Abstract
NMDA receptor activity is modulated by various compounds, including sulfhydryl redox agents and Zn2+. In addition to a slow and persistent component of redox modulation common to all NMDA receptors, NR1/NR2A receptors uniquely have a rapid and reversible component that has been variously attributed to redox or Zn2+ effects. Here we show that this rapid modulatory effect can be described by two time constants with relatively fast (∼6 sec) and intermediate (60 sec) half lives, and it is likely to be attributable to both redox agents and Zn2+. Using site-directed mutagenesis, we identified three pairs of cysteine residues that underlie the various kinetic components of redox modulation of NMDA-evoked currents inXenopus oocytes expressing NR1/NR2A receptors: (1) Cys 87 and Cys 320 in NR2A underlie the fast component, (2) Cys 79 and Cys 308 in NR1 underlie the intermediate component, and (3) Cys 744 and Cys 798 in NR1 underlie the persistent component. Mutation of these redox-sensitive cysteine residues also affects high-affinity, voltage-independent Zn2+ inhibition that is specific to NR1/NR2A receptors. Exposure to methanethiosulfonate agents that modify cysteine residues did not block the Zn2+inhibition. Thus, these cysteine residues do not appear to coordinate Zn2+ directly. Instead, the redox status of these cysteine residues may modulate the sensitivity of the receptor to Zn2+.
Keywords: NMDA receptor, glutamate, zinc, redox, recombinant cDNA, cysteine, dithiothreitol
The NMDA subtype of glutamate receptor has been implicated in neuronal development (Constantine-Paton, 1990), long-term potentiation (Bliss and Collingridge, 1993), and excitotoxicity (Choi, 1988; Meldrum and Garthwaite, 1990; Lipton and Rosenberg, 1994). Hence, precise regulation of NMDA receptor activity is critical, and several endogenous modulators of NMDA receptor activity have been reported (Dingledine et al., 1999). Redox modulation of the NMDA receptor is defined by the effect of sulfhydryl reducing agents, such as dithiothreitol (DTT), which enhance NMDA-evoked responses, and by oxidizing agents, such as 5–5′-dithio-bis-2-nitrobenzoic acid (DTNB), which decrease NMDA responses (Aizenman et al., 1989). In addition, endogenous redox agents such as glutathione (Gilbert et al., 1991;Sucher and Lipton, 1991) and lipoic acid (Tang and Aizenman, 1993a) have been shown to modulate NMDA receptor function via redox modulatory sites. We previously reported that two cysteine residues in the NR1 subunit (Cys 744 and Cys 798) are responsible for the slow, persistent component of redox modulation of recombinant NR1/NR2B, NR1/NR2C, and NR1/NR2D receptors (Sullivan et al., 1994). The potentiation of NR1/NR2A receptors by DTT, however, has an additional rapid, reversible component, which quickly disappears after washout (Köhr et al., 1994).
Similar to redox modulation, Zn2+ inhibits NMDA-evoked currents in various neuronal preparations (Peters et al., 1987; Westbrook and Mayer, 1987). Zn2+appears to inhibit NMDA responses by a dual mechanism; voltage-independent Zn2+ inhibition is mediated by a decrease in the frequency of channel opening, and voltage-dependent inhibition involves channel block resembling that produced by Mg2+ (Mayer et al., 1989;Christine and Choi, 1990; Legendre and Westbrook, 1990). Recombinant NR1/NR2A and NR1/NR2B receptors exhibit similar voltage-dependent block, but voltage-independent inhibition occurs with much higher apparent affinity in NR1/NR2A than in NR1/NR2B receptors (Williams, 1996; Chen et al., 1997; Paoletti et al., 1997). The observation that heavy metal chelators potentiate NMDA-evoked currents in NR1/NR2A receptors led to the suggestion that the rapid/reversible component of DTT potentiation, which is unique to NR1/NR2A receptors, might be caused by chelation of trace amounts of Zn2+ rather than to a redox-based mechanism (Paoletti et al., 1997).
In the present study, using the Xenopus oocyte expression system and site-directed mutagenesis, we have identified three pairs of cysteine residues, two pairs in the NR1 subunit and one pair in the NR2A subunit, as the structural determinants that confer dual properties of redox and Zn2+ modulation to NR1/NR2A receptors (Fig. 1). Recently,Choi and Lipton (1999) and Fayyazuddin et al. (2000) reported that multiple histidine residues on NR2A constitute the high-affinity Zn2+-binding site of recombinant NR1–1a/NR2A receptors. Here, we suggest that the redox status of these three pairs of cysteine residues determines the sensitivity of NMDA receptors to high-affinity Zn2+ inhibition rather than contributing to the Zn2+-binding site. Taken together, these findings suggest the presence of a novel network of amino acid residues that affect high-affinity Zn2+ inhibition of the NMDA receptor by either binding Zn2+ (represented by the histidine residues of NR2A) or modulating Zn2+action without actually binding (represented by three pairs of redox-sensitive cysteine residues on NR1/NR2A).
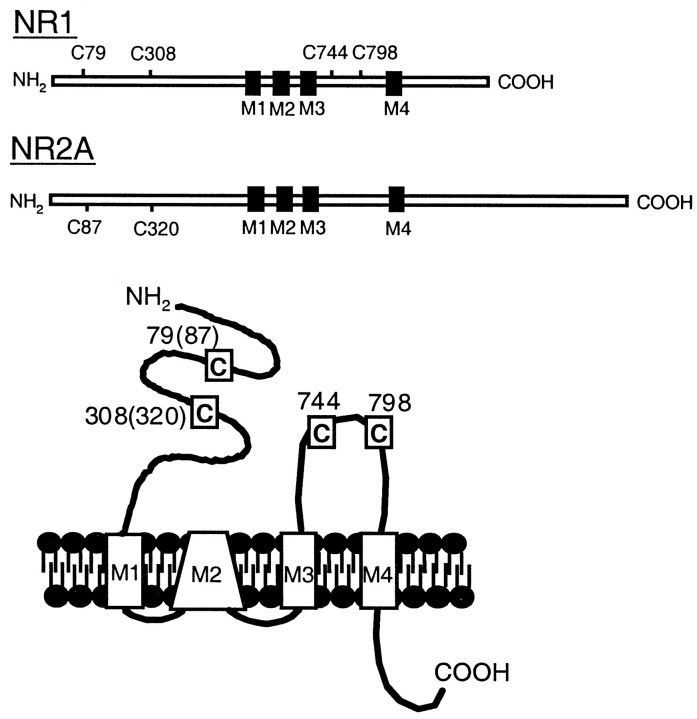
Fig. 1.
Relative positions of cysteine residues in NR1 and NR2A that are important in redox modulation. A schematic outline of the NR1–1a and the NR2A subunits. Four putative membrane associated segments M1–M4 are indicated by solid boxes.Inset, Positions of the cysteine residues in the context of the proposed transmembrane topology of NMDA receptor subunits with an extracellular N terminus, an intracellular C terminus, three transmembrane domains, and an M2 region forming a re-entrant loop (Hollmann et al., 1994; Wo and Oswald, 1994; Wood et al., 1995).Numbers indicate the position of the cysteine residues in NR1–1a, and numbers in parentheses are the homologous cysteine residues in NR2A.
MATERIALS AND METHODS
Site-directed mutagenesis. Mutants were generated using the Chameleon double-stranded site-directed mutagenesis kit based on T7 DNA polymerase (Stratagene, La Jolla, CA). NMDA receptor subunit cDNA templates were pJS1 for NR1–1a [a gift from S. F. Heinemann (Sullivan et al., 1994)], and NR2A [a gift from P. H. Seeburg (Monyer et al., 1992)]. All experiments were performed with the NR1 splice variant that lacked exon 5 and contained exon 21 and exon 22, which is designated herein NR1–1a (Hollmann et al., 1993). Mutants were verified by sequencing. NR1–1a mutants, NR1–1a(C79S), NR1–1a(C308S), NR1–1a(C744A), and NR1–1a(C798A) were made available to us by J. M. Sullivan. NR1–1a constructs were cloned into the high expression vector pGEMHE (Liman et al., 1992) to maximize the expression of NR1–1a receptor protein in Xenopus oocytes. Multiple cysteine mutants were generated as necessary by restriction enzyme digestion and subcloning of relevant fragments.
cRNA synthesis. The template was prepared from a circular plasmid cDNA by linearizing the 3′ untranslated region withNheI (for NR1–1a), or EcoRV (for NR2A). cRNA, which incorporates the 5′ cap analog (m7G(5′)ppp(5′)G), was transcribed from 1 μg of linearized template in vitro by T7 (for NR1–1a) or T3 (for NR2A) RNA polymerase according to the mMessage mMachine protocol (Ambion, Austin, TX). cRNA concentrations were determined by measuring the optical density at 260 nm and by agarose gel electrophoresis.
Preparation of oocytes and injection of cRNA. Xenopusoocytes were prepared as previously described (Sullivan et al., 1994;Choi and Lipton, 1999). Frog oocytes of stages V or VI were surgically removed from the ovaries of Xenopus laevis. Lumps of ∼100 oocytes were incubated with 580 U/ml (2 mg/ml) collagenase type I (Sigma, St. Louis, MO) for 2 hr in Ca2+-free frog Ringer's solution (in mm: 82.5 NaCl, 2 KCl, 1 MgCl2, and 5 HEPES, pH 7.5, with NaOH). After slow agitation to remove the follicular cell layer, the oocytes were washed extensively with Ca2+-free frog Ringer's solution. Oocytes were maintained at 18°C in frog Ringer's solution (in mm: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 mm HEPES, pH 7.5, with NaOH) supplemented with 550 mg/l sodium pyruvate as a carbon source and 100 μg/ml gentamycin. Twenty-four hr later, oocytes were injected with up to 10 ng of cRNA of each subunit using a 10 μl Drummond microdispenser under visual control with a dissecting microscope.
Two-electrode voltage-clamp recording. Two to seven days after injection, oocytes were recorded in frog Ringer's solution under two-electrode voltage clamp at −80 mV, except during experiments to determine Zn2+ inhibition curves when the holding potential was held at −40 mV to separate more clearly voltage-independent and voltage-dependent inhibition. Recordings were performed at room temperature with an Oocyte Clamp OC-725b amplifier (Warner Instrument Corporation, Hamden, CT) using MacLab version 3.5 software (ADInstruments, Milford, MA). Voltage-sensing electrodes had a resistance of 1–4 MΩ, and current-injecting electrodes had a resistance of 0.5–1 MΩ. Both were filled with 3m KCl. Oocytes were continuously superfused with a solution containing 90 mm NaCl, 1 mm KCl, 10 mm HEPES, 1.5 mm BaCl2, and 100 μm glycine, pH 7.5. Barium was used as the divalent cation, rather than calcium, to minimize secondary activation of calcium-activated Cl− current (Leonard and Kelso, 1990). Drugs dissolved in frog Ringer's solution were applied by superfusion at a flow rate of ∼2 ml/min in a 100 μl chamber with an array of pipettes similar to the “sewer pipe” system used in patch-clamp recording to achieve relatively rapid solution exchange. The sewer pipe superfusion system we used is a relatively fast mechanism for solution exchange in a chamber similar to the one previously described by Vyklicky et al. (1990). Using a concentration jump paradigm with Mg2+, we measured a time constant of <3 sec for complete solution exchange in the recording chamber.
Zn2+dose–response experiments. Zn2+ dose–response curves were generated by measuring steady-state currents at −40 mV after serial application of various concentrations of Zn2+ in the presence of a saturating concentration of NMDA (200 μm) plus glycine (100 μm) at pH 7.3. As described in Paoletti et al. (1997), for [Zn2+] ≤ 1 μm, Zn2+ solutions buffered with tricine (10 mm) were used because of the apparent high affinity of NR1–1a/NR2A receptors for Zn2+. Tricine-containing solutions were used to obtain Zn2+ inhibition curves (as in Fig. 3C). “0” Zn2+solution refers to a nominally Zn2+-free solution containing tricine (10 mm) and no added Zn2+. The solutions were prepared by adding 0.1, 0.3, 1.0, 3.0, 10.0, 29.3, and 91.7 μm of Zn2+ to 10 mm tricine to yield estimated free Zn2+ concentrations of 1, 3, 10, 30, 100, 300, and 1000 nm, respectively.
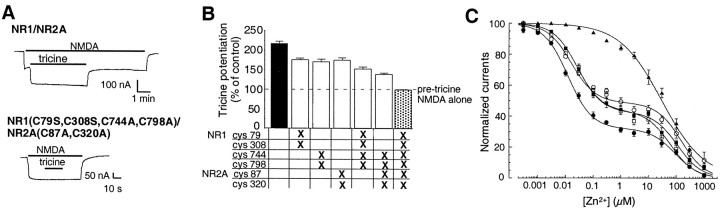
Fig. 3.
The same three pairs of cysteine residues involved in potentiation of NMDA-evoked currents by DTT also mediate potentiation by tricine and voltage-independent Zn2+inhibition. A, Current tracings showing potentiation of NMDA-evoked (200 μm NMDA plus 100 μmglycine) currents by tricine (10 mm) in oocytes expressing wild-type NR1–1a/NR2A receptors (top trace), and absence of potentiation by tricine in oocytes expressing NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) mutant receptors (bottom trace). Unlike potentiation by DTT (Fig.2A), there was no slow component of potentiation by tricine (10 mm). B, Potentiation of NMDA-evoked currents by tricine in the various cysteine mutant combinations expressed as a percentage of the response to NMDA alone (mean ± SEM; n = 6–21 for each group). Thedashed line represents the absence of potentiation of NMDA-evoked currents. The amplitude of potentiation was measured 15 sec after the onset of tricine and NMDA coapplication (similar to DTT potentiation). Tricine potentiation for all mutants was significantly different from that of wild-type NR1–1a/NR2A receptors, and potentiation of NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors was significantly different from that of the other mutants (p < 0.01, ANOVA followed by Fisher's PLSD test). C, NMDA-evoked (200 μm NMDA plus 100 μm glycine) currents expressed as a percentage of the current recorded in the presence of the Zn2+chelator tricine (10 mm). Currents were recorded at a holding potential of −40 mV and pH 7.3 for the following subunit compositions: NR1–1a/NR2A (closed circles), NR1–1a(C744A, C798A)/NR2A (open circles), NR1–1a(C79S, C308S)/NR2A (open squares), NR1–1a/NR2A(C87A, C320A) (closed squares), and NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) (closed triangles). Each point represents the mean ± SEM of the responses obtained from 4–10 oocytes. For IC50 values and Hill coefficients, see Table2.
RESULTS
Mutation of three pairs of cysteines in NR1–1a and NR2A abolishes DTT potentiation
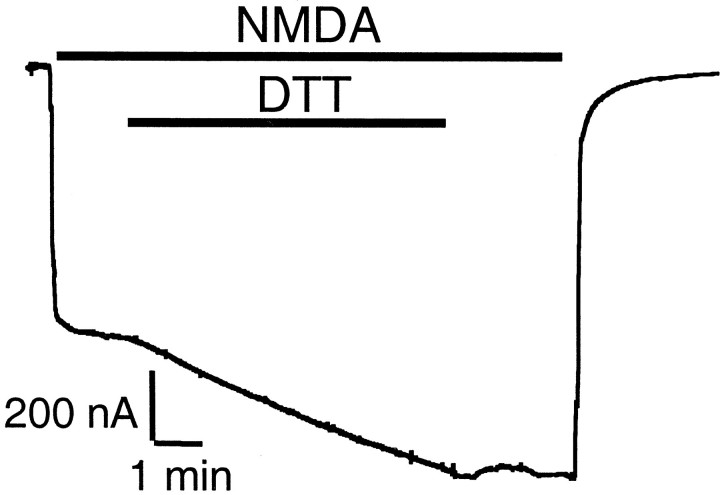
Previous studies suggested that there are two kinetic components to potentiation by DTT of wild-type NR1–1a/NR2A receptor responses (Köhr et al., 1994): a relatively rapid effect of transient duration and a slow component with delayed onset and relatively persistent duration (Fig.2A). Although mutation of NR1(C744,C798) in NR1–1a/NR2A receptors led to abolition of the slow component, the rapid effect of DTT potentiation was still present (Fig. 2A). These results suggested to us that there were additional molecular determinants underlying the rapid effect. However, Paoletti et al. (1997) reported that the rapid effect of DTT was attributable to its ability to chelate Zn2+ rather than to a redox effect. Therefore, we tested DTT potentiation of NR1–1a/NR2A receptors in a nominally Zn2+-free solution containing tricine (10 mm) and no added Zn2+.
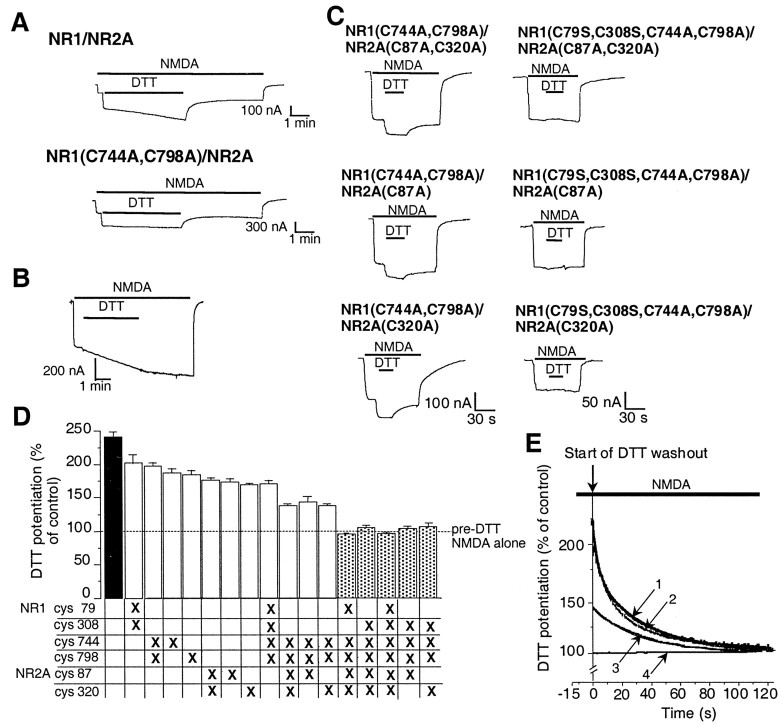
Fig. 2.
Identification of three pairs of cysteine residues involved in potentiation of NMDA-evoked currents by DTT.A, DTT potentiation of NMDA-evoked (200 μmNMDA plus 100 μm glycine) currents in an oocyte-expressing NR1–1a/NR2A receptors or NR1–1a(C744A, C798A)/NR2A receptors. DTT (3 mm) produced potentiation of rapid onset followed by a slow component of further potentiation in wild-type NR1–1a/NR2A receptors. Mutations of Cys 744 and Cys 798 in NR1 led to abolition of the slow component. However, the rapid potentiation by DTT was still present in NR1–1a(C744A, C798A)/NR2A receptors. Similar results were obtained from five oocytes for each group.B, DTT potentiation of NR1–1a/NR2A receptors in nominally Zn2+-free solution. In the presence of tricine (10 mm), added to the recording medium to produce a virtually Zn2+-free (0 Zn2+) solution, the slow component of DTT potentiation was still present, but the rapid effect of DTT was largely eliminated. C,Current tracings showing decreased DTT potentiation in oocytes expressing NR1–1a(C744A, C798A)/NR2A(C87A, C320A) receptors and complete absence of DTT potentiation in oocytes expressing NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors. Mutating either NR2A C87 or C320 alone had the same effect as mutating both cysteines together. D, Potentiation of NMDA-evoked currents by DTT in various cysteine mutant combinations expressed as a percentage of the response to NMDA alone (mean ± SEM,n = 4–9 for each group). The dashed line represents the absence of potentiation of NMDA-evoked currents. To ensure that predominantly the rapid component of DTT potentiation was considered, the NMDA-evoked currents were measured 15 sec after the onset of DTT and NMDA coapplication. DTT potentiation of all mutant combinations was significantly different from that of wild-type NR1–1a/NR2A, and potentiation of NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors was significantly different from that of all other mutant combinations [p < 0.01, ANOVA followed by Fisher's protected least significant difference (PLSD) test]. E, Washout phase of DTT potentiation of NMDA-evoked currents in wild-type or mutant NR1–1a/NR2A receptors. Raw data are shown (traces 1–4) with exponential fits superimposed. After a 15 sec application of DTT, washout (beginning at t = 0) was observed for 120 sec. For display purposes, current traces are normalized to the NMDA response of each oocyte at 120 sec (arbitrarily defined as 100%). This plotting procedure does not display the slow, persistent component of redox modulation, which manifests a washout time constant of >10 min and which we have already shown is attributable to NR1 Cys 744 and Cys 798 (Sullivan et al., 1994). The first 120 sec of the washout phase of DTT potentiation in NR1–1a/NR2A receptors could be well fitted by the sum of two exponential kinetic components (trace 1 with time constants τ1and τ2; see Results for values). While mutation of NR1 Cys 744 and Cys 798 (trace 2) abolished the slow, persistent component (which is not manifest in this figure), it did not have any effect on the fast or the intermediate components (evidenced by the fact that traces 1 and2 are virtually superimposable). Additional mutations of NR2A Cys 87 and Cys 320 eliminated the fast component, but not the intermediate component (trace 3; which was well fitted by a single exponential curve with time constant, τ; see Results for value). If we also mutated NR1 Cys 79 and Cys 308, the intermediate component was also eliminated (trace 4; which can be fit by a straight line).
Zn2+ chelation by tricine largely eliminated the rapid effect of DTT potentiation (Fig.2B). This result supports the notion that rapid potentiation by DTT is caused by direct chelation of Zn2+ by its thiol groups. Similar results were obtained when we used, in contrast to DTT, a non-thiol-based reducing agent, tris-(2-carboxyethyl)-phosphine, which was previously shown to affect NMDA receptor redox state (Burns, et al., 1991; Gozlan et al., 1995). The fact that various reducing agents, however, may also bind Zn2+, at least to some degree, leaves open the question of whether cysteine residues on the NMDA receptor that influence the Zn2+effect may also participate in redox signaling. It occurred to us, for example, that there might be more than one underlying molecular basis for the rapid component of DTT potentiation and that both Zn2+ chelation and redox modulation might contribute. If this were indeed the case, then different kinetics of action might be observed for each molecular process. Additionally, if the same cysteine residues influence the effect of Zn2+ and mediate a component of redox modulation, then site-directed mutagenesis of these cysteines would abrogate not only the effect of Zn2+ but also that of oxidizing agents such as DTNB that do not chelate Zn2+. These issues are therefore addressed experimentally below.
In chimeric studies, Köhr et al. (1994) localized the rapid component of DTT potentiation to the N-terminal 370 amino acid residues of NR2A. Therefore, we mutated the three cysteine residues in this N-terminal region of NR2A (Cys 87, Cys 231, and Cys 320) to alanines either individually, in pairs, or as a triple mutant, and expressed each mutant with the wild-type NR1–1a subunit in oocytes. Mutation of NR2A Cys 231 had no effect on DTT potentiation, but the double mutant, NR2A(C87A,C320A), made the amplitude of rapid potentiation by DTT when coexpressed with the wild-type NR1–1a subunit smaller (181 ± 3% of NMDA alone, n = 5 vs 241 ± 8%,n = 8 for wild-type NR1–1a/NR2A; mean ± SEM). Mutating either of these cysteines alone had the same effect as mutating both cysteines together (Fig. 2C,D). Previously, we had shown that mutating either NR1 Cys 744 or Cys 798 also had the same effect as mutating both cysteines together, i.e., to eliminate the slow and persistent component of DTT potentiation (Sullivan et al., 1994). Mutation of the cysteine residues in the NR1 subunit (Cys 79 and Cys 308) that are homologous to Cys 87 and Cys 320 of NR2A also made the rapid component of DTT potentiation smaller (202 ± 11%; n = 4), and mutating either cysteine alone had the same effect as mutating both cysteines together. Finally, expression of NR1–1a/NR2A receptors with mutations in all three of these pairs of cysteines, NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A), completely abrogated DTT potentiation (96 ± 1%, n = 5; Fig.2C,D). DTT potentiation was also abolished in NR1-1a(C79S,C744A,C798A)/NR2A(C87A,C320A) receptors (95 ± 1%;n = 5), NR1–1a(C308S,C744A,C798A)/NR2A(C87A) receptors (102 ± 3%; n = 4), and NR1–1a(C308S,C744A,C798A)/NR2A(C320A) receptors (105 ± 3%;n = 5). Therefore, it appears that NR1 C79 and C308 form a pair of cysteines, and NR2A C87 and C320 form another pair of cysteines, with mutation of either cysteine of a pair yielding the same phenotype.
Because of the fast onset of the “rapid” component of DTT potentiation of NR1–1a/NR2A receptors and the fact that “onset” is composed of both microscopic “on” and “off” rate constants, the kinetics of the rapid DTT effect could be more accurately quantified during the washout phase, which is more representative of the off rate constant (Fig. 2E). We found that the first 120 sec of DTT washout could be best fit with two exponentials of fast (τ1 = 6.1 ± 1.0 sec) and intermediate (τ2 = 54.4 ± 9.3 sec) time constants (n = 6). We already knew that mutation of NR1 Cys 744 and Cys 798 abolished the slow, persistent component with a washout time constant (τ3) >10 min (Sullivan et al., 1994), but these NR1(C798A,C744A) mutations did not have a significant effect on the fast or intermediate components of washout in this analysis. In contrast, mutation of NR2A Cys 87 and Cys 320 eliminated the fast component, whereas the intermediate component remained (τ = 63.6 ± 6.6 sec; n = 4). If we also mutated NR1 Cys 79 and Cys 308, the intermediate component was also eliminated. Thus, NR2A(C87,C320) underlie the fast, NR1(C79,C308) the intermediate, and NR1(C744,C798) the slow, persistent components of the DTT effect. The fact that Zn2+ washout manifests a single kinetic component (Paoletti et al., 1997), and the relatively rapid component of the DTT effect displays two time constants (with τ of ∼6 and 60 sec) suggests that Zn2+ chelation alone cannot completely account for the rapid effects of DTT. Thus, we investigated these cysteine mutants further.
Effect of cysteine mutations on NMDA receptor agonist and channel properties
To determine whether mutation of these cysteine residues produced extensive structural changes affecting multiple NMDA receptor properties, we constructed NMDA and glycine dose–response curves for wild-type and mutant NR1–1a/NR2A receptors. In the presence of a saturating concentration of glycine (100 μm), the EC50 for NMDA was only sixfold lower for NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A) than for wild-type NR1–1a/NR2A receptors (Table 1). This shift of the NMDA dose–response curve was mainly accounted for by the NR1 Cys 744 and Cys 798 mutation, as we had shown previously (Sullivan et al., 1994). Similarly, in the presence of a saturating concentration of NMDA (200 μm), the EC50 for glycine was only sixfold lower for NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A) than for wild-type NR1–1a/NR2A receptors (Table 1). The shift of the glycine dose–response curve was mainly attributable to the presence of the NR2A Cys 87 and Cys 320 mutations. Additionally, the voltage-dependent blockade by Mg2+ of NR1–1a(C79S, C308S,C744A,C798A)/NR2A(C87A,C320A) receptors was indistinguishable from that of wild-type NR1–1a/NR2A receptors (data not shown). These results indicate that the agonist sites and channel pore of the NMDA receptor complex were relatively unchanged by mutations of these cysteine residues.
Table 1.
EC50 and Hill coefficients for NMDA and glycine in wild-type and cysteine mutant NR1-1a/NR2A receptors
| NMDA | Glycine | Imax(nA) | |||
|---|---|---|---|---|---|
| EC50(μm) | n | EC50(μm) | n | ||
| NR1-1a/NR2A | 39.4 ± 1.7 | 1.82 ± 0.15 | 2.48 ± 0.12 | 1.19 ± 0.06 | 539 ± 78 |
| NR1-1a(C744A,C798A)/NR2A | 12.4 ± 0.6 | 1.17 ± 0.06 | 1.73 ± 0.13 | 1.03 ± 0.07 | 721 ± 95 |
| NR1-1a(C79S,C308S)/NR2A | 48.9 ± 2.3 | 2.00 ± 0.20 | 2.62 ± 0.09 | 1.26 ± 0.05 | 342 ± 57 |
| NR1-1a/NR2A(C87A,C320A) | 24.2 ± 1.5 | 1.55 ± 0.12 | 0.92 ± 0.04 | 1.31 ± 0.07 | 378 ± 122 |
| NR1-1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A) | 5.95 ± 0.22 | 1.47 ± 0.08 | 0.43 ± 0.03 | 1.17 ± 0.07 | 131 ± 31 |
NMDA-evoked currents were measured in wild-type or mutant NR1-1a/NR2A receptors expressed in oocytes. EC50values ± SD were calculated from dose–response curves generated by fitting the data with the equation I =Imax/[1 + (EC50/[agonist])
], wheren is the Hill slope, and Imax is the maximum response normalized to 100%.
Mutation of the same three pairs of cysteines in NR1–1a and NR2A affects high-affinity, voltage-independent Zn2+inhibition
Next, we tested whether mutation of cysteine residues in NR1–1a/NR2A receptors had any effect on potentiation of NMDA-evoked currents by heavy metal chelators. In our experiments, tricine (10 mm) potentiated NMDA-evoked currents in NR1–1a/NR2A receptors (Fig. 3A). However, unlike potentiation by DTT (shown in Fig. 2A), potentiation by tricine of NR1–1a/NR2A receptor responses was completely reversed by washout and had no slow (τ ∼ 10 min) or intermediate (τ ∼ 50–60 sec) component. Interestingly, we found that the same three pairs of cysteine residues involved in DTT potentiation also underlie potentiation of NMDA-evoked currents by tricine. Potentiation by tricine was abolished in NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A)receptors (108 ± 3% of NMDA alone; n = 8) compared to that of wild-type NR1–1a/NR2A receptors (220 ± 6%, n = 20; Fig. 3A,B).
The above results with tricine suggest that these mutations in cysteine residues should also have an effect on inhibition of NMDA receptors by exogenous Zn2+. Therefore, we constructed Zn2+ dose–response curves for wild-type and mutant NR1–1a/NR2A receptors (Fig. 3C). Because voltage-independent Zn2+ inhibition of NR1–1a/NR2A receptors occurs with high affinity (IC50 in the nanomolar range; Chen et al., 1997; Paoletti et al., 1997), we performed Zn2+ dose–response experiments in tricine-buffered solutions. At −40 mV, the Zn2+ dose–response curve for NR1–1a/NR2A receptors was well fit with a two-binding-site isotherm, yielding IC50 values of 12 nm and 92 μm (with relative weights of 68 and 32%, representing high-affinity voltage-independent and low-affinity, voltage-dependent inhibition, respectively). Dose–response curves for NR1–1a/NR2A receptors bearing mutations in only one pair of cysteine residues revealed that the relative weight of the high-affinity, voltage-independent inhibition decreased compared to that of wild-type NR1–1a/NR2A receptors with only a small effect on the IC50 of voltage-independent inhibition (approximately twofold increase; Table2). However, the dose–response curve for receptors bearing mutations of all three pairs of cysteines, NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A), did not segregate into two well separated regions of inhibition and could be fit with a single-binding site isotherm yielding an IC50value of 31 μm (Fig. 3C). Thus, we show that mutation of the same three pairs of cysteine residues that underlie DTT potentiation also abrogates the apparent high-affinity, voltage-independent component of Zn2+inhibition of NR1–1a/NR2A receptors.
Table 2.
IC50 and relative weight for high-affinity, voltage-independent and low-affinity, voltage-dependent Zn2+ inhibition in wild-type and cysteine mutant NR1-1a/NR2A receptors
| High-affinity, voltage-independent | Low-affinity, voltage-dependent | |||
|---|---|---|---|---|
| IC50 (nm) | Relative weight | IC50 (μm) | Relative weight | |
| NR1-1a/NR2A | 12.3 ± 1.0 | 0.68 ± 0.01 | 92.2 ± 24.5 | 0.32 ± 0.01 |
| NR1-1a(C744A,C798A)/NR2A | 16.2 ± 3.1 | 0.51 ± 0.02 | 149 ± 53 | 0.49 ± 0.05 |
| NR1-1a(C79S,C308S)/NR2A | 26.7 ± 5.0 | 0.56 ± 0.02 | 62.6 ± 22.4 | 0.44 ± 0.04 |
| NR1-1a/NR2A(C87A,C320A) | 27.3 ± 0.3 | 0.57 ± 0.01 | 111 ± 28 | 0.43 ± 0.03 |
Zn2+ inhibition of NMDA-evoked currents was measured in wild-type or mutant NR1-1a/NR2A receptors expressed in oocytes. IC50 values ± SD were calculated from dose–response curves generated by fitting the data with a two-binding-site isothermy = 1 − ((a/(1 + IC50(1)/[Zn2+])) + (b/(1 + IC50(2))/[Zn2+]))), where y is the relative current, a and b are the respective weights of each isotherm, a + b = 1, and IC50(1) or IC50(2) is the concentration producing 50% inhibition of each component.
Mutation of three pairs of cysteines in NR1–1a and NR2A also abolishes DTNB inhibition
Because DTT can chelate Zn2+ (Cornell and Crivaro, 1972) as well as act as a sulfhydryl reducing agent on the NMDA receptor (Aizenman et al., 1989), it was not clear what contribution to the Zn2+ and redox effects were made by these pairs of cysteine residues. To address this question, we used a sulfhydryl-specific oxidizing agent, DTNB, which does not chelate Zn2+. DTNB inhibited NMDA-evoked currents in oocytes expressing NR1–1a/NR2A receptors (to 85 ± 1.0% of control; n = 5). In NR1–1a/NR2A receptors, DTNB inhibition was completely abolished only when all three pairs of cysteines were mutated [in NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A) receptors, responses were 98 ± 1.0% of control, n = 16; Figure4]. Mutation of any one or two of the three pairs of cysteine residues was not sufficient to completely block the effect of DTNB (Fig. 4). This result suggests that all three pairs of cysteine residues also contribute to redox modulation in NR1–1a/NR2A receptors.
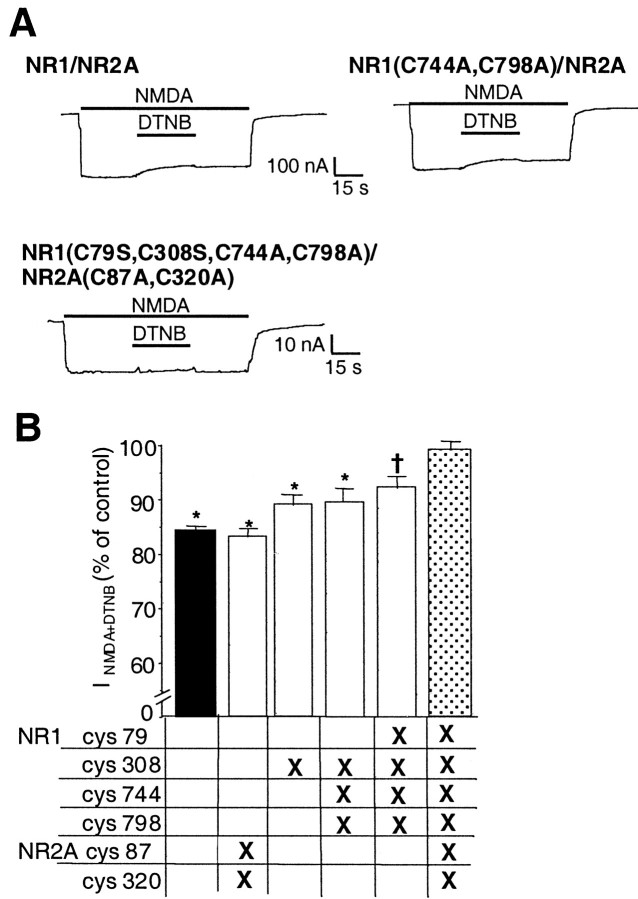
Fig. 4.
DTNB inhibition of NMDA-evoked currents in wild-type and mutant NR1–1a/NR2A receptors. A,NMDA-evoked currents (200 μm NMDA plus 100 μm glycine) in oocytes expressing NR1–1a/NR2A, NR1–1a(C744A, C798A)/NR2A, and NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors. DTNB (0.5 mm) still inhibited NMDA-evoked currents in NR1–1a/NR2A and NR1–1a(C744A, C798A)/NR2A receptors, but inhibition was abolished in NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors. B,DTNB inhibition of NMDA-evoked currents from wild-type NR1–1a/NR2A receptors and various cysteine mutant combinations expressed as a percentage of the response to NMDA alone (mean ± SEM,n = 5–16). The amplitude of inhibition was measured 30 sec after the onset of DTNB and NMDA coapplication. DTNB inhibition of NMDA-evoked currents in NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors was significantly different from that observed in wild-type NR1–1a/NR2A or subunit combinations containing only one or two pairs of mutated cysteine residues. (*p < 0.01 and †p < 0.03, ANOVA followed by Fisher's PLSD test).
Methanethiosulfonate agents, which modify cysteine residues, do not block Zn2+ inhibition
Our group (Choi and Lipton, 1999) as well as others (Fayyazuddin et al., 2000; Low et al., 2000) recently found by mutational analysis that multiple histidine residues on the NR2A subunit comprise the high-affinity Zn2+-binding site of recombinant NR1–1a/NR2A receptors. To test whether the three pairs of cysteine residues, in addition to these histidine residues, are involved in forming the Zn2+-binding site (Christianson, 1991; Vallee and Falchuk, 1993), we used a methanethiosulfonate agent, 2-(trimethy-lammonium) ethyl methanethiosulfonate (MTSET), to modify the cysteine residues. This agent is known to interact with free thiols rapidly and specifically to form mixed disulfides (Akabas et al., 1992). Exposure to MTSET (3 min, 500 μm) abolished subsequent DTNB inhibition of NR1–1a/NR2A receptor responses, suggesting that MTSET has access to the critical cysteine residues (data not shown). However, such treatment with MTSET did not abolish high-affinity, voltage-independent Zn2+ inhibition (IC50 for voltage-independent inhibition before MTSET: 12.9 ± 1.2 nm, relative weight 70% vs after MTSET: 6.0 ± 0.8 nm, relative weight 71%,n = 5; Fig. 5). This result argues against the idea that these three pairs of cysteine residues coordinate Zn2+ at its binding site. Alternatively, these cysteines may act in pairs to form reversible disulfide bridges (Armstrong et al., 1998), in which case MTSET would not be able to react with them. Mechanism notwithstanding, our findings suggest that these cysteine residues can modulate the Zn2+ effect without actually binding Zn2+.
Fig. 5.
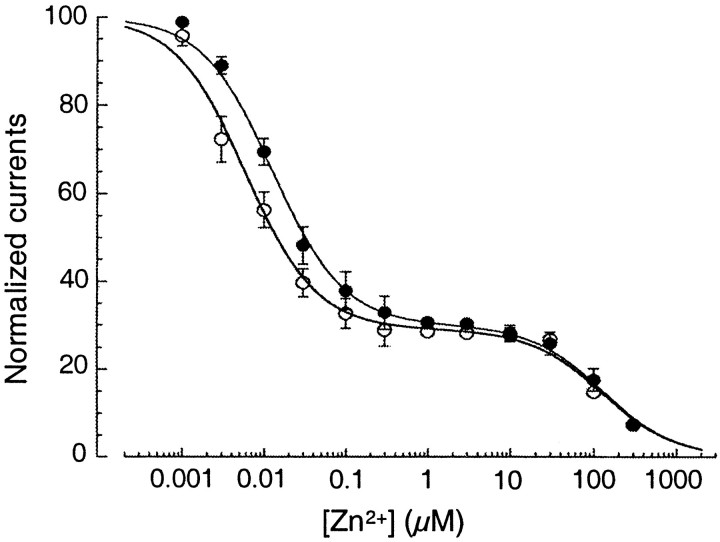
Zn2+ dose–response curves for NR1–1a/NR2A receptors after exposure to MTSET. NMDA-evoked (200 μm NMDA plus 100 μm glycine) currents were expressed as a percentage of the current recorded in the presence of the Zn2+ chelator tricine (10 mm). Currents were recorded at a holding potential of −40 mV and pH 7.3. Each point represents the mean ± SEM of the responses obtained from four or five oocytes. The absence of an error bar indicates that the SEM was smaller than the symbol diameter for that value. Zn2+ dose–response curves before (open circles) and after (closed circles) exposure to MTSET (3 mm, 2 min). There was a 15 sec wash after MTSET treatment.
Histidine mutations that disrupt high-affinity Zn2+ binding also influence redox potentiation
Next, we tested DTT potentiation in histidine mutants that largely abolished high-affinity Zn2+ inhibition of the NMDA receptor (Choi and Lipton, 1999). Here we found that the rapid, reversible effects of DTT potentiation, composed of both a fast component and intermediate component as quantified during washout of the effect (with washout time constants of ∼6 and 60 sec, respectively), were totally eliminated in NR1–1a/NR2A(H42G,H44S) mutant receptors, and the slow component may have decreased somewhat as well (Fig. 6). On the surface, this result might be interpreted to support the hypothesis that the rapid potentiation of NR1/NR2A by DTT is primarily attributable to Zn2+ chelation, but Zn2+ binds to a single high-affinity binding site, and empirically is a bimolecular process described by a single time constant for washout (Paoletti et al., 1997; Choi and Lipton, 1999). Hence, an effect of DTT on Zn2+ chelation alone would not account for the fact that the histidine mutants completely eliminated not just the fast component but both kinetic components of rapid DTT potentiation, composed of a fast and an intermediate time constant.
Fig. 6.
DTT potentiation of NR1–1a/NR2A(H42G, H44S) receptors. Mutation of His 42 and His 44 of NR2A, which form the critical “short-spacer” element of the high-affinity Zn2+-binding site (Choi and Lipton, 1999), entirely eliminated the fast (τ1 ∼6 sec) and the intermediate (τ2 = 60 sec) components of rapid DTT potentiation; the slow component of redox modulation, although still present in this figure, appeared decreased in n = 4 oocytes.
DISCUSSION
In this study, we identified three pairs of cysteine residues, Cys 79 and Cys 308 of NR1, Cys 744 and Cys 798 of NR1, and Cys 87 and Cys 320 of NR2A, as the molecular determinants of redox modulation of NR1–1a/NR2A receptors. Additionally, these three pairs of cysteines influence high-affinity, voltage-independent Zn2+ inhibition of NR1–1a/NR2A receptor responses.
Based on the observations concerning Zn2+chelation by the sulfhydryl reducing agent, DTT, made by Paoletti et al. (1997), and our result showing that the rapid-onset potentiation by DTT is largely eliminated in tricine-buffered 0 Zn2+ solution, it is likely that the predominant effect of rapid-onset potentiation by DTT of NR1/NR2A receptors is caused by the chelation of Zn2+. However, one can alternatively interpret the result as evidence that rapid-onset potentiation by DTT is a result of relief of high-affinity voltage-independent Zn2+ inhibition after changing the properties of the Zn2+-binding site by redox agents, as discussed below. To clearly separate a redox-based mechanism from Zn2+ chelation, one needs to use compounds that are powerful reducing agents on the NMDA receptor but poor Zn2+ chelators. At this time, no perfect reagent with these properties exists, and DTT is clearly both a chelator of Zn2+ and a reducing agent. Thus, we would like to point out that any discussion of whether the action of DTT on NR1/NR2A responses can be attributed to Zn2+ chelation, to redox effects, or to both is not particularly instructive.
Instead, we want to address the question of whether these three pairs of cysteines, which are responsible for DTT potentiation, play a role in both Zn2+ and redox modulation. Previously, we and our colleagues had shown that Cys 744 and Cys 798 of NR1 mediate redox modulation of NR1–1a/NR2B-D receptors that manifest only a slow kinetic component of potentiation after exposure to DTT (Sullivan et al., 1994). Here we further show that Cys 744 and Cys 798 of NR1 are not only involved in redox modulation of NR1–1a/NR2A receptors but that they also affect voltage-independent Zn2+ inhibition when Zn2+ levels are systematically varied by chelation with tricine (Zheng et al., 1998). Similarly, we show that the other two pairs of cysteine residues of NR1 and NR2A mentioned above are also important for both redox and Zn2+ effects. Admittedly, inherent limitations in the Xenopus oocyte expression system preclude us from performing precise quantification of the kinetics of DTT action. For example, the kinetics of DTT washout might encompass more complex or rapid processes than those ascribed here, such as an effect of Zn2+ on the gating kinetics of the receptor-channel complex. Nonetheless, in our semiquantitative approach, we show that there are at least three components to the washout phase of DTT; and from our mutational analysis, we demonstrate that each of the three pairs of cysteine residues underlies a distinct kinetic component. Two of these kinetic components have relatively rapid half lives (with τ on the order of seconds). In contrast,Paoletti et al. (1997) showed only one apparent binding site for high-affinity voltage-independent Zn2+inhibition, whose off-rate could be fit by a single exponential of relatively rapid τ. In our hands, the kinetics of tricine potentiation also reveal only a single, very fast Zn2+-mediated process. Therefore, the three component kinetics of DTT washout, and more specifically, the two most rapid components, cannot be simply explained by the action of DTT as a Zn2+ chelator. In addition, we used the sulfhydryl oxidizing agent DTNB, which does not chelate Zn2+, to probe for redox modulatory effects. Experiments with DTNB revealed that mutations of Cys 744 and Cys 798 of NR1 were not sufficient to completely abolish the effect of this oxidizing agent. In fact, it was necessary to mutate all three pairs of cysteine residues to totally abrogate redox modulation by DTNB of receptors containing NR1–1a and NR2A subunits. Moreover, experiments with the NR2A histidine mutants showed that corruption of the high-affinity Zn2+-binding site led to elimination not only of the fast component of DTT modulation but also the intermediate component (and affected the slow component as well). This finding leads us to believe that Zn2+and redox effects are closely related; it appears that one affects the other, similar to the link between proton and Zn2+ inhibition previously suggested by the experiments of Traynelis et al. (1998) and Choi and Lipton (1999). The exact mechanism whereby the three components of redox modulation are affected by Zn2+ binding must await future investigations. One unifying pathway for allosteric modulation would hold that these cysteine residues play a key role in gating of NMDA receptor channels, and thus modulate both Zn2+ and redox effects. Moreover, if Zn2+ affects channel gating, then elimination of high-affinity Zn2+inhibition through mutation of it binding site would also affect redox modulation.
Histidine residues, rather than cysteine residues, have recently been suggested to be necessary for high-affinity Zn2+ inhibition by constituting the Zn2+-binding site (Choi and Lipton, 1999;Fayyazuddin et al., 2000; Low et al., 2000). Mutating cysteines could potentially cause a major structural change in the receptor, thus nonspecifically disrupting Zn2+-binding sites as well as other receptor properties. Mutations of the three pairs of cysteine residues under consideration resulted in a sixfold decrease in the EC50 for both NMDA and glycine. In contrast, amino acid residues that have been shown to be important for agonist binding by mutational analysis increase the EC50 on the order of 1000-fold (Kuryatov et al., 1994; Wafford et al., 1995; Hirai et al., 1996; Laube et al., 1997;Anson et al., 1998). Moreover, although the glutamate-binding site is thought to be located on the NR2 subunit (Laube et al., 1997;Anson et al., 1998), the decrease in EC50 for NMDA observed in NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A,C320A) receptors is mainly attributable to the mutations of NR1 Cys 744 and Cys 798. Similarly, although the glycine-binding site is thought to be located on the NR1 subunit (Kuryatov et al., 1994; Hirai et al., 1996; but see Wafford et al., 1993, 1995), the decrease in EC50 for glycine observed in NR1–1a(C79S,C308S,C744A,C798A)/NR2A(C87A, C320A) receptors is mainly caused by the mutations of NR2A Cys 87 and Cys 320. Therefore, mutations of these pairs of cysteine residues are unlikely to disrupt the glutamate and glycine-binding sites to any significant degree, but instead may possibly cause changes in channel gating, as discussed below.
One possible mechanism of how these three pairs of cysteine residues might affect high-affinity, voltage-independent Zn2+ inhibition is that the degree of this Zn2+ inhibition can be influenced by the redox status of the NMDA receptor. In our experiments on neurons or oocytes not previously exposed to redox agents, the magnitude of potentiation by the reducing agent, DTT, was severalfold greater than the magnitude of inhibition by the oxidizing agent, DTNB, suggesting that the native redox state of NMDA receptors is closer to the fully oxidized than the fully reduced state. In the oxidized state, these three pairs of cysteine residues appear to form disulfide bonds, and the conformational changes presumably initiated by Zn2+ binding are transduced into a greater effect on voltage-independent inhibition of NMDA-evoked currents than in the chemically reduced state. In contrast, in the reduced state that favors free thiol groups, or in cysteine mutants in which disulfide bonds cannot be formed, Zn2+ binding is apparently “uncoupled” from the inhibition of NMDA-evoked currents, and the effect of Zn2+ is far less (Fig.7). Therefore, the redox status of these three pairs of cysteine residues appear to be crucial in determining Zn2+ sensitivity of the NMDA receptor, and thus form a series of molecular “cysteine switches.” Our results could also be interpreted to show that these cysteine mutations eliminate redox modulation (e.g., by DTNB) and at the same time selectively distort the high-affinity Zn2+-binding site without much affecting other properties of the NMDA receptor, such as glutamate and glycine binding. In either case, the findings show that the redox status of these three pairs of cysteine residues influence modulation by both low concentrations of Zn2+ and by specific redox agents such as DTNB.
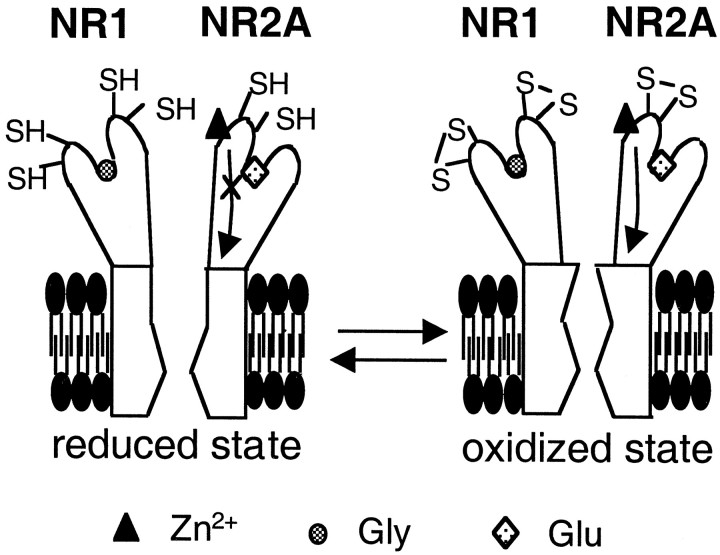
Fig. 7.
Proposed model for the redox-sensitive cysteine residues of the NMDA receptor underlying modulation of voltage-independent Zn2+ inhibition. It has been proposed that the glutamate-binding site is on the NR2A subunit (Laube et al., 1997; Anson et al., 1998), whereas the glycine-binding site is on the NR1 subunit (Kuryatov et al., 1994; Wafford et al., 1995; Hirai et al., 1996). In the oxidized state (right panel), three pairs of critical cysteine residues form disulfide bonds. In the oxidized state, the conformational change initiated by voltage-independent Zn2+ binding is transduced into greater inhibition of NMDA-evoked currents than in the reduced state. An arrow represents the transduction process mediating the inhibitory effect on channel activity after voltage-independent Zn2+ binding. In contrast, in the reduced state (left panel), disulfide bonds are broken to form free thiol groups. In mutated receptors lacking the critical cysteine residues, disulfide bonds also cannot be formed. Under these conditions, voltage-independent Zn2+binding is uncoupled from inhibition of NMDA-evoked currents. Uncoupling is diagrammed as a cross through thearrow representing the transduction process. Thus, there is less Zn2+ inhibition of NMDA current under reducing conditions. The observed results are counterintuitive if Zn2+ binding to cysteine residues were responsible for the inhibitory effect of Zn2+, because Zn2+ coordinates to free thiol and not to disulfide; Zn2+ would be expected to exert a greater inhibitory effect in the chemically reduced state of the receptor if Zn2+ binding to thiol were responsible for the effect, the opposite of the empirical result.
Previously, several modulators of NMDA receptors had been shown to exert their effects through common molecular determinants. For example, the glycine-independent form of spermine potentiation of NMDA receptors lacking exon 5 in NR1 (designated NR1–1a) was reported to be attributable to relief from tonic proton inhibition (Traynelis et al., 1995). Additionally, mutation of NR1–1a Cys 744 and Cys 798 abolished not only DTT potentiation but also glycine-independent spermine potentiation and shifted the sensitivity of NR1–1a/NR2B receptors to protons (Sullivan et al., 1994). More recently, tyrosine kinase Src-induced potentiation was shown to be attributable to the relief of Zn2+ inhibition (Zheng et al., 1998), and a link between proton inhibition and zinc inhibition has been suggested (Traynelis et al., 1998; Choi and Lipton, 1999). Here we report that three pairs of cysteine residues mediate modulation by both redox agents and Zn2+. The observation that pH, spermine, Zn2+ (the voltage-independent component), nitric oxide, and redox modulation all affect the frequency of channel opening (Christine and Choi, 1990; Legendre and Westbrook, 1990; Traynelis and Cull-Candy, 1991; Rock and Macdonald, 1992; Tang and Aizenman, 1993b; Lipton et al., 1998) leads one to speculate that the effects of these various modulators may be transduced via common downstream molecular determinants on the NMDA receptor. Our findings suggest that high-affinity Zn2+ inhibition may occur only on the oxidized form of the NR1/NR2A receptor and that the redox status of the three pairs of cysteines may be crucial in transducing the Zn2+-binding “signal” to channel gating. Thus, the three pairs of cysteine residues that we have identified may constitute, at least in part, these common downstream determinants that play a substantial role in gating of the channel. If that is indeed the case, sulfhydryl redox agents may be uniquely capable of influencing and possibly occluding effects of the other modulators by changing the redox status of these critical cysteine residues. Regulation of these molecular cysteine switches may also offer a novel therapeutic approach to curtail excessive NMDA receptor activity under pathological conditions.
Footnotes
This work was supported in part by National Institutes of Health Grants P01 HD29587, R01 EY05477 (S.A.L.), and MH12255 (Y.-B.C.), and by the American Heart Association (H.-S.V.C.). S.A.L. was a consultant to Allergan (Irvine, CA) and Neurobiological Technologies (Richmond, CA) in the field of NMDA receptor antagonists. We thank Mala Rastogi and Amy D. Brideau for technical assistance and Posina V. Rayudu for helpful discussions. We also thank an anonymous reviewer for insightful comments concerning the interpretation and summary of our data.
Correspondence should be addressed to Stuart A. Lipton, Center for Neuroscience and Aging, The Burnham Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037. E-mail: slipton@burnham.org.
REFERENCES
- 1.Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- 2.Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine- substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- 3.Anson LC, Chen PE, Wyllie D, Colquhoun D, Schoepfer R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J Neurosci. 1998;18:581–589. doi: 10.1523/JNEUROSCI.18-02-00581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Burns JA, C BJ, Moran J, Whitesides GM. Selective reduction of disulfides by tris-(2-carboxyethyl)-phosphine. J Org Chem. 1991;56:2648–2650. [Google Scholar]
- 7.Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- 8.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 9.Choi Y-B, Lipton SA. Mechanism of action and identification of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 1999;23:171–180. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 10.Christianson DW. Structural biology of zinc. Adv Protein Chem. 1991;42:281–355. doi: 10.1016/s0065-3233(08)60538-0. [DOI] [PubMed] [Google Scholar]
- 11.Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantine-Paton M. NMDA receptor as a mediator of activity-dependent synaptogenesis in the developing brain. Cold Spring Harb Symp Quant Biol. 1990;55:431–443. doi: 10.1101/sqb.1990.055.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Cornell NW, Crivaro KE. Stability constant for the zinc-dithiothreitol complex. Anal Biochem. 1972;47:203–208. doi: 10.1016/0003-2697(72)90293-x. [DOI] [PubMed] [Google Scholar]
- 14.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 15.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert KR, Aizenman E, Reynolds IJ. Oxidized glutathione modulates N-methyl-d-aspartate- and depolarization-induced increases in intracellular Ca2+ in cultured rat forebrain neurons. Neurosci Lett. 1991;133:11–14. doi: 10.1016/0304-3940(91)90045-u. [DOI] [PubMed] [Google Scholar]
- 17.Gozlan H, Khazipov R, Diabira D, Ben-Ari Y. In CA1 hippocampal neurons, the redox state of NMDA receptors determines LTP expressed by NMDA but not by AMPA receptors. J Neurophysiol. 1995;73:2612–2617. doi: 10.1152/jn.1995.73.6.2612. [DOI] [PubMed] [Google Scholar]
- 18.Hirai H, Kirsch J, Laube B, Betz H, Kuhse J. The glycine binding site of the N-methyl-d-aspartate receptor subunit NR1: identification of novel determinants of co-agonist potentiation in the extracellular M3–M4 loop region. Proc Natl Acad Sci USA. 1996;93:6031–6036. doi: 10.1073/pnas.93.12.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- 20.Hollmann M, Maron C, Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13:1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 21.Köhr G, Eckardt S, Luddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 22.Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 23.Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 24.Legendre P, Westbrook GL. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. J Physiol (Lond) 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard JP, Kelso SR. Apparent desensitization of NMDA responses in Xenopus oocytes involves calcium-dependent chloride current. Neuron. 1990;4:53–60. doi: 10.1016/0896-6273(90)90443-j. [DOI] [PubMed] [Google Scholar]
- 26.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–71. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 27.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 28.Lipton SA, Rayudu PV, Choi Y-B, Sucher NJ, Chen H-SV. Redox modulation of the NMDA receptor by NO-related species. Prog Brain Res. 1998;118:73–82. doi: 10.1016/s0079-6123(08)63201-x. [DOI] [PubMed] [Google Scholar]
- 29.Low C-M, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular determinants of coordinated proton and zinc inhibition of N-methyl-d-aspartate NR1/NR2A receptors. Proc Natl Acad Sci USA. 2000;97:11062–11067. doi: 10.1073/pnas.180307497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer ML, Vyklicky LJ, Westbrook GL. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol (Lond) 1989;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 32.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 33.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-d-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 35.Rock DM, Macdonald RL. The polyamine spermine has multiple actions on N-methyl-d-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992;41:83–88. [PubMed] [Google Scholar]
- 36.Sucher NJ, Lipton SA. Redox modulatory site of the NMDA receptor-channel complex: regulation by oxidized glutathione. J Neurosci Res. 1991;30:582–591. doi: 10.1002/jnr.490300316. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 38.Tang LH, Aizenman E. Allosteric modulation of the NMDA receptor by dihydrolipoic and lipoic acid in rat cortical neurons in vitro. Neuron. 1993a;11:857–863. doi: 10.1016/0896-6273(93)90115-8. [DOI] [PubMed] [Google Scholar]
- 39.Tang LH, Aizenman E. The modulation of N-methyl-D-aspartate receptors by redox and alkylating reagents in rat cortical neurones in vitro. J Physiol (Lond) 1993b;465:303–323. doi: 10.1113/jphysiol.1993.sp019678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traynelis SF, Cull-Candy S. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol (Lond) 1991;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 42.Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Vyklicky LJ, Benveniste M, Mayer ML. Modulation of N-methyl-d-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol (Lond) 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wafford KA, Bain CJ, Le Bourdelles B, Whiting PJ, Kemp JA. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. NeuroReport. 1993;4:1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
- 46.Wafford KA, Kathoria M, Bain CJ, Marshall G, Le BB, Kemp JA, Whiting PJ. Identification of amino acids in the N-methyl-d-aspartate receptor NR1 subunit that contribute to the glycine binding site. Mol Pharmacol. 1995;47:374–380. [PubMed] [Google Scholar]
- 47.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 48.Williams K. Separating dual effects of zinc at recombinant N-methyl-d-aspartate receptors. Neurosci Lett. 1996;215:9–12. doi: 10.1016/s0304-3940(96)12924-4. [DOI] [PubMed] [Google Scholar]
- 49.Wo ZG, Oswald RE. Transmembrane topology of two kainate receptor subunits revealed by N-glycosylation. Proc Natl Acad Sci USA. 1994;91:7154–7158. doi: 10.1073/pnas.91.15.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood MW, VanDongen HM, VanDongen AM. Structural conservation of ion conduction pathways in K channels and glutamate receptors. Proc Natl Acad Sci USA. 1995;92:4882–4886. doi: 10.1073/pnas.92.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]