Abstract
Recently, we identified four kinetically distinct voltage-gated K+ currents, IAf,IAs,IK, andISS, in rat superior cervical ganglion (SCG) neurons and demonstrated thatIAf and IAs are differentially expressed in type I (IAf,IK,ISS), type II (IAf,IAs,IK,ISS), and type III (IK,ISS) SCG cells. In addition, we reported that IAf is eliminated in most (∼70%) SCG cells expressing Kv4.2W362F, a Kv4 subfamily-specific dominant negative. The molecular correlate(s) of the residualIAf, as well as that ofIAs,IK, andISS, however, are unknown. The experiments here were undertaken to explore the role of Kv1 α-subunits in the generation of voltage-gated K+currents in SCG neurons. Using the Biolistics Gene Gun, cDNA constructs encoding a Kv1 subfamily-specific dominant negative, Kv1.5W461F, and enhanced green fluorescent protein (EGFP) were introduced into SCG neurons. Whole-cell recordings from EGFP-positive Kv1.5W461F-expressing cells revealed a selective decrease in the percentage of type I cells and an increase in type III cells, indicating that IAf is gated by Kv1 α-subunits in a subset of type I SCG neurons.IAf is eliminated in all SCG cells expressing both Kv1.5W461F and Kv4.2W362F.IAf τdecay values in Kv1.5W461F-expressing and Kv4.2W362F-expressing type I cells are significantly different, revealing that Kv1 and Kv4 α-subunits encode kinetically distinct IAf channels. Expression of Kv1.5W461F increases excitability by decreasing action potential current thresholds and converts phasic cells to adapting or tonic firing. Interestingly, the molecular heterogeneity ofIAf channels has functional significance because Kv1- and Kv4-encoded IAf play distinct roles in the regulation of neuronal excitability.
Keywords: K+ channels, I A , Kv1 α-subunits, Kv4 α-subunits, Kv1.5W461F, transgenics, gene gun, neuronal excitability, repetitive firing patterns
K+channels are important determinants of membrane excitability, and differences in K+ channel expression influence action potential waveforms, repetitive firing patterns, and synaptic integration (Pongs, 1999; Hausser et al., 2000). Electrophysiological studies have revealed that most mammalian neurons express multiple types of voltage-gated K+current components with distinct time- and voltage-dependent properties (Rudy, 1988; Storm, 1990). In addition, a number of voltage-gated K+ (Kv) channel pore-forming (α) and accessory (Kvβ and KChIP) subunits that underlie these channels have been identified (Coetzee et al., 1999; Pongs, 1999; An et al., 2000), and there is presently considerable interest in identifying the molecular correlates and defining the functional roles of the various voltage-gated K+ currents in mammalian neurons.
Recently, we reported that sympathetic neurons isolated from the rat superior cervical ganglion (SCG) express multiple, kinetically distinct, voltage-gated K+ current components: two transient A-type currents,IAf andIAs; a delayed rectifier current,IK; and a steady-state non-inactivating current, ISS (Malin and Nerbonne, 2000). Although IK andISS are present in all rat SCG neurons, IAf andIAs are differentially distributed;IAf is detected in ∼90% of cells and, in a subset of these cells (∼20%),IAs is also expressed (Malin and Nerbonne, 2000). Based on these expression patterns, three cell types were distinguished: type I cells (IAf,IK,ISS), type II cells (IAf,IAs,IK,ISS), and type III cells (IK,ISS) (Malin and Nerbonne, 2000). In addition, we exploited a molecular genetic strategy to assess directly the role of Kv4 α-subunits in the generation of voltage-gated K+ currents in these cells. These experiments revealed that expression of Kv4.2W362F, a Kv4 subfamily-specific dominant negative (Barry et al., 1998), results in the elimination of IAf in most (∼70%) SCG cells (Malin and Nerbonne, 2000). The molecular correlate(s) of the residual IAf, as well as of IAs,IK, andISS, have not been defined.
Previous studies have documented the expression of mRNAs encoding several Kv α- and β-subunits in SCG neurons, including several members of the Kv1 subfamily, Kv1.1, Kv1.2, Kv1.4, and Kv1.5 (Dixon and McKinnon, 1996; Pankevych et al., 1999). Interestingly, previous studies have shown that heterologous expression of Kv1 α-subunits alone or in combination with Kvβ subunits gives rise to voltage-gated K+ currents with a wide range of time- and voltage-dependent properties (Coetzee et al., 1999). In addition, it has recently been demonstrated that Kv1 α-subunits encode distinct types of voltage-gated K+ currents in myocardial cells (Feng et al., 1997; Bou-Abboud and Nerbonne, 1999; Guo et al., 1999, 2000; London et al., 2001). In mouse ventricular myocytes, for example, Kv1.4 encodes the slow transient K+ current,Ito,s (Guo et al., 1999, 2000), and Kv1.5 underlies one component of the slow delayed rectifier currentIK,slow (London et al., 2001). These observations led us to hypothesize that Kv1 α-subunits might contribute to the formation of one or more voltage-gated K+ currents in rat SCG neurons, and the experiments here were undertaken to test this hypothesis directly.
MATERIALS AND METHODS
SCG neurons in vitro. Sympathetic neurons were isolated from the SCGs of embryonic day 21 to postnatal day 1 Long–Evans rat pups using a procedure similar to that described by Chang et al. (1990). Briefly, after anesthesia with 5% halothane, animals were decapitated, and the SCGs were removed. Ganglia were successively incubated for 30 min periods in collagenase and trypsin at room temperature; isolated SCG neurons were obtained by trituration and subsequent centrifugation. Dissociated SCG cells were resuspended in growth medium (Earle's Minimum Essential Medium with 10% fetal calf serum, 0.14 mml-glutamine, 100 U/ml penicillin–streptomycin, and 0.05 mm NGF) and plated at a density of 2.5 × 104 cells per square centimeter on glial monolayers (prepared as in Raff et al., 1979). Cells were maintained at 37°C in a 95% O2/5% CO2 incubator, and the medium was exchanged with fresh growth medium approximately every 48 hr.
Immunohistochemistry. The affinity-purified rabbit polyclonal anti-Kv1.2 antibody used here was raised against residues 468–486 in the C terminus of Kv1.2 and has been shown to reliably and specifically detect Kv1.2 (Barry et al., 1995). The Kv1.4-specific antibody was targeted against residues 13–37 in the N-terminal region of Kv1.4 and has been shown previously to detect Kv1.4 with no detectable cross-reactivity to other Kv1 subfamily members (Barry et al., 1995). The anti-Kv1.5 antibody, purchased from UBI (Lake Placid, NY; catalog #06-463), was generated against amino acid residues 542–602 in the C terminus of Kv1.5 and has been demonstrated to be specific for Kv1.5 (Barry et al., 1995). For immunohistochemistry, cells were fixed in 4% paraformaldehyde for 30 min, incubated in blocking buffer (PBS containing 5% normal goat serum, 0.02% Triton X-100, and 0.1% NaNH3) for 1 hr, and exposed to one of the Kv1 α-subunit-specific primary antibodies (1:100 dilution) at 4°C overnight. After washing with PBS, cultures were incubated first with a biotinylated goat anti-rabbit antibody (1:200 dilution; Chemicon, Temecula, CA) for 1 hr at room temperature, washed again, and then exposed to an avidin-conjugated horseradish peroxidase for 1 hr at room temperature (ABC kit; Vector Laboratories, Burlingame, CA). The cultures were washed again before incubation with 0.05% diaminobenzidine in PBS. The progress of the reaction was monitored under the microscope, and the reactions were quenched after ∼5 min by washing with PBS. A mouse monoclonal anti-myc antibody (Calbiochem, La Jolla, CA) was used at a 1:100 dilution to detect Kv1.5W461F-myc protein expression in transfected SCG neurons. Cultures were fixed, blocked, and incubated in primary antibody as described above. After washing, cultures were incubated with a Cy3-conjugated rabbit anti-mouse IgG secondary antibody (Chemicon), and labeled cells were visualized under epifluorescence illumination.
Transfection of isolated SCG neurons. In control experiments, 1.6 μm gold beads were coated with pCMV-EGFP (Clontech, Cambridge, UK) and propelled (450 psi; 2 mm carrier distance) into SCG neurons at 4 d in vitro using the Gene Gun (Bio-Rad, Hercules, CA), a biolistic projectile system. After transfections, the cultures appeared healthy, and expression of EGFP was readily detected 24 hr later (see Results). In experiments aimed at examining the effects of Kv1.4, Kv1.5W461F or Kv4.2W362F + Kv1.5W461F expression in SCG neurons, the gold particles were coated with pBK-CMV-Kv1.4 and pCMV-EGFP (4:1 ratio); pBK-CMV-Kv1.5W461F-myc and pCMV-EGFP (4:1 ratio); or pBK-CMV-Kv4.2W362F-FLAG, pBK-CMV-Kv1.5W461F-myc and pCMV-EGFP (4:4:1 ratio). In all cases, a total of 10 μg of DNA was used in coating the beads. Because EGFP expression was used to identify transfected neurons for subsequent electrophysiological characterization, immunohistochemical experiments were performed to determine whether EGFP-expressing cells also expressed the Kv1.5W461F-myc construct (see Results).
Electrophysiological recording. Whole-cell recordings were obtained from isolated SCG neurons at room temperature (22–25°C). Data were collected using an Axopatch-1B patch-clamp amplifier, and experiments were controlled with a P5–120 Gateway 2000 computer through a TL-1 DMA interface using the pClamp7 software package (Axon Instruments, Foster City, CA). Electrodes were fabricated from soda-lime glass (Chase 2502) with a two-stage puller, and the shanks were coated with a silicone elastomer (Sylgard; Dow Corning, Midland, MI). Pipette resistances were 1.5–3 MΩ after fire-polishing. For voltage-clamp recordings, the bath solution routinely contained (in mm): 140 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 5 glucose, 0.001 TTX, and 0.1 CdCl2, pH 7.5, 300 mOsm. Recordings were also obtained from SCG neurons in the current-clamp mode, and the TTX and CdCl2 were omitted from the bath for these studies. The pipette solution for both current- and voltage-clamp recordings contained (in mm): 135 KCl, 10 HEPES, 5 glucose, 3 MgATP, 0.5 NaATP, 2 EGTA, 1.1 CaCl2, pH 7.5, 300 mOsm. Series resistances, estimated from the decays of the uncompensated capacitative transients, were 2–5 MΩ and were compensated electronically by ∼80–90%. Because current amplitudes were <10 nA, the voltage errors resulting from the uncompensated series resistance were always <10 mV and were not corrected. Voltage-gated K+ currents were evoked during 125 msec or 6 sec depolarizing voltage steps to test potentials between −40 mV and +50 mV from a holding potential of −90 mV. Single action potentials and action potential trains were recorded in response to brief (1.5 msec) 200–400 pA and prolonged (500 msec) 20–200 pA depolarizing current injections.
Data analysis. Data were compiled and analyzed using pClamp7 and Excel (Microsoft, Seattle, WA) software and are presented as means ± SEM. The decay phases of the capacitative transients were analyzed, and only cells in which >90% of the amplitude of the capacitative transient decayed with a single exponential time course were included in this study. Only data obtained from cells with input resistances >100 MΩ were analyzed and included here. The mean ± SEM input resistance and capacitance of EGFP-expressing SCG neurons were 0.34 ± 0.03 GΩ and 32 ± 2 pF (n = 43), respectively. Leak currents were <100 pA (at −70 mV) and were not subtracted. To determine the amplitudes and the decay time constants of the individual components of the total depolarization-activated outward K+currents in SCG neurons, the inactivation phases of the currents recorded during prolonged (6 sec) depolarizations were analyzed using the equation: y =A1e−t/τ1+A2e−t/τ2+A3e−t/τ3+ C, where A1,A2, andA3 (measured in picoamperes per picoFarad) are the amplitudes of the inactivating current components (IAf,IAs, andIK) that decay with time constants τ1, τ2, and τ3 (measured in milliseconds), respectively, and C is the steady-state current (measured in picoamperes per picoFarad) remaining at the end of the 6 sec depolarizations (see Results). Fits were obtained using CLAMPFIT6, and best fits were determined by eye (in all cases, ς < 30 pA). All current-clamp recordings were obtained from cells with overshooting action potentials and stable resting membrane potentials negative to −40 mV. Action potential durations were measured at 50% (APD50) and 90% (APD90) repolarization. Statistical significance was examined with the Student's t test; where appropriate, p values are presented in the text.
RESULTS
Heterogeneity of outward K+ current waveforms in SCG neurons
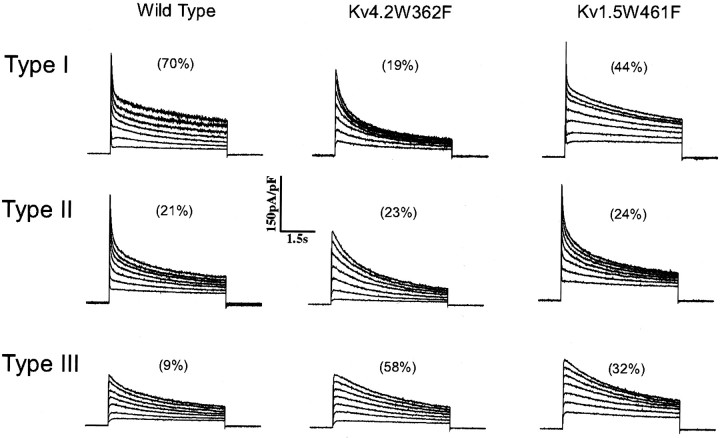
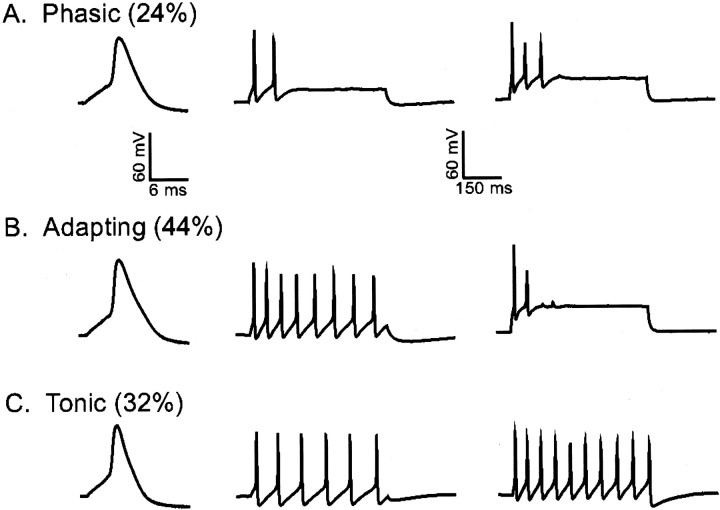
Previously, we have shown that the waveforms of the Ca2+-independent, depolarization-activated K+ currents in isolated rat SCG neurons are highly stereotyped (Malin and Nerbonne, 2000). The records presented in Figure 1, left panel, are representative of the three distinct phenotypes observed; they are referred to as types I, II, and III. Most (>90%) wild-type SCG cells express a prominent rapid component of current decay, consistent with the presence of the fast transient “A-type” current, referred to asIAf (Malin and Nerbonne, 2000). The exception is type III cells, which express only a slowly decaying outward K+ current,IK, and a non-inactivating current,ISS (Fig. 1, left panel; Table 1). In type I SCG cells (Fig. 1,left panel), the decay phases of the currents are well fitted by the sum of two exponentials, reflecting the presence of IAf (mean ± SEM τdecay = 121 ± 14 msec),IK (mean ± SEM τdecay = 2560 ± 187 msec), and the non-inactivating current ISS (Table1). In addition to IAf,IK, andISS, a more slowly inactivating, transient outward K+ current, referred to as IAs, (mean ± SEM τdecay 480 ± 21 msec) is present in type II SCG cells (Fig. 1, left panel; Table 1). The decay properties of the individual current components are similar in types I, II, and III SCG cells, although there are marked differences in current densities (Table 1).
Fig. 1.
Distinct effects of expression of Kv4.2W362F or Kv1.5W461F on voltage-gated outward K+currents in SCG neurons. Whole-cell voltage-gated outward K+ currents were recorded from isolated SCG neurons in response to 6 sec depolarizing voltage steps to test potentials between −10 and +50 mV from a holding potential of −90 mV. Experiments were conducted as described in Materials and Methods with 1 μm TTX and 100 μm CdCl2 in the bath solution to block voltage-gated inward Na+ and Ca2+ currents, respectively. The records in theleft, middle, and right panels were recorded from wild-type, Kv4.2W362F-expressing, and Kv1.5W461F-expressing cells, respectively. There are distinct and stereotyped differences in the waveforms of the currents in wild-type I, II, and III SCG cells (see Results). There is, for example, a prominent rapid component of current decay,IAf, in type I and II cells that is not evident in type III cells. The numbers given above the records in each column reflect the percentages of cells studied under each experimental condition that display the type I, II, or III phenotype. Expression of either Kv4.2W362F (middle) or Kv1.5W461F (right) decreases the percentage of type I cells and increases the percentage of type III cells. Kv4.2W362F expression also eliminatesIAf in type II cells (see Results) (Malin and Nerbonne, 2000).
Table 1.
Outward K+ currents in wild-type SCG neurons expressing Kv4.2W362F and/or Kv1.5W461F 1-a
| Wild type | Kv4.2W362F1-b | Kv1.5W461F | Kv1.5W461F + Kv4.2W362F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type III | Type I | Type II | Type III | Type I | Type II | Type III | Type II | Type III | |
| Peak current density (pA/pF) | 252 ± 20 | 259 ± 22 | 211 ± 18 | 244 ± 35 | 289 ± 31 | 162 ± 21 | 241 ± 22 | 260 ± 33 | 239 ± 28 | 219 ± 42 | 218 ± 35 |
| IAf | |||||||||||
| Density (pA/pF) | 81 ± 11 | 111 ± 21 | 64 ± 20 | 67 ± 6 | 83 ± 9 | ||||||
| τ (msec) | 121 ± 14 | 95 ± 8 | 190 ± 18* | 97 ± 101-160 | 72 ± 8 | ||||||
| IAs | |||||||||||
| Density (pA/pF) | 45 ± 3 | 67 ± 15 | 40 ± 8 | 56 ± 14 | |||||||
| τ (msec) | 480 ± 21 | 490 ± 31 | 585 ± 47 | 482 ± 61 | |||||||
| IK | |||||||||||
| Density (pA/pF) | 108 ± 12 | 64 ± 9 | 136 ± 11 | 115 ± 7 | 157 ± 25 | 111 ± 22 | 90 ± 13 | 74 ± 10 | 171 ± 36 | 102 ± 44 | 135 ± 60 |
| τ (msec) | 2560 ± 187 | 2800 ± 193 | 2200 ± 176 | 2368 ± 197 | 2473 ± 254 | 2050 ± 235 | 3127 ± 197 | 3125 ± 438 | 3089 ± 234 | 2789 ± 363 | 2473 ± 254 |
| ISS | |||||||||||
| Density (pA/pF) | 74 ± 5 | 45 ± 5 | 79 ± 6 | 68 ± 15 | 72 ± 15 | 65 ± 9 | 52 ± 8 | 48 ± 6 | 84 ± 7 | 65 ± 9 | 86 ± 12 |
| n | 30 | 9 | 4 | 6 | 7 | 18 | 15 | 8 | 11 | 3 | 10 |
All values are mean ± SEM; current densities and τdecay values were determined from currents recorded on depolarizations to +50 mV; n= number of cells.
Data from Malin and Nerbonne (2000).
Values in Kv4.2W362F-expressing cells are significantly (p < 0.04) different from those recorded in wild-type cells.
F1-160: Values in Kv1.5W461F-expressing cells are significantly (p < 0.001) different from those recorded in Kv4.2W362F-expressing cells.
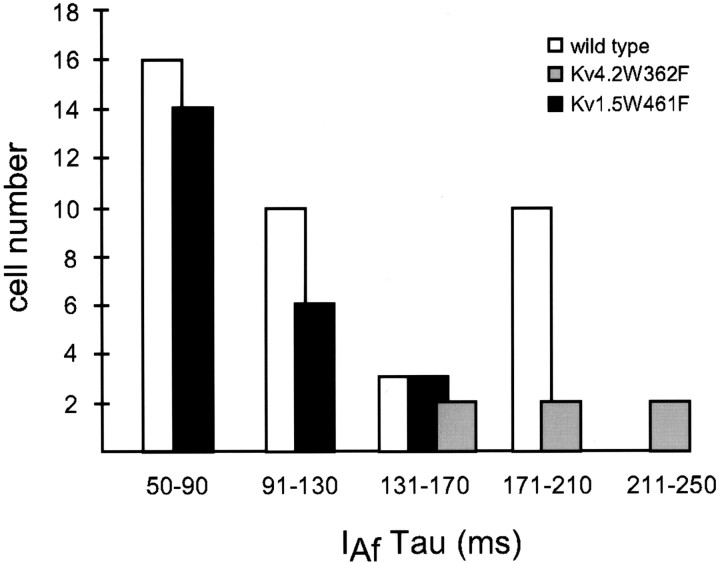
In previous studies, we also demonstrated that expression of a pore mutant of Kv4.2, Kv4.2W362F, which functions as a Kv4-specific dominant negative (Barry et al., 1998), eliminatesIAf in most (∼70%) SCG neurons (Malin and Nerbonne, 2000). The expression of Kv4.2W362F (Fig. 1,middle panel) results in a marked increase in type III cells, which express only IK andISS, and reveals a novel phenotype (i.e., one not seen in wild-type SCG cells) in which onlyIAs,IK, andISS are present (Malin and Nerbonne, 2000). In a subset (∼20%) of type I SCG cells expressing Kv4.2W362F (Fig. 1, middle panel, top), however,IAf is evident (Table 1). These observations suggested either that Kv4.2W362F expression was insufficient to eliminate IAf in some cells or, alternatively, that the fast transient outward K+ current in a subset of type I SCG neurons is not encoded by α-subunits of the Kv4 subfamily. The mean density of IAf (64 ± 20 pA/pF) in type I cells expressing Kv4.2W362F is not significantly different from the mean IAf density (81 ± 11 pA/pF) in wild-type I cells (Table 1). Analysis of the decay phases of the currents, however, revealed that the mean ± SEM inactivation time constant (190 ± 18 msec) of the residualIAf is significantly larger than in wild-type (I or II) SCG cells (Table 1) (Malin and Nerbonne, 2000). In addition, examination of the distribution ofIAf inactivation time constants (Fig.2) in wild-type I SCG cells suggested the presence of two distinct IAfcomponents: one with τdecay values between 50 and 130 msec, and another with τdecay values between 131 and 210 msec (Fig. 2). Interestingly, expression of Kv4.2W362F dramatically shifts the distribution of τdecay values in the remaining type I cells; the range of τdecay values in Kv4.2W362F-expressing type I cells is 138–249 msec, with a mean of 190 ± 18 msec (Fig. 2) (Malin and Nerbonne, 2000). These observations were interpreted as suggesting that, in a subset of type I SCG cells, IAf is not encoded by α-subunits of the Kv4 subfamily (Malin and Nerbonne, 2000).
Fig. 2.
Distinct components ofIAf inactivation in SCG neurons. The time constants of inactivation of the rapid component of current decay,IAf, were determined in wild-type, Kv4.2W362F-expressing, and Kv1.5W461F-expressing SCG cells as described in Materials and Methods. The τdecay values were binned in 40 msec increments for comparison purposes and, as is evident, the distributions of τdecay values in wild-type, Kv4.2W362F-expressing, and Kv1.5W461F-expressing SCG cells are distinct.
Expression of Kv1 α-subunits in SCG neurons
Although the previous studies clearly documented a role for Kv4 α-subunits in the generation of IAfin the majority of SCG neurons (Malin and Nerbonne, 2000), the molecular correlate(s) of the residualIAf, as well asIAs,IK, andISS, remain to be identified. Previous molecular studies have demonstrated that several Kv α-subunits, in addition to Kv4.2 and Kv4.3, as well as several Kv β-subunits are expressed in rat SCG neurons (Dixon and McKinnon, 1996; Pankevych et al., 1999). In heterologous systems, expression of Kv1 α-subunits, alone or in combination with accessory β-subunits, gives rise to voltage-gated K+ currents with diverse kinetic and voltage-dependent properties, suggesting that Kv1 subfamily members may contribute to one or more outward K+ currents in rat SCG (and other) neurons. Importantly, previous studies have shown that several Kv1 α-subunits, including Kv1.1, Kv1.2, Kv1.4, and Kv1.5, and Kv β-subunits are expressed at the message level in SCG neurons (Dixon and McKinnon, 1996; Pankevych et al., 1999), suggesting that Kv1 α-subunits likely play a role in the generation of voltage-gated K+ currents in these cells. To examine Kv1 α-subunit protein expression in SCG cells, therefore, immunohistochemical experiments with anti-Kv1 α-subunit-specific antibodies were undertaken. These experiments revealed that Kv1 α-subunits are readily detected in isolated SCG neurons at the protein level (Fig. 3). As is illustrated in Figure 3B, for example, Kv1.4 expression is readily detected in the cell bodies of isolated SCG neurons. The expression pattern for Kv1.2 is distinct (from that of Kv1.4) in that Kv1.2 staining is seen throughout the processes of isolated SCG neurons, whereas Kv1.4 is only detected in cell bodies and proximal processes (Fig. 3A,B). In addition, all SCG cells are labeled with the anti-Kv1.2 antibody (Fig. 3A), whereas only a subset of cells express Kv1.4 (Fig. 3B). In contrast, Kv1.5 protein expression was not detected in SCG neurons (Fig. 3C).
Fig. 3.
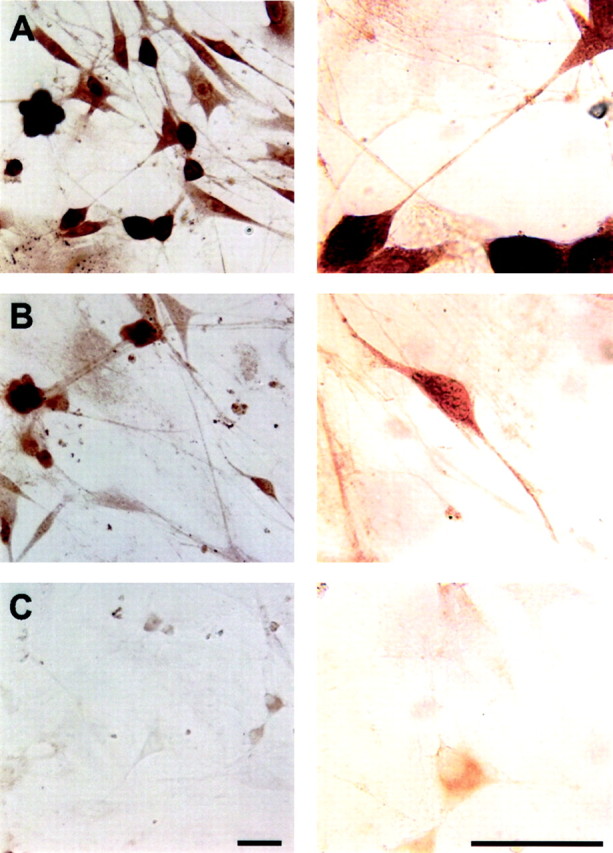
Expression of Kv1 α-subunits in SCG neurons. Isolated wild-type SCG neurons were fixed and probed with polyclonal antibodies generated against Kv1.2 (A), Kv1.4 (B), or Kv1.5 (C) 48 hr after plating, as described in Materials and Methods. As is evident, the Kv1.2 and Kv1.4 α-subunits are readily detected in SCG neurons, although the staining patterns of these subunits are distinct. Scale bars, 50 μm.
Expression of Kv1.5W461F eliminates IAfin a subset of SCG neurons
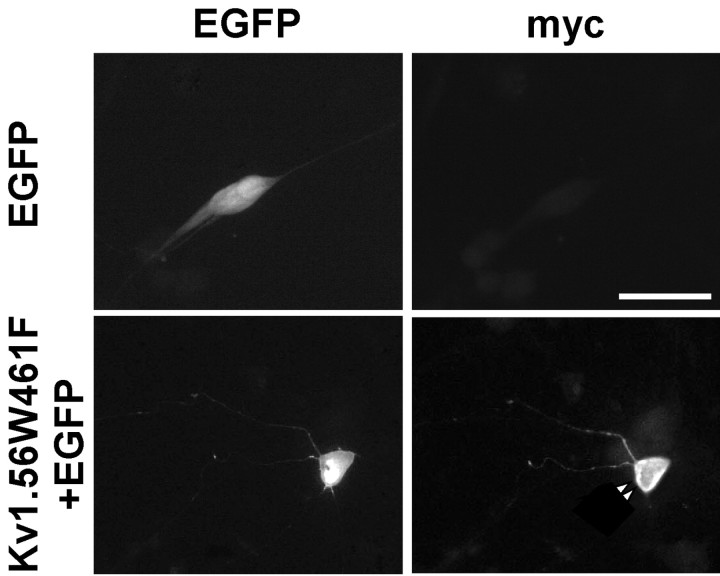
To explore directly the role of Kv1 α-subunits, the Kv1 subfamily-specific dominant negative construct, Kv1.5W461F-myc (Li et al., 1999), was used. Previous studies have shown that heterologous co-expression [in human embryonic kidney (HEK)-293 cells] of Kv1.5W461F-myc with either Kv1.5 or Kv1.4 attenuates current amplitudes relative to cells expressing (wild-type) Kv1.5 or Kv1.4 alone (Li et al., 1999). To examine the effects of Kv1.5W461F expression on the outward K+ currents in SCG neurons, beads were coated with cDNA constructs encoding Kv1.5W461F-myc and EGFP, and SCG cells were transfected using the Biolistic Gene Gun (see Materials and Methods). Within ∼24 hr of transfection, EGFP was readily detected under epifluorescence illumination (Fig.4); ∼10% of the cells in these cultures were EGFP-positive. To determine whether EGFP-positive SCG cells also express the Kv1.5W461F transgene, the cultures were fixed ∼48 hr after transfection and probed with an anti-mycantibody. These experiments revealed that all EGFP-positive cells (n = 100) also express Kv1.5W461F-myc. An example of an EGFP-positive, Kv1.5W461F-myc-positive SCG cell is shown in Figure 4, (bottom panels). Themyc staining appears to be predominantly on the cell surface (arrowheads), whereas EGFP expression is detected in the cytosol (Fig. 4, bottom).
Fig. 4.
Expression of Kv1.5W461F-myc in transfected SCG neurons. Isolated SCG neurons, transfected with EGFP alone (top) or with Kv1.5W461F-myc and EGFP (bottom) using the gene gun, were fixed and stained 24 hr later (see Materials and Methods). The top andbottom panels show EGFP fluorescence (left) and Cy3 fluorescence (right) images of the same field. Anti-myc staining is only evident in cultures transfected with Kv1.5W461F-myc (compare right panels,top and bottom). In addition, EGFP expression correlates with Kv1.5W461F (bottom, compare left and right panels). Scale bar, 50 μm.
Representative whole-cell voltage-gated outward K+ current waveforms recorded from Kv1.5W461F-expressing SCG cells are presented in Figure 1 (right panel). In Kv1.5W461F-expressing type I cells (Fig. 1,right panel), IAf is evident, and the mean ± SEM current density (57 ± 6 pA/pF) is similar to that seen in wild-type I cells (Table 1). Mean ± SEM IK (90 ± 13 pA/pF) andISS (52 ± 8 pA/pF) densities in Kv1.5W461F-expressing type I cells are also similar to the densities in wild-type I cells (Table 1). The percentage (44%; 15 of 34) of type I SCG neurons, however, is substantially lower than seen in recordings from wild-type cells, in which 70% of cells are classified as type I (Fig. 1, left panel). In parallel with the decrease in the percentage of type I cells, there is a marked increase in the percentage of type III cells (Fig. 1, compare left andright panels), which express onlyIK andISS. In contrast, the percentage of Kv1.5W461F-expressing cells classified as type II is similar to the percentage of wild-type cells displaying this phenotype (Fig. 1, compare left and right panels). The densities of the currents and mean ± SEM τdecay values for IAf (72 ± 8 msec),IAs (585 ± 47 msec), andIK (3125 ± 438 msec) in Kv1.5W461F-expressing type II cells are not significantly different from those in wild-type II cells (Table 1). These observations suggest that Kv1 α-subunits do not contribute measurably to the voltage-gated outward K+ currents in wild-type II SCG cells (see Discussion).
The concomitant decrease in type I cells and the increase in type III cells with the expression of Kv1.5W461F suggests that the functional removal of channels encoded by Kv1 α-subunits converts ∼25% of type I cells to type III by specific elimination ofIAf. The expression of Kv1.5W461F also results in a shift in the distribution of τdecay values forIAf (Fig. 2). In cells expressing Kv1.5W461F, τdecay values range from 54 to 150 msec, with a mean ± SEM value of 88 ± 8 msec (Table 1). This value is significantly (p < 0.001) smaller than the mean ± SEM τdecay (190 ± 18 msec) for IAf remaining in Kv4.2W362F-expressing cells (Table 1, Fig. 2), consistent with the hypothesis that there are two molecularly distinct components ofIAf.
IAf is eliminated in all SCG cells co-expressing Kv4.2W362F and Kv1.5W461F
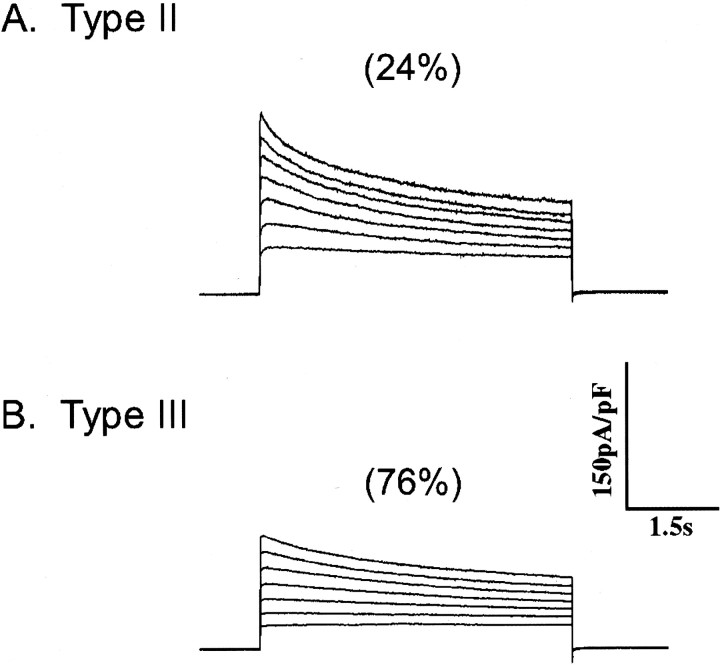
To test the hypothesis that there are two populations ofIAf channels encoded by distinct Kv subfamilies, SCG cells were cotransfected with Kv1.5W461F and Kv4.2W362F (and EGFP). Representative outward K+ currents recorded from EGFP-positive cells are presented in Figure 5. As is evident, the rapidly inactivating IAf, prominent in wild-type SCG cells (Fig. 1, left panels), is not evident in cells expressing Kv4.2W362F and Kv1.5W461F (Fig. 5). The majority (∼75%) of Kv4.2W362F–Kv1.5W461F-expressing cells are classified as type III (Fig. 5). This is a significant increase in type III cells, compared with wild-type, Kv4.2W362F-expressing, or Kv1.5W461F-expressing cells (Fig. 1). The mean ± SEM densities ofIK (135 ± 60 pA/pF) andISS (86 ± 12 pA/pF) in the Kv1.5W461F–Kv4.2W362F-expressing type III cells are not significantly different from the densities of these currents determined in wild-type III cells (Table 1). Analysis of the outward K+ currents in the remaining (∼25%) cells reveals the presence of IAs,IK, andISS, suggesting that these are type II cells lacking IAf (Table 1).
Fig. 5.
Coexpression of both Kv1.5W461F and Kv4.2W362F eliminates IAf. Isolated SCG neurons were transfected with Kv1.5W461F, Kv4.2W362F, and EGFP using the Biolistic Gene Gun (as described in Materials and Methods), and outward K+ currents were recorded from EGFP-positive cells as described in the legend to Figure 1. Two distinct current waveforms were evident in these recordings: the vast majority (76%) of cells were found to express only IK andISS and were classified as type III (B); the remaining cells (24%) expressIAs,IK, andISS, and, therefore, are type II cells lacking IAf (A). The fast transient current IAf was not detected in any of these cells (n = 15). Analysis of the decay phases of the currents revealed that the densities ofIAs,IK, andISS in Kv1.5W461F + Kv4.2W362F-expressing type II and III cells are indistinguishable from the currents in wild-type II and III cells (Table 1).
Expression of Kv1.5W461F increases excitability of SCG cells
Subsequent experiments were aimed at determining the effects of eliminating the Kv1-mediated IAf on the waveforms of individual action potentials and the repetitive firing properties of SCG neurons. As reported previously, current-clamp recordings from wild-type SCG cells reveal that ∼45% of the cells are “phasic,” firing one or two action potentials in response to a prolonged (500 msec) depolarizing current injection (Malin and Nerbonne, 2000). Increasing the amplitude of the injected current does not markedly influence the number of action potentials recorded (Malin and Nerbonne, 2000). Approximately 30% of wild-type SCG cells display the “adapting” phenotype. In these cells, low-amplitude current injections produce repetitive firing, and the firing frequency decreases over time. At higher amplitude current injections, adapting cells cease firing and are refractory (Malin and Nerbonne, 2000). Adapting cells are further distinguished from phasic cells by increased input resistances and decreased current thresholds for action potential generation (Table 2). The remaining (∼25%) wild-type SCG neurons are “tonic,” regular-spiking cells that fire at a fixed frequency during prolonged current injections up to 300 pA (Malin and Nerbonne, 2000). In addition, individual action potentials in tonic cells are brief (Table 2), and the firing frequency increases with the amplitude of the injected current (Malin and Nerbonne, 2000). Briefer action potential durations and the lack of adaptation (Table 2) clearly distinguish tonic from phasic and adapting SCG neurons.
Table 2.
Effects of Kv1.5W461F and Kv4.2W362F expression on SCG neuron firing properties 2-a
| Rin(GΩ) | Vm (mV) | APThresh(pA) | APA (mV) | APD50 (msec) | APD90(msec) | n | ||
|---|---|---|---|---|---|---|---|---|
| Wild type | All cells | 0.34 ± 0.03 | −48 ± 1 | 46 ± 5 | 82 ± 4 | 3.66 ± 0.14 | 5.92 ± 0.20 | 28 |
| Phasic | 0.26 ± 0.02 | −45 ± 2 | 67 ± 1 | 80 ± 5 | 4.18 ± 0.24 | 6.55 ± 0.34 | 12 | |
| Adapting | 0.60 ± 0.06 | −48 ± 1 | 24 ± 2 | 87 ± 3 | 3.57 ± 0.20 | 5.96 ± 0.20 | 9 | |
| Tonic | 0.22 ± 0.02 | −52 ± 1 | 42 ± 2 | 91 ± 3 | 2.97 ± 0.03 | 4.87 ± 0.20 | 7 | |
| Kv1.5W461F | All cells | 0.38 ± 0.04 | −48 ± 1 | 32 ± 3 | 89 ± 8 | 3.08 ± 0.14 | 5.26 ± 0.25 | 25 |
| Phasic | 0.30 ± 0.03 | −49 ± 2 | 43 ± 62-160 | 90 ± 3* | 3.16 ± 0.18* | 5.36 ± 0.37* | 6 | |
| Adapting | 0.48 ± 0.06 | −47 ± 2 | 24 ± 2 | 89 ± 3 | 3.25 ± 0.24 | 5.51 ± 0.45 | 11 | |
| Tonic | 0.30 ± 0.05 | −50 ± 1 | 36 ± 5 | 92 ± 2 | 2.77 ± 0.21 | 4.84 ± 0.41 | 8 | |
| Kv4.2W362F | All cells | 0.68 ± 0.08 | −49 ± 1 | 28 ± 2 | 90 ± 2 | 3.54 ± 0.27 | 5.59 ± 0.22 | 25 |
| Phasic | 0.64 ± 0.142-160 | −47 ± 2 | 30 ± 72-160 | 82 ± 3 | 3.63 ± 0.44 | 6.07 ± 0.33 | 6 | |
| Adapting | 0.76 ± 0.12 | −47 ± 1 | 26 ± 3 | 89 ± 2 | 3.47 ± 0.14 | 5.76 ± 0.23 | 13 | |
| Tonic | 0.60 ± 0.092-160 | −53 ± 3 | 27 ± 22-160 | 99 ± 4 | 2.90 ± 0.09 | 4.90 ± 0.20 | 6 | |
| Kv1.5W461F + Kv4.2W362F | All cells | 1.08 ± 0.172-160 | −44 ± 2 | 35 ± 4 | 88 ± 3 | 3.07 ± 0.19* | 5.11 ± 0.30* | 38 |
| Phasic | 0.36 ± 0.04* | −43 ± 4 | 38 ± 72-160 | 90 ± 7 | 2.76 ± 0.242-160 | 4.71 ± 0.312-160 | 5 | |
| Adapting | 1.28 ± 0.17* | −42 ± 2 | 25 ± 2 | 81 ± 6 | 3.75 ± 0.43 | 6.11 ± 0.66 | 17 | |
| Tonic | 0.47 ± 0.042-160 | −47 ± 2 | 31 ± 5 | 94 ± 4 | 2.65 ± 0.24 | 4.54 ± 0.39 | 16 |
All values are means ± SEM. Rin, Input resistance;Vm, resting membrane potential; APthresh, current required to reach the threshold for generating an action potential; APA, action potential amplitude.
Mean values are significantly different from those in wild-type cells at the
p < 0.03 and
F2-160: p < 0.005 levels.
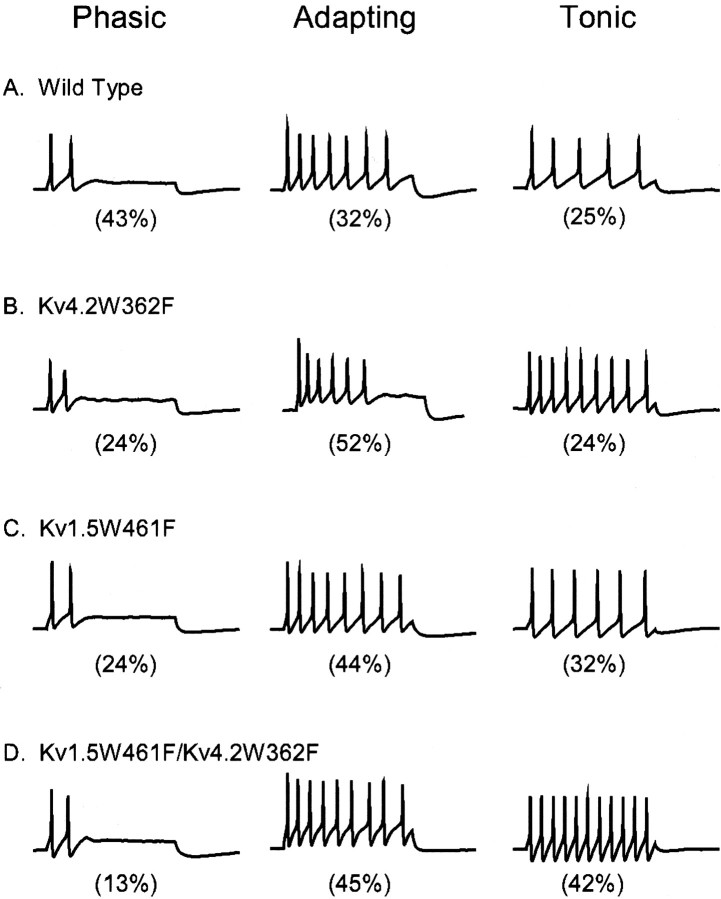
The phasic, adapting, and tonic firing patterns are also seen in recordings obtained from Kv1.5W461F-expressing SCG cells (Fig.6). The distribution of SCG cell firing patterns, however, is markedly affected by Kv1.5W461F expression. Functional elimination of Kv1-encodedIAf channels reduces the number of phasic cells (Fig. 6A) from 43% of wild-type to 24% of Kv1.5W461F-expressing cells (Table 2). In contrast, the percentage of adapting cells (Fig. 6B) increases from 32% of wild-type to 44% of Kv1.5W461F-expressing cells (Table 2). The percentage of tonic cells (Fig. 6C) is also increased slightly (from 25 to 32%) with Kv1.5W461F expression (Table 2). Thus, the selective removal of the Kv1-mediatedIAf converts many (∼40%) phasic cells to the adapting or tonic firing patterns (Fig. 6, Table 2). Interestingly, the percentage of type I cells with Kv1-encodedIAf channels (∼25%) is very similar to the percentage of cells (∼20%) converted from phasic firing with Kv1.5W461F expression, suggesting that type I cells with Kv1-encodedIAf channels are phasic (see Discussion). In contrast to the findings with Kv4.2W362F, however, expression of Kv1.5W461F does not affect the input resistances of phasic or tonic cells (Table 2). Thus, the properties of Kv1- and Kv4-encoded IAf channels are distinct, in that Kv1-encoded IAf channels are not open at rest and, therefore, do not contribute to the resting input resistances of SCG neurons.
Fig. 6.
Expression of Kv1.5W461F increases the percentage of adapting cells. Isolated SCG neurons were transfected with Kv1.5W461F (and EGFP), and action potentials and repetitive firing patterns were recorded in response to brief or prolonged depolarizing current injections, as described in Materials and Methods. Current-clamp recordings from (3) representative Kv1.5W461F-expressing cells are shown in A, B, andC. In each cell, single action potentials were elicited by 1.5 msec current injections (left), and repetitive firing patterns were recorded in response to 100 pA (middle) or 200 pA (right) 500 msec current injections. On the basis of the response(s) to the 500 msec current injections, cells were classified as phasic (A), adapting (B), or tonic (C) (Table 2). With Kv1.5W461F expression, the percentages of adapting and tonic cells are increased, and the percentage of phasic cells is decreased relative to the firing pattern distribution seen in wild-type cells (Table 2).
Analysis of the properties of the phasic firing cells remaining with Kv1.5W461F expression revealed that the mean ± SEM current threshold for action potential generation in the phasic cells lacking Kv1-encoded IAf (i.e., the remaining phasic cells) is significantly (p < 0.005) lower than the mean determined in wild-type phasic cells (Table 2). In addition, the mean ± SEM action potential amplitude in the remaining phasic cells is significantly (p < 0.03) larger than in wild-type phasic cells (Table 2). These observations are consistent with the suggestion that there are two distinct populations of phasic SCG cells and, further, that the current thresholds for action potential generation are lower and action potential amplitudes are higher in the subset of phasic cells lacking Kv1-encoded IAf channels. Action potential durations in the remaining phasic cells are also decreased significantly (p < 0.005) compared with wild-type phasic cells (Table 2). This unexpected observation suggests that there are additional repolarizing currents activated in phasic cells lacking Kv1-encoded IAfchannels, perhaps because of the increased action potential amplitudes in these cells (Table 2). The mean ± SEM current threshold values for action potential generation, as well as action potential amplitudes and durations, in adapting and tonic SCG cells, in contrast, are not affected by Kv1.5W461F expression (Table 2), consistent with the suggestion that Kv1-encoded IAfchannels specifically tune excitability in phasic cells (see Discussion).
Complete removal of IAf markedly increases excitability and further reduces the percentage of phasic cells
In SCG neurons transfected with both the Kv1.5W461F and the Kv4.2W362F dominant negative constructs, the distribution of repetitive firing patterns is altered markedly (Fig.7D) compared with wild-type cells (Fig. 7A), as well as with cells expressing Kv4.2W362F (Fig. 7B) or Kv1.5W461F (Fig. 7C) alone. After the complete removal of IAf, few phasic cells remain (13%) (Fig. 7D), and the percentages of adapting and tonic cells are increased. These effects appear to be the sum of the Kv1- and Kv4-mediated changes; removal of either the Kv1- or the Kv4-encoded IAf channels alone increases the percentage of adapting cells at the expense of phasic cells, and removal of Kv1 channels also increases tonic cell number (Fig. 7). As seen with the expression of Kv4.2W362F alone, the mean ± SEM input resistance of SCG cells expressing Kv1.5W461F and Kv4.2W362F is significantly (p < 0.005) increased compared with wild-type cells (Table 2). These findings are expected because expression of Kv4.2W362F alone significantly affects the mean ± SEM input resistance (Table 2). Expression of Kv1.5W461F and Kv4.2W362F, like expression of either Kv1.5W461F or Kv4.2W362F alone, also decreases the current thresholds for action potential generation (Table 2).
Fig. 7.
Elimination of the Kv1- and Kv4-encodedIAf increases the percentage of adapting SCG neurons. Action potentials and repetitive firing patterns were recorded from isolated SCG neurons 24 hr after transfection with EGFP alone (A), EGFP and Kv4.2W362F (B), EGFP and Kv1.5W461F (C), or EGFP, Kv1.5W461F and Kv4.2W362F (D), as described in the legend to Figure 6. The phasic (left), adapting (middle), and tonic (right) firing patterns were evident in recordings obtained under all of these experimental conditions. In recordings from cells with reduced IAf density, the percentages of phasic cells are lower, and the percentages of adapting and tonic cells are higher than seen in recordings from wild-type cells (Tables 2, 4, 5).
Increasing Kv1-mediated IAf converts adapting cells to phasic firing
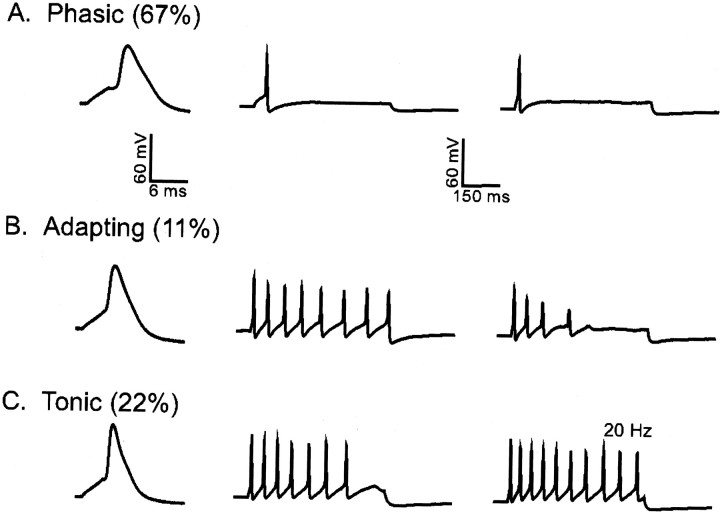
The experiments described above demonstrate a role for Kv1 α-subunits in the generation of IAfin a subset of type I phasic-firing SCG neurons. Of the Kv1 α-subunits, only Kv1.4 produces rapidly inactivating outward K+ currents when expressed alone in heterologous expression systems (Coetzee et al., 1999). In addition, Kv1.4 has recently been shown to underlie the slow transient outward K+ current in mammalian cardiac cells (Guo et al., 1999, 2000), and Kv1.4 is expressed at the protein level in SCG neurons (Fig. 3). Subsequent experiments, therefore, were aimed at examining the effects of “over-expression” of Kv1.4 on the outward K+ currents and the firing properties of SCG neurons. Representative current-clamp recordings from Kv1.4-expressing cells are shown in Figure8. The percentage of phasic cells (Fig.8A) is increased markedly (to 67%) with Kv1.4 expression, relative to the percentage (43%) of wild-type phasic SCG cells (Table 3). The fraction of adapting cells is decreased (Fig. 8B), whereas the percentage of tonic cells is unchanged (Fig. 8C, Table 3). Indeed, of the 18 cells examined, only two adapting cells were detected, suggesting that Kv1.4 expression converts most adapting cells to phasic firing. Consistent with the increase in phasic cells and the decrease in adapting cells, the mean ± SEM current threshold for action potential generation is increased significantly (p < 0.005) in all Kv1.4-expressing SCG cells (Table 3). These data support our hypothesis that the current threshold for action potential generation is determined largely byIAf density and is linked to firing pattern. Expression of Kv1.4 also significantly (p < 0.04) decreases mean ± SEM APD50 values in phasic cells, whereas action potential durations in Kv1.4-expressing tonic and adapting cells are not significantly different from those in wild-type tonic and adapting cells (Table 3).
Fig. 8.
Expression of Kv1.4 increases the percentage of phasic firing SCG cells. Action potentials and repetitive firing patterns, recorded as described in the legend to Figure 6, were obtained from isolated SCG neurons 24 hr after transfection with wild-type Kv1.4 and EGFP. As in wild-type cells, the phasic (A), adapting (B), and tonic (C) firing patterns were seen in recordings from cells transfected with Kv1.4. The percentage of adapting cells, however, is lower and the percentage of phasic cells is higher in Kv1.4-expressing cells than seen in recordings from wild-type or Kv1.5W461F-expressing SCG cells (Figs. 6, 7, Tables 2, 4).
Table 3.
Expression of Kv1.4 decreases the excitability of SCG neurons 3-a
| Rin (GΩ) | Vm(mV) | APThresh (pA) | APA (mV) | APD50(msec) | APD90 (msec) | n | |
|---|---|---|---|---|---|---|---|
| All cells | 0.40 ± 0.09 | −47 ± 2 | 80 ± 123-150 | 84 ± 3 | 3.24 ± 0.23 | 5.50 ± 0.36 | 18 |
| Phasic | 0.39 ± 0.12 | −46 ± 3 | 100 ± 153-160 | 79 ± 3 | 3.29 ± 0.323-160 | 5.53 ± 0.53 | 12 |
| Adapting | 0.71 | −47 | 20 | 91 | 3.72 | 6.15 | 2 |
| Tonic | 0.29 ± 0.05 | −52 ± 2 | 50 ± 9 | 95 ± 3 | 2.86 ± 0.08 | 5.10 ± 0.14 | 4 |
All values are means ± SEM.
Values are significantly different from those in wild-type cells at the
F3-150: p < 0.005 and
F3-160: p< 0.04 levels (Table 2).
Representative voltage-clamp recordings obtained from SCG neurons expressing Kv1.4 (and EGFP) are shown in Figure9. Analysis of the decay phases of the currents revealed that IAf density is increased significantly (p < 0.02) to a mean ± SEM value of 141 ± 9 pA/pF in Kv1.4-expressing type I cells. In addition, the mean ± SEM τdecay value forIAf (169 ± 10 msec) in Kv1.4-expressing type I cells is significantly larger than the mean time constant for IAf (121 ± 14 msec) in wild-type I cells (Table 1). Interestingly, over-expression of Kv4.2 reduces the mean ± SEM τdecay forIAf (Table 3), revealing that Kv1.4 and Kv4.2 encode kinetically distinct K+currents when expressed in sympathetic neurons. These differences are also reflected in the ranges of IAfτdecay values seen. In wild-type I cells, for example, IAfτdecay values range from 40 to 209 msec (Fig.9D), whereas in SCG cells expressing Kv1.4,IAf τdecayvalues range from 142 to 205 msec. This change is distinct from that seen in SCG cells expressing Kv4.2, in which τdecay values range from 50 to 128 msec (Fig.9D, Table 4). Thus, exogenous Kv1.4 encodes a fast transient current in SCG neurons with inactivation kinetics similar to the component eliminated with Kv1.5W461F expression (Fig. 1, Tables 1, 4). Furthermore, this component is kinetically distinct from that encoded by Kv4.2 (Fig. 9D, Table 4).
Fig. 9.
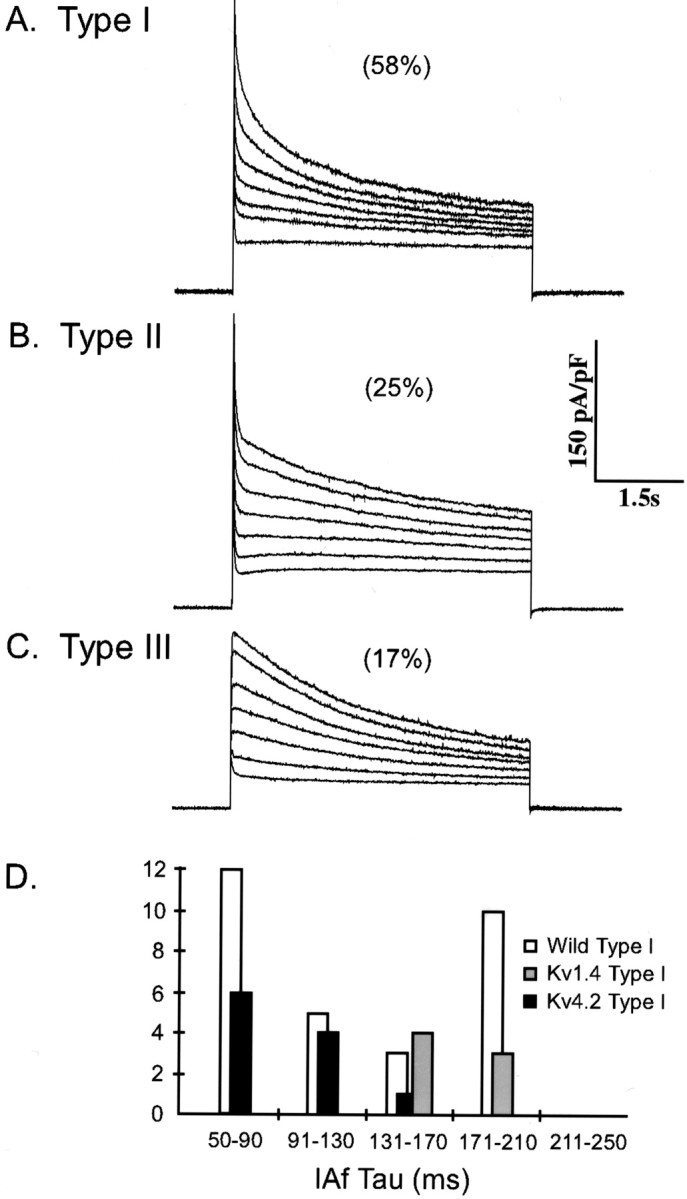
Expression of Kv1.4 increasesIAf only in type I SCG neurons. Isolated SCG neurons were transfected with Kv1.4 and EGFP using the Biolistic Gene Gun (as described in Materials and Methods), and outward K+ currents were recorded from EGFP-expressing cells as described in the legend to Figure 1. Analysis of the decay phases of the currents provided the mean ± SEM densities ofIAf,IAs,IK, andISS (Table 1) and allowed classification of Kv1.4-expressing cells as type I (A), type II (B), or type III (C). The distribution of (type I, type II, and type III) cells is unaffected by Kv1.4 expression, and the mean ± SEMIAf density is increased significantly (p < 0.001) in type I SCG cells, as compared with wild-type I cells (Table 1). Interestingly, type II and type III cells are unaffected by expression of Kv1.4. The distributions of IAf τdecay values in wild-type, Kv4.2-expressing, and Kv1.4-expressing type I cells are distinct, consistent with the hypothesis that Kv4.2 and Kv1.4 encode kinetically distinct fast transient currents in SCG cells (D).
Table 4.
SCG neurons transfected with wild-type Kv4.2 or Kv1.4 4-a
| Kv4.24-b | Kv1.4 | ||||
|---|---|---|---|---|---|
| Type I | Type II | Type I | Type II | Type III | |
| Peak current density | 249 ± 27 | 291 ± 37 | 281 ± 39 | 275 ± 34 | 156 ± 45 |
| IAf | |||||
| Density (pA/pF) | 127 ± 94-150 | 136 ± 16 | 141 ± 94-150 | 83 ± 8 | |
| τ (msec) | 87 ± 10 | 70 ± 8 | 169 ± 104-160 | 66 ± 10 | |
| IAs | |||||
| Density (pA/pF) | 57 ± 21 | 30 ± 7 | |||
| τ (msec) | 472 ± 8 | 557 ± 107 | |||
| IK | |||||
| Density (pA/pF) | 78 ± 17 | 67 ± 2 | 78 ± 14 | 101 ± 31 | 119 ± 36 |
| τ (msec) | 2126 ± 518 | 2151 ± 195 | 2050 ± 261 | 3588 ± 344 | 4031 ± 466 |
| ISS | |||||
| Density (pA/pF) | 40 ± 6 | 38 ± 17 | 54 ± 12 | 74 ± 9 | 43 ± 13 |
| n | 11 | 5 | 7 | 3 | 2 |
All values are mean ± SEM; current densities and τdecay values were determined for currents evoked at +50 mV; n = number of cells.
Data from Malin and Nerbonne, (2000).
F4-150: Values are significantly (p < 0.02) higher than in wild-type I SCG cells (see Table 1);
F4-160: values are significantly (p < 0.001) larger than in Kv4.2-transfected cells.
In contrast, mean IK andISS densities, as well as the kinetics of IK decay, are not significantly different in Kv1.4-expressing and wild-type I cells (compare Tables 1,4). Interestingly, Kv1.4 expression has no effect onIAf density (83 ± 8 pA/pF) in type II cells (Fig. 9B, Table 4). The other currents,IAs,IK, andISS, in type II cells are also unaffected by Kv1.4 expression (Table 4). Similarly, the densities and properties of IK andISS in type III cells expressing Kv1.4 are indistinguishable from those seen in wild-type III cells (Tables 1,4). Together, these results suggest that functional Kv1-encodedIAf channels are not expressed in type II or type III cells even when the (wild-type) Kv1.4 α-subunit is exogenously introduced (see Discussion).
DISCUSSION
Kv1 α-subunits underlie IAf in a subset of type I SCG neurons
The results of the experiments reported here demonstrate that expression of Kv1.5W461F results in the elimination ofIAf in a subset of type I SCG cells and a corresponding increase in the percentage of type III cells (Fig.1, Table 1). The properties and the densities of the currents in Kv1.5W461F-expressing type II and type III cells are indistinguishable from the currents in wild-type II and III cells, suggesting that Kv1 α-subunits do not make a significant contribution toIAf in type II cells or toIK andISS in type II and III cells. Importantly, the experiments here also demonstrate that the residualIAf present in Kv4.2W362F-expressing type I SCG cells (Malin and Nerbonne, 2000) does not reflect incomplete suppression of Kv4-encoded IAfchannels. Rather, the residual IAf in the Kv4.2W362F-expressing SCG cells reflects Kv1-encodedIAf channels. In addition, the mean ± SEM IAfτdecay values determined in Kv1.5W461F- and Kv4.2W362F-expressing cells are significantly (p< 0.001) different (Fig. 2), consistent with the hypothesis that there are two types of IAf channels in wild-type SCG neurons that reflect the expression of distinct gene products. Because co-expression of Kv1.5W461F and Kv4.2W362F eliminatesIAf in all SCG cells (Fig. 5, Table1), it is also possible to conclude that Kv1 and Kv4 α-subunits must encode all of the fast transient current (IAf) in these cells.
Previous studies have demonstrated the presence of distinct types of transient outward K+ currents in neurons and muscle cells and shown that these are encoded by Kv1 and Kv4 α-subunits (Solc et al., 1987; Tsunoda and Salkoff, 1995; Guo et al., 1999, 2000). In contrast to the findings here, however, the transient outward K+ currents inDrosophila neurons and myocytes (Solc et al., 1987; Tsunoda and Salkoff, 1995), as well as those in mammalian cardiac myocytes (Guo et al., 1999, 2000), display markedly different time- and voltage-dependent properties and are readily distinguished electrophysiologically. Interestingly, however, the identification of two molecularly distinct components of a “single” electrophysiologically defined current has been reported previously forIKr in Xenopus spinal neurons (Ribera, 1996) and for IK, slow in mouse ventricular myocytes (Xu et al., 1999). These findings corroborate the power of the molecular pharmacological approach exploited here and in these previous studies (Ribera, 1996; Xu et al., 1999). The Kv α-subunit-specific dominant negative strategy selectively eliminates individual current components, allowing the characterization of detailed properties of the currents remaining (as well as of the currents eliminated). In the case ofIAf in SCG neurons, experiments completed on cells expressing Kv1.5W461F or Kv4.2W362F revealed the presence of two current components that had not previously been distinguished using conventional electrophysiological and/or pharmacological methods. The molecular pharmacological approach exploited here also allowed determination of the functional roles of the individual Kv1- and Kv4-encoded components ofIAf in shaping the waveforms of individual action potentials and repetitive firing patterns in SCG neurons.
Functional roles of IAf in SCG neurons
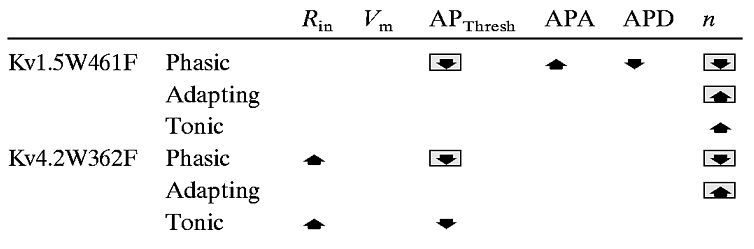
The experiments completed here reveal that the functional consequences of eliminating the Kv1- or the Kv4-encodedIAf channels in SCG cells are distinct, suggesting that the molecular heterogeneity inIAf has physiological significance. Elimination of Kv1-encoded IAfincreases excitability by decreasing the current threshold for action potential generation (Table 5), without measurably affecting cell input resistances. Removal of Kv4-encodedIAf channels also increases excitability by lowering current thresholds, although, in this case, input resistances are also increased (Table 5) (Malin and Nerbonne, 2000). In addition, the Kv1- and Kv4-encodedIAf channels play different roles in controlling repetitive firing in SCG neurons. Although elimination of either Kv1- or Kv4-encoded IAf reduces (by ∼50%) the number of phasic cells (Table 5), removal of the Kv4-encoded IAf converts phasic cells to adapting firing, whereas removal of the Kv1-encodedIAf converts some phasic cells to adapting and others to tonic firing (Fig. 7, Table 2). Interestingly, co-expression of Kv1.5W461F and Kv4.2W362F dramatically reduces, but does not eliminate, the number (percentage) of phasic cells. This finding suggests that, although IAf is an important contributor, it is not the sole determinant of phasic firing. In addition, expression of Kv4.2W362F significantly (p < 0.02) increases the mean (±SEM) firing rates in tonic cells (in response to 200 pA current injections) to 29 ± 2 Hz from the wild-type value of 15 ± 1 Hz. There is also an increase in the mean ± SEM tonic firing rate in Kv1.5W461F-expressing cells, although, in this case, the increase (to 20 ± 6 Hz) is modest (∼30%) and most Kv1.5W461F-expressing tonic cells fire at frequencies well within the wild-type range (Fig.7).
Table 5.
Phenotypic consequences of eliminating the Kv1- or Kv4-encoded components of IAf
 |
|---|
 indicates changes common to Kv1- and Kv4-encoded IAfelimination.
indicates changes common to Kv1- and Kv4-encoded IAfelimination.
As noted above, the experiments here revealed that the voltage-clamp effects of Kv1.5W461F expression are specific to type I cells; expression of Kv1.5W461F removes IAfin ∼25% of SCG cells, all of which are type I (Fig. 1, Table 1). In addition, expression of Kv1.5W461F converts ∼50% of phasic firing rat SCG cells to the adapting or tonic firing patterns (Fig. 7, Table5). Thus, we can conclude that ∼50% of the phasic cells are type I. The experiments here also revealed that the mean (±SEM) active and passive membrane properties of the phasic cells remaining with Kv1.5W461F expression are distinct from those of wild-type phasic cells (Table 3). Specifically, action potential amplitudes are greater, and action potential durations are shorter in the phasic cells lacking Kv1-encoded IAf channels (i.e., the phasic cells remaining with Kv1.5W461F expression). In contrast, action potential amplitudes and durations in Kv4.2W362F-expressing and wild-type phasic cells are indistinguishable (Table 5). These observations suggest that in the phasic cells expressing Kv1-encodedIAf channels, action potential amplitudes are lower, and action potential durations are longer than in phasic cells expressing Kv4-encodedIAf channels.
The distinct functional roles of Kv1- and Kv4-encodedIAf likely reflect differences in the voltage dependences of activation of the currents. The results of experiments completed here suggest, for example, that the Kv4-encodedIAf channels are open at more hyperpolarized potentials (than the Kv1-encoded channels) and affect neuronal excitability by decreasing resting SCG cell input resistance (Malin and Nerbonne, 2000). The Kv1-encodedIAf channels, in contrast, likely are only activated at more depolarized potentials and influence excitability, therefore, by opposing the action potential upstroke; Kv1-encoded IAf channels do not contribute to resting input resistance in SCG cells. In addition to this functional diversity, the expression of molecularly distinctIAf channels encoded by Kv1 and Kv4 α-subunits also provides a means for differential modulation of channel, and therefore, cellular, functioning through interactions with distinct transmitters and/or intracellular messenger pathways (Roeper et al., 1997; Wang et al., 1997; Pan et al., 2000). Clearly, experiments aimed at exploring the molecular mechanisms involved in the regulation and modulation of Kv1- and Kv4-encoded neuronalIAf channels will be of considerable interest.
Footnotes
This work was supported by a National Science Foundation predoctoral fellowship to S.A.M. and the National Institutes of Health Grant NS-30676.
Correspondence should be addressed to Dr. Jeanne M. Nerbonne, Washington University Medical School, 660 South Euclid Avenue, Box 8130, St. Louis, MO 63110. E-mail:jnerbonn@pcg.wustl.edu.
REFERENCES
- 1.An WF, Bowlby MR, Betty M, Cao J, Ling H-P, Mendoza G, Hinson JW, Mattson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 2.Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels? Circ Res. 1995;77:361–369. doi: 10.1161/01.res.77.2.361. [DOI] [PubMed] [Google Scholar]
- 3.Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knock-out of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 α-subunit. Circ Res. 1998;83:560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- 4.Bou-Abboud E, Nerbonne JM. Molecular correlates of the calcium-independent depolarization-activated K+ currents in rat atrial myocytes. J Physiol (Lond) 1999;517:407–420. doi: 10.1111/j.1469-7793.1999.0407t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JY, Martin DP, Johnson EM., Jr Interferon suppresses sympathetic neuronal cell death caused by nerve growth factor deprivation. J Neurochem. 1990;55:436–445. doi: 10.1111/j.1471-4159.1990.tb04155.x. [DOI] [PubMed] [Google Scholar]
- 6.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Meira E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JE, McKinnon D. Potassium channel mRNA expression in prevertebral and paravertebral sympathetic neurons. Eur J Neurosci. 1996;8:183–191. doi: 10.1111/j.1460-9568.1996.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Wible B, Li G-R, Wang Z, Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, Xu H, London B, Nerbonne JM. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol (Lond) 1999;521:587–599. doi: 10.1111/j.1469-7793.1999.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of Ito,f and Ito,s: early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 α-subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 11.Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Guo W, Nerbonne JM. Functional consequences of cardiac-specific expression of wild-type or mutant (dominant negative) Kv1.5 α-subunits in mouse ventricular myocytes. Circulation. 1999;102:II-261. [Google Scholar]
- 13.London B, Guo W, Pan X-H, Lee JS, Shusterman V, Rocco CJ, Logothetis DA, Nerbonne JM, Hill JA. Targeted replacement of Kv1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of IK,slow and resistance to drug-induced QT prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 14.Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of Kv4.2W362F: molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan S-J, Sumners C, Gelband CH. Kv1.4 underlies angiotensin II-mediated inhibition of neuronal A-type K+ current. Biophys J. 2000;78:450A. [Google Scholar]
- 16.Pankevych H, Kristufek D, Huck S. Perinatal and postnatal regulation of Shaker-related genes in rat superior cervical ganglion. Soc Neurosci Abstr. 1999;25:739.1. [Google Scholar]
- 17.Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999;452:31–35. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 18.Raff MC, Fields KL, Hakomori S-I, Mirsky R, Pruss RM, Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979;174:283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- 19.Ribera AB. Homogeneous development of electrical excitability via heterogeneous ion channel expression. J Neurosci. 1996;16:1123–1130. doi: 10.1523/JNEUROSCI.16-03-01123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel Kv1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;24:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 23.Solc CK, Zagotta WN, Aldrich RW. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987;236:1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- 24.Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw and Shab encode most embryonic potassium currents. J Neurosci. 1995;15:1741–1754. doi: 10.1523/JNEUROSCI.15-03-01741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Sumners C, Posner P, Gelband CH. A-type K+ current in neurons cultured from neonatal hypothalamus and brain stem: modulation by angiotensin II. J Neurophysiol. 1997;78:1021–1029. doi: 10.1152/jn.1997.78.2.1021. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 α-subunit. Circ Res. 1999;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]