Abstract
Cyclin-dependent kinase 5 (Cdk5) null mice exhibit a unique phenotype characterized by perinatal mortality, disrupted cerebral cortical layering attributable to abnormal neuronal migration, lack of cerebellar foliation, and chromatolytic changes of neurons in the brainstem and the spinal cord. Because Cdk5 is expressed in both neurons and astrocytes, it has been unclear whether this phenotype is primarily attributable to defects in neurons or in astrocytes. Herein we report reconstitution of Cdk5 expression in neurons in Cdk5 null mice and its effect on the null phenotype. Unlike the Cdk5 null mice, the reconstituted Cdk5 null mice that express the Cdk5 transgene under the p35 promoter (TgKO mice) were viable and fertile. Because Cdk5 expression is mainly limited to neurons in these mice and rescues the defects in the nervous system of the Cdk5 null phenotype, it clearly demonstrates that Cdk5 activity is necessary for normal development and survival of p35-expressing neurons.
Keywords: Cdk5, cerebrum, cerebellum, neuron, astrocyte, phosphorylation, neurodegeneration, transgenic mice
Cyclin-dependent kinase 5 (Cdk5) is a member of the Cdk family of serine/threonine kinases and is so named because of its sequence homology to other Cdks (Hellmich et al., 1992;Lew et al., 1992; Meyerson et al., 1992). Unlike other Cdks that are involved in cell cycle control, Cdk5 is mainly involved in phosphorylation of target proteins in postmitotic neurons (Shetty et al., 1993). It phosphorylates cytoskeletal components such as high-molecular weight neurofilament protein (NF-H) and microtubule-associated proteins (MAP) tau and MAP1B (Mandelkow et al., 1992; Kobayashi et al., 1993) and is implicated in regulation of neuronal migration, neurite outgrowth (Nikolic et al., 1996), and axon patterning (Connell-Crowley et al., 2000).
The activity of Cdk5 is regulated in two ways, by its binding with neuron-specific activator proteins p35, p25, and p39, and by phosphorylation (Sharma et al., 1999; Zukerberg et al., 2000). The activator proteins p35 and p39 are noncyclin proteins (Lew et al., 1994; Tsai et al., 1994; Tang et al., 1995), with p25 being a proteolyzed fragment of p35 (Lew et al., 1994). Cdk5 is ubiquitously expressed and is most abundant in the nervous system (Hellmich et al., 1992; Tsai et al., 1993). p35 and p39 are mostly expressed in the nervous system, with p35 predominating in the cerebral cortex and p39 in the cerebellum (Lew et al., 1994; Tsai et al., 1994; Matsushita et al., 1996; Tomizawa et al., 1996; Delalle et al., 1997; Zheng et al., 1998). p35 protein is also expressed in testis. However, p39 expression is restricted to the nervous system (Cai et al., 1997; Zheng et al., 1998; Honjyo et al., 1999). Cdk5 kinase activity is primarily restricted to the nervous system, apparently because of the restricted distribution of its activator proteins p35, p25, and p39. However, Cdk5 activity has also been demonstrated in muscle during myoblast differentiation (Lazaro et al., 1997; Philpott et al., 1997).
We have reported previously Cdk5 null phenotype associated with perinatal mortality, abnormal neuronal migration, cerebellar defoliation, accumulation of NF-H, and chromatolytic changes in motor neurons in brainstem and spinal cord (Ohshima et al., 1996b; Gilmore et al., 1998). Interestingly, p35 null mice also have an inverted cerebral cortical layering pattern and aberrant axonal trajection, but these mice are viable (Chae et al., 1997; Kwon and Tsai, 1998; T. Ohshima and A. B. Kulkarni, unpublished results). The cerebellum of the p35 null mice shows subtle phenotypic changes, but the brainstem, spinal cord, and peripheral nervous system are unaffected (Chae et al., 1997;Kwon and Tsai, 1998).
The present investigation was undertaken to determine whether targeted reconstitution of Cdk5 expression in the neurons of Cdk5 null mice leads to reversal of the phenotype and perinatal mortality. Using a tissue-specific expression strategy, we restored Cdk5 expression only in the regions expressing p35 and rescued the Cdk5 null mice. The functional significance of Cdk5 in neurons for embryonic development and survival is discussed.
MATERIALS AND METHODS
Cdk5 overexpression mice driven by p35 promoter (TgCdk5 mice). The murine Cdk5 and p35 genes with their promoter regions were cloned and characterized as reported previously (Ohshima et al., 1995, 1996a). The 1.2 kb p35 promoter region consisting of anEcoRI-NotI fragment (Ohshima et al., 1996a) was ligated with the 4.8 kb Cdk5 gene consisting of aNotI-XhoI fragment (Ohshima et al., 1995) in pGEM 9Z(−) plasmid. XbaI-XbaI fragment containing p35 promoter and Cdk5 gene was subcloned into pUC18 plasmid, and a 45 bp tag derived from SV40 was inserted into SpeI site downstream of the poly(A+) signal (Fig.1A). The tag contains an XhoI restriction site for genotyping. For microinjection, the 6 kb fragment was excised from the pUC18 plasmid and purified. Transgenic mice were produced by pronuclear injection of the transgene (Sreenath et al., 1999). Homozygous transgenic mice (TgCdk5 mice) were identified by Southern blot analysis.
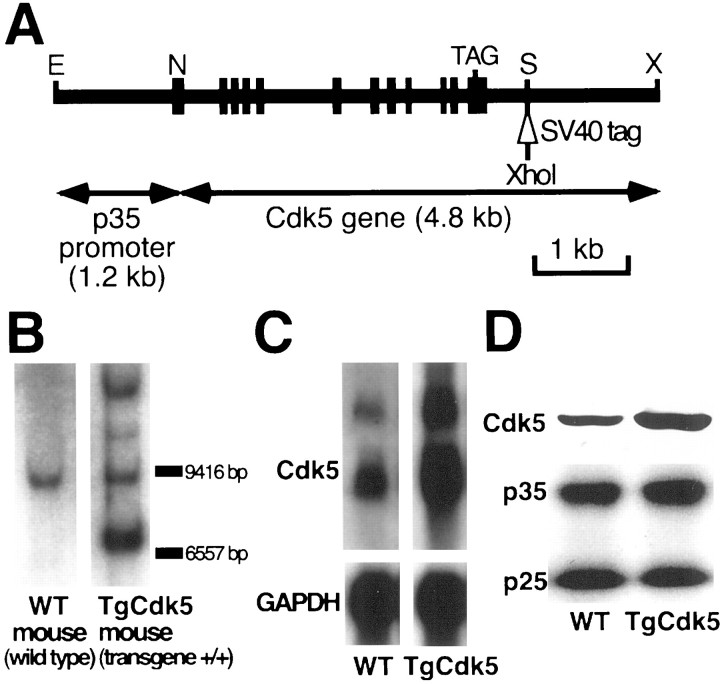
Fig. 1.
Generation of transgenic mice overexpressing Cdk5 using the p35 promoter (TgCdk5 mice). A, Diagrammatic representation of the transgene construct showing p35 promoter region (1.2 kb) ligated to the Cdk5 gene (4.8 kb). A 45 bp tag derived from SV40 containing XhoI site is inserted intoSpeI site. Sites for XbaI (X), NotI (N), EcoRI (E), and SpeI (S) are indicated. B, Genotyping of tail DNA from WT and TgCdk5 mouse by Southern blot analysis. Additional bands derived from the transgene were revealed in TgCdk5 mice. C, Northern blot analysis of Cdk5 mRNA from the entire brain showing a marked increase of Cdk5 mRNA level in the TgCdk5 mouse. D, Western blot analysis of Cdk5 protein from the entire brain showing a marked increase of Cdk5 protein in the TgCdk5 mouse. Note that the level of both the p35 and p25 proteins remain unaltered in TgCdk5 compared with the corresponding WT control.
Transgenic mice in which Cdk5 is expressed only in p35 region (TgKO mice). TgCdk5 mice were crossed with Cdk5 +/− mice. Of the F1 generation, the mice with genotype of Cdk5 +/− and transgene +/− were identified by Southern blot analysis. These mice were intercrossed, and of the F2 generation, the mice with the genotype of endogenous Cdk5 −/− and transgene +/+ were identified by Southern blot analysis. All of the transgenic mice were housed in a temperature-controlled animal care unit and kept on a 12 hr light/dark cycle. Animals were maintained, and the studies were performed in accordance with the National Institutes of Health Guidelines.
Southern blot analysis. Genomic DNA extracted from a mouse tail was digested with EcoRI (for endogenous Cdk5 genotyping) or XhoI (for transgenic Cdk5 genotyping) overnight, electrophoresed on a horizontal 0.8% agarose gel, and transferred to Nytran (Schleicher & Schuell, Keene, NH) membranes. The membrane was hybridized with a random-primed32P-labeled probe at 42°C overnight. The probe for Cdk5 genotyping was the 0.5 kbSpeI-NotI fragment from the 5′-flanking sequence (Ohshima et al., 1996b). The probe for TgCdk5 transgene genotyping was the entire 6 kb transgene. The membranes were washed in 2× SSC, 0.1% SDS at 45°C for 10 min, 0.2× SSC, and 0.1% SDS at 65°C for 30–60 min, and exposed to x-ray film.
RNA analysis. Total RNA was prepared from the mouse cerebrum, cerebellum, spinal cord, heart, liver, kidney, testis, ovary, and muscle by column purification (Rneasy; Qiagen, Valencia, CA). For Northern blot analysis, RNA was size-separated on 1% denaturing agarose gels and transferred to a Nytran membrane. The membrane was hybridized with 32P-labeled 2.1 kb cDNA of Cdk5 at 42°C overnight in the presence of 50% formamide, 10× Denhardt's solution, 5× SSPE, 0.1% SDS, 100 μg/ml ssDNA. The membrane was washed in 2× SSC, 0.1% SDS at 45°C for 10 min, 0.2× SSC, and 0.1% SDS at 65°C for 30–60 min, and exposed to x-ray film. After stripping the probe, the same membrane was used for hybridization with the mouse glyceraldehyde-3-phosphate dehydrogenase probe.
Expression and purification of the recombinant p35.glutathione S-transferase (GST)-p35 and GST-p25 in pGEX4T-2 were constructed by a PCR method using the oligonucleotides 5′-GAGATCCATGGG-CACG- GTGCTG-3′ (p35) and 5′-GTCCGGATCCGCCCAGCCCCCG-CCG-3′ (p25) as forward primers, 5′-GTGATGAATTCTGGATCACCG-ATC-3′ as a reverse primer, and DNA from 6X His-tagged p35 construct (a gift from L. H. Tsai, Harvard School of Medicine, Boston, MA) as a template. The PCR products were digested with BamHI and EcoRI, and the resulting fragments were cloned into BamHI-EcoRI cut pGEX 4T-2. GST-p35 and GST-p25 fusion proteins were expressed and purified as described previously (Pant et al., 1997).
Preparation of the tissue extracts. The cerebrum, cerebellum, spinal cord, heart, liver, kidney, testis, and muscle from the age-matched wild-type (WT) and the transgenic mice were removed surgically, frozen in dry ice, and stored at −80°C. Frozen tissues were further processed as described previously to obtain protein extracts, except for the addition of microcystine LR 2 μm to the homogenization buffer used in these extractions (Veeranna et al., 1996). Protein estimation was performed using the BCA method as described by the manufacturer (Pierce, Rockford, IL).
Electrophoresis and Western blot analysis. Ten to 15 μg of protein was loaded per lane for SDS-PAGE using Novex (San Diego, CA) 10–20% gradient gels. Electrotransfer and immunoblotting were performed as described previously (Shetty et al., 1995) using rabbit polyclonal anti-Cdk5 C-terminal antibody C-8 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-p35 C-terminal antibody C-19 (Santa Cruz Biotechnology). The immunoblots were developed by the ECL method as described by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ). Alternatively membranes were developed using alkaline phosphatase (AP)-based 5-brom-4-chlor-indolyl-phosphate/ nitroblue-tetrazolium-chloride (BCIP/NBT) single reagent (Kirkegaard & Perry Laboratories, Gaithersburg, MD).
Immunoprecipitation and kinase assay. Immunoprecipitation of Cdk5 and the kinase assays using the immunoprecipitates from the tissues of TgCdk5, TgKO, and wild-type mice were performed as described previously using C-8 rabbit polyclonal antibody, which specifically reacts with Cdk5 protein (Veeranna et al., 1996; Pant et al., 1997). Evaluation of the kinase activity in the immunoprecipitates obtained from the Cdk5 overexpression mice and the corresponding controls were performed by the addition of 7.5 μg of bacterially expressed GST-p35 or GST-p25 activator fusion proteins (Pant et al., 1997). The expressed proteins used in these experiments were partially purified, soluble, and active when tested with recombinant Cdk5 (N. D. Amin and H. Pant, unpublished observations).
Tissue preparation for in situ hybridization, histology, and immunohistochemistry. A minimum of three transgenic and two wild-type mice were examined at each time point of 1, 2, 3, 5, and 7 months of age. For paraffin sections of the mouse brains, adult mice were perfused transcardially with 0.1 mPBS, pH 7.4 and 4% paraformaldehyde (PFA) in PBS. The brain and spinal cord were removed and immersion-fixed in 4% PFA in PBS for 24 hr at 4°C. The embryos were removed by hysterotomy from their dams, decapitated, and immersion-fixed in 4% PFA in PBS for 24 hr at 4°C. After fixation, the tissues were dehydrated and embedded in paraffin. Six micrometer paraffin sections were cut and stained with hematoxylin and eosin and Nissl stains by standard methods. For thionine staining, two sets of TgKO mice and one set of wild-type control mouse at the ages of 2, 3, 5, and 7 months were perfused transcardially with wash solution (0.8% NaCl, 0.4% dextrose, 0.8% sucrose, 0.023% CaCl2, and 0.034% sodium cacodylate). The mice were then transcardially perfused with fixative (4% PFA, 4% sucrose, and 1.4% sodium cacodylate) and immersed in the same fixative for 3–5 d. Seventy-one frozen coronal serial sections with slice thickness of 30 μm covering the entire brain were made for each brain and stained with thionine.
In situ hybridization. Digoxigenin (DIG)-labeled antisense and sense riboprobes were generated by in vitrotranscription of pBSK plasmid DNA containing a Cdk5 cDNA insert using T7 and T3 RNA polymerase to generate cRNA probes. Sections were deparaffinized and hydrated through xylenes and a graded ethanol series. After a brief wash in Tris-buffered saline I [(TBS I) 100 mm Tris Cl and 150 mm NaCl, pH7.5], the sections were treated with 10 μg/ml proteinase K in 50 mm Tris Cl, pH7.5, at 37°C for 30 min and then washed in TBS I for three times for 5 min each. The slides were immersed in 0.1 m triethanolamine buffer with 0.25% acetic anhydride at room temperature for 10 min to prevent nonspecific probe binding and washed in 2× SSC for 5 min twice. Prehybridization was done by applying 100 μl of prehybridization solution (50% formamide, 4× SSC, 0.1% SDS, 1× Denhardt's solution, and 400 μg/ml denatured ssDNA) for 1 hr at 45°C, and subsequently sections were hybridized with 30 μl of prehybridization solution with 5–10 ng/μl of either sense or antisense probes. The slides were heated at 65°C for 5 min to denature the target, and hybridization was performed at 45°C for 18 hr in the moist chamber. After three 10 min washes in 2× SSC to remove excess probe, the sections were treated with 100 μl of 40 μg/ml RNase A in 500 mmNaCl and 1 mm EDTA, pH 8.0, at 37°C for 30 min to remove unhybridized probe. Stringent washes were performed with 2× SSC for 30 min at 50°C followed by 0.2× SSC for 30 min at 60°C. After a wash in TBS I, sections were blocked for 30 min with 5% normal goat serum in TBS I containing 0.05% Tween 20 (TBS-T). Subsequently, 100 μl of anti-DIG-AP conjugate (Roche Molecular Biochemical, Indianapolis, IN) solution (1.5 U/ml in TBS I) was applied for 1 hr. After three washes in TBS-T, sections were immersed in TBS II (100 mm Tris-Cl and 150 mm NaCl, pH 9.5) for 5 min at room temperature to activate the alkaline phosphatase, and colorimetric detection was performed with BCIP/NBT (Roche Molecular Biochemicals) at room temperature for 3 hr. The slides were washed in a stream of distilled water and mounted (Kadkol et al., 1999).
Immunofluorescent staining of cells. Mixed cultures of neurons and astrocytes were prepared from embryonic day 17.5 (E17.5) embryos as described previously (Vicario-Abejon et al., 1998). Cells were fixed with 4% PFA for 15 min at room temperature. After blocking for 30 min in PBS with 0.1% Triton X-100 (PBST) and 5% normal goat serum, cultures were incubated with the primary antibody [monoclonal anti-TuJ1 (Covance, Princeton, NJ), monoclonal anti-GFAP (ICN, Costa Mesa, CA), and polyclonal anti-Cdk5 (Santa Cruz Biotechnology)] for 4 hr at room temperature. After two washes in PBST, the secondary antibody [rhodamine-coupled anti-mouse (Jackson ImmunoResearch, West Grove, PA) and biotinylated anti-rabbit (Vector Laboratories, Burlingame, CA)] was applied at a 100-fold dilution at room temperature. One hour later, cultures were washed with PBST and incubated with avidin-coupled FITC (Vector Laboratories) diluted 50-fold in PBS. Cells were mounted in 70% glycerol in PBS with 2% 1,4 diazabicyclo-(2,2,2)-octane (Sigma, St. Louis, MO) after two washes in PBS.
Immunohistochemistry. The following primary antibodies were used in the immunohistochemical study. Rabbit polyclonal anti-Cdk5 C-terminal antibody (C-8) was used at a dilution of 1:100. Rabbit polyclonal anti-cow GFAP antibody (Dako, Carpinteria, CA) was used at a dilution of 1:500. Mouse monoclonal antibody SMI-31 (Sternberger Monoclonals, Lutherville, MD) was used at a dilution of 1:1000. After deparaffinization, the slides were washed in PBS for 5 min twice and immersed in 90% methanol containing 0.3% H2O2 for 30 min. After washing in distilled water, the slides were incubated in PBST for 5 min. The slides were incubated with blocking solution (normal goat serum diluted with PBS for anti-rabbit antibodies and normal horse serum diluted with PBS for anti-mouse antibodies) (Vectastain Elite ABC kit; Vector Laboratories) for 30 min and then incubated in primary antibody diluted with the blocking solution at 4°C overnight. After three washes in PBST, they were incubated in diluted biotinylated secondary antibody solution (PBS containing normal goat serum and anti-rabbit-IgG antibody or PBS containing normal horse serum and anti-mouse-IgG antibody) (Vectastain Elite ABC kit) for 30 min. Subsequent color development with the ABC and DAB reagents (Vector Laboratories) was performed according to the instructions of the manufacturer.
RESULTS
Generation and analysis of transgenic mice overexpressing Cdk5 driven by p35 promoter (TgCdk5 mice)
We first confirmed the genotypes of mice generated by the F2 cross. Figure 1B shows the genotype analysis of tail DNA from the WT and TgCdk5 mice by Southern blot analysis, in which the entire transgene was used as a probe. Additional bands derived from the transgene as well as the band derived from endogenous Cdk5 were revealed in the TgCdk5 mouse. Expression of mRNA and protein in the brain was confirmed by Northern and immunoblot analysis, respectively. There was a twofold to fourfold overexpression of Cdk5 mRNA and protein in the brains of TgCdk5 mice over WT mice (Fig.1C,D). The overexpression of Cdk5 in TgCdk5 did not influence the expression levels of p35 and p25, as shown in Figure1D.
The Cdk5 transgene is functional
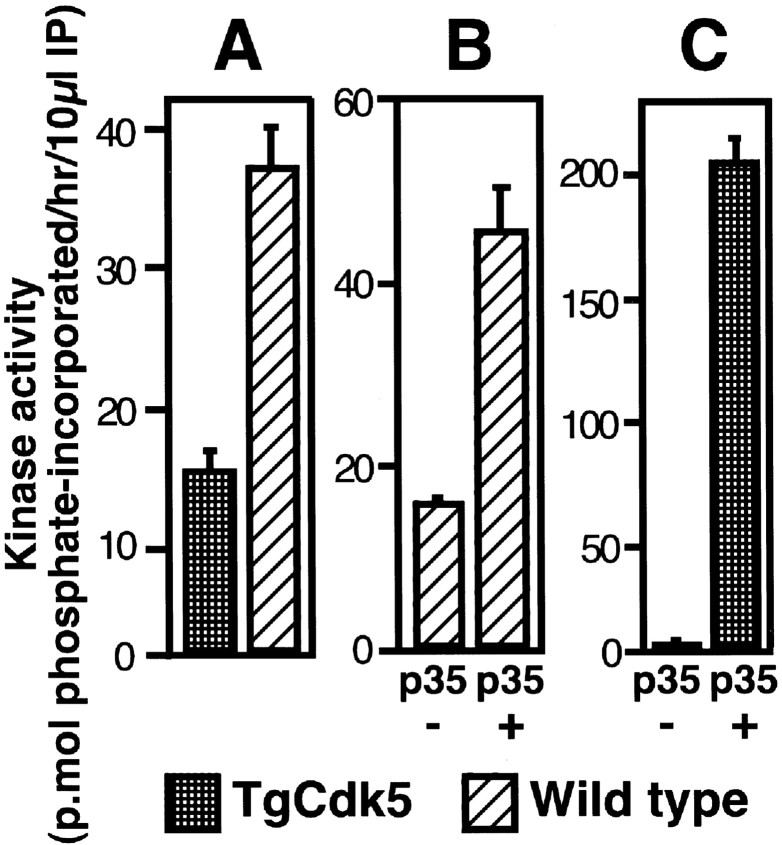
The level of Cdk5 kinase activity was determined in immunoprecipitates of Cdk5 from TgCdk5 and WT mouse brains. An unexpected reduction in Cdk5 kinase activity was observed in Cdk5 immunoprecipitates from TgCdk5 mice overexpressing Cdk5 (Fig.2A). To rule out a functionally inactive Cdk5 transgene product as a cause for the reduction in kinase activity in TgCdk5 mice, the in vitrokinase assays were performed after the addition of the bacterially expressed p35 protein to the kinase assay mixture containing Cdk5 immunoprecipitates from TgCdk5 and WT mice. There was a marked increase in Cdk5 activity upon addition of exogenous p35 to the kinase assay mixtures derived from both the TgCdk5 and WT mice, indicating that the product of the Cdk5 transgene was functional (Fig.2B,C). Addition of bacterially expressed p35 to the kinase assay mixture of WT mouse showed a 2.5-fold increase in kinase activity over the corresponding control without addition of exogenous p35 (Fig. 2B). A similar experiment performed using an immunoprecipitate from the TgCdk5 mouse revealed a 47-fold enhancement in the kinase activity upon addition of exogenous p35 (Fig. 2C). Thus, exogenous addition of p35 enhances Cdk5 activity in vitro, indicating that the transgenic Cdk5 protein is functional. Experiments performed by the exogenous addition of p25, an activator of Cdk5 derived from proteolytic cleavage of p35, yielded similar results (data not shown).
Fig. 2.
Cdk5 activity in TgCdk5 and WT brains.A, Analysis of the Cdk5 kinase activity in the Cdk5 immunoprecipitates from TgCdk5 versus WT mouse brains showing that the kinase activity of TgCdk5 mouse is 40% of the WT mouse.B, WT mouse in the absence and presence of added GST-p35. Note that the addition of p35 resulted in a 2.5-fold increase in kinase activity. C, TgCdk5 mouse in the absence and presence of GST-p35. Note that the exogenous addition of GST-p35 led to a dramatic increase in kinase activity by 47-fold.
Generation and analysis of transgenic mice in which Cdk5 is expressed only in tissues that express p35 (TgKO mice)
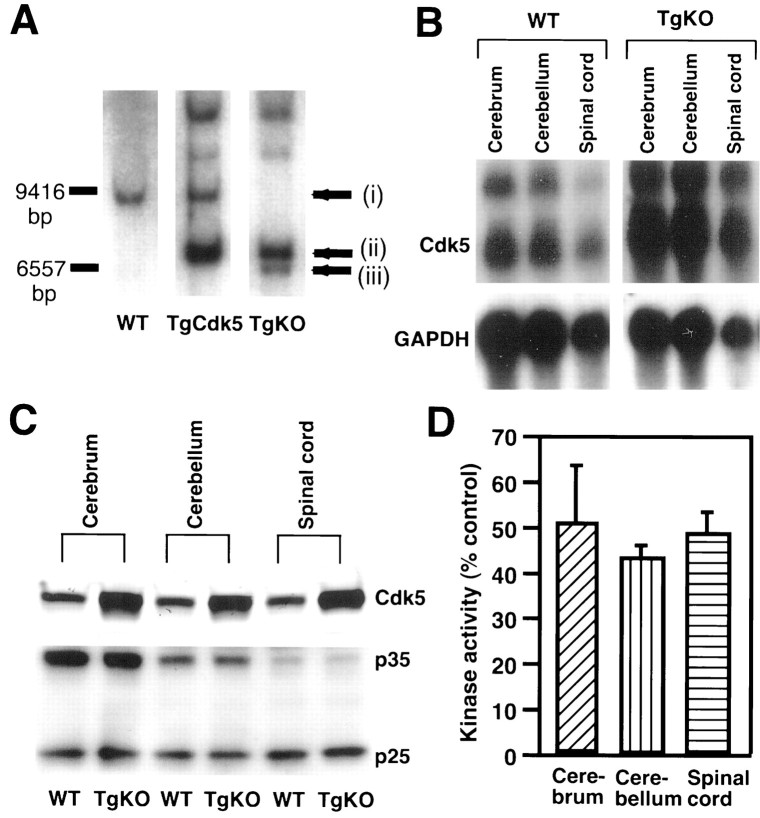
To determine whether expression of Cdk5 in p35-expressing areas was sufficient for reversing lethality of Cdk5 null mice, we crossed the TgCdk5 line to the Cdk5 knock-out line to generate mice lacking endogenous Cdk5 but with the Cdk5 transgene under the control of the p35 promoter. Of the F1 generation, the mice with a Cdk5 +/− and transgene +/− genotype were identified by Southern blot analysis. These mice were intercrossed and, of the F2 generation, the mice with the genotype of endogenous Cdk5 −/− and transgene +/+ (TgKO mice) were identified by Southern blot analysis. Figure3A shows additional bands derived from the transgene and the mutant Cdk5 allele, and lack of endogenous Cdk5-derived band in TgKO mouse. The TgKO mice were born with normal sex ratio. There was no difference in the number of the live newborn mice from that of the wild-type mice. They did not exhibit any developmental abnormalities. Analysis of mRNA from the TgKO mice showed a marked increase of Cdk5 in the cerebrum, cerebellum, spinal cord (Fig. 3B), and testis (data not shown). The heart, liver, kidney, ovary, and muscle from TgKO mice showed trace amounts of Cdk5 mRNA (data not shown). These findings are compatible with the fact that, in the adult mice, p35 is expressed selectively in neurons (Lew et al., 1994; Tsai et al., 1994; Matsushita et al., 1996; Tomizawa et al., 1996; Delalle et al., 1997; Zheng et al., 1998) and testis (T. Tanaka and A. B. Kulkarni, unpublished observations) and at extremely low levels in other tissues. Cdk5 protein was seen in regions in which p35 is normally expressed (Fig. 3C). The liver, kidney, and muscle showed minimal expression, the heart showed moderate expression, and the testis revealed a high level of Cdk5 protein expression (data not shown). Cdk5 mRNA levels corresponded to Cdk5 protein levels in all the organs analyzed except the heart, in which moderate levels of Cdk5 protein were present despite low Cdk5 mRNA levels, which is suggestive of either faster mRNA degradation or slower protein degradation. Although Cdk5 protein levels were higher in TgKO compared with WT mice (Fig. 3C), the kinase activity was ∼40–60% lower in TgKO compared with WT in cerebrum, cerebellum, and spinal cord (Fig. 3D). No activity was detectable in the other tissues analyzed, including liver, heart, kidney, and muscle. However, a negligible level of activity was observed in the testis (data not shown).
Fig. 3.
Generation of TgKO mice. A, Genotyping of tail DNA from WT, TgCdk5, and TgKO mouse by Southern blot analysis. In the TgKO mouse, a band (arrow i) from endogenous Cdk5 was missing, and additional bands derived from the transgene (arrow ii) and mutant allele (arrow iii) were revealed. B, Northern blot analysis of Cdk5 mRNA from cerebrum, cerebellum, and spinal cord of TgKO mouse and WT mouse. Cdk5 mRNA in these brain regions from the TgKO mouse is markedly increased. C, Western blot analysis of Cdk5 protein from TgKO mouse and WT mouse. Cdk5 was overexpressed in cerebrum, cerebellum, and spinal cord in TgKO compared with the WT mouse, whereas the p35 and p25 levels are similar in both the TgKO and WT mouse. D, Cdk5 kinase activity of TgKO mouse compared with the WT mouse. In the TgKO mouse, the level of Cdk5 activity in the cerebrum, cerebellum, and spinal cord is approximately half of the corresponding WT mouse. Note that the kinase activity in the cerebrum, cerebellum, and spinal cord of WT mouse is considered as 100%.
Spatial expression pattern of Cdk5 in the TgKO mouse brain
Because Cdk5 expression is under the control of a p35 promoter in TgKO mice, Cdk5 expression is expected only in p35-expressing regions. Therefore, we performed in situhybridization using 5-month-old TgKO mice and corresponding WT mice to analyze whether the pattern of Cdk5 transgene expression in TgKO mice was similar to that of the wild-type p35 expression pattern. Figure4 shows the results of in situhybridization with Cdk5 riboprobes. The antisense Cdk5 probe yielded high levels of signals in the adult CNS (Fig.4A,C,E), whereas sense probes revealed no specific hybridization (data not shown). A specific hybridization signal was present in perikarya of the neurons throughout the cerebral cortex (Fig. 4A), whereas a high level of Cdk5 mRNA expression was observed in hippocampal pyramidal cells and in granule cells in the dentate gyrus (Fig.4C). Granule cells and Purkinje cells in the cerebellum showed a moderate level of Cdk5 expression (Fig. 4E). Thus, the expression pattern of Cdk5 in the TgKO mouse was confined to the areas in which p35 is expressed.
Fig. 4.
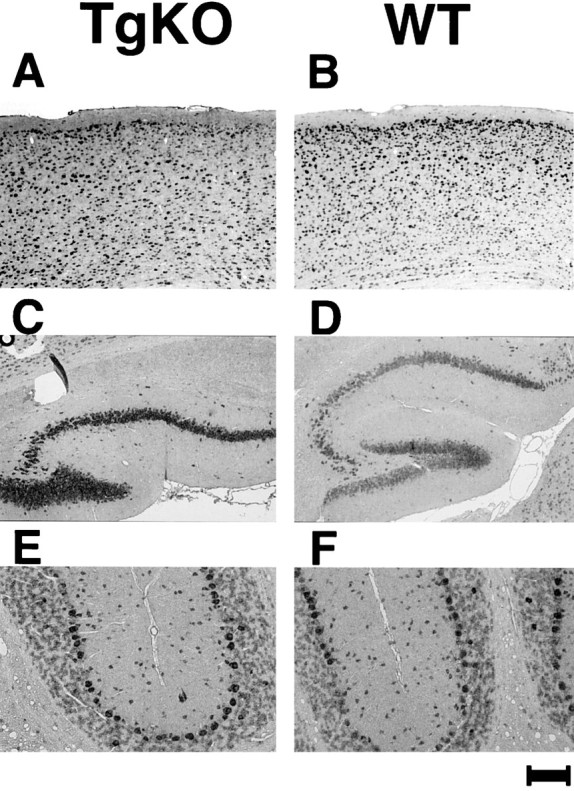
In situ hybridization of brains from 5-month-old TgKO mouse (A, C,E) and corresponding wild-type mouse (B,D, F) with DIG-labeled Cdk5 cRNA antisense probe. A, The signal was revealed in perikarya of the neurons throughout the cerebral cortex. C, A high level of Cdk5 expression was observed in hippocampal pyramidal cells and granule cells in dentate gyrus. E, The somata of the Purkinje cells were highly stained, and the granule cells and the molecular cells were stained moderately. Scale bar: A,B, 175 μm; C, D, 200 μm; E, F, 34 μm.
Transgenic Cdk5 protein is not expressed in astrocytes
Double immunocytochemistry was performed for TuJ1, GFAP, and Cdk5 in mixed cultures of astrocytes and neurons prepared from E17.5 embryos of WT and TgKO mice (Fig. 5) to confirm that Cdk5 expression in TgKO is restricted to p35-expressing cells in the nervous system. Cdk5 was expressed in astrocytes derived from WT mice (Fig. 5A,B) but was absent in astrocytes derived from TgKO mice (Fig.5C,D) as seen by double staining with GFAP, an astrocytic marker, and Cdk5. On the other hand, the double staining of neurons in these cultures with TuJ1 and Cdk5 revealed the expression of Cdk5 in neurons derived from both TgKO and the wild-type mice (Fig.5E–H). However, the level of Cdk5 expression was higher in the neurons derived from TgKO mice compared with the neurons derived from the WT mice (Fig. 5, compare A,G).
Fig. 5.
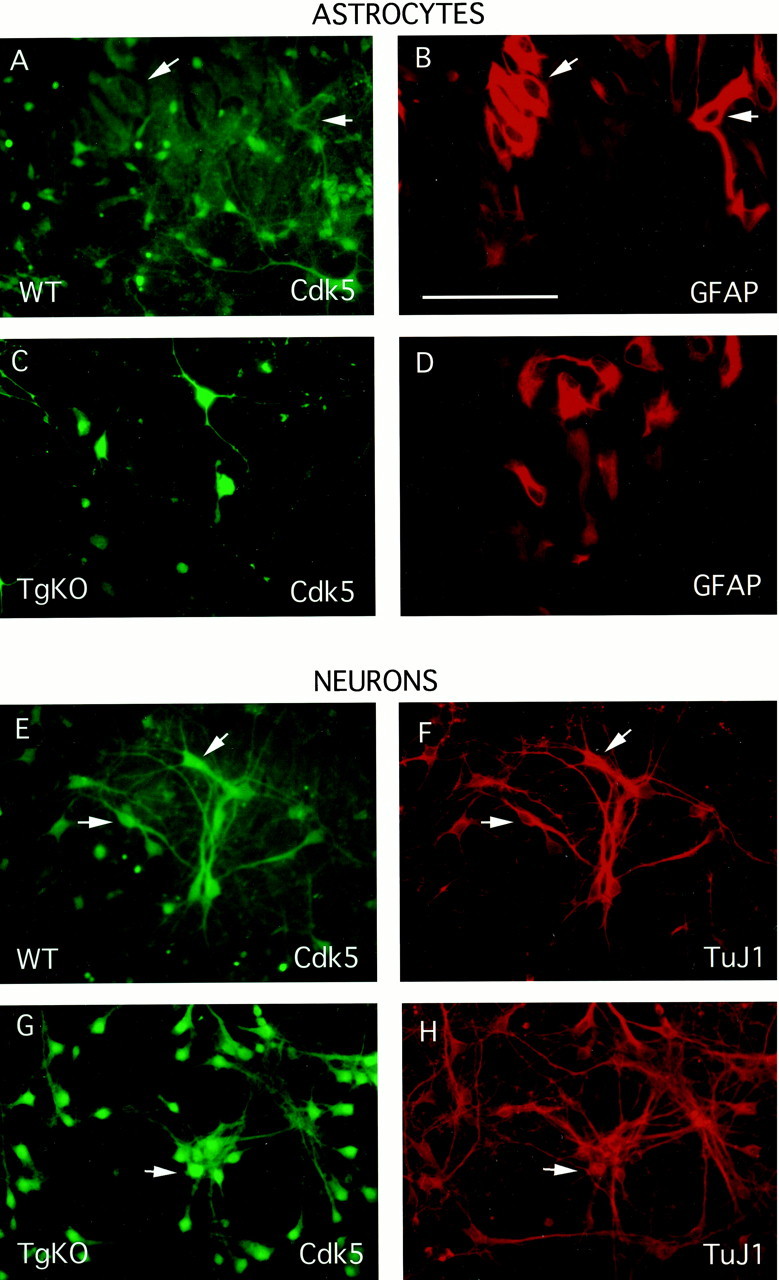
Mixed neuronal–astrocytic cultures were prepared from E17.5 WT and TgKO mice embryos as described by Vicario-Abejon et al. (1998), with minor modifications. After fixation with 4% PFA, double immunocytochemistry was performed on the cultures with either GFAP (an astrocytic marker) and Cdk5 or TuJ1 (a neuronal marker) and Cdk5. Panels on the left (FITC,green) show the staining pattern of Cdk5 with the corresponding GFAP or TuJ1 (rhodamine, red) staining to the right. WT mice show Cdk5 staining in neurons as well as astrocytes (A, B,E, F), whereas there is no Cdk5 seen in the astrocytes of TgKO mice (C,D, G, H). In addition, the levels of transgenic Cdk5 in TgKO neurons is higher than that seen in neurons derived from WT mice (compare E,G). Scale bar (in B), 100 μm.
Morphological analysis of brain development in TgKO mice
When the brains of Cdk5 null mice are analyzed perinatally, they reveal abnormal cortical layering, lack of cerebellar foliation, and ballooning of neurons in brainstem and spinal cord (Ohshima et al., 1996b). We analyzed the brains of E18.5 embryos from TgKO mice and WT mice. In contrast to the Cdk5 null embryos (Fig.6C,F), age-matched TgKO mice that express Cdk5 in p35-expressing regions show normal brain morphology with normal cortical layering and cerebellar foliation (Fig. 6A,D). There were no macroscopic differences in the brain between age-matched TgKO and WT adult mice. Nissl-stained coronal sections of the cerebral cortex from TgKO adult mouse showed a well-defined cortical layering pattern (Fig.6G), which was similar to that of WT mouse (Fig.6H). The hippocampal formation of the TgKO adult mouse was well organized, and the pyramidal cell layer and granule cell layer were clearly defined (data not shown). The cerebellum from the TgKO adult mouse had normal foliation (Fig. 6I), which was identical to that of WT (Fig. 6J). The Purkinje cell layer, molecular layer, and granular layer of the cerebellum from the TgKO mouse was clearly defined and well organized. Thionine-stained coronal serial frozen sections obtained from two sets of TgKO mice and one set of WT mouse at the ages of 2, 3, 5, and 7 months were examined. Coronal serial sections from each of the above aged brains revealed no abnormalities in cerebral and cerebellar cortical layering pattern in TgKO mice. By immunohistochemical analysis, cerebral cortical neurons, cerebellar Purkinje cells, and granule cells expressed Cdk5 when analyzed with anti-Cdk5 antibody (data not shown), which was identical to that of WT mice. Unlike Cdk5 null mice, TgKO mice did not display ballooned neurons in spinal cord and brainstem (data not shown). Thus, all of these data indicate that the TgKO mice are similar to the WT mice in morphological parameters analyzed in the CNS.
Fig. 6.
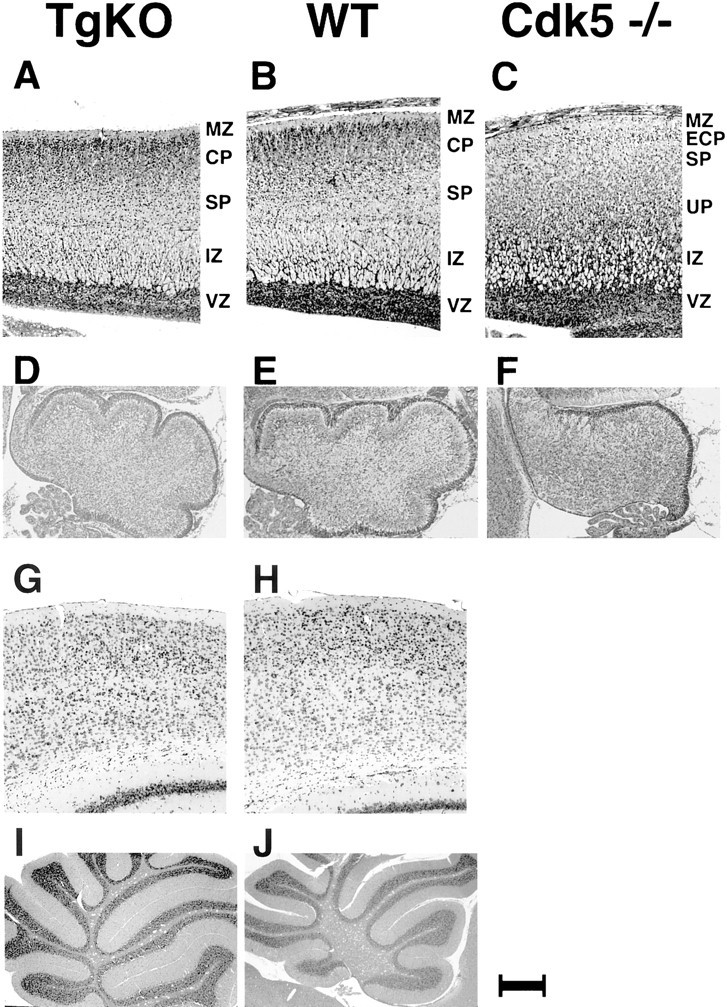
Histological analysis of TgKO mice brain compared with WT and Cdk5 −/− mouse brains from E18.5 embryos (A–F) and adult mice (G–J). A, The sagittal section of the cerebral cortex from the TgKO mouse embryo at E18.5. The thick cortical plate (CP) beneath the marginal zone (MZ) is clearly distinguished. Below the cortical plate, the subplate (SP) and the intermediate zone (IZ) are clearly recognized. B, The sagittal section of the cerebral cortex from the WT mouse embryo at E18.5. The cortex consists of the marginal zone (MZ), the cortical plate (CP), the subplate (SP), the intermediate zone (IZ), and the ventricular zone (VZ). C, The sagittal section of the cerebral cortex from the Cdk5 −/− mouse embryo at E18.5. The marginal zone (MZ) is followed by the early cortical plate (ECP), the subplate (SP), the underplate (UP), the intermediate zone (IZ), and the ventricular zone (VZ) (Gilmore et al., 1998). D, The cerebellum from the TgKO mouse embryo at E18.5 showing developmentally appropriate foliation.E, The cerebellum from the WT mouse embryo at E18.5 showing foliation for comparison. F, The cerebellum from the Cdk5 −/− E18.5 mouse embryo showing lack of foliation.G, Nissl-stained coronal section of the cerebral cortex from the TgKO adult mouse (5 months of age) showing a well defined cortical layering pattern. H, Nissl-stained coronal section of the cerebral cortex from the WT adult mouse (5 months of age). I, The sagittal section of the cerebellum from the TgKO adult mouse showing normal foliation. J, The sagittal section of the cerebellum from the WT adult mouse. Scale bar:A–C, 160 μm; D–F, 206 μm;G, H, 77 μm; I,J, 740 μm.
Aberrant phosphorylation of the cytoskeleton in Cdk5 null mice is corrected in TgKO mice
Cdk5 is involved in cytoskeletal protein phosphorylation and may lead to NF-tangle formation, a critical change associated with neurodegenerative diseases (Mandelkow et al., 1992; Baumann et al., 1993; Nakamura et al., 1997; Julien and Mushynski, 1998; Bajaj et al., 1999). A comparative study of the phosphorylation status of cytoskeletal elements shown previously to be phosphorylated by Cdk5 was performed with SMI-31 antibody. SMI-31 specifically reacts with phospho-epitopes of high-molecular weight NF, MAP, and tau proteins. Cdk5 null mice showed densely stained neuronal cell bodies in the brainstem, indicating a hyperphosphorylated status of the cytoskeleton resembling that seen in neurodegenerative disorders (Ohshima et al., 1996b). In contrast, TgKO mice (Fig.7A) did not reveal such changes but exhibited axonal staining patterns similar to the WT (Fig.7B). Thus, restoration of Cdk5 expression corrected the aberrant phosphorylation of cytoskeletal elements in the soma of brainstem neurons in TgKO mice.
Fig. 7.
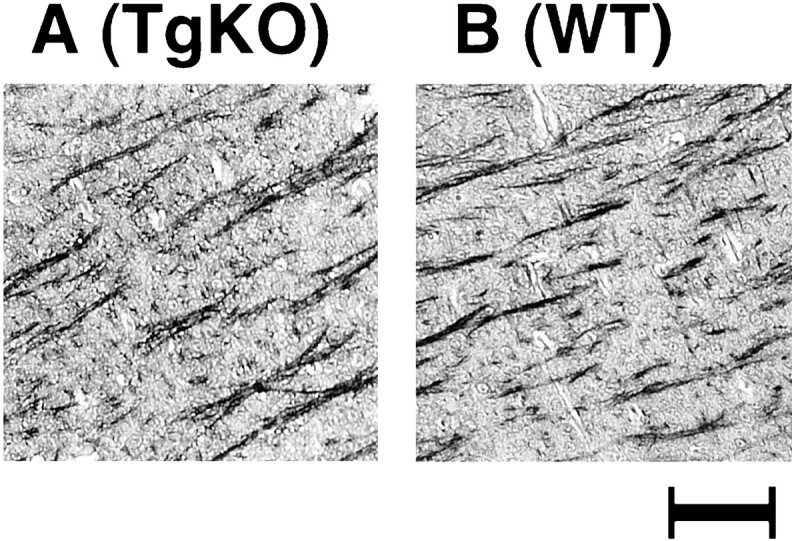
Comparative study of the neuronal phosphorylation in TgKO and WT mice. Sagittal sections of the brainstem region from the TgKO mouse embryo (A) and the WT mouse embryo (B) at E18.5, stained with phospho-epitope-specific monoclonal antibody (SMI-31). Note the resemblance between the TgKO (A) and the WT (B) mouse, showing similar axonal staining. Scale bar, 1 mm.
DISCUSSION
Targeted disruption of Cdk5 in mice leads to embryonic lethality and associated defects, such as abnormal cortical layering, lack of cerebellar foliation, and ballooning of neurons in brainstem and spinal cord (Ohshima et al., 1996b). Ballooning of neurons may result from hyperphosphorylation of cytoskeletal proteins in cell bodies, reminiscent of neurodegenerative disorders. Although neuronal death in vital brainstem regions may be responsible for lethality in Cdk5 null mice, the absence of Cdk5 and its activity in other tissues such as muscle and heart may also contribute to embryonic lethality. In this study, we sought to determine whether limited re-expression of Cdk5 in the nervous system was sufficient to reverse the abnormalities and lethality. Because expression of p35 is predominantly restricted to the nervous system, transgenic mice were generated in which a Cdk5 transgene was expressed under the control of a p35 promoter.
Cdk5 kinase activity in the brain from the TgCdk5 mice is lower than that of WT mice
Cdk5 activity was analyzed in whole-brain lysates from TgCdk5 mice that overexpress Cdk5. Unexpectedly, TgCdk5 lysates showed a markedly lower Cdk5 kinase activity compared with wild-type controls. Northern and Western blot analysis showed robust expression of Cdk5 mRNA and protein, ruling out lack of expression as a cause of decreased Cdk5 activity. To determine whether a relative deficiency of activator protein p35 caused the decreased Cdk5 activity, we added bacterially expressed p35 to the kinase assay mixture. Addition of p35 augmented Cdk5 activity in TgCdk5 mice by 47-fold compared with a 2.5-fold enhancement in wild-type mice. Hence, the lower level of Cdk5 activity in TgCdk5 mice is indeed attributable to relative deficiency of p35. The other possibility is that the higher level of transgenic Cdk5 protein may interfere with the transcription of p35 and reduce its production. This could lead to a decreased level of Cdk5 activity observed in TgCdk5 mice compared with the WT. Indeed, this is not true, because the Western blot analysis of p35 in TgCdk5 mice revealed no difference in the p35 protein levels compared with the WT mice. Together, our results confirm the active nature of the Cdk5 protein in TgCdk5 mice. These initial results were the basis for the generation of TgKO mice lacking endogenous Cdk5 but expressing transgenic Cdk5 under the control of p35 promoter.
Cdk5 is restricted to p35 regions in TgKO mice
Restricted expression of Cdk5 in the regions in which p35 is expressed helped us to understand the importance of Cdk5 in peripheral areas that do not express p35. Because astrocytes lack p35 (Tsai et al., 1994; Honjyo et al., 1999), our observation that astrocytes from TgKO mice lack Cdk5 is consistent with the expected results. TgKO mice clearly showed the Cdk5 mRNA transcript in the nervous system and testis. The Northern blot analysis also showed an additional band despite high-stringency wash conditions indicative of the fact that the Cdk5 cDNA probe hybridized with a closely related sequence. This transcript might represent an alternatively spliced Cdk5 or a transcript closely related to Cdk5. Analysis of Cdk5 kinase activity in cerebrum, cerebellum, and spinal cord of TgKO mice revealed a 60% reduction in kinase activity compared with the WT controls. This reduction in kinase activity is similar to that of TgCdk5 mice that show a decreased kinase activity, probably because of an autoinhibitory phenomenon caused by excess concentration of the Cdk5, competing for a limited amount of the activator protein p35. The absence of Cdk5 kinase activity in the peripheral tissues analyzed such as heart, in which Cdk5 is expressed, could be attributable to the absence of the activator p35. The low level of Cdk5 activity observed in the testis despite the presence of abundant quantity of both the Cdk5 and p35 proteins in TgKO mice strongly argues for the presence of an unknown inhibitor of Cdk5.
Cdk5 is a key molecule critical for survival and neuronal development
Cdk5 null mice are embryonic lethal, but p35 null mice survive with abnormal brain development (Chae et al., 1997; Kwon and Tsai, 1998). p35 and other activators such as p39, an isoform of p35 encoded on a separate gene, influence Cdk5 activity (Tang et al., 1995; Zheng et al., 1998). The lack of embryonic lethality of the p35 null mice suggests that p35 is not essential for embryonic survival, although p35 null mice still display neuronal migration defects. This is possibly attributable to the redundancy in the activator system, such as p39 expression. The level of Cdk5 kinase activity in the cerebral cortex and cerebellum of the p35 null mice is ∼5 and 20%, respectively, compared with the WT mice (Ohshima and Kulkarni, unpublished observations). This difference in the level of Cdk5 kinase activity in cerebrum and cerebellum is consistent with the level of p39 in the adult mice (Zheng et al., 1998). This might represent a minimal level of Cdk5 activity in the brain that may be necessary for survival but yet might be insufficient for normal neuronal migration because migration defects are seen in p35 null mice. The survival of p35 null mice also suggests that p39 by itself is capable of supporting the survival and may not be involved in neuronal migration defects observed in the p35 null mice. It is possible that disruption of both activators p35 and p39 would lead to lethality and phenotype similar to Cdk5 null mice because it may result in total loss of kinase activity. Our results with TgKO mice clearly support the indispensability of Cdk5 for survival. Our results also make it clear that the expression of Cdk5 in p35-expressing regions is sufficient for neuronal development and survival.
Cdk5 outside of the p35-expressing regions is not critical for survival
TgKO mice do not express Cdk5 in the liver, kidney, and ovary, among the organs analyzed, whereas WT mice have Cdk5 expression in such regions. On the other hand, the astrocytes in TgKO mice do not express Cdk5, whereas astrocytes from WT mice do express Cdk5. This suggests that neuronal expression of Cdk5 is sufficient for survival. Cdk5 outside of the p35-expressing regions does not seem to play a critical role for survival, although it may have more local actions in peripheral tissues. Although Cdk5 is expressed abundantly in the testis, the fact that the castrated animals survive rules out possible importance of its role in testis for survival. The heart of the Cdk5 null mice is normal. However, the functional significance of Cdk5 expression in the heart is yet unknown. Thus, these data indicate that Cdk5 expression in other tissues is either dispensable or has redundant functions with other Cdks.
Cdk5 plays a key role in modulation of cellular signals
Although a clear pathway delineating the upstream and downstream effectors of Cdk5 is yet to be uncovered, mounting evidence suggests that Cdk5 is a key molecule in mediating important signals involved in developmental and functional regulation of neurons. Integrins augment p35 expression, leading to enhanced Cdk5 activity facilitating neurite outgrowth (Pigino et al., 1997; Paglini et al., 1998). Brain-derived neurotrophic factor enhances Cdk5 activity leading to enhanced NF-H phosphorylation during synapse formation in cortical neurons (Tokuoka et al., 2000). Calcium-activated protease calpain cleaves p35 into p25 leading to augmented Cdk5 activity. This phenomenon has been implicated in apoptosis of neurons in culture (Kusakawa et al., 2000). Cdk5 is also reported to be involved in signaling in dopaminergic neurons, in which it phosphorylates DARPP-32 (dopamine and cAMP-regulated phospho-protein; relative molecular mass of 32,000) leading to an inhibition of PKA activity (Bibb et al., 1999). Interestingly, Cdk5 also downregulates the activity of protein phosphatase-I by phosphorylating inhibitor-I at serine 67 leading to its activation (Huang and Paudel, 2000). Cadherin-mediated cell adhesion is influenced by Cdk5 (Kwon et al., 2000), because the aggregation of cortical neurons is higher in the p35 null mice compared with the WT mice because of the absence of Cdk5–p35–β catenin complex. In muscle, Cdk5 is required for the activity of transcription factors responsible for the synthesis of muscle-specific proteins. Transfection of a dominant negative Cdk5 in myoblasts leads to failure of their development into myotubes (Lazaro et al., 1997). Together with our findings, Cdk5 plays a central role in mediating important physiological signals and it is irreplaceable in neurons.
Footnotes
We acknowledge Drs. Harold Gainer, Philip Grant, Pankaj Qasba, and Joseph G. Gleeson for critical reading of this manuscript, David M. Jacobowitz for his technical help with histology, and Shrihari Kadkol for his technical help with in situ hybridization.
Drs. Tanaka and Veeranna contributed equally to this work.
Correspondence should be addressed to Dr. Ashok B. Kulkarni, Functional Genomics Unit, National Institute of Dental and Craniofacial Research, National Institutes of Health, Building 30, Room 529, Bethesda, MD 20892. E-mail: ak40m@nih.gov.
Dr. Ohshima's present address: Laboratory of Developmental Biology, Brain Science Institute, RIKEN, 2–1 Hirosawa, Wako, Saitama, Japan.
REFERENCES
- 1.Bajaj NP, al-Sarraj ST, Leigh PN, Anderson V, Miller CC. Cyclin dependent kinase-5 (CDK-5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) and is present in affected motor neurones in ALS. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:833–850. doi: 10.1016/s0278-5846(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 2.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin- dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 3.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 4.Cai XH, Tomizawa K, Tang D, Lu YF, Moriwaki A, Tokuda M, Nagahata S, Hatase O, Matsui H. Changes in the expression of novel Cdk5 activator messenger RNA (p39nck5ai mRNA) during rat brain development. Neurosci Res. 1997;28:355–360. doi: 10.1016/s0168-0102(97)00063-1. [DOI] [PubMed] [Google Scholar]
- 5.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 6.Connell-Crowley L, Le Gall M, Vo DJ, Giniger E. The cyclin-dependent kinase cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol. 2000;10:599–602. doi: 10.1016/s0960-9822(00)00487-5. [DOI] [PubMed] [Google Scholar]
- 7.Delalle I, Bhide PG, Caviness VS, Jr, Tsai LH. Temporal and spatial patterns of expression of p35, a regulatory subunit of cyclin-dependent kinase 5, in the nervous system of the mouse. J Neurocytol. 1997;26:283–296. doi: 10.1023/a:1018500617374. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honjyo Y, Kawamoto Y, Nakamura S, Nakano S, Akiguchi I. Immunohistochemical localization of CDK5 activator p39 in the rat brain. NeuroReport. 1999;10:3375–3379. doi: 10.1097/00001756-199911080-00022. [DOI] [PubMed] [Google Scholar]
- 11.Huang KX, Paudel HK. Ser67-phosphorylated inhibitor 1 is a potent protein phosphatase 1 inhibitor. Proc Natl Acad Sci USA. 2000;97:5824–5829. doi: 10.1073/pnas.100460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol. 1998;61:1–23. doi: 10.1016/s0079-6603(08)60823-5. [DOI] [PubMed] [Google Scholar]
- 13.Kadkol SS, Gage WR, Pasternack GR. In situ hybridization: theory and practice. Mol Diagn. 1999;4:169–183. doi: 10.154/MODI00400169. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi S, Ishiguro K, Omori A, Takamatsu M, Arioka M, Imahori K, Uchida T. A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 1993;335:171–175. doi: 10.1016/0014-5793(93)80723-8. [DOI] [PubMed] [Google Scholar]
- 15.Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S. Calpain-dependent proteolytic cleavage of the p35 CDK5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- 16.Kwon YT, Tsai LH. A novel disruption of cortical development in p35(−/−) mice distinct from reeler. J Comp Neurol. 1998;395:510–522. doi: 10.1002/(sici)1096-9861(19980615)395:4<510::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YT, Gupta A, Zhou Y, Nikolic M, Tsai LH. Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr Biol. 2000;10:363–372. doi: 10.1016/s0960-9822(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 18.Lazaro JB, Kitzmann M, Poul MA, Vandromme M, Lamb NJ, Fernandez A. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J Cell Sci. 1997;110:1251–1260. doi: 10.1242/jcs.110.10.1251. [DOI] [PubMed] [Google Scholar]
- 19.Lew J, Winkfein RJ, Paudel HK, Wang JH. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem. 1992;267:25922–25926. [PubMed] [Google Scholar]
- 20.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 21.Mandelkow EM, Drewes G, Biernat J, Gustke N, Van Lint J, Vandenheede JR, Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992;314:315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita M, Tomizawa K, Lu YF, Moriwaki A, Tokuda M, Itano T, Wang JH, Hatase O, Matsui H. Distinct cellular compartment of cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activator protein (p35nck5a) in the developing rat cerebellum. Brain Res. 1996;734:319–322. [PubMed] [Google Scholar]
- 23.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura S, Kawamoto Y, Nakano S, Ikemoto A, Akiguchi I, Kimura J. Cyclin-dependent kinase 5 in Lewy body-like inclusions in anterior horn cells of a patient with sporadic amyotrophic lateral sclerosis. Neurology. 1997;48:267–270. doi: 10.1212/wnl.48.1.267. [DOI] [PubMed] [Google Scholar]
- 25.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T, Nagle JW, Pant HC, Joshi JB, Kozak CA, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse cyclin-dependent kinase 5 gene. Genomics. 1995;28:585–588. doi: 10.1006/geno.1995.1194. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima T, Kozak CA, Nagle JW, Pant HC, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse gene encoding cyclin-dependent kinase 5 regulatory subunit p35. Genomics. 1996a;35:372–375. doi: 10.1006/geno.1996.0370. [DOI] [PubMed] [Google Scholar]
- 28.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996b;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paglini G, Pigino G, Kunda P, Morfini G, Maccioni R, Quiroga S, Ferreira A, Caceres A. Evidence for the participation of the neuron-specific CDK5 activator P35 during laminin-enhanced axonal growth. J Neurosci. 1998;18:9858–9869. doi: 10.1523/JNEUROSCI.18-23-09858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pant AC, Veeranna, Pant HC, Amin N. Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5). Brain Res. 1997;765:259–266. doi: 10.1016/s0006-8993(97)00561-1. [DOI] [PubMed] [Google Scholar]
- 31.Philpott A, Porro EB, Kirschner MW, Tsai LH. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev. 1997;11:1409–1421. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- 32.Pigino G, Paglini G, Ulloa L, Avila J, Caceres A. Analysis of the expression, distribution and function of cyclin dependent kinase 5 (cdk5) in developing cerebellar macroneurons. J Cell Sci. 1997;110:257–270. doi: 10.1242/jcs.110.2.257. [DOI] [PubMed] [Google Scholar]
- 33.Sharma P, Sharma M, Amin ND, Albers RW, Pant HC. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc Natl Acad Sci USA. 1999;96:11156–11160. doi: 10.1073/pnas.96.20.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty KT, Kaech S, Link WT, Jaffe H, Flores CM, Wray S, Pant HC, Beushausen S. Molecular characterization of a neuronal-specific protein that stimulates the activity of Cdk5. J Neurochem. 1995;64:1988–1995. doi: 10.1046/j.1471-4159.1995.64051988.x. [DOI] [PubMed] [Google Scholar]
- 36.Sreenath TL, Cho A, MacDougall M, Kulkarni AB. Spatial and temporal activity of the dentin sialophosphoprotein gene promoter: differential regulation in odontoblasts and ameloblasts. Int J Dev Biol. 1999;43:509–516. [PubMed] [Google Scholar]
- 37.Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 38.Tokuoka H, Saito T, Yorifuji H, Wei F, Kishimoto T, Hisanaga S. Brain-derived neurotrophic factor-induced phosphorylation of neurofilament-H subunit in primary cultures of embryo rat cortical neurons. J Cell Sci. 2000;113:1059–1068. doi: 10.1242/jcs.113.6.1059. [DOI] [PubMed] [Google Scholar]
- 39.Tomizawa K, Matsui H, Matsushita M, Lew J, Tokuda M, Itano T, Konishi R, Wang JH, Hatase O. Localization and developmental changes in the neuron-specific cyclin-dependent kinase 5 activator (p35nck5a) in the rat brain. Neuroscience. 1996;74:519–529. doi: 10.1016/0306-4522(96)00136-4. [DOI] [PubMed] [Google Scholar]
- 40.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 41.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 42.Veeranna, Shetty KT, Amin N, Grant P, Albers RW, Pant HC. Inhibition of neuronal cyclin-dependent kinase-5 by staurosporine and purine analogs is independent of activation by Munc-18. Neurochem Res. 1996;21:629–636. doi: 10.1007/BF02527763. [DOI] [PubMed] [Google Scholar]
- 43.Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]