Abstract
Desensitization of 5-HT1A receptors could be involved in the long-term therapeutic effect of anxiolytic and antidepressant drugs. Pretreatment of rats with the 5-HT2A/2C agonist DOI induces an attenuation of hypothalamic 5-HT1Areceptor–Gz-protein signaling, measured as the ACTH and oxytocin responses to an injection of the 5-HT1A agonist 8-OH-DPAT. We characterized this functional heterologous desensitization of 5-HT1A receptors in rats and examined some of the mechanisms that are involved. A time course experiment revealed that DOI produces a delayed and reversible reduction of the ACTH and oxytocin responses to an 8-OH-DPAT challenge. The maximal desensitization occurred at 2 hr, and it disappeared 24 hr after DOI injection. The desensitization was dose-dependent, and it shifted the oxytocin and ACTH dose–response curves of 8-OH-DPAT to the right (increased ED50) with no change in their maximal responses (Emax). The 5-HT2A receptor antagonist MDL 100,907 prevented the DOI-induced desensitization, indicating that 5-HT2Areceptors mediate the effect of DOI. Analysis of the components of the 5-HT1A receptor–Gz-protein signaling system showed that DOI did not alter the level of membrane-associated Gz-proteins in the hypothalamus. Additionally, DOI did not alter the binding of [3H]8-OH-DPAT or the inhibition by GTPγS of [3H]8-OH-DPAT binding in the hypothalamus. In conclusion, the activation of 5-HT2Areceptors induces a transient functional desensitization of 5-HT1A receptor signaling in the hypothalamus, which may occur distal to the 5-HT1A receptor–Gz-protein interface.
Keywords: neuroendocrine, serotonin, oxytocin, ACTH, Gz-protein, [3H]8-OH-DPAT binding, GTPγS
Among the seven families of serotonin receptors, 5-HT1A and 5-HT2A receptors have an important role in mood disorders (Stockmeier et al., 1997; Staley et al., 1998; Olivier et al., 1999; Sargent et al., 2000). Selective serotonin reuptake inhibitors (SSRIs) produce a desensitization of 5-HT1A receptors (Li et al., 1996; Berlin et al., 1998; Raap et al., 1999; Bosker et al., 2001) and alter the functioning of 5-HT2A receptors (Tilakaratne et al., 1995;Bonson et al., 1996; Raap and Van de Kar, 1999). Desensitization of 5-HT1A receptors could contribute to the therapeutic effects of SSRIs. Because chronic treatment with SSRIs results in long-term elevation in the levels of 5-HT in the synapse, SSRIs could produce a heterologous desensitization of 5-HT1A receptors by the activation of other 5-HT receptors.
5-HT1A receptors and 5-HT2receptors interact via their signaling proteins (Katada et al., 1985;Berg et al., 1998; Tournois et al., 1998; Evans et al., 2001). In Chinese hamster ovary (CHO) cells stably expressing the human 5-HT1A receptors and 5-HT2Creceptors, the activation of 5-HT2C receptors induces a heterologous desensitization of 5-HT1Areceptors via protein kinase C and a cyclooxygenase-dependent metabolite of arachidonic acid (Evans et al., 2001). 5-HT2A receptors are coupled also via Gq/11 proteins to phospholipase C and phospholipase A2 signaling pathways (Garcia and Kim, 1997; Grotewiel and Sanders-Bush, 1999). Thus 5-HT2A receptors could cross-talk to 5-HT1A receptors to produce a heterologous desensitization of 5-HT1A receptors.
Functional interactions between 5-HT1A receptors and 5-HT2A/2C receptors have been demonstrated in behavioral studies. These studies either examined the effect of 5-HT1A receptor activation on 5-HT2A/2C receptor-mediated behaviors (Darmani et al., 1990; Krebs-Thomson and Geyer, 1998) or determined the impact of 5-HT2A/2C receptor desensitization on the behavioral and temperature responses to 5-HT1Areceptor activation (Hensler and Truett, 1998). To our knowledge, only one group has examined the impact of 5-HT2A/2Creceptor activation on the reproductive behavioral response to 8-OH-DPAT (Maswood and Uphouse, 1997).
5-HT1A receptors labeled with [3H]8-OH-DPAT and 5-HT2A/2C receptors labeled with [I125]DOI are found in the hypothalamic paraventricular nucleus (PVN) (Appel et al., 1990; Li et al., 1997), where oxytocin and corticotropin-releasing factor (CRF) cells are located (Sawchenko and Swanson, 1985). Evidence indicates that 8-OH-DPAT-induced release of oxytocin and adrenocorticotropic hormone (ACTH) is mediated by 5-HT1Areceptor–Gz-protein signaling in the hypothalamic paraventricular nucleus (Serres et al., 2000). Activation of hypothalamic 5-HT2A receptors also mediates oxytocin and ACTH release (Bagdy 1996; Van de Kar et al., 2001). Thus the 8-OH-DPAT-induced increase in the plasma levels of ACTH and oxytocin is a useful index of the sensitivity of hypothalamic 5-HT1A receptors and can be used to examine the interaction between 5-HT2A and 5-HT1A receptors in the hypothalamus.
The present study is the first in vivo characterization of a functional heterologous desensitization of 5-HT1Areceptors after 5-HT2A receptor activation with DOI. In addition, the hypothalamic levels of membrane-associated Gz-protein and the coupling of hypothalamic 5-HT1A receptors to G-proteins were examined as possible underlying mechanisms for this heterologous desensitization.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (225–275 gm) were purchased from Harlan Sprague Dawley (Indianapolis, IN). The rats were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on from 7:00 A.M. to 7:00 P.M.). Food and water were available ad libitum. All procedures were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by Loyola University Institutional Animal Care and Use Committee.
Drugs
±8-Hydroxy-2-(di-n-propylamino) tetralin hydrobromide ([3H]8- OH-DPAT) and (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCl (DOI) were purchased from Research Biochemicals (Natick, MA). (±)-α-(2,3-Dimethoxyphenyl)-1-(2-fluorophenylethyl-4-piperidinemethanol (MDL 100,907) was donated generously by Hoechst Marion Roussel Research Institute (Cincinnati, OH). 8-OH-DPAT was dissolved in 0.9% saline at four concentrations (0.03, 0.05, 0.1, and 0.5 mg/ml). DOI was dissolved in 0.9% saline at three concentrations (0.5, 2.5, and 5 mg/ml). MDL 100,907 was dissolved in a minimal volume of 0.01N HCl and diluted with saline to the final concentration of 0.01 mg/ml. All solutions were made fresh before injections and injected at a volume of 1 ml/kg.
Experimental protocols
After their arrival, the rats were housed two per cage for at least 1 week, followed by 4 d of handling. On the day of the experiments the rats were assigned randomly to different experimental groups (n = 8 per group) to receive drug treatments. Cage mates were assigned to the same treatment group. After receiving different drug treatments, the rats were decapitated. The trunk blood was collected in centrifuge tubes containing a 0.5 ml solution of 0.3m EDTA, pH 7.4. After centrifugation the plasma was stored at −70°C for radioimmunoassays of plasma hormone concentrations. The hypothalamus was dissected, immediately frozen in liquid nitrogen, and later stored at −70°C for immunoblot analysis and receptor binding assays.
Time course of the effect of DOI on hormone responses to an 8-OH-DPAT challenge. The 5-HT2A/2C receptor agonist DOI (2.5 mg/kg, i.p.) or saline was administered to rats. At different time points after DOI injection (1, 2, 4, 24 hr) the rats were challenged with the 5-HT1A agonist 8-OH-DPAT (0.05 mg/kg, s.c.) or saline. Then 15 min after the 8-OH-DPAT injection the rats were decapitated.
Effect of pretreatment with increasing doses of DOI on hormone responses to an 8-OH-DPAT challenge. Rats were injected with increasing doses of DOI (0.5, 2.5 and 5 mg/kg, i.p.) or saline. At 2 hr later the rats were challenged with 8-OH-DPAT (0.05 mg/kg, s.c.) or saline. Then 15 min after the 8-OH-DPAT challenge the rats were decapitated.
Dose–response to an 8-OH-DPAT challenge in rats pretreated with DOI. Rats were injected with DOI (2.5 mg/kg, i.p.) or saline. At 2 hr later the rats were challenged with saline or increasing doses of 8-OH-DPAT (0.03, 0.05, 0.1, 0.5 mg/kg, s.c.). Then 15 min after the 8-OH-DPAT challenge the rats were decapitated.
Pretreatment with the 5-HT2A antagonist MDL 100,907.Rats first received an injection of vehicle or the 5-HT2A antagonist MDL 100,907 (0.01 mg/kg, s.c.). At 15 min after this injection the rats received an injection of DOI (2.5 mg/kg, i.p.) or saline. Then 2 hr after the DOI injection the rats were challenged with 8-OH-DPAT (0.05 mg/kg, s.c.) or saline. The rats were decapitated 15 min after the 8-OH-DPAT challenge.
Radioimmunoassay
Plasma oxytocin and ACTH were determined by radioimmunoassays as described previously in detail (Li et al., 1993, 1997).
Immunoblot analysis of Gz-proteins
Tissue preparation. All procedures were conducted at 4°C. Briefly, the hypothalamic tissues were homogenized in 0.4 ml of 50 mm Tris buffer, pH 7.4, containing 150 mm NaCl, 10% sucrose, and 0.5 mmphenylmethanesulfonyl fluoride (PMSF) and additional protease inhibitors purchased as a cocktail [containing 4-(2-aminoethyl)benzenesulfonyl fluoride, pepstatin A,trans-epoxysuccinyl-l-leucyl-amido(4-guanidino)butane, bestatin, leupeptin, and aprotinin] from Sigma (1.5 μl/30 mg tissue; St. Louis, MO). After centrifugation at 20,000 × g for 60 min the pellets were collected and resuspended by sonication in a 20 mm Tris buffer, pH 8 [containing (in mm) 1 EDTA, 100 NaCl, 1 dithiothreitol, and 1% sodium cholate] plus the protease inhibitory cocktail (1.5 μl cocktail/30 mg tissue) in a ratio of 3 μl buffer/mg tissue. The resuspended pellets were incubated while shaking for 1 hr and then centrifuged at 100,000 × g for 60 min. The supernatant was collected for the immunoblot analysis of membrane-bound Gz-protein levels. The protein concentration was measured with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL).
Quantification of Gz-protein. Immunoblot analysis of membrane-associated Gz-proteins has been described previously in detail (Raap et al., 1999; Serres et al., 2000). Briefly, the solubilized proteins (2 μg/lane) were resolved by SDS-PAGE and then transferred electrophoretically to nitrocellulose membranes. After incubation with a blocking buffer (PBS containing 0.2% casein and 0.1% Tween 20), the nitrocellulose membranes were probed overnight at 4°C with polyclonal antisera for Gz (I-20, 1:6000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Then the membranes were incubated with a secondary antibody (goat anti-rabbit serum, 1:25,000 dilution for 1 hr; Cappell, Organon Teknika, Durham, NC), followed by an incubation with rabbit peroxidase anti-peroxidase (1:5000 dilution for 1 hr; Cappell, Organon Teknika). Finally, the membranes were incubated with the ECL chemiluminescence substrate solution (Amersham, Arlington Heights, IL) and then exposed to Kodak x-ray film. Films were analyzed densitometrically with the Scion Image program (Frederick, MD). The data for each sample were the means of three replications and were calculated as integrated optical densities (IOD)/μg protein. The percentile changes of the mean IOD/μg protein values of each sample were calculated with respect to the average of IOD/μg protein values of saline-treated samples.
[3H]8-OH-DPAT binding
All procedures were performed on ice. The hypothalamic tissues were homogenized quickly in 2 ml Tris buffer (50 mm), pH 7.7, using a Polytron, and were centrifuged at 35,000 ×g for 10 min. The pellets were resuspended with 2 ml of 50 mm Tris buffer, and the procedure was repeated four times. Then the samples were diluted to a final concentration of 20 mg of tissue per milliliter for the receptor binding assay. [3H]8-OH-DPAT binding (specific activity, 124.9 mCi/ml, 0.8 nm) was performed in 1 ml of 50 mm Tris buffer, pH 7.7, containing 0.5 mm EDTA, 10 mmMgSO4, 0.8 nm[3H]8-OH-DPAT, 2 mg hypothalamic tissue, and different concentrations of GTPγS (from 10−9 to 10−5m). The specificity of [3H]8-OH-DPAT binding to 5-HT1A receptors was defined in the presence of 1 μm WAY100635. The ability of GTPγS at both IC50 (0.02 μm) andEmax (1 μm) concentrations to inhibit [3H]8-OH-DPAT labeling of 5-HT1A receptors was examined in hypothalamic homogenates obtained from saline- and DOI-treated (2.5 mg/kg, i.p.) rats. The protein concentration was measured with a BCA protein assay kit (Pierce). 5-HT1A receptor binding was expressed as femtomoles per milligram of protein.
Statistical analyses
The data are presented as group means (n = 8) and the SEM. Except for immunoblot data, all other data were analyzed by two-way or three-way ANOVA. Group means were compared by a Newman–Keuls multiple range test (Steel and Torrie, 1960). The immunoblot data were analyzed by a Student's t test. A computer program (GBSTAT, Silver Spring, MD) was used for all of the statistical analyses.
RESULTS
Time course of the effect of DOI on hormone responses to a challenge with a 5-HT1A agonist
The 5-HT2A/2C receptor agonist DOI (2.5 mg/kg, i.p.) was injected at different time points (1, 2, 4, 24 hr) before 8-OH-DPAT challenge. DOI alone did not alter plasma oxytocin and ACTH levels significantly at these time points. 8-OH-DPAT significantly increased the plasma levels of oxytocin and ACTH by 731 and 930%, respectively. The time course effects of DOI on oxytocin (Fig.1A) and ACTH (Fig.1B) showed a delayed onset and reversible inhibition, by DOI, of the hormone responses to the subsequent challenge with 8-OH-DPAT. For oxytocin the three-way ANOVA indicated no significant main effects of time (F(3,107) = 0.04;p > 0.1) but a significant main effect of DOI (F(1,107) = 9.96; p < 0.01) and 8-OH-DPAT (F(1,107) = 270.51; p < 0.01). The interaction between time and DOI was not significant. However, there was a significant interaction between DOI and 8-OH-DPAT (F(1,107) = 10.55; p < 0.01). For ACTH the three-way ANOVA indicated that there was a significant main effect of 8-OH-DPAT (F(1,106) = 319.56; p< 0.01). The main effect of time (F(3,106) = 2.45; p > 0.05) and the main effect of DOI (F(1,106) = 3.23; p > 0.05) were not significant. However, both the interactions between time and DOI (F(3,106) = 4.75;p < 0.05) and between DOI and 8-OH-DPAT (F(1,106) = 10.48; p< 0.05) were significant. The Newman–Keuls test indicated that DOI significantly decreased both plasma oxytocin and ACTH responses to the 8-OH-DPAT challenge at 2 and 4 hr, with a maximal effect at 2 hr post-DOI administration (47% reduction for oxytocin; 51% reduction for ACTH) (Fig. 1). At 1 hr after treatment with DOI there was no inhibition of the effect of 8-OH-DPAT on either ACTH or oxytocin levels (Fig. 1). These observations indicate that activation of 5-HT2A/2C receptors produces a transient and delayed onset attenuation of 5-HT1Areceptor-mediated secretion of oxytocin and ACTH.
Fig. 1.
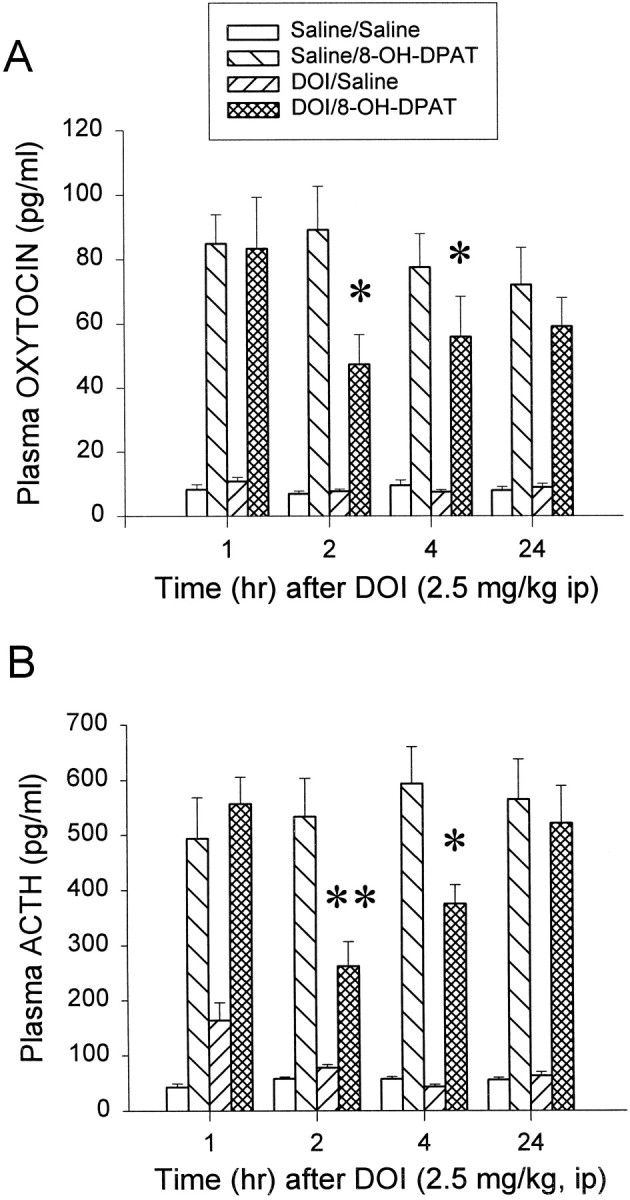
Time course of DOI-induced reduction of the oxytocin (A) and the ACTH (B) responses to an 8-OH-DPAT (0.05 mg/kg, s.c.) challenge. The data represent the means ± SEM of six to eight rats per group. *Significant difference from saline/8-OH-DPAT group (p < 0.05); **significant difference from saline/8-OH-DPAT group (p < 0.01); three-way ANOVA and Newman–Keuls multiple range test.
Effect of pretreatment with increasing doses of DOI on hormone responses to 8-OH-DPAT
DOI was administered at three doses: 0.5, 2.5, and 5 mg/kg intraperitoneally, respectively. Basal plasma levels of oxytocin and ACTH in saline-challenged rats were not altered significantly by DOI at these doses. However, DOI inhibited the oxytocin (Fig.2A) response to 8-OH-DPAT challenge in a dose-dependent manner. For plasma oxytocin the two-way ANOVA indicated a significant main effect of both DOI (F(3,55) = 2.92; p < 0.05) and 8-OH-DPAT (F(1,55) = 71.08;p < 0.01). There was a significant interaction between DOI and 8-OH-DPAT (F(3,55) = 3.08;p < 0.05). The Newman–Keuls test indicated that DOI significantly reduced the effect of 8-OH-DPAT on plasma oxytocin levels at the doses of 2.5 mg/kg (by 40%; p < 0.05) and 5 mg/kg (by 58%; p < 0.01). For plasma ACTH in this experiment the two way ANOVA indicated a significant main effect of 8-OH-DPAT (F(1,54) = 119.82;p < 0.01), but no significant main effect of DOI (F(3,54) = 0.25; p > 0.1) and no significant interaction between DOI and 8-OH-DPAT (F(3,54) = 1.47; p > 0.1). However, baseline levels of ACTH were elevated from 43.1 ± 3.4 to 99.6 ± 12.8. Subtracting the baseline levels from 8-OH-DPAT-stimulated ACTH release would indicate that the additive effects of DOI on basal ACTH levels could have masked its desensitizing effects (saline/DPAT group, 382.2; DOI 0.5/DPAT group, 326.6; DOI 2.5/DPAT group, 271.2; DOI 5/DPAT group, 228.4 pg/ml). Hence the highest dose of DOI produced a >40% inhibition of the ACTH response to 8-OH-DPAT.
Fig. 2.
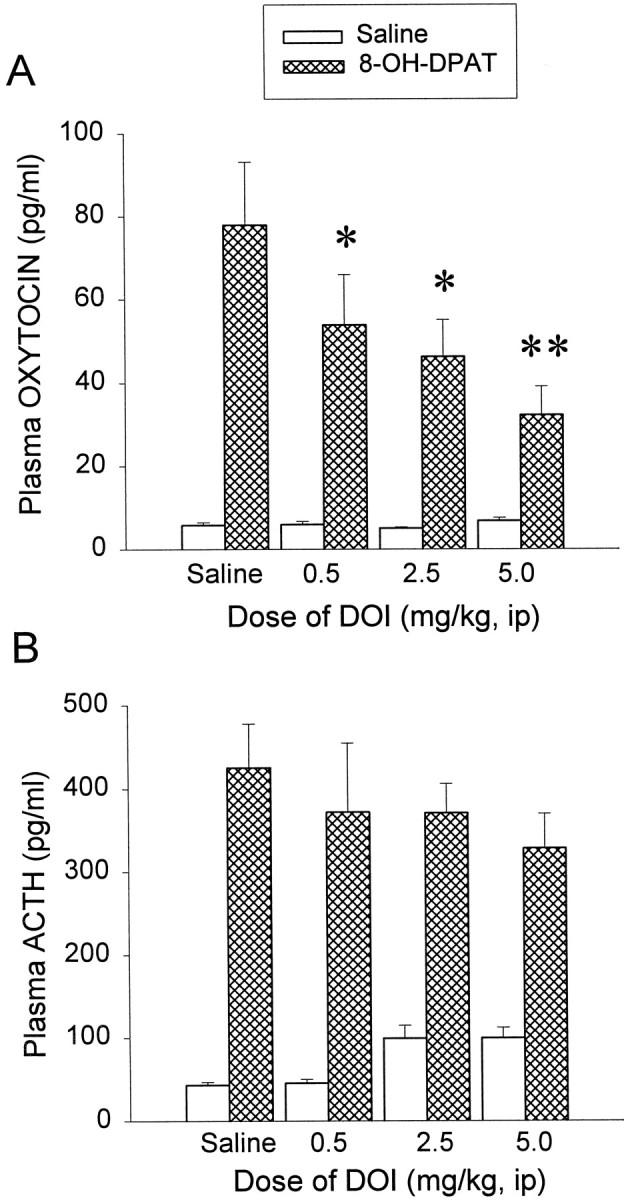
Examination of the doses of DOI that reduce the oxytocin (A) and the ACTH (B) responses to an 8-OH-DPAT (0.05 mg/kg, s.c.) challenge. The data represent the means ± SEM of seven to eight rats per group. *Significant difference from saline/8-OH-DPAT group (p < 0.05); **significant difference from saline/8-OH-DPAT group (p < 0.01); two-way ANOVA and Newman–Keuls multiple range test.
Dose–response effect of 8-OH-DPAT in rats pretreated with DOI
Increasing doses of 8-OH-DPAT (0.03, 0.05, 0.1, 0.5 mg/kg, s.c.) or saline were administered 2 hr after DOI (2.5 mg/kg, i.p.) or saline treatments. In saline-pretreated rats, 8-OH-DPAT significantly increased plasma oxytocin and ACTH levels in a dose-dependent manner. At the doses of 0.05 and 0.1 mg/kg, 8-OH-DPAT increased plasma oxytocin to 53 and 80% of maximal response, respectively. However, ACTH is a more amplified hormone. At the dose of 0.05 mg/kg, 8-OH-DPAT produced a maximal increase in plasma ACTH levels. DOI treatment shifted both oxytocin (Fig. 3A) and ACTH (Fig. 3B) dose–response curves to the right, with no change in Emax. For plasma oxytocin the two-way ANOVA showed a significant main effect of DOI (F(1,67) = 7.26; p < 0.01) and a significant main effect of 8-OH-DPAT (F(4,67) = 120.00; p< 0.01). The interaction between DOI and 8-OH-DPAT was also significant (F(4,67) = 3.72;p < 0.01). The Newman–Keuls test indicated that, in saline-pretreated rats, 8-OH-DPAT at the dose of 0.03 mg/kg produced no significant effect. At the doses of 0.05 and 0.1 mg/kg, 8-OH-DPAT significantly elevated plasma oxytocin levels. Basal plasma oxytocin level in saline-challenged rats was not altered by DOI. However, DOI significantly lowered the oxytocin responses to 8-OH-DPAT at the challenge doses of 0.05 mg/kg (p < 0.05) and 0.1 mg/kg (p < 0.01), but not at the highest dose of 0.5 mg/kg (Fig. 3A). For plasma ACTH the two-way ANOVA indicated a significant main effect of DOI (F(1,60) = 4.67; p < 0.05) and a significant main effect of 8-OH-DPAT (F(4,60) = 47.7; p < 0.01). There was a significant interaction between DOI and 8-OH-DPAT (F(4,60) = 6.45; p < 0.01). The Newman–Keuls test indicated that 0.03 mg/kg 8-OH-DPAT produced no significant effect on plasma ACTH. However, a dose of 0.05 mg/kg 8-OH-DPAT already increased plasma ACTH to its maximal level. Basal plasma ACTH level in saline-challenged rats was not altered by DOI. However, DOI significantly lowered the ACTH response to 8-OH-DPAT at the challenge doses of 0.05 mg/kg (p < 0.01) and 0.1 mg/kg (p < 0.05), respectively, but not at the dose of 0.5 mg/kg (Fig. 3B).
Fig. 3.
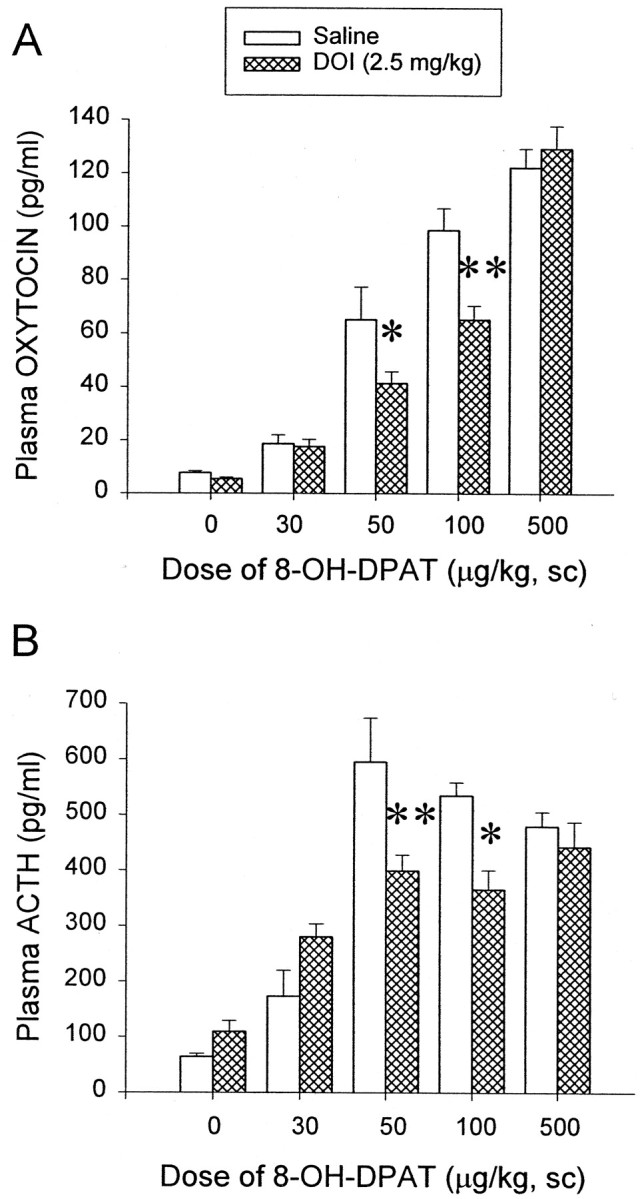
DOI shifts the oxytocin (A) and ACTH (B) dose–response curve of 8-OH-DPAT effects to the right, with no change inEmax. The data represent the means ± SEM of six to eight rats per group. *Significant difference from saline/8-OH-DPAT group (p < 0.05); **significant difference from saline/8-OH-DPAT group (p < 0.01); two-way ANOVA and Newman–Keuls multiple range test.
Pretreatment with the 5-HT2A antagonist MDL 100,907 prevents DOI-induced reduction of hormone responses to 8-OH-DPAT
The basal plasma levels of oxytocin and ACTH were not altered by MDL 100,907 or by DOI. The 8-OH-DPAT challenge significantly elevated both plasma levels of oxytocin (Fig.4A) and ACTH (Fig.4B). The three-way ANOVA indicated that there was no significant main effect of MDL 100,907 for oxytocin (F(1,55) = 2.74; p > 0.1) and ACTH (F(1,55) = 1.08;p > 0.1). For oxytocin there were significant interactions between MDL 100,907 and DOI (F(1,55) = 7.17; p < 0.01) as well as among MDL 100,907, DOI, and 8-OH-DPAT (F(1,55) = 6.41; p < 0.05). For ACTH the main effect of 8-OH-DPAT was significant (F(1,55) = 109.11; p< 0.01), but neither the interaction between MDL 100,907 and DOI nor the interaction among MDL 100,907, DOI, and 8-OH-DPAT was significant. However, the Newman–Keuls test indicated that MDL 100,907 blocked the ability of DOI to inhibit the effect of 8-OH-DPAT on plasma levels of oxytocin (Fig. 4A) and ACTH (Fig.4B). Thus the effect of DOI on both hormone responses to 8-OH-DPAT challenge is mediated predominantly by the activation of 5-HT2A receptors.
Fig. 4.
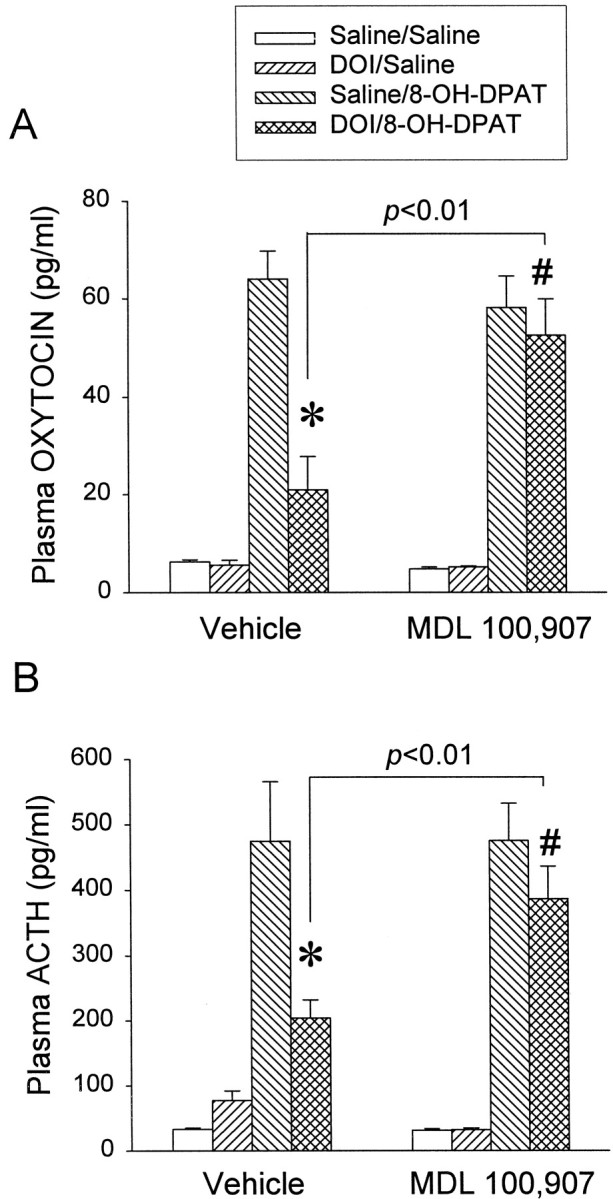
The 5-HT2A receptor antagonist MDL 100,907 reverses the inhibitory effect of DOI on the oxytocin (A) and the ACTH (B) responses to an 8-OH-DPAT (0.05 mg/kg, s.c.) challenge. The data represent the means ± SEM of 7–10 rats per group. *Significant difference from saline/8-OH-DPAT group (p < 0.01); #significant difference from DOI/8-OH-DPAT group (p < 0.01); three-way ANOVA and Newman–Keuls multiple range test.
DOI does not change the level of membrane-associated Gz-proteins in the hypothalamus
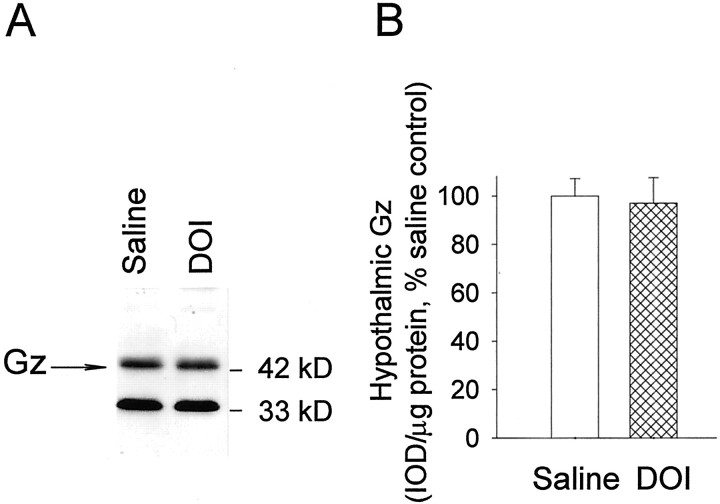
The concentration of membrane-associated Gz-proteins in the hypothalamus of DOI- and saline-treated rats was measured by immunoblot analysis. The specificity of the antibody for Gz-protein has been verified in a previous study (Raap et al., 1999). An example of an immunoblot of membrane-bound Gz-proteins is shown in Figure 5A. The result of the densitometric analysis is presented in Figure 5B. A Student's t test indicates that hypothalamic levels of membrane-associated Gz-proteins were not reduced significantly by 2.5 mg/kg DOI intraperitoneally, a dose that significantly reduces hormone responses to an 8-OH-DPAT challenge.
Fig. 5.
DOI does not change the level of membrane-associated Gz-protein in the hypothalamus.A, Immunoblot of Gz-proteins in the particulate fraction of the hypothalamic from DOI- and saline-treated rats. B, Results of the densitometric analysis of the membrane-bound Gz-proteins in the hypothalamus. The data represent the means ± SEM of eight hypothalamic samples per group. The data for each sample are the average of three replicates and were analyzed with a Student's t test.
DOI does not change the GTPγS-induced inhibition of [3H]8-OH-DPAT binding in the hypothalamus
5-HT1A receptors in the hypothalamus were labeled with [3H]8-OH-DPAT (0.8 nm). The specific binding of [3H]8-OH-DPAT was essentially the same when 1 μm 5-HT or 1 μm WAY100635 was used to define nonspecific binding. Increasing concentrations of GTPγS (from 10−9 to 10−5m) gradually inhibited [3H]8-OH-DPAT binding with an EC50 ≈0.02 μm and anEmax concentration ≈1 μm (Fig.6A).
Fig. 6.
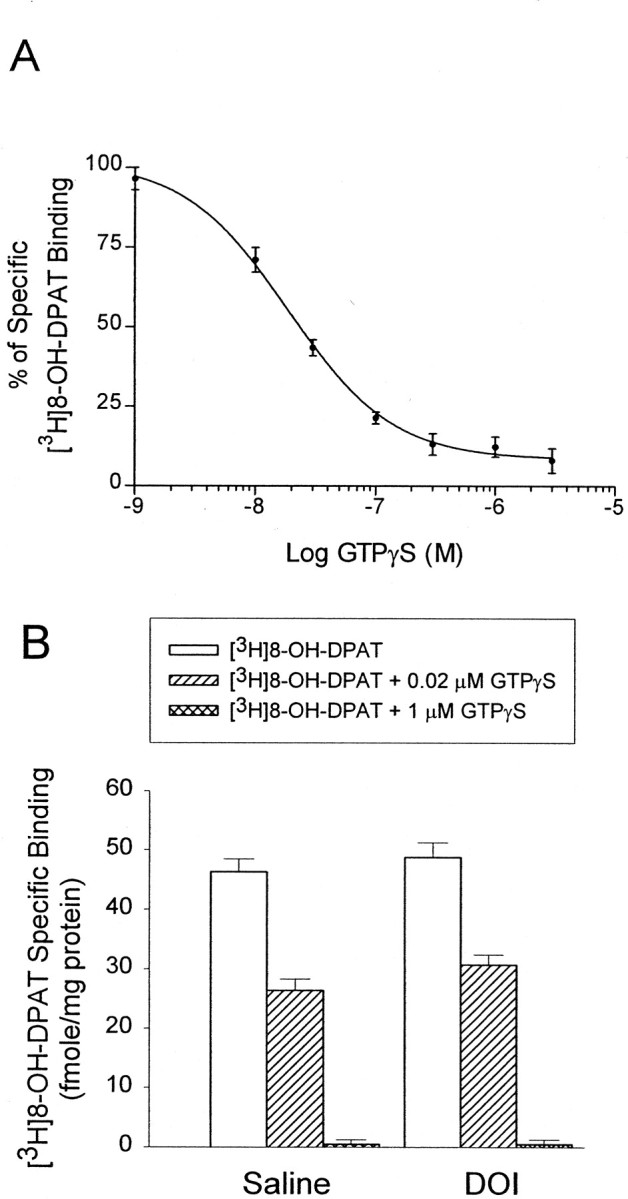
DOI does not change the coupling of 5-HT1A receptors to G-proteins in the hypothalamus.A, Inhibition of [3H]8-OH-DPAT (0.8 nm) binding in hypothalamic homogenates with increasing concentration of GTPγS (from 10−9to10−5m). Each datapoint represents the mean of three assays. Specific binding is defined by WAY100635 (1 μm). B, DOI does not alter the GTPγS-induced inhibition of [3H]8-OH-DPAT binding at the GTPγS concentrations of 0.02 μm (EC50) and 1 μm (Emax). The data represent the means ± SEM of [3H]8-OH-DPAT binding from eight hypothalamic samples per group; two-way ANOVA and Newman–Keuls multiple range test.
We used 0.02 μm (IC50) and 1 μm (Emax) concentrations of GTPγS to examine the degree of inhibition of [3H]8-OH-DPAT (0.8 nm) binding in the hypothalamic homogenates from saline- and DOI-treated rats. The two-way ANOVA indicated that there was a significant main effect of GTPγS (F(42,2) = 374.54; p< 0.01), but no significant main effect of DOI (F(42,1) = 2.45; p > 0.1). The interaction between GTPγS and DOI was not significant (F(42,2) = 0.74; p > 0.1). The Newman–Keuls test indicated that DOI treatment did not alter the ability of both concentrations of GTPγS (0.02 or 1 μm) to inhibit the binding of [3H]8-OH-DPAT in hypothalamic homogenates (Fig. 6B). These data suggest that DOI treatment does not reduce the coupling of 5-HT1Areceptors to G-proteins in the hypothalamus.
DISCUSSION
The present study provides the first in vivodemonstration of a functional heterologous desensitization of hypothalamic 5-HT1A receptors after activation of 5-HT2A receptors. The main findings are that (1) activation of 5-HT2A receptors with DOI produces a delayed and transient reduction of 5-HT1Areceptor-mediated secretion of oxytocin and ACTH, with an increase in ED50 and no change inEmax, and that (2) DOI treatment does not change the level of membrane-associated Gz-protein or the coupling of 5-HT1A receptors to G-proteins in the hypothalamus.
8-OH-DPAT, the prototypical 5-HT1A receptor agonist, has a high affinity for 5-HT1A receptors and from 10- to 100-fold lower affinity for other 5-HT receptor subtypes (Hoyer et al., 1994). The effect of 8-OH-DPAT on the secretion of ACTH and oxytocin is inhibited by the 5-HT1Aantagonists WAY100635, NAN-190, and pindolol (Bagdy and Kalogeras, 1993; Critchley et al., 1994; Meller and Bohmaker, 1994; Vicentic et al., 1998). Thus the changes in plasma levels of ACTH and oxytocin after an 8-OH-DPAT challenge reflect the functional state of the 5-HT1A receptor system.
DOI is a 5-HT2A/2C agonist with a similar affinity for 5-HT2A and 5-HT2C receptors (Hoyer, 1988). Therefore, we used MDL 100,907 to determine whether 5-HT2A or 5-HT2C receptors mediate the DOI-induced desensitization of 5-HT1A receptors. MDL 100,907 is a selective antagonist with >100-fold higher affinity for 5-HT2A than for 5-HT2Creceptors (Johnson et al., 1996; Kehne et al., 1996). The dose of MDL 100,907 (0.01 mg/kg) used in the present study was selected to avoid occupancy of 5-HT2C receptors (Dekeyne et al., 1999; Smith et al., 1999). Thus the ability of MDL 100,907 to block the effect of DOI indicates that 5-HT2A receptors mediate the DOI-induced desensitization of hypothalamic 5-HT1A receptors.
Although evidence exists for receptor reserve in 5-HT1A receptor-induced release of ACTH (Meller and Bohmaker, 1994), oxytocin is a more direct indicator of the functioning of the hypothalamic 5-HT1A receptors after 8-OH-DPAT challenge (Serres et al., 2000). A dose of 0.05 mg/kg 8-OH-DPAT produces a maximal release of ACTH but only 50% of the maximal response of oxytocin (Fig. 3). This difference in amplification may explain our observation that DOI attenuates the oxytocin response to 8-OH-DPAT in a dose-dependent manner (Fig. 2A), whereas the inhibition of the ACTH response to 8-OH-DPAT is less dramatic (Fig. 2B).
DOI increases the secretion of oxytocin and ACTH by activating 5-HT2A receptors in the hypothalamic paraventricular nucleus (Van de Kar et al., 2001). It could be argued that a reduction of hormone responses to a subsequent 8-OH-DPAT challenge is attributable to a hormone-depleting effect of DOI. However, the time course experiment indicates that, 1 hr after treatment with DOI, there is no desensitization of the oxytocin or ACTH responses to 8-OH-DPAT even if one would subtract from the ACTH response the effect of DOI on baseline ACTH levels (Fig. 1). If the pretreatment with DOI would have depleted oxytocin, CRF, or ACTH stores, there should have been a reduced response to 8-OH-DPAT at 1 hr after DOI treatment. In addition, DOI pretreatment did not reduce the maximal hormone responses to 8-OH-DPAT challenge (Fig. 3). This indicates that DOI pretreatment has not depleted hormone stores. Thus DOI-induced attenuation of hormone responses to the 8-OH-DPAT challenge is more likely to be attributable to a heterologous desensitization of hypothalamic 5-HT1A receptors.
The hypothalamic paraventricular nucleus is crucial for the serotonergic stimulation of ACTH and oxytocin release (Liposits et al., 1987; Saphier, 1991; Kawano et al., 1992; Bagdy and Makara, 1994;Rittenhouse et al., 1994; Van de Kar et al., 1995). Autoradiographic studies indicate a substantial density of [3H]8-OH-DPAT-labeled 5-HT1A receptors in the medial parvocellular divisions (containing CRF neurons) and the ventrolateral magnocellular divisions (containing oxytocin neurons) of the hypothalamic paraventricular nucleus (Li et al., 1997). The mRNA coding for 5-HT1A receptors also has been detected in the paraventricular nucleus (Wright et al., 1995). Moreover, the pertussis toxin-resistant Gz-protein and Gz mRNA are expressed in the hypothalamic paraventricular nucleus (Serres et al., 2000). The 5-HT1A receptor-mediated release of oxytocin and ACTH is not inhibited by pertussis toxin, but it is inhibited by Gz antisense oligodeoxynucleotides that reduce the level of Gz-protein in the paraventricular nucleus (Serres et al., 2000). Accordingly, 8-OH-DPAT increases the secretion of oxytocin and ACTH by activating 5-HT1A receptor–Gz-protein signaling systems in the hypothalamic paraventricular nucleus.
Mechanical destruction of the hypothalamic paraventricular nucleus prevents the DOI-induced increase in plasma levels of oxytocin and ACTH (Bagdy, 1996). Autoradiographic studies indicate the presence of 5-HT2A receptors in the hypothalamic paraventricular nucleus (Appel et al., 1990). In addition, in situ hybridization data indicate mRNA coding for 5-HT2A receptors in the hypothalamic paraventricular nucleus (Wright et al., 1995; Gundlah et al., 1999). Activation of 5-HT2A receptors with DOI increases Fos expression in oxytocin and CRF-expressing neurons, an effect that is blocked by the 5-HT2A antagonist MDL 100,907 (Van de Kar et al., 2001). Combined, it is likely that 5-HT1A receptors and 5-HT2Areceptors are colocalized on CRF and oxytocin cells, mediating oxytocin and ACTH release. Therefore, DOI-induced desensitization of the oxytocin and ACTH responses to a challenge with 8-OH-DPAT may represent a heterologous desensitization of 5-HT1Areceptor–Gz-protein signaling by activation of the 5-HT2A receptors. However, there is no evidence showing that 5-HT1A receptors or 5-HT2A receptors are coexpressed in oxytocin or CRF cells, nor is there evidence indicating that 5-HT1A receptors and 5-HT2A receptors are colocalized in the same cells in the paraventricular hypothalamic nucleus. Thus an alternative explanation is that DOI may activate 5-HT2A receptors on interneurons in a complex neuronal circuit, resulting in a desensitization of hypothalamic 5-HT1A receptors.
The lack of reduction in the maximal response to 8-OH-DPAT after treatment with DOI suggests no downregulation of 5-HT1A receptors. Consistent with this conclusion is the observation that [3H]8-OH-DPAT binding to hypothalamic tissues was not reduced by DOI. The time interval after DOI treatment (2 hr) might be too short for a reduction in the density of 5-HT1A receptors.
DOI-induced desensitization of hypothalamic 5-HT1A receptor–Gz-protein signaling is characterized by delayed onset and short duration. These characteristics support a hypothesis of post-translational modification mechanisms such as phosphorylation, myristoylation, or palmitoylation (Hallak et al., 1994; Morales et al., 1998). Myristoylation and/or palmitoylation provide anchorage for Gz-proteins to associate with the plasma membrane and transmit extracellular signals through the receptor down the intracellular signal transduction cascade (Hallak et al., 1994;Beck et al., 1997). Because no change in membrane-associated Gz-proteins was observed, it is not likely that a change of the palmitoylation or myristoylation state of Gz-proteins is involved in DOI-induced desensitization of 5-HT1A receptors. Phosphorylation plays an important role in short-term receptor desensitization (Chuang et al., 1996; Freedman and Lefkowitz, 1996). Cell culture studies demonstrate that activation of protein kinase C induces phosphorylation of 5-HT1A receptors and desensitizes 5-HT1A receptor-mediated inhibition of forskolin-stimulated cAMP accumulation (Raymond, 1991; Evans et al., 2001). Phosphorylation of Gz-proteins by protein kinase C prolongs the time during which Gzα stays in its uncoupled monomeric state (Fields and Casey, 1995) and reduces the ability of RGSZ1 proteins to potentiate the GTPase activity of Gzα-proteins (Glick et al., 1998; Wang et al., 1998). Moreover, DOI has been reported to increase protein kinase C activity in several brain regions (Wang and Friedman, 1990; Rahimian and Hrdina, 1995). Therefore, it is possible that DOI activates 5-HT2A receptors to increase the activity of protein kinase C, leading to phosphorylation of 5-HT1A receptors and/or Gz-proteins and to desensitization of 5-HT1A receptors in the hypothalamus.
DOI treatment does not reduce the coupling of 5-HT1A receptors to G-proteins in the hypothalamus. This conclusion is supported by the observation that DOI did not reduce the binding of [3H]8-OH-DPAT to 5-HT1A receptors at a concentration close to its EC50 (0.8 nm). In addition, GTPγS-induced inhibition of [3H]8-OH-DPAT binding to 5-HT1A receptors was not altered by DOI. However, 5-HT1A receptors also have a high affinity for Gi1-, Gi2-, Gi3-, and Go-proteins (Butkerait et al., 1995; Albert et al., 1996). GTPγS uncouples all Gi/o-proteins from 5-HT1Areceptors with no preference for Gz. Thus it is possible that an uncoupling of 5-HT1A receptors from Gz-proteins in the hypothalamic paraventricular nucleus could be masked by a lack of change in G-protein coupling in other hypothalamic nuclei. Similarly, we measured only the level of membrane-associated Gz-proteins in the whole hypothalamus and could not rule out the possibility that DOI may alter Gz-proteins in the hypothalamic paraventricular nucleus.
In conclusion, the activation of 5-HT2A receptors with DOI induces a delayed onset and transient functional heterologous desensitization of 5-HT1A receptor signaling that is not attributable to a change in the coupling of 5-HT1A receptors to G-proteins and is not attributable to a change in the level of membrane-associated Gz-proteins in the hypothalamus. This desensitization might be attributable to changes in signaling components distal to 5-HT1Areceptor–Gz-protein interaction.
Footnotes
This work was supported in part by United States Public Health Service Grants RO1 NS34153 and RO1 MH58448 (L.D.V.), RO1 NS38059 (N.A.M.), and RO1 MH60687 (G.B.) and by the National Alliance for Research on Schizophrenia and Depression (D.K.R.). We are grateful to Heather Patel for her technical assistance, to Dr. Lanny C. Keil from the National Aeronautics and Space Administration Ames Research Center (Moffat Field, CA) for the oxytocin antiserum, and to Hoechst Marion Roussel Research Institute (Cincinnati, OH) for the sample of MDL 100,907.
Correspondence should be addressed to Dr. Louis D. Van de Kar, Department of Pharmacology, Loyola University of Chicago, Stritch School of Medicine, 2160 South First Avenue, Maywood, IL 60153. E-mail:lvandek@lumc.edu.
REFERENCES
- 1.Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teiteler M, de Souza EB. Autoradiographic characterization of (±)-1-(2,5-dimethoxy-4-[125I]iodophenyl-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 3.Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A, and 5-HT2C receptor-mediated oxytocin, prolactin, and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- 4.Bagdy G, Kalogeras KT. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain Res. 1993;611:330–332. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- 5.Bagdy G, Makara GB. Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin, and corticosterone responses. Endocrinology. 1994;134:1127–1131. doi: 10.1210/endo.134.3.8119151. [DOI] [PubMed] [Google Scholar]
- 6.Beck HI, Chan JS, Wong YH. Receptor-induced βγ release from fatty acylation-deficient mutants of Gαz. NeuroReport. 1997;8:937–940. doi: 10.1097/00001756-199703030-00024. [DOI] [PubMed] [Google Scholar]
- 7.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 8.Berlin I, Warot D, Legout V, Guillemant S, Schöllnhammer G, Puech AJ. Blunted 5-HTlA-receptor agonist-induced corticotropin and cortisol responses after long-term ipsapirone and fluoxetine administration to healthy subjects. Clin Pharmacol Ther. 1998;63:428–436. doi: 10.1016/S0009-9236(98)90038-8. [DOI] [PubMed] [Google Scholar]
- 9.Bonson KR, Buckholtz JW, Murphy DL. Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology. 1996;14:425–436. doi: 10.1016/0893-133X(95)00145-4. [DOI] [PubMed] [Google Scholar]
- 10.Bosker FJ, Cremers TI, Jongsma ME, Westerink BHC, Wikström VH, Den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine1A receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Butkerait P, Zheng Y, Hallak H, Graham TE, Miller HA, Burris KD, Molinoff PB, Manning DR. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. Reconstitution of a coupled phenotype by coexpression of mammalian G-protein subunits. J Biol Chem. 1995;270:18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- 12.Chuang TT, Iacovelli L, Sallese M, De Blasi A. G-protein-coupled receptors: heterologous regulation of homologous desensitization and its implications. Trends Pharmacol Sci. 1996;17:416–421. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]
- 13.Critchley DJP, Childs KJ, Middlefell VC, Dourish CT. Inhibition of 8-OH-DPAT-induced elevation of plasma corticotrophin by the 5-HT1A receptor antagonist WAY100635. Eur J Pharmacol. 1994;264:95–97. doi: 10.1016/0014-2999(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 14.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 15.Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 600175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- 16.Evans KL, Cropper JD, Berg KA, Clarke WP. Mechanisms of regulation of agonist efficacy at the 5-HT1A receptor by phospholipid-derived signaling components. J Pharmacol Exp Ther. 2001;297:1025–1035. [PubMed] [Google Scholar]
- 17.Fields TA, Casey PJ. Phosphorylation of Gzα by protein kinase C blocks interaction with the βγ complex. J Biol Chem. 1995;270:23119–23125. doi: 10.1074/jbc.270.39.23119. [DOI] [PubMed] [Google Scholar]
- 18.Freedman NJ, Lefkowitz RJ. Desensitization of G-protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–353. [PubMed] [Google Scholar]
- 19.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 20.Glick JL, Meigs TE, Miron A, Casey PJ. RGSZ1, a Gz-selective regulator of G-protein signaling whose action is sensitive to the phosphorylation state of Gzα. J Biol Chem. 1998;273:26008–26013. doi: 10.1074/jbc.273.40.26008. [DOI] [PubMed] [Google Scholar]
- 21.Grotewiel MS, Sanders-Bush E. Differences in agonist-independent activity of 5-HT2A and 5-HT2C receptors revealed by heterologous expression. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:21–27. doi: 10.1007/pl00005318. [DOI] [PubMed] [Google Scholar]
- 22.Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A, and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- 23.Hallak H, Brass LF, Manning DR. Failure to myristoylate the α-subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no effect on phosphorylation by protein kinase C. J Biol Chem. 1994;269:4571–4576. [PubMed] [Google Scholar]
- 24.Hensler JG, Truett KA. Effect of chronic serotonin-2 receptor agonist or antagonist administration on serotonin-1A receptor sensitivity. Neuropsychopharmacology. 1998;19:354–364. doi: 10.1016/S0893-133X(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer D. Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci. 1988;9:89–94. doi: 10.1016/0165-6147(88)90174-5. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–204. [PubMed] [Google Scholar]
- 27.Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:205–209. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- 28.Katada T, Gilman AG, Watanabe Y, Bauer S, Jakobs KH. Protein kinase C phosphorylates the inhibitory guanine nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985;151:431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawano S, Osaka T, Kannan H, Yamashita H. Excitation of hypothalamic paraventricular neurons by stimulation of the raphe nuclei. Brain Res Bull. 1992;28:573–579. doi: 10.1016/0361-9230(92)90105-7. [DOI] [PubMed] [Google Scholar]
- 30.Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PLM, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Pre-clinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- 31.Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2 receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Brownfield MS, Battaglia G, Cabrera TM, Levy AD, Rittenhouse PA, Van de Kar LD. Long-term treatment with the antidepressants fluoxetine and desipramine potentiates endocrine responses to the serotonin agonists 6-chloro-2-[1-piperazinyl]-pyrazine (MK-212) and (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCI (DOI). J Pharmacol Exp Ther. 1993;266:836–844. [PubMed] [Google Scholar]
- 33.Li Q, Muma NA, Van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and Go proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- 34.Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- 35.Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin-releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- 36.Maswood N, Uphouse L. Modulation of the behavioral effects of 8-OH-DPAT by estrogen and DOI. Pharmacol Biochem Behav. 1997;58:859–866. doi: 10.1016/s0091-3057(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 37.Meller E, Bohmaker K. Differential receptor reserve for 5-HT1A receptor-mediated regulation of plasma neuroendocrine hormones. J Pharmacol Exp Ther. 1994;271:1246–1252. [PubMed] [Google Scholar]
- 38.Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of Gαz requires two signals. Mol Biol Cell. 1998;9:1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivier B, Soudijn W, van Wijngaarden I. The 5-HT1A receptor and its ligands: structure and function. Prog Drug Res. 1999;52:103–165. doi: 10.1007/978-3-0348-8730-4_3. [DOI] [PubMed] [Google Scholar]
- 40.Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65:1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- 41.Raap DK, Evans S, Garcia F, Li Q, Muma NA, Wolf WA, Battaglia G, Van de Kar LD. Daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J Pharmacol Exp Ther. 1999;288:98–106. [PubMed] [Google Scholar]
- 42.Rahimian R, Hrdina PD. Possible role of protein kinase C in regulation of 5-hydroxytryptamine 2A receptors in rat brain. Can J Physiol Pharmacol. 1995;73:1686–1691. doi: 10.1139/y95-731. [DOI] [PubMed] [Google Scholar]
- 43.Raymond JR. Protein kinase C induces phosphorylation and desensitization of the human 5-HT1A receptor. J Biol Chem. 1991;266:14747–14753. [PubMed] [Google Scholar]
- 44.Rittenhouse PA, Bakkum EA, Levy AD, Li Q, Carnes M, Van de Kar LD. Evidence that ACTH secretion is regulated by serotonin2A/2C (5-HT2A/2C) receptors. J Pharmacol Exp Ther. 1994;271:1647–1655. [PubMed] [Google Scholar]
- 45.Saphier D. Paraventricular nucleus magnocellular neuronal responses following electrical stimulation of the midbrain dorsal raphe. Exp Brain Res. 1991;85:359–363. doi: 10.1007/BF00229413. [DOI] [PubMed] [Google Scholar]
- 46.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin-1A receptor binding measured by positron emission tomography with [11C]WAY100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 47.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc. 1985;44:221–227. [PubMed] [Google Scholar]
- 48.Serres F, Li Q, Garcia F, Raap DK, Battaglia G, Muma NA, Van de Kar LD. Evidence that Gz-proteins couple to hypothalamic 5-HT1A receptors in vivo. J Neurosci. 2000;20:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology (Berl) 1999;144:248–254. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- 50.Staley JK, Malison RT, Innis RB. Imaging of the serotonergic system: interactions of neuroanatomical and functional abnormalities of depression. Biol Psychiatry. 1998;44:534–549. doi: 10.1016/s0006-3223(98)00185-1. [DOI] [PubMed] [Google Scholar]
- 51.Steel RGD, Torrie JH. Principles and procedures of statistics with special reference to the biological sciences. McGraw-Hill; New York: 1960. [Google Scholar]
- 52.Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 53.Tilakaratne N, Yang ZL, Friedman E. Chronic fluoxetine or desmethylimipramine treatment alters 5-HT2 receptor-mediated c-fos gene expression. Eur J Pharmacol. 1995;290:263–266. doi: 10.1016/0922-4106(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 54.Tournois C, Mutel V, Manivet P, Launay JM, Kellermann O. Cross-talk between 5-hydroxytryptamine receptors in a serotonergic cell line: involvement of arachidonic acid metabolism. J Biol Chem. 1998;273:17498–17503. doi: 10.1074/jbc.273.28.17498. [DOI] [PubMed] [Google Scholar]
- 55.Van de Kar LD, Rittenhouse PA, Li Q, Levy AD, Brownfield MS. Hypothalamic paraventricular, but not supraoptic, neurons mediate the serotonergic stimulation of oxytocin secretion. Brain Res Bull. 1995;36:45–50. doi: 10.1016/0361-9230(94)00161-s. [DOI] [PubMed] [Google Scholar]
- 56.Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY100635 inhibits 8-OH-DPAT stimulated oxytocin, ACTH, and corticosterone, but not prolactin secretion. Eur J Pharmacol. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]
- 58.Wang H-Y, Friedman E. Central 5-hydroxytryptamine receptor-linked protein kinase C translocation: a functional postsynaptic signal transduction system. Mol Pharmacol. 1990;37:75–79. [PubMed] [Google Scholar]
- 59.Wang J, Ducret A, Tu YP, Kozasa T, Aebersold R, Ross EM. RGSZ1, a Gz-selective RGS protein in brain: structure, membrane association, regulation by Gαz phosphorylation, and relationship to a Gz GTPase-activating protein subfamily. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- 60.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]