Abstract
The long‐term survival of osteosarcoma patients with metastatic or recurrent disease remains dismal, and new therapeutic options are urgently needed. The purpose of our study was to compare the efficacy of CD47 mAb plus doxorubicin combination therapy in mouse models of osteosarcoma with CD47 mAb and doxorubicin monotherapy. Forty‐eight NOD scid gamma (NSG) mice with intratibial MNNG/HOS tumors received CD47 mAb, doxorubicin, combination therapy, or control IgG treatment. Twenty‐four mice (n = 6 per group) underwent pre‐ and post‐treatment magnetic resonance imaging (MRI) scans with the macrophage marker ferumoxytol, bioluminescence imaging, and histological analysis. Tumor ferumoxytol enhancement, tumor flux, and tumor‐associated macrophages (TAM) density were compared between different groups using a one‐way ANOVA. Twenty‐four additional NSG mice underwent survival analyses with Kaplan–Meier curves and a log‐rank (Mantel–Cox) test. Intratibial osteosarcomas demonstrated significantly stronger ferumoxytol enhancement and significantly increased TAM quantities after CD47 mAb plus doxorubicin combination therapy compared to CD47 mAb (P = 0.02) and doxorubicin monotherapy (P = 0.001). Tumor‐bearing mice treated with CD47 mAb plus doxorubicin combination therapy demonstrated significantly reduced tumor size and prolonged survival compared to control groups that received CD47 mAb (P = 0.03), doxorubicin monotherapy (P = 0.01), and control IgG (P = 0.001). In conclusion, CD47 mAb plus doxorubicin therapy demonstrates an additive therapeutic effect in mouse models of osteosarcomas, which can be monitored with an immediately clinically applicable MRI technique.
Keywords: bone sarcoma, CD47, doxorubicin, ferumoxytol, macrophages, MR imaging
Abbreviations
- BLI
bioluminescence imaging
- MRI
magnetic resonance imaging
- MSME
multislice multiecho
- NSG
NOD.Cg‐Prkdc scid Il2rg tm1Wjl/SzJ
- TAM
tumor‐associated macrophages
1. Introduction
Therapy of high‐grade osteosarcomas includes chemotherapy with doxorubicin, cisplatin, and methotrexate and resection of the primary tumor (Bielack et al., 2002; Link et al., 1986; Marina et al., 2016). Unfortunately, the outcome of patients with metastatic disease remains dismal, with survival rates of 15–30% (Aljubran et al., 2009; Marina et al., 2016). Therefore, new therapy options for patients with metastasized osteosarcomas are urgently needed (Aljubran et al., 2009).
Tumor‐associated macrophages (TAMs) have been recognized as a new target for cancer immunotherapies (Cassetta and Kitamura, 2018). TAMs in high‐grade osteosarcoma are associated with reduced metastasis and improved survival (Buddingh et al., 2011). Recent clinical therapies have employed the use of TAM to directly attack tumor cells (Sikic et al., 2019; Takimoto et al., 2019; Yang and Zhang, 2017). Many osteosarcoma cells overexpress the surface marker CD47, which acts as a ‘don't eat me signal’ for TAM. Recent work in animal models has demonstrated that anticancer activity from TAM can be activated in sarcomas by blocking CD47 (Xu et al., 2015). Treatment with monoclonal antibodies against CD47 resulted in sarcoma cell phagocytosis by TAM and significant tumor growth reduction in mouse models (Edris et al., 2012; Herrmann et al., 2012). However, CD47 mAb monotherapy could not cure sarcoma‐bearing mice (Mohanty et al., 2019). As with other systemic therapies, CD47 mAb therapy will have to be combined with other therapies to maximize its efficacy.
Doxorubicin is established for the treatment of osteosarcomas and acts on a common mechanistic pathway with CD47 mAb by inducing immunogenic cell death (Apetoh et al., 2007; Casares et al., 2005). Doxorubicin induces the expression of calreticulin on the cell surface of sarcoma cells that binds to low‐density lipoprotein receptor‐related protein 1 and functions as a prophagocytic ‘eat me’ signal for TAM (Chao et al., 2010; Fucikova et al., 2011). Previous studies have shown mixed findings for combination effects of anthracyclines with CD47 mAb. Suppression of CD47 chemosensitized hepatocellular carcinoma through blockade of CTSS/PAR2 signaling (Lee et al., 2014; Lo et al., 2016). In a different study, it has been shown that targeting CD47 enhanced the effect of doxorubicin chemotherapy in vivo by reducing tumor growth and metastatic spread by activation of an antitumor innate immune response (Feliz‐Mosquea et al., 2018; Iribarren et al., 2019). In contrast, Hermann et al. showed that CD7 mAb treatment did not further enhance macrophage‐mediated phagocytosis of anthracycline pretreated rhabdomyosarcoma cells (Herrmann et al., 2012). Anthracyclines have also been reported to downregulate CD47 levels in pancreatic cancer (Liu et al., 2017). It has been shown that in presence of CD47 binding peptide, camptothecin and doxorubicin‐induced proapoptotic activity was considerably inhibited (Rath et al., 2006). To our knowledge, antitumor effects of doxorubicin and CD47 mAb combination therapy have not been explored in osteosarcoma. We hypothesize that combined therapy of osteosarcomas with doxorubicin and CD47 mAb will significantly increase the efficacy of either drug alone (Fig. 1A–C).
Figure 1.
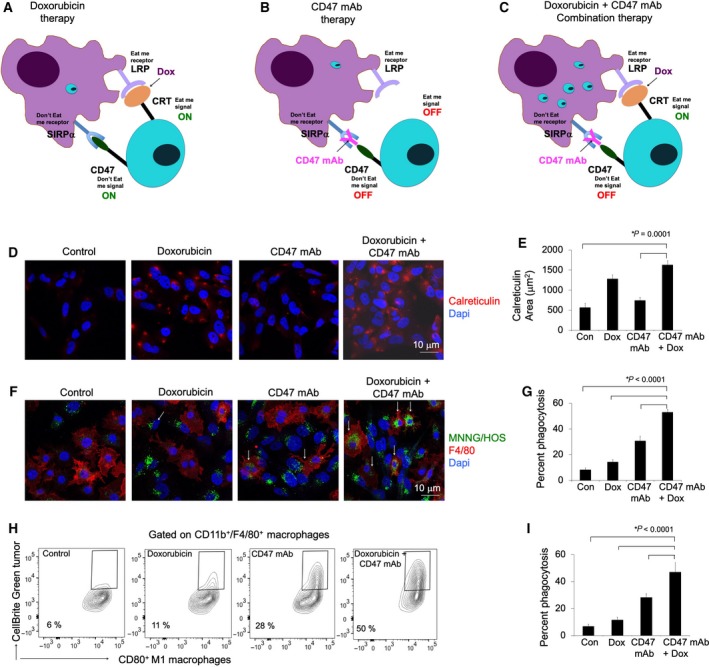
Doxorubicin enhances the phagocytic efficacy of CD47 mAb in osteosarcomas. Schematic demonstration of macrophage‐mediated tumor phagocytosis: (A) Doxorubicin therapy: Doxorubicin induces calreticulin (CRT) on the surface of tumor cells, which ‘turns on’ an eat‐me signal and enables binding to an eat‐me receptor on macrophages. However, CD47 expression on tumor cells counteracts calreticulin‐mediated phagocytosis, (B) CD47 mAb therapy: CD47 mAb block the interaction of tumor CD47 with SIRPα and ‘turns off’ the don't eat‐me signal, (C) combined doxorubicin and CD47 mAb combination therapy synergized by turning the eat‐me signal ‘on’ and don't eat‐me signal ‘off’, and inducing macrophage‐mediated tumor cell phagocytosis. (D) Representative calreticulin staining of MNNG/HOS tumor cells treated with IgG, doxorubicin (0.5 μm), CD47 mAb (10 μg·mL−1), and combination therapy. (E) Corresponding quantitative area of calreticulin staining of control and treated tumor cells. (F) For phagocytosis assays, MNNG/HOS tumor cells were cocultured with murine bone marrow‐derived M1 macrophages for 6 h. Confocal images of CellBrite green‐labeled MNNG/HOS tumor cells and F4/80+ macrophages in the presence of different therapeutics. Cells exposed to combination therapy show an increased quantity of phagocytized tumor cells in macrophages (arrows; scale bar 10 μm) compared to monotherapy. (G) Corresponding relative phagocytosis, calculated as the number of macrophages with phagocytized cancer cell divided by total macrophages per five high‐power field × 100%. (H) Flow cytometry contour plots of M1 macrophages uptaking control IgG and doxorubicin plus CD47mAb‐treated MNNG/HOS tumor cells and (I) corresponding charts showing tumor cell phagocytosis in control and treated sets. Data are displayed as means ± SD of n = 5 experiments per group, P value as indicated, one‐way ANOVA.
To evaluate the effect of doxorubicin and CD47 mAb on TAM in osteosarcomas, we can leverage a new imaging technique that can noninvasively monitor the phagocytic activity of TAM in vivo. The approach relies on the FDA‐approved iron oxide nanoparticle compound ferumoxytol, which is phagocytosed by TAM and can be detected with clinical standard magnetic resonance imaging (MRI) technology. We previously showed that ferumoxytol‐MRI could detect TAM in osteosarcomas in mouse models (Mohanty et al., 2019) and in patients (Aghighi et al., 2018). We also showed that ferumoxytol‐MRI can monitor TAM response to immune‐modulating cancer therapies (Daldrup‐Link et al., 2011).
Thus, the purpose of our study was to compare the efficacy of CD47 mAb plus doxorubicin combination therapy in mouse models of osteosarcoma with CD47 mAb and doxorubicin monotherapy.
2. Materials and Methods
2.1. In vitro studies
MNNG/HOS cells (ATCC, Manassas, VA, USA) were grown in EMEM (ATCC, Manassas, VA, USA) supplemented with 10% FBS, 100 units·mL−1 of penicillin, and 100 mg·mL−1 of streptomycin. Cell lines used were authentic and confirmed to be mycoplasma negative using the MycoAlert Mycoplasma Activity kit (Lonza, Slough, UK). MNNG/HOS cells were engineered with luciferase‐td Tomato lentivirus. Lentiviral production and concentration were performed according to standard procedure. Osteosarcoma cells were transduced for 12 h at 37 °C, 5% CO2, with lentivirus containing 4 μg·mL−1 polybrene (Nitta et al., 2015). After 12 h, cells were washed repeatedly to remove extracellular lentivirus. Td Tomato‐positive osteosarcoma cells were sorted using a BD FACS ARIA (Becton Dickinson, Franklin Lakes, NJ, USA).
Calreticulin protein level in control and treated cells was evaluated by immunofluorescence staining as described previously (Mohanty et al., 2019; Zanganeh et al., 2016).
To evaluate macrophage‐mediated tumor phagocytosis (Mohanty et al., 2019; Zhang et al., 2016), in the presence of doxorubicin plus CD47 mAb combination therapy, MNNG/HOS osteosarcoma cells were labeled with 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindodicarbocyanine (CellBrite™ Green; Biotium, Fremont, CA, USA) according to the manufacturer's protocol and incubated at a 1 : 1 ratio with bone marrow‐derived M1 mouse macrophages in serum‐free IMDM, with 10 μg·mL−1 CD47 mAb, 500 nm doxorubicin, or both at 37 °C for 6 h. M1 polarization of macrophages was performed with previously established protocol (Mohanty et al., 2019). F4/80‐stained macrophages were assayed using Leica SP8 confocal microscopy. Tumor phagocytosis was calculated as the percentage of macrophages positive for phagocytized CellBrite™ Green+ cells. Tumor phagocytosis was also confirmed with flow cytometry on a BD FACS ARIA II flow cytometer. Fluorescently labeled antibodies targeting macrophage markers (CD11b, F4/80 and CD80) were used to identify the M1 population (11). Phagocytosis was quantified by the percentage of CellBrite™ Green events among CD11b+F4/80+CD80+ macrophage events.
2.2. In vivo studies
Animal studies were approved by the Administrative Panel on Laboratory Animal Care at Stanford University (Protocol 24965). To establish orthotopic osteosarcomas, 48 NOD scid gamma (NSG) mice were injected intratibially with luciferase‐td Tomato expressing MNNG/HOS (5 × 105) suspended in 20 μL PBS. Tibias of anesthetized mice were cleaned and injected with 20 μL of tumor cell suspension using a 27G tuberculin syringe (Fig. 2A). Tumor growth was confirmed with bioluminescence imaging (BLI). Twenty‐four xenografted mice were randomly divided into four groups (n = 6 mice/group): Group 1 received a combination therapy of intravenous doxorubicin (Sigma, St. Louis, MO, USA) via the tail vein at a dose of 1 mg·kg−1 on days 0, 2, and 4 (3× per week, low‐dose schedule for minimal side effects) and intraperitoneal injections of CD47 mAb (clone B6H12; BioXcell, Lebanon, NH, USA) at a dose of 10 mg·kg−1 on days 1, 3, and 5 (3× per week). These doses were chosen based on the previous studies (Mohanty et al., 2019; Ren et al., 2008; Wang et al., 2010; Zhang et al., 2016). Group 2 received doxorubicin only and Group 3 received CD47 mAb only, using the same protocols as described above. Group 4 received intraperitoneal injections of control IgG (MOPC‐21; BioXcell) on days 1, 3, and 5 (3× per week).
Figure 2.
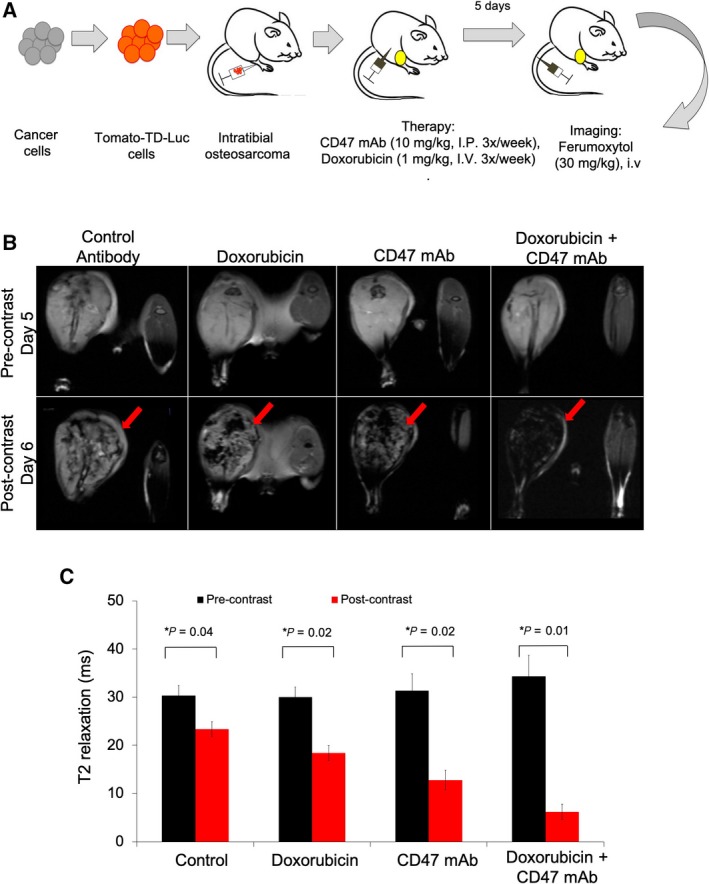
Ferumoxytol‐MRI shows increased T2 contrast in osteosarcomas after CD47 mAb combination therapy compared to monotherapy. (A) Schematic representation of experimental design: MNNG/HOS osteosarcoma cells were transfected with Tomato‐Td‐luciferase construct and implanted into the tibia of NSG mice (n = 6/group). Tumor‐bearing mice were treated with CD47 mAb (10 mg·kg−1, 3× per week) or doxorubicin (1 mg·kg−1, 3× per week) or combination therapy. Five days after therapy, MRI was performed prior to and 24‐h post‐ferumoxytol administration (i.v.). (B) Representative T2‐weighted MR images of MNNG/HOS tumors before (upper row) and at 24 h after (lower row) intravenous injection of the macrophage marker ferumoxytol. Ferumoxytol enhancement is demonstrated by dark (negative) tumor enhancement on T2‐weighted MR images (red arrows). (C) T2 relaxation times of control and treated tumors. T2 relaxation times (quantitative measures of dark tumor ferumoxytol enhancement) were measured on T2 maps, which were generated based on multiecho T2 MSME sequences. All results are represented as mean ± SD from six tumors per experimental group, P‐value as indicated, one‐way ANOVA.
Twenty‐four additional NOD scid gamma (NSG) mice with MNNG/HOS intratibial tumors were treated with doxorubicin plus CD47 mAb, doxorubicin only, CD47 mAb only or IgG, using the same protocols as above (n = 6 per group). Animal survival was evaluated from the first day of treatment until death. Body weight was measured twice a week. Animals were euthanized when turning moribund according to the above‐mentioned predefined criteria rapid weight loss, loss of ability to ambulate, labored respiration, or inability to drink or feed to avoid animal suffering.
2.3. MR imaging
After completion of control IgG, doxorubicin, CD47 mAb, CD47 mAb + doxorubicin therapy, all mice underwent MRI on a 7T MR scanner (Bruker‐Agilent Technologies‐General Electric Healthcare, Billerica, MA, USA) before intravenous injection of ferumoxytol and at 24 h after injection of ferumoxytol via the tail vein (Feraheme™; AMAG Pharmaceuticals, Waltham, MA, USA, 30 mg·kg−1). The following pulse sequences were used: T2‐weighted fast spin echo sequences with a repetition time (TR) of 4500 ms, an echo time (TE) of 42 ms, and a flip angle α: 90° and T2‐weighted multislice multiecho (MSME) sequences with a TR of 3000 ms, a TE of 8, 16, 24, 32, 40, 48, 56, 64, 72, 80, 88, and 96 ms and α: 90°. A field of view of 2 cm × 2 cm and a slice thickness of 0.5 mm for the MRI acquisitions. MSME images were used to create T2 maps and measure T2 relaxation times of the whole tumor with osirix software (Pixmeo, Geneva, Switzerland).
2.4. Bioluminescent imaging
Bioluminescent imaging was performed before therapy (day 0) as well as on day 5 (= directly after completion of therapy) and day 10 (= 5 days after completion of therapy), using an IVIS Spectrum scanner (Perkin Elmer Caliper Life Science, Waltham, MA, USA). D‐luciferin (firefly) potassium salt solution (Biosynth, Itasca, IL, USA; 15 mg·mL−1) was injected intraperitoneally (0.139 g luciferin per kg body weight), and mice were imaged until peak radiance was achieved. Total flux (photons per second) of osteosarcomas was measured by a single operator who was blinded to the experimental groups, using living image 4.0 software (Mohanty et al., 2017; Zanganeh et al., 2016). Total tumor flux (y‐axis) was plotted against different time points (x‐axis) to measure tumor growth overtime.
2.5. Immunocytochemistry
After completion of all imaging procedures, primary decalcified tumors and the bilateral lungs were explanted, fixed in 10% formalin, embedded in paraffin, cut at 5‐μm thickness, and processed for histology. Decalcification with 10% EDTA was performed as described previously (Belluoccio et al., 2013). For Prussian blue iron staining, tissues sections were deparaffinized with xylene, rehydrated, and stained according to the manufacturer's recommendation with the Sigma‐Aldrich Accustain Iron Stain Kit (Sigma‐Aldrich, St. Louis, MO, USA) (Mohanty et al., 2017). The DAB‐Quanto kit (Thermo Scientific, Waltham, MA, USA) was used to enhance Prussian blue stains. Sections were counterstained with nuclear fast red (Fisher Scientific, Hampton, NH, USA). For immunofluorescence, tumors were fixed in 4% paraformaldehyde and embedded in OCT embedding medium. Primary tumor sections were permeabilized with 0.1% Triton X‐100 in PBS for 10 min, washed 3 × 5 min in PBS, and blocked with 3% BSA in PBS for 30 min and stained for macrophages by incubation with F4/80 (1 : 100 dilution; R&D), CD80 (1 : 50 dilution; R&D, Minneapolis, MN, USA), iNOS (1 : 200 dilution; Abcam, Cambridge, MA, USA), calreticulin (1 : 200 dilution; Abcam) primary antibodies overnight at 4 °C before being washed 3× in PBS for 5 min each. Alexa Fluor 647‐conjugated secondary antibodies (1 : 200 dilution; Invitrogen) were added for 2 h in the dark and washed 3 × 5 min in PBS before samples were mounted with DAPI mounting media (Invitrogen, Carlsbard, CA, USA). Immunofluorescence images were acquired with a Leica SP8 confocal microscope using leica af software (Leica software, Leica Microsystems, Buffalo Groves, IL, USA) at 20× and 40× objectives. The percent area covered by Prussian Blue was quantitated using freely available imagej software (U. S. National Institutes of Health, Bethesda, MD, USA), using color thresholding followed by particle analysis (Aghighi et al., 2018). The percent area covered by F4/80+/CD80+/iNOS‐positive macrophages and calreticulin area in tumor xenografts was calculated by velocity software (Mohanty et al., 2019).
For histological analysis of lung metastases, coronal sections of the right and left lung were cut and stained with hematoxylin and eosin (H&E). Representative images were captured using an AxioImager Widefield Fluorescence Microscope (Zeiss, Thornwood, NY, USA) with a 20× objective for whole‐slide imaging. The summed area of metastases in the lungs (Krupnick et al., 2012) was measured with imagej software by one investigator, who was blinded to the treatment of the animals.
2.6. Statistical analysis
Tumor T2 relaxation time, BLI total flux measurements, the summed tumor area of metastases in the lungs, as well as the percent area of Prussian blue‐positive nanoparticles and F4/80+, CD80+, iNOS+ macrophages in primary tumors and calreticulin expression in tumors were compared between different experimental groups using exact one‐way analysis of variance (ANOVA). Kaplan–Meier survival curves were compared between different treatment groups using the log‐rank (Mantel–Cox) test. Statistical analyses were performed using graphpad prism (GraphPad, San Diego, CA, USA) software. The level of significance was set at P < 0.05 for all analyses.
3. Results
3.1. Combination treatment with doxorubicin and CD47 mAb enhances phagocytic elimination of osteosarcoma cells
Doxorubicin is known to be an inducer of immunogenic cell death (Apetoh et al., 2007) by triggering the surface expression of the tumor antigen calreticulin (Fucikova et al., 2011), which functions as an ‘eat me’ signal for TAM. To evaluate whether doxorubicin treatment induces calreticulin expression on MNNG/HOS tumor cells, we applied a calreticulin stain. We found increased calreticulin staining on the cell surface of MNNG/HOS tumor cells undergoing doxorubicin or combination treatment compared to PBS‐treated or CD47 mAb‐treated tumor cells (Fig. 1D,E, P = 0.0001).
To evaluate, whether calreticulin expression increases the phagocytic efficacy of CD47 mAb therapy, we cocultured MNNG/HOS tumor cells with bone marrow‐derived M1 macrophages in presence of doxorubicin, CD47 mAb, and combination treatment. We observed that tumor cells receiving combination treatment showed marked increase of phagocytosis compared with doxorubicin (3.6‐fold, P < 0.0001) or CD47 mAb treatments (1.7‐fold, P < 0.0001) both by confocal microscopy (Fig. 1F,G) and by FACS (Fig. 1H,I and Fig. S1).
3.2. Osteosarcomas treated with doxorubicin in combination with CD47 mAb showed increased ferumoxytol enhancement compared to monotherapy
Ferumoxytol serves as a TAM biomarker, which can be detected with MRI. To evaluate the in vivo TAM response to different immune‐modulating therapies, we performed ferumoxytol‐enhanced MRIs at day 6 after doxorubicin plus CD47 mAb combination therapy as well as doxorubicin, CD47 mAb and IgG monotherapy. In all treatment groups, precontrast MR images demonstrated bright (hyperintense) T2 signal of the primary tumor compared to skeletal muscle (Fig. 2B). At 24 h after injection of ferumoxytol, MR images demonstrated variable hypointense (dark) T2‐enhancement of the tumor tissue (Fig. 2B): Compared to precontrast images, quantitative T2 relaxation times were significantly decreased by 1.3‐fold after IgG treatment (P = 0.04), 1.6‐fold after doxorubicin treatment (P = 0.02), 2‐fold after CD47 treatment (P = 0.02), and 5.3‐fold decreased after doxorubicin plus CD47 mAb combination therapy (P = 0.01), indicating increasing nanoparticle retention in TAM (Fig. 2B,C).
3.3. Osteosarcomas treated with combination therapy contained increased quantities of iron nanoparticles and macrophages compared to monotherapy
To determine, whether the increased ferumoxytol‐MRI enhancement in osteosarcomas after combination therapy corresponded to increased quantities of activated macrophages in the tumor tissue, we performed immunohistochemistry stains against the iron oxide nanoparticles and TAMs. We found significantly larger summed areas of DAB‐Prussian blue iron staining in tumors treated with doxorubicin plus CD47 mAb combination therapy as compared to tumors treated with doxorubicin alone, CD47 mAb alone or control IgG alone (Fig. 3A,B, P = 0.0001). Immunofluorescence staining further showed increased quantities of F4/80+ macrophages in tumors treated with combination therapy when compared to doxorubicin alone (P = 0.001), CD47 mAb alone (P = 0.02), or control IgG alone (P = 0.001; Fig. 3A,B). We also performed immunofluorescence staining for M1 TAM activation markers‐CD80 and iNos and eat‐me receptor calreticulin on tumor cells. We observed that tumors receiving combination therapy demonstrated marked increase of CD80+ (P = 0.05, 0.01, ns) and iNos+ TAMs (P = 0.003, P = 0.015, ns) when compared to control, doxorubicin, or CD47 mAb‐treated tumors, respectively (Fig. 3C,D). We also observed increased staining intensity for calreticulin in tumors receiving combination therapy compared to control and CD47 mAb‐treated tumors (Fig. 3C,D, P = 0.002).
Figure 3.
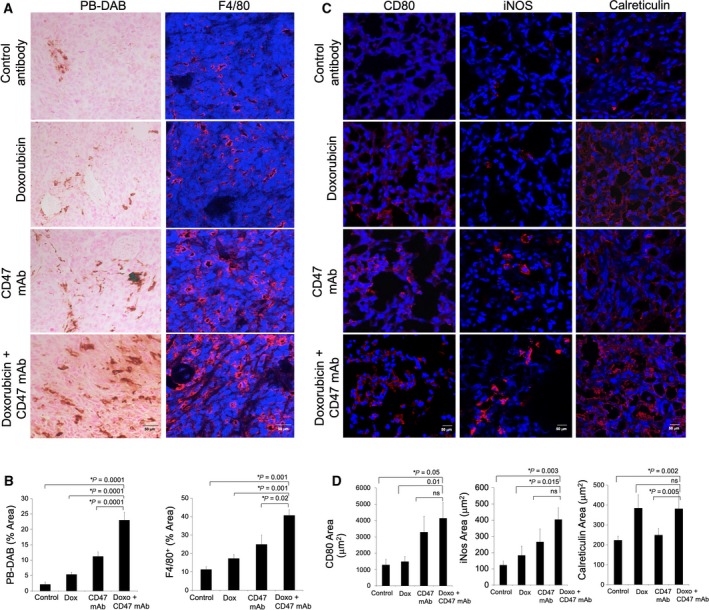
Histopathology shows M1 macrophage activation after doxorubicin and CD47 mAb therapy. (A) Representative Prussian blue‐DAB (scale bar 50 μm) iron stains and immunofluorescent F4/80 confocal images (scale bar 50 μm) of MNNG/HOS tumors show increasing quantities of iron oxide nanoparticles and macrophages in tumors treated with control Ab, CD47 mAb, doxorubicin, and combination therapies. (B) Corresponding quantitative area of Prussian blue‐DAB and F4/80‐positive macrophages in control and treated tumors. (C) Confocal immunofluorescent images and (D) Corresponding quantitative area of CD80, iNOS, and calreticulin staining in control and treated tumors (scale bar 50 μm). All results are represented as mean ± SD from six tumors per experimental group, P‐value as indicated, one‐way ANOVA.
3.4. Doxorubicin plus CD47 mAb combination therapy significantly inhibited the growth of intratibial osteosarcomas and pulmonary metastases
Bioluminescence imaging data showed a significantly decreased tumor burden in mice treated with doxorubicin plus CD47 mAb as compared to mice treated with doxorubicin only (P = 0.01), CD47 mAb only (P = 0.03), or control IgG (P = 0.001, Fig. 4A,B). Combining CD47 mAb with doxorubicin reduced the tumor flux 7‐fold compared with doxorubicin only and fourfold compared with CD47 mAb therapy only.
Figure 4.
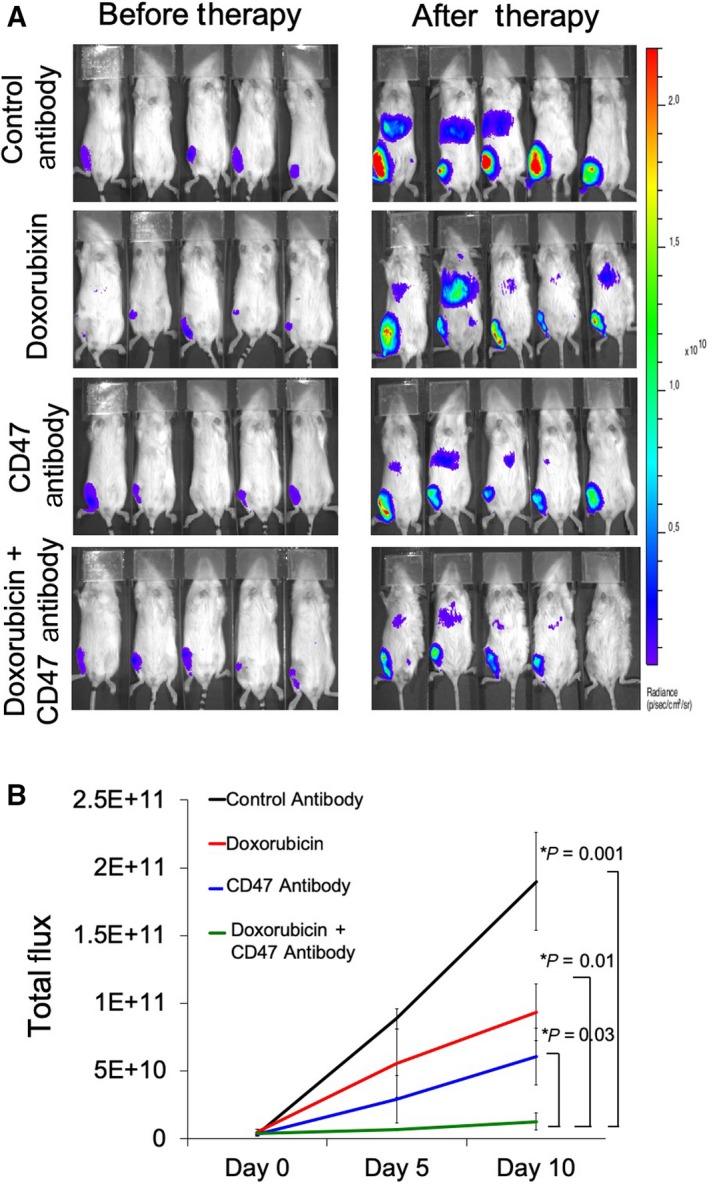
Bioluminescence imaging shows decreased tumor growth of osteosarcomas after doxorubicin and CD47 mAb combination therapy. (A) Bioluminescent in vivo images of mice with intratibial MNNG/HOS osteosarcomas before and after therapy with IgG, CD47 mAb, doxorubicin, and combination therapy. (B) Total quantified flux of MNNG/HOS osteosarcomas at different time points after intravenous treatment with IgG, doxorubicin, CD47 mAb, or combination therapy. Results are represented as mean ± SD from six tumors per experimental group, P‐value as indicated, one‐way ANOVA.
Since MNNG/HOS intratibial tumors spontaneously metastasize to the lungs (Ren et al., 2015; Wan et al., 2009), we compared the tumor burden of lung metastasis in mice treated with doxorubicin plus CD47 mAbs to that of control groups. Lung sections from control IgG‐treated animals showed signs of metastasis from primary tumors (Fig. 5A,B). Compared to IgG‐treated controls, the summed tumor area on histopathological lung sections was not significantly different in doxorubicin‐treated mice (P = 0.05). However, the summed tumor area was 1.8‐fold reduced in CD47 mAb‐treated mice (P = 0.01) and 5‐fold reduced in doxorubicin plus CD47 mAb‐treated mice (P = 0.002) (Fig. 5A,B).
Figure 5.
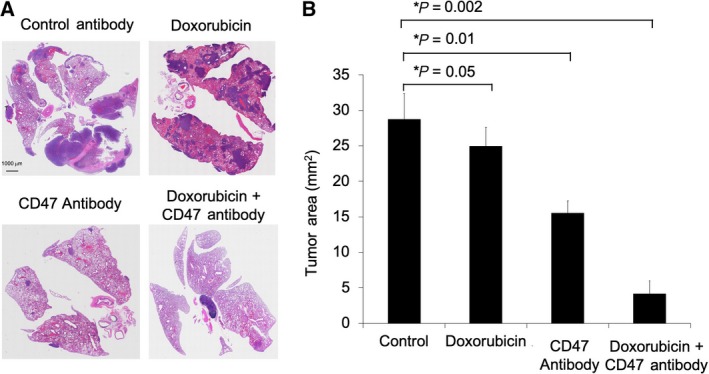
Doxorubicin plus CD47 mAb combination therapy prevents pulmonary metastasis in osteosarcoma‐bearing mice. (A) Low‐power (10×, H&E stain) view of the lungs showed metastasis from the primary tumor. (B) Corresponding summed tumor area of pulmonary metastases in mice treated with control IgG, doxorubicin, CD47 mAb, and combination therapy. Results are represented as mean ± SD from six animals per experimental group, P‐value as indicated, one‐way ANOVA.
3.5. Combination therapy significantly improved survival of osteosarcoma‐bearing mice compared to monotherapy
Kaplan–Meier survival curves showed that animals treated with doxorubicin plus CD47 mAb had significantly prolonged survival compared to animals treated with monotherapy (Fig. 6A). Compared to untreated controls, doxorubicin or CD47 mAb monotherapy survival was prolonged significantly in doxorubicin plus CD47 mAb‐treated mice (P = 0.007, Fig. 6A). In addition, ferumoxytol‐MRI could predict favorable outcomes. The degree of primary tumor ferumoxytol‐MRI enhancement at the end of combination therapy correlated with overall survival (r = −0.9, P = 0.01, Spearman rank correlation, Fig. 6B). These results suggest that ferumoxytol tumor enhancement can be used as a predictive biomarker for tumor response to doxorubicin and CD47 mAb therapy.
Figure 6.
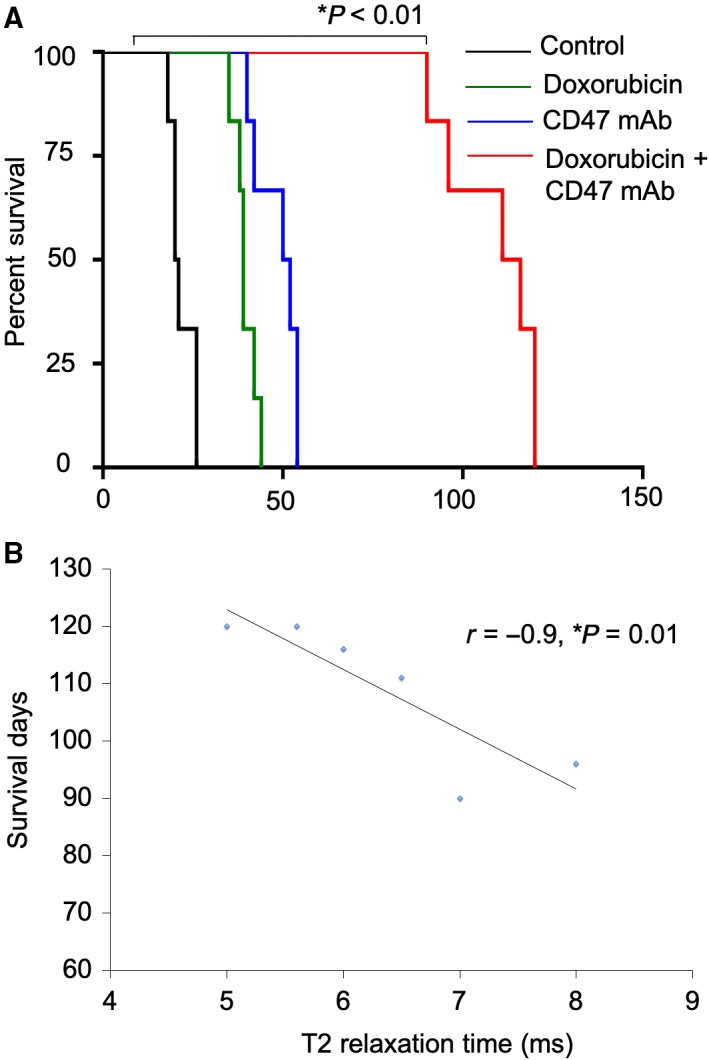
Doxorubicin plus CD47 mAb combination therapy improves survival in osteosarcoma‐bearing mice. (A) Kaplan–Meier survival curves demonstrate a significant survival benefit of combination therapy as compared to control, doxorubicin, and anti‐CD47 alone, log‐rank Mantel–Cox test (log‐rank Mantel–Cox test). (B) Tumor T2 relaxation times, obtained from ferumoxytol‐MRI, correlate with survival outcomes of mice receiving combination therapy (r = −0.9, P = 0.01, Spearman rank correlation, n = 6).
4. Discussion
Our data showed a strong additive effect of doxorubicin plus CD47 mAb combination therapy in mouse models of osteosarcomas, which led to significantly inhibited tumor growth and significantly improved survival of tumor‐bearing mice compared to CD47 mAb alone and doxorubicin alone. We postulate that macrophage‐mediated phagocytosis of tumor cells in the presence of CD47 mAb supports macrophage‐activating effects of doxorubicin. The NOD scid gamma (NSG) mouse strain is B‐ and T‐cell deficient, but has an intact macrophage response (Hu et al., 2011). This model allowed us to study macrophage responses to human xenografts, although it has to be recognized that results of CD47 mAb and doxorubicin combination therapies might be different in fully immunocompetent subjects. Several investigators, including Xu et al. (2015), and our own team (Mohanty et al., 2019) previously reported overexpression of CD47 on osteosarcoma cells and efficacy of CD47 mAb against human osteosarcomas in mouse models. Our team introduced ferumoxytol‐enhanced MRI as a new imaging biomarker for CD47 mAb‐mediated changes in TAM quantities and phagocytic activity in mouse models of MNNG/HOS osteosarcomas. We found that CD47 mAb triggered macrophage phagocytosis and tumoricidal effects in osteosarcomas and reduced tumor burden in vivo. We also found that the majority of cancer cells were phagocytosed alive and subsequently died in macrophages, while a smaller number of tumor cells died first and were secondarily phagocytosed (Mohanty et al., 2019). Studies by Xu et al. show that blockade of CD47 by specific Abs suppresses the invasive ability of osteosarcoma tumor cells and further inhibits spontaneous pulmonary metastasis of KRIB osteosarcoma cells in vivo. Although CD47mAb inhibited osteosarcoma growth in the above studies, combination therapy with antitumor chemotherapies is warranted to improve survival and reduce metastasis (Xu et al., 2015).
Combination therapy of doxorubicin and CD47 mAb has shown success in different cancers (Feliz‐Mosquea et al., 2018; Iribarren et al., 2019; Li et al., 2018; Wu et al., 2018) but their efficacy in osteosarcoma remains unknown. To our knowledge, our study is the first to evaluate the efficacy of combination therapy with doxorubicin in osteosarcomas. This is important, as monotherapies cannot reach complete tumor regression and future clinical translations will require integration of CD47 mAb therapies with classical chemotherapy.
Previous investigators studied other combinations of conventional cytotoxic drugs and immunotherapies to achieve enhanced effects and overcome tumor resistance to classical chemotherapy (Yan et al., 2018). Since cytotoxic drugs may be either immunostimulatory or immunosuppressive (Nowak et al., 2006), it is important to find synergistic combinations and recognize antagonistic combinations. In osteosarcomas, doxorubicin treatment increased the efficacy of immunotherapy with dendritic cells (Kawano et al., 2016) through similar mechanisms of HSP 70 and calreticulin activation, observed in our study. On the other hand, combining doxorubicin with PD‐L1 inhibitors showed a minimal survival advantage over PD‐L1‐monotherapy in K7M2 osteosarcoma model (Lussier et al., 2015). In this K7M2 metastatic osteosarcoma model, the authors show that α‐PD‐L1 mAb‐treated mice demonstrated an adaptive resistance mechanism in the microenvironment where the tumor or tumor microenvironment may be using CTLA‐4 ligation as an alternative pathway to escape immune destruction. Thus, combining doxorubicin chemotherapy with PD‐L1 blockade immunotherapy did not appear to have additional beneficial effects on tumor control. On the other hand, in patients with lymphoma, a recent phase 1b study showed that CD47 mAb in concert with rituximab showed enhanced effects by enhancing macrophage‐mediated antibody‐dependent cellular phagocytosis (Advani et al., 2018). The authors achieved 33% complete response in patients with diffuse large B‐cell lymphoma and 43% complete response in patients with follicular lymphoma (Advani et al., 2018). In a mouse model of breast cancer, Feliz‐Mosquea et al. (2018) found significantly reduced metastasis after doxorubicin and CD47 mAb combination therapy.
With the introduction of new TAM‐modulating immunotherapies to the clinic, it becomes increasingly important to identify and stratify responders and nonresponders to these therapies. Since tumor response to new immunotherapies does not lead to a decline in tumor size, at least not in the immediate post‐treatment phase, new tools for treatment monitoring are required. Various diagnostic tools for TAM detection and quantification have been developed, including gene expression analyses, immunohistochemistry, and fluorescent magnetic nanoparticle labeling (Almatroodi et al., 2016; Kung et al., 2017; Sikandar et al., 2017). However, a major disadvantage of these methods is their invasive nature and lack of clinical availability. We established ferumoxytol‐MRI as a new imaging biomarker for tumor response to TAM‐modulating therapies in mouse models of breast cancer (Daldrup‐Link et al., 2011), brain cancer (Mohanty et al., 2017), and osteosarcomas (Mohanty et al., 2019). In addition, we recently showed that ferumoxytol‐MRI can quantify TAM quantities in patients with osteosarcoma and lymphoma (Aghighi et al., 2018) and brain cancer (Iv et al., 2018). A major side effect of anti‐CD47 treatment is anemia. Ferumoxytol is an FDA‐approved iron supplement for anemia treatment and might counteract this side effect (Daldrup‐Link et al., 2011; Muehe et al., 2015). Ferumoxytol can rarely lead to allergic and anaphylactic reactions. Fortunately, we have not encountered major side effects thus far in our patients with osteosarcomas (Muehe et al., 2015). We carefully screen our patients for any history of allergic reactions and exclude patients with risk of allergies from ferumoxytol exposure.
5. Conclusions
CD47 mAb plus doxorubicin combination therapy improves survival in osteosarcoma‐bearing mice.
Ferumoxytol‐MRI can monitor TAM responses of human osteosarcomas to CD47 mAb plus doxorubicin combination therapy in vivo, in a mouse model.
The described new therapy approach and imaging tool for monitoring treatment response are immediately clinically translatable.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
SM and HED‐L designed the experiments. SM, MA, KY, and JLT performed the in vitro and in vivo experiments. SM, MA, KY, and HED‐L analyzed the data and prepared the figures. SM and HED‐L wrote the manuscript. HED‐L reviewed the data and edited the manuscript.
Supporting information
Fig. S1. Gating strategy for macrophage‐mediated tumor phagocytosis.
Acknowledgements
This study was supported by a grant from the National Cancer Institute (R21CA190196 to HED‐L). JLT was supported by a stipend from the German Cancer Aid (Deutsche Krebshilfe, P‐91650709 to JT). The authors thank members of the Small Animal Imaging Facility, Neuroscience Microscopy Service, Cell Sciences Imaging Facility (Beckman Centre), and Stanford Cancer Institute for providing the equipment and infrastructure for this project. We thank Eileen Misquez for the administrative support of our research. Infrastructure support was provided by a Support Grant for the Stanford Cancer Institute (5P30CA124435‐10). SM was in part supported by a grant from the CCNE‐TD U54CA199075 to Sanjiv Sam Gambhir. JT was supported by a stipend from the German Cancer Aid (Deutsche Krebshilfe, P‐91650709 to JT).
Contributor Information
Suchismita Mohanty, Email: suchi30@gmail.com.
Heike E. Daldrup‐Link, Email: H.E.Daldrup-Link@stanford.edu.
References
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP et al (2018) CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med 379, 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghighi M, Theruvath AJ, Pareek A, Pisani L, Alford R, Muehe AM, Sethi TK, Holdsworth SJ, Hazard FK, Gratzinger D et al (2018) Magnetic resonance imaging of tumor associated macrophages: clinical translation. Clin Cancer Res 24, 4110–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljubran A, Griffin A, Pintilie M and Blackstein M (2009) Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol 20, 1136–1141. [DOI] [PubMed] [Google Scholar]
- Almatroodi SA, McDonald CF, Darby IA and Pouniotis DS (2016) Characterization of M1/M2 tumour‐associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P et al (2007) Toll‐like receptor 4‐dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13, 1050–1059. [DOI] [PubMed] [Google Scholar]
- Belluoccio D, Rowley L, Little CB and Bateman JF (2013) Maintaining mRNA integrity during decalcification of mineralized tissues. PLoS One 8, e58154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielack SS, Kempf‐Bielack B, Gn D, Exner GU, Flege S, Helmke K, Kotz R, Salzer‐Kuntschik M, Werner M and Winkelmann W (2002) Prognostic factors in high‐grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20, 776–790. [DOI] [PubMed] [Google Scholar]
- Buddingh EP, Kuijjer ML, Duim RA, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC and Lankester AC (2011) Tumor‐infiltrating macrophages are associated with metastasis suppression in high‐grade osteosarcoma: a rationale for treatment with macrophage‐activating agents. Clin Cancer Res 17, 2110–2119. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas‐Stubbs S and Obeid M (2005) Caspase‐dependent immunogenicity of doxorubicin‐induced tumor cell death. J Exp Med 202, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L and Kitamura T (2018) Targeting tumor‐associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Jaiswal S, Weissman‐Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T and Park CY (2010) Calreticulin is the dominant pro‐phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2, 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup‐Link HE, Golovko D, Ruffell B, DeNardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF and Corot C (2011) MRI of tumor‐associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res 17, 5695–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edris B, Weiskopf K, Volkmer AK, Volkmer J‐P, Willingham SB, Contreras‐Trujillo H, Liu J, Majeti R, West RB and Fletcher JA (2012) Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA 109, 6656–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliz‐Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Meléndez GC, Schwartz AL, Chen QR, Griner LM, Guha R et al (2018) Combination of anthracyclines and anti‐CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat 172, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J and Spisek R (2011) Human tumor cells killed by anthracyclines induce a tumor‐specific immune response. Cancer Res 71, 4821–4833. [DOI] [PubMed] [Google Scholar]
- Herrmann D, Seitz G, Fuchs J and Armeanu‐Ebinger S (2012) Susceptibility of rhabdomyosarcoma cells to macrophage‐mediated cytotoxicity. Oncoimmunology 1, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Van Rooijen N and Yang YG (2011) Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood 118, 5938–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iribarren K, Buque A, Mondragon L, Xie W, Lévesque S, Pol J, Zitvogel L, Kepp O and Kroemer G (2019) Anticancer effects of anti‐CD47 immunotherapy in vivo. Oncoimmunology 8, 1550619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iv M, Samghabadi P, Holdsworth S, Gentles A, Rezaii P, Harsh G, Li G, Thomas R, Moseley M, Daldrup‐Link HE et al (2018) Quantification of macrophages in high‐grade gliomas by using ferumoxytol‐enhanced MRI: a pilot study. Radiology 290, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Tanaka K, Itonaga I, Iwasaki T, Miyazaki M, Ikeda S and Tsumura H (2016) Dendritic cells combined with doxorubicin induces immunogenic cell death and exhibits antitumor effects for osteosarcoma. Oncol Lett 11, 2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick AS, Tidwell VK, Engelbach JA, Alli VV, Nehorai A, You M, Vikis HG, Gelman AE, Kreisel D and Garbow JR (2012) Quantitative monitoring of mouse lung tumors by magnetic resonance imaging. Nat Protoc 7, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung WH, Yu CF, Lee AC, Yang CD, Liu YC, Chen FH and Huang HD (2017) Gene expression profiling of tumor‐associated macrophages after exposure to single‐dose irradiation. Comput Biol Chem 69, 138–146. [DOI] [PubMed] [Google Scholar]
- Lee TKW, Cheung VCH, Lu P, Lau EYT, Ma S, Tang KH, Tong M, Lo J and Ng IOL (2014) Blockade of CD47‐mediated cathepsin S/protease‐activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology 60, 179–191. [DOI] [PubMed] [Google Scholar]
- Li F, Lv B, Liu Y, Hua T, Han J, Sun C, Xu L, Zhang Z, Feng Z, Cai Y et al (2018) Blocking the CD47‐SIRPα axis by delivery of anti‐CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 7, e1391973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR and Kirkpatrick JA (1986) The effect of adjuvant chemotherapy on relapse‐free survival in patients with osteosarcoma of the extremity. N Engl J Med 314, 1600–1606. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Li L, Liu XJ, Li Y, Zhao CY, Wang RQ and Zhen YS (2017) Mithramycin‐loaded mPEG‐PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int J Nanomed 12, 5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J, Lau EYT, So FTY, Lu P, Chan VSF, Cheung VCH, Ching RHH, Cheng BYL, Ma MKF, Ng IOL et al (2016) Anti‐CD 47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int 36, 737–745. [DOI] [PubMed] [Google Scholar]
- Lussier DM, Johnson JL, Hingorani P and Blattman JN (2015) Combination immunotherapy with α‐CTLA‐4 and α‐PD‐L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, Hook JM, Arndt C, van den Berg H and Brennan B (2016) Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high‐grade osteosarcoma (EURAMOS‐1): an open‐label, international, randomised controlled trial. Lancet Oncol 17, 1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S, Chen Z, Li K, Morais GR, Klockow J, Yerneni K, Pisani L, Chin FT, Mitra S, Cheshier S et al (2017) A novel theranostic strategy for MMP‐14 expressing glioblastomas impacts survival. Mol Cancer Ther 16, 1909–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S, Yerneni K, Theruvath JL, Graef CM, Nejadnik H, Lenkov O, Pisani L, Rosenberg J, Mitra S and Cordero AS (2019) Nanoparticle enhanced MRI can monitor macrophage response to CD47 mAb immunotherapy in osteosarcoma. Cell Death Dis 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehe AM, Feng D, von Eyben R, Luna‐Fineman S, Link MP, Muthig T, Huddleston AE, Neuwelt EA and Daldrup‐Link HE (2015) Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Invest Radiol 51, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta M, Muragaki Y, Maruyama T, Ikuta S, Komori T, Maebayashi K, Iseki H, Tamura M, Saito T and Okamoto S (2015) Proposed therapeutic strategy for adult low‐grade glioma based on aggressive tumor resection. Neurosurg Focus 38, E7. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Lake RA and Robinson BW (2006) Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev 58, 975–990. [DOI] [PubMed] [Google Scholar]
- Rath GM, Schneider C, Dedieu S, Rothhut B, Soula‐Rothhut M, Ghoneim C, Sid B, Morjani H, El Btaouri H and Martiny L (2006) The C‐terminal CD47/IAP‐binding domain of thrombospondin‐1 prevents camptothecin‐and doxorubicin‐induced apoptosis in human thyroid carcinoma cells. Biochim Biophys Acta 1763, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Ren W, Korchin B, Lahat G, Wei C, Bolshakov S, Nguyen T, Merritt W, Dicker A, Lazar A and Sood A (2008) Combined vascular endothelial growth factor receptor/epidermal growth factor receptor blockade with chemotherapy for treatment of local, uterine, and metastatic soft tissue sarcoma. Clin Cancer Res 14, 5466–5475. [DOI] [PubMed] [Google Scholar]
- Ren L, Mendoza A, Zhu J, Briggs JW, Halsey C, Hong ES, Burkett SS, Morrow J, Lizardo MM, Osborne T et al (2015) Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget 6, 29469–29481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar SS, Kuo AH, Kalisky T, Cai S, Zabala M, Hsieh RW, Lobo NA, Scheeren FA, Sim S, Qian D et al (2017) Role of epithelial to mesenchymal transition associated genes in mammary gland regeneration and breast tumorigenesis. Nat Commun 8, 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S and Papadopoulos K (2019) First‐in‐human, first‐in‐class phase I trial of the anti‐CD47 antibody Hu5F9‐G4 in patients with advanced cancers. J Clin Oncol 37, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto CH, Chao MP, Gibbs C, McCamish MA, Liu J, Chen JY, Majeti R and Weissman IL (2019) The macrophage ‘do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol 30, 486–489. [DOI] [PubMed] [Google Scholar]
- Wan X, Kim SY, Guenther LM, Mendoza A, Briggs J, Yeung C, Currier D, Zhang H, Mackall C, Li WJ et al (2009) Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene 28, 3401–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, An T, Wang J, Zhao J, Wang Z, Zhuo M, Bai H, Yang L, Zhang Y and Wang X (2010) Potential clinical significance of a plasma‐based kras mutation analysis in patients with advanced non–small cell lung cancer. Clin Cancer Res 16, 1324–1330. [DOI] [PubMed] [Google Scholar]
- Wu L, Yu GT, Deng WW, Mao L, Yang LL, Ma SR, Bu LL, Kulkarni AB, Zhang WF, Zhang L et al (2018) Anti‐CD47 treatment enhances anti‐tumor T‐cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology 7, e1397248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J‐F, Pan X‐H, Zhang S‐J, Zhao C, Qiu B‐S, Gu H‐F, Hong J‐F, Cao L, Chen Y and Xia B (2015) CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 6, 23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Kumar AB, Finnes H, Markovic SN, Park S, Dronca RS and Dong H (2018) Combining immune checkpoint inhibitors with conventional cancer therapy. Front Immunol 9, 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L and Zhang Y (2017) Tumor‐associated macrophages: from basic research to clinical application. J Hematol Oncol 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro‐inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, Liu J, Achrol AS, Richard C, Sommerkamp P et al (2016) Anti‐CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS One 11, e0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gating strategy for macrophage‐mediated tumor phagocytosis.


