Abstract
Understanding the nuances of AmpC β-lactamase–mediated resistance can be challenging, even for the infectious diseases specialist. AmpC resistance can be classified into 3 categories: (1) inducible chromosomal resistance that emerges in the setting of a β-lactam compound, (2) stable derepression due to mutations in ampC regulatory genes, or (3) the presence of plasmid-mediated ampC genes. This review will mainly focus on inducible AmpC resistance in Enterobacteriaceae. Although several observational studies have explored optimal treatment for AmpC producers, few provide reliable insights into effective management approaches. Heterogeneity within the data and inherent selection bias make inferences on effective β-lactam choices problematic. Most experts agree it is prudent to avoid expanded-spectrum (ie, third-generation) cephalosporins for the treatment of organisms posing the greatest risk of ampC induction, which has best been described in the context of Enterobacter cloacae infections. The role of other broad-spectrum β-lactams and the likelihood of ampC induction by other Enterobacteriaceae are less clear. We will review the mechanisms of resistance and triggers resulting in AmpC expression, the species-specific epidemiology of AmpC production, approaches to the detection of AmpC production, and treatment options for AmpC-producing infections.
Keywords: Enterobacter cloacae, antimicrobial resistance, Citrobacter freundii, Serratia marcescens
This review summarizes mechanisms of resistance resulting in AmpC expression, triggers of AmpC expression, the species-specific epidemiology of AmpC β-lactamase production, approaches to the detection of AmpC production, and treatment approaches for infections caused by AmpC β-lactamase–producing bacteria.
AmpC β-lactamases are class C enzymes under the Ambler classification scheme, containing serine residues at their active site for catalysis [1]. Mechanisms of AmpC β-lactamase resistance can be divided into 3 categories: (1) inducible resistance via chromosomally encoded ampC genes (eg, Enterobacter cloacae, Serratia marcescens, Citrobacter freundii, Pseudomonas aeruginosa, etc.), (2) noninducible chromosomal resistance due to promoter and/or attenuator mutations (eg, Escherichia coli, Shigella species, Acinetobacter baumannii), (3) or plasmid-mediated resistance (eg, Klebsiella pneumoniae, E. coli, Salmonella species, etc.) [2].
Exposure to β-lactams can trigger a cascade of events leading to significant AmpC production and β-lactam resistance, even for infections caused by initially susceptible isolates. The risk of inducing AmpC production varies by β-lactam and by species, complicating treatment decisions. This review will focus mostly on inducible, chromosomally encoded AmpC β-lactamase–mediated resistance and provide the necessary knowledge required to make rational treatment decisions in an increasingly complex multidrug-resistant gram-negative world.
MECHANISMS OF RESISTANCE
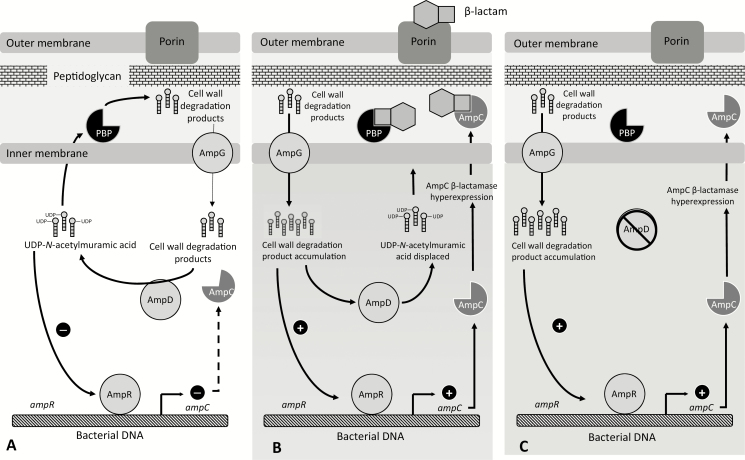
Chromosomally encoded ampC genes can be induced in the appropriate environment [3]. Normally, the regulatory protein AmpR reduces AmpC β-lactamase expression to very low levels [4]. Certain β-lactams induce the production of cell-wall degradation products (eg, N-acetylglucosamine-1,6-anhydro-N-acetylmuramic acid oligopeptides) [5]. As these peptides accumulate, they compete with uridine diphosphate (UDP)–N-acetylmuramic acid peptides for binding to AmpR, the negative regulator of AmpC [6]. With decreased UDP-N-acetylmuramic acid peptide binding to AmpR, AmpR undergoes conformational changes that disable its function, increasing production of AmpC enzymes [7]. As an example, this sequence of events increases C. freundii AmpC expression by more than 11-fold in an in vitro model [8].
A second recycling protein, AmpD, is responsible for cleavage of residues off cell-wall degradation products, reducing their ability to bind to AmpR but still allowing them to be recycled back into the cell-wall synthesis pathway [7, 9]. AmpG transports oligopeptides involved in peptidoglycan recycling and AmpC regulation into the cytosol [10]. As concentrations of degradation products increase, AmpD is unable to cleave all of the necessary peptides, leading to binding of these products to AmpR, decreasing AmpR repression and subsequently increasing ampC transcription [9]. After β-lactam exposure ceases, AmpC production levels generally return to baseline. However, if mutations occur in regulatory genes (in order of most to least common: ampD > ampR > ampG), stable derepression can ensue resulting in overtranscription of ampC, even in the absence of a β-lactam trigger [9, 11]. Figure 1 is a schematic illustration of the regulation of chromosomally mediated AmpC expression.
Figure 1.
A simplified illustration of AmpC β-lactamase expression. A, Basal AmpC β-lactamase production. B, Increased transcription of ampC in the presence of an inducing β-lactam antibiotic that increases cell-wall degradation production to levels beyond the capacity of AmpD cleavage. Cell-wall degradation products accumulate and compete with UDP-N-acetylmuramic acid peptides for binding to AmpR. With decreased binding of UDP-N-acetylamuramic acid to AmpR, AmpR undergoes conformational changes resulting in increased AmpC production. C, An ampD mutation resulting in inactivation and subsequent stable derepression of AmpC. Abbreviations: PBP, penicillin binding protein; UDP, uridine diphosphate.
High-level AmpC expression (ie, hyperexpression) appears to confer a fitness cost to an organism because of the cytoplasmic accumulation of degradation products [12, 13]. Despite this, in the face of a persistent stimulus (eg, β-lactam exposure) this phenotype may be sustained. In addition, by eliminating susceptible (non-derepressed) subpopulations, β-lactam therapy can select for stably resistant, derepressed mutants, further contributing to the isolation of organisms no longer susceptible to specific β-lactams.
TRIGGERS OF AmpC HYPEREXPRESSION
Antibiotics recognized as potent inducers of the previously described pathway of AmpC production include the aminopenicillins, amoxicillin-clavulanate, narrow-spectrum (ie, first-generation) cephalosporins, and the cephamycins [5, 14]. Because common AmpC producers such as E. cloacae complex, C. freundii, and S. marcescens can easily hydrolyze these agents even at basal AmpC expression levels, they are intrinsically resistant to these potent inducers. Piperacillin-tazobactam (TZP), aztreonam, and expanded-spectrum (ie, third-generation) cephalosporins are weak inducers of AmpC hyperproduction but can be hydrolyzed if enough β-lactamase is made, translating to increased drug-specific minimum inhibitory concentrations (MICs) [5]. Cefepime has the advantage of being a weak inducer while withstanding hydrolysis by AmpC β-lactamases because of the formation of a stable acyl enzyme complex [15]. Imipenem is a potent inducer of AmpC production, but it remains stable against hydrolysis by also forming an acyl enzyme complex [14].
The rates of development of resistance to ceftriaxone, ceftazidime, and cefepime for 10 E. cloacae isolates were evaluated by daily transfer to medium containing 2-fold serial dilutions of these antibiotics [16]. The emergence of resistance was significantly higher for ceftazidime and ceftriaxone compared with cefepime [16]. Although emergence of resistance to β-lactams during therapy can occur with any agent, available clinical data appear to be in agreement with in vitro data, suggesting that this risk is by far the greatest with expanded-spectrum cephalosporins [17–23]. Table 1 summarizes data from available observational studies demonstrating the risk of emergence of resistance during exposure to specific β-lactams due to putative AmpC production. The activity of cefepime and carbapenems consistently approaches 100% against isolates that appear to be AmpC producers in the absence of other relevant β-lactamase enzymes (eg, coproduction of extended-spectrum β-lactamases [ESBLs], carbapenemases, etc.). Data from in vitro and animal models suggest that TZP less frequently selects for TZP-resistant Enterobacter species isolates compared with the frequency of expanded-spectrum cephalosporin resistance during expanded-spectrum cephalosporin exposure [24–27].
Table 1.
Select Observational Studies Quantifying the Risk of Emergence of Resistance of Specific Enterobacteriaceae to β-Lactam Agents due to Putative AmpC Production During Exposure to These Agents
| First author, year | Organism | Piperacillin- Tazobactam, % | Expanded- Spectrum Cephalosporins,a % | Cefepime, % | Carbapenems, % | Notes |
|---|---|---|---|---|---|---|
| Chow, 1991 [19] | Enterobacter spp. | … | 19 | … | … | Nineteen percent (6/31) of patients treated with expanded-spectrum cephalosporin for susceptible Enterobacter bloodstream isolates developed expanded-spectrum cephalosporin resistance during therapy. |
| Jacobson, 1995 [21] | Enterobacter spp. | … | 21 | … | … | Species-specific resistance emergence: E. cloacae, 16% (11/70); Klebsiella (formerly Enterobacter) aerogenes, 6% (4/70) |
| Kaye, 2001 [20] | Enterobacter spp. | … | 19 | … | … | Species-specific resistance emergence: E. cloacae, 9% (31/343); K. aerogenes, 17% (18/108) |
| Lee, 2002 [42] | Enterobacter spp. | … | 3 | … | … | Three percent (5/198) of patients treated with expanded-spectrum cephalosporins for susceptible Enterobacter bloodstream isolates developed expanded-spectrum cephalosporin resistance during therapy. |
| Choi, 2007 [43] | Serratia spp. | … | 7 | … | … | Seven percent (3/42) of patients treated with expanded-spectrum cephalosporins for susceptible Serratia spp. bloodstream isolates developed expanded-spectrum cephalosporin resistance during therapy. |
| Hilty, 2013 [44] | Enterobacter cloacae | 20 | 67 | … | … | Sixty-seven percent (2/3) of patients treated with expanded-spectrum cephalosporins for E. cloacae developed expanded-spectrum cephalosporin resistance during therapy; 20% (1/5) of patients treated with TZP for E. cloacae bloodstream isolates developed resistance to TZP during therapy. |
| Choi, 2008 [18] | Overall | 2 | 5 | 0 | 0 | Species-specific resistance emergence: E. cloacae, 8% (10/121); K. aerogenes, 12% (8/65); E. agglomerans, 0% (0/4); E. asburiae, 0% (0/1) |
| Enterobacter spp. | … | 8 | … | … | ||
| Citrobacter freundii | … | 3 | … | … | ||
| Serratia marcescens | … | 0 | … | … | ||
| Morganella morganii | … | 0 | … | … |
Abbreviations: spp., species; TZP, piperacillin-tazobactam.
aAll included studies used the pre-2010 Clinical Laboratory and Standards Institute third-generation cephalosporin susceptibility breakpoint of 8 μg/mL; the ceftriaxone and cefotaxime susceptibility breakpoints have since been lowered to 1 μg/mL and the ceftazidime susceptibility breakpoint for Enterobacteriaceae has since been lowered to 4 μg/mL.
SPECIES-SPECIFIC EPIDEMIOLOGY OF AmpC PRODUCTION
Chromosomally encoded ampC genes can be identified in a number of gram-negative organisms, including E. cloacae, Klebsiella (formerly Enterobacter) aerogenes, C. freundii, S. marcescens, Providencia stuartii, P. aeruginosa, Hafnia alvei, and Morganella morganii [2]. A number of acronyms have been used to represent these organisms (eg, ESCPM, SPICE, SPACE, etc.). The hallmark phenotypic pattern of these organisms is that they appear to be susceptible to third-generation cephalosporins if AmpC production is not induced (ie, in the absence of AmpC production), but resistance can develop upon β-lactam exposure as early as a single day after drug initiation [21]. The extent of β-lactam resistance conferred by these enzymes is associated with the regulatory gene network of the organism and its ability to control expression levels [28]. As an example, although ampC is chromosomally encoded in E. coli, it lacks the necessary inducible mechanisms for expressing this β-lactamase at a high-enough level to be clinically significant in the absence of rare promotor and/or attenuator mutations [4, 29]. On the contrary, if plasmid-mediated ampC genes are present in E. coli (eg, blaCMY, blaFOX, blaDHA,blaACC, blaACT,blaMIR, blaMOX, etc.), AmpC production is constitutive, leading to resistance to expanded-spectrum cephalosporins, as is evident by in vitro susceptibility testing.
Some frequently encountered Enterobacteriaceae are conspicuous by the absence of a chromosomal ampC gene such as K. pneumoniae, Klebsiella oxytoca, Salmonella species, and Proteus mirabilis [2]. Even species in the same genus as some of the ESCPM organisms may not possess chromosomal ampC genes, such as Citrobacter amalonaticus or Citrobacter koseri [2].
Quantifying the likelihood of AmpC induction across bacterial species would best be defined by identifying organisms initially susceptible to certain β-lactams (eg, ceftriaxone) that, on subsequent isolation (and after β-lactam exposure), become resistant—with molecular genotyping and expression studies to confirm that the same organism was recovered rather than acquisition of or selection for a different organism of the same species. Unfortunately, few such studies exist. However, available observational studies can still be helpful in providing some insight into this risk (Table 1).
A landmark study by Chow and colleagues [19] in 1991 highlighted this concern clinically. Evaluating the outcomes of 31 patients with Enterobacter species bloodstream infections for which initial ceftriaxone susceptibility was demonstrated, emergence of ceftriaxone resistance occurred in 19% of isolates. A 2001 study by Kaye and colleagues [20] also found that 19% of patients treated with ceftriaxone for susceptible Enterobacter spp. infections developed resistance during therapy. Choi et al [18] showed that 8% of patients with Enterobacter species infections developed expanded-spectrum cephalosporin resistance during exposure to these agents. In light of these findings, expanded-spectrum cephalosporins for the treatment of infections caused by Enterobacter species is discouraged, except in uncomplicated urinary tract infections where it is hypothesized that a rapid bactericidal effect is likely to be achieved prior to the selection of hyperproducing mutants [30].
The Enterobacteriaceae are not homogenous in their level of expression of AmpC production (Table 1). Derepressed S. marcescens, M. morganii, and P. stuartii strains express AmpC levels that are ~10-fold lower than derepressed E. cloacae or C. freundii isolates [31, 32].
METHODS OF AmpC β-LACTAMASE DETECTION
Inhibitors known to confirm AmpC production include cloxacillin or boronic acid, with specificity generally in the range of 70–90% [33–39]. As an example, ETEST (BioMerieux, Durham, North Carolina) gradient strips consisting of cefotetan or cefoxitin on both sides with a constant concentration of cloxacillin on a single side are interpreted as positive tests in the setting of a reduction in the cephamycin MIC of at least 3 dilutions or a deformation of the ellipse of inhibition (ie, “phantom zone”) in the presence of cloxacillin [37]. Boronic acid can be added to an inert disk near a cephamycin or third-generation cephalosporin disk (ie, double-disk synergy test), added directly to the β-lactam disk and compared with an unmodified β-lactam disk (disk potentiation test), or evaluated using broth microdilution approaches to identify AmpC β-lactamase production [35, 36, 38, 39]. Phenotypic assays are unable to distinguish between AmpC production due to derepression of a chromosomal ampC gene or the presence of a plasmid-associated ampC gene. Molecular approaches to identifying common plasmid-mediated ampC genes are available but their use is generally limited to research settings [40]. Clinical and Laboratory Standards Institute–endorsed criteria for AmpC detection in clinical isolates do not currently exist [41].
Even in the absence of targeted testing to identify AmpC production, clinicians can still evaluate Enterobacteriaceae antimicrobial susceptibility test results to putatively distinguish likely AmpC production from ESBL production. Table 2 demonstrates anticipated susceptibility results for an E. cloacae strain producing various β-lactamase enzymes. Although both AmpC β-lactamase and ESBL producers are generally resistant to expanded-spectrum cephalosporins, AmpC producers—in contrast to ESBL producers—are reliably resistant to cephamycins [45]. Cefepime can overcome inactivation by AmpC β-lactamases, making cefepime activity almost always retained in the presence of AmpC production [46, 47]. ESBL producers most often demonstrate elevated cefepime MICs and are interpreted as susceptible dose-dependent or resistant to cefepime. The susceptibility patterns of other β-lactams are less reliable.
Table 2.
Anticipated Enterobacter cloacae Antibiotic Susceptibility Patterns in the Setting of Common β-Lactamase Enzymes
| Antibiotic | Wild typea | AmpC β-lactamases | ESBL | KPC | NDMb | OXA-48-like carbapenemasesc |
|---|---|---|---|---|---|---|
| Ampicillin | R | R | R | R | R | R |
| Piperacillin/tazobactam | S | S/R | S/R | R | R | R |
| Cefoxitin | S | R | S | R | R | R |
| Ceftriaxone | S | R | R | R | R | S |
| Cefepime | S | S/SDD | S/SDD/R | R | R | S |
| Ceftazidime/avibactam | S | S | S | S | R | S |
| Aztreonam | S | S/R | R | R | S | S |
| Ertapenem | S | S | S | R | R | S/R |
| Meropenem | S | S | S | S/R | R | S/R |
| Meropenem/vaborbactam | S | S | S | S | R | R |
Abbreviations: ESBL, extended-spectrum β-lactamases; KPC, Klebsiella pneumoniae carbapenemases; NDM, New Delhi metallo-β-lactamases; R, resistant; S, susceptible; SDD, susceptible dose-dependent.
a Enterobacter cloacae isolates are resistant to ampicillin due to the production of narrow-spectrum β-lactamases.
bNDM-producing organisms generally co-produce ESBLs, AmpCs β-lactamases, or other carbapenemases. Although aztreonam can withstand hydrolysis by NDM-producing organisms, it will generally be inactive in the presence of these other β-lactamases.
cOXA-48-like carbapenemases generally co-produce ESBLs, AmpC β-lactamases, or other carbapenemases. Although aztreonam, expanded-spectrum cephalosporins, and cefepime can generally withstand hydrolysis by OXA-48-like carbapenemases, they will often be inactive in the presence of these other β-lactamases.
To further complicate matters, for any Enterobacteriaceae with AmpC production either due to induction/derepression of a chromosomal ampC gene or the presence of a plasmid-associated ampC gene, additional β-lactamases may also be produced by the same organism (eg, ESBLs, carbapenemases), resulting in complex susceptibility patterns and making phenotypic interpretation challenging [48]. On the basis of available data, largely summarized in Table 1, we would suggest that it is reasonable for clinical microbiology laboratories to consider withholding third-generation cephalosporin results for invasive Enterobacter species infections (ie, infections outside of the urinary tract) or include a disclaimer citing the potential for emergence of resistance to these agents. However, there are insufficient data at the present time to expand this guidance to other β-lactams that may be impacted by AmpC β-lactamase activity (ie, aztreonam, TZP) or other Enterobacteriaceae in the "SPACE" organisms group.
TREATMENT OPTIONS
Concerns regarding sustained antibiotic potency against organisms with high levels, or the potential for high levels, of AmpC expression complicate treatment decisions. A survey of infectious diseases specialists practicing in Australia, New Zealand, and Singapore found that more than half of clinicians preferred to treat presumed AmpC-producing infections with carbapenems (58%), with the remainder using either cefepime (19%) or, less commonly, TZP (8%) [49]. Although carbapenems provide reliable coverage against AmpC hyperproducers, carbapenem overuse is not without significant clinical consequences. The relative efficacy of TZP and cefepime remains debatable because of heterogeneity across studies. Studies published to date are observational (ie, risk of bias in treatment assignment), are generally small, rarely include confirmation as to whether the included isolates were indeed hyperproducers of AmpC β-lactamases, and have inconsistencies regarding the use of combination therapy, durations of therapy administered, source of infection, and source control measures.
Newer β-lactamase inhibitors (βLIs), such as avibactam and vaborbactam, have the most potent activity of the βLIs against AmpC production [50]. The high cost of the newer βL/βLIs and limited access to these agents in certain regions of the world preclude their routine use for presumed AmpC producers. Perhaps even more important, the continued crisis of gram-negative resistance reminds us that these agents should be reserved for circumstances when alternatives do not exist, which is not the case with AmpC-producing organisms (in the absence of additional carbapenem resistance mechanisms) [51]. Because they do not have a β-lactam ring and do not provide a substrate for AmpC-mediated hydrolysis, fluoroquinolones and trimethoprim-sulfamethoxazole, when susceptibility is demonstrated in vitro, are well suited as either oral step-down therapy or for the treatment of mild to moderate infections due to presumed AmpC-producing organisms. Below we will focus on the data evaluating TZP and cefepime for the treatment of infections by presumed AmpC-producing Enterobacteriaceae.
Piperacillin-tazobactam
Available clinical data suggest that the risk of emergence of resistance to TZP during the treatment of AmpC-producing Enterobacteriaceae infections is low [18–20]. To highlight one such study, Cheng and colleagues [52] conducted a case-control study in hospitalized patients with Enterobacter species, Serratia species, or Citrobacter species bloodstream infections from 2009 to 2015 to better understand the role of TZP therapy. After propensity-score matching, there were 41 patients in the TZP group and 41 patients treated with either cefepime or meropenem. No differences in clinical failures, 7-day or 30-day mortality, or persistent bacteremia were observed between the groups. However, given the small sample size, the possibility of a type II error exists, particularly because 30-day mortality in the TZP group was double that of the cefepime/meropenem group [53]. Table 3 summarizes additional comparative effectiveness studies evaluating the role of TZP for AmpC-producing infections.
Table 3.
Select Observational Comparative Effectiveness Studies Evaluating β-Lactams for the Treatment of Enterobacteriaceae With Putative AmpC Expression
| First author, year, country | Population | Phenotypic Testing for AmpC Hyperproduction | Agent 1 | Agent 2 | Outcomes Comparing Agent 1 vs 2 | Conclusion |
|---|---|---|---|---|---|---|
| Marcos, 2008, Spain [69] | Enterobacter spp. BSI from 1991–2006 | None | TZP (n = 19) | Carbapenem (n = 57) | 30-d mortality: 11% vs 18% | TZP is not inferior to carbapenems in an adjusted analysis. |
| O’Neal, 2012, USA [65] | Enterobacter spp. BSI from 2006–2008 | None | Cefepime (n = 12) | PTZ (n = 6); carbapenem (n = 30) | In-hospital mortality: 8% vs 17% vs 47% | Cefepime and TZP are not inferior to carbapenems in an unadjusted analysis. |
| Hilty, 2013, Switzerland [44] | Enterobacter cloacae BSI from 2008–2011 | Disk approximation assay | Cefepime (n = 18) | TZP (n = 4); carbapenem (n = 5) | 28-d mortality: 11% vs 25% vs 20% | Cefepime is not inferior to carbapenems in an unadjusted analysis. Investigators note they are unable to draw conclusions about TZP. |
| Tamma, 2013, USA [63] | Enterobacter spp., Citrobacter spp., or Serratia spp. BSI; pneumonia; or intra-abdominal infections from 2010–2011 | Cefotetan-boronic acid disk test and cefotetan-cloxacillin Etest (positive by both for inclusion) | Cefepime (n = 32) | Carbapenem (n = 32) | 30-d mortality: 31% vs 34% | Cefepime is not inferior to carbapenems in a propensity-score–matched cohort. |
| Blanchette, 2014, USA [70] | Enterobacter spp., Citrobacter spp., or Serratia spp. BSI; pneumonia; intra-abdominal infection; urinary tract infection; or skin and soft tissue infection from 2009–2012 | None | Cefepime (n = 32) | Ertapenem (n = 16) | Treatment success: 89% vs 69% | Cefepime is not inferior to carbapenems in an unadjusted analysis. |
| Chaubey, 2014, Canada [66] | Enterobacter spp., Citrobacter spp., Hafnia alvei, Morganella morganii, Providencia spp., or Serratia spp. BSI from 2000–2008 | None | Cefepime (n = 4) | TZP (n = 22); carbapenem (n = 45) | In-hospital mortality: 25% vs 46% vs 11% | TZP is inferior to cefepime or carbapenems. |
| Huh, 2014, South Korea [17] | Enterobacter spp. BSI from 2004–2011 | None | Cefepime (n = 5) | TZP (n = 18); carbapenem (n = 20) | 30-d mortality: 13% vs 6% vs 30% | Cefepime and TZP are not inferior to carbapenems in an adjusted analysis. |
| Siedner, 2014, USA [64] | Enterobacter spp. BSI from 2005–2011 | None | Cefepime (n = 12) | Carbapenem (n = 10) | In-hospital mortality: 17% vs 30% | Cefepime is not inferior to carbapenems in a propensity-score–matched cohort. |
| Lin, 2015, Taiwan [68] | Morganella morganii BSI from 2003–2012 | None | Cefepime (n = 49) | TZP (n = 41); carbapenem (n = 14) | 14-d mortality: 18% vs 20% vs 29% | Cefepime and TZP are not inferior to carbapenems in an unadjusted analysis. |
| Lee, 2015, Taiwan [67] | E. cloacae BSI from 2008–2012 | None | Cefepime (n = 72) | Carbapenem (n = 72) | 30-d mortality: 26% vs 22% | Cefepime is not inferior to carbapenems overall in an adjusted analysis. Cefepime is inferior to carbapenems for cefepime MICs 4–8 μg/mL, but when confirmed ESBLs were removed, there were no differences in outcomes. |
| Cheng, 2017, USA [52] | Enterobacter spp., Citrobacter spp., or Serratia spp. BSI from 2009–2015 | None | TZP (n = 41) | Cefepime or meropenem (n = 41) | 30-d mortality: 15% vs 7% | TZP is not inferior to cefepime or carbapenems in propensity-score–matched cohort. |
Abbreviations: BSI, bloodstream infection; ESBL, extended-spectrum β-lactamase; MIC, minimum inhibitory concentration; spp., species; TZP, piperacillin-tazobactam.
Outcomes associated with TZP therapy for AmpC-producing bloodstream infections were also the focus of a meta-analysis [54]. Eight observational studies conducted between 1980 and 2014 were included and unadjusted outcomes data were compared between patients receiving TZP (or ticarcillin-clavulanate, which is no longer available for clinical use in the United States) versus carbapenem therapy. In the definitive therapy group, 30-day mortality was similar between the 179 patients receiving TZP and the 474 patients receiving carbapenem therapy at 18% and 14%, respectively. Mortality was also not significantly different between the 232 patients receiving empiric TZP therapy (10%) and the 107 patients receiving empiric carbapenem therapy (21%). On closer inspection, twice as many people died in the carbapenem group, making selection bias a concern. Although this analysis sought to evaluate patients with bloodstream infections due to Enterobacter species, Serratia species, Citrobacter species, Providencia species, and Morganella species, the vast majority of infections were due to Enterobacter species, making meaningful conclusions about the other organisms difficult. Notwithstanding the limitations of the available observational data evaluating the role of TZP for the treatment of AmpC-producing infections, they do not indicate a clear safety signal that patients who receive TZP have poorer outcomes than those prescribed carbapenem therapy.
In light of the drawbacks of the available data, a pilot randomized controlled trial (MERINO II) comparing TZP (4.5 g IV every 6 hours) and meropenem (1 g IV every 8 hours) for adults with bloodstream infections caused by Enterobacter species, C. freundii, M. morganii, Providencia species, or S. marcescens is currently underway (ClinicalTrials.gov Identifier: NCT02437045). The investigators intend to recruit 100 patients (50 in each arm) to establish the safety and efficacy of TZP for bacteremia caused by AmpC producers and to lay the groundwork for a future, sufficiently powered clinical trial.
Cefepime
Cefepime has a net neutral charge (ie, zwitterion similar to imipenem), which provides the advantage of rapidly penetrating outer membranes compared with other cephalosporins with a net positive charge (eg, ceftriaxone, cefotaxime), enhancing access to its enzymatic target and evading β-lactamase inactivation [55]. Moreover, the low affinity and limited turnover to a number of class C β-lactamases generally enable it to maintain stability in the setting of even high quantities of AmpC β-lactamases [56, 57]. This has not been consistent across studies, with some in vitro studies demonstrating cefepime resistance in the setting of high organism burden (ie, >105 CFU/mL in sites other than urine) [58, 59]. In an in vitro model, every 12-hour cefepime dosing allowed for E. cloacae mutant selection that was not observed with every 8-hour cefepime dosing [60]. Isolated cases of clinical failure with the use of cefepime for Enterobacter species infections have also been reported, generally in association with high organism burden infections and every 12-hour dosing strategies [61]. It is unclear, however, if coproduction of ESBLs may have occurred in cases of reported failures. When resistance to cefepime does occur, it is most likely attributable to the selection of porin mutations [16, 62]. Observational studies, however, have generally not reinforced theoretical concerns regarding the use of cefepime for organisms prone to AmpC production (Table 3).
Sanders and colleagues [63] reported on a series of 16 patients with expanded-spectrum cephalosporin-resistant Enterobacter species infections at a variety of body sites who achieved favorable clinical responses with cefepime, and with no emergence of cefepime resistance observed. An observational study evaluated hospitalized patients with bloodstream infections, pneumonia, or intra-abdominal infections caused by Enterobacter species, Serratia species, or Citrobacter species treated with either cefepime or meropenem therapy [64]. Patients were only included if their isolates were positive for AmpC production on the basis of agreement by 2 different phenotypic methods. Although a single-center observational experience and limited by its sample size, in a propensity-score matched cohort, differences in clinical outcomes between the 32 patients treated with cefepime (1–2 g every 8 hours) versus the 32 patients treated with meropenem (1 g every 8 hours) were not present [64]. These findings have been replicated in several observational studies [65–68]. The aforementioned meta-analysis evaluated 7 observational studies comparing cefepime and carbapenems for the treatment of presumed AmpC-producing bloodstream infections and found that the unadjusted 30-day mortality was similar [54].
Investigators from Taiwan sought to explore the outcomes of patients with E. cloacae bloodstream infections treated with cefepime with susceptible dose-dependent MICs (ie, cefepime MICs of 4–8 μg/mL) [67]. Evaluating 33 patients infected with E. cloacae with cefepime MICs of 4–8 μg/mL, patients treated with cefepime had less favorable outcomes compared with patients treated with carbapenems, regardless of the cefepime administration strategy used (ie, 2 g every 12 hours, 1 g every 8 hours, or 2 g every 8 hours). However, when excluding the 16 patients with phenotypically confirmed ESBL production, differences in outcomes were not observed [67]. It remains unclear whether excluding the 16 patients reduced the ability to detect a difference in outcomes if one truly existed, or if this report serves as a cautionary reminder that for E. cloacae (and other "SPACE" organisms) with elevated cefepime MICs, ESBL production/coproduction and associated cefepime failure is likely.
CONCLUSIONS
In summary, organisms with the potential for AmpC production play an important role in management decisions. Well-designed studies to better quantify the risk of AmpC production, to identify easy-to-perform and accurate diagnostic approaches for AmpC production, and to inform appropriate treatment recommendations for AmpC-producing organisms are needed. Although many lingering questions exist that are ripe for further investigation, there are 2 key points that infectious diseases practitioners should be aware of. First, Enterobacter species (and K. aerogenes) are the most problematic pathogens in their potential for AmpC β-lactamase induction. For serious Enterobacter species infections, it is prudent to avoid the use of expanded-spectrum cephalosporins, even if susceptible in vitro. Because AmpC production is less prominent for other "SPACE" organisms, for these organisms it is reasonable to base treatment decisions on in vitro susceptibility results while using optimal drug administration strategies, ensuring source control measures, and monitoring patients closely to evaluate for appropriate clinical responses. Second, although available observational studies are informative, studies that are adequately powered, interventional, and include diverse species are necessary but lacking. The extent to which TZP or cefepime can be used to treat presumed AmpC producers is largely unsettled. Available observational data indicate that both agents are acceptable treatment options for invasive AmpC-producing infections, with more data supporting the use of cefepime (preferentially administered every 8 hours) compared to TZP. The results of the MERINO II study will likely provide some important insights into the role of TZP in the management of AmpC-producing infections. Although convincing clinical data indicating that carbapenems are superior to either cefepime or TZP for AmpC-producing infections are lacking, for severely ill patients with complex foci of infection, while awaiting the results of more definitive interventional studies, carbapenem therapy can be a reasonable treatment option on a case-by-case basis.
Notes
Antibacterial Resistance Leadership Group members. P. D. Tamma, Y. Doi, R. A. Bonomo.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number UM1AI104681). The work was also supported in part by the National Institutes of Health (grant numbers K23-AI127935 [to P. D. T.]; R01-AI104895, R21-AI123747, and R21-AI135522 [to Y. D.]; R01-AI100560, R01-AI063517 and R01-AI072219 [R. A. B.]; and R21-AI130608 [P. J. S.]).
Potential conflicts of interest. Y. D. reports grants from Accelerate Diagnostics and The Medicines Company and personal fees from Meiji, Achaogen, Allergan, Curetis, The Medicines Company, Roche, Gilead, Pfizer, Tetraphase, Geom, Merck, Astellas, and Recida, all outside the submitted work. P. J. S. reports grants and personal fees from Accelerate Diagnostics; grants from BD Diagnostics, Inc, bioMerieux, Inc, Check-Points Diagnostics BV, and Hardy Diagnostics; and personal fees from Roche Diagnostics, Opgen, Inc, and Oxford Nanopore, all outside the submitted work. P. D. T. reports grants from Merck, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Antibacterial Resistance Leadership Group:
P D Tamma, Y Doi, and R A Bonomo
References
- 1. Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 2010; 54:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bennett PM, Chopra I. Molecular basis of beta-lactamase induction in bacteria. Antimicrob Agents Chemother 1993; 37:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Honoré N, Nicolas MH, Cole ST. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J 1986; 5:3709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber DA, Sanders CC. Diverse potential of beta-lactamase inhibitors to induce class I enzymes. Antimicrob Agents Chemother 1990; 34:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs C, Frère JM, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell 1997; 88:823–32. [DOI] [PubMed] [Google Scholar]
- 7. Guérin F, Isnard C, Cattoir V, Giard JC. Complex regulation pathways of AmpC-mediated β-lactam resistance in Enterobacter cloacae complex. Antimicrob Agents Chemother 2015; 59:7753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindberg F, Westman L, Normark S. Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc Natl Acad Sci U S A 1985; 82:4620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidtke AJ, Hanson ND. Model system to evaluate the effect of ampD mutations on AmpC-mediated beta-lactam resistance. Antimicrob Agents Chemother 2006; 50:2030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korfmann G, Sanders CC. AmpG is essential for high-level expression of AmpC beta-lactamase in Enterobacter cloacae. Antimicrob Agents Chemother 1989; 33:1946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaneko K, Okamoto R, Nakano R, Kawakami S, Inoue M. Gene mutations responsible for overexpression of AmpC beta-lactamase in some clinical isolates of Enterobacter cloacae. J Clin Microbiol 2005; 43:2955–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folkesson A, Eriksson S, Andersson M, Park JT, Normark S. Components of the peptidoglycan-recycling pathway modulate invasion and intracellular survival of Salmonella enterica serovar Typhimurium. Cell Microbiol 2005; 7:147–55. [DOI] [PubMed] [Google Scholar]
- 13. Moya B, Juan C, Albertí S, Pérez JL, Oliver A. Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2008; 52: 3694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanders CC, Bradford PA, Ehrhardt AF, et al. Penicillin-binding proteins and induction of AmpC beta-lactamase. Antimicrob Agents Chemother 1997; 41:2013–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hancock RE, Bellido F. Antibacterial in vitro activity of fourth generation cephalosporins. J Chemother 1996; 8(Suppl 2):31–6. [PubMed] [Google Scholar]
- 16. Fung-Tomc JC, Gradelski E, Huczko E, Dougherty TJ, Kessler RE, Bonner DP. Differences in the resistant variants of Enterobacter cloacae selected by extended-spectrum cephalosporins. Antimicrob Agents Chemother 1996; 40:1289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huh K, Kang CI, Kim J, et al. Risk factors and treatment outcomes of bloodstream infection caused by extended-spectrum cephalosporin-resistant Enterobacter species in adults with cancer. Diagn Microbiol Infect Dis 2014; 78:172–7. [DOI] [PubMed] [Google Scholar]
- 18. Choi SH, Lee JE, Park SJ, et al. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob Agents Chemother 2008; 52:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 1991; 115:585–90. [DOI] [PubMed] [Google Scholar]
- 20. Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob Agents Chemother 2001; 45:2628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobson KL, Cohen SH, Inciardi JF, et al. The relationship between antecedent antibiotic use and resistance to extended-spectrum cephalosporins in group I beta-lactamase-producing organisms. Clin Infect Dis 1995; 21:1107–13. [DOI] [PubMed] [Google Scholar]
- 22. Weinstein RA. Endemic emergence of cephalosporin-resistant Enterobacter: relation to prior therapy. Infect Control 1986; 7:120–3. [DOI] [PubMed] [Google Scholar]
- 23. Nicolle LE. Prior antimicrobial therapy and resistance of Enterobacter Citrobacter and Serratia to third generation cephalosporins. J Hosp Infect 1988; 11:321–7. [DOI] [PubMed] [Google Scholar]
- 24. Lister PD. Beta-lactamase inhibitor combinations with extended-spectrum penicillins: factors influencing antibacterial activity against Enterobacteriaceae and Pseudomonas aeruginosa. Pharmacotherapy 2000; 20:213S–8S; discussion 224S–8S. [DOI] [PubMed] [Google Scholar]
- 25. Stearne LE, van Boxtel D, Lemmens N, Goessens WH, Mouton JW, Gyssens IC. Comparative study of the effects of ceftizoxime, piperacillin, and piperacillin-tazobactam concentrations on antibacterial activity and selection of antibiotic-resistant mutants of Enterobacter cloacae and Bacteroides fragilis in vitro and in vivo in mixed-infection abscesses. Antimicrob Agents Chemother 2004; 48:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akova M, Yang Y, Livermore DM. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed class I beta-lactamases. J Antimicrob Chemother 1990; 25:199–208. [DOI] [PubMed] [Google Scholar]
- 27. Higashitani F, Nishida K, Hyodo A, Inoue M. Effects of tazobactam on the frequency of the emergence of resistant strains from Enterobacter cloacae, Citrobacter freundii, and Proteus vulgaris (beta-lactamase derepressed mutants). J Antibiot (Tokyo) 1995; 48:1027–33. [DOI] [PubMed] [Google Scholar]
- 28. Livermore DM. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol 1987; 6:439–45. [DOI] [PubMed] [Google Scholar]
- 29. Forward KR, Willey BM, Low DE, et al. Molecular mechanisms of cefoxitin resistance in Escherichia coli from the Toronto area hospitals. Diagn Microbiol Infect Dis 2001; 41:57–63. [DOI] [PubMed] [Google Scholar]
- 30. Livermore DM, Brown DF, Quinn JP, Carmeli Y, Paterson DL, Yu VL. Should third-generation cephalosporins be avoided against AmpC-inducible Enterobacteriaceae? Clin Microbiol Infect 2004; 10:84–5. [DOI] [PubMed] [Google Scholar]
- 31. Power P, Galleni M, Ayala JA, Gutkind G. Biochemical and molecular characterization of three new variants of AmpC beta-lactamases from Morganella morganii. Antimicrob Agents Chemother 2006; 50:962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohlmann R, Bähr T, Gatermann SG. Species-specific mutation rates for ampC derepression in Enterobacterales with chromosomally encoded inducible AmpC β-lactamase. J Antimicrob Chemother 2018; 73:1530–6. [DOI] [PubMed] [Google Scholar]
- 33. Weston GS, Blázquez J, Baquero F, Shoichet BK. Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J Med Chem 1998; 41:4577–86. [DOI] [PubMed] [Google Scholar]
- 34. Jacoby GA, Walsh KE, Walker VJ. Identification of extended-spectrum, AmpC, and carbapenem- hydrolyzing beta-lactamases in Escherichia coli and Klebsiella pneumoniae by disk tests. J Clin Microbiol 2006; 44:1971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitout JD, Le PG, Moore KL, Church DL, Gregson DB. Detection of AmpC beta-lactamases in Escherichia coli, Klebsiella spp., Salmonella spp. and Proteus mirabilis in a regional clinical microbiology laboratory. Clin Microbiol Infect 2010; 16:165–70. [DOI] [PubMed] [Google Scholar]
- 36. Black JA, Thomson KS, Buynak JD, Pitout JD. Evaluation of beta-lactamase inhibitors in disk tests for detection of plasmid-mediated AmpC beta-lactamases in well-characterized clinical strains of Klebsiella spp. J Clin Microbiol 2005; 43:4168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polsfuss S, Bloemberg GV, Giger J, Meyer V, Böttger EC, Hombach M. Practical approach for reliable detection of AmpC beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol 2011; 49:2798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yagi T, Wachino J, Kurokawa H, et al. Practical methods using boronic acid compounds for identification of class C beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol 2005; 43:2551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song W, Jeong SH, Kim JS, et al. Use of boronic acid disk methods to detect the combined expression of plasmid-mediated AmpC beta-lactamases and extended-spectrum beta-lactamases in clinical isolates of Klebsiella spp., Salmonella spp., and Proteus mirabilis. Diagn Microbiol Infect Dis 2007; 57:315–8. [DOI] [PubMed] [Google Scholar]
- 40. Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 2002; 40:2153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clinical and Laboratory Standards Ins titute. Performance standards for antimicrobial susceptibility testing. 28th ed CLSI document M100. Wayne, PA; 2018. [Google Scholar]
- 42. Lee SO, Kim YS, Kim BN, Kim MN, Woo JH, Ryu J. Impact of previous use of antibiotics on development of resistance to extended-spectrum cephalosporins in patients with Enterobacter bacteremia. Eur J Clin Microbiol Infect Dis 2002; 21:577–81. [DOI] [PubMed] [Google Scholar]
- 43. Choi SH, Lee JE, Park SJ, et al. Prevalence, microbiology, and clinical characteristics of extended-spectrum beta-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganiiin Korea. 2007; 26:557–61. [DOI] [PubMed] [Google Scholar]
- 44. Hilty M, Sendi P, Seiffert SN, et al. Characterisation and clinical features of Enterobacter cloacae bloodstream infections occurring at a tertiary care university hospital in Switzerland: is cefepime adequate therapy? Int J Antimicrob Agents 2013;41:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reuland EA, Hays JP, de Jongh DM, et al. Detection and occurrence of plasmid-mediated AmpC in highly resistant gram-negative rods. PLoS One 2014; 9:e91396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomson KS. Extended-spectrum-beta-lactamase, AmpC, and carbapenemase issues. J Clin Microbiol 2010; 48:1019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pfaller MA, Jones RN, Marshall SA, et al. Inducible ampC beta-lactamase producing gram-negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE). Diagn Microbiol Infect Dis 1997; 28:211–9. [DOI] [PubMed] [Google Scholar]
- 48. Alvarez M, Tran JH, Chow N, Jacoby GA. Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother 2004; 48:533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harris PN, Alder L, Paterson DL. Antimicrobial susceptibility reporting and treatment selection for AmpC-producing Enterobacteriaceae: what do microbiologists and infectious disease practitioners actually practice? Pathology 2015; 47:386–8. [DOI] [PubMed] [Google Scholar]
- 50. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 2019; 68:519–24. [DOI] [PubMed] [Google Scholar]
- 51. Tamma PD, Hsu AJ. Defin ing the role of novel β-lactam agents targeting carbapenem-resistant gram-negative organisms. J Pediatric Infect Dis Soc 2019; pii:piz002. Epub ahead of print. doi:10.1093/jpids/piz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng L, Nelson BC, Mehta M, et al. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aitken SL, Foolad F, McDaneld PM. Should piperacillin-tazobactam be used as definitive therapy against Ent erobacteriaceae harboring inducible AmpC beta-lactamases? Antimicrob Agents Chemother 2017;61:e01160-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harris PN, Wei JY, Shen AW, et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother 2016; 71:296–306. [DOI] [PubMed] [Google Scholar]
- 55. Hancock RE, Bellido F. Factors involved in the enhanced efficacy against gram-negative bacteria of fourth generation cephalosporins. J Antimicrob Chemother 1992; 29(Suppl A):1–6. [DOI] [PubMed] [Google Scholar]
- 56. Pechère JC, Vladoianu IR. Development of resistance during ceftazidime and cefepime therapy in a murine peritonitis model. J Antimicrob Chemother 1992; 29:563–73. [DOI] [PubMed] [Google Scholar]
- 57. Johnson CC, Livornese L, Gold MJ, Pitsakis PG, Taylor S, Levison ME. Activity of cefepime against ceftazidime-resistant gram-negative bacilli using low and high inocula. J Antimicrob Chemother 1995; 35:765–73. [DOI] [PubMed] [Google Scholar]
- 58. Kang CI, Pai H, Kim SH, et al. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. J Antimicrob Chemother 2004; 54:1130–3. [DOI] [PubMed] [Google Scholar]
- 59. Negri MC, Baquero F. In vitro selective concentrations of cefepime and ceftazidime for AmpC beta-lactamase hyperproducer Enterobacter cloacae variants. Clin Microbiol Infect 1999; 5(Suppl 1):25–8. [DOI] [PubMed] [Google Scholar]
- 60. Limaye AP, Gautom RK, Black D, Fritsche TR. Rapid emergence of resistance to cefepime during treatment. Clin Infect Dis 1997; 25:339–40. [DOI] [PubMed] [Google Scholar]
- 61. Fernández-Cuenca F, Rodríguez-Martínez JM, Martínez-Martínez L, Pascual A. In vivo selection of Enterobacter aerogenes with reduced susceptibility to cefepime and carbapenems associated with decreased expression of a 40 kDa outer membrane protein and hyperproduction of AmpC beta-lactamase. Int J Antimicrob Agents 2006; 27:549–52. [DOI] [PubMed] [Google Scholar]
- 62. Sanders WE Jr, Tenney JH, Kessler RE. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis 1996; 23:454–61. [DOI] [PubMed] [Google Scholar]
- 63. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis 2013; 57:781–8. [DOI] [PubMed] [Google Scholar]
- 64. Siedner MJ, Galar A, Guzmán-Suarez BB, et al. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis 2014; 58:1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O’Neal CS, O’Neal HR, Daniels TL, Talbot TR. Treatment outcomes in patients with third-generation cephalosporin-resistant Enterobacter bacteremia. Scand J Infect Dis 2012; 44:726–32. [DOI] [PubMed] [Google Scholar]
- 66. Chaubey VP, Pitout JD, Dalton B, Gregson DB, Ross T, Laupland KB. Clinical and microbiological characteristics of bloodstream infections due to AmpC β-lactamase producing Enterobacteriaceae: an active surveillance cohort in a large centralized Canadian region. BMC Infect Dis 2014; 14:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee NY, Lee CC, Li CW, et al. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother 2015; 59:7558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin TY, Chan MC, Yang YS, et al. Clinical manifestations and prognostic factors of Morganella morganii bacteremia. Eur J Clin Microbiol Infect Dis 2015; 34:231–6. [DOI] [PubMed] [Google Scholar]
- 69. Marcos M, Iñurrieta A, Soriano A, Martínez JA, Almela M, Marco F, Mensa J. Effect of antimicrobial therapy on mortality in 377 episodes of Enterobacter spp. bacteraemia. J Antimicrob Chemother 2008; 62:397–403. [DOI] [PubMed] [Google Scholar]
- 70. Blanchette LM, Kuti JL, Nicolau DP, Nailor MD. Clinical comparison of ertapenem and cefepime for treatment of infections caused by AmpC beta lactamase- producing Enterobacteriaceae. Scand J Infect Dis 2014; 46:803–8. [DOI] [PubMed] [Google Scholar]