrations in SP and intracellular TFV-dp concentrations in SMCs during E/C/F/TAF treatment. Secondary objectives included the assessments of HIV-1 RNA suppression in SP and changes in semen quality (according to the World Health Organization recommendations for the examination and processing of human semen) 12 weeks after the switch to E/C/F/TAF.
Procedures and Assessments
Study visits were scheduled at baseline and at weeks 4 and 12. At baseline (before switching to E/C/F/TAF) and at the week 12 visit, paired blood and semen samples were collected for analyses of HIV-1 RNA and drug concentrations. Blood and semen samples were obtained by peripheral venous puncture and self-masturbation, respectively. Participants were advised to take their ART at the same time each morning. After confirming that the last dose had been taken correctly and as scheduled, blood and semen samples were collected at the end of the dosing interval (24 hours post-dose ± 1 hour and before the next dose; C24). Semen quality was assessed before the switch and after 12 weeks on E/C/F/TAF. Due to the high variability usually observed in semen quality tests, 2 semen samples collected 4 weeks apart were evaluated before the ART switch and another 2 samples were collected after week 12. Participants were instructed to abstain from sexual activity for at least 72 hours before the collection of each semen sample.
CD4+ lymphocyte counts and hematology and chemistry tests (liver function, renal function, electrolytes, and lipids) were performed at baseline and at weeks 4 and 12. Clinical events and ART-related adverse events were recorded at each study visit.
Laboratory Methods
Sample Processing
Blood and semen samples were processed within 2 hours of collection, as described elsewhere [15].
Blood samples were collected in ethylenediaminetetraacetic acid–containing tubes, and plasma was separated by centrifugation (2600 rpm for 15 minutes at 4ºC). Plasma samples were transferred to cryogenic vials and stored frozen at −80ºC until analysis.
Peripheral blood mononuclear cells (PBMCs) were isolated using cell preparation tubes (CPTs; Becton-Dickinson, Franklin Lakes, NJ) containing sodium citrate. Each CPT, containing 8 mL of whole blood, was centrifuged at 2700 rpm for 20 minutes at room temperature. The contents of the CPT, isolated above the gel, were poured into a 15 mL conical tube. Approximately 2 mL of phosphate-buffered saline (PBS) was added to the CPT to resuspend any remaining monocytes, and PBS was then added to the 15 mL conical tube. The conical tube was then centrifuged at 1300 rpm at room temperature for 10 minutes. The supernatant was discarded and the cell pellet was resuspended in 100 mL of PBS and placed on ice. Mononuclear cells were counted using a hemocytometer with trypan blue exclusion. The remaining cells were lysed for approximately 15 minutes with 200 mL of 100% methanol. Cell debris was removed by centrifugation at 4ºC for 15 minutes at 3000 rpm. The supernatant was transferred to a cryogenic vial and stored at −80ºC until analysis.
Semen samples were transported in sterile containers. Specimens were left to liquefy at room temperature for 30 minutes. SP was separated by centrifugation (20 minutes at 2000–2600 rpm in a conical tube at 4ºC), transferred to cryogenic vials, and stored at −80º C until analysis. The cell pellet was resuspended in 8 mL of PBS, layered onto 4 mL of Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Uppsala, Sweden), and centrifuged at 2000 rpm for 20 minutes at room temperature. The SMCs found at the interface between the PBS and Ficoll were carefully removed, placed in 10 mL of PBS, and centrifuged. The cell pellet was resuspended to 100 mL. SMCs were counted using a hemocytometer and trypan blue exclusion. Cells were lysed for approximately 15 minutes with 200 mL of 100% methanol. Cell debris was removed by centrifugation for 15 minutes at 3000 rpm at 4ºC. The supernatant was aliquoted into cryogenic vials and stored at −80º C until analysis.
Human Immunodeficiency Virus–1 RNA Determinations
HIV-1 RNA levels in BP and SP were measured using a real-time polymerase chain reaction assay (Abbott RealTime HIV-1) with a quantification limit of 40 copies/mL.
Drug Concentrations
TFV and TFV-dp concentrations were measured at the University of North Carolina Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core, using a validated liquid chromatography-tandem mass spectrometry method. TFV was extracted from BP and SP samples using protein precipitation, with an isotopically-labeled internal standard (TFV13C5), and eluted from a Waters Atlantis T3 analytical column (2.1×50 mm, 3 μm particle size for BP; 2.1×100 mm, 3 μm particle size for SP). TFV-dp was extracted from cell lysate using protein precipitation, with an isotopically-labeled internal standard (TFVdp13C5), and eluted from a Thermo Scientific BioBasic AX T3 analytical column (2.1×50 mm, 5 μm particle size). All analytes were detected on an AB Sciex API-5000 triple quadrupole mass spectrometer. Standards and quality controls were prepared in duplicate, and a calibration curve was generated using weighted linear regression of analyte, as the internal standard peak area ratio versus the concentration. Concentrations in the quality controls and study samples were calculated from this calibration curve using Sciex Analyst Chromatography software. The acceptance criteria for the assay was ±15% (BP and SP) or ±20% (cell lysate) of the nominal concentration for standards and quality controls. The quantifiable ranges were 1-4000 ng/mL for BP, 5-5000 ng/mL for SP, and 0.02–20 ng/ml for cell lysate.
Semen Quality Tests
The semen quality was evaluated according to the World Health Organization laboratory manual for the examination and processing of human semen (fifth edition, 2010) [19]. Sperm concentration and motility were analyzed using an automated computer-aided semen analysis system, which uses a phase-contrast microscope and a standard counting chamber (2 μL semen sample and 10 μm of depth). Sperm vitality and sperm morphology were calculated using an optical microscope at x1000 magnification on smears stained with eosin and Papanicolaou, respectively.
The results of the semen quality tests were evaluated and interpreted by an expert (E. P.). For the analysis, we calculated the mean values for each parameter of the 2 semen samples obtained before the ART switch (while on E/C/F/TDF) and the 2 semen samples obtained after 12 weeks on E/C/F/TAF.
Other Laboratory Tests
Urine samples were tested for Chlamydia trachomatis and Neisseria gonorrhoeae by polymerase chain reaction (Xpert CT/NG, Cepheid, Solna, Sweden) at baseline and at week 12 to rule out concomitant asymptomatic urethral infections.
Statistical Methods
Data are presented as the median (range). The statistical analysis was performed using the nonparametric Wilcoxon signed-rank test. All analyses were carried out using the IBM SPSS statistics software for Windows (Version 19; IBM, Armonk, NY).
Ethics
The study protocol was approved by the ethics review committee at Bellvitge University Hospital, in accordance with the principles of the 2008 Declaration of Helsinki and the Spanish regulatory authorities. Written informed consent was obtained from all participants before any study procedures were performed. This study was registered at the European Union Clinical Trials Registry (EudraCT 2016-001371-69).
RESULTS
In total, 14 participants, with a median age of 42 years (range 34–58), completed all the study procedures. The median time on ART was 10.5 years (range 1–20), with 82 months (range 15–197) of plasma HIV-1 RNA suppression and 18 months (range 7–53) receiving E/C/F/TDF. The median CD4+ T cell count at baseline was 632 cells/μL (range 309–1742).
Urine tests for Chlamydia trachomatis and Neisseria gonorrhoeae were negative in all participants at baseline and at week 12. All individuals had HIV-1 RNA <40 copies/mL in both BP and SP at baseline (while on E/C/F/TDF).
The primary objective was to evaluate TFV concentrations in SP and intracellular TFV-dp concentrations in SMCs during E/C/F/TAF treatment.
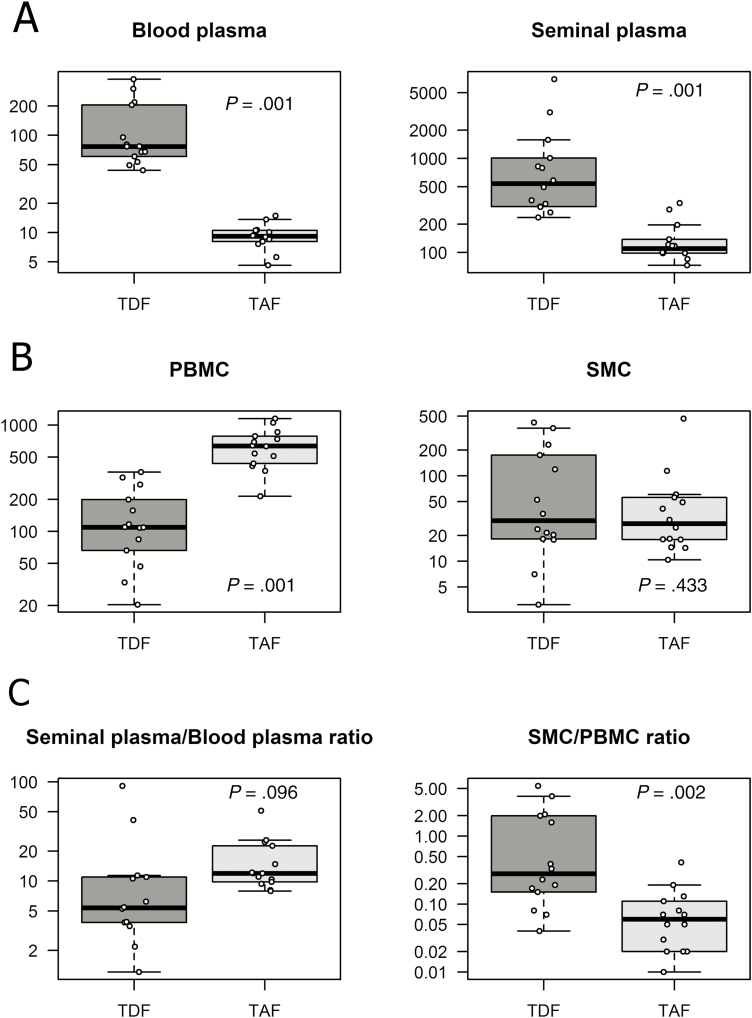
TFV and TFV-dp concentrations are shown in Table 1 and Figure 1 (Supplementary Table). With TAF, the median TFV C24 in SP was 110 ng/mL (range 73–336 ng/mL), which is 11.9 times higher than TFV C24 in BP (9.17 ng/mL; range 4.6–14.9). The median TFV SP:BP ratio was higher with TAF than with TDF, although this difference did not reach statistical significance (11.96 ng/mL vs 5.37 ng/mL; P = .096). However, the TFV C24 in SP achieved with TAF was significantly lower than the TFV C24 in SP with TDF dosing (110 ng/mL vs 540 ng/mL; P = .001).
Table 1.
Extracellular Tenofovir and Intracellular Tenofovir-diphosphate Concentrations
| Median (Range) | P Value | ||
|---|---|---|---|
| TFV in BP, ng/mL | TDF | 76.8 (43.7–378) | .001 |
| TAF | 9.17 (4.6–14.9) | ||
| TFV in SP, ng/mL | TDF | 540 (236–6980) | .001 |
| TAF | 110 (73–336) | ||
| TFV-dp in PBMCs, fmol/106 cells | TDF | 109.07 (20.34–361.34) | .001 |
| TAF | 637.29 (213.65–1154.36) | ||
| TFV-dp in SMCs, fmol/106 cells | TDF | 29.83 (3.10–421.14) | .433 |
| TAF | 27.55 (10.40–468.68) | ||
| TFV SP/BP ratio | TDF | 5.37 (1.21–91.00) | .096 |
| TAF | 11.96 (7.92–51.16) | ||
| TFV-dp SMC/PBMC ratio | TDF | 0.28 (0.04–5.48) | .002 |
| TAF | 0.06 (0.01–0.41) |
Abbreviations: BP, blood plasma; dp, diphosphate; PBMCs, peripheral blood mononuclear cells; SMCs, seminal mononuclear cells; SP, seminal plasma; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
Figure 1.
A, Extracellular tenofovir concentrations (C24h ng/mL), with TDF and TAF; B, intracellular tenofovir-diphosphate concentrations (C24h fmol/106 cells), with TDF and TAF; and C, tenofovir and tenofovir-diphosphate semen:blood ratio, with TDF and TAF. Abbreviations: PBMC, peripheral blood mononuclear cell; SMC, seminal mononuclear cell; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
TAF yielded a 23-fold lower median TFV-dp concentration in SMCs than in PBMCs (27.55 fmol/106cells vs 637.29 fmol/106cells). Although the median TFV-dp SMC:PBMC ratio was significantly lower with TAF than with TDF (0.06 vs 0.27; P = .002), the median TFV-dp C24h achieved with TAF and TDF were comparable in SMCs (27.55 fmol/106cells vs 29.83 fmol/106cells; P = .433).
The secondary objectives were to asses HIV-1 RNA suppression in SP and changes in semen quality 12 weeks after the switch to E/C/F/TAF.
All individuals maintained HIV-1 RNA suppression <40 copies/mL in both BP and SP at week 12 after switching to E/C/F/TAF.
The semen quality parameters prior to the switch (while on E/C/F/TDF) and 12 weeks after the switch (while on E/C/F/TAF) are shown in Table 2. Statistically significant differences were observed in progressive motility (better with TAF) and morphology (better with TDF), but they cannot be considered clinically relevant.
Table 2.
Semen Quality
| Patients | TDF | TAF | P Value |
|---|---|---|---|
| Sperm concentration, x106 per mL | 33.6 (1.1–144.1) | 31.9 (7.1–82.9) | .152 |
| Progressive motility, rapid progression + slow progression, % | 8.3 (1.8–58.1) | 23.3 (2.2–53.6) | .019 |
| Vitality, live spermatozoa, % | 61.5 (13–81) | 66 (48–85) | .230 |
| Sperm morphology, normal forms, % | 5 (2–16) | 3 (2.5–6) | .049 |
Lower reference limits (fifth percentiles and 95% confidence intervals) for semen characteristics [19] are: sperm concentration (x106 per mL), 15 (12–16); progressive motility (%), 32 (31–34); vitality (live spermatozoa, %), 58 (55–63); and sperm morphology (normal forms, %), 4 (3–4).
Abbreviations: TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
DISCUSSION
HIV-1 RNA suppression in the MGT might be limited by the ability of ARV drugs to penetrate this HIV reservoir. Few studies to date have analyzed the seminal distribution of TFV and TFV-dp or seminal HIV-1 RNA suppression in individuals treated with TAF-containing regimens. In this study, we found that extracellular/intracellular TFV distribution differs between TAF and TDF.
Due to its intracellular metabolism, TAF yields higher concentrations of TFV-dp in target blood lymphoid cells, and it also yields 90% lower plasma TFV exposure than TDF [16]. This intracellular/extracellular distribution pattern, however, is not reproduced in semen. We observed that TAF yielded lower intracellular TFV-dp concentrations in SMCs than in PBMCs, and higher TFV concentrations in SP than in BP. It is noteworthy, however, that even though the TFV SP:BP ratio did not differ significantly between TAF and TDF, the TFV concentration achieved with TAF in SP was significantly lower than that achieved with TDF. Nevertheless, TAF yielded a median 9.6-fold higher TFV C24h than TFV IC50 for wild-type HIV-1 (11.5 ng/mL) [20]. By contrast, the TFV-dp SMC:PBMC ratio was significantly lower with TAF than with TDF, but no statistically significant differences were observed between TAF and TDF for their median TFV-dp C24h in SMCs. The median TFV-dp C24h in SMCs was below the TFV-dp EC50 (36.7 fmol/million CD4 cells) [20] with both TAF and TDF. However, the lymphocyte population in SMCs is mostly monocyte-derived, and data from previous studies indicate that competing endogenous nucleotide pools are lower in comparison with CD4+ T lymphocytes [21, 22]. Moreover, TFV interacts synergistically with emtricitabine and elvitegravir/cobicistat [23]. Taking these considerations into account, TFV-dp concentrations below the TFV EC50 for CD4 cells does not necessarily mean low efficacy in SMCs.
A previous study by Dumond et al [17] assessed extracellular and intracellular seminal TFV and TFV-dp concentrations, respectively, in HIV-infected individuals receiving F/TDF- and F/TAF-containing ART regimens and in HIV-negative volunteers receiving F/TDF [17]. Despite the heterogeneity and lower sample size of this study, the intracellular and extracellular TFV distribution observed with TDF and TAF was comparable to that seen in our study. A noteworthy aspect of our study is that the same individuals received TDF and TAF at different times, and combined with the same ARV drugs (E/C/F/TDF and E/C/F/TAF). Both studies show a different extracellular/intracellular TFV distribution pattern in PB and semen for both TDF and TAF, in addition to a different extracellular and intracellular seminal TFV distribution pattern. Although more studies are needed to study the mechanisms involved in the different drug distribution pattern in the MGT observed with TDF and TAF, we could hypothesize that differences in drug transporter affinities and differences in cathepsin A between PBMC and SMC might explain these findings.
Despite the differences in seminal penetration, both TAF and TDF, in combination with E/C/F, maintained HIV-1 RNA suppression in semen.
Although the extracellular/intracellular distribution pattern of TAF in semen is different than in blood, our results suggest that the seminal tenofovir concentrations achieved with TAF are sufficient to contribute to the suppression of HIV-1 replication in this compartment. These data of TAF exposure in MGT are also of interest to define the role of TAF in pre-exposure prophylaxis (PrEP). However, TAF is not currently recommended for PrEP, and data from clinical trials (eg, the ongoing trial NCT02842086) are needed to demonstrate the efficacy and safety of TAF in this preventive strategy.
ARV drug penetration in the MGT might have an impact on semen quality and ART efficacy [18, 24]. We found statistically-significant differences in progressive semen motility and morphology in semen quality tests performed during treatment with TDF and TAF. These results must be interpreted with caution, however, as semen analysis has some limitations, including, in our case, a small sample size and high interindividual variability. In addition, the differences between the 2 treatments were not consistent: progressive motility was better with TAF, while morphology was better with TDF. Finally, differences are only considered to be clinically relevant in the case of a change from severe asthenozoospermia (progressive motility ≤5%) or severe teratozoospermia (normal forms ≤1) to normal reference values, or vice versa. The differences detected thus cannot be considered to be clinically relevant.
Our study has some limitations. As in other studies assessing pharmacokinetics in reservoirs, the sample size was small, and this makes results more prone to influences from interindividual and intraindividual variability. Nevertheless, it should be noted that we studied TFV concentrations in individuals who first received E/C/F/TDF and then switched to E/C/F/TAF. Moreover, our results are comparable with those reported by Dumond et al [17] in participants with different characteristics. Larger studies are needed to elucidate the potentially differential effects of TAF and TDF on semen quality. Another consideration is that we only measured HIV-1 RNA in SP. We did not, therefore, study the role of continued HIV-1 RNA production by long-lived, infected cells in semen. Nonetheless, HIV-1 RNA in SP is the most widely-used parameter to assess viral suppression in the MGT [25, 26].
In summary, the present study shows differences in the extracellular and intracellular distribution of TFV in semen between individuals treated with TAF and TDF. Nevertheless, both drugs, used in combination with elvitegravir, cobicistat, and emtricitabine, maintained HIV-1 RNA suppression in semen, and no clinically-relevant differences were observed in semen quality parameters.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 25th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 4–7 March 2018. Abstract number: 478.
Author contributions. A. I. and D. P. designed the study; A. I., J. M. T., and D. P. recruited participants; A. I., J. M. T., and B. G conducted the study visits; J. N. performed the microbiological procedures; M. L. C. and A. D. M. K. performed liquid chromatography–tandem mass spectrometry to measure tenofovir (TFV) and TFV-diphosphate concentrations; E. P. performed and interpreted the semen quality tests; S. M. assisted in data collection and study coordination; A. I. performed the statistical analysis; A. I., J. M. T., and D. P. analyzed and interpreted the results; A. I. drafted the manuscript; and J. M. T., M. L. C., A. D. M. K., and D. P. reviewed the manuscript. All authors revised the manuscript for important intellectual content and contributed to the final version.
Acknowledgments. The authors thank all the patients who participated in this study.
Disclaimer. Gilead Sciences was given the opportunity to review a preliminary version of this article for factual accuracy. Gilead Sciences provided financial support to this work. The authors are solely responsible for the study design, interpretation of results, and final content of the article.
Financial support. This work was supported by Gilead Sciences; the RD16/0025/0003 project within the Plan Nacional Research, Development and Innovation; Institute of Health Carlos III-Subdirección General de Evaluación; the European Regional Development Fund; and the University of North Carolina at Chapel Hill Center for AIDS Research, a National Institutes of Health–funded program (P30 AI50410).
Potential conflicts of interest. A. I. has received financial compensation for lectures, consultancy work, and educational activities and funds for research from Abbvie, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dome, and ViiV Healthcare. J. M. T. has received financial compensation for lectures, consultancies, and educational activities, as well as research funding from AbbVie, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dome, and ViiV Healthcare. D. P. has received research grants and/or honoraria for advisories and/or conferences from ViiV Healthcare, Bristol-Myers Squibb, Abbott, Gilead, Janssen-Cilag, and Merck Sharp & Dome. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS 2016; 11:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galvin SR, Cohen MS. Genital tract reservoirs. Curr Opin HIV AIDS 2006; 1:162–6. [DOI] [PubMed] [Google Scholar]
- 3. Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:335–43. [DOI] [PubMed] [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sax PE, Wohl D, Yin MT, et al. ; GS-US-292-0104/0111 Study Team Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–15. [DOI] [PubMed] [Google Scholar]
- 6. Eron JJ, Orkin C, Gallant J, et al. ; AMBER study group A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS 2018; 32:1431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orkin C, Molina JM, Negredo E, et al. ; EMERALD study group Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV 2018; 5:e23–34. [DOI] [PubMed] [Google Scholar]
- 8. Imaz A, Podzamczer D. Tenofovir alafenamide, emtricitabine, elvitegravir, and cobicistat combination therapy for the treatment of HIV. Expert Rev Anti Infect Ther 2017; 15:195–209. [DOI] [PubMed] [Google Scholar]
- 9. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 19 November 2018. [Google Scholar]
- 10. European AIDS Clinical Society (EACS) Guidelines. Clinical management and treatment of HIV infected adults in Europe Version 9.1. Available at: http://www.europeanaidsclinicalsociety.org. Accessed 19 November 2018.
- 11. AIDS Study Group (GESIDA) of the Spanish Society of Infectious Diseases, Clinical Microbiology, the National AIDS Plan. GESIDA/National AIDS plan consensus document on antiretroviral therapy in adults infected by the human immunodeficiency virus Available at: http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2016-tar.pdf. Accessed 1 June 2018.
- 12. Mills A, Arribas JR, Andrade-Villanueva J, et al. ; GS-US-292-0109 team Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. [DOI] [PubMed] [Google Scholar]
- 13. Post FA, Tebas P, Clarke A, et al. Brief report: switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected adults with renal impairment: 96-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr 2017; 74: 180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huhn GD, Tebas P, Gallant J, et al. A randomized, open-label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment-experienced HIV-1-infected adults. J Acquir Immune Defic Syndr 2017; 74:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr 2008; 47:329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63:449–55. [DOI] [PubMed] [Google Scholar]
- 17. Dumond JB, Greene SA, Prince HM, et al. Differential extracellular, but similar intracellular, disposition of two tenofovir formulations in the male genital tract. Antivir Ther 2018. doi: 10.3851/IMP3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Leeuwen E, Wit FW, Repping S, et al. Effects of antiretroviral therapy on semen quality. AIDS 2008; 22:637–42. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 20. Gilead Sciences. Viread [Package Insert]. Foster City, CA: Gilead Sciences, Inc, 2010. [Google Scholar]
- 21. Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in Men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gavegnano C, Kennedy EM, Kim B, Schinazi RF. The impact of macrophage nucleotide pools on HIV-1 reverse transcription, viral replication, and the development of novel antiviral agents. Mol Biol Int 2012; 2012:625983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kulkarni R, Hluhanich R, McColl DM, Miller MD, White KL. The combined anti-HIV-1 activities of emtricitabine and tenofovir plus the integrase inhibitor elvitegravir or raltegravir show high levels of synergy in vitro. Antimicrob Agents Chemother 2014; 58:6145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowe SH, van Leeuwen E, Droste JA, et al. Semen quality and drug concentrations in seminal plasma of patients using a didanosine or didanosine plus tenofovir containing antiretroviral regimen. Ther Drug Monit 2007; 29:566–70. [DOI] [PubMed] [Google Scholar]
- 25. Baeten JM, Kahle E, Lingappa JR, et al. ; Partners in Prevention HSV/HIV Transmission Study Team Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson JA, Ping LH, Dibben O, et al. ; Center for HIV/AIDS Vaccine Immunology HIV-1 populations in semen arise through multiple mechanisms. PLOS Pathog 2010; 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.