Abstract
NMDA receptors, an ionotropic subtype of glutamate receptors (GluRs) forming high Ca2+-permeable cation channels, are composed by assembly of the GluRζ subunit (NR1) with any one of four GluRε subunits (GluRε1–4; NR2A-D). In the present study, we investigated neuronal functions in mice lacking the GluRε1 subunit. GluRε1 mutant mice exhibited a malfunction of NMDA receptors, as evidenced by alterations of [3H]MK-801 binding as well as 45Ca2+ uptake through the NMDA receptors. A postmortem brain analysis revealed that both dopamine and serotonin metabolism were increased in the frontal cortex and striatum of GluRε1 mutant mice. The NMDA-stimulated [3H]dopamine release from the striatum was increased, whereas [3H]GABA release was markedly diminished in GluRε1 mutant mice. When (+)bicuculline, a GABAA receptor antagonist, was added to the superfusion buffer, NMDA-stimulated [3H]dopamine release was significantly increased in wild-type, but not in the mutant mice. GluRε1 mutant mice exhibited an increased spontaneous locomotor activity in a novel environment and an impairment of latent learning in a water-finding task. Hyperlocomotion in GluRε1 mutant mice was attenuated by treatment with haloperidol and risperidone, both of which are clinically used antipsychotic drugs, at doses that had no effect in wild-type mice. These findings provide evidence that NMDA receptors are involved in the regulation of behavior through the modulation of dopaminergic and serotonergic neuronal systems. In addition, our findings suggest that GluRε1 mutant mice are useful as an animal model of psychosis that is associated with NMDA receptor malfunction and hyperfunction of dopaminergic and serotonergic neuronal systems.
Keywords: NMDA receptor, GluRε1 subunit, dopaminergic neuronal system, serotonergic neuronal system, hyperlocomotion, schizophrenia
NMDA receptors, a subtype of glutamate receptors (GluRs), play an important role in excitatory neurotransmission, synaptic plasticity, and brain development. They are inherent ligand-gated cation channels with high Ca2+ permeability, which are composed by assembly of the GluRζ subunit (NR1) with any one of four GluRε subunits (GluRε1–4; NR2A-D). Although the GluRζ subunit exists in the brain at all developmental stages, GluRε subunits are expressed in distinct temporal and spatial patterns (Mayer and Westbrook, 1987;Hollmann and Heinemann, 1994; Nakanishi and Masu, 1994).
The diverse functions of NMDA receptor subunits have been demonstrated in mice lacking particular subunits by a gene-targeting recombination technique. GluRζ mutant mice showed a deficit of all NMDA receptors and perinatal death (Forrest et al., 1994; Li et al., 1994), suggesting that the GluRζ subunit is an essential molecule in NMDA receptors and in brain development. Perinatal death was also found in GluRε2 mutant mice (Kutsuwada et al., 1996) but not in GluRε4 mutant mice that showed a reduced spontaneous activity (Ikeda et al., 1995). Mice lacking postnatal GluRε1 or GluRε3 are viable: impairments of hippocampal long-term potentiation (LTP) and spatial learning were observed in GluRε1 mutant mice (Sakimura et al., 1995), whereas GluRε3 mutant mice exhibited no deficiencies (Ebralidze et al., 1996). These findings suggest that GluRε subunits are major determinants of the functional roles of NMDA receptors.
Pharmacological studies have revealed that blockade of NMDA receptorsin vivo causes behavioral abnormalities accompanied by the functional alteration of monoaminergic neuronal systems. For example, noncompetitive NMDA receptor antagonist MK-801 or phencyclidine (PCP) induces characteristic behavioral syndromes in animals, including hyperlocomotion and stereotypy, which are accompanied by an increase in dopaminergic and serotonergic neuronal activities in various brain regions (Hiramatsu et al., 1989; Loscher et al., 1991). Genetic evidence has recently been obtained in mice with reduced GluRζ subunit (Mohn et al., 1999). The mutant mice display behavioral abnormalities, which are similar to those observed in rodents treated with NMDA receptor antagonists. The behavioral alterations in the mutant mice were ameliorated by treatment with haloperidol or clozapine, antipsychotic drugs that block dopaminergic and serotonergic receptors. Accordingly, the mutant mice with reduced GluRζ subunit are proposed as a new animal model for schizophrenia, which has been hypothesized to be associated with NMDA receptor dysfunction (Javitt and Zukin, 1991; Tamminga, 1998) and the hyperfunction of dopaminergic and serotonergic neuronal systems (Seeman et al., 1976; Meltzer, 1991).
In the present study, we investigated the alteration of neuronal functions in mice lacking the GluRε1 subunit. The [3H]MK-801 binding and the45Ca2+ uptake through the NMDA receptors were measured to examine the functional alterations of NMDA receptors in GluRε1 mutant mice. To demonstrate the modulatory effect of NMDA receptors on monoaminergic neuronal systems, monoamine metabolism was evaluated from the tissue contents of monoamines and their metabolites. The NMDA-stimulated [3H]dopamine and [14C]serotonin releases were also measured in GluRε1 mutant mice. Finally, we assessed the behavioral alteration and the effects of neuroleptics on this alteration in GluRε1 mutant mice.
MATERIALS AND METHODS
Animals. Mutant mice lacking the GluRε1 subunit of NMDA receptors were provided by Sakimura et al. (1995). The homozygous GluRε1 mutant mice (−/−; 3-months-old) and the wild-type mice (+/+; 3-months-old) used in this study were obtained by crossing F13 heterozygous GluRε1 mutant mice (+/−) having a 99.99% pure C57BL/6 genetic background. The genotypes of mice were determined by tail biopsy and PCR, using primers E1P1, 5′-TCTGGGGCCTGGTCTTCAACA-ATTCTGTGC-3′ (the nucleotide residues 1766–1795 of GluRε1 cDNA), E1P2, 5′-CTTCTTGTCACTGAGGCCAGTCACTTGGTC-3′ (complementary to the residues 1921–1950), and NeoP1, 5′-GCCTGCTTGCCGAATATCATGGTGGAAAAT-3′. The animals were housed in plastic cages and were kept in a regulated environment (24 ± 1°C, 50 ± 5% humidity), with a 12 hr light/dark cycle (lights on at 9:00 A.M.). Food and tap water were available ad libitum. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Nagoya University School of Medicine. The procedures involving animals and their care were conducted in conformity with the international guidelines Principles of Laboratory Animal Care (National Institutes of Health publication 85–23, revised 1985).
[3H]MK-801 binding. The GluRε1 mutant mice and the wild-type mice were killed by decapitation, and brains were quickly removed and placed on an ice-cold glass plate. The forebrain (minus the cerebellum and brainstem) was rapidly dissected out, frozen, and stored in a deep freezer at −80°C until assayed. [3H]MK-801 binding was measured as described previously (Yoneda and Ogita, 1989, 1991), with a minor modification. Briefly, frozen samples were thawed at room temperature and homogenized in 40 volumes of 50 mm Tris-acetate buffer, pH 7.4, containing 1 mm EDTA using a Physcotron homogenizer. All further procedures were performed at 4°C. The homogenates were centrifuged at 40,000 × g for 30 min, and resultant pellets were washed three times with the same volume of 50 mm Tris-acetate buffer, pH 7.4. The final pellets were suspended in 30 volumes of 0.32 m sucrose, and the suspensions were frozen at −80°C for no longer than 1 week until use. On the day of the experiments, the frozen suspensions were thawed at room temperature and treated with 0.08% Triton X-100 at 4°C (an approximate protein concentration of 0.32 mg/ml) for 10 min with gentle stirring. The treatment was terminated by centrifugation at 40,000 × g for 30 min, and pellets were washed five times with 40 volumes of 50 mm Tris-acetate buffer, pH 7.4, followed by centrifugation at 40,000 ×g for 30 min. For determination of [3H]MK-801 binding, an aliquot (0.3 mg of protein) of the membrane preparations was incubated, in the presence or absence of glutamate (10 μm), glycine (10 μm), and spermidine (1 mm), with 5 nm(+)[3-3H]MK-801 (22.5 Ci/mmol; NEN Life Science Products, Boston, MA) in a total volume of 0.5 ml of 50 mm Tris-acetate buffer, pH 7.4, at 30°C for 16 hr. The incubation was terminated by the addition of 3 ml of ice-cold 50 mm Tris-acetate buffer and subsequent filtration through a Whatman GF/B glass filter under a constant vacuum. The filter was rinsed with the same volume of ice-cold 50 mm Tris-acetate buffer three times within 10 sec. Radioactivity retained on the filter was measured by liquid scintillation spectrophotometry, at a counting efficiency of 57–59%. Nonspecific binding was defined with 0.1 mm cold (+)MK-801 (Sigma, St. Louis, MO), and the specific binding accounted for >60% of the total binding found in the absence of cold (+)MK-801.
45Ca2+uptake. The GluRε1 mutant mice and the wild-type mice were killed by decapitation, the brains were quickly removed, and the forebrain was dissected out on an ice-cold glass plate. The forebrains were homogenized in 20 volumes of ice-cold 0.32 msucrose at 4°C in a Teflon glass homogenizer. All further procedures were performed at 4°C. The homogenates were centrifuged at 1000 × g for 10 min. The supernatants were collected and then diluted 1:1 with basal buffer of the following composition (in mm): 135 NaCl, 5 KCl, 1 CaCl2, and 10 HEPES, pH-adjusted to 7.4 with Tris base, and centrifuged at 10,000 × g for 15 min. The pellets were resuspended in basal buffer and used for the45Ca2+ uptake assay. The synaptosome suspension (0.5 mg of protein) was preincubated in a total volume of 450 μl of basal buffer, in the presence or absence of (+)MK-801 (100 μm), at 37°C for 10 min. The45Ca2+ uptake assay was initiated by adding 50 μl of prewarmed basal buffer containing 1 μCi/ml45CaCl2 (18.1 mCi/mg; NEN Life Science Products), in the presence or absence of NMDA (100 μm), glycine (10 μm), and spermidine (1 mm) or high K+ (45 mm; isomolar replacement of NaCl with KCl). The reaction was terminated after 5 min by adding 3 ml of ice-cold basal buffer. The mixture was rapidly filtered under vacuum over Whatman GF/B glass filters, and the filters were rinsed twice with 3 ml of basal buffer. The radioactivity was determined by liquid scintillation spectrophotometry at a counting efficiency of 90%. Ca2+ uptake was defined by subtracting the uptake at 4°C.
Monoamine metabolism. The GluRε1 mutant mice and the wild-type mice were killed by focused microwave irradiation for 1.5 sec at 5 kW, the brains were quickly removed, and the prefrontal cortex, striatum, hippocampus, and thalamus were dissected out on an ice-cold glass plate according to the method of Glowinski and Iversen (1966). Each brain region was rapidly frozen and stored in a deep freezer at −80°C until assayed. The contents of monoamines and their metabolites were determined using an HPLC system with an electrochemical detector (Eicom, Kyoto, Japan), as described by Noda et al. (1998). Briefly, each frozen brain sample was weighed and homogenized with an ultrasonic processor in 350 μl of 0.2m perchloric acid containing isoproterenol as an internal standard. The homogenates were placed in ice for 30 min and centrifuged at 20,000 × g for 15 min at 4°C. The supernatants were mixed with 1 m sodium acetate to adjust the pH to 3.0 and injected into an HPLC system equipped with a reversed-phase ODS column (Eicompak MA-5 ODS; 4.6 × 150 mm; Eicom) and an electrochemical detector. The column temperature was maintained at 25°C, and the detector potential was set at +750 mV. The mobile phase was 0.1 m citric acid and 0.1m sodium acetate, pH 3.6, containing 14% methanol, 180 mg/l sodium-l-octanesulfonate and 5 mg/l EDTA, and the flow rate was set at 1 ml/min. The turnover of monoamines was calculated from the content of each monoamine and their metabolites.
[3H]Dopamine, [14C]serotonin, and [3H]GABA release. The frontal cortex and striatum in the GluRε1 mutant and the wild-type mice were dissected and sliced in two directions at a thickness of 300 μm in a McIlwain tissue chopper (Yamada et al., 1993). The cortical and striatal slices were incubated at 37°C for 30 min in 2.5 ml of Krebs'–Ringer's solution buffer containing 1 μm[3H]dopamine (60.0 Ci/mmol; NEN Life Science Products), 1 μm[14C]serotonin (52.3 mCi/mmol; NEN Life Science Products), and 10 μm pargyline (Sigma). For [3H]GABA release, the striatal slices were incubated in Krebs'–Ringer's solution buffer containing 1 μm[3H]GABA (36.2 Ci/mmol; NEN Life Science Products) and 100 μm amino-oxyacetic acid (Sigma). The composition of the Krebs'–Ringer's solution buffer was (in mm): 125 NaCl, 4.8 KCl, 25 NaHCO3, 1.2 KH2PO4, 1.2 CaCl2, 10 glucose, and 0.57 ascorbic acid, gassed with 95% O2 and 5% CO2. After three washes, the cortical and striatal slices were transferred to superfusion chambers and superfused with Krebs'–Ringer's solution buffer at a rate of 0.5 ml/min. After 60 min of superfusion (t = 60 min), 25 successive 2 min fractions were collected (t = 60 min to t = 110 min). The cortical and striatal slices were exposed to two separate 2 min stimuli with Krebs'–Ringer's solution buffer containing high K+ (25 mm; isomolar replacement of NaCl with KCl) starting at t = 70 min and Krebs'–Ringer's solution buffer containing NMDA (100 μm) starting at t = 90 min. After the superfusion, the radioactive material remaining in the tissue slices was extracted with 0.1 m HCl. The radioactivity was then determined by liquid scintillation spectrophotometry, followed by the fractional efflux rate of each fraction.
The NMDA-stimulated [3H]dopamine release from striatal slices was also measured in the presence of (+)bicuculline, a GABAA receptor antagonist. The [3H]dopamine-labeled striatal slices were superfused with Krebs'–Ringer's solution buffer containing 10 μm (+)bicuculline (Sigma) until the end of the experiment. After 60 min of superfusion, the striatal slices were stimulated by NMDA (100 μm) for 2 min starting att = 90 min, as described above.
Behavioral analyses. To measure locomotor activity in a novel environment, a mouse was placed in a transparent acrylic cage with a black frosting Plexiglas floor (45 × 26 × 40 cm), and locomotion and rearing were measured every 5 min for 120 min using digital counters with infrared sensors (Scanet SV-10; Toyo Sangyo, Toyama, Japan).
The water-finding task was performed as described previously (Ichihara et al., 1989, 1993; Nabeshima and Ichihara, 1993). Briefly, the apparatus consisted of an open field (30 × 50 × 15 cm) with an alcove (10 × 10 × 10 cm) in the middle of one of the long walls of the enclosure. The inside was painted gray, and the floor of the open field was divided into 15 identical squares with black lines. A drinking tube, identical to that used in the home cage, was inserted into the center of the alcove ceiling with its tip 5 cm (in the training trial) or 7 cm (in the test trial) above the floor. The task consisted of two trials; a training trial (the 1st day) and a test trial (the 2nd day). In the training trial, a mouse not deprived of water was placed in one corner of the open field and allowed to freely explore the training apparatus for 3 min. During this time, the frequency of touching, sniffing, or licking of the drinking tube in the alcove (number of approaches) was recorded. It should be noted that water was not delivered from the drinking tube in the training trial. Animals that did not begin exploring within 3 min or did not make contact with the drinking tube during exploration were omitted from the test trial. One of 13 GluRε1 mutant mice tested was excluded because of this criterion, whereas none of wild-type mice (n = 12) were excluded. The mouse was immediately returned to the home cage after the training trial and was deprived of water for 24 hr before the test trial. Nontrained mice were prepared for comparison with the trained mice in terms of their ability to find the water source in the same apparatus. In the test trial, the trained mouse or a nontrained mouse was placed in the same corner of the test apparatus. The time until the mouse moved out of the corner and the time until the mouse entered the alcove were measured as the starting latency and the entering latency, respectively. In addition, the time between entering the alcove and drinking the water (finding latency) was measured. Thus, latent learning was assessed by recording the number of approaches in the training trial and starting, entering, and finding latencies in the test trial.
Drugs. Haloperidol was purchased from Sigma. Risperidone was supplied by Janssen Kyowa (Tokyo, Japan). Haloperidol and risperidone were dissolved in distilled water containing two equivalent volumes of tartaric acid. Haloperidol (0.003 or 0.01 mg/kg, p.o.) or risperidone (0.01 or 0.03 mg/kg, p.o.) was administered 60 min before the measurement of locomotor activity in a novel environment.
Statistical analysis. All data were expressed as the mean ± SEM. Statistical differences between the GluRε1 mutant and the wild-type mice were determined with Student's tcomparison test. In the analysis of locomotion and rearing curves, statistical differences between the GluRε1 mutant and the wild-type mice were determined by an ANOVA with repeated measures. In the behavioral analysis using pharmacological agents, statistical differences among values for individual groups were determined by ANOVA, followed by the Student–Newmann–Keuls multiple comparisons test when F ratios were significant (p < 0.05).
RESULTS
Function of NMDA receptors in GluRε1 mutant mice
A previous study has shown that NMDA receptor channel current and LTP in the hippocampal CA1 region are reduced in GluRε1 mutant mice (Sakimura et al., 1995). These findings suggest that the targeted disruption of the GluRε1 subunit gene results in an impairment of NMDA receptor function. To demonstrate the functional alterations of NMDA receptors in GluRε1 mutant mice, we first performed a radioligand-binding assay using a noncompetitive NMDA receptor antagonist, [3H]MK-801 (Fig.1). The binding of [3H]MK-801 was determined in forebrain synaptic membranes treated with Triton X-100 to deplete endogenous amino acids (Yoneda and Ogita, 1989, 1991). There was no difference in the basal specific binding of [3H]MK-801 between wild-type and GluRε1 mutant mice. The specific binding of [3H]MK-801 in both wild-type and GluRε1 mutant mice was markedly increased when the assay was performed in the presence of 10 μm glutamate, glutamate plus 10 μm glycine, or glutamate plus glycine plus 1 mm spermidine. Under the stimulated conditions, there was significantly less specific binding of [3H]MK-801 in GluRε1 mutant mice than wild-type mice. The addition of glycine or spermidine alone did not change the [3H]MK-801 binding in either of the mice (data not shown).
Fig. 1.
[3H]MK-801 binding in forebrain synaptic membranes of GluRε1 mutant mice. Triton-treated forebrain synaptic membranes were incubated with 5 nm[3H]MK-801 at 30°C for 16 hr, in the presence or absence of 10 μm glutamate (Glu), Glu plus 10 μm glycine (Gly), or Glu plus Gly plus 1 mm spermidine (SPD). Each column represents the mean ± SEM (n = 4). *p < 0.05; **p < 0.01 versus wild (+/+).
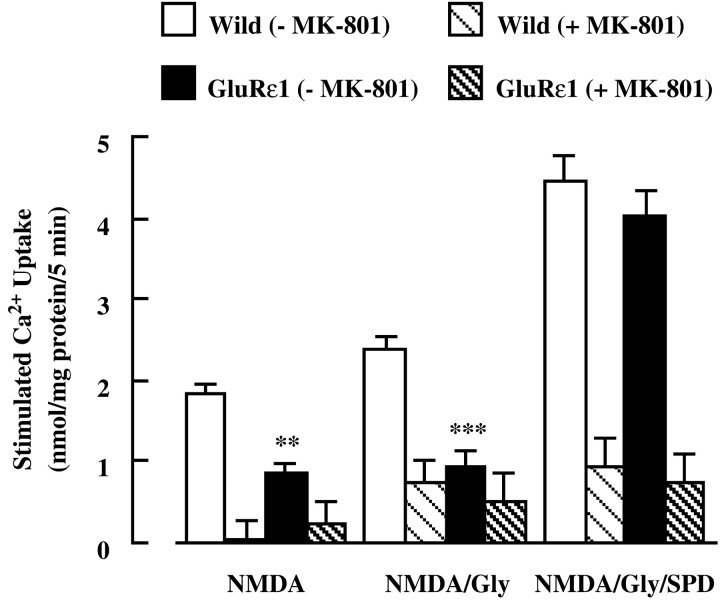
We next measured45Ca2+ uptake into forebrain synaptosomes through NMDA receptors (Fig.2). There was no difference in the basal45Ca2+ uptake into synaptosomes between wild-type and GluRε1 mutant mice, the value being 12.7 ± 0.1 and 13.2 ± 0.2 nmol/mg of protein per 5 min, respectively. When the assay was performed in the presence of 100 μm NMDA, NMDA plus 10 μm glycine, or NMDA plus glycine plus 1 mm spermidine,45Ca2+ uptake was increased in both groups. However,45Ca2+ uptake in GluRε1 mutant mice was significantly lower than that in wild-type mice under the stimulated conditions with NMDA or NMDA plus glycine. There was no difference in45Ca2+ uptake between the two groups when the assay was performed in the presence of NMDA, glycine, and spermidine. The NMDA, glycine, and/or spermidine-stimulated45Ca2+ uptake in both groups was antagonized by the addition of 100 μmMK-801. In contrast, there was no difference in high K+ (45 mm)-stimulated45Ca2+ uptake between wild-type (6.5 ± 0.2 nmol/mg of protein per 5 min) and GluRε1 mutant mice (6.1 ± 0.3 nmol/mg of protein per 5 min). These results on the [3H]MK-801 binding and the NMDA-stimulated45Ca2+ uptake suggest the malfunction of NMDA receptors in GluRε1 mutant mice.
Fig. 2.
NMDA-stimulated45Ca2+ uptake into forebrain synaptosomes of GluRε1 mutant mice. The forebrain synaptosomes were preincubated at 37°C for 10 min, in the presence or absence of 100 μm MK-801. The assay was initiated by adding prewarmed buffer containing 1 μCi/ml 45CaCl2 for 5 min, in the presence of 100 μm NMDA, NMDA plus 10 μm Gly, or NMDA plus Gly plus 1 mm SPD. Each column represents the mean ± SEM (n = 6). **p < 0.01, ***p < 0.001 versus wild (−MK-801).
Monoaminergic neuronal activities in GluRε1 mutant mice
To investigate whether the targeted disruption of the GluRε1 subunit gene would affect the function of monoaminergic neuronal systems, monoamine metabolism was assessed from the tissue contents of monoamines and their metabolites in various regions of the brain in GluRε1 mutant mice. As shown in Figure3, A and B, the ratios of homovanillic acid (HVA) to dopamine (DA) in the frontal cortex and of 3,4-dihydroxyphenylacetic acid (DOPAC) to DA in the striatum were significantly increased to 125.4 and 124.9%, respectively, in GluRε1 mutant mice compared with in wild-type mice. Moreover, both in the frontal cortex and striatum, the ratio of 5-hydroxyindoleacetic acid (5-HIAA) to serotonin (5-HT) was increased in GluRε1 mutant mice, to 126.1 and 125.4%, respectively. On the other hand, the ratio of 3-methoxy-4-hydroxyphenylglycol (MHPG) to norepinephrine (NE) was decreased in the hippocampus of GluRε1 mutant mice (Fig. 3C). No changes in monoamine metabolism were observed in the thalamus (Fig. 3D).
Fig. 3.
Monoamine metabolism in various brain regions of GluRε1 mutant mice. The tissue contents of monoamine and its metabolite in various brain regions were measured by HPLC with an electrochemical detector. a, MHPG/NE; b,DOPAC/DA; c, HVA/DA; d, 5-HIAA/5-HT. Each column represents the mean ± SEM (n = 7–8). *p < 0.05; **p < 0.01 versus wild (+/+).
We next examined whether [3H]DA and [14C]5-HT release induced by the activation of NMDA receptors is altered in GluRε1 mutant mice (Fig.4). The 100 μmNMDA-stimulated [3H]DA release from striatal slices was significantly increased in GluRε1 mutant mice, whereas no change in basal release was observed (Fig.4B). The [3H]DA release from cortical slices of the mutant mice did not differ from that in wild-type mice (data not shown). The NMDA-stimulated [14C]5-HT release from cortical slices, but not striatal slices (data not shown), showed a tendency to increase in GluRε1 mutant mice (Fig. 4A; p = 0.067). There was no difference in high K+(25 mm)-stimulated [3H]DA and [14C]5-HT release between wild-type and GluRε1 mutant mice (data not shown). To clarify the mechanism of the enhanced NMDA-stimulated [3H]DA release in the striatum of GluRε1 mutant mice, we examined the role of the GABAergic neuronal system because it has been demonstrated that GABAergic neurons exert an inhibitory effect on the NMDA-evoked DA release in the striatum (Krebs et al., 1993). The NMDA-stimulated [3H]DA release in wild-type mice was significantly increased in the presence of 10 μm (+)bicuculline, a GABAA receptor antagonist, although there was no change in GluRε1 mutant mice. As a result, NMDA-stimulated [3H]DA release in the presence of (+)bicuculline was significantly reduced in GluRε1 mutant mice compared with in wild-type mice (Fig. 4C). Accordingly, it is suggested that in wild-type mice, NMDA-stimulated [3H]DA release is tonically suppressed by the concomitant release of GABA, the effect being mediated through GABAA receptors. In the mutant mice, NMDA-stimulated GABA release may be decreased because of the malfunction of NMDA receptors, and as a result, the NMDA-stimulated [3H]DA release was not affected by treatment with (+)bicuculline. To prove this, we measured the NMDA-stimulated [3H]GABA release in the striatum. As we expected, the NMDA-stimulated [3H]GABA release from striatal slices was markedly reduced in GluRε1 mutant mice compared with wild-type mice (Fig. 4D).
Fig. 4.

NMDA-stimulated [3H]dopamine, [14C]serotonin, and [3H]GABA release in GluRε1 mutant mice. The cortical and striatal slices were incubated with 1 μm[3H]DA, 1 μm[14C]5-HT, and 10 μm pargyline at 37°C for 30 min. For [3H]GABA release, the striatal slices were incubated with 1 μm[3H]GABA and 100 μm amino-oxyacetic acid. After washes, the cortical and striatal slices were superfused with Krebs'–Ringer's solution buffer at 37°C and exposed to 25 mm KCl at t = 70 min and then to 100 μm NMDA at t = 90 min for 2 min. For [3H]DA release in the presence of (+)bicuculline (Bicu), the [3H]DA-labeled striatal slices were superfused with Krebs'–Ringer's solution buffer containing 10 μm (+)bicuculline until the end of the experiment. Each column represents the mean ± SEM (n = 7–8). *p < 0.05; ***p < 0.001 versus corresponding wild (+/+). #p < 0.05 versus NMDA-stimulated [3H]DA release in wild (+/+).
These findings suggest that the disruption of the GluRε1 subunit results in an enhancement of dopaminergic and serotonergic neuronal activities, because of disinhibition of the GABAergic input.
Locomotor activity in GluRε1 mutant mice
Locomotor activity in animals is regulated by the monoaminergic neuronal systems, particularly dopaminergic and serotonergic neuronal activities (Giros et al., 1996; Gainetdinov et al., 1999). To clarify the behavioral influences of the altered monoaminergic neuronal functions in GluRε1 mutant mice, we assessed locomotor activity in a novel environment. The locomotor activity of GluRε1 mutant mice in a novel environment was recorded by counting the number of infrared sensor crossings for a 120 min observation period (Fig.5). During the first 60 min observation period, GluRε1 mutant mice showed an increase in horizontal (locomotion; Fig. 5A) and vertical (rearing; Fig.5B) activities compared with wild-type mice [ANOVA analysis with repeated measures; F(1,22) = 3.470, p = 0.0002 (locomotion);F(1,22) = 2.028, p = 0.0265 (rearing)]. However, the increase that occurred during the first 60 min period was reduced to the level of wild-type mice during the next 60 min period.
Fig. 5.
Locomotor activity in a novel environment in GluRε1 mutant mice. Locomotor activity and the number of rearing events in a novel environment were measured every 5 min for 120 min. Each column represents the mean ± SEM (n = 12). An ANOVA with repeated measures revealed a significant difference in locomotion (F(1,22) = 3.470;p = 0.0002) and rearing curves (F(1,22) = 2.028; p= 0.0265). **p < 0.01 versus wild (+/+).
To test the involvement of dopaminergic and serotonergic neuronal systems in the increased locomotor activity, we examined the effects of a potent DA receptor antagonist, haloperidol, and a DA/5-HT receptor antagonist, risperidone, in GluRε1 mutant mice (Fig.6). Both drugs reduced the locomotor activity in wild-type and GluRε1 mutant mice in a dose-dependent manner. Haloperidol (0.003 mg/kg) and risperidone (0.01 mg/kg) ameliorated the hyperlocomotion of GluRε1 mutant mice, at doses that had no effect in wild-type mice [ANOVA analysis;F(5,43) = 18.806, p < 0.0001 (locomotion-haloperidol);F(5,43) = 8.598, p < 0.0001 (rearing-haloperidol); F(5,51)= 15.205, p < 0.0001 (locomotion-risperidone);F(5,51) = 8.111, p < 0.0001 (rearing-risperidone)]. These results suggest that the increase in dopaminergic and serotonergic neuronal activities contributes to the enhanced locomotor activity in GluRε1 mutant mice.
Fig. 6.
Effects of haloperidol and risperidone on the increased locomotor activity in GluRε1 mutant mice. Haloperidol (0.003 or 0.01 mg/kg, p.o.) or risperidone (0.01 or 0.03 mg/kg, p.o.) was administered 60 min before the measurement of locomotor activity in a novel environment. Each column represents the mean ± SEM (n = 8–10). ANOVA analysis:F(5,43) = 18.806, p< 0.0001 (locomotion-haloperidol);F(5,43) = 8.598, p< 0.0001 (rearing-haloperidol);F(5,51) = 15.205, p< 0.0001 (locomotion-risperidone);F(5,51) = 8.111, p< 0.0001 (rearing-risperidone). *p < 0.05 versus corresponding vehicle-treated wild (+/+). #p < 0.05; ##p < 0.01; ###p < 0.001 versus corresponding vehicle-treated GluRε1 (−/−).
Latent learning in GluRε1 mutant mice
Previous studies indicated that spatial and contextual learning in GluRε1 mutant mice is impaired as manifested by performance in the Morris water maze and the contextual fear conditioning tasks (Sakimura et al., 1995; Kiyama et al., 1998). To further explore a possible change in cognitive function in GluRε1 mutant mice, we examined performance in the water-finding task. Because mice were not reinforced either positively or negatively by water in the training trials of the water-finding task, their performance in the test trial is dependent on latent learning, and selective attention underlies the acquisition of latent learning (Cheal, 1980; Ichihara et al., 1993). There was no significant difference in the number of approaches between wild-type (n = 12; 4.9 ± 0.6) and GluRε1 mutant mice (n = 12; 4.1 ± 0.4). Figure7 shows the performance in the test trial of the water-finding task in wild-type and GluRε1 mutant mice. No measurable difference was observed between the two groups of nontrained mice (Fig. 7A). In wild-type mice that were subjected to the training trial 24 hr earlier (trained wild-type mice), the starting, entering, and finding latencies were shorter than those in the corresponding nontrained wild-type mice. The shortening of starting and entering latencies, but not finding latency, was observed in trained GluRε1 mutant mice compared with nontrained animals (Fig.7A). When we compared the performance of trained GluRε1 mutant mice with that of trained wild-type mice, we found the starting, entering, and finding latencies to be significantly longer in trained GluRε1 mutant mice than trained wild-type mice (Fig. 7B). Accordingly, these results suggest that the latent learning associated with selective attention to the drinking tube is impaired in GluRε1 mutant mice.
Fig. 7.
Latent learning in the water-finding task in GluRε1 mutant mice. The starting, entering, and finding latencies were measured in the test trial 24 hr after the training trial of the water-finding task. The starting, entering, and finding latencies in trained wild-type mice (+/+) were 2.1 ± 0.3, 9.0 ± 0.8, and 67.5 ± 17.9 sec, respectively. Each column represents the mean ± SEM (n = 12). *p< 0.05; **p < 0.01 versus corresponding nontrained group. #p < 0.05; ##p < 0.01 versus trained wild (+/+).
DISCUSSION
The NMDA receptor is distinguished by several characteristic properties, including modulation by glycine, activation by polyamines such as spermidine and spermine, inhibition by Mg2+, Zn2+and specific open-channel blockers (MK-801 and PCP), and high permeability of Ca2+ (Mayer and Westbrook, 1987; Hollmann and Heinemann, 1994; Nakanishi and Masu, 1994). Thus, the NMDA receptor is considered a receptor-channel complex consisting of at least four major domains: (1) a glutamate (specific exogenous ligand NMDA) recognition domain, (2) a glycine recognition domain insensitive to strychnine, (3) a polyamine recognition domain, and (4) a channel formation domain permeable to Ca2+. In the present study, we investigated the function of NMDA receptors in GluRε1 mutant mice by examining [3H]MK-801 binding and45Ca2+ uptake through the receptors under various conditions. When both assays were conducted in the presence of glutamate (or NMDA), glycine and/or spermidine, partial impairment of NMDA receptor function was evident in the mutant mice.
It has been demonstrated that GluRε1 mutant mice exhibit a reduction in hippocampal LTP (Sakimura et al., 1995). The reduction may be attributable to a diminution of Ca2+influx through the NMDA receptors, because Ca2+ influx through NMDA receptors is critical to the induction of hippocampal LTP (Lynch et al., 1983;Malenka et al., 1988). This inference was more directly proved by the malfunction of NMDA receptors in GluRε1 mutant mice observed in the present study. Furthermore, Kiyama et al. (1998) have reported that although the threshold for LTP induction is increased in GluRε1 mutant mice, normal LTP formation is seen after a stronger tetanic stimulation. Consistent with the finding, a reduction of45Ca2+ uptake in GluRε1 mutant mice was not observed any more when NMDA receptors were strongly activated by NMDA, glycine, and spermidine. Accordingly, it is suggested that the disruption of the GluRε1 subunit results in a reduction, but not the loss, of NMDA receptor function.
The pharmacological blockade of NMDA receptors in vivocauses behavioral abnormalities such as hyperlocomotion and stereotypy and functional alterations of monoaminergic neuronal systems, particularly dopaminergic and serotonergic neuronal systems (Hiramatsu et al., 1989; Loscher et al., 1991). Locomotor activity is mainly regulated by the dopaminergic neuronal system, and the activation of this system induces hyperlocomotion (Giros et al., 1996; Gainetdinov et al., 1999). The serotonergic neuronal system is also involved in the modulation of locomotor activity, being localized downstream of the dopaminergic neuronal system, because serotonergic neurotransmission can modulate behavioral alteration without producing concurrent changes of dopaminergic neurotransmission (Gainetdinov et al., 1999). Activation of the serotonergic neuronal system is inhibitory to hyperlocomotion (Geyer, 1996; Lucki, 1998). Accordingly, it has been considered that the blockade of NMDA receptors in vivocauses hyperlocomotion by either directly or indirectly activating dopaminergic neuronal function (Imperato et al., 1990; Miller and Abercrombie, 1996). In the present study, we demonstrated that GluRε1 mutant mice with genetically reduced NMDA receptor function exhibited hyperlocomotion, which is associated with an increase in dopaminergic and serotonergic neuronal activities in the frontal cortex and striatum. The increased serotonergic neuronal activity in vivo in GluRε1 mutant mice may be a result of delicate homeostatic alterations after the increase in dopaminergic neuronal activity to maintain a balance between the neuronal systems for locomotor activity.
We observed an increase in NMDA-stimulated [3H]DA release in the striatum, but not the frontal cortex, of GluRε1 mutant mice, although DA metabolism was enhanced in both regions in vivo. The NMDA-stimulated [14C]5-HT release in the frontal cortex and striatum of the mutant mice did not differ from that in wild-type mice, although 5-HT metabolism was enhanced in both regions in vivo. Thus, it is likely that not only direct effects, but also indirect effects, of the reduced NMDA receptor function are involved in the increased dopaminergic and serotonergic neuronal activity in vivo. We demonstrated in the present study that the enhancement of NMDA-stimulated [3H]DA release in GluRε1 mutant mice is attributable to the disinhibition of the dopaminergic neuronal system from inhibitory regulation by the GABAergic neuronal system. Alternatively, it has been demonstrated that PCP and its analog ketamine cause an increase in glutamate release and activation of glutamatergic neurotransmission via non-NMDA and metabotropic glutamate receptors. This enhancement of glutamatergic neurotransmission results in an increase in DA release (Moghaddam et al., 1997; Adams and Moghaddam, 1998; Moghaddam and Adams, 1998). Therefore, it is necessary to measure the release of various neurotransmitters in GluRε1 mutant mice to clarify the neurochemical mechanism that underlies the hyperactivity of dopaminergic and serotonergic neuronal systems.
Schizophrenia is one of the diseases that have been hypothesized to be associated with NMDA receptor dysfunction (Javitt and Zukin, 1991;Tamminga, 1998) and the hyperfunction of dopaminergic and serotonergic neuronal systems (Seeman et al., 1976; Meltzer, 1991). Several lines of evidence suggest that dysfunction of glutamatergic and dopaminergic neuronal mechanisms contributes to the pathophysiology of schizophrenia. For example, PCP produces schizophrenia-like symptoms in healthy people (Luby et al., 1959), and preexisting symptoms in patients with schizophrenia are exacerbated by its psychotomimetic properties (Javitt and Zukin, 1991; Malhotra et al., 1997). Clinical doses of antipsychotic drugs in treating schizophrenia are correlated with their affinities for D2 receptors (Seeman et al., 1976; Leysen et al., 1994). Antipsychotic DA receptor antagonists are also effective in preventing PCP-induced abnormal behavior in animals such as hyperlocomotion and stereotyped behavior (Kitaichi et al., 1994; Noda et al., 1995). Therefore, animals treated with NMDA receptor antagonists have been used as a model for schizophrenia, and the amelioration of hyperlocomotion in this animal model is known as a screening test for the efficacy of antipsychotic drugs (Carlsson and Carlsson, 1990; Corbett et al., 1993, 1995; Moghaddam and Adams, 1998).
In the present study, we examined the effects of haloperidol (D2 receptor antagonist) and risperidone (D2 and 5-HT2 receptor antagonist) on the hyperlocomotion in GluRε1 mutant mice, because our neurochemical experiments suggested that dopaminergic and serotonergic neuronal activities are increased in the mutant mice. Haloperidol and risperidone are administered to schizophrenic patients as typical and atypical antipsychotic drugs, respectively, the former having marked extrapyramidal side effects (EPS) at effective doses (Hoffman and Donovan, 1995) and the latter being able to suppress psychotic symptoms without EPS (Ereshefsky et al., 1989; Gerlach, 1991). Haloperidol and risperidone were effective in attenuating the hyperlocomotion of GluRε1 mutant mice at doses that did not affect the locomotor activity in wild-type mice.
GluRε1 mutant mice also showed an impairment of performance in the test trial of the water-finding task (i.e., an increase of starting, entering, and finding latencies), although their performance in the training trial did not differ from that of wild-type mice. Therefore, it is unlikely that the impairment of the mutant mice is attributable to altered anxiety processes (for example, avoidance of open field). Because the mice were not reinforced either positively or negatively by water in the training trial, their performance in the test trial is dependent on latent learning, and selective attention underlies the acquisition of latent learning (Cheal, 1980; Ichihara et al., 1993). Thus, cognitive function, including latent learning associated with selective attention, spatial learning (Sakimura et al., 1995), and contextual learning (Kiyama et al., 1998), is impaired in GluRε1 mutant mice. The impairment of latent learning in the mutant mice may be attributable to the increased dopaminergic neuronal activity, because activation of dopaminergic neuronal function by treatment with apomorphine and methamphetamine results in an impairment of performance in the water-finding task (Ichihara et al., 1993; Nabeshima et al., 1994). Moreover, PCP-treated mice, which have been used as a pharmacological animal model for schizophrenia, were impaired in latent learning in the water-finding task (our unpublished observations).
Collectively, GluRε1 mutant mice exhibit several behavioral abnormalities related to schizophrenia, including hyperlocomotion and cognitive impairment (Schildkraut, 1965; Snyder et al., 1974; Ban et al., 1984). These findings suggest that GluRε1 mutant mice, which have hypofunction of the glutamatergic system, as well as hyperactivity of the dopaminergic and serotonergic neuronal systems, may be useful as an animal model for schizophrenia. However, further experiments to characterize the behavioral alteration and the effects of neuroleptics on this behavioral alteration are necessary to establish GluRε1 mutant mice as a new genetic animal model of schizophrenia.
In summary, mice lacking the GluRε1 subunit display behavioral abnormalities probably caused by the hyperfunction of dopaminergic and serotonergic neuronal systems as a consequence of NMDA receptor malfunction. In addition, our findings suggest that GluRε1 mutant mice are useful as an animal model of psychosis such as schizophrenia.
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research (10044260) and COE Research from the Ministry of Education, Science, Sports, and Culture of Japan, by the Health Sciences Research Grants for Research on Pharmaceutical and Medical Safety from the Ministry of Health and Welfare of Japan, and by Special Coordination Funds for Promoting Science and Technology, Target-Oriented Brain Science Research Program from the Ministry of Science and Technology of Japan.
Correspondence should be addressed to Dr. Toshitaka Nabeshima, Department of Neuropsychopharmacology and Hospital Pharmacy, Nagoya University Graduate School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya 466-8560, Japan. E-mail: tnabeshi@med.nagoya-u.ac.jp.
REFERENCES
- 1.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban TA, Guy W, Wilson WH. Description and distribution of the subtypes of chronic schizophrenia based on Leonhard's classification. Psychiatr Dev. 1984;2:179–199. [PubMed] [Google Scholar]
- 3.Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia: implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 4.Cheal ML. Disruption of selective attention by apomorphine, but not amphetamine, in the Mongolian gerbil. Psychopharmacology (Berl) 1980;69:93–100. doi: 10.1007/BF00426528. [DOI] [PubMed] [Google Scholar]
- 5.Corbett R, Hartman H, Kerman LL, Woods AT, Strupczewski JT, Helsley GC, Conway PC, Dunn RW. Effects of atypical antipsychotic agents on social behavior in rodents. Pharmacol Biochem Behav. 1993;45:9–17. doi: 10.1016/0091-3057(93)90079-9. [DOI] [PubMed] [Google Scholar]
- 6.Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K, Dunn RW. Antipsychotic agents antagonize non-competitive N-methyl-d-aspartate antagonist-induced behaviors. Psychopharmacology (Berl) 1995;120:67–74. doi: 10.1007/BF02246146. [DOI] [PubMed] [Google Scholar]
- 7.Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ereshefsky L, Watanabe MD, Tran-Johnson TK. Clozapine: an atypical antipsychotic agent. Clin Pharmacol. 1989;8:691–709. [PubMed] [Google Scholar]
- 9.Forrest D, Yuzaki M, Soares HD, Hg L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 11.Gerlach J. New antipsychotics: classification, efficacy, and adverse effects. Schizophr Bull. 1991;17:289–309. doi: 10.1093/schbul/17.2.289. [DOI] [PubMed] [Google Scholar]
- 12.Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 13.Giros B, Jaber M, Jones SR, Wightmann RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 14.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. the disposition of [3H] norepinephrine, [3H] dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu M, Cho AK, Nabeshima T. Comparison of the behavioral and biochemical effects of the NMDA receptor antagonists, MK-801 and phencyclidine. Eur J Pharmacol. 1989;166:359–366. doi: 10.1016/0014-2999(89)90346-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extarpyramidal side effect liability. Psychopharmacology (Berl) 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- 17.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 18.Ichihara K, Nabeshima T, Kameyama T. Differential effects of pimozile and SCH23390 on acquisition of learning in mice. Eur J Pharmacol. 1989;164:189–195. doi: 10.1016/0014-2999(89)90458-5. [DOI] [PubMed] [Google Scholar]
- 19.Ichihara K, Nabeshima T, Kameyama T. Dopaminergic agonists impair latent learning in mice: possible modulation by noradrenergic function. J Pharmacol Exp Ther. 1993;264:122–128. [PubMed] [Google Scholar]
- 20.Ikeda K, Araki K, Takayama C, Inoue Y, Yagi T, Aizawa S, Mishina M. Reduced spontaneous activity of mice defective in the ε4 subunit of the NMDA receptor channel. Mol Brain Res. 1995;33:61–71. doi: 10.1016/0169-328x(95)00107-4. [DOI] [PubMed] [Google Scholar]
- 21.Imperato A, Scrocco MG, Bacchi S, Angelucci L. NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur J Pharmacol. 1990;187:555–556. doi: 10.1016/0014-2999(90)90387-l. [DOI] [PubMed] [Google Scholar]
- 22.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 23.Kitaichi K, Yamada K, Hasegawa T, Furukawa H, Nabeshima T. Effects of risperidone on phencyclidine-induced behaviors: comparison with haloperidol and ritanserin. Jpn J Pharmacol. 1994;66:181–189. doi: 10.1254/jjp.66.181. [DOI] [PubMed] [Google Scholar]
- 24.Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ε1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs MO, Kemel ML, Gauchy C, Desban M, Glowinski J. Local GABAergic regulation of the NMDA-evoked release of dopamine is more important in striosomes than in matrix of the rat striatum. Neuroscience. 1993;57:249–260. doi: 10.1016/0306-4522(93)90060-s. [DOI] [PubMed] [Google Scholar]
- 26.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 27.Leysen JE, Janssen PMF, Megens AAHP, Schotte A. Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994;55 [Suppl]:5–12. [PubMed] [Google Scholar]
- 28.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knock-out mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 29.Loscher W, Annies R, Honack D. The N-methyl-d-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats. Neurosci Lett. 1991;128:191–194. doi: 10.1016/0304-3940(91)90258-u. [DOI] [PubMed] [Google Scholar]
- 30.Luby ED, Cohen BD, Rosenbaum F, Gottlieb J, Kelley R. Study of a new schizophrenomimetic drug, Sernyl. Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 31.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 32.Lynch G, Larson J, Kelso S, Barrionuevo G, Schotter F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Neuron. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 33.Malenka RC, Kauer JA, Zuker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 35.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 36.Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- 37.Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- 38.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 39.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 41.Nabeshima T, Ichihara K. Measurement of dissociation of amnesic and behavioral effects of drugs in mice. In: Conn PM, editor. Paradigms for the study of behavior, methods in neurosciences. Vol. 14. Academic; San Diego: 1993. pp. 217–229. [Google Scholar]
- 42.Nabeshima T, Nakayama S, Ichihara K, Yamada K, Shiotani T, Hasegawa T. Effects of nefiracetam on drug-induced impairment of latent learning in mice in a water finding task. Eur J Pharmacol. 1994;255:57–65. doi: 10.1016/0014-2999(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 44.Noda Y, Miyamoto Y, Mamiya T, Kamei H, Furukawa H, Nabeshima T. Involvement of dopaminergic system in phencyclidine-induced place preference in mice pretreated with phencyclidine repeatedly. J Pharmacol Exp Ther. 1998;286:44–51. [PubMed] [Google Scholar]
- 45.Noda Y, Yamada K, Furukawa H, Nabeshima T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol. 1995;116:2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 47.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 48.Seeman P, Lee T, Chau-Wong, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 49.Snyder SH, Banerjee SP, Yamamura HI, Greenberg D. Drugs, neurotransmitters, and schizophrenia. Science. 1974;184:1243–1253. doi: 10.1126/science.184.4143.1243. [DOI] [PubMed] [Google Scholar]
- 50.Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K, Teraoka T, Morita S, Hasegawa T, Nabeshima T. Neuropharmacological characterization of voltage-sensitive calcium channels: possible existence of neomycin-sensitive, ω-conotoxin GVIA- and dihydropyridine-resistant calcium channels in the rat brain. Jpn J Pharmacol. 1993;63:423–432. doi: 10.1254/jjp.63.423. [DOI] [PubMed] [Google Scholar]
- 52.Yoneda Y, Ogita K. Labeling of NMDA receptor channels by [3H]MK-801 in brain synaptic membranes treated with Triton X-100. Brain Res. 1989;499:305–314. doi: 10.1016/0006-8993(89)90779-8. [DOI] [PubMed] [Google Scholar]
- 53.Yoneda Y, Ogita K. Heterogeneity of the N-methyl-d-aspartate receptor ionophore complex in rat brain, as revealed by ligand binding techniques. J Pharmacol Exp Ther. 1991;259:86–96. [PubMed] [Google Scholar]