Abstract
The stratum griseum superficiale (SGS) of the superior colliculus contains a high concentration of the recently described GABAC receptor. In a previous study, it was postulated that activation of these receptors on inhibitory interneurons functions to disinhibit projection cells that relay visual information to the thalamus and brainstem. To test this model, we usedin vitro whole-cell patch-clamp methods to measure effects of GABA and muscimol on EPSCs and IPSCs evoked in rat SGS by electrical optic layer stimulation. The neurons were filled with biocytin for later morphological characterization. As expected, bath applications of GABA and muscimol always strongly depressed evoked PSCs at concentrations of >100 and >1 μm, respectively. However, at lower agonist concentrations, which most likely activate GABAC but not GABAA receptors, effects were not uniform. Evoked responses were suppressed by both agonists in 48% of the neurons, whereas the remaining cells exhibited enhanced responses with increased evoked EPSCs, decreased evoked IPSCs, or both types of change. Most morphologically identified cells with suppressed responses (14 of 17 cells) had morphological characteristics of putative GABAergic interneurons, whereas almost all cells with enhanced responses (8 of 10 cells) had morphological characteristics of projection cells. Finally, all effects of GABA and muscimol at low concentrations were blocked by (1,2,5,6-tetrahydropyridine-4-yl) methylphosphinic acid, a specific GABAC receptor antagonist, but not by the specific GABAA receptor antagonist bicuculline. Taken together, these results indicate that in SGS, GABACreceptors are predominantly expressed by GABAergic neurons and that activation of these receptors leads to disinhibition of SGS projection cells.
Keywords: GABA, ionotropic GABA receptors, GABAAreceptors, GABAC receptors, GABAergic circuits, interneurons, patch clamp, superior colliculus
GABA is the most frequently used inhibitory neurotransmitter in the mammalian CNS (Nicoll et al., 1990). At least three different receptor subtypes, the ionotropic GABAA and GABAC receptors and the metabotropic GABAB receptors, mediate inhibitory actions of GABA. These three GABA receptors can be distinguished pharmacologically by their selective responses to various receptor agonists and antagonists (Johnston, 1996a,b; Chebib and Johnston, 1999; Bormann, 2000). Most physiological and pharmacological studies of GABAergic neurotransmission have focused on GABAA and GABAB receptors, whereas GABAC receptors have been identified and studied primarily in the retina, where they are heavily expressed (Enz et al., 1995, 1996; Qian et al., 1997; Koulen et al., 1998).
Although GABAC receptors were at first assumed to be functionally important only in the retina (Lukasiewicz, 1996), evidence is increasing that they are expressed at significant levels in nonretinal structures as well (Boué-Grabot et al., 1998; Wegelius et al., 1998; Enz and Cutting, 1999). Specifically, in mammals, expression of GABAC receptor ρ subunit mRNA has now been demonstrated in the superior colliculus (SC), dorsal lateral geniculate nucleus (dLGN), and cerebellar Purkinje cells (Boué-Grabot et al., 1998; Wegelius et al., 1998). In addition, bicuculline-resistant and baclofen-independent GABA effects have been reported in cerebellum (Drew et al., 1984; Drew and Johnston, 1992), SC (Arakawa and Okada, 1988; Platt and Withington, 1998), dLGN (Zhu and Lo, 1999), and amygdala (Delaney and Sah, 1999).
In the rat SC, immunocytochemical detection demonstrates that GABAC receptor ρ subunits are restricted to the superficial gray layer, the stratum griseum superficiale (SGS) (Pasternack et al., 1999). Moreover, application of GABA and muscimol at low concentrations that affect GABACbut not GABAA receptors (Bormann and Feigenspan, 1995; Johnston, 1996b; Chebib and Johnston, 1999; Bormann, 2000) significantly increases field potential (FP) amplitudes evoked in SGS by electrical stimulation of the optic layer, stratum opticum (SO). In contrast, higher concentrations of GABA and muscimol, which also affect GABAA receptors (Bormann, 1988; Sieghart, 1995), strongly attenuate FP amplitudes in guinea pigs (Arakawa and Okada, 1988) and rats (Pasternack et al., 1999). Because FP amplitude increases at low concentrations could be blocked by application of the GABAC receptor antagonist (1,2,5,6-tetrahydropyridine-4-yl) methylphosphinic acid (TPMPA), the simplest explanation for these results is a specific expression of GABAC receptors by a population of local inhibitory interneurons. Inhibition of these interneurons with GABA or muscimol at low concentrations would lead to a disinhibition of other cells, including projection cells, and to a resulting increase in the evoked FP (Pasternack et al., 1999).
To test these ideas at the single-cell level, we used in vitro whole-cell patch-clamp methods to measure the effects of GABAC receptor activation on responses evoked in rat SGS cells by electrical SO stimulation. After the physiological experiments, we reconstructed the morphology of the sampled neurons to relate intercellular differences in response properties to morphologically defined cell classes. Our results support the conclusion that GABAC receptors are specifically expressed by GABAergic interneurons in SGS, and that their activation disinhibits the evoked responses of projection cells.
MATERIALS AND METHODS
Living brains slices were obtained from 3- to 6-week-old Wistar rats (used at Duke University) or Long–Evans hooded rats (used at Ruhr-Universität). No qualitative or quantitative differences were found between results obtained from animals of either of the two strains or from animals of different ages within the range used. Therefore, we pooled our data from all animals. All experimental procedures were in accordance with Institutional Animal Care and Use Committee Protocol guidelines. Animals were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (Nembutal, 50 mg/kg), and 300 μm-thick coronal slices were prepared by standard techniques (Edwards et al., 1989; Plant et al., 1995). The slices were kept in oxygenated artificial CSF (ACSF) containing (in mm): 123 NaCl, 2.5 KCl, 1 NaH2PO4, 1.3 MgSO4, 26.2 NaHCO3, 11 glucose, and 2.5 CaCl2, to which 2.0 mm kynurenic acid was added. For recording, slices were transferred to a submerged type recording chamber and superfused at 3 ml/min with kynurenic acid-free ACSF at room temperature.
Whole-cell recordings from SGS neurons were performed under visual guidance using borosilicate micropipettes (impedance 5–8 MΩ) filled with internal solution composed of (in mm): 130 potassium gluconate, 2 sodium gluconate, 20 HEPES, 4 MgCl2, 4 Na2ATP, 0.4 NaGTP, and 0.5 EGTA, to which 0.5% biocytin (Molecular Probes, Eugene, OR) was added shortly before recording. The measured membrane potentials were corrected for the junction potential of −10 mV.
Postsynaptic responses were evoked with an array of eight stainless steel wire electrodes (NB Labs, Denison, TX) placed in SO. Stimuli were 5–100 μA in amplitude and had a duration of 500 μsec. Neurons recorded in the SGS were dorsal to the stimulus electrode array. At Duke University, the neuronal signals were amplified and filtered using a PC501-A amplifier (Warner Instruments, Hamden, CT), digitized at 20 kHz with a DigiData 1200 interface (Axon Instruments, Foster City, CA) and displayed, stored, and analyzed using pClamp6 software (Axon Instruments). At Ruhr-Universität, an EPC9 amplifier (Heka, Lambrecht, Germany) was used for data acquisition, and Pulse/Pulsefit software (Heka) was used for data storage and analysis. Unless otherwise stated, postsynaptic current responses evoked by SO stimuli were averaged over three consecutive stimulus applications.
All GABA receptor-related drugs were bath-applied, and a 10 min application time proved sufficient to achieve stable responses. The drugs applied were GABA, muscimol, bicuculline methiodide, (Sigma, St. Louis, MO), 2-amino-5-phosphonopentanoic acid (APV), 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), TPMPA, and [3-[[(3,4-dichlorophenyl)-methyl]amino]propyl](diethoxymethyl)phosphinic acid (CGP 52432) (Tocris Cookson, Bristol, UK). Because the GABAC receptor antagonist TPMPA also acts as a weak agonist at GABAB receptors (Ragozzino et al., 1996; Pasternack et al., 1999), it was always coapplied with the selective GABAB receptor antagonist CGP 52432 to isolate its influences on the GABAC receptor.
Usually five or six single SC slices were obtained from each experimental animal. To avoid a possible overlap of the dendritic trees of labeled neurons, which would have complicated cell reconstruction, only one cell was recorded in each slice. After recording, slices were fixed in 4% phosphate-buffered formaldehyde for at least 24 hr before they were further processed to visualize the biocytin label that diffused into the cell from the patch-clamp pipette (Hall and Lee, 1993; Lee and Hall, 1995). In brief, after inactivation of endogenous peroxidases with 10% methanol and 1% hydrogen peroxide in 0.1m PBS, the slices were cryoprotected by immersion in increasing concentrations of dimethylsulfoxide (up to 20% in PBS) before they were quickly frozen using a mixture of acetone and dry ice. After thawing and reimmersing in PBS, the slices were incubated for 2 hr each in avidin and horseradish peroxidase solutions (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA). Labeled cells were visualized after incubation of slices in 0.05% 3′3′-diaminobenzidine and 0.003% H2O2 for 10–20 min. Slices were then dehydrated and mounted onto slides with DePeX (Crescent, Hauppauge, NY). Cells were drawn at ×500 magnification using a camera lucida attached to a microscope.
RESULTS
Cell types in SGS
Whole-cell recordings were made from 70 neurons in the SGS under voltage-clamp conditions. Electrical stimulation of the subjacent SO elicited two different response patterns in SGS neurons. One group of cells (n = 41) responded with short-latency, single-peak EPSCs followed by an IPSC with a longer duration (Fig.1). Cells of the second group (n = 29) responded with single- or multiple-peak EPSCs but did not exhibit significant IPSCs, even when the membrane potential was clamped at more positive values (Fig.2).
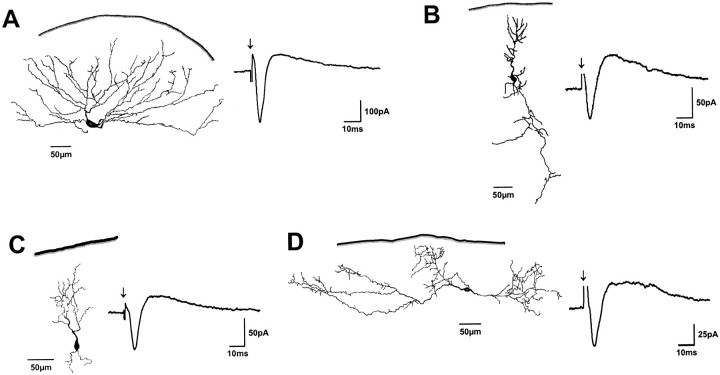
Fig. 1.
Representative examples of cells that responded with an EPSC followed by an IPSC to electrical SO stimulation. Dendritic morphology and postsynaptic currents are shown for a wide-field cell (A), two narrow-field cells (B, C), and a horizontal cell (D).Solid lines indicate the dorsal collicular surface.Arrows indicate SO stimulus onset.
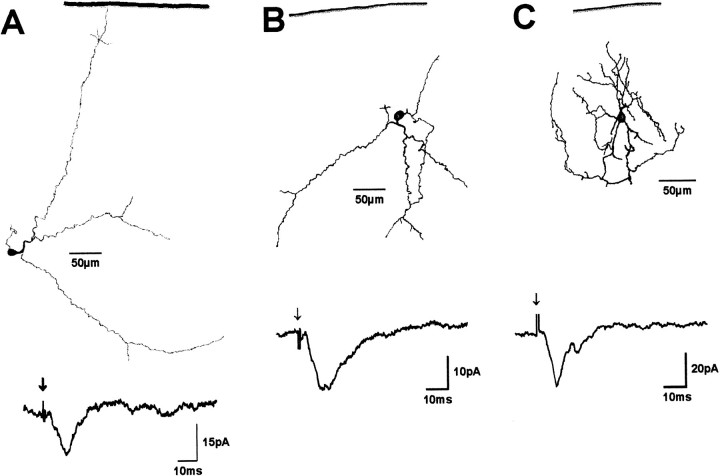
Fig. 2.
Representative examples of cells that responded only with an EPSC to SO stimulation. Dendritic morphology and postsynaptic currents are shown for two piriform cells (A, B) and a stellate cell (C). Solid lines indicate the dorsal collicular surface.Arrows indicate SO stimulus onset.
After biocytin histochemistry, 30 of the neurons sampled contained sufficient intracellular label to allow morphological classification according to criteria introduced by Langer and Lund (1974). Wide-field cells (n = 4) (Fig. 1A) usually had large oval (long axis up to 28 μm) and vertically oriented cell bodies. Their dendritic trees also were oriented vertically and extended up to 350 μm laterally in diameter. Narrow-field cells (n = 9) (Fig. 1B,C) were characterized by smaller (long axis of <15 μm) vertically oriented cell bodies and vertically oriented dendritic fields with a lateral extension of <150 μm. Horizontal cells (n = 6) (Fig.1D) had large oval (long axis up to 30 μm) cell bodies that were oriented parallel to the SC surface. Their dendrites were oriented horizontally and frequently gave rise to small caliber arborizations with numerous dendritic spines. Dendritic fields of horizontal cells extended up to 800 μm laterally. Piriform cells (n = 7) (Fig. 2A,B) had small, round cell bodies (diameter, 15–20 μm) and dendritic trees with variable orientations that extended up to 300 μm laterally. Stellate cells (n = 4) (Fig. 2C) had small round cell bodies (diameter, 8–10 μm) and radially oriented dendritic trees. They gave rise to numerous local axonal arborizations that overlapped within their dendritic fields.
In all wide-field cells (four of four), in five of nine narrow-field cells, in all horizontal cells (six of six), and in one of four stellate cells, SO stimulation evoked an EPSC followed by a significant IPSC. In the remaining narrow-field cells (four of nine) and stellate cells (three of four), and in all piriform cells (seven of seven), only EPSCs were observed after SO stimulation.
Pharmacological characterization of evoked responses
In the first set of experiments, we characterized excitatory and inhibitory influences on SGS cell responses evoked by SO stimulation. In particular, we were interested in the extent to which postsynaptic currents depended on synaptic transmission through glutamate receptors and to what extent GABAA and GABAB receptors contributed to the inhibitory currents.
First, we determined the dependence of the evoked PSCs on synaptic transmission through ionotropic glutamate receptors. When the specific AMPA receptor antagonist CNQX (40 μm) was coapplied with the specific NMDA receptor antagonist APV (100 μm), all PSCs were completely blocked (Fig.3A). This result indicates that all of the evoked IPSCs in our experiments depended on excitatory synaptic transmission, and therefore that no inhibitory synapses were monosynaptically activated by the electrical stimulation of the SO axons.
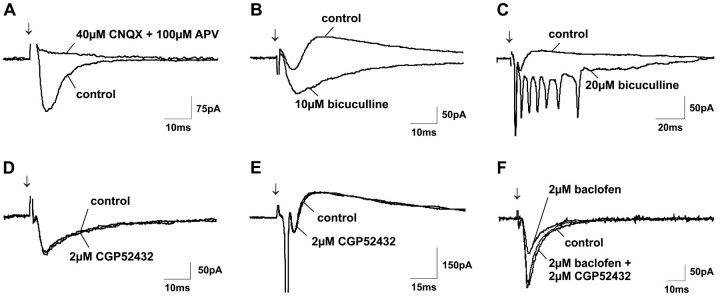
Fig. 3.
Characterization of EPSCs and IPSCs in SGS neurons evoked by SO stimulation. A, When excitatory synaptic transmission through glutamate receptors was blocked by coapplication of the AMPA receptor antagonist CNQX and the NMDA receptor antagonist APV, all PSCs disappeared. B, C, Application of the GABAA receptor antagonist bicuculline strongly enhanced EPSC amplitudes and prolonged EPSC durations, thereby frequently leading to firing of numerous unclamped spikes. D, E, In contrast to bicuculline, application of the GABAB receptor antagonist CGP 52432 did not change evoked responses. However, CGP 52432 could completely block GABAB receptor activation induced by bath application of 2 μm baclofen (F). Arrows indicate SO stimulus onset.
Bath application of the specific GABAA receptor antagonist bicuculline at concentrations of 10 or 20 μmcompletely eliminated IPSCs. As a result, EPSCs were significantly prolonged and EPSC amplitudes were strongly increased (Fig.3B). This enhancement of postsynaptic excitation frequently led to the appearance of multiple unclamped action potentials (Fig.3C).
In contrast to the strong effects obtained with bicuculline, bath application of the specific GABAB receptor antagonist CGP 52432 (2 μm) changed neither the amplitudes nor the durations of evoked PSCs (Fig. 3D,E). The ineffectiveness of CGP 52432, however, was not attributable either to a paucity of GABAB receptors or to an insufficient concentration of CGP 52432. Thus, application of the specific GABAB receptor agonist baclofen (2 μm) strongly decreased EPSC amplitudes, demonstrating the presence of GABAB receptors (Fig. 3F). Moreover, coapplication of 2 μm CGP 52432 was able to completely block this depressive effect of baclofen (Fig. 3F), indicating that 2 μm CGP 52432 is sufficient to block GABAB receptor activation.
Effects of GABA receptor agonists on evoked responses
Because GABAC receptors have a higher affinity for both GABA and muscimol than do GABAAreceptors (Bormann and Feigenspan, 1995; Johnston, 1996b; Chebib and Johnston, 1999; Bormann, 2000), we asked whether these two agonists have different effects when applied at different concentrations. These experiments were designed to examine, at the single-cell level, the mechanisms responsible for the previous observation that GABA and muscimol increase evoked field potentials in SGS at low concentrations, but decrease their amplitudes at higher concentrations (Pasternack et al., 1999).
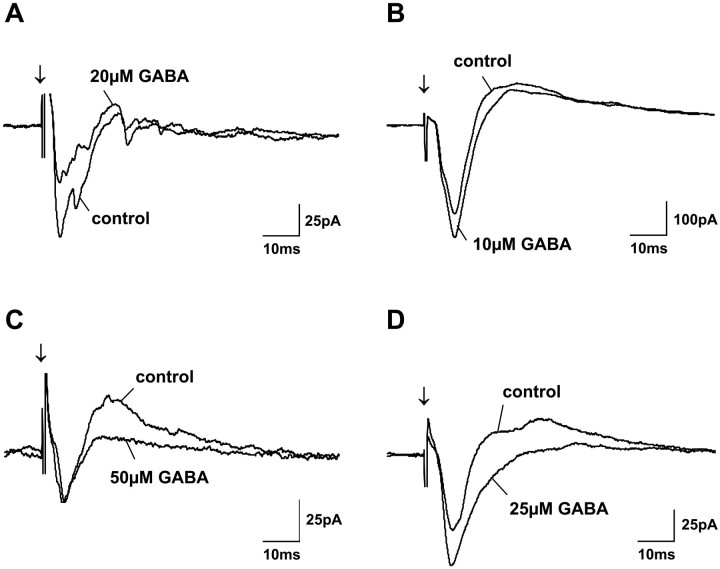
The lowest concentration of GABA that significantly changed amplitudes of EPSCs evoked by SO electrical stimulation was 10 μm. Two effects were observed when we used GABA at concentrations between 10 and 50 μm. For 54% of the cells tested (14 of 26), EPSC amplitudes were decreased (Fig.4A). For the remaining cells (46%; 12 of 26), application of GABA at these low concentrations either enhanced EPSC amplitudes (Fig. 4B), decreased IPSC amplitudes (Fig. 4C), or, at the same time, both enhanced EPSC amplitudes and decreased IPSC amplitudes (Fig.4D). Whether only EPSCs were increased or IPSCs were decreased or whether EPSC increases and IPSC decreases occurred at the same time did not depend on the GABA concentration. Thus, for example, application of 50 μm GABA only increased EPSCs in one cell, only decreased IPSCs in two cells, and increased EPSCs and decreased IPSCs at the same time in two other cells. In contrast to these variable effects that were observed with GABA concentrations up to 50 μm, increasing the GABA concentration in the bath to >100 μm resulted in similar effects for all tested cells; specifically, a large decrease of all evoked currents was observed (Fig. 5). The effectiveness of GABA applied at low concentrations varied only slightly between different slices. Once we had established 10–50 μm as the low concentration range in which GABA was effective, we used 25 μm as the standard low GABA concentration. If, however, no significant effect was observed, we increased the GABA concentration to 50 μm; in all cases, 50 μmGABA proved to be sufficient to elicit significant effects.
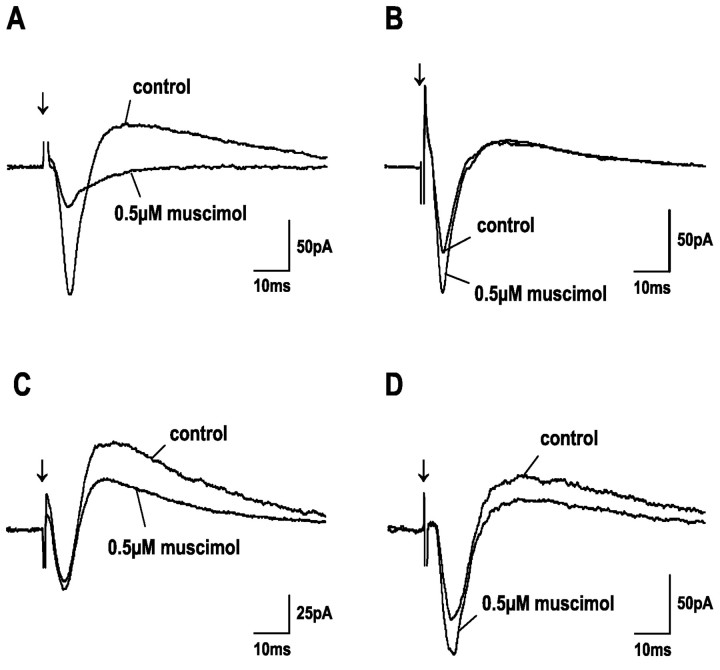
Fig. 4.
Effect of bath-applied GABA on postsynaptic currents of four different SGS neurons. Each graph shows PSCs before (control) and during application of GABA at low concentrations (as indicated). Different effects of GABA can be observed for different cells. GABA either reduced EPSC amplitudes (A), or increased EPSC amplitudes (B), or decreased IPSC amplitudes (C), or increased EPSC and decreased IPSC amplitudes at the same time (D).Arrows indicate SO stimulus onset.
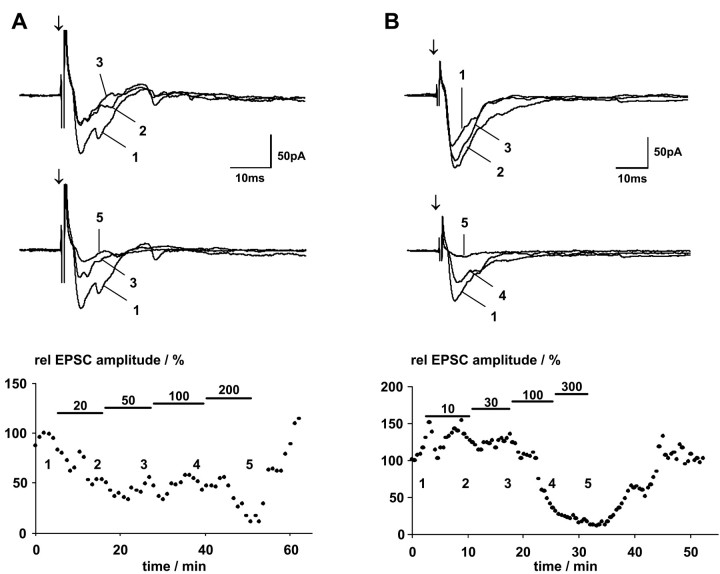
Fig. 5.
Effect of increasing GABA concentrations on postsynaptic currents of two different SGS neurons. In thebottom panels, normalized EPSC peak amplitudes are plotted versus time. Black bars indicate application of GABA at various concentrations. The numbers denote the time at which the individual currents shown in the top panels were recorded. For the cell shown inA, identified as a piriform cell, the lowest GABA concentration tested (10 μm) decreased the EPSC amplitude. Increasing GABA concentrations initially did not change this decrease, until a GABA concentration of 200 μm further reduced EPSP amplitudes. For the cell shown in B, identified as a narrow-field cell, GABA increased EPSP amplitudes at 10 and 30 μm, whereas higher concentrations strongly depressed EPSCs. Arrows indicate SO stimulus onset.
When muscimol was used as an agonist instead of GABA, we obtained similar results. Low concentrations of muscimol (<1 μm) resulted in decreases in the evoked EPSCs for 41% (9 of 22) of the tested cells (Fig. 6A). For the remaining cells (59%; 13 of 22), muscimol at the same concentrations either enhanced EPSC amplitudes (Fig.6B), decreased IPSC amplitudes (Fig. 6C), or at the same time both enhanced EPSC amplitudes and decreased IPSC amplitudes (Fig. 6D). Because we used muscimol only at concentrations of 0.5 and 1 μm, we cannot comment in detail on the extent to which the effects of muscimol on EPSCs and/or IPSCs were concentration-dependent. As was the case for GABA applications, increasing the muscimol concentration to values >2 μm led to strong depression of all evoked responses in all cells (Fig. 7).
Fig. 6.
Effect of bath-applied muscimol on postsynaptic currents of four different SGS neurons. Each graph shows PSCs before (control) and during application of 0.5 μm muscimol. The effect of muscimol differs for different cells. Similar to results obtained with GABA, at low concentrations muscimol either reduced EPSC amplitudes (A), increased EPSC amplitudes (B), decreased IPSC amplitudes (C), or increased EPSC and decreased IPSC amplitudes at the same time (D).Arrows indicate SO stimulus onset.
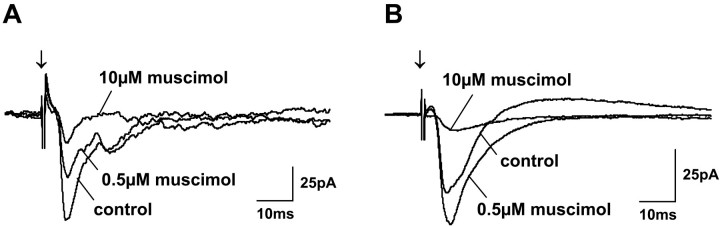
Fig. 7.
Concentration dependence of muscimol effects on postsynaptic currents. Traces show PSCs before (control) and during application of 0.5 and 10 μm muscimol. For the cell shown in A, muscimol reduced EPSC amplitudes at both concentrations. The cell inB shows increased EPSC and decreased IPSC amplitudes during 0.5 μm muscimol application and strongly reduced PSCs during 10 μm muscimol application.Arrows indicate SO stimulus onset.
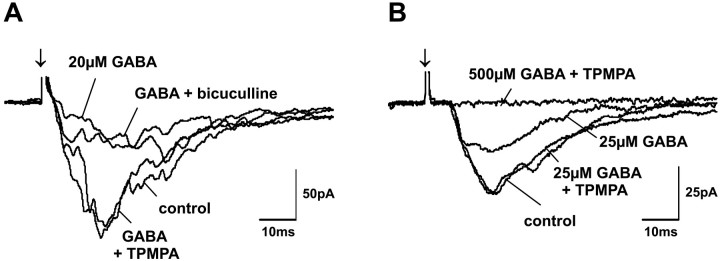
We used TPMPA and bicuculline as specific antagonists to GABAC and GABAAreceptors, respectively, to further distinguish between the influences of GABAC and GABAAreceptors. Because muscimol acts as an agonist at both GABAA and GABACreceptors, but not at GABAB receptors, it was unlikely that GABAB receptors mediated the specific effects that we observed when applying GABA and muscimol at low concentrations. However, because TPMPA acts as a weak agonist at GABAB receptors (Ragozzino et al., 1996) it was always coapplied with the selective GABABreceptor antagonist CGP 52432 (2 μm). Because 2 μm CGP 52432 did not influence evoked PSCs (Fig.3C,D), although it could block GABABreceptor activation at this concentration (Fig. 3E), and because 50 μm TPMPA together with 2 μm CGP 52432 also did not change evoked responses (data not shown), we conclude that GABAB receptor-mediated currents were negligible in our experiments.
Because of the higher affinity of GABACreceptors to both GABA and muscimol, it seemed reasonable to propose that the effects of GABA and muscimol at low concentrations were GABAC receptor-mediated. We tested this by comparing GABA and muscimol effects before and after the GABAC receptor antagonist TPMPA was added to the bath solution. Coapplication of TPMPA completely blocked the changes in EPSC amplitude that we achieved with either GABA or muscimol at low concentrations in 68% (13 of 19) of the cells tested. The block of GABA and muscimol effects by TPMPA was complete, regardless of whether the low concentration of agonist had decreased (Fig.8A,C) or increased (Fig. 8B,D) the EPSC amplitudes when applied alone.
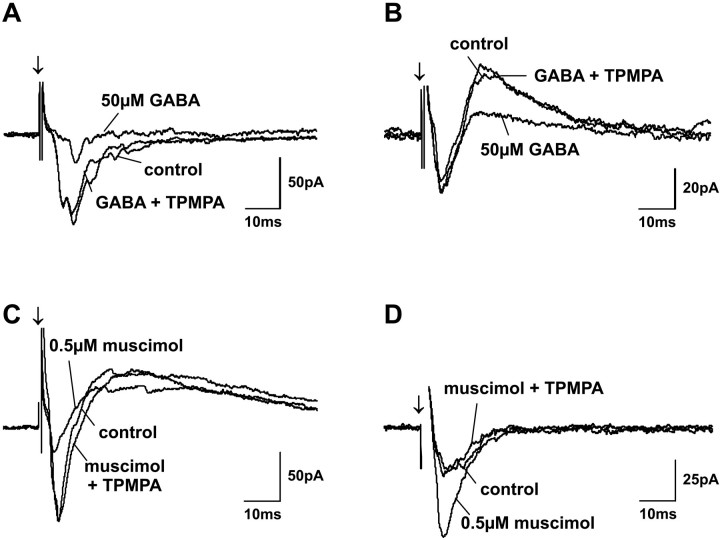
Fig. 8.
Selective block of GABA and muscimol effects on postsynaptic currents with TPMPA. Traces show PSCs before application (control), during application of 50 μm GABA (A, B) and 0.5 μmmuscimol (C, D), and during coapplication of 50 μm TPMPA with GABA and muscimol. Regardless of whether GABA or muscimol reduced (A, C) or enhanced (B, D) postsynaptic currents at low concentrations, coapplication of TPMPA completely blocked the agonist effect in all cases. Because TPMPA acts as a weak GABAB receptor agonist, the GABAB receptor antagonist CGP 52432 was always coapplied with TPMPA. The neuron in A was identified as a stellate cell, and the neuron in C was identified as a horizontal cell. Arrows indicate SO stimulus onset.
If the effects observed while applying GABA and muscimol at low concentrations were indeed mediated by GABAC receptors, the specific GABAA receptor antagonist bicuculline should not block these effects, because bicuculline is not effective at GABAC receptors (Bormann and Feigenspan, 1995; Johnston, 1996b; Bormann, 2000). We tested this prediction by comparing effects of low concentrations of GABA and muscimol before and after the addition of TPMPA to the bath solution and then after substitution of TPMPA by bicuculline. Although a complete block was achieved when TPMPA was coapplied, adding bicuculline to the bath solution did not block the low concentration effects of GABA or muscimol (Fig. 9A).
Fig. 9.
Interaction of TPMPA and bicuculline with GABA-induced effects on postsynaptic currents. EPSC amplitude reductions induced by low GABA concentrations could be completely blocked by coapplication of 50 μm TPMPA. In contrast, coapplication of 20 μm bicuculline was unable to change the depressive effects of low GABA concentrations (A) (cell identified as a piriform cell). On the other hand, although coapplication of 50 μm TPMPA could completely block effects of low GABA concentrations, EPSC reductions induced by high GABA concentrations remained unaffected during TPMPA coapplication (B). Arrows indicate SO stimulus onset.
Finally, if TPMPA at the concentration used in our experiments was not acting at GABAA receptors, it should not block the effects of GABA and muscimol at the higher concentrations that activate GABAA receptors in addition to GABAC receptors. To test this prediction, we compared the influence of TPMPA on GABA and muscimol effects at high concentrations. Although TPMPA had blocked the effects of low concentrations of GABA and muscimol, the depressive effects on evoked responses induced by application of higher GABA and muscimol concentrations remained unaltered during coapplication of TPMPA (Fig.9B).
Correlation with cell morphology
Low concentrations of GABA and muscimol suppressed EPSCs in all identified horizontal cells, six of seven piriform cells, one of four wide-field cells, two of seven narrow-field cells, and two of three stellate cells. It should also be noted that when these cells showed IPSCs, the IPSCs were completely blocked by low concentrations of GABA and muscimol. The remaining wide-field cells (three of four), narrow-field cells (five of seven), piriform cell, and stellate cell all showed increased EPSC amplitudes only, decreased IPSC amplitudes only, or increased EPSC amplitudes together with decreased IPSC amplitudes after the application of GABA and muscimol at low concentrations.
DISCUSSION
Concentration dependence of GABA and muscimol effects
We examined the effects of GABA and muscimol on responses of rat SGS neurons evoked by electrical SO stimulation. Bath application of both agonists changed EPSC and IPSC amplitudes in a concentration-dependent manner. At high concentrations, above 100 and 1 μm for GABA and muscimol, respectively, these agonists decreased EPSC and IPSC amplitudes for all cells tested. At lower concentrations, GABA and muscimol reduced EPSC amplitudes in about half of the tested cells (proportion differences for GABA and muscimol not statistically significant). For the remaining cells, their effect was an enhancement rather than a depression of postsynaptic excitation; that is, either EPSC amplitudes were increased, IPSC amplitudes were decreased, or an EPSC increase occurred together with an IPSC decrease.
A similar concentration dependence of GABA and muscimol effects was observed on FPs evoked by electrical SO stimulation in guinea pig SGS (Arakawa and Okada, 1988). Bath-applied GABA and muscimol increased FP amplitudes <1 mm and 10 μm, respectively, whereas higher concentrations strongly decreased FP amplitudes. Because muscimol is not active at GABAB receptors and because application of the GABAB receptor agonist baclofen only decreased FP amplitudes, it was concluded that GABAB receptors were not involved. Instead, the existence of excitatory GABA receptors was proposed that would be activated by lower concentrations of GABA and muscimol than are GABAA receptors (Arakawa and Okada, 1988).
Evidence for the presence of GABAC receptors
More recently, it was proposed that the concentration dependence of GABA and muscimol effects on electrically evoked FPs in rats reflects an involvement of GABAC receptors (Pasternack et al., 1999). This proposal was based on the following evidence. First, GABAC receptors exhibit a 10-fold higher affinity to both agonists than do GABAA receptors (Bormann and Feigenspan, 1995;Johnston, 1996b; Chebib and Johnston, 1999; Bormann, 2000). Second, TPMPA, a specific GABAC receptor antagonist (Ragozzino et al., 1996), blocks FP amplitude increases achieved with low agonist concentrations, but amplitude decreases achieved with higher concentrations are unaffected (Pasternack et al., 1999). Third, results from both in situ hybridization (Boué-Grabot et al., 1998; Wegelius et al., 1998) and immunocytochemistry (Pasternack et al., 1999) suggest that rat SGS contains GABAC receptor-specific ρ subunits at high density (Cutting et al., 1991; Enz and Cutting, 1998).
Data from the present study provide support at the cellular level for the proposal that GABAC receptors are responsible for the FP amplitude enhancement. The concentration dependence of GABA and muscimol effects most likely results from different agonist affinities of GABAC and GABAA receptors. Thus, at concentrations <100 and 1 μm, respectively, GABA and muscimol activate GABAC but not GABAAreceptors. Moreover, these effects are blocked by TPMPA, but not by the selective GABAA receptor antagonist bicuculline. Finally, TPMPA blocks low-concentration GABA and muscimol effects, but not inhibitory effects achieved with higher concentrations.
These results all support the conclusion that GABAA and GABACreceptors serve different functions in SGS. Activation of GABAA receptors by high agonist concentrations suppressed evoked responses in all cells tested. Because GABAA receptor density is high in SGS (Bowery et al., 1987; Mize, 1992; Mize and Butler, 1997), this finding is not surprising. In fact, strong depressive effects of GABAA receptor activation on rat SGS neuronal responses have been shown both in vivo (Binns and Salt, 1997) and in vitro (Özen et al., 2000).
Activation of GABAC receptors affects SGS circuitry differently. The depressive effects of GABA and muscimol at low concentrations observed in half of the sampled cells can be explained by a direct action. Because GABAC receptors are ligand-gated Cl− channels (Bormann and Feigenspan, 1995; Johnston, 1996b), their activation will directly induce inhibition in neurons that express them. In contrast, enhanced responses observed in the remaining neurons can be explained by an indirect action. If GABAC receptors are primarily or exclusively expressed by local inhibitory interneurons (Fig. 10), enhanced responses in SGS projection cells could result from the reduced activity of inhibitory neurons. Because we not only found increased EPSC amplitudes but also decreased IPSC amplitudes, we conclude that GABAC receptors mediate disinhibition for about half of the SGS neurons sampled. This conclusion is consistent with FP amplitude increases after application of GABA and muscimol at low concentrations (Pasternack et al., 1999). The finding that GABAC receptor activation either increases EPSC amplitudes and/or decreases IPSC amplitudes most likely reflects different proportions of excitatory versus inhibitory input to individual neurons.
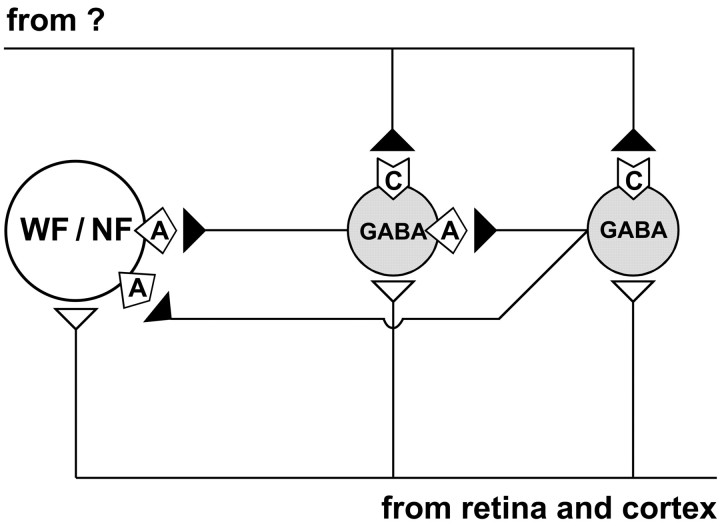
Fig. 10.
Schematic drawing of SGS circuitry as proposed from our results. All cell types recorded [i.e., narrow-field cells (NF), wide-field cells (WF), horizontal cells, piriform cells, and stellate cells] receive excitatory input (open triangles) from retinal and/or cortical fibers, and therefore respond with EPSCs to SO stimulation. GABAergic horizontal, piriform, and/or stellate cells provide inhibitory input (filled triangles) to narrow-field and wide-field cells mainly through GABAA receptors (
 ). Some GABAergic cells, most likely horizontal cells, are also contacted by other local GABAergic cells, again mainly through GABAA receptors. Therefore, SO stimulation also elicits IPSCs in narrow-field, wide-field, and horizontal cells. Only GABAergic horizontal, piriform, and stellate cells receive an extrinsic inhibitory input of unknown origin that operates through GABAC receptors (
). Some GABAergic cells, most likely horizontal cells, are also contacted by other local GABAergic cells, again mainly through GABAA receptors. Therefore, SO stimulation also elicits IPSCs in narrow-field, wide-field, and horizontal cells. Only GABAergic horizontal, piriform, and stellate cells receive an extrinsic inhibitory input of unknown origin that operates through GABAC receptors (
 ).
).
Correlation of responses to SO stimulation with morphological cell types
Our sample of well-stained cells included nine narrow-field cells, four wide-field cells, six horizontal cells, seven piriform cells, and four stellate cells. GABA accumulation or GABA-like immunoreactivity have been shown for horizontal, piriform, and stellate cells, and all of these populations of morphologically defined cell types are likely to include local GABAergic interneurons (Mize et al., 1982; Mize, 1988,1992). However, cells with stellate morphology can also show glutamate-like immunoreactivity, and therefore this morphological class may also include non-GABAergic projection neurons (Jeon et al., 1997). Narrow-field cells and wide-field cells are not GABAergic and are regarded as projection neurons. Their main efferent targets are the subjacent stratum griseum intermediale of SC, dorsal and ventral LGN, pretectal nuclear complex, parabigeminal nucleus, and lateral posterior thalamic nucleus (Huerta and Harting, 1984; Lee and Hall, 1995).
We found that postsynaptic currents evoked by SO stimulation consisted either of a single- or multiple-peak EPSC or of a single-peak EPSC followed by an IPSC. These two response types were clearly correlated with morphological cell classes. Most narrow-field cells (five of nine), all wide-field cells (four of four), and all horizontal cells (six of six) exhibited an EPSC followed by a longer-lasting IPSC. Cells that belong to these morphological classes therefore seem to receive a delayed inhibitory input in addition to the excitatory input conveyed to them directly by afferent fibers traveling in SO. IPSCs evoked in these cells are probably achieved by feedforward inhibitory mechanisms activated by retinal and/or cortical inputs through local GABAergic interneurons (Mize, 1992) (Fig. 10, left GABAergic cell). This possibility is in agreement with data from electron microscopic studies, which demonstrated GABA-immunoreactive presynaptic dendrites forming dendro-dendritic contacts with non-GABAergic cells as well as with horizontal cells (Mize et al., 1991; Mize, 1992). Moreover, bath application of bicuculline transforms responses of projection cells from a transient to a more sustained pattern (Özen et al., 2000). In contrast, pure excitatory responses consisting of single- or multiple-peak EPSCs without IPSCs were observed for all piriform cells (seven of seven) and almost all stellate cells (three of four). The lack of IPSCs indicates that these cells do not receive feedforward inhibition of retinal or cortical origin (Fig. 10, right GABAergic cell). A consistent finding from rabbit SC is that electrical optic chiasm stimulation elicits EPSPs followed by IPSPs in narrow-field cells but only EPSPs in stellate cells (Takahashi and Ogawa, 1978).
Cell type-specific expression of GABAC receptors
GABA and muscimol effects also were strongly correlated with the morphological class to which an individual neuron belonged. Evoked EPSCs of all identified horizontal cells (six of six) and of almost all piriform cells (six of seven) and stellate cells (three of four) were significantly decreased by low agonist concentrations, an effect that we propose is mediated by their GABACreceptors. IPSCs evoked in horizontal cells were also strongly reduced, indicating a reduced inhibitory input to these cells during GABAC receptor activation.
In contrast, evoked responses of the majority of narrow-field cells (five of seven) and wide-field cells (three of four) were enhanced by low GABA and muscimol concentrations, because they generated increased EPSC amplitudes, decreased IPSC amplitudes, or a combination of the two effects. The simplest interpretation consistent with all of these results is that GABAC receptor activation strongly reduced the inhibitory input to narrow-field and wide-field cells; thus direct excitatory inputs were facilitated.
Conclusions
In conclusion, our results provide further evidence for a preferential expression of GABAC receptors by local GABAergic interneurons in SGS (Fig. 10). During GABAC receptor activation, responses of these interneurons are depressed, and, as a result, responses of non-GABAergic projection cells postsynaptic to interneurons are enhanced. Thus, in contrast to GABAA and GABAB receptor function, which directly inhibits evoked responses, activating GABACreceptors leads to an indirect, interneuron-mediated disinhibition of SGS projection cells.
What might be the source of a GABAergic input to GABAergic interneurons that is specifically mediated by GABACreceptors? One clue comes from the observation that piriform cells and most stellate cells did not show stimulus-evoked IPSCs, although they were inhibited by GABAC receptor activation. For at least these two classes of putative interneurons, the GABAC receptor-mediated input was not activated by SO stimulation. Because nearly all SGS neurons respond to SO stimulation, an intrinsic presynaptic source would seem unlikely. Thus, we postulate that any input responsible for GABAC receptor-mediated disinhibition would be extrinsic to SGS (Fig. 10). This extrinsic GABAergic input source might terminate on interneurons in an arrangement similar to that proposed for the dLGN (Zhu and Lo, 1999). In fact, axon terminals that exhibit GABA-like immunoreactivity contact GABAergic postsynaptic profiles in rat, rabbit, and monkey SGS (Pinard et al., 1991; Mize et al., 1991, 1994).
An extrinsic GABAergic input could either locally or globally disinhibit SGS projection neurons at specific behavioral conditions. Recognized sources of long-range GABAergic inputs to SGS are the pretectal nuclear complex and the ventral LGN (Nunes Cardozo et al., 1994; Moore et al., 2000). Future studies will have to determine whether inhibition from one of those sources is mediated via GABAC receptors.
Footnotes
This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 509 “Neurovision,” TP A8, and a Heisenberg fellowship to M.S.) and by National Institutes of Health Grant EY08233. We thank M. C. Helms and P. Lee for helpful discussions and technical support during the course of this work and for comments on this manuscript.
Correspondence should be addressed to Dr. Matthias Schmidt, Allgemeine Zoologie und Neurobiologie, Ruhr-Universität Bochum, ND 6/25, D-44780 Bochum, Germany. E-mail:mschmidt@neurobiologie.ruhr-uni-bochum.de.
REFERENCES
- 1.Arakawa T, Okada Y. Excitatory and inhibitory action of GABA on synaptic transmission in slices of guinea pig superior colliculus. Eur J Pharmacol. 1988;158:217–224. doi: 10.1016/0014-2999(88)90070-2. [DOI] [PubMed] [Google Scholar]
- 2.Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol (Lond) 1997;504:629–639. doi: 10.1111/j.1469-7793.1997.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 4.Bormann J. The “ABC” of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 5.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 6.Boué-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor subunits in rat brain. J Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowery NG, Hudson AL, Prince GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 8.Chebib M, Johnston GAR. The “ABC” of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- 9.Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH. Cloning of the γ-aminobutyric (GABA) 1 cDNA: a GABA receptor highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney AJ, Sah P. GABA receptors inhibited by benzodiazepines mediate fast inhibitory transmission in the central amygdala. J Neurosci. 1999;19:9698–9704. doi: 10.1523/JNEUROSCI.19-22-09698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drew CA, Johnston GAR. Bicuculline- and baclofen-insensitive γ-aminobutyric acid binding to rat cerebellar membranes. J Neurochem. 1992;58:1087–1092. doi: 10.1111/j.1471-4159.1992.tb09366.x. [DOI] [PubMed] [Google Scholar]
- 12.Drew CA, Johnston GAR, Weatherby RP. Bicuculline-insensitive GABA receptors: studies on the binding of (−)-baclofen to rat cerebellar membranes. Neurosci Lett. 1984;52:317–321. doi: 10.1016/0304-3940(84)90181-2. [DOI] [PubMed] [Google Scholar]
- 13.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 14.Enz R, Cutting GR. Molecular composition of GABAC receptors. Vision Res. 1998;78:1431–1441. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 15.Enz R, Cutting GR. GABAC receptor ρ subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur J Neurosci. 1999;11:41–50. doi: 10.1046/j.1460-9568.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 16.Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor subunits ρ1 and ρ2 in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 17.Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAC receptor ρ subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. I. The superficial gray layer. J Comp Neurol. 1993;332:213–223. doi: 10.1002/cne.903320206. [DOI] [PubMed] [Google Scholar]
- 19.Huerta MF, Harting JK. The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H, editor. Comparative neurology of the optic tectum. Plenum; New York: 1984. pp. 687–773. [Google Scholar]
- 20.Jeon CJ, Gurski MR, Mize RR. Glutamate containing neurons in the cat superior colliculus revealed by immunocytochemistry. Vis Neurosci. 1997;14:387–393. doi: 10.1017/s0952523800011500. [DOI] [PubMed] [Google Scholar]
- 21.Johnston GAR. GABAA receptor pharmacology. Pharmacol Ther. 1996a;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnston GAR. GABAC receptors: relatively simple transmitter-gated ion channels? Trends Pharmacol Sci. 1996b;17:319–323. [PubMed] [Google Scholar]
- 23.Koulen P, Brandstätter JH, Enz R, Bormann J, Wässle H. Synaptic clustering of GABAC receptor ρ subunits in the rat retina. Eur J Neurosci. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Langer TP, Lund RD. The upper layers of the superior colliculus of the rat: a Golgi study. J Comp Neurol. 1974;158:418–435. doi: 10.1002/cne.901580404. [DOI] [PubMed] [Google Scholar]
- 25.Lee P, Hall WC. Interlaminar connections of the superior colliculus in the tree shrew. II. Projections from the superficial gray to the optic layer. Vis Neurosci. 1995;12:573–588. doi: 10.1017/s0952523800008464. [DOI] [PubMed] [Google Scholar]
- 26.Lukasiewicz PD. GABAC receptors in the vertebrate retina. Mol Neurobiol. 1996;12:181–194. doi: 10.1007/BF02755587. [DOI] [PubMed] [Google Scholar]
- 27.Mize RR. Immunocytochemical localization of γ-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol. 1988;276:169–187. doi: 10.1002/cne.902760203. [DOI] [PubMed] [Google Scholar]
- 28.Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- 29.Mize RR, Butler GD. The distribution of the GABAA β2,β3 subunit receptor in the cat superior colliculus using antibody immunocytochemistry. Neuroscience. 1997;79:1121–1135. doi: 10.1016/s0306-4522(96)00667-7. [DOI] [PubMed] [Google Scholar]
- 30.Mize RR, Spencer RF, Sterling P. Two types of GABA-accumulating neurons in the superficial gray layer of the cat superior colliculus. J Comp Neurol. 1982;206:180–192. doi: 10.1002/cne.902060207. [DOI] [PubMed] [Google Scholar]
- 31.Mize RR, Jeon CJ, Hamada OL, Spencer RF. Organization of neurons labeled by antibodies to γ-aminobutyric acid (GABA) in the superior colliculus of the Rhesus monkey. Vis Neurosci. 1991;6:75–92. doi: 10.1017/s0952523800000924. [DOI] [PubMed] [Google Scholar]
- 32.Mize RR, Whitworth RH, Nunes-Cardozo B, van der Want J. Ultrastructural organization of GABA in the rabbit superior colliculus revealed by quantitative postembedding immunocytochemistry. J Comp Neurol. 1994;341:273–287. doi: 10.1002/cne.903410211. [DOI] [PubMed] [Google Scholar]
- 33.Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 35.Nunes Cardozo B, Mize RR, Van der Want JJ. GABAergic and non-GABAergic neurons in the nucleus of the optic tract project to the superior colliculus: an ultrastructural retrograde tracer and immunocytochemical study in the rabbit. J Comp Neurol. 1994;350:646–656. doi: 10.1002/cne.903500410. [DOI] [PubMed] [Google Scholar]
- 36.Özen G, Augustine GJ, Hall WC. Contribution of superficial layer neurons to premotor bursts in the superior colliculus. J Neurophysiol. 2000;84:460–471. doi: 10.1152/jn.2000.84.1.460. [DOI] [PubMed] [Google Scholar]
- 37.Pasternack M, Boller M, Pau B, Schmidt M. GABAA and GABAC receptors have contrasting effects on excitability in superior colliculus. J Neurophysiol. 1999;82:2020–2023. doi: 10.1152/jn.1999.82.4.2020. [DOI] [PubMed] [Google Scholar]
- 38.Pinard R, Benfares J, Lanoir J. Electron microscopic study of GABA-immunoreactive neuronal processes in the superficial gray layer of the rat superior colliculus: their relationships with degenerating retinal nerve endings. J Neurocytol. 1991;20:262–276. doi: 10.1007/BF01235544. [DOI] [PubMed] [Google Scholar]
- 39.Plant D, Eilers J, Konnerth A. Patch-clamp technique in brain slices. In: Boulton A, Baker G, Walz W, editors. Patch-clamp applications and protocols, Neuromethods, Vol 26. Humana; Totowa, NJ: 1995. pp. 233–258. [Google Scholar]
- 40.Platt B, Withington DJ. GABA-induced long-term potentiation in the guinea-pig superior colliculus. Neuropharmacology. 1998;37:1111–1122. doi: 10.1016/s0028-3908(98)00100-2. [DOI] [PubMed] [Google Scholar]
- 41.Qian H, Hyatt G, Schanzer A, Hazra R, Hackam AS, Cutting GR, Dowling JE. A comparison of GABAC and ρ subunit receptors from the white perch retina. Vis Neurosci. 1997;14:843–851. doi: 10.1017/s0952523800011585. [DOI] [PubMed] [Google Scholar]
- 42.Ragozzino D, Woodward RM, Murata Y, Eusebi F, Overman LE, Miledi R. Design and in vitro pharmacology of a selective γ-aminobutyric acidC receptor antagonist. Mol Pharmacol. 1996;50:1024–1030. [PubMed] [Google Scholar]
- 43.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 44.Takahashi Y, Ogawa T. Electrophysiological properties of morphologically identified neurons in the rabbit's superior colliculus. Exp Neurol. 1978;60:254–266. doi: 10.1016/0014-4886(78)90081-x. [DOI] [PubMed] [Google Scholar]
- 45.Wegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saarma M, Reeben M. Distribution of GABA receptor ρ subunit transcripts in the rat brain. Eur J Neurosci. 1998;10:350–357. doi: 10.1046/j.1460-9568.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu JJ, Lo FS. Three GABA receptor-mediated postsynaptic potentials in interneurons in the rat lateral geniculate nucleus. J Neurosci. 1999;19:5721–5730. doi: 10.1523/JNEUROSCI.19-14-05721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]