Abstract
The fruitless gene in Drosophilaproduces male-specific protein (FRUM) involved in the control of courtship. FRUM spatial and temporal patterns were examined in fru mutants that exhibit aberrant male courtship. Chromosome breakpoints at the locus eliminated FRUM. Homozygous viable mutants exhibited an intriguing array of defects. In fru1males, there were absences of FRUM-expressing neuronal clusters or stained cells within certain clusters, reductions of signal intensities in others, and ectopic FRUMexpression in novel cells. fru2 males exhibited an overall decrement of FRUM expression in all neurons normally expressing the gene.fru4 andfrusat mutants only produced FRUM in small numbers of neurons at extremely low levels, and no FRUM signals were detected infru3 males. This array of abnormalities was inferred to correlate with the varying behavioral defects exhibited by these mutants. Such abnormalities include courtship among males, which has been hypothesized to involve anomalies of serotonin (5-HT) function in the brain. However, double-labeling uncovered no coexpression of FRUMand 5-HT in brain neurons. Yet, a newly identified set of sexually dimorphic FRUM/5-HT-positive neurons was identified in the abdominal ganglion of adult males. These sexually dimorphic neurons (s-Abg) project toward regions of the abdomen involved in male reproduction. The s-Abg neurons and the proximal extents of their axons were unstained or absent in wild-type females and exhibited subnormal or no 5-HT immunoreactivity in certainfru-mutant males, indicating thatfruitless controls the formation of these cells or 5-HT production in them.
Keywords: fruitless transposons, chromosome aberrations, brain neurons, ventral nerve cord, sexual dimorphism, serotonergic neurons
Courtship in Drosophila melanogaster is regulated by a somatic sex-determination hierarchy. One of the “downstream” genes functioning within this hierarchy is fruitless (for review, see Goodwin, 1999;Yamamoto and Nakano, 1999). fru mutations cause the most sharply defined effects on male courtship, compared with behaviorally mutant phenotypes associated with other downstream genes (Villella and Hall, 1996; Finley et al., 1997). fruitless produces male- and female-specific transcripts under the control of a distal promoter (P1) located ∼100 kb from the bulk of the open reading frame of the gene (Ryner et al., 1996). The male-specific proteins (FRUMs) encoded by P1-controlled mRNAs are likely to be involved in the regulation of courtship or the development of the neural substrates for male reproductive behavior (Goodwin 1999). In females, P1-produced transcripts are not translated into detectable FRU protein (Lee et al., 2000; Usui-Aoki et al., 2000).
One approach toward understanding how fru regulates male courtship is to compare patterns of FRUMexpression in the CNS of various fruitless mutants that display behavioral phenotypes ranging from mildly to severely defective (Villella et al., 1997; Goodwin et al., 2000). The courtship subnormalities and bisexual behavior caused by fru mutations could be understood in terms of where FRUMis expressed in the CNS (or not expressed, as the case may be) in a given mutant.
The fact that several fru-mutant types court other males might be attributable to subnormal levels of serotonin (5-HT) in relevant brain cells. This hypothesis suggested itself because of the anomalous inter-male courtships that are induced by ectopic expression of the white (w+) gene (Zhang and Odenwald, 1995; Hing and Carlson, 1996).white encodes a tryptophan–guanine transporter; because tryptophan is a precursor of 5-HT, inducedw+ expression all over the brain could cause subnormal 5-HT levels in neurons that normally produce it. Drug-induced 5-HT reductions can induce homosexual behavior of male mammals (for review, see Gessa and Tagliamonte, 1974; Fratta et al., 1977). fru mutants, which are predicted to exhibit deficits in male-specific transcription factors encoded by this gene (Goodwin, 1999; Goodwin et al., 2000), could also be deficient in 5-HT if its production is downstream of FRUMfunction. Moreover, the actions of both ectopic-w+ andfruitless have been suggested to be in the same pathway because of a blockade of the behavioral effects of ectopic-w+ by a frumutation that, by itself, causes very low levels of male courtship (Nilsson et al., 2000). Based on these suppositions, we analyzed the relationship between the spatial distribution of 5-HT andfru gene products in normal and mutant CNSs.
Along with demonstrating that elements of 5-HT production are downstream of fru functioning (although not in CNS regions that one might have expected), this feature of the study provided the first information on axonal projections of certain FRUM cells. Assessing the subcellular localization of FRUM alone gives no insight into this matter; FRU immunoreactivities are nuclear (Lee et al., 2000), consistent with the supposition that these proteins are gene regulators.
MATERIALS AND METHODS
Strains and culturing. Stocks and progeny from crosses of D. melanogaster were reared in 12 hr light/dark (LD) cycles at 25°C, 70% relative humidity, on a sucrose–cornmeal–yeast medium containing the mold inhibitor Tegosept. A Canton-S strain was used as the wild-type control.
The fruitless mutant stocksfru1, fru2,fru3, fru4, and frusat were maintained as described in Villella et al. (1997) and Goodwin et al. (2000). The following homozygous-lethal fru variants, missing all or part of the locus, were combined in pairwise crosses (see Table 1):Df(3R)ChaM5, Df(3R)P14,Df(3R)frusat15,Df(3R)fruw24, andDf(3R)fru4–40 (Gailey and Hall, 1989;Ito et al., 1996; Ryner et al., 1996; Anand et al., 2001). These deletions will be referred to asDf-ChaM5, Df-P14,Df-sat15,Df-fruw24, andDf-fru4–40. The lethal variantsfruw12 andfruw27, each carrying single breakpoints within the locus, were crossed to each other or to certainDf-bearing flies to generate severely lesioned genotypes (see Table 1). The Df-fru or fru-lethal stocks were balanced with In(3LR)TM6B, Tb,In(3LR)TM3, Sb, or Tp(3)MKRS, Sb.
Table 1.
Lack of male-specific FRUM protein in severely affected fruitless mutants
| Genotype | Relative FRUM immunostaining, % (n pupal CNS specimens) |
|---|---|
| WT | 100 (10) |
| fru3/fru3 | 0 (6) |
| fruw12/fru3 | 0 (3) |
| Df-fruw24/fru3 | 0 (5) |
| fruw27/fru3 | 0 (5) |
| fru3/Df-ChaM5 | 0 (7) |
| fruw12/Df-ChaM5 | 0 (7) |
| fruw27/Df-ChaM5 | 0 (5) |
| fruw12/Df-fruw24 | 0 (3) |
| fruw12/fruw27 | 0 (3) |
| Df-fruw24/fruw27 | 0 (3) |
| Df-ChaM5/Df-sat15 | 0 (5) |
| Df-fru4-40/Df-sat15 | 0 (5) |
Immunohistochemistry using anti-FRUM was performed to assess expression of the male forms of FRU protein in the CNSs of 2-d-old male pupae from a wild-type (WT) stock, afru3-bearing strain, and pupal progeny resulting from crosses of fru-breakpoint variants to each other or certain such variants to fru3 (the only homozygous-viable mutant used here). The w-including genotypes are chromosome aberrations, each involving a breakpoint within the fru locus; most of the Df-including genotypes are deletions, each of which has one breakpoint at this locus and thus is missing part of the locus (Df-fruw24removes the entire locus). Nearly all breakpoint combinations, with respect to the chromosome aberrations depicted in Figure 1, were generated, except forfruw24/Df-ChaM5 (which die as embryos). For the results column, the wild-type staining level was simply set at 100, because there was no apparent FRUMimmunostaining in pupae carrying any of these mutant orfru-breakpoint variant specimens.
To obtain 2-d-old pupae homozygous for a fru mutation or carrying a given transheterozygous combination, flies fromTM6B-balanced stocks were crossed to each other. This was necessary because of the homozygous lethality or sterility associated with fru variants, with the exception offru2, which is homozygous-viable and fertile (permitting pupae to be obtained from a true-breeding stock). For the other strains, balancer-over-fruheterozygotes were crossed, and animals not expressing the pupal markerTb were selected. To stage these developing animals, white prepupae were selected, sexed by gonadal size, and maintained on a Petri dish with a wet filter paper at 25°C, 70% relative humidity, on a 12 hr LD cycle for 2 d. To collect homozygous or transheterozygous fru-mutant adults,TM3- or MKRS-balanced fru stocks were used; progeny not expressing the Sb marker were selected.
Immunohistochemistry and in situ hybridization.Polyclonal anti-FRUM, designed to detect male-specific proteins encoded by male-specific frutranscripts stemming from the action of the sex-specific P1 promoter, was generated in a rat as described in Lee et al. (2000). Anti-FRUM-mediated staining was effected using whole-mounts of dissected CNSs from 2-d-old pupae and 4- to 7-d-old adults. The antibody was applied at a dilution of 1:300. To detect cell and tissue labeling mediated by such application, two different secondary antibodies were used. (1) Horseradish peroxidase-conjugated anti-rat serum (made in donkey; Jackson ImmunoResearch, West Grove, PA) was applied at a dilution of 1:200. For the color reaction, the tissues were incubated at room temperature in the dark for 20 min in phosphate buffer containing 0.5 mg/ml 3,3′-diaminobenzidine tetrahydrochloride; 0.0015% hydrogen peroxide solution was added, and the color development was monitored under a dissecting microscope. After sufficient color had developed, the reaction was terminated by rinsing tissues with distilled water. Stained tissues were rinsed three times in phosphate buffer, dehydrated, cleared in glycerol, and mounted in 100% glycerol under glass coverslips. (2) For fluorescent immunostaining, fluorescein isothiocyanate (FITC)-conjugated secondary IgG (made in donkey; Jackson ImmunoResearch) was used at a dilution of 1:200. CNSs labeled in this manner were mounted with 2% n-propyl gallate in 80% glycerol in phosphate buffer, pH 7.4. Preparations were observed under a Zeiss Axiophot microscope or an MRC600 laser-scanning confocal microscope (Bio-Rad, Richmond, CA).
To apply anti-5-HT (made in rabbit; Protos Biotech, New York, NY) for immunohistochemistry, CNSs were fixed in a solution of 4% paraformaldehyde including 7.5% picric acid for 1 hr at room temperature; this antibody was used at a dilution of 1:500. The secondary antisera, FITC-conjugated anti-rabbit IgG (made in donkey; Jackson ImmunoResearch), was used at a dilution of 1:200, applying the same procedures used for anti-FRUMimmuno-histochemistry.
Immunofluorescent double-labelings were performed on whole-mounted CNSs; anti-FRUM (from rat) and anti-5-HT (from rabbit) were applied to whole-mounted CNSs of wild-type adult males. Dissection, fixation, and washes were performed as described above for anti-5-HT, except for the fixation, which was done on ice. FITC-conjugated anti-rat IgG for anti-FRUMand lissamine rhodamine sulfonyl chloride-conjugated anti-rabbit IgG (from donkey; Jackson ImmunoResearch) for anti-5-HT were used as secondary antisera. Preparations were viewed in the confocal microscope described above, which is equipped with an argon–krypton laser and dual-channel scanning. Colocalization was verified by merging the two channels.
For in situ hybridization with whole-mounted CNSs, an antisense probe from the P1 region of the fru locus (see Fig. 1) was applied to CNSs dissected from 1-d-oldDf-fru4–40/Df-sat15or Df-ChaM5/Df-sat15(double-deletion) male pupae. The particular probe (called P1.S1; see Fig. 1) and the labeling procedures were as described in Lee et al. (2000).
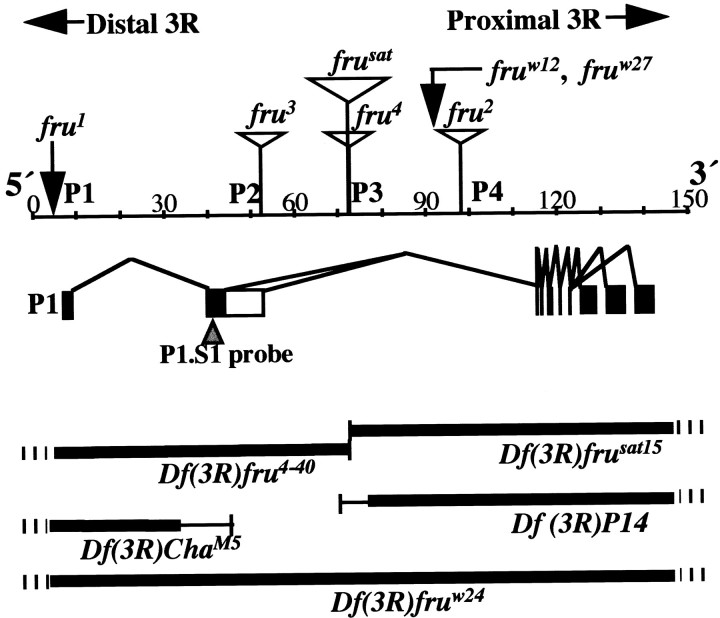
Fig. 1.
The fruitless gene and genetic variations at the locus. This large (∼130 kb) gene contains at least four promoters (Ps); the 5′-most promoter (P1,left) controls the production of primary transcripts that are sex-specifically spliced with respect to the second exon (Ryner et al., 1996; Goodwin et al., 2000). P1-promoted RNA species are diagrammed in the middle panel. The principal alternative splicings of interest occur near the 5′ (left) end. (There are additional such splicings near the 3′ end, designated by three black rectangles on theright that result in different kinds of Zn-finger pairs near the C termini of FRU protein isoforms.) The 5′ sex-specific splicings result in a male mRNA of which relatively 5′ coding sequences (5′ ORF) are translated to produce 101 male-specific amino acids bound to the remainder of the residues that are encoded by relatively 3′ sequences emanating from the right part of the gene. This protein is called FRUM, which is specifically detected by an antibody generated against the male-specific N-terminal residues (Lee et al., 2000). In females, the 5′ ORF runs into a stop codon after nucleotides encoding 94 amino acids (because of the alternative splicing referred to above). A ∼280-nucleotide probe applied in this study to detect sex-specifically spliced transcripts (cf. Lee et al., 2000) corresponds to sequences in the second exon, designated by a black rectangle (middle left) and pointed to by an inverted triangle. Sex-nonspecific promoters (P2, P3, andP4) are used to generate transcripts that lack the male-specific 101 residues and are believed to be associated with vital fru functions that operate in both sexes (Ryner et al., 1996; Anand et al, 2001). Such functions are inferred (in part) from the effects of fruw12 andfruw27, which are translocation and inversion breakpoints that cause late-developmental lethality when they are homozygous or heterozygous for a deletion that eliminates the entire locus. fruw24 is such a deletion (Df), indicated by the thick black line (this and other such lines designate deleted material); hash marks (for this and the otherDfs) indicate that the deletion extends well beyond the locus. The four additional Dfs that were applied have breakpoints within the locus (Ito et al., 1996; Ryner et al., 1996;Anand et al, 2001), as indicated by thin vertical lines(the thin horizontal portions of these Dfindicators imply not-quite-certain breakpoint determinations). Homozygous-viable fruitless mutants are caused, in one case, by an inversion breakpoint (fru1) and, in the remaining cases, by transposon inserts (open triangles) inserted at intragenic locations as determined by Ito et al. (1996), Ryner et al., (1996), and Goodwin et al. (2000). These and other features of the diagram are based on results in these three reports, as well as information obtained by interrogating theDrosophila genome database at www.fruitfly.org with sequences of various fru cDNAs (Lee et al., 2000) and molecular determination of the fru1inversion breakpoint, which was found to be 3.3 kb upstream (to theleft) of the transcription start site for RNAs generated under the control of the P1 promoter. The beginning of a 7 bp consensus-sequence for the latter starts 28 bp downstream of the transcription start (T. Carlo, S. F. Goodwin, J.-C. Billeter, L. C. Ryner, B. S. Baker, and J. C. Hall, unpublished observations).
Scoring of staining intensities. To analyze the levels of FRUM in CNSs of viable frumutants and wild-type males, fluorescently immunostained signals from various FRUM-expressing neuronal clusters in whole-mounted CNS were quantified as described in Lee et al. (2000). We focused on three such clusters within the superior protocerebrum in analyzing the wild type and the fru mutants. These and other FRUM cell groups had staining intensities assessed for animals carrying the various fru genotypes (see Table 2 legend). The FRUM immunostaining quantifications were performed on whole-mounted brains dissected from 2-d-old pupae, which show the highest level of CNS expression by this protein (Lee et al., 2000), and from 4- to 7-d-old adults, which are active courters. The specimens to be compared (in terms of genotype or life-cycle stage) were processed simultaneously to minimize signal variations that could occur for nonsubstantive reasons. At least five CNSs were sampled from animals of each genotype at both of the different stages. The dorsoanterior brain region that contains both neuronal clusters was imaged at 100× magnification by confocal microscopy (2 or 4 μm optical sections). Staining intensities for nuclei of cells within these two clusters were obtained (as pixel values) from at least five individual brains by applying an Adobe PhotoShop (3.0) tool called Histogram. This permitted an average value to be computed from several strongly stained nuclei in the cells of the two nearby clusters; for a given brain, such an average value was obtained for only the left or right hemisphere. The relevant FRUM signal values ranged from black to white (of 256 gray values); “whitest” represents maximal protein expression (see Table 2 for additional details).
Table 2.
Staining intensities of FRUM-expressing cells in fruitless mutants
| Genotype (Sex) | Behavioral changes | % Relative staining intensity (digitized value ± SEM) | Remarks on staining patterns | ||
|---|---|---|---|---|---|
| n:Pu/Ad | Pupa | Adult | |||
| WT (M) | N.A. | 5/5 or 1 | 100 (232 ± 52-a, 235 ± 42-b, 236 ± 22-c) | 100 (150 ± 122-a, 1302-c) | Twenty clusters of FRUM-containing neurons, within several discrete brain regions and throughout most of the VNC; all cells show similar levels of immunostaining |
| WT (F) | N.A. | 5/6 | 0 | 0 | No immunoreactive cells visible |
| fru1 (M) | Court F; sings; sterile; vig. M-M | 7/5 | 24 (55 ± 92-a), 21 (49 ± 82-b), 100 (235 ± 122-c) | 92 (120 ± 112-c) | Staining intensities variable among immunoreactive cells; levels of FRUM normal or nearly so in several cells, but reduced in most, including to 0 in fru-mAL and ASP1 cluster (Fig. 4); ectopic expression in many non-FRUM cells within brain and VNC (Fig. 4) |
| fru2 (M) | Court F; sings; fertile; mild M-M | 5/5 | 67 (156 ± 32-a) | 60 (89 ± 72-a) | Non-severe and apparently uniform decrement in staining intensity |
| fru3 (M) | Weak court F; mute; sterile; mild M-M | 5/5 | 0 | 0 | No immunoreactive cells visible |
| fru4 (M) | Court F; mute; sterile; mild M-M | 5/5 | 4 (9 ± 12-b) | 0 | A few immunoreactive cells visible with extremely low intensities infru-P and pSP2 clusters (Fig. 3) |
| frusat (M) | Very weak court F; mute; sterile; mild M-M | 5/5 | 9 (20 ± 22-b) | 0 | Immunoreactive cells visible with low signal intensities in fru-aSP3, Lv, AL, P, SP brain cluster; and PrMs, MsMt, and Ab VNC ones (Fig.3) |
In the genotype column, M designates male and F designates female. The behavioral column briefly summarizes the defects and anomalies reported for these five fruitless mutants by Villella et al. (1997) and Goodwin et al. (2000). N.A., Not applicable; court (or weak court) F, mutant male courts females (or does so weakly as the case may be); mute, performs wing extension when oriented toward or following female, but produces no courtship song; vig. (or mild) M-M, mutant males court each other vigorously (or at relatively low levels, but above that of wild-type inter-male courtship-like interactions). For the immunohistochemical results tabulated here, brains were dissected from animals at two life-cycle stages: 2-d-old pupae and 4 to 7-d-old adults. In the n column, numbers of pupal specimens (Pu) are indicated on the left, numbers of adult brains (Ad) on the right. For wild-type (WT) and fru1 pupae, immunostaining levels in the brain-neuronal clustersfru-aSP2, fru-P, and fru-mcAL (Figs.3, 4A,C,E) were analyzed. The fru1mutant showed variable staining intensities among cells or within clusters, as shown in Figure 4; here, this variability is exemplified by quantifying FRUM levels in three brain-cell groups of pupae and one such (fru-mcAL) in adults. Forfru2 pupae and adults, the comparisons relative to WT are for the fru-aSP2 cluster. Forfru4 and frusat, cells infru-P clusters were analyzed (Fig. 3H,I), these being one of only two brain regions retaining FRUMimmunostaining in these mutants (in pupae only). The mean digitized signal levels (± SEM) are within parentheses in the first two data columns (no SEM for fru-mcAL in WT adult, in that only one specimen was quantified for this cluster, whereas five WT male brains were analyzed for fru-aSP2). The nominal maximal values for wild-type pupal and adult brains were set at 100%, and the mutant percentages quoted are relative to that maximum.
fru-aSP2.
fru-P.
fru-mcAL.
To assess levels of FRUM expression in CNSs of 2-d-old pupae that carried transheterozygous Dfs or were homozygous for the fru3mutation, protein expression was scored subjectively in whole-mounts (3–10 individual specimens for each genotype) using a fluorescent microscope at 40× magnification. A representative image for each genotype was obtained using confocal microscopy afterward. To assess staining intensities from CNSs subjected to P1-fru-probe in situ hybridization, at least six specimens were subjectively evaluated using brightfield microscopy. A representative image for an animal of a given fru genotype was obtained at 30× magnification.
5-HT uptake. In attempts to determine whether the lack of 5-HT immunostaining in the abdominal ganglion of thefru3 mutant (see Results) is attributable to the absence of the relevant cells (those that contain signal in wild type) or a dearth of serotonin synthesis, ventral nerve cords (VNCs) of 4- to 5-d-old adult males were dissected and exposed to exogenously applied 5-HT, essentially as described in Vallés and White (1986). Tissues were incubated in Drosophila Ringer's [(in mm) 130 NaCl, 4.7 KCl, 1.8 CaCl2, 0.74 KH2PO4, and 0.35 Na2HPO4] containing a 5-HT/creatine-sulfate (Sigma, St. Louis, MO) at the following concentrations: 1, 5, 10, 100, and 500 μm. The VNCs were rinsed three times for 15 min each in ice-cold Ca2+-free Ringer's, then fixed with 4% paraformaldehyde with 7.5% picric acid for 1 hr at room temperature. Application of primary anti-5-HT and subsequent immunohistochemical procedures were as described above (see Immunohistochemistry andin situ hybridization).
RESULTS
Male-specific fru products in the CNS of chromosome-breakpoint variants
Among the most severely defective fru variants in terms of courtship behavioral subnormalities are those expressing the effects of chromosome breakpoints within the locus. We suspected that animals carrying most or all of these genotypes would lack detectable FRUM. This expectation is based on the molecular characterization of these chromosome aberrations (Fig.1) against a background of thefru transcript-types that are produced under the control of a given promoter (Ryner et al., 1996; Goodwin et al., 2000). In fact, two of the fru variants in question (Fig. 1) were shown to be null for FRUM immunostaining: theDf-ChaM5/Df-P14 andDf-fru4–40/Df-P14 double-deletion types (Lee et al., 2000). These assessments were performed principally as a control for specificity of the antibody.
Drosophila carrying theDf-ChaM5/Df-P14 orDf-fru4–40/Df-P14 deletions develop into viable adults, as can be rationalized by their ability to transcribefru mRNAs under the control of one or more promoters located downstream of P1 (Fig. 1). These transcripts encode FRU protein isoforms that are produced in both males and females (Lee et al., 2000); absence of these products, caused by radiation-inducedfru-locus lesions (Ryner et al., 1996) associated with chromosomal breakpoints located between the P1 and P4 promoters (Fig.1), leads to near lethality of males and females (Anand et al., 2001). The Df-ChaM5/Df-P14 andDf-fru4–40/Df-P14 combinations [in which P4 and possibly P3 are active, but P1 is deleted (Fig. 1)] allow for normal viability; males of these genotypes exhibit severely subnormal levels of courtship and do not mate (Villella et al., 1997;Anand et al., 2001).
Two further double-deletion types cause similar subnormalities of male courtship:Df-ChaM5/Df-sat15 andDf-fru4–40/Df-sat15, whose levels of courtship directed at females are nearly zero (Anand et al, 2001). As expected from the positions of the intra-frubreakpoints associated with these three deletions (Fig. 1), neither P1-promoted transcripts (Fig.2A) nor FRUM protein (Table1) was detected in males of these two genotypes. Actually, it could be that a transcript fragment containing sequences from the 5′ end of P1-promoted mRNA would have been labeled by the probe applied (Fig. 1); and that the male-specific, N-terminal residues encoded by these 5′ sequences would be present as an anti-FRUM-labeled oligopeptide (Fig. 1). That no signals were detected by the nucleic acid or the antibody probe (Fig. 2A; Table 1) indicates that the truncated forms of neither fru transcript nor FRU protein accumulate to levels detectable by in situ hybridization or immunohistochemistry. These results are consistent with previous results obtained from histological analyses ofDf-ChaM5/Df-P14 andDf-fru4–40/Df-P14 males (Lee et al., 2000).
Fig. 2.
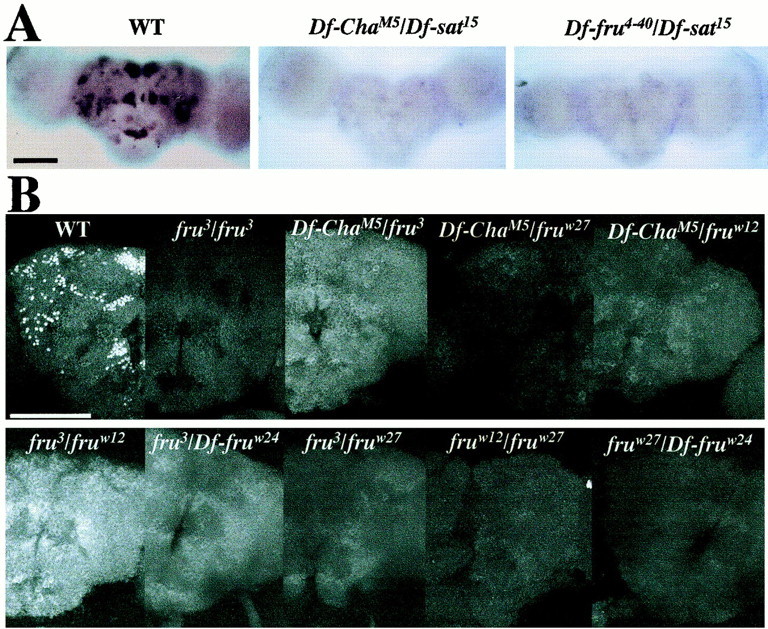
Lack of sex-specific fruitlessexpression in the CNS of fru-breakpoint mutants. A, In situ hybridizations performed on pupal progeny resulting from crosses involving three of the deletions depicted in Figure 1; 1-d-old male pupae had afru-derived riboprobe (Fig. 1) applied to whole-mounted CNSs of wild type (WT, n = 11) and these twoDf/Df types (Df-ChaM5/Df-sat15,n = 6;Df-fru4–40/Df-sat15,n = 6); no signals were elicited by this nucleic-acid probe in any of the 12 double-deletion specimens.B, Anti-FRUM immunohistochemistry performed on pupal progeny resulting from crosses of various deletions and other breakpoint variants (Fig. 1); heterozygotes involving certain of the chromosome aberrations and one of the frutransposon mutants were included as a negative control (compare Fig. 3; Table 2); antibody against the male-specific form of the protein was applied to whole-mounted CNSs dissected from 2-d-old male pupae. Summaries of these immuno-histochemical results (including numbers of samples per genotype) are given in Table 1. Signals (or the absence thereof) were examined by confocal microscopy, and representative images were made for specimens of the various genotypes at 40×.A, B, anterior views of the brains.B, The brain image offru3/fruw12(lower left panel) shows a whitishgeneral background staining that was not detected in other specimens of this genotype (cf. Table 1) and bears no relation to the WT pattern. Scale bars: A, 100 μm; B, 50 μm.
There is an additional category of intralocus lesions associated with the fru gene: inversion and translocation breakpoints calledfruw12 andfruw27. These are located between P1 and the 3′-fru ORF, relatively close to the latter (Ryner et al., 1996) (Fig. 1). When fruw27 orfruw12 is heterozygous with a deletion that removes relatively 5′ sequences (Df-ChaM5 orDf-fru4–40), the result is a viable adult that (as a male) exhibits almost no courtship (Ryner et al., 1996; Anand et al, 2001. When either of these proximally located breakpoints is heterozygous with a full fru deletion (Fig.1, Df-fruw24), or in a transheterozgote carrying the two lesions, the result is late developmental lethality (Ryner et al., 1996). All of thesefruw27- andfruw12-including genotypes would be expected to eliminate FRUM protein, provided that the aforementioned N-terminal oligopeptide cannot accumulate to detectable levels. These expectations were met (Fig.2B; Table 1).
FRUM in the CNS of homozygous-viablefruitless mutants
The other genetic variations involving the fruitlessgene are homozygous viable mutants, most of which are caused by transposons inserted within the locus (Fig. 1). The transposons in the four relevant mutants (Ito et al., 1996; Ryner et al., 1996; Goodwin et al., 2000) are inserted between the P1 promoter and the bulk of the open reading frame of fru (Fig. 1). Thus, the P1 promoter itself should be active in these mutants. Indeed, in situhybridizations using a sex-specific probe (like that applied in the current study) (Fig. 1) revealed signal patterns in these mutants similar to that of wild type (Goodwin et al., 2000). Therefore, transcriptional activity of the P1 promoter per se seems unimpaired in these mutants. Yet these P-element-derived inserts cause aberrant splicing of sex-specific fru transcripts into acceptor sites present within the transposons (Goodwin et al., 2000). This splice-trapping results in anomalous P1-promoted transcripts in Northern blottings stemming from extracts offru2, fru3,fru4, andfrusat adults (Goodwin et al., 2000). In such blots, normal, full-length mRNAs generated by action of P1 were undetectable. However, reverse transcription-PCR assessments were able (with difficulty) to detect low levels of sex-specificfru+-like transcripts in males homozygous for a given fru variant, although the nonquantitative nature prevented comparison of residual P1-mRNA levels among the four mutant types (Goodwin et al., 2000). In any case, these results suggest that some sex-specificfru+ transcripts bypass the aberrant splicing caused by the insertions. Therefore, FRUM might be detectable in certain of these mutants.
However, none of these issues regarding the male-specific FRU protein has been empirically examined in the fru transposon mutants. Thus, in a given mutant, how much, if any, FRUM would be detectable, and where would it be found within the CNS? To address these matters, anti-FRUM immunohistochemistry was performed on the CNSs of 2-d-old male pupae and adults carrying the transposon mutations. A principal goal was to correlate FRUM expression levels with the behavioral impairments of mutants, which are summarized in Table2. For example, we suspected that this protein would be present at least in fru2because such males exhibit the mildest courtship abnormalities among the four transposon mutants used in this study (Table 2).
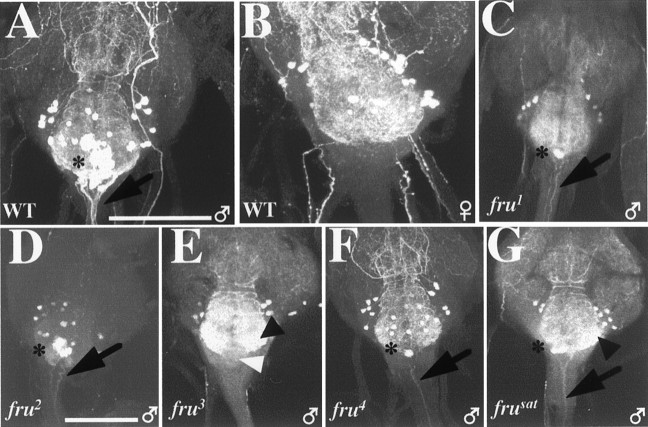
Homozygous mutants and those heterozygous for a transposon insert and a given recessive-lethal fru variant were tested by CNS dissections and application of anti-FRUM(Figs. 2B, 3, 4; Tables 1, 2).fru2 males exhibited readily detectable staining. In this fertile fru mutant (Gailey and Hall, 1989), there were only mild (and less than across-the-board) reductions in numbers of FRUM neurons, compared with the array of signals observed in the CNS of normal males (Fig.3, compare F with A, B) (Table 3). The immunohistochemical signals were 30–40% lower than normal in pupal and adult CNS specimens from fru2 males (Fig. 3F; Table 2). Therefore, the absence of readily detectable P1 transcripts (in Northern blots) is especially misleading for this mutant.
Fig. 3.
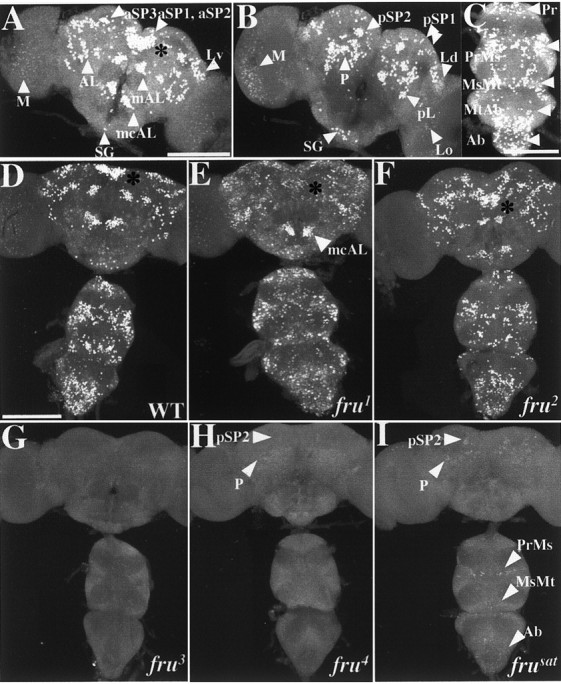
Effects of viable fruitlessmutations on FRUM expression in the CNS. Pupal progeny of crosses involving heterozygous male parents (for most of these mutant genotypes, for which homozygosity causes sterility) had CNSs dissected and subjected to immunostaining. All specimens shown are from 2-d-old pupae, the images for which were obtained by confocal microscopy at 20×. The definitions and approximate intra-CNS locations of the FRUM-expressing neuronal clusters designated bywhite arrowheads (e.g., aSP3, AL, mcAL) are specified in Table 3 (also see Lee et al., 2000). A,B, Anterior and posterior views, respectively, of wild-type male brains (representative of 36 specimens exposed to anti-FRUM). C, Ventral nerve cord from a wild-type (WT) male, as viewed (in the microscope) from the dorsal side of these ganglia but shown as a projection of stacked optical sections through the whole ventral cord. InA–C, groups of FRUM-containing view of the WT male pattern. CNS neurons are designated by white arrowheads. D, Overall view of the WT male pattern. This image (and most of those inE–I) is a projection from stacked optical sections starting from the anterior side of the brain and the ventral side of the VNC. E–I, Similar views of CNSs from pupae homozygous for each of the five viable mutations. These brain-plus-VNC images are representative of the following numbers of specimens: E,fru1 (n = 7);F, fru2(n = 5); G,fru3 (n = 15);H, fru4(n = 5); and I,frusat (n = 5). Two nearby dorsal brain clusters, fru-aSP1 andfru-aSP2 (see A), have signal-containing locations indicated by asterisks in D–F.H, I, The arrowheads near the top point to the locations of a few brain cells (compared with WT) that stained within two brain groups (cf. B) of these two mutants. I, The three arrowheads at the bottom point to a few VNC cells that stained within the fru thoracic- and abdominal-ganglionic groups (cf.C). Scale bars, 100 μm.
Fig. 4.
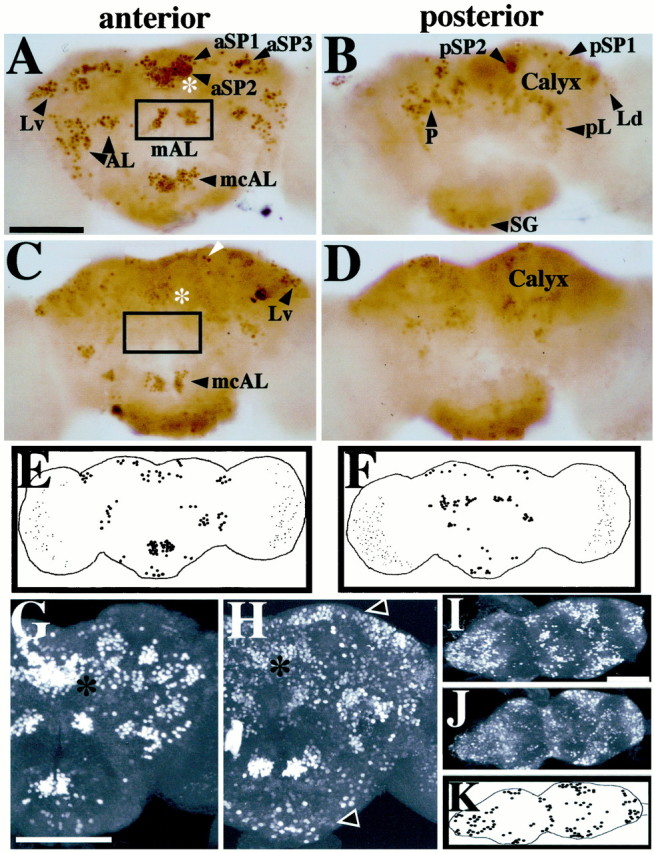
Nonrandom spatial effects offru1 on expression of FRUM in the brain. Pupal progeny of males heterozygous for this (recessive sterilizing) mutation had CNSs dissected (from 2-d-old pupae) and subjected to whole-mount anti-FRUM histochemistry. A,B, Control brightfield micrographs (from PhotoShop assemblies of four to five consecutive focal-plane images each) obtained from a wild-type (WT) brain, representative of 20 specimens stained by peroxidase-mediated color reactions (Fig. 3, compareA, B). C,D, fru1 anterior- and posterior-brain patterns, respectively, from scrutiny of 12 mutant specimens (processed and photographed as in A andB). Only a few cells with low-intensity staining were detected within the fru-aSP1 and fru-aSP2 clusters of fru1 brains (asterisks, C vs A). C, The boxed area designates the absence of the normally stained fru-mAL cluster (cf. box in A). Certain neurons showed no apparent staining-intensity decrement compared with WT; this is exemplified by neurons pointed to byarrowheads in C (also seeE and F). Other FRUM cells or clusters were absent or exhibited significantly decreased staining intensities in this mutant, e.g., in the vicinity of the mushroom-body calyx(D); these qualitative and quantitative anomalies of sex-specific fru expression are consistent with those obtained by in situ hybridizations using later-stagefru1 pupae (Goodwin et al., 2000).E, F, Diagrams of representative anterior and posterior fru1 brain views, respectively, showing cells or clusters with relatively strong staining intensities in brain regions that apparently correspond to those expressing FRUM in WT. G,H, Confocal images showing, respectively, anterior views of 2-d-old male pupal brains from WT andfru1 males (representative of 36 and 7 specimens, respectively, processed in this manner for animals of the two genotypes). Asterisks denote the location of the nearby fru-aSP1 and fru-aSP2 clusters that exhibit subnormal numbers and intensities of stained neurons infru1(H). Brains from this mutant also contain FRUM immunostaining in regions not labeled in WT. Such ectopic signals in fru1 are widely distributed and show low-intensity staining.Arrowheads point to examples of such ectopic-expression regions. I, J, Confocal images showing ventral views of ventral nerve cords dissected from WT andfru1 2-d-old male pupae.K, Diagram of FRUM-containing VNC neurons that gave relatively high-intensity staining. These cells are in posterior CNS regions that apparently correspond to the locations of such neuronal groups, although numbers of signal-containing cells are reduced within a given VNC region of the mutant. Scale bars, 100 μm.
Table 3.
Counts of FRUM-expressing cells in the pupal CNS of fru mutants
| Neuronal cluster | WT | fru2/fru2 | fru3/fru3 | fru4/fru4 | frusat/frusat | fru1/fru3 | fru1/fru4 | fru1/fruw24 |
|---|---|---|---|---|---|---|---|---|
| Anterior brain | ||||||||
| fru-aSP1 | 16 ± 1 | 16 ± 1 (4) | 0 (10) | 0 (4) | 0 (4) | 7 ± 1 (5) | 6 ± 1 (3) | 0 (4) |
| fru-aSP2 | 57 ± 2 | 44 ± 3 (5) | 0 (10) | 0 (4) | 0 (4) | 19 ± 1 (5) | 28 ± 1 (3) | 16 ± 1 (4) |
| fru-aSP3 | 40 ± 3 | 36 ± 2 (4) | 0 (10) | 0 (4) | 5 ± 1 (3) | 24 ± 3 (5) | 38 ± 1 (3) | 6 ± 2 (3) |
| fru-Lv | 17 ± 1 | 18 ± 1 (4) | 0 (10) | 0 (4) | 5 ± 2 (2) | 15 ± 1 (5) | 20 ± 4 (3) | 9 ± 1 (4) |
| fru-mAL | 29 ± 1 | 22 ± 1 (4) | 0 (10) | 0 (4) | 0 (4) | 7 ± 1 (5) | 10 ± 2 (3) | 1 ± 1 (3) |
| fru-AL | 54 ± 3 | 46 ± 1 (5) | 0 (10) | 0 (4) | 9 ± 2 (4) | 46 ± 4 (7) | 50 ± 2 (4) | 29 ± 6 (2) |
| fru-mcAL | 30 ± 1 | 25 ± 4 (2) | 0 (10) | 0 (4) | 0 (4) | 32 ± 2 (5) | 33 ± 2 (4) | 27 ± 2 (3) |
| Spanning portions of both anterior and posterior brain | ||||||||
| fru-SG | 12 ± 1 | 16 ± 2 (4) | 0 (10) | 0 (4) | 0 (4) | 7 ± 1 (3) | 9 ± 2 (4) | 7 ± 4 (2) |
| fru-M | 164 ± 8 | N.A. | 0 (10) | 0 (4) | 0 (4) | N.A. | N.A. | N.A. |
| fru-Ld | 50 ± 4 | N.A. | 0 (10) | 0 (4) | 0 (4) | N.A. | N.A. | N.A. |
| fru-Lo | 34 ± 1 | N.A. | 0 (10) | 0 (4) | 0 (4) | N.A. | N.A. | N.A. |
| Posterior brain | ||||||||
| fru-pSP1 | 7 ± 1 | 7 ± 1 (2) | 0 (10) | 0 (4) | 0 (4) | 6 ± 1 (3) | 7 ± 1 (4) | 4 ± 1 (3) |
| fru-pSP2 | 16 ± 1 | 16 ± 2 (4) | 0 (10) | 4 ± 1 (4) | 7 ± 1 (3) | 15 ± 2 (3) | 14 ± 1 (4) | 6 ± 2 (2) |
| fru-P | 73 ± 4 | 62 ± 1 (4) | 0 (10) | 5 ± 1 (4) | 20 ± 4 (4) | 87 ± 13 (3) | 76 ± 4 (4) | 59 ± 6 (2) |
| fru-pL | 12 ± 2 | 11 ± 2 (4) | 0 (10) | 0 (4) | 0 (4) | 11 ± 2 (3) | 12 ± 2 (5) | 14 ± 1 (2) |
| Thoracic ganglia | ||||||||
| fru-Pr | 21 ± 1 | 17 ± 1 (4) | 0 (10) | 0 (4) | 0 (4) | 15 ± 1 (4) | 14 ± 2 (5) | 9 ± 2 (2) |
| fru-PrMs | 83 ± 1 | 71 ± 1 (4) | 0 (10) | 0 (4) | 10 ± 1 (4) | 49 ± 3 (3) | 57 ± 3 (3) | 31 ± 2 (2) |
| fru-MsMt | 52 ± 4 | 35 ± 2 (4) | 0 (10) | 0 (4) | 4 ± 1 (4) | 25 ± 3 (3) | 29 ± 2 (3) | 27 ± 9 (2) |
| fru-MtAb | 14 ± 1 | 8 ± 2 (4) | 0 (10) | 0 (4) | 0 (4) | 10 ± 1 (3) | 11 ± 1 (3) | 14 ± 2 (3) |
| Abdominal ganglion | ||||||||
| fru-Ab | 91 ± 3 | 83 ± 2 (4) | 0 (10) | 0 (4) | 8 ± 1 (4) | 44 ± 2 (3) | 49 ± 5 (3) | 24 ± 3 (3) |
Immunostaining was mediated by application of anti-FRUM to the CNS of 2-d-old pupal males of various homozygous or heterozygous fru types (including homozygousfru+ = wild-type = WT). Allfru variants indicated are mutant alleles exceptw24, which designates a fru-locus deletion. Numerical data from the fru1 mutant are not included, because there are too many weakly stained, ectopically located cells to count them accurately; and the more intensely labeled cells in the various ganglia of this mutant (putative subsets of the WT patterns) could not necessarily be distinguished on a cell-by-cell basis from those with “weak” signals (Figs. 3-5). Numbers (mean ± SEM) of signal-containing neurons were counted within a given neuronal group for one side of the brain or the VNC (see below);n values for these hemi-ganglia are in parentheses. The neuronal groupings (leftmost column) were classified as in Lee et al. (2000), and in fact the numbers of FRUM cells within the various WT neuronal clusters (leftmost data column) are from that report (although in it, results of the cell counts were quoted as mean ± range). The complete absence of staining within a given CNS region is indicated by a “zero” count. For most specimens, counts of immunostained neurons were made for the cluster in question within the left or (bilaterally symmetrical) right side of the brain or ventral nerve cord; when both sides of a CNS were used, the left and right counts were treated independently. Values in boldindicate that there was at least an approximately twofold difference between the mean mutant value compared with that of WT (bold not used for the obvious “zero” mutant cases). Despite the appearance (under the microscope) of signal-containing neurons in brain clustersfru-M, fru-Ld, and fru-Lo in three of the mutant types, cell counts were not performed because of extremely low staining levels in these regions for pupae of these genotypes (thus, N.A., data not available).
The other three transposon mutants are sterile and exhibit more severe courtship defects than does fru2(Villella et al., 1997; Goodwin et al., 2000; Nilsson et al. 2000). We suspected that at least one of these behaviorally sterile mutants, such as frusat, which is nearly courtless, might be a FRUM-null variant. In the immunohistochemical assays, however,frusat showed small numbers of cells, albeit with extremely low levels of staining; the results fromfru4 were similar (Fig. 3H,I; Table 3). Such minimal signals were in partly overlapping regions of the CNS of these two mutants: in brain clusters calledfru-pSP2 and fru-P (Fig. 3, compare H, I with A–C for wild type; also see Tables 2, 3). In addition to fru expression in these two portions offrusat brains, weakly stained neurons were found in three anterior brain regions fru-aSP3, Lv, and AL) as well as in certain ventral cord regions:fru-PrMs, MsMt, and Ab clusters (Fig. 3, compareC, I; see also Tables 2, 3). In the VNC of pupae,fru4 was blank. No staining was detected in the CNS of frusat orfru4 adults. The effects offru3 were the most severe because no confocally observable immunohistochemical signals were observed in the CNS of either pupae or adults (Fig. 3G; Tables 2, 3). The absence of detectable FRUM infru3 specimens was also observed in males heterozygous for that mutation and either of two frudeletions (Fig. 2B).
The final homozygous-viable fruitless mutant examined wasfru1. Such males court females vigorously, although they do not mate with them, and they exhibit by far the most dramatic inter-male courtships of all fruitlessmutant types (Table 2). fru1 is caused by an inversion breakpoint within the locus (Gailey and Hall, 1989) that is located ∼3 kb upstream of the transcription-start site for P1-promoted mRNAs (Fig. 1). In Northern blots offru1 extracts (Goodwin et al., 2000), probed with nucleic acids from the same region used in the currentin situ hybridizations (Fig. 1), all of the usual sex-specific transcripts were present in both sexes offru1 homozygotes (there are three such P1-promoted mRNA types because of alternative splicings at the 3′ end of the primary transcripts). However, this mutant exhibited anomalies in the spatial expression of P1 transcripts examined in the CNS of pharate adults (Goodwin et al., 2000). It is as if the chromosomal lesion in fru1, which occurred in a 5′-flanking region of the locus (Fig. 1) and thus may have damaged regulatory sequences, causes qualitatively altered expression of sex-specific mRNAs at the level of transcription. This is in contrast to the effects of the transposon mutations, the effects of which are post-transcriptional (see above).
Immunohistochemical results from CNSs of 2-d-old pupal males homozygous for fru1 are shown in Figures3E and 4 and summarized in Tables 2 and 3. Major differences were observed when compared with the wild-type pattern. First, certain clusters, or neurons within a given cluster, were absent in the CNS of the fru1. In particular, regionsfru-mAL and aSP1 were devoid of staining. Other brain regions that are stained by anti-FRUM in wild-type males, such as fru-aSP3, Lv, and mcAL, had reasonably clear signals in corresponding portions offru1 brains (Fig.4, compare A, C). Second, most of the FRUM neurons of this mutant showed weaker staining intensity when compared with that of wild type. However, few cells, in particular within the fru-aSP3 and fru-Lv brain clusters, exhibited nearly normal levels of FRUM immunostaining (Fig.4A–D). Third, another difference from the norm involves novel cells within the fru1brain that express the male-specific protein (Fig.4H). Such ectopic expression of FRUM within numerous cells of the CNS made it difficult to determine whether certain normal clusters or cells in a given cluster are missing in fru1. The three kinds of fru1 versus wild-type differences just enumerated were also observed in the ventral CNS of male pupae (Fig. 4I,J). Also, fru1-associated reductions in numbers of neurons were discernible in most of the VNC clusters, such as fru-Ab, PrMs, and MsMt (Fig.4I,J,K). In the fru1 abdominal ganglion, FRUM immunostaining (within the relevantfru-Ab) neurons was diminished (Fig. 4, compareI, J). This is dealt with in more detail in the next section.
These immunohistochemical results from the five viable frumutants permit certain rationalizations of variations among their extents and types of courtship defects. These mutants can be categorized into two groups. One consists offru1 andfru2, which court rather vigorously (Table 2). These mutants exhibited decrements in FRUM expression in the CNS, but overall are nowhere near the amorphic state for P1-encoded proteins.fru1 showed strong immunohistochemical decreases in certain FRUM neurons, whereasfru2 was more uniformly hypomorphic. The other mutant group consists of fru3,fru4, andfrusat, which exhibit no FRUM expression or immunostaining in very small numbers of neurons. These three sterile mutants court females at lower to much-lower levels than those characteristic offru1 andfru2 male behavior (Table 2).
Subnormalities of FRUM expression in the VNC of fru mutants are likely to be connected with their courtship-song defects. In this regard,frusat males, which are mute (Goodwin et al., 2000), exhibited thoracic-ganglionic FRUM signals in only a few neurons of the prothorax and mesothorax. The residual VNC expression in this mutant is insufficient for singing to occur. The songlessfru3 andfru4 types provide no putative neural-dissection information because these mutants are devoid of detectable FRUM throughout the thoracic ganglia. The song-enabled fru1 mutant shows approximately one-third of the normal number of FRUM prothoracic and mesothoracic neurons with ostensibly normal staining intensities; many other such neurons exhibit significantly reduced immunostaining (Fig. 4, compareI,J), as if they may not be involved in basic singing ability. Adding rather robustfruitless expression to these prothoracic/mesothoracic neurons in the fru2 mutant, such that these males express FRUM within the majority of the cells in this VNC region, instead of only one-third of them as in fru1, makes no apparent difference. fru2 males sing vigorously but exhibit the same mild defect as dofru1 males (Villella et al., 1997).
fru3, fru4, andfrusat males do not attempt copulation and lack a male-specific abdominal muscle called the Muscle of Lawrence (MOL; Gailey et al., 1991; Ito et al., 1996; Villella et al., 1997). The near-to-complete absence of FRUM abdominal ganglionic signals in these mutants (Table 3) is likely to underlie such defects. Some information is provided as to which abdominal neurons may differentially control these two phenotypes, in that frusatmales retain a small proportion of the normal abdominal ganglionic pattern, but such FRUM cells are insufficient for any MOL formation or abdominal bending toward the genitalia of the female. However, there is a problem with one element of this supposition: the overall courtship offrusat males, and that offru3 as well, is so diminished beyond the early orientation and female-following stages (Villella et al., 1997;Goodwin et al., 2000) that the absence of a late-stage behavior such as attempted copulation is not as meaningful as in the case of a vigorous mutant courter. Thus, the courtship performed byfru1 males, for which attempted copulation is also utterly absent, is potentially more interesting in this regard, an issue taken up in the next section. With regard to the abdominal MOL, fru1 possesses these male-specific structures, albeit in diminished form (Gailey et al., 1991). MOL formation during the metamorphosis of this mutant is likely to be controlled by certain of the relatively few neurons that robustly express the protein in fru1 abdominal ganglia (Fig. 4; cf. Lawrence and Johnston, 1986; Currie and Bate, 1995). fru2 causes MOL abnormalities as well (Gailey et al., 1991), but it is less quantitatively subnormal in abdominal ganglionic expression of FRUMcompared with fru1 (let alone the severely depleted transposon mutants). However, a spatially nonrandom subnormality in a few of the CNS cells may be sufficient to impinge on MOL formation in fru2. Despite the mild and generalized FRUM decrements infru2 (Table 2), including within the VNC, these males routinely attempt copulation.
Perhaps the most dramatic courtship anomaly exhibited by afruitless mutant involves the fact thatfru1 males court other males in an extremely vigorous manner, compared with the levels of such “courtship chaining” behavior that are caused by any of the other mutations, let alone the complete absence of such behavior in groups of wild-type males (Villella et al., 1997). It is reasonable to presume that the neural etiology of courtship chaining (and the anomalously high levels of inter-male courtships observed when twofruitless individuals are paired) is in the brains of the mutants. The abnormalities of FRUMexpression in that part of the CNS are also unique infru1, in the sense that several brain regions exhibited nearly normal distributions and apparent levels of the protein, but a limited number of other regions showed severe decrements in staining (Table 2; Fig. 4). A comparison of the FRUM brain-expression pattern infru1 with the more severe and global decrements in staining observed for certain of the other mutantsprovides an explanation for the dramatically varying degrees of sex-recognition breakdown among the differentfruitless mutants (see Discussion).
FRUM in semifertilefru-mutant transheterozygotes
Males homozygous for fru1,fru3, orfru4 do not attempt copulation and are sterile; but fru1/fru3and fru1/fru4 males are fertile, albeit in lower than normal proportions (Castrillon et al., 1993; Villella et al., 1997). To examine whether the ability of the transheterozygous males to bend their abdomens toward the female's genitalia correlate with novel FRUM-expression phenotypes, immunohistochemistry on whole-mounted CNSs was performed. Tissues were dissected from 2-d-old male pupae offru1/fru3 orfru1/fru4 and compared with specimens from wild type, the parental homozygous types, andfru1/Df-fruw24; the latter type is heterozygous for the fru1mutation and a complete deletion of the locus, a genotype that causes male behavioral sterilty (Anand et al., 2001).
Several interesting FRUM immunostaining differences were revealed among these mutant types. First, there was an ∼30% decrease in apparent protein expression levels in the transheterozygotes compared with wild type (Fig.5, examples of quantified results in legend). FRUM levels in the sterilefru1/Df-fruw24 male type were apprehended to be as low as infru1/fru3 andfru1/fru4 (staining intensities not quantified forfru1/Df-fruw24). Overall expression levels seemed to be uniform throughout CNS in the three heterozygous types just described. Second, there were marked reductions in the numbers of stained cells in several clusters within the CNS of fru1/fru3and fru1/fru4 males, such as fru-aSP1, aSP2, aSP3, mAL (brain),fru-PrMs, MsMt, and Ab (ventral nerve cord) (Fig. 5). Depending on the neuronal group (among the seven just indicated), the number of FRUMcells decreased approximately two- or threefold (Table 3). In other CNS regions, the numbers of stained neurons were nearly normal (Table 3). The reductions, or lack thereof, were quite consistent when comparing the signals and counts fromfru1/fru3 to those from fru1/fru4 (Fig.5; Table 3). Third, anti-FRUM staining patterns for thesefru1/fru3 andfru1/fru4 males were somewhat similar to those of fru1homozygotes. The distributions of signal-containing neurons in these transheterozygotes resembled those of strongly stained cells in males homozygous for fru1 (Fig. 4, compare with Fig. 5J, K, L). However, weakly stained FRUM cells (and ectopic ones, see below) were not detectable infru1/fru3 orfru1/fru4 males.
Fig. 5.
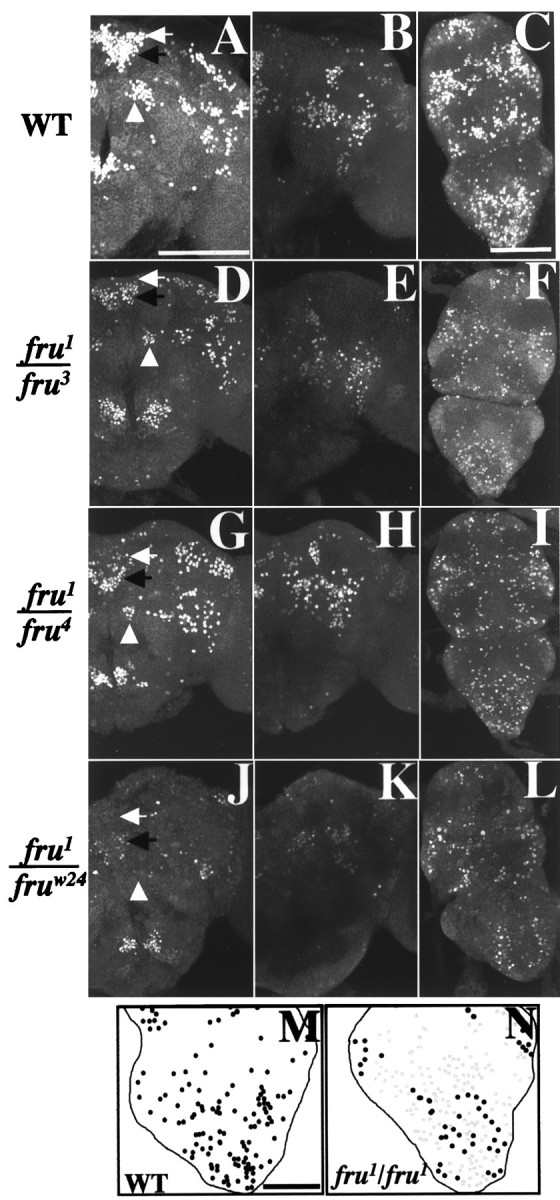
FRUM expression in the CNS of quasi-fertile fruitless mutant combinations. CNSs from 2-d-old male pupae carryingfru1/fru3(n = 14),fru1/fru4(n = 12), wild type (WT, n = 36), andfru1/Df-fruw24(n = 6) were subjected to anti-FRUM immunohistochemistry. WT andfru1/Df-fruw24were used as normal and fully mutant controls, respectively. The resulting representative images were prepared with confocal microscopy to show both anterior and posterior views of the brain and whole projection of the VNC for WT (A–C),fru1/fru3(D–F),fru1/fru4(G–I), andfru1/Df-fruw24(J–L). A, D,G, J, White arrows, fru-aSP1 neuronal cluster; black arrows, fru-aSP2; andwhite arrowheads, fru-mAL.fru1/fru3 andfru1/fru4 samples were stained with relatively low intensities overall; for example, thefru-aSP2 cluster of FRUM-containing brain neurons infru1/fru3 andfru1/fru4 males (D, G, black arrows) gave staining intensities (see Materials and Methods) of 160 ± 14 (mean ± SEM; n = 3) and 164 ± 9 (n = 3), respectively, whereas the corresponding WT value for aSP2 (black arrow in A) was 217 ± 7 (n = 3). However, the micrographs shown do not reflect such staining-intensity differences, because these confocal images were in saturation to maximize viewability of signal-containing CNS regions. M, N, Drawings of fluorescent signals viewed confocally from the ventral side of the abdominal ganglion to produce both representative diagrams for WT and fru1-homozygous males, respectively. Infru1/fru1, fewer cells than normal stained in a relatively intense WT-like manner (black dots); other neurons, possibly representing further subsets of the normal pattern but including many ectopically expressing cells, stained weakly (gray dots). These numerous ectopic expressing cells could not be revealed in the low-magnification micrographs for this CNS region in this mutant (Figs.3E, 4J). Scale bars:A–L, 100 μm; M,N, 50 μm.
Now we focus on abdominal ganglion expression of FRUM in fertilefru1/fru3 andfru1/fru4 males compared with sterilefru1/fru1 andfru1/Df-fru24 males, against a background of the likelihood that the male's copulation attempts are controlled by this posterior-most region of the CNS. In the abdominal ganglion, ∼50% of the normal numbers of FRUM cells were stained infru1/fru3 andfru1/fru4, and ∼25% in fru1/Df-fruw24males (Table 3). This difference in the FRUM cell number might be responsible for the lack of attempted copulation byfru1/Df-fruw24 males. However, the twofold decrement in the transheterozygotes (compared with wild-type) is still compatible with routine mating ability. Thesefru1/fru3 andfru1/fru4 males did not show any ectopic expression of FRUM, of the kind that fru1 homozygotes exhibit in the abdominal ganglion (Fig. 5, compareN,M) as well as in other CNS ganglia (see previous section of Results). This homozygous-sterile mutant type also exhibits a decrement in the number of heavily staining abdominal ganglionic neurons (Fig. 5, N vsM; compare Fig. 3, E vs D, and Fig. 4,J vs I) similar to the paucity shown byfru1/Df-fruw24 males (see above; Fig. 5, compareL,N).
In general, the three male types that each carried only one copy of thefru1 mutation gave protein-expression patterns similar to one another, althoughfru1/Df-fruw24hemizygous males were farther from wild type, compared with the fertile but FRUM-subnormal transheterozygotes (Fig. 5; Table 3). Nevertheless, the similarities amongfru1/fru3,fru1/fru4, andfru1/Df-fruw24 could be explained by an allele-dosage effect. TheDf-fru24 deletion generates no gene product, and homozygosity for fru3or fru4 eliminates most or all FRUM expression (Fig. 3). Thus, FRUM production in the three heterozygous types being considered would seem mostly to come from the one dose of the fru1 allele in common among them. One reason for the perception of an overall reduction of FRUM, under the influence of this mutation, could be that the weak and ectopically expressing neurons found infru1 homozygotes are below detection levels in each of the (one-dose) heterozygotes. However, thisfru1-dosage effect does not explain why more FRUM cells were observed and counted within certain CNS regions of the fru-mutant transheterozygous types, compared with the near absence of staining infru1 homozygotes, e.g., for thefru-aSP1 and mAL brain clusters. About one-third to one-half the normal numbers of stained neurons were observed in these locations within the CNS offru1/fru3 orfru1/fru4 males (Fig.5; Table 3), whereas almost no cellular signals were detected in the corresponding brain regions offru1/fru1 males (Fig.4). Therefore, another factor that may point to an explanation of the differences among these three types, which are most dramatic in terms of fertility, is that there are special kinds of gene interactions between the fruitless alleles themselves whenfru1 is heterozygous withfru3 orfru4. This kind of phenomenon would involve something other than the amount of final gene product (in this case male-specific FRU protein) produced according to the dosage of the mutant alleles in question. Instead, as suggested originally byCastrillon et al. (1993), some sort of mutual correction may occur between the alleles on the separate third chromosomes at the level of primary gene expression infru1/fru3 andfru1/fru4 males. There is, however, no way that a current understanding of the primary transcripts or the array of mature mRNAs encoded by this gene (Ryner et al., 1996; Goodwin et al., 2000) can rationalize the complementing manner by which these mutant alleles may interact.
fru effects on sexually dimorphic serotonergic abdominal cells
To examine the possible relationship between fruitlessfunction and 5-HT (see introduction), we double-labeled whole-mounted CNSs with antibodies against FRUM and the neuromodulator. The results are presented in Figure 6. We found 5-HT-immunoreactive neurons broadly distributed throughout the brain (n = 12, data not shown), the thoracic ganglia, and the abdominal ganglion of adult males (Fig. 6A). Previously, Vallés and White (1986, 1988) identified nine groups of serotonergic neurons in the adult brain and five groups in the ventral nervous system of Drosophila [see Nässel (1988, 1996) and Monastrioti (1999) for reviews of serotonergic labeling in the CNS of this and other insects]. Against this background, we stained serotonergic neurons in the CNS of adult flies that were genetically normal, compared with those expressingfru mutations.
Fig. 6.
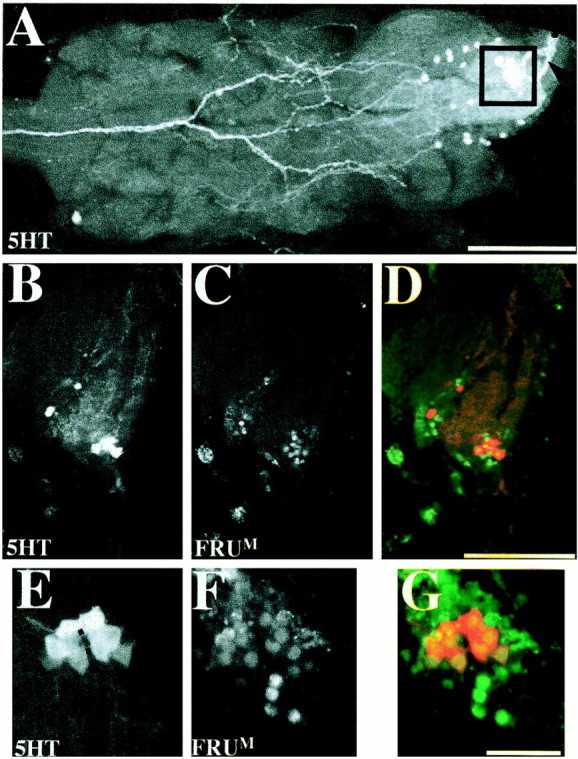
Neurons coexpressing FRUM and serotonin in the abdominal ganglion. These cells, dubbed s-Abg, were revealed in the posterior tip of the male VNC by anti-5-HT single-labeling and double-labeling application of that antibody along with anti-FRUM. Twelve 4- to 7-d-old wild-type males were used for anti-5-HT immunohistochemistry alone and 10 separate such males for double-labeling. A, Confocal image showing a dorsal view of serotonergic neurons in the thoracic and abdominal ganglia. Two different sizes of anti-5-HT-labeled neurons appear in the abdominal ganglion; the boxed area shows a cluster of eight s-Abg neurons; the black arrowhead points to the proximal portion of axons projecting posteriorly from these cells into the median trunk nerve. B, C, Neurons expressing 5-HT- and FRUM neurons in an adult-male abdominal ganglion. These sagittal views of the s-Abg neurons show the cell bodies to be located dorsally (toward the right of each panel). D, Combined image of B andC, depicting coexpression of the two antigens. 5-HT immunoreactivity was observed mostly within (and throughout) the cell bodies (red), and FRUM immunoreactivity was detected only in nuclei (green). E, F, Higher-magnification dorsal view of s-Abg neurons labeled by anti-5-HT and anti-FRUM, respectively. Six consecutive 1.2 μm focal planes were combined to show all s-Abg neurons; thedotted line in E was drawn to indicate the symmetrically paired structure of the s-Abg neuronal clusters.G, Combined image of E andF (5-HT in red, FRUMin green). Scale bars: A–D, 100 μm;E–F, 25 μm.
We assume that fruitless mutations and ectopic expression of the white gene (Zhang and Odenwald, 1975) cause males to court other such flies because of anomalous brain function (possible involvement of the VNC is counterintuitive). Ectopic expression (and probably overexpression) ofw+ in the brain could deplete 5-HT levels in cells that normally express the fru gene, mutations of which can easily be found to cause a similar neurochemical deficit (see introduction). Thus FRUM and 5-HT would be coexpressed in at least some of the neurons that normally contain these substances. However, within the brain of wild-type males, no FRUM neurons whatsoever were double-labeled with anti-5-HT (n = 10, data not shown). The usual locations of cells and processes immunoreactive for this substance were observed (see above). The number of 5-HT neurons is not particularly large, reinforcing the possibility that global uptake of a serotonin precursor throughout the brain could deplete levels of this substance in their usual locations. However, if ectopic expression ofw+ is mechanistically related to fru-mutational effects via 5-HT, the current results indicate that there is a need to formulate a hypothesis different from one involving direct intracellular effects of the latter genotypes. Perhaps white and the tryptophan transporter it encodes cause this neuromodulator to be anomalously present in FRUM cells or other neurons that directly interact with them; such effects might derangefru-controlled brain functions insofar as sex recognition is regulated. Another possibility, not mutually exclusive, is that ectopicw+ leads to anomalous 5-HT levels in cells that interact with FRUMneurons, deranging brain functions that are not directly controlled by fruitless but are components of the neural substrates for courtship. In any case, the lack of a simple relationship between fruitless gene products and serotonergic neurons, which would have bolstered the notion that both abnormal genotypes cause their courtship effects via 5-HT depletion in the same key brain cells, suggests that ectopic-white males are made to behave in a manner that caricatures the phenotype offruitless mutants.
In the course of these double-labeling tests, we scrutinized signals elicited by anti-FRUM and anti-5-HT in all CNS ganglia. Within the male's ventral cord, the great majority offru-expressing neurons in the four pairs of ganglia (cf. Lee et al., 2000) contained no detectable 5-HT. There was, however, an exception within one VNC region. It involves certain newly identified serotonergic cells in the abdominal ganglion (Fig. 6). For these neurons, coexpression of FRUM and 5-HT was observed in a total of eight cells at the posterior tip of the VNC (Fig. 6). These serotonergic-abdominal giant neurons (s-Abg) are located close to one another in a relatively dorsal side of the abdominal ganglion and have conspicuously large cell bodies (Fig.6A–E). Larval serotonergic neurons in the developing nervous system are reorganized during metamorphosis (Vallés and White, 1988; Monastrioti, 1999). In this respect, putative precursors of the s-Abg neurons were not detected in the third-instar larval CNS (n = 6) or in the abdominal ganglion of 2-d-old male pupae (n = 5, data not shown). Therefore, these s-Abg neurons in Drosophila may form during metamorphosis (cf.Thorn and Truman 1994a,b), or they may have been born earlier and taken on their final neurochemical quality during late stages of development (cf. Tublitz and Sylwester, 1990).
With regard to the projection patterns of the s-Abg cells that were revealed by 5-HT-immunostaining (Fig. 6A), each neuron appeared to have more than one neurite. In most specimens, the s-Abg neurons were closely clumped together. A few preparations exhibited fairly clear bilaterality of these cell bodies and their posterior projections. These 5-HT-immunoreactive neurites also appear to be within the median trunk (which is known to innervate posterior abdominal segments), genital segments, and internal reproductive organs (Hertweck, 1931).
The putatively fru-related function of these cells and their processes would seem to involve aspects of male reproduction because the patterns of 5-HT immunoreactivity being described were not observed in or posterior to the abdominal ganglion of adult females (Fig. 7, compare A,B). Whether these cells exist in females, as opposed to being present but devoid of 5-HT, is unknown. In this regard, bear in mind that there is no FRUMimmunostaining anywhere in the CNS of females (Lee et al., 2000).
Fig. 7.
Abnormal sex-specific serotonin expression in the abdominal ganglia of fru mutant males. These images are whole (A, B, D,F) or partial (C,E, G) projections of stacked images through a given ganglion, viewed in the confocal microscope from the ventral side. A, B, Abdominal ganglia of 4- to 7-d-old wild-type adult male and female, respectively; in the latter (representative of five female VNCs processed), there were no anti-5-HT-mediated signals like those observed in this region of the male CNS, whose cluster of s-Abg cells (compare Fig. 6) are designated by an asterisk in A (representative of 12 male VNCs observed); the arrow in Apoints to axonal projections from male s-Abg neurons.C–G, Serotonergic neurons in the abdominal ganglia offru mutants. C, Infru1 (n = 5), s-Abg cell bodies (asterisk) and processes (arrow) were weakly stained (uniformly among the specimens, in contrast to fru4) (see below). D, In fru2(n = 8), the s-Abg neuronal cluster (asterisk) and projections from such cells (arrow) were normal or nearly so (in terms of numbers of cell bodies and staining intensities) among the several specimens. The image shown depicts strong signals, although in this one there were weak signals in a serotonergic neuropil that usually gives strong staining (E, G, black arrowheads) in abdominal ganglia of all frugenotypes (including fru2).E, In fru3(n = 6), no 5-HT immunostaining in s-Abg cells or neurites was observed; the white arrowhead points to where the cell bodies should be. F, Infru4 (n = 5), two specimens showed no 5-HT immunoreactivity in s-Abg neurons, whereas three gave weak staining in one to three s-Abg cell bodies and their processes (as exemplified in the case shown and itsasterisk for cell bodies and black arrowfor processes). G, Infrusat (n = 5), relatively few s-Abg neurons were stained by anti-5-HT (although more than in fru4), and the cell bodies and processes in which signals were elicited (asterisk, arrow) gave weak signals (uniformly among the specimens). Scale bars: A, B, 50 μm;C–G, 100 μm.
In fru-mutant males, anti-5-HT immunoreactivity in the s-Abg neurons as well as the axons projecting from them was absent or defective (Fig. 7). fru1 andfrusat showed low levels of transmitter staining in some of the s-Abg neurons and their process (Fig. 7,C and G, respectively).
In fru3, there was no detectable 5-HT immunoreactivity in s-Abg neurons or their axons (Fig. 7E). At best, fru4 mutant males presented weakly detectable 5-HT immunoreactivity in these structures (Fig.7F). fru2 males were normal with respect to numbers of s-Abg neurons and their projections (as stained by anti-5-HT), although the levels of staining intensity in both subcellular compartments of these neurons appeared to be lower than in wild type (Fig. 7D).
For fru3, the most severely subnormal mutant in terms of FRUM and 5-HT expression in the abdominal ganglion, it was not immediately possible to determine whether the general absence of both kinds of immunoreactivity is caused by an absence of s-Abg neurons or by the lack of serotonin production in these cells. To address this question, 5-HT-uptake experiments were performed. These were based on the fact that exogenously applied 5-HT was found to be absorbed selectively by serotonergic neurons in the CNSs of third-instar larvae that expressed late-developmentally lethal Dopa decarboxylase(Ddc) mutations (Vallés and White, 1988); relatively severe (but viable) Ddc variants cause severe decrements in 5-HT synthesis (Livingstone and Tempel, 1983). Ventral nerve cords from adult fru3 males were exposed to a series of 5-HT-creatinine concentrations (n = 3 VNCs at 1 μm, n = 4 at 5 μm, n = 5 at 10 μm, n = 2 at 100 μm, and n = 5 at 500 μm). The resulting immunostaining led to the following patterns. In wild-type VNCs (n = 3, data not shown), we observed lowered endogenous 5-HT levels in the serotonergic neurons that are undisturbed by this fru mutation; we infer that the incubation procedure necessary for 5-HT uptake is the major cause of this depletion. In fru3 VNCs, at the lowest concentration applied to fru3specimens (1 μm, data not shown), neither s-Abg-like cell bodies nor neurites could be recognized; but as incubations with increasing 5-HT concentrations were performed, there were increasing numbers of immunostained cells along with stronger signal strengths (Fig.8A–C). At the highest concentration of 5-HT applied, a subset of these structures in the ganglion of the mutant exhibited what appeared to be the appropriate immunoreactivity (Fig. 8D). The signals associated with the VNC cell bodies and processes in question appeared similar to those of genetically normal s-Abgs in their size, shape, and intraganglionic location. Thus, it seems as if at least some of these VNC cells are retained in this mutant and able to take up serotonin. However, it was not possible to determine unambiguously whether the normal fru/5-HT-expressing cells and their projections were labeled in the fru3 ganglia. Therefore, it remains an open question as to whether these neurons are eliminated by a developmental effect of this mutation, or whether, if present, the cells are unable to absorb exogenously applied 5-HT in the conditions used.
Fig. 8.
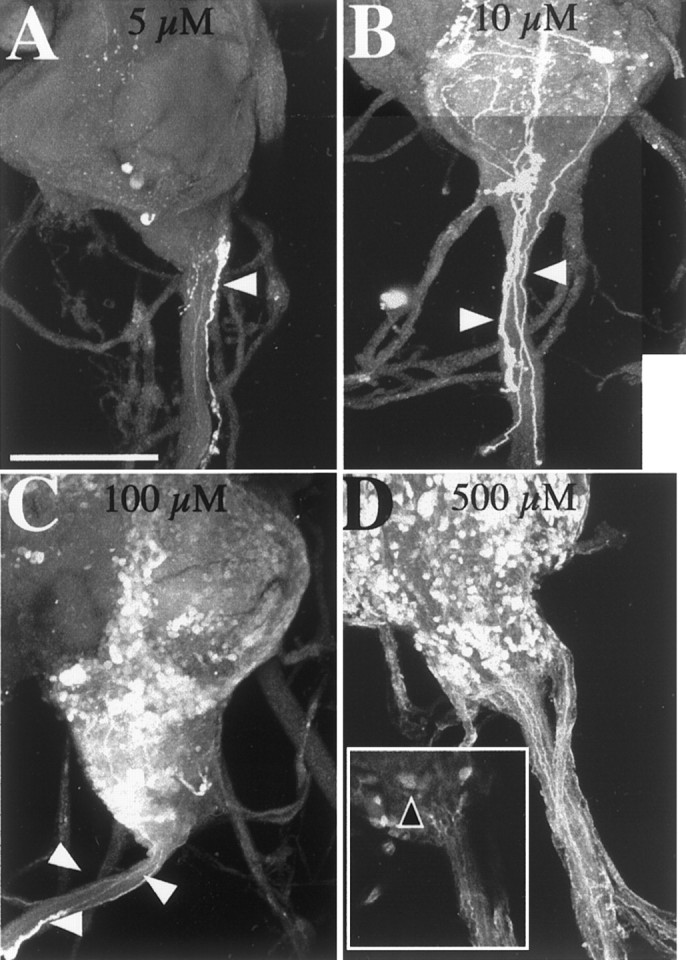
Serotonin immunoreactivity in the abdominal ganglion resulting from exogenous application of 5-HT. Immunohistochemistry with anti-5-HT was performed with dissected VNCs of fru3 adult males that were incubated in 5-HT solutions. Immunofluorescently labeled tissues were examined by confocal microscopy, and stacked images of abdominal ganglia were obtained from specimens exposed to the range of concentrations indicated. A–D, Representative immunostaining after application of 5-HT at 5 μm(n = 4) (A), 10 μm (n = 5) (B), 100 μm (n = 2) (C), and 500 μm(n = 5) (D). No s-Abg-like 5-HT-immunoreactive neurons or their projections were observed to result from the lowest two concentrations of 5-HT used (A, B). Thus, the thin neurite signal inA, which runs down the center of the median trunk, was traced back to certain cell bodies in the posterior tip of the abdominal ganglion, but they were too small to be s-Abg cell bodies. Another feature of A is a cell body and fiber (white arrowhead) that appears to be outside of the main abdominal nerve. (In association with these signals are varicosities that suggest the structures are neurohemal fibers that could not be projections from s-Abg cells.) B, None of the stained fibers (white arrowheads) in the main nerve could be traced back to s-Abg-like cell bodies. D, At the highest concentration, a few cells that putatively took up exogenous 5-HT appeared to be similar to s-Abg neurons in their size and shape (black arrowhead within the box; image of the box is based on one focal plane); no such cells exhibit intrinsically derived immunostaining in these cells in this mutant (Fig. 7E). The putative s-Abg cell bodies, to which 5-HT was supplied in thefru3 abdominal ganglion shown (D), are in a dorsomedial region of the posterior CNS tip. This is the location of such neurons in more definitively identifiable circumstances (Fig.7A,C,D). Scale bars, 100 μm.
DISCUSSION
Correlations between FRUM expression andfru-mutant phenotypes
The various fruitless mutants exhibit striking behavioral defects and differences among one another (Goodwin, 1999). The degrees and kinds of anti-FRUMstaining abnormalities found for these five viable mutants were argued (in Results) to correlate with their courtship subnormalities and anomalies. However, the expression/behavioral correlations are not always tight. For example, frusat males are nearly courtless, but they exhibit FRUM signals in a few cells (Fig.3F). fru3 males court more vigorously but have no detectable FRUM (Table 2), equivalent to the effects of chromosome breakpoints within the gene (Table 1). Behaviorally, these breakpoint variants are nearly courtless (Villella et al., 1997;Anand et al., 2001). We suspect that fru3males generate low levels of FRUM protein, more than in the breakpoint variants, but undetectable by the antibody.
A further supposition presented in conjunction with descriptions of the anti-FRUM results for the viable mutants is that expression defects in relatively posterior regions of the VNC are the neural etiology of the courtship-song abnormalities that are exhibited to one degree or another by all five mutants. It is almost certainly not a coincidence that this sex-specific singing behavior depends on the presence of genetically male neurons in the thoracic ganglia (for review, see Greenspan, 1995).
With regard to FRUM-immunoreactive neurons in the most posterior region of the VNC, expression defects in the mutants were hypothesized (in Results) to be connected with the inability of four of them (except fru2) to attempt copulation, on the one hand, and to develop a normal male-specific abdominal muscle, on the other. The patterns of FRUM cells in the abdominal ganglia are potentially most interesting forfru1/fru3 andfru1/fru4 males, which are partly fertile (Castrillon et al., 1993; Villella et al., 1997). Many FRUM-positive cells were observed in the abdominal ganglia of transheterozygotes (Fig. 5), although only ∼50% the wild-type number (Table 3). We propose that certain of these neurons regulate attempted copulation.fru1 homozygotes exhibit fewer abdominal-ganglionic cells that are intensely stained by anti-FRUM, compared with the transheterozygotes; and males homozygous for this mutation never bend their abdomens to attempt copulation (Hall, 1978). If a certain subset of the FRUM abdominal ganglionic pattern is critical for this behavior, it could be that such cells are missing or protein-null in fru1, yet present infru1/fru3 andfru1/fru4. Another possibility is that ectopic FRUM cells in the abdominal ganglion in fru1 males influence phenotypes involving the abdominal ganglion. Infru1/fru3 orfru1/fru4 males, no ectopic FRUM expression was apparent (Fig.5), which might be the reason for restoration of fertility in the transheterozygotes compared with the behavior offru1 homozygotes (fru1/Df-fruw24also showed no ectopic FRUM neurons, but this is probably a false negative with respect to the detectability of these weakly expressing cells because of the single copy of the functional fru allele). In males for which the onlyfruitless allele is fru1, ectopically expressed protein could alter the function of this part of the CNS such that the neuromuscular control of attempted copulation is ruined. This would be a distinctly different etiology than the abdominal-ganglionic FRUM-lessness (Table3) that very likely underlies the inability of males homozygous for either fru3 orfru4 to perform copulation attempts.
Inter-male courtship and FRUMexpression defects
We now consider the expression of fruitless in the context of sexual orientation. Wild-type males show female-oriented courtship, whereas fru mutants show varying levels of decreased orientation toward females and anomalous interest in other males. fru2 is informative in this regard because males homozygous for this mutation prefer females (Villella et al., 1997), and this mutant exhibits the least severe FRUM expression defects in the brain (Fig.3B; Table 2). However, simple correlations break down for other genotypes. Thus, in single-pair tests,frusat males court other males at lower levels compared with the degrees of homosexual courtships displayed byfru3 andfru4; all three of these mutants court other males in group situations at only about one-fourth the level displayed by fru1 (Villella et al., 1997;Goodwin et al., 2000). Males homozygous forfru3, fru4, or frusat are similarly depleted of brain FRUM (Table 2).fru1, the most vigorous courter of other males, cannot have this anomalous behavior explained by gross subnormalities of FRUM levels. One etiology of the unique inter-male courtship displayed by this mutant may be the ectopically expressing FRUMneurons found within the brain (discussed above in another context). However, it is also important to consider thatfru1 males are depleted of the protein nonrandomly (Fig. 4; Table 2). In particular, no FRUM was detectable within a brain region near the antennal lobes called fru-mAL, although a much more dorsal region (aSP1) was also devoid of signals. The part of the brain near (and very likely including) mAL is provocative because this region has been implicated in sexual recognition; genetically feminized brain sites in the vicinity of the antennal lobes cause such males to court other males (Ferveur et al., 1995). The same behavioral effect could result from these brain sites being inadequately masculinized, if that is the consequence of a lack of FRUM in this region.
The other viable mutants that exhibit homosexual courtship are immunohistochemically similar to fru1 in that all lack FRUM in sites near the antennal lobes (Figs. 3, 4). It is possible that thefru3, fru4, and frusat mutants court other males at relatively low levels compared with the behavior offru1 because the latter mutant retains FRUM in several brain regions necessary for any courtship. In other words, the brakes would be off in fru1, absent mAL expression of FRUM (Fig. 4); but the product of the gene must be elsewhere in the brain for that effect to manifest itself in terms of robust inter-male courtship. At least one such region is very likely the dorsoposterior region that does contain FRUM cells (Lee et al., 2000; and this report) and requires the presence of genetically male neurons in order for any male-like courtship to occur (for review, seeGreenspan, 1995). The fact that the fru3mutant courts other flies of either sex more vigorously than dofru-deletion males is probably attributable to the (aforementioned) supposition that this transposon mutant generates modest levels of FRUM, albeit indistinguishably above the zero immunostaining observed in the double-deletion types (Table 1). Their near-courtlessness is consistent with the idea that some FRUM is required if an appreciable level of male courtship is to be directed toward another fly, be it male or female.
Possible male-specific functions of fru-affected serotonergic neurons
Our discovery of sexually dimorphic s-Abg neurons in the abdominal ganglion (Figs. 6, 7) could provide an anatomical link tofru+-dependent sex-specific phenotypes not yet known to be influenced by this gene. The s-Abg neurite signals elicited by anti-5-HT also provide the first information on a projection pattern for fru-expressing cells. These findings indicate that the formation of the s-Abg neurons or production of 5-HT in them is male-specific and under frucontrol. The 5-HT-uptake results (Fig. 8) suggest that s-Abg cells are present but are unable to synthesize this transmitter in the FRUM-lessfru3 mutant.
Innervation by 5-HT/FRUM neurons of abdominal muscles influenced by fru-gene action is unlikely. This is because glutamate is the canonical neuromuscular transmitter inDrosophila (e.g., DiAntonio et al., 1999), although comprehensive information is understandably lacking as to whether this molecule is responsible for intercellular communication at all such synapses. In addition to the muscles in this body region, there are male-specific organs that have better-appreciated structures (compared with unknown muscles hypothetically devoted to attempted copulation) and functions (compared with the MOL) for which reproductive significance is unknown. In this regard, the s-Abg neurons seem to send their axonal projections into the median-trunk nerve (Hertweck, 1931), the terminal branches of which innervate the genital segments and internal reproductive organs as well as certain abdominal muscles (Miller, 1950)
5-HT has been suggested to play a role in altered sexual orientation inDrosophila (Zhang and Odenwald, 1995). A potentially close connection between the action of FRUM and serotonin in terms of inter-male courtship would have been worthy of deeper consideration if these factors had been found to be coexpressed. But the current results uncovered no overt 5-HT link tofru-expressing brain neurons. The fact that there is a distinctly separate colocalization of FRUMand serotonin at the opposite end of the CNS (Figs. 6-8) suggests that regulation of the presence and action of this neurotransmitter can be a downstream target of fru function, an unexpected connection between the control of sexual differentiation and this piece ofDrosophila neurochemistry. This bonus was but one of the results of being able to monitor the presence of fruitlessgene products at high resolution.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants NS-33352 and GM-21473, and NIH Neuroscience Training Grant 5T32-NS-07292. We thank Jae H. Park, Adriana Villella, Stephen F. Goodwin, Jean-Christophe Billeter, Bruce S. Baker, and Barbara J. Taylor for helpful discussions, and we appreciate comments on this manuscript from Barbara J. Taylor, Margit Foss, and Ralph J. Greenspan. We thank Edward Dougherty and Maki Kaneko for confocal and photographic assistance, and Barbara Beltz and Kalpana White for advice about anti-5-HT immunohistochemistry.
Correspondence should be addressed to Jeffrey C. Hall, Department of Biology, MS-008, Brandeis University, Waltham, MA 02454-9110. E-mail: hall@brandeis.edu.
Dr. Lee's present address: Department of Biochemistry and Cellular and Molecular Biology, University of Tennessee, Knoxville, TN 37996-0840.
REFERENCES
- 1.Anand A, Villella A, Morales A, Ryner LC, Carlo T, Goodwin SF, Song H-S, Gailey DA, Hall JC, Baker BS, Taylor BJ (2001) The sex determination gene fruitless, in addition to controlling male sexual behaviors, has sex-nonspecific vital functions. Genetics, in press. [DOI] [PMC free article] [PubMed]
- 2.Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P-element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie DA, Bate M. Innervation is essential for the development and differentiation of a sex-specific adult muscle in Drosophila melanogaster. Development. 1995;121:2549–2557. doi: 10.1242/dev.121.8.2549. [DOI] [PubMed] [Google Scholar]
- 4.DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferveur J-F, Störtkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 6.Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fratta W, Biggio G, Gessa GL. Homosexual mounting behavior induced in male rats and rabbits by a tryptophan-free diet. Life Sci. 1977;21:379–383. doi: 10.1016/0024-3205(77)90518-5. [DOI] [PubMed] [Google Scholar]
- 8.Gailey DA, Hall JC. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics. 1989;121:773–785. doi: 10.1093/genetics/121.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gailey DA, Taylor BJ, Hall JC. Elements of the fruitless locus regulate development of the muscle of Lawrence, a male-specific structure in the abdomen of Drosophila melanogaster adults. Development. 1991;113:879–890. doi: 10.1242/dev.113.3.879. [DOI] [PubMed] [Google Scholar]
- 10.Gessa GL, Tagliamonte A. Possible role of brain serotonin and dopamine in controlling male sexual behavior. In: Costa E, Gessa GL, Sandler M, editors. Advances in biochemical psychopharmacology, Vol 11. Raven; New York: 1974. pp. 217–228. [PubMed] [Google Scholar]
- 11.Goodwin SF. Molecular neurogenetics of sexual differentiation and behaviour. Curr Opin Neurobiol. 1999;9:759–765. doi: 10.1016/s0959-4388(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin SF, Taylor BJ, Villella A, Foss M, Ryner LC, Baker BS, Hall JC. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics. 2000;154:725–745. doi: 10.1093/genetics/154.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenspan RJ. Understanding the genetic construction of behavior. Sci Am. 1995;272(4):72–78. doi: 10.1038/scientificamerican0495-72. [DOI] [PubMed] [Google Scholar]
- 14.Hall JC. Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav Genet. 1978;8:125–141. doi: 10.1007/BF01066870. [DOI] [PubMed] [Google Scholar]
- 15.Hertweck H. Anatomie und Variabilitat des nervensystems und der sinnesorgane von Drosophila melanogaster (Meigen). Z Wiss Zool. 1931;139:559–663. [Google Scholar]
- 16.Hing A, Carlson JR. Male–male courtship behavior induced by ectopic expression of the Drosophila white gene: role of sensory function and age. J Neurobiol. 1996;30:327–335. doi: 10.1002/(SICI)1097-4695(199608)30:4<454::AID-NEU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci USA. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence PA, Johnston P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell. 1986;45:505–513. doi: 10.1016/0092-8674(86)90282-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila CNS. J Neurobiol. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Livingstone M, Tempel B. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- 21.Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. Wiley; New York: 1950. pp. 481–495. [Google Scholar]
- 22.Monastrioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Nässel DR. Serotonin and serotonin-immunoreactive neurons in the nervous system of insects. Prog Neurobiol. 1988;30:1–85. doi: 10.1016/0301-0082(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 24.Nässel DR. Neuropeptides, amines and amino acids in an elementary insect ganglion: functional and chemical anatomy of the unfused abdominal ganglion. Prog Neurobiol. 1996;48:325–420. doi: 10.1016/0301-0082(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson EE, Asztalos Z, Lukacsovich T, Awano W, Usui-Aoki K, Yamamoto D. fruitless is in the regulatory pathway by which ectopic mini-white and transformer induce bisexual courtship in Drosophila. J Neurogenet. 2000;13:213–232. doi: 10.3109/01677060009084495. [DOI] [PubMed] [Google Scholar]
- 26.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 27.Thorn RS, Truman JW. Sexual differentiation in the CNS of the moth, Manduca sexta. I. Sex and segment-specificity in production, differentiation, and survival of the imaginal midline neurons. J Neurobiol. 1994a;25:1039–1053. doi: 10.1002/neu.480250902. [DOI] [PubMed] [Google Scholar]
- 28.Thorn RS, Truman JW. Sexual differentiation in the CNS of the moth, Manduca sexta. II. Target dependence for the survival of the imaginal midline neurons. J Neurobiol. 1994b;25:1054–1066. doi: 10.1002/neu.480250903. [DOI] [PubMed] [Google Scholar]
- 29.Tublitz N, Sylwester AW. Postembryonic alteration of transmitter phenotype in individually identified peptidergic neurons. J Neurosci. 1990;10:161–168. doi: 10.1523/JNEUROSCI.10-01-00161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usui-Aoki K, Ito H, Ui-Yei K, Takahashi J, Lukacsovitch T, Awano W, Nakata H, Piao ZF, Nillson EE, Tomida J, Yamamoto D. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol. 2000;2:500–506. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- 31.Vallés AM, White K. Development of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin. J Neurosci. 1986;6:1482–1491. doi: 10.1523/JNEUROSCI.06-05-01482.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallés AM, White K. Serotonin-containing neurons in Drosophila melanogaster: development and distribution. J Comp Neurol. 1988;268:414–428. doi: 10.1002/cne.902680310. [DOI] [PubMed] [Google Scholar]
- 33.Villella A, Hall JC. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–334. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto D, Nakano Y. Sexual behavior mutants revisited: molecular and cellular basis of Drosophila mating. Cell Mol Life Sci. 1999;56:634–646. doi: 10.1007/s000180050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S-D, Odenwald WF. Misexpression of the white gene triggers male–male courtship in Drosophila. Proc Natl Acad Sci USA. 1995;92:5525–5529. doi: 10.1073/pnas.92.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]