Abstract
Aerobic capacity, which is expressed as peak oxygen consumption (VO2peak), is well-known to be an independent predictor of all-cause mortality and cardiovascular prognosis. This is true even for people with various coronary risk factors and cardiovascular diseases. Although exercise training is the best method to improve VO2peak, the guidelines of most academic societies recommend 150 or 75 min of moderate- or vigorous- intensity physical activities, respectively, every week to gain health benefits. For general health and primary and secondary cardiovascular prevention, high-intensity interval training (HIIT) has been recognized as an efficient exercise protocol with short exercise sessions. Given the availability of the numerous HIIT protocols, which can be classified into aerobic HIIT and anaerobic HIIT [usually called sprint interval training (SIT)], professionals in health-related fields, including primary physicians and cardiologists, may find it confusing when trying to select an appropriate protocol for their patients. This review describes the classifications of aerobic HIIT and SIT, and their differences in terms of effects, target subjects, adaptability, working mechanisms, and safety. Understanding the HIIT protocols and adopting the correct type for each subject would lead to better improvements in VO2peak with higher adherence and less risk.
Keywords: High-intensity interval training, Exercise, Training, Coronary artery disease, Chronic heart failure, Prevention, Lifestyle, Health, Peak O2 consumption, Aerobic capacity
Core tip: There are numerous of high-intensity interval training (HIIT) protocols, which can be classified into aerobic HIIT and anaerobic HIIT [usually called sprint interval training (SIT)]. Professionals in health-related fields, including primary physicians and cardiologists, may find it confusing when selecting an appropriate protocol for their patients. This review describes the classifications of aerobic HIIT and SIT, and their differences in terms of effects, target subjects, adaptability, working mechanisms, and safety. Understanding the HIIT protocols and adopting the correct type for each patient would lead to better improvements in VO2peak with higher adherence and less risk.
INTRODUCTION
Accumulated evidence suggests that aerobic capacity (VO2peak) is the strongest predictor of future health, all-cause mortality[1-3], and cardiovascular risks[4,5]. Moreover, several studies have suggested that people with established coronary vascular disease (CVD) risk factors (such as high body mass index, hypertension, or diabetes) and high cardiorespiratory fitness have a highly attenuated risk of CVD and premature mortality[4,5]. Thus, it has become a major goal in the medical field to improve VO2peak in patients with lifestyle-related diseases with (as a secondary prevention strategy) or without (as a primary prevention strategy) cardiac disorders. For improvement in public health, performing regular physical exercise is indispensable together with a nutritional approach. Healthy young and middle-aged people can select from the many choices of exercise training methods, including recreational sports, in daily life. In contrast, people with lifestyle-related disease and/or elderly people are often sedentary and physically unfit. Thus, some useful techniques and limitations exist when encouraging exercise training with adequate safety and high adherence in these people. High-intensity interval training (HIIT) has been recognized as an alternative and more efficient protocol than moderate-intensity continuous training (MCT), which is the gold standard recommended in several guidelines[6-8]. HIIT and sprint interval training (SIT) for 6-8 wk increase VO2peak more than or at least comparable to MCT. In this comprehensive review, many protocols of HIIT and SIT for improving aerobic and metabolic capacity were evaluated for their effects in patients with sedentary lifestyle-related diseases with or without cardiac disease to determine appropriate protocol recommendations for different patient populations. General practitioners and cardiologists should pay more attention to exercise and physical activity rather than to the prescription of drugs.
EXERCISE IS MEDICINEⓇ (EIM) ENCOURAGES PEOPLE TO FORM EXERCISE HABITS
To improve primary and secondary prevention methods in cardiovascular medicine, physical activity should be promoted as a first-line strategy despite new drug developments in the medical treatment field.
Although the value of exercise for improving health is well recognized world-wide[9], widespread adoption of exercise habits has not been adequately achieved, especially in highly developed countries where the use of automobiles is highly prevalent. In a recent study from the World Health Organization[10], about 27.5% of the population in 2016 was recognized as sedentary (i.e., with insufficient physical activity). In this context, EIM is a global health initiative promoted worldwide by the American College of Sports Medicine[11]. EIM encourages primary care physicians and other health-care providers to include physical activity when designing treatment plans, and to offer evidenced-based exercise programs to their patients or refer their patients to qualified exercise professionals. EIM is committed to the belief that physical activity promotes optimal health, is integral in the prevention and treatment of many medical conditions, and should be regularly assessed and included as part of health care. Irrespective of disease severity, exercise can bring improvements in aerobic and metabolic capacity as well as cardiac function if performed with an optimal dose, frequency, and intensity. Despite the continuous recommendations by the American College of Sports Medicine and related pro-fessional societies worldwide, the effects of such recommendations on public awareness have been very limited. Many kinds of wearable heart rate monitors and accelerometers are commercially available. Although these state-of-the art products could motivate sedentary people and increase their frequency of exercise training or participation in sports events, more efficient and effective exercise training strategies are still required.
For the success of EIM, professionals who can encourage target people to exercise in a planned way according to detailed exercise protocols, functioning as an intermediary between physicians and patients, would be very important.
GUIDELINE RECOMMENDATION: MCT AS A CLASSIC AND SIMPLE PROTOCOL
The current guidelines on physical activity for health recommend that adults should engage in at least 150 min of moderate-intensity activity or 75 min of vigorous-intensity activity per week, or any combination of activities that amount to the same total energy expenditure[6,12]. Similarly, in the field of cardiac rehabilitation, MCT has been the gold standard for many years for patients with cardiac diseases[13]. The current guidelines on cardiac rehabilitation/exercise training recommend endurance exercises with a moderate intensity at 50%-85% (mostly 70%-85%) of the peak heart rate or anaerobic threshold level for patients with CVD or chronic heart failure (CHF)[7,8,14]. The latest guidelines suggest HIIT as an alternative protocol to improve aerobic capacity and cardiac function. However, the adoption of HIIT in the cardiac rehabilitation setting is still controversial among researchers. In Japan, only a few studies describing the effects of HIIT have been published[15-17]. On the other hand, MCT has been used as a control strategy in randomized controlled trials (RCTs) that evaluated HIIT or SIT. Thus, evidence for the same amount of MCT has been accumulated. In representative MCTs such as walking or jogging, each workout is time consuming and usually monotonous and boring. Therefore, although MCT has become a classic protocol based on evidence from RCTs, it remains difficult for most people, with lack of time being cited as a common hindrance[18].
HIGH INTENSITY IS THE KEY ELEMENT OF EFFICIENT EXERCISE PROTOCOLS: HIIT AND SIT
HIIT
The inclusion of “adapted” high intensity (relative to a subject’s current physical ability) in the exercise protocol is a key component for exercise to be more efficient as a “medicine.” The clinical and physiological benefits of HIIT compared with those of MCT are shown in Table 1. In multiple RCTs, a wide range of targets, including skeletal muscles[19-22], risk factors[21], vasculature[19-22], respiration[22,23], autonomic function[24], cardiac function[20,22,25-27], exercise capacity[26], inflammation[27], quality of life[27], physiological markers such as VO2peak, and endothelial function, showed better improvements with HIIT than with MCT.
Table 1.
Variables improved by high-intensity interval training
| Variables | Target |
| Skeletal muscle biopsy | |
| PGC-1α | |
| Mitochondrial function in lateral vastus | O2 consumption |
| Fatty acid transporter in the vastus lateralis and FAS (a key lipogenic enzyme) | |
| IR β subunit in skeletal muscle (peripheral insulin sensitivity) | Metabolic |
| Re-uptake of Ca2+ into the salcoplasmic reticulum | |
| Physiological test | |
| Exercise test | |
| Improvement of ventilatory efficiency (increased value of PETCO2) | Respiratory function |
| Oxygen consumption at the first ventilator threshold | Cardiac function |
| Oxygen pulse | Cardiac function |
| Parasympathetic activity (HR recovery) | Autonomic function |
| Duration of exercise time | Autonomic function |
| Distance walked during the 6-min walk | Work capacity |
| Ultrasonography | |
| Cardiac function | |
| Reversed LV re-modelling (LV end diastolic and systolic volumes) | Cardiac function |
| Ea | |
| Diastolic function (e′, E, E/ e′, E/A ratio, higher proportion of e′ > 8 cm/s, E improvement during exercise), | |
| Systolic function after 12 wk at rest and during exercise) | |
| E reduction | |
| Deceleration time increase | |
| Left atrial volume | |
| Reduced-plasma BNP | |
| Vascular | |
| Endothelial dysfunction (FMD) | Vascular function |
| Coronary plaque necrotic core reduction in defined coronary segments | Vascular function |
| Laboratory test | |
| Myeloperoxidase | Anti-oxidant |
| High sensitivity CRP | Inflammation |
| Interleukin-6 | |
| insulin sensitivity (HOMA index) | Metabolic |
| HbA1C | |
| Clinico-social data | |
| Increased Short Form-36 physical/mental component scores and decreased Minnesota Living with Heart Failure questionnaire score | Quality of life |
| Frequency of metabolic syndrome | Risk factor |
HOMA: Homoestasis model assessment; IR: Insulin receptor; PGC: Peroxisome-proliferator activated receptor γcoactivator; FMD: Flow mediated dilation; FAS: Fatty acid synthase; PETCO2: End-tidal carbon dioxide; HR: Heart rate; LV: Left ventricular; BNP: Brain natriuretic peptide.
High-intensity exercise consists of aerobic HIIT and anaerobic SIT.
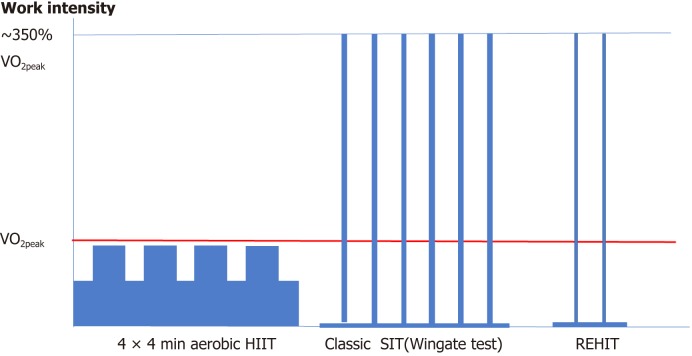
Figure 1 illustrates the representative protocols of aerobic HIIT and 2 anaerobic SITs, as well as a comparison of their intensities, duration, and frequencies. These exercise protocols require a shorter exercise duration to obtain the same benefit as that provided by moderate-intensity exercises. Although maintaining a high intensity exercise workout for a longer duration could be preferred, high-intensity exercise can be realistically tolerated by people with sedentary lifestyle, obesity, old age, or cardiac disease only in the form of interval training. In this regard, HIIT consists of brief, intermittent bursts of vigorous activity (less than VO2peak but usually involves < 100% [70%-90%] of VO2peak or 85%-95% of the peak heart rate) interspersed with active rest periods[22,28,29], whereas SIT is classically a Wingate-type protocol (all-out, vigorous-intensity exercise involving approximately 350% of VO2peak[30]) interspersed with longer complete rest periods. These high-intensity protocols are demanding for the subjects even though the intensity is adapted to the individual’s aerobic capacity and the rest period. Although the most popular and evidence-rich protocols are the Wingate test[31] for SIT, and the 4 × 4 min[28,32] or 10 × 1 min protocol for HIIT, many other protocols can be applied by modifying the workout duration, rest interval (work/rest ratio[33]), workout intensity, and workout frequency. The difference between HIIT and SIT is that SIT refers to anaerobic supramaximal VO2max (all-out) intensity and HIIT refers to aerobic submaximal VO2max intensity. The peak power output (PPO) of SIT is about 350% of the power output at VO2max[30]. Meanwhile, the common elements between the two protocols are the high work intensity adapted to the current aerobic capacity of the individual, and the aim of improving both aerobic capacity (VO2peak) and metabolic capacity. However, the risk of these protocols has also been a concern, and more studies are warranted before these protocols are adopted to more common use. A supervised workout is mandatory to maintain high-intensity adherence until the participants become accustomed to the intensity and to heart rate measurements during physical activity by using a wearable heart rate monitoring device. Home-based HIIT is also possible if experienced management programs are provided by renowned centers[34].
Figure 1.
Schema of high-intensity interval training (HIIT) protocols. Adapted from Ito S. EC Cardiology 6.3 (2019): 196-200. HIIT is classified into two types: submaximal aerobic HIIT and all-out anaerobic HIIT [sprint interval training (SIT)]. Reduced-exertion HIIT (REHIT) is a low-dose and shorter SIT that is modified from SIT but is still an all-out anaerobic exercise. 4 × 4 min HIIT: four 4-min intervals at 90%-95% of maximal heart rate separated by 3-min active recovery periods of moderate intensity at 60%-70% of the maximal heart rate. Classic SIT: repeated (6-8) all-out bouts at vigorous intensity ~350% of VO2peak of short duration (30 s) followed by a long complete rest (2-5 min). REHIT: 10-min cycling session at 25 W interspersed with 1 (first session) or 2 (all remaining sessions) Wingate-type cycle-sprints against a constant torque of 0.65 Nm・kg lean mass−1. Sprints last 10 s in sessions 1-4, 15 s in sessions 5-12, and 20 s in the remaining 12 sessions.
Definitions of HIIT and SIT
Unfortunately, the definition of “HIIT” varies across different studies. This review uses the recently suggested definition, which describes HIIT as high-intensity exercise with aerobic intervals, with the target intensity existing in submaximal VO2max between 85% and 95% of the peak heart rate[35]. This definition is distinct from that of SIT, which involves low-volume supramaximal (i.e., all-out) performance[36]. Often, the term “aerobic HIIT” is used for HIIT with sub-VO2max intensity. In this regard, in this review, SIT was evaluated separately from HIIT because its intensity is about 3.5-fold (350% VO2max) the intensity of HIIT; thus, SIT is a very demanding exercise protocol and has been deemed adaptable only to young healthy people in previous studies[36,37]. Elderly people, those with lifestyle-related diseases other than diabetes mellitus, and patients with CVDs have been excluded from the target subjects of SIT.
Representative HIIT protocols
The exercise duration of HIIT has been defined as 30 s to several minutes. This type of HIIT has been adapted for people with lifestyle-related diseases with or without cardiac diseases. There have been RCTs comparing HIIT and MCT for patients with coronary artery diseases (Table 2, showing positive[28,38-41] and negative[19,23,42,43] results) and CHF (Table 3, showing positive[22,25,26,44] and negative[24,45-47] results), with the aim of improving aerobic capacity[48]. The protocols of HIIT and the number of studies showing the superiority of HIIT over MCT in each protocol are shown in Table 4. In both groups, the 4 × 4 min protocol was the most frequently used, showing positive rate of 70.2% in the coronary artery disease group and 75% in the CHF group. The other protocols with exercise durations of 30 s, 2 min, and 3 min were also effective in a limited number of studies.
Table 2.
Mode, intensity, and VO2peak increment in high-intensity interval training versus moderate-intensity continuous training in randomized controlled trials (coronary artery disease)
| Study | Published yr | Sample | n | HIIT | MCT | Duration | Mode | VO2peak pre | VO2peak %increase | ||
| HIIT | MCT | HIIT (%) | MCT (%) | ||||||||
| 1 Rognmo et al[28] | 2004 | CAD | 17 (HIIT = 8) | 3 d/wk 4 x 4 min@80%-90% VO2peak total 33min | 3 d/wk 41 min@50%-60% VO2peak isoload to HIIT | 10 wk | TM | 31.8 | 32.1 | 17.9a | 7.9 |
| 2 Warbur-ton et al[41] | 2005 | CAD (previous CABG or AP) | 14 (HIIT = 7) | 2 d/wk, 2 min@90%VO2R, 2 min recovery, 30 min total | 2 d/wk 30 min @65%VO2R, average training volume similar to HIIT | 16 wk | TM etc1 | 22 | 21 | 31.8a | 9.5 |
| 3 Tjønna et al[21] | 2008 | Metabolic syndrome | 28 (HIIT = 9) | 3 d/wk 4 × 4 min@90%HRmax, 3 min active recovery @70% HRmax 40 min total | 3 d/wk 47 min @70% HRmax, equalized training volume | 16 wk | TM | 33.6 | 36 | 35a | 16 |
| 4 Moholdt et al[43] | 2009 | post CABG | 59 (HIIT = 28) | 5 d/wk 4 × 4 min@90%HRpeak, 3 min recovery | 5 d/wk 46 min + Aerobic group exercise, iso energic to HIIT | 4 wk | TM | 27.1 | 26.2 | 12.1 | 8.8 |
| 5 Moholdt et al[40] | 2011 | post MI | 89 (HIIT = 30) | 2 d/wk 4 × 4 min@85%-95%HRpeak, 3 min recovery | 2 d/wk 60 min@58% PPO | 12 wk | TM1 | 31.6 | 32.2 | 14.6a | 7.8 |
| 6 Rocco et al[23] | 2012 | CAD | 37 (HIIT = 17) | 3 d/wk 7 × 3 min@RCP, 7×3 min recovery@VAT total 42 min | 3 d/wk 50 min@VAT | 3 mo | TM | 18 | 17.9 | 23.3 | 24.6 |
| 7 Currie et al[51] | 2013 | recent event CAD post PCI, CABG, etc | 22 (HIIT = 11) | 2 d/wk 10 × 1 min@89% (80%-104%) PPO, 1 min recovery@10%PPO, 1 d/wk home-based @similar intensity | 2 d/wk 30-50 min @58% PPO, 1d/wk home-based @similar intensity not isocaloric | 12 wk | bike | 19.8 | 18.7 | 24 | 19 |
| 8 Keteyian et al[38] | 2014 | Stable CAD (post MI CABG and/or PCI) | 28 (HIIT = 15) | 3 d/wk 4 × 4 min@80%-90%HHR | 3 d/wk 30 min@60%-70%HRR | 10 wk | TM | 22.4 | 21.8 | 16a | 8 |
| 9 Madssen et al[39] | 2014 | CAD with stents | 36 (HIIT = 16) | 3 d/wk 4 × 4 min@85%-95%HRpeak, 3 min active recovery@70%HRpeak | 3 d/wk 46 min@ 70%HRmax, isocaloric | 12 wk | TM | 31.2 | 29.8 | 10.6a | 6.7 |
| 10 Conraads et al[19] | 2015 | CAD | 173 (HIIT = 85) | 3 d/wk 4 × 4 min@90%-95%HRpeak, 3 min active recovery | 3 d/wk 37 min@ 70%-75% %HRmax | 12 wk | bike | 23.5 | 22.2 | 22.7 | 20.3 |
Adapted from Ito S et al. Internal Medicine. 2016; 55: 2329-2336.
in VO2peak % increase raw: There is significant difference in % increase of VO2peak between HIIT and MCT. 4 × 4 min means 4 × 4 min intervals per one HIIT training session. Study 2: a data shown is VO2 at anaerobic threshold. Data is shown in figure without exact value at VO2peak (30+ in HIIT 30 in MCT)., and %increase at peak exercise is similar. TM etc1 means TM or stair climber,or, upper leg ergometer. Study 4: There was no difference at 4 wk: Increase of VO2peak between 4 wk and 6 mo was significant within HIIT and between HIIT and MCT. The participant attended additional sessions with various intensity at the center with their choice. Exercise was performed at center for 4 wk and at home for 6 mo. Study 5: TM1 means TM or aerobic exercise. AP: Angina pectoris; bike: Cycle ergometer; Cont: Continuous; CABG: Coronary artery bypass graft; CAD: Coronary artery disease; TM: Treadmill; HIIT: High-intensity interval training; HRpeak: Peak heart rate; HRR: Heart rate reserve; MCT: Moderate-intensity continuous training; PPO: Peak power output; RCP: Respiratory compensation point; VAT: Ventilator anaerobic threshold; VO2R: VO2 reserve; WRp: Peak work rate.
Table 3.
Mode, intensity, and VO2peak increment in high-intensity interval training versus moderate-intensity continuous training (congestive heart failure or diastolic dysfunction) in randomized
| Study | Published yr | Sample | n | HIIT | MCT | Duration | Mode | VO2peak pre | VO2peak %increase | ||
| HIIT | MCT | HIIT (%) | MCT (%) | ||||||||
| 1 Dimo-poulos et al[24] | 2006 | CHF | 24 (HIIT = 10) | 3 d/wk, 30 seconds@100% WRp, 30 s rest | 3 d/wk, 40 mins@50%WRp | 36 sessions | bike | 15.4 | 15.5 | 7.8 | 5.8 |
| 2 Wisloff et al[22] | 2007 | CHF, Post MI | 27 (HIIT = 9) | 3 d (2 d supervised)/wk 4 × 4 min @90%-95%HRpeak, 3 min active recovery 50%-70% HRpeak, total 38 min | 3 d (2 d supervised)/wk, 47 min@70%-75% HRpeak, isoload to HIIT | 12 wk | TM | 13 | 13 | 46a | 14 |
| 3 Roditis et al[46] | 2007 | CHF | 21 (HIIT = 11) | 3 d/wk 30 secc @WRpeak 30 s rest, total of 40 min | 3 d/wk 40 min@50%WRpeak, equal to total work of HIIT | 36 sessions | bike | 14.2 | 15.3 | 8.5 | 8.5 |
| 4 Smart et al[47] | 2012 | CHF (LVEF< 35%) | 20 (HIIT = 10) | 3 d/wk 30 × 1 min @70% VO2peak, 1 min recovery | 3 d/wk 30 min@70%VO2peak, same absolute volume of work | 16 wk | bike | 12.2 | 12.4 | 21 | 13 |
| 5 Freyssin et al[26] | 2012 | CHF (LVEF< 40%) | 26 (HIIT = 12) | 5 d/wk 12 × 30 sec@50% (4 wk) + 80% (4 wk) of maximum powerb 1 min @ complete rest | 5 d/wk 45 min@HRVT1c | 8 wk | Bike (HIIT), bike + TM (MCT) | 10.7 | 10.8 | 27.1a | 1.9 |
| 6 Fu et al[44] | 2013 | CHF (LVEF≦40%) NYHA II, III | 45 (HIIT = 15) | 3 d/wk 5 × 3 min@80%VO2peak 3 min recovery@40% VO2peak | 3 d/wk 60 min @60% VO2peak, isoload to Int | 12 wk | bike | 16 | 15.9 | 22.5b | 0.6 |
| 7 Iellamo et al | 2013 | CHF with OMI (LVEF< 40%) | 20 (HIIT = 10) | 2-5 d/wk 2-4 × 4 min@75%-80%HRR, 3 min active pause walk@45%-50%HRR | 2-5 d/wk 30-45 min @45%-60%HRR, equated training load (TRIMPi) | 12 wk | TM | 18.7 | 18.4 | 8 22 | 4 22 |
| 8 Hollekim-Strand et al[20] | 2014 | diastolic dysfunction with Diabetes mellitus | 37 (HIIT = 20) | 3 d/wk 4 × 4 min @90%-95%HRpeak, total 40 min | Current guideline 10 min/bout 210 min/wk) | 12 wk, Home-based thereafter | unknown | 31.5 | 33.2 | 13.0a | 3.6 |
| 9 Angadi et al[25] | 2015 | CHF with preserved EF | 15 (HIIT = 9) | 3 d/wk 4 × 4 min @85%-90%HRpeak, 3 min active recovery | 3 d/wk 30 min@70%HRpeak | 4 wk | 19.2 | 16.9 | 9.4a | 0 | |
| 10 Ellingsen et al[45] | SMARTex-HF, 2017 | Stable CHF (NYHA2-3) EF≦35% | 200 (3 arms) (HIIT=77) | 25 sessions 4 × 4 min@90%-95% HRpeak, 3 min active recovery 50%-70% HRpeak total 38 min | 25 sessions, 47 min@60-70%HRpeak | 12 wk | bike or TM | 0.9 | 1.1 | 5.4 | 6.8 |
| 11 Suchy C et al | OptimEX-CLIN, Ongoing | HFpEF | 180 (HIIT 60) | 3 d/wk 4 × 4 min@ 90%-95% HR peak, 3 min active recovery 50%-70% HRpeak, total 38 min | 5 d/wk 40 min@60%-70%HRpeak | 3, 12 mo, home-based after 3 mo | bike | ? | ? | ? | ? |
Controlled Trials Adapted from Ito S et al. Internal Medicine. 2016; 55: 2329-2336.
ain pre VO2peak % increase raw: There is significant difference in % increase of VO2peak between HIIT and MCT. Study 5: b each training session consisted of 3 series (12 repetitions of 30 s of exercises, separated by 5 minutes of rest); c half of the MCT was on a treadmill and half on a bike. Study 6: b pre versus post (not between groups). Study 7: Study hypothesis is similar adaptation in HIIT and MCT. Study 9: a evaluated by standardized effect size (d = 0.94) Bike: Cycle ergometer; CAD: Coronary artery disease; CHF: Congestive heart failure; EF: Ejection fraction; HRpeak: Peak heart rate; HIIT: High intensity interval training; HRVT1: Heart rate at the first ventilator threshold; HRR: Heart rate reserve; LVEF: Left ventricular ejection fraction; MCT: Moderate-intensity continuous training; MI: Myocardial infarction; min, minute; NYHA: New York Heart Association; RCP: Respiratory compensation point; VAT: Ventilator anaerobic threshold; PPO: Peak power output; TM: Treadmill; VO2peakR: VO2peak reserve; VT1: First ventilator threshold; WRp: Peak work rate.
Table 4.
High-intensity interval training (HIIT) protocol and superiority of HIIT to moderate-intensity continuous training in VO2peak improvement
| Protocol | No. of study | More improvement of VO2peak in HIIT than in MCT | |
| Coronary artery disease | 10 × 1 min | 1 | 0/1 |
| 8 × 2 min | 1 | 1/1 | |
| 7 × 3 min | 1 | 0/1 | |
| 4 × 4 min | 7 | 5/7 (70.2%) | |
| Chronic heart failure | 40 × 30 s | 3 | 1/3 |
| 30 × 1 min | 1 | 0/1 | |
| 5 × 3 min | 1 | 1/1 | |
| 4 × 4 min | 6 | 3/4 (75%) | |
| 56% (5/9) 2 studies ongoing |
Randomized controlled trials comparing improvement of VO2peak after exercise between HIIT and MCT in patients with CAD or CHF are shown. The protocols of HIIT and incidence of superiority of HIIT to MCT in each protocol are shown. In both groups 4×4 min was most frequently used showing positive rate 70.2% in the coronary artery disease group and 75% in the chronic heart failure group. The other protocols with 30 s, 2 min, and 3 min exercise duration are also effective in the limited number of studies. HIIT: High-intensity interval training; MCT: Moderate-intensity continuous training; CAD: Coronary artery disease; CHF: Chronic heart failure.
The 4 × 4 min protocol is popularly used in patients with lifestyle-related plus cardiac disease, and was initially adapted for cardiac disease by Wisløff and Rognomo et al[22,28]. In the first RCT on HIIT in a clinical setting, Rognmo et al[28] evaluated the effects of HIIT compared with those of MCT, with the same total training load, and found that HIIT produced a higher increase of VO2peak in patients with stable coronary artery disease than MCT. This trial adapted the 4 × 4 min method for patients with cardiac disease for the first time, using the same protocol as that used by the same group for young football players[32]. Leading researchers have reported the positive effects of HIIT on aerobic and metabolic capacity in single-center RCTs and meta-analyses. According to several RCTs, HIIT was superior in improving VO2peak in 60% (6/10) of patients with coronary artery disease and in 45.6% of those with CHF (Table 4). The effect of HIIT depends on the workout duration/rest ratio. In contrast, the latest multicenter RCT [Study of Myocardial Recovery After Exercise Training in Heart Failure: (SMARTEX)] showed a negative result using the 4 × 4 min method for patients with CHF with reduced left ventricular dysfunction[45] despite many other studies reporting positive results[22,25,26,44]. Furthermore, this study clarified a problem in this protocol: A low adherence to the exercise intensity. There was a large overlap in the intensity between the HIIT and MCT groups, and this could be a key factor explaining the lack of a difference in the increase of VO2peak between groups[45,49].
Although the 4 × 4 min aerobic HIIT protocol has been used in many studies, it did not consistently yield good results. Some researchers do not recommend this protocol because they believe that the load is excessive and the workout duration is too long for patients with sedentary/cardiac diseases, suggesting that it is a clinically unrealistic training method.
The aerobic 10 × 1 min HIIT protocol has also been developed by Gibala’s group for broader targets including people with obesity and a sedentary lifestyle by decreasing the intensity from all-out performance to approximately VO2max and by increasing each workout duration from 30 s to 60 s[29,50]. The number of repetitions was increased from 4-6 to 8-12 during the training course. This led to concomitant doubling of the total external energy expenditure. This protocol was utilized for patients with coronary artery disease by Currie et al[42,51] and in patients with CHF by Smart and Steele[47]. In an RCT comparing the 10 × 1 min HIIT and MCT, HIIT was not found to be superior in improving VO2peak. The intensity of exercise was similar to that of the 4 × 4 min aerobic HIIT protocol. Each workout duration was as short as 1 min, but the frequency was higher than that of the 4 × 4 min. There are fewer studies about the 10 × 1 min HIIT protocol than those on the 4 × 4 min protocol. The duration of 1 min at 89% (80%-104%) PPO[51] might be rather short because the target heart rate cannot be attained within that time.
RCTs that compare the superiority of multiple different HIIT protocols in improving aerobic and metabolic parameters are limited[52,53]. Thus, researchers tend to select the protocol based on their experience, or they modify the exercise parameters (work and rest time). The effects of varying interval training intensities on the 40-km time-trial performance of trained cyclists were evaluated in a single study by Stepto et al[53], in which well-trained male cyclists were randomly assigned to 1 of 5 groups with different HIIT protocols (12 × 30 s at 175% PPO, 12 × 1 min at 100% PPO, 12 × 2 min at 90% PPO, 8 × 4 min at 85% PPO, and 4 × 8 min at 80% PPO). The cyclists completed 6 HIIT sessions over a 3-week period in addition to their habitual aerobic base training. The groups that followed the 12 × 30 s and 4 × 8 min protocols showed better improvement with respect to speed.
Unique HIIT protocols
The aim of recent exercise trends is to obtain benefits with the lowest and shortest workload.
Several groups have tried to establish shorter protocols in HIIT and SIT. These seem to be beneficial to the physical structure and fitness even in targets with lifestyle-related diseases, old age, or cardiac disorders. To overcome the criticisms of the 4 × 4 min protocol, a couple of finely tuned HIIT protocols, in which the frequency, workload, and work duration are initially set at low levels and altered during the training course, have been reported by several researchers[15,54-57].
Matsuo et al[15]: The Japanese high-intensity interval aerobic training (J-HIAT) program: 3 sets of 2–3-min cycling at vigorous intensity (first and second sets: 3 min at 85%-90% VO2peak, third set: 3 min at 80%-85% VO2max) with 2-min active rest at 50%VO2peak between each set (healthy, sedentary young 20–30-year-old adults)[15]. This protocol was developed to control energy expenditure for astronauts participating in long-term space missions.
Osuka et al[58]: The elderly Japanese male version of high-intensity interval aerobic training (EJ-HIAT): 3 sets of 2–3 min cycling at 75%-85% VO2peak (first set: 3 min at 85% VO2peak, second set: 2 min at 80% VO2peak, and third set: 2 min at 75% VO2peak) with 1–2-min active rest at 50% VO2peak (first set: 2 min, second set: 1 min) (60–69-year-old sedentary elderly men; mean age, 67.6 ± 1.8 years). A gradually decreasing load was planned for 2-3 wk, aiming at the protocol described above. A significant aerobic and metabolic response was attained by the shorter protocol than the 4 × 4 min protocol with a completion rate of 100%.
Alvarez et al[54,55]: During all training sessions, patients were instructed by the exercise specialists to jog/run and walk at a steady pace, which should be controlled by maintaining a score of 15–17 (jogging/running) and < 9 (walking) in the 15-point rating of perceived exertion scale. The goal was to reach 90%-100% and 70% of their predicted reserve heart rate at the end of the jogging/running and walking intervals, respectively. The progressive HIIT protocol started (1–2 wk) with 8 jogging/running intervals of approximately 30 s interspersed with approximately 120 s of low-intensity walking. To promote sufficient workloads for eliciting improvements throughout the 12-wk follow-up, there was a 7%-10% increase in the high-intensity interval duration and a 4% decrease in the recovery interval duration every 2 wk. There was also an increase of 2 exercise intervals every 4 wk of follow-up. The total workout duration increased from 4 to 13.5 min (weeks 1–16). The total recovery duration ranged from 18 to 24 min (weeks 1–16). The number of intervals ranged from 8 to 14 (weeks 1–16). The exercise duration ranged from 30 to 58 s. The target subjects were overweight/obese adult women aged 35–55 years with type 2 diabetes (T2D).
SIT
Classic SIT (Wingate test)
Because SIT is the highest-intensity workout program that needs an intensity more than the VO2peak, the protocol is characterized by a short duration (30 s workout), followed by a long complete rest (2-5 min). This causes acute hemodynamic changes, such as abrupt blood pressure and heart rate increases, which may lead to a disruption of plaque and visceral organ ischemia by blood flow redistribution. Thus, SIT should be adapted only for young sedentary/recreationally active subjects but not for patients with hypertension, chronic kidney disease, and CVDs under the classic SIT protocol. Allemeier et al[59] demonstrated that VO2max can be improved by approximately 14% by as little as three repeated Wingate sprints per training session. The classic SIT protocol incorporating up to six repeated 30-s Wingate sprints was first used in a study by Barnett et al[60], who reported an 8% increase in VO2max and a 42% increase in maximal citrate synthase activity after 8 wk of SIT. This protocol was subsequently used by Gibala’s group with minor modification, to investigate the aerobic adaptation associated with classic SIT[36,37]. Although classic Wingate protocols[31] use “4-6” repeated 30-s sprints, none of the studies provided a specific justification for the use of this method. Thus far, no studies have attempted to justify the 4-6 × 30 s Wingate sprints as an optimal SIT protocol[61].
The effects of 8-10 × 30-s Wingate sprints in the 1980s and 1990s included the following wide-ranging parameters: Maximal glycolytic and mitochondrial enzyme activity[62,63], purine metabolism[64], pulmonary and muscle gas exchange[65], muscle metabolism and ion regulation[66], muscle buffering capacity[67], erythrocyte characteristics[68], and improvement of VO2max[63,65,66,68].
Concept of “low-volume/shorter” SIT for adaptation to a wide range of sedentary/ recreationally active people
It has been consistently shown that a single 30-s Wingate sprint can reduce muscle glycogen stores in the vastus lateralis by 20%-30%[61,69-72]. What is intriguing, however, is that glycogenolysis is only activated during the first 15 s of the sprint and is then strongly attenuated during the final 15 s[72]. Moreover, activation of glycogenolysis is inhibited in subsequent repeated sprints[72]. This suggests that the classic SIT (4-6 repeated 30-s Wingate sprints) may be unnecessarily strenuous, as similar glycogen depletion may be achieved with 1–2 sprints of a shorter duration (15-20 s)[61,73,74]. In turn, this would make the training sessions more time efficient, less strenuous, and more applicable to the sedentary general population. Hazell et al[75] directly compared the impact of reducing the sprint duration in the classic SIT protocol from 30 s to 10 s, and reported similar increases in VO2max with the 10-s protocol. Similarly, Zelt et al[76] reported no significant difference in the VO2max response to the classic SIT protocol with 30-s sprints (4%) and a modified protocol with 15-s sprints (8%). Similar to a reduction in the sprint duration, a reduction in the number of sprint repetitions was evaluated in two studies. Allemeier et al[59] and Ijichi et al[16] demonstrated robust improvements in VO2max after a protocol involving three repeated 30-s Wingate sprints. The protocol in these two studies had longer passing interval durations of 20 and 10 min, respectively.
One possible alternative strategy could be to define the minimum volume of exercise required to improve health indices with the aim of increasing exercise adherence. Vollaard et al[77] reviewed SIT protocols with the shortest duration and least amount of work. They also constructed a modified SIT aiming for the most time-efficient and effective protocol with high adherence for sedentary subjects and diabetic patients[61,74,78]. To date, this training protocol, named reduced-exertion HIIT (REHIT) (10-min SIT sessions, 3 sessions a week for 6 wk, involving only two 20-s Wingate sprints), represents the smallest volume of exercise (when considered per session) that has been shown to induce positive effects on health. This protocol was sufficient to improve VO2max by 10%-13%[61,74]. Vollaard et al[77]also found that after performing only two maximal sprint intervals, each additional sprint in a training session reduced the overall improvement in fitness by around 5%. It is important to remember that these findings are only applicable to supramaximal exercise, which requires specialized exercise bikes that enable extremely high intensity exercise. This result might raise questions about the previously held “common sense” idea that performing more repetitions of high-intensity exercise would produce greater improvements in cardiorespiratory fitness. Ruffino et al[78] compared the effects of REHIT and moderate-intensity walking on health markers in patients with T2D in a counterbalanced crossover study. Sixteen men with T2D (mean age: 55 ± 5 years) completed 8 wk of REHIT and 8 wk of moderate-intensity walking (five 30-min sessions/wk at an intensity corresponding to 40%-55% of the heart rate reserve), with a 2-mo washout period between interventions. They concluded that REHIT was superior to a 5-fold larger volume of moderate-intensity walking in improving aerobic fitness but had a similar result in terms of improving insulin sensitivity or glycemic control in patients with T2D in the short term. In studies evaluating REHIT, subjects with age > 60 years, uncontrolled hypertension, liver dysfunction, and renal dysfunction were excluded. Although evidence for patients with these comorbidities are lacking, the REHIT protocol might have a potential application for patients with some lifestyle-related diseases if careful attention is paid to hemodynamic changes, especially blood pressure spikes.
FEASIBILITY OF AND LIMITATIONS IN ADOPTING HIIT AND SIT FOR SEDENTARY/OBESE/ELDERLY/DISEASED SUBJECTS
HIIT
Even in exercise training with submaximal aerobic HIIT, adequate adherence to the target intensity and frequency was not achieved in multicenter RCTs[8,45,49]. Another clear limitation is the large dropout rate during follow-up after the supervised exercise period[79]. HIIT has been accepted for patients with cardiac diseases, as shown in the protocols in Table 4. Although the target heart rate was as high as 90%-95% of the peak heart rate, the intensity was calculated from an individual’s peak heart rate, and these aerobic HIIT protocols could be utilized for a wide range of targets subjects including the elderly and patients with diseases.
SIT
The tolerability and adherence of SIT for non-athletes and sedentary people is low.
The target subjects in previous studies on SIT include young people who are healthy and/or recreationally active. The number of subjects in each study was very small, and there might be bias in the selection of study subjects. It was possible that subjects who have no or little experience in sports/exercise training, irrespective of age, may have had difficulties in performing the all-out exercises. In this regard, REHIT may widen the target subjects owing to its smallest volume of exercise among the available protocols. Furthermore, it can be adapted for all age groups of sedentary, recreationally active, and of course highly trained people, but not in those who are sedentary, aged > 60 years, and with CVD (personal communication with Dr. Vollaard).
POTENTIAL RISKS OF THE HIIT AND SIT PROTOCOLS
HIIT
Previously reported studies on HIIT had small numbers of subjects and contained limited reference about the safety and injury risk of this training protocol in the general population. A Norwegian group observed only two knee injuries in extremely overweight patients[21]. Levinger et al[80] published a systematic review about adverse events during or immediately after HIIT. They found that the incidence of adverse responses during or 24 h after HIIT, as acute responses to a single session of HIIT, in patients with cardiometabolic diseases was around 8%, which was somewhat higher than the previously reported risk during MCT[80]. Rognmo et al[81] examined the risk of cardiovascular events during organized HIIT and MCT among 4846 patients with coronary heart disease in 3 Norwegian cardiac rehabilitation centers. In a total of 175820 exercise training hours, during which all patients performed both types of training, 1 fatal cardiac arrest during moderate-intensity exercise (129456 exercise hours) and 2 nonfatal cardiac arrests during HIIT (46364 exercise hours) were reported. No myocardial infarctions were reported. They concluded that the risk of a cardiovascular event was low after both HIIT and MCT in a cardiovascular rehabilitation setting[81].
SIT and low-dose/shorter SIT
Systematic reviews on the safety and injury risk of SIT are very limited. Supramaximal sprints used in protocols such as the Wingate protocol are associated with a short but sharp increase in blood pressure as well as an increase in blood flow, which could pose a risk of dislodging unstable plaques. Redistribution of blood flow (increased flow in muscle followed by decreased flow in visceral organs) might pose a risk to patients with CVD and chronic kidney disease. However, SIT or shorter/low-dose SIT has been adopted only in healthy, sedentary, and usually young people. For these subjects, the cardiovascular risk could be very low because the incidence of hypertension and/or atheroscrelotic disease is low. For individuals with lifestyle-related diseases and/or CVD, the potential risk of the SIT/REHIT protocol has not been evaluated. Thus, currently, it should not be adopted for these individuals. Ruffino et al[78] investigated REHIT for patients with T2D, and neither risk nor cardiovascular event was reported.
INTRODUCTION OF THE OPTIMAL INTENSITY/DOSE OF ACTIVITY IN DAILY LIFE: PERSONAL ACTIVITY INDEX
Other than supervised exercise training using sophisticated exercise protocols, non-supervised daily training and activity could also be useful to improve aerobic capacity. For activity counseling and promotion of physical activity, providing some feedback to individuals with personalized and meaningful information would be beneficial to motivate them to increase or maintain their physical activity[73,82]. Goals such as “10000 steps per day” or “30 min of activity per day,” which are the same for all people, are easily understandable but do not reflect the body’s response to each activity. The goal “10000 steps” has a different meaning for each individual [e.g., what speed, where (uphill or downhill)]. The most personalized, accurate way to track and measure the body’s response to activity is by monitoring the heart rate. Changes in heart rate reflect the body’s response to physical activity regardless of the activity type. Because there has never been a simple way to convert heart rate to a metric, Nes et al[83] developed a new single metric called the Personalized Activity Index (PAI). PAI can be integrated in self-assessment heart rate devices and defines a weekly beneficial heart rate pattern during physical activity. Furthermore, PAI could translate into reduced long-term risk of premature CVD and all-cause mortality, according to the epidemiologic study (HUNT)[83-85] performed in Nord-Trøndelag county in Norway, which analyzed a large, apparently healthy, general population cohort (n = 29950, aged ≥ 20 years). Obtaining a score of ≥ 100 weekly PAI has been shown to reduce the risk of premature CVD death in healthy subjects as well as in individuals with known CVD risk factors, regardless of whether or not the current physical activity recommendations were met[86]. PAI could inform potential users of how much physical activity is needed to reduce the risk of premature CVD death[83]. PAI users could also identify know the exercise intensity and time of exercise that are effective and efficient for appropriate exercise/physical activity according to their own daily experience followed by feedback. For example, exercising at very vigorous intensities may yield high PAI scores and higher VO2peak, even with considerably lower total exercise time than expressed in the current recommendations[84]. As a simple pattern, exercise once a week is also effective[85] if the exercise intensity is enough to improve VO2peak.
WORKING MECHANISMS OF HIIT AND SIT
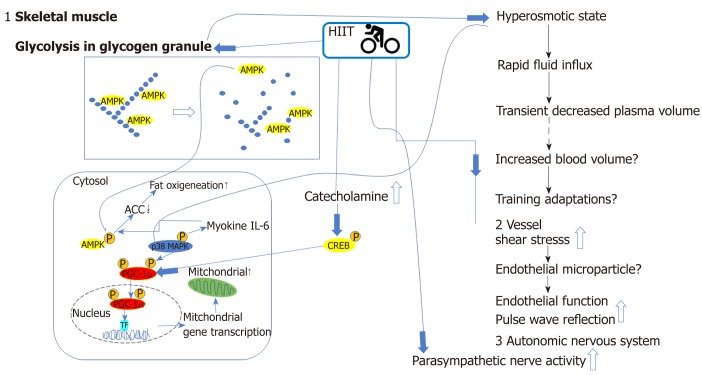
The mechanisms involved in the superiority of HIIT to MCT have not been clearly elucidated. However, there are several potential mechanisms[48] (Figure 2). The first reason for the improvement in the aerobic capacity with HIIT can be explained by the following intracellular signaling sequence[87]: Muscular stimulus by HIIT → increase in 5’-AMP-activated protein kinase (AMPK) activity in muscle cells → increase in peroxisome proliferator activated receptor-γcoactivator-1α (PGC-1α) mRNA and protein → increase in the mRNA and protein expression of the mitochondrial oxygenation enzyme → improvement in physical fitness (aerobic capacity)[88]. Secondly, it is reasonable to speculate that the higher shear stress in HIIT during exercise bouts may trigger greater responses at the cellular and molecular levels, leading to a partial recovery from endothelial dysfunction. Thirdly, Hanssen et al[89]recently reported another potential reason for the benefits of HIIT; they reported the acute effects of interval versus continuous-endurance training on pulse wave reflection in healthy young men. Although initially higher after HIIT, the augmentation index at a set heart rate declined in the 24-h follow-up period, indicating favorable effects on pulse-wave reflection compared with that after MCT. The possible mechanism of the REHIT protocol using two (but not three or more) repeated bouts of supramaximal 20-s workout was proposed by Vollaard et al[77,90]. The adaptations to SIT for VO2max may be peripheral in origin owing to improved skeletal muscle oxygen extraction because of mitochondrial density[77]. Vollaard et al[77]proposed that both increased blood volume and increased mitochondrial density could plausibly be explained by the rapid glycogen depletion associated with supramaximal exercise[73]. Glycogen breakdown during repeated supramaximal sprints has been shown to be completely attenuated by the time of the third sprint. Thus, it is plausible, according to the two speculated mechanisms[77,90] below, that performing only two repeated supramaximal sprints is sufficient to saturate the adaptive response.
Figure 2.
Graphic representation of beneficial cardiovascular and metabolic effects and relevant mechanisms activated by high-intensity interval training. Glycolysis of glycogen granules in the skeletal muscle, catecholamine release, increased shear stress in the vessels, and increased autonomic nerve activity by HIIT are related to increased aerobic and metabolic capacities. Activity in skeletal muscle cells and arteries are increased during HIIT. The decrease in glycogen content by glycolysis results in the release of the AMP-activated protein kinase (AMPK) from the glycogen particle, resulting in greater activity and altered localization. In addition, exercise in a low-glycogen state after glycolysis leads to the phosphorylation and activation of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α). Finally, the osmotic stress associated with a rapid change in glycogen content and increased glucose concentration can activate mitogen-activated protein kinases (MAPKs) such as p38, which can phosphorylate and activate PGC-1. Another target of p38 is interleukin 6 (IL-6), which targets AMPK as one of the potential targets. These alterations in muscle signaling also result in improved circulating fatty acid (FA) utilization. The increased catecholamine level promotes an increase in fat metabolism by activating heat shock protein through protein kinase A. An additional cellular target of catecholamine is the cAMP response element-binding protein (CREB). HIIT can increase the phosphorylation and activation of CREB in both exercised muscle and muscles that were not recruited during the exercise due to the central effects of elevated central nervous system activity. One of the targets of CREB is PGC-1α. An increase in PGC-1α mRNA and protein with co-activation of the transcription factor results in the increase in the mRNA and protein of the mitochondrial oxygenation enzyme, and finally, improvements in physical fitness (aerobic capacity). HIIT increases cardiac output, leading to shear stress in arteries and resulting in improvements in endothelial function and pulse wave reflection potentially through endothelial microparticles.ACC: Acetyl CoA carboxylase; AMPK: AMP-activated protein kinase; CREB: cAMP response element-binding protein; HIIT: High-intensity interval training; IL-6: Interleukin 6; MAPK: Mitogen-activated protein kinases; PGC1α: Peroxisome proliferator-activated receptor γ coactivator 1-α; TF: Transcription factor.
The first mechanism is as follows: maximal rates of glycogenolysis in the initial 15s of a supramaximal sprint → accumulation of metabolic derivatives → hypertonic intramyocellular environment → influx of water to the myocardium → transient approximately 15%-20% drop in plasma volume within a time span of only a few minutes. This severe disturbance of circulatory homeostasis could be a stimulus for the body to increase blood volume in response to repeated SIT sessions.
The second mechanism is as follows: Glycogenolysis → release and activation of glycogen-bound AMPK[91] → downstream signaling pathway involving PGC-1α → increased mitochondrial density.
FUTURE PERSPECTIVES
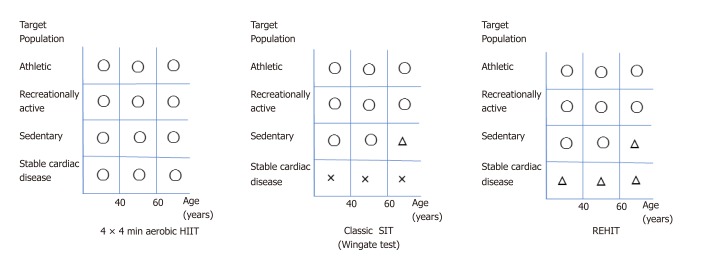
More studies are warranted to establish the most efficient protocol for each target subject according to clinical characteristics and fitness level, to improve aerobic capacity and to establish higher adherence. Thus far, aerobic HIIT (submaximal intensity) could be feasible and has a low risk for people with lifestyle-related diseases, obesity, sedentary lifestyle, old age, or cardiac disorders when performed, at their own individual intensity. In contrast, classic SIT (supramaximal) is applicable only for healthy young people. A smaller-dose and shorter SIT such as the 2 × 20 s protocol (REHIT) could be utilized for sedentary young/middle-aged targets. The feasibility and safety of REHIT for elderly and sedentary people, patients with stable ischemic heart disease and CHF, and patients with chronic kidney disease have not been evaluated. Figure 3 shows a personal proposal of HIIT protocols for target people stratified by age, exercise habits, and cardiovascular disease. Although the increased application of HIIT in the health and medical fields is expected, its feasibility and safety should be further evaluated in the near future.
Figure 3.
Personal proposal of high-intensity interval training (HIIT) protocols for target people stratified by age, exercise habits, and cardiovascular disease. 4 × 4 min HIIT: Can be adopted for all subjects, with the intensity maintained at 85%-95% of an individual ’s peak heart rate. Classic sprint interval training (SIT): The feasibility and safety of this protocol for patients complicated with cardiovascular disease have not been evaluated. Reduced-exertion HIIT (REHIT): Its feasibility and safety for patients complicated with cardiovascular disease have not been evaluated. Because REHIT is much less strenuous than classic SIT, future research on this protocol is expected for patients with stable cardiovascular diseases besides high-risk patients, such as those with refractory hypertension and coronary heart disease with atherosclerotic plaque. O: adaptable for all target subjects; Δ: potentially adaptable for target subjects without risk; ×: should be prohibited for all target subjects.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Invited manuscript
Peer-review started: February 12, 2019
First decision: March 15, 2019
Article in press: July 17, 2019
P-Reviewer: Kaypaklı O, Tousoulis D S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
Specialty type: Cardiac and cardiovascular systems
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
References
- 1.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 2.Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, Ekelund U, Katzmarzyk PT, Blair SN. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45:504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 3.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 4.DeFina LF, Haskell WL, Willis BL, Barlow CE, Finley CE, Levine BD, Cooper KH. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015;57:324–329. doi: 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57:306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 7.Piepoli MF, Corrà U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, Dendale P, Doherty P, Gaita D, Höfer S, McGee H, Mendes M, Niebauer J, Pogosova N, Garcia-Porrero E, Rauch B, Schmid JP, Giannuzzi P. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol. 2014;21:664–681. doi: 10.1177/2047487312449597. [DOI] [PubMed] [Google Scholar]
- 8.JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012) Circ J. 2014;78:2022–2093. doi: 10.1253/circj.cj-66-0094. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16 Suppl 1:3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 10.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 11.Lobelo F, Stoutenberg M, Hutber A. The Exercise is Medicine Global Health Initiative: a 2014 update. Br J Sports Med. 2014;48:1627–1633. doi: 10.1136/bjsports-2013-093080. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 13.Hambrecht R, Niebauer J, Fiehn E, Kälberer B, Offner B, Hauer K, Riede U, Schlierf G, Kübler W, Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25:1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 14.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo T, Saotome K, Seino S, Shimojo N, Matsushita A, Iemitsu M, Ohshima H, Tanaka K, Mukai C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med Sci Sports Exerc. 2014;46:42–50. doi: 10.1249/MSS.0b013e3182a38da8. [DOI] [PubMed] [Google Scholar]
- 16.Ijichi T, Hasegawa Y, Morishima T, Kurihara T, Hamaoka T, Goto K. Effect of sprint training: training once daily versus twice every second day. Eur J Sport Sci. 2015;15:143–150. doi: 10.1080/17461391.2014.932849. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara H, Ueda SY, Miyamoto T. Low-frequency severe-intensity interval training improves cardiorespiratory functions. Med Sci Sports Exerc. 2015;47:789–798. doi: 10.1249/MSS.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 18.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, Denollet J, Frederix G, Goetschalckx K, Hoymans VY, Possemiers N, Schepers D, Shivalkar B, Voigt JU, Van Craenenbroeck EM, Vanhees L. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. 2015;179:203–210. doi: 10.1016/j.ijcard.2014.10.155. [DOI] [PubMed] [Google Scholar]
- 20.Hollekim-Strand SM, Bjørgaas MR, Albrektsen G, Tjønna AE, Wisløff U, Ingul CB. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J Am Coll Cardiol. 2014;64:1758–1760. doi: 10.1016/j.jacc.2014.07.971. [DOI] [PubMed] [Google Scholar]
- 21.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL. Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 23.Rocco EA, Prado DM, Silva AG, Lazzari JM, Bortz PC, Rocco DF, Rosa CG, Furlan V. Effect of continuous and interval exercise training on the PETCO2 response during a graded exercise test in patients with coronary artery disease. Clinics (Sao Paulo) 2012;67:623–628. doi: 10.6061/clinics/2012(06)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimopoulos S, Anastasiou-Nana M, Sakellariou D, Drakos S, Kapsimalakou S, Maroulidis G, Roditis P, Papazachou O, Vogiatzis I, Roussos C, Nanas S. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:67–73. doi: 10.1097/01.hjr.0000198449.20775.7c. [DOI] [PubMed] [Google Scholar]
- 25.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) 2015;119:753–758. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 26.Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. 2012;93:1359–1364. doi: 10.1016/j.apmr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sports Med. 2013;43:313–338. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 28.Rognmo Ø. Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 29.Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol (1985) 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 31.Sands WA, McNeal JR, Ochi MT, Urbanek TL, Jemni M, Stone MH. Comparison of the Wingate and Bosco anaerobic tests. J Strength Cond Res. 2004;18:810–815. doi: 10.1519/13923.1. [DOI] [PubMed] [Google Scholar]
- 32.Helgerud J, Engen LC, Wisloff U, Hoff J. Aerobic endurance training improves soccer performance. Med Sci Sports Exerc. 2001;33:1925–1931. doi: 10.1097/00005768-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Kavaliauskas M, Aspe RR, Babraj J. High-Intensity Cycling Training: The Effect of Work-to-Rest Intervals on Running Performance Measures. J Strength Cond Res. 2015;29:2229–2236. doi: 10.1519/JSC.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 34.Aamot IL, Karlsen T, Dalen H, Støylen A. Long-term Exercise Adherence After High-intensity Interval Training in Cardiac Rehabilitation: A Randomized Study. Physiother Res Int. 2016;21:54–64. doi: 10.1002/pri.1619. [DOI] [PubMed] [Google Scholar]
- 35.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48:1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 36.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keteyian SJ, Hibner BA, Bronsteen K, Kerrigan D, Aldred HA, Reasons LM, Saval MA, Brawner CA, Schairer JR, Thompson TM, Hill J, McCulloch D, Ehrman JK. Greater improvement in cardiorespiratory fitness using higher-intensity interval training in the standard cardiac rehabilitation setting. J Cardiopulm Rehabil Prev. 2014;34:98–105. doi: 10.1097/HCR.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 39.Madssen E, Moholdt T, Videm V, Wisløff U, Hegbom K, Wiseth R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol. 2014;114:1504–1511. doi: 10.1016/j.amjcard.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Moholdt T, Aamot IL, Granøien I, Gjerde L, Myklebust G, Walderhaug L, Hole T, Graven T, Stølen T, Mølmen-Hansen HE, Støylen A, Skogvoll E, Slørdahl SA, Wisløff U. Long-term follow-up after cardiac rehabilitation: a randomized study of usual care exercise training versus aerobic interval training after myocardial infarction. Int J Cardiol. 2011;152:388–390. doi: 10.1016/j.ijcard.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Warburton DE, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, Chan SY. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95:1080–1084. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 42.Currie KD, Bailey KJ, Jung ME, McKelvie RS, MacDonald MJ. Effects of resistance training combined with moderate-intensity endurance or low-volume high-intensity interval exercise on cardiovascular risk factors in patients with coronary artery disease. J Sci Med Sport. 2015;18:637–642. doi: 10.1016/j.jsams.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Moholdt TT, Amundsen BH, Rustad LA, Wahba A, Løvø KT, Gullikstad LR, Bye A, Skogvoll E, Wisløff U, Slørdahl SA. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158:1031–1037. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, Liu MH, Chiang CL, Wang JS. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167:41–50. doi: 10.1016/j.ijcard.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 45.Ellingsen Ø. Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A; SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group. High-Intensity Interval Training in Patients With Heart Failure With Reduced Ejection Fraction. Circulation. 2017;135:839–849. doi: 10.1161/CIRCULATIONAHA.116.022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roditis P, Dimopoulos S, Sakellariou D, Sarafoglou S, Kaldara E, Venetsanakos J, Vogiatzis J, Anastasiou-Nana M, Roussos C, Nanas S. The effects of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2007;14:304–311. doi: 10.1097/hjr.0b013e32808621a3. [DOI] [PubMed] [Google Scholar]
- 47.Smart NA, Steele M. A comparison of 16 weeks of continuous vs intermittent exercise training in chronic heart failure patients. Congest Heart Fail. 2012;18:205–211. doi: 10.1111/j.1751-7133.2011.00274.x. [DOI] [PubMed] [Google Scholar]
- 48.Ito S, Mizoguchi T, Saeki T. Review of High-intensity Interval Training in Cardiac Rehabilitation. Intern Med. 2016;55:2329–2336. doi: 10.2169/internalmedicine.55.6068. [DOI] [PubMed] [Google Scholar]
- 49.Karlsen T. Aamot IL, Haykowsky M, Rognmo Ø, High Intensity Interval Training for Maximizing Health Outcomes. Prog Cardiovasc Dis. 2017;60:67–77. doi: 10.1016/j.pcad.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Currie KD, Dubberley JB, McKelvie RS, MacDonald MJ. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc. 2013;45:1436–1442. doi: 10.1249/MSS.0b013e31828bbbd4. [DOI] [PubMed] [Google Scholar]
- 52.Bækkerud FH. Solberg F, Leinan IM, Wisløff U, Karlsen T, Rognmo Ø. Comparison of Three Popular Exercise Modalities on V˙O2max in Overweight and Obese. Med Sci Sports Exerc. 2016;48:491–498. doi: 10.1249/MSS.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 53.Stepto NK, Hawley JA, Dennis SC, Hopkins WG. Effects of different interval-training programs on cycling time-trial performance. Med Sci Sports Exerc. 1999;31:736–741. doi: 10.1097/00005768-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez C, Ramírez R, Flores M, Zúñiga C, Celis-Morales CA. [Effect of sprint interval training and resistance exercise on metabolic markers in overweight women] Rev Med Chil. 2012;140:1289–1296. doi: 10.4067/S0034-98872012001000008. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, Mancilla R, Flores-Opazo M, Cano-Montoya J, Ciolac EG. Low-Volume High-Intensity Interval Training as a Therapy for Type 2 Diabetes. Int J Sports Med. 2016;37:723–729. doi: 10.1055/s-0042-104935. [DOI] [PubMed] [Google Scholar]
- 56.Matsuo T, Ohkawara K, Seino S, Shimojo N, Yamada S, Ohshima H, Tanaka K, Mukai C. An exercise protocol designed to control energy expenditure for long-term space missions. Aviat Space Environ Med. 2012;83:783–789. doi: 10.3357/asem.3298.2012. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo T, Saotome K, Seino S, Eto M, Shimojo N, Matsushita A, Iemitsu M, Ohshima H, Tanaka K, Mukai C. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: VO₂max, cardiac mass, and heart rate recovery. Eur J Appl Physiol. 2014;114:1963–1972. doi: 10.1007/s00421-014-2917-7. [DOI] [PubMed] [Google Scholar]
- 58.Osuka Y, Matsubara M, Hamasaki A, Hiramatsu Y, Ohshima H, Tanaka K. Development of low-volume, high-intensity, aerobic-type interval training for elderly Japanese men: a feasibility study. Eur Rev Aging Phys Act. 2017;14:14. doi: 10.1186/s11556-017-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allemeier CA, Fry AC, Johnson P, Hikida RS, Hagerman FC, Staron RS. Effects of sprint cycle training on human skeletal muscle. J Appl Physiol (1985) 1994;77:2385–2390. doi: 10.1152/jappl.1994.77.5.2385. [DOI] [PubMed] [Google Scholar]
- 60.Barnett C, Carey M, Proietto J, Cerin E, Febbraio MA, Jenkins D. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004;7:314–322. doi: 10.1016/s1440-2440(04)80026-4. [DOI] [PubMed] [Google Scholar]
- 61.Metcalfe RS, Babraj JA, Fawkner SG, Vollaard NB. Towards the minimal amount of exercise for improving metabolic health: beneficial effects of reduced-exertion high-intensity interval training. Eur J Appl Physiol. 2012;112:2767–2775. doi: 10.1007/s00421-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs I, Esbjörnsson M, Sylvén C, Holm I, Jansson E. Sprint training effects on muscle myoglobin, enzymes, fiber types, and blood lactate. Med Sci Sports Exerc. 1987;19:368–374. [PubMed] [Google Scholar]
- 63.MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol (1985) 1998;84:2138–2142. doi: 10.1152/jappl.1998.84.6.2138. [DOI] [PubMed] [Google Scholar]
- 64.Stathis CG, Febbraio MA, Carey MF, Snow RJ. Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol (1985) 1994;76:1802–1809. doi: 10.1152/jappl.1994.76.4.1802. [DOI] [PubMed] [Google Scholar]
- 65.McKenna MJ, Heigenhauser GJ, McKelvie RS, Obminski G, MacDougall JD, Jones NL. Enhanced pulmonary and active skeletal muscle gas exchange during intense exercise after sprint training in men. J Physiol. 1997;501(Pt 3):703–716. doi: 10.1111/j.1469-7793.1997.703bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol (1985) 2000;89:1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- 67.Sharp RL, Costill DL, Fink WJ, King DS. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Int J Sports Med. 1986;7:13–17. doi: 10.1055/s-2008-1025727. [DOI] [PubMed] [Google Scholar]
- 68.Katz A, Sharp RL, King DS, Costill DL, Fink WJ. Effect of high intensity interval training on 2,3-diphosphoglycerate at rest and after maximal exercise. Eur J Appl Physiol Occup Physiol. 1984;52:331–335. doi: 10.1007/BF01015222. [DOI] [PubMed] [Google Scholar]
- 69.Esbjörnsson-Liljedahl M, Bodin K, Jansson E. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J Appl Physiol (1985) 2002;93:1075–1083. doi: 10.1152/japplphysiol.00732.1999. [DOI] [PubMed] [Google Scholar]
- 70.Esbjörnsson-Liljedahl M, Sundberg CJ, Norman B, Jansson E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol (1985) 1999;87:1326–1332. doi: 10.1152/jappl.1999.87.4.1326. [DOI] [PubMed] [Google Scholar]
- 71.Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol (1985) 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 72.Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJ. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Physiol. 1999;277:E890–E900. doi: 10.1152/ajpendo.1999.277.5.E890. [DOI] [PubMed] [Google Scholar]
- 73.Metcalfe RS, Koumanov F, Ruffino JS, Stokes KA, Holman GD, Thompson D, Vollaard NB. Physiological and molecular responses to an acute bout of reduced-exertion high-intensity interval training (REHIT) Eur J Appl Physiol. 2015;115:2321–2334. doi: 10.1007/s00421-015-3217-6. [DOI] [PubMed] [Google Scholar]
- 74.Metcalfe RS, Tardif N, Thompson D, Vollaard NB. Changes in aerobic capacity and glycaemic control in response to reduced-exertion high-intensity interval training (REHIT) are not different between sedentary men and women. Appl Physiol Nutr Metab. 2016;41:1117–1123. doi: 10.1139/apnm-2016-0253. [DOI] [PubMed] [Google Scholar]
- 75.Hazell TJ, Macpherson RE, Gravelle BM, Lemon PW. 10 or 30-s sprint interval training bouts enhance both aerobic and anaerobic performance. Eur J Appl Physiol. 2010;110:153–160. doi: 10.1007/s00421-010-1474-y. [DOI] [PubMed] [Google Scholar]
- 76.Zelt JG, Hankinson PB, Foster WS, Williams CB, Reynolds J, Garneys E, Tschakovsky ME, Gurd BJ. Reducing the volume of sprint interval training does not diminish maximal and submaximal performance gains in healthy men. Eur J Appl Physiol. 2014;114:2427–2436. doi: 10.1007/s00421-014-2960-4. [DOI] [PubMed] [Google Scholar]
- 77.Vollaard NBJ, Metcalfe RS, Williams S. Effect of Number of Sprints in an SIT Session on Change in V˙O2max: A Meta-analysis. Med Sci Sports Exerc. 2017;49:1147–1156. doi: 10.1249/MSS.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 78.Ruffino JS, Songsorn P, Haggett M, Edmonds D, Robinson AM, Thompson D, Vollaard NB. A comparison of the health benefits of reduced-exertion high-intensity interval training (REHIT) and moderate-intensity walking in type 2 diabetes patients. Appl Physiol Nutr Metab. 2017;42:202–208. doi: 10.1139/apnm-2016-0497. [DOI] [PubMed] [Google Scholar]
- 79.Tjønna AE, Stølen TO, Bye A, Volden M, Slørdahl SA, Odegård R, Skogvoll E, Wisløff U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116:317–326. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- 80.Levinger I, Shaw CS, Stepto NK, Cassar S, McAinch AJ, Cheetham C, Maiorana AJ. What Doesn't Kill You Makes You Fitter: A Systematic Review of High-Intensity Interval Exercise for Patients with Cardiovascular and Metabolic Diseases. Clin Med Insights Cardiol. 2015;9:53–63. doi: 10.4137/CMC.S26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rognmo Ø. Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126:1436–1440. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 82.Peacock OJ, Western MJ, Batterham AM, Stathi A, Standage M, Tapp A, Bennett P, Thompson D. Multidimensional individualised Physical ACTivity (Mi-PACT)--a technology-enabled intervention to promote physical activity in primary care: study protocol for a randomised controlled trial. Trials. 2015;16:381. doi: 10.1186/s13063-015-0892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nes BM, Gutvik CR, Lavie CJ, Nauman J, Wisløff U. Personalized Activity Intelligence (PAI) for Prevention of Cardiovascular Disease and Promotion of Physical Activity. Am J Med. 2017;130:328–336. doi: 10.1016/j.amjmed.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 84.Nes BM, Janszky I, Aspenes ST, Bertheussen GF, Vatten LJ, Wisløff U. Exercise patterns and peak oxygen uptake in a healthy population: the HUNT study. Med Sci Sports Exerc. 2012;44:1881–1889. doi: 10.1249/MSS.0b013e318258b443. [DOI] [PubMed] [Google Scholar]
- 85.Wisløff U, Nilsen TI, Drøyvold WB, Mørkved S, Slørdahl SA, Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? 'The HUNT study, Norway'. Eur J Cardiovasc Prev Rehabil. 2006;13:798–804. doi: 10.1097/01.hjr.0000216548.84560.ac. [DOI] [PubMed] [Google Scholar]
- 86.Zisko N, Skjerve KN, Tari AR, Sandbakk SB, Wisløff U, Nes BM, Nauman J. Personal Activity Intelligence (PAI), Sedentary Behavior and Cardiovascular Risk Factor Clustering - the HUNT Study. Prog Cardiovasc Dis. 2017;60:89–95. doi: 10.1016/j.pcad.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Terada S, Kawanaka K, Goto M, Shimokawa T, Tabata I. Effects of high-intensity intermittent swimming on PGC-1alpha protein expression in rat skeletal muscle. Acta Physiol Scand. 2005;184:59–65. doi: 10.1111/j.1365-201X.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- 88.Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab. 2004;286:E208–E216. doi: 10.1152/ajpendo.00051.2003. [DOI] [PubMed] [Google Scholar]
- 89.Hanssen H, Nussbaumer M, Moor C, Cordes M, Schindler C, Schmidt-Trucksäss A. Acute effects of interval versus continuous endurance training on pulse wave reflection in healthy young men. Atherosclerosis. 2015;238:399–406. doi: 10.1016/j.atherosclerosis.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 90.Vollaard NBJ, Metcalfe RS. Research into the Health Benefits of Sprint Interval Training Should Focus on Protocols with Fewer and Shorter Sprints. Sports Med. 2017;47:2443–2451. doi: 10.1007/s40279-017-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab. 2012;302:E1343–E1351. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]