Abstract
Rheumatoid arthritis is characterized by erosive inflammation of the joints, new bone proliferation, and ankylosis, leading to severely reduced locomotion and intense chronic pain. In a model of this disease, adjuvant-induced polyarthritis in the rat, neurons involved in pain transmission and control undergo plastic changes, especially at the spinal level. These changes affect notably neurons that contain opioids, such as enkephalins deriving from preproenkephalin A (PA) precursor protein. Using recombinant herpes simplex virus containing rat PA cDNA, we enhanced enkephalin synthesis in sensory neurons of polyarthritic rats. This treatment markedly improved locomotion and reduced hyperalgesia. Furthermore, the progression of bone destruction slowed down, which is the most difficult target to reach in the treatment of patients suffering from arthritis. These data demonstrate the therapeutic efficacy of enkephalin overproduction in a model of systemic inflammatory and painful chronic disorder.
Keywords: proenkephalin A overproduction, dorsal root sensory ganglia neurons, polyarthritic rats, reduced hyperalgesia, improved polyarthritis-related disability, limited joint destruction
Rheumatoid arthritis is a systemic autoimmune disease primarily manifested by chronic erosive inflammation of the joints associated with intense pain (Pearson, 1956; Colpaert, 1987; Harris, 1990). Its etiology is still unknown, but significant insights into its physiopathology have been obtained from experimental animal models. Although none of these models has all of the characteristics of the human disease, adjuvant-induced polyarthritis in the rat shares numerous behavioral and biochemical characteristics with rheumatoid arthritis (Pearson, 1956; Calvino et al., 1987; Colpaert, 1987).
Proenkephalin A (PA)-derived peptides are involved in the control of pain, and an antihyperalgesic action of overexpressed PA was reported recently in experimentally induced acute inflammatory pain in the mouse (Wilson et al., 1999). Numerous data indicate that opioids, in addition to acting at central sites, modulate pain and inflammatory processes by acting at peripheral sites (Stein and Yassouridis, 1997; Houghton et al., 1998). At the periphery, opioid peptides are thought to originate mainly from inflammatory cells (Schäfer et al., 1994); however, although PA is expressed in a relatively slight proportion of cell bodies of sensory neurons (Pohl et al., 1994), which are located in dorsal root ganglia (DRG), enkephalin-containing axons and terminals present within glabrous skin (Carlton and Coggeshall, 1997) and soft tissue of joints (El Hassan et al., 1998) might represent an additional source of peripheral opioids. Interestingly, in polyarthritic rats, PA expression drops in lumbar DRG that contain cell bodies of sensory nerves from hindlimbs (especially affected by the disease) (Pohl et al., 1997), and the levels of the main PA-derived peptide, met-enkephalin (ME), are reduced in the soft tissue of ankle joints (El Hassan et al., 1998).
Altogether, these findings led us to assess whether PA overexpression in sensory neurons of the hindlimbs could have a beneficial effect in chronically suffering polyarthritic rats. We used herpes simplex virus type 1 (HSV-1)-derived vectors, particularly suitable for transgene transfer into sensory neurons (Geller and Breakefield 1988; Davar et al., 1994; Smith et al., 1995; Goins et al., 1999). We demonstrated recently these vectors to be highly efficient to drive PA gene expression in DRG neurons of healthy rats (Antunes-Bras et al., 1998,2001). Here, we generated recombinant, rat PA cDNA containing thymidine kinase-defective HSV-1 vectors (HSVLatEnk) to prevent possible viral replication and spread.
MATERIALS AND METHODS
HSV-derived vector construction. The pLatEnk plasmid was constructed by subcloning under the Lat-long terminal repeat (LTR) promoter (Lokensgard et al., 1994) the rat PA coding region (derived from pYSEAI; Yoshikawa et al., 1984) into theHindIII-Eco47III-linearized pLat-LTR-LacZ vector (Antunes-Bras et al., 1998). Thymidine kinase gene (UL23) in KOS HSV-1 DNA, bearing the Lat-LTR-LacZ transcriptional units in gC locus (Tk+HSVLatβ-gal; Antunes-Bras et al., 1998), was disrupted by homologous recombination with p23d plasmid containing deleted UL23 gene (520 bpNsiI to SacI fragment). Tk−HSVLatβ-gal was generated by cotransfecting 5 μg of linearized p23d DNA, using the calcium phosphate precipitation method, with 5 μg of HSVLatβ-gal into Vero cells grown in 1× DMEM (Life Technologies, Gaithersburg, MD) containing 10 U/ml of penicillin and streptomycin, 7.5 mm sodium bicarbonate, 2 mmglutamine, and 10% fetal calf serum. Cell cultures were treated 4 hr later with 15% glycerol (v/v) and incubated for 4 d at 37°C in M199 medium (Life Technologies) until the cytopathic effect had spread throughout the cellular monolayer. Cellular debris were spun down, and new confluent Vero cells were infected with the resulting supernatant and incubated at 37°C in the presence of 10 μm acyclovir. Lysis plaques resistant to acyclovir were picked and separated in two aliquots. One aliquot was saved, and DNA was extracted from the second one and screened for the presence of deleted UL23 gene using PCR analysis. Thirty PCR cycles (96°C 1 min; 60°C 0.5 min; and 72°C 2 min) were made with 40 pmol of primers (primer A, 5′GCGCTCCTCGTACCAGCGAAG3′; primer B, 5′CCAGCGTCTTGTCAT TGGCG3′) (Fig.1) in a mixture containing 10 mm each dNTP, 25 mmMgSO4, and 0.5 U of Taq DNA polymerase (Eurobio, Les Ulis, France), in 1× reaction buffer containing 2.5% formamide. Single plaque-isolated Tk−HSVLatβ-gal (HSVLatβ-gal) were then amplified on Vero cells. HSVLatEnk was generated by pLatEnk DNA homologous recombination with HSVLatβ-gal DNA in Vero cells as indicated above. Recombinant HSVLatEnk were isolated by PCR analysis and purified by successive limiting dilutions. The presence of both the disrupted TK gene and the Lat-LTR-pEnk transcriptional unit (primer 1, 5′CTGACTGTGTTTCTGTAT- TTG3′; primer 2, 5′TAGCCAAGAAGTATGGAGGG3′; primer 3, 5′TGATAGTCCATC CACCACTCG3′) was confirmed by PCR analysis (using the same experimental protocol as above) on purified HSVLatEnk DNA obtained from plaque isolates (Fig. 1). Finally, HSVLatEnk DNA was further analyzed by Southern blot hybridization with [32P]-labeled cDNA probe corresponding to the entire coding region of rat PA mRNA (Fig. 1). Briefly, 10 μg of purified HSVLatEnk DNA, 10 μg of HSVLatβ-gal, and 0.1 μg of pLatEnk plasmid DNA were BamHI digested and separated on 0.8% agarose gel. After DNA denaturation and gel neutralization, DNA was electrically transferred onto Hybond N nylon membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). After prehybridization (3 hr, 42°C) in 50 mm sodium phosphate buffer, pH 6.5, containing 50% formamide, 0.5% SDS, 5× SSC, 5× Denhardt's solution, and 0.2 mg/ml denatured herring sperm DNA, the blot was hybridized overnight at 42°C in the same solution with ∼1.5 × 106 cpm/ml [32P]-labeled cDNA probe. Membrane was gradually washed with 2× SSC containing 0.5% SDS at room temperature and then at 45°C, followed by 0.1% SSC at 65°C. The blot was finally exposed to x-ray film (Hyperfilm MP; Amersham Pharmacia Biotech) for 3–5 hr at room temperature.
Fig. 1.
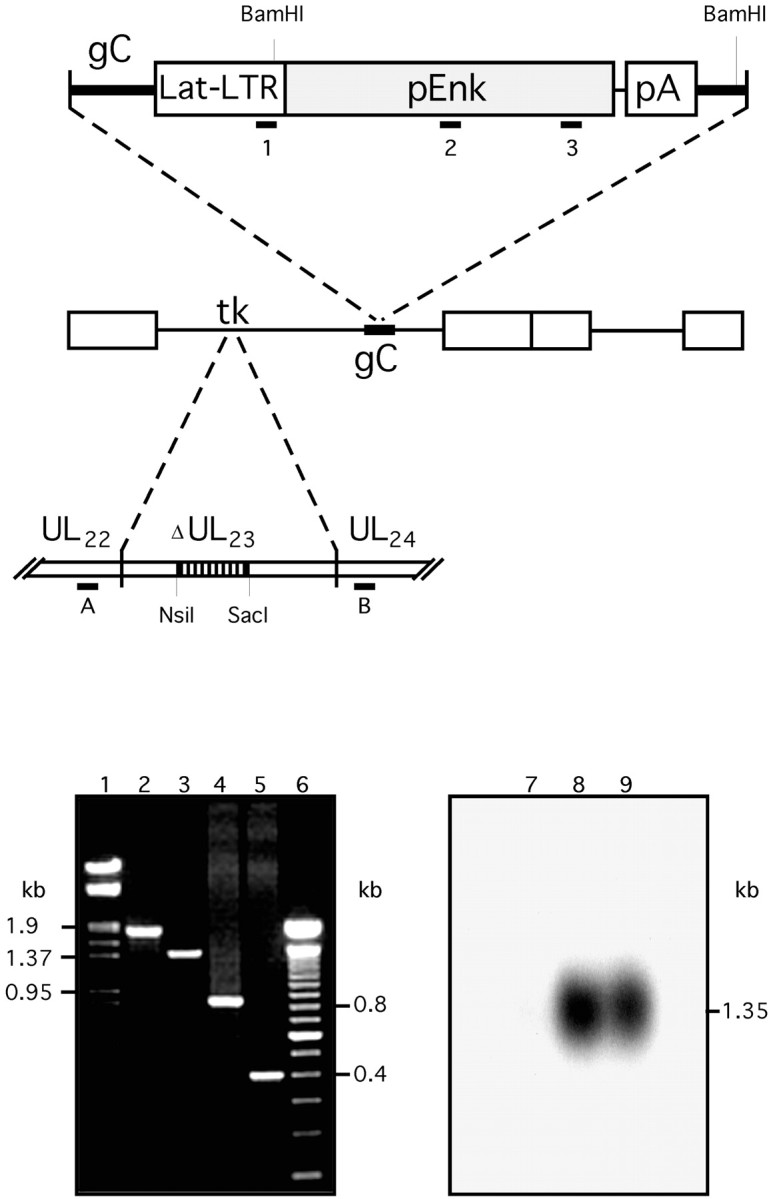
Schematic representation of recombinant HSVLatEnk vector and its molecular characterization. Thymidine kinase gene (UL23) in KOS-derived Tk+HSVLatβ-gal DNA [bearing β-galactosidase reporter gene downstream of the Lat-LTR promoter inserted into the gC locus (Antunes-Bras et al., 1998)] was disrupted by homologous recombination with p23d plasmid DNA containing NsiI toSacI deleted HSV TK gene to generate TK-negative HSVLatβ-gal vector (HSVLatβ-gal). HSVLatEnk recombinant was then created as described previously (Antunes-Bras et al., 1998), by inserting Lat-LTR-pEnk transcriptional unit into the gC gene of HSVLatβ-gal by homologous recombination. Purified HSVLatEnk DNA was analyzed by PCR, and subsequent PCR products were separated on ethidium bromide-stained 1.2% agarose gel for the presence of both the TK-deleted gene and the Enk transgene. Positions of respective primers used are indicated on the diagram. Lanes 1 and6 show the Lambda DNA/HindIII-EcoRI and 100 bp molecular weight standards (given in kilobases), respectively. Lane 2, Approximately 1.8 kb PCR product generated by amplification, using primers A/B, of wild-type HSV DNA; lane 3, ∼1.3 kb PCR product generated using the same primers A/B on HSVLatEnk DNA.Lanes 4 and 5 show the PCR amplification products obtained with the set of primers 1/3 and 2/3 on HSVLatEnk DNA, respectively. HSVLatEnk DNA was further analyzed by Southern hybridization. Ten micrograms of HSVLatβ-gal DNA (lane 7), 10 μg of HSVLatEnk DNA (lane 8), and 0.1 μg of pLatEnk DNA (lane 9) were digested withBamHI, applied on 0.8% agarose gel, electrotransferred on nylon membrane, and hybridized with a [32P]-labeled cDNA probe corresponding to the rat pEnk coding region. Whereas no hybridization signal was apparent on HSVLatβ-gal (lane 7), positively labeled DNA fragments of ∼1.35 kb were generated on HSVLatEnk DNA (lane 8) and pLatEnk DNA (lane 9). This size corresponds to the expected size of DNA fragment resulting from theBamHI digestion of both HSVLatEnk and pLatEnk DNA.
HSVLatEnk and HSVLatβ-gal were concentrated by ultracentrifugation (50,000 × g; 4°C; 1 hr). Viruses were resuspended in 10% sucrose, and their titers were determined using standard plaque assay on Vero cells. The HSVLatEnk and HSVLatβ-gal titers were of 5 × 108 pfu/ml and 2 × 109 pfu/ml, respectively. Functional efficacy of HSVLatEnk was characterized in vitro on either Vero cells or human neuroblastoma cells (SK-N-MC) by searching for the presence of PA encoding mRNA and ME-like material (MELM) in both infected (with either HSVLatEnk or HSVLatβ-gal) and uninfected cells. Briefly, 48 hr after infection of cells at a multiplicity of infection of 1, total RNA was extracted using the acid–guanidinium method (Chomczynski and Sacchi, 1987) and treated with DNase. RNA was then recovered by phenol–chloroform extraction and ethanol precipitation. Proenkephalin A mRNA was detected by reverse transcription (RT)-PCR on 0.5 μg of total RNA according to the Access RT-PCR system instructions (Promega, Madison, WI) using 40 pmol of each PA-specific primer (5′TAGCCAAGAAGTATGGAGGG3′; and 5′GACTATCAGGTAGGTGGTGAGC3′). Synthesis of ME-like material was revealed using immunohistochemistry with a monoclonal anti-ME antibody (1:1000; Valbiotech, Paris, France). Forty-eight hours after infection, cells on poly-d-lysine-coated coverslips were fixed with 4% paraformaldehyde in PBS at room temperature. After washing, cells were preincubated in PBS containing 0.1% Triton X-100 and 6% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and then processed as described in the protocol for immunohistochemistry (see below).
Animals and treatments. All experiments were performed in accordance with institutional guidelines that are in compliance with national and international law and policies for use of animals in neuroscience research (Council directive number 87848, Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale). All animals were maintained under the same conditions (22 ± 1°C; 60 ± 10% relative humidity; 12 hr light/dark cycle; food and water availablead libitum). Polyarthritis was induced by an intradermal injection at the base of the tail of 0.05 ml of Freund's adjuvant (Gouret et al., 1976) in 6-week-old male Sprague Dawley rats. In addition to healthy animals, three homogeneous groups of polyarthritic rats were constituted and deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) for virus or vehicle administration. The first and second group of polyarthritic rats were infected bilaterally on slightly scarified hind footpads with ∼5 × 106 pfu of either HSVLatEnk or HSVLatβ-gal, respectively. Treatment of sham-infected polyarthritic rats in the third group consisted of vehicle (10 μl of 10% sucrose in 0.9% NaCl) application onto scarified footpads.
Previous studies showed that the resulting increase in the concentrations of MELM in rat lumbar DRG is maximum 3 weeks after infection with HSVLatEnk (Antunes-Bras et al., 1998). Because most of the polyarthritis-associated symptoms peak 3–5 weeks after induction of the disease (Calvino et al., 1987), rats were infected 2 weeks after polyarthritis induction, and subsequent experiments were generally performed 3 weeks later. In particular, animals used for radioimmunoassay, immunohistochemical, or in situhybridization procedures were killed by decapitation 3 weeks after infection, i.e., 5 weeks after polyarthritis induction. The lumbar enlargement of the spinal cord and adjacent DRG (L1–L6) were dissected at 0–4°C. The spinal cord was divided into its ventral and dorsal parts (except when used for in situ hybridization experiments), and left and right ganglia were pooled. Tissue pieces for RNA analyses were frozen in liquid nitrogen and stored at −80°C. Radioimmunological determinations of tissue levels of ME, calcitonin gene-related peptide (CGRP), and substance P were performed as described previously (Cesselin et al., 1980; Pohl et al., 1990).
Hindpaw diameter measurements and radiological analyses.Animals were anesthetized with chloral hydrate (300 mg/kg, i.p.) 2 weeks (i.e., just before infection with either HSVLatβ-gal or HSVLatEnk) and 5 weeks after polyarthritis induction. The diameter of their hindpaws was measured using a digital micrometer (Mitutoyo). The presence and severity of lesions were evaluated on radiographs (made 2, 3, and 5 weeks after polyarthritis induction) by an expert rheumatologist, who was unaware of the treatments. A four-degree rating scale was used for the bilateral evaluation of ankle and metatarsus toe joints: 0, no obvious lesions; 1, doubtful or mild lesions; 2, medium lesions with joint space narrowing or disappearance; and 3, severe lesions with joint destruction and mild periostitis. To take into account as precisely as possible the development of periostitis, which is an important variable in joint lesions (and can be widely developed despite relatively medium joint destruction), each score value was raised by one if extensive periostitis was present. Accordingly, the maximum rate was 16. Because no differences in hindpaw diameters or severity scores of lesions were observed between sham-infected and HSVLatβ-gal-infected polyarthritic rats, animals in these two groups are referred to as control polyarthritic rats.
Behavioral studies. Pain-related behavior was assessed by measuring the latency of foot withdrawal elicited by noxious radiant heat (Ugo Basile Plantar test, intensity 7; Ugo Basile, Comerio, Italy) applied to hindpaw plantar surface (Galbraith et al., 1993). Three measures at 1 hr intervals were performed bilaterally, and the mean was considered as one value for each animal.
Spontaneous locomotor activity was measured in an open field, the floor of which was divided into five compartments with black lines. The open field was in a red-lighted room in which animals were introduced 12 hr before the beginning of the experiments. Each animal was placed into the open field, and 1 min later its locomotor activity was video monitored and tape recorded. The number of line crosses and rearings were counted for a 7 min period by two independent observers in a distant room.
Performances of the different groups of polyarthritic rats were first assessed 3 weeks after infection and compared with those of healthy controls. The next day, animals were anesthetized with chloral hydrate (300 mg/kg, i.p.). The skin was incised at the level of the scapula, an Alzet osmotic minipump (delivery rate of 1 μl/hr; 2001 model; AlzaScientific Products, Palo Alto, CA) was implanted subcutaneously, and the incision was then sutured. Following the instructions of the manufacturer, osmotic minipumps were filled with either naloxone or naloxone methiodide to administer each of these drugs at the dose of 3 mg · kg−1 · d−1. “Sham-”treated animals were implanted with saline-delivering minipumps. Behavioral studies were performed on the fourth day after minipump implantation. Observers were blinded to the group of polyarthritic rats (either controls or HSVLatEnk-infected rats) and to the drug delivered. Similar conditions were used to assess the performances of animals 5 and 8 weeks after infection. Behavioral experiments did not reveal any differences between sham-infected and HSVLatβ-gal-infected polyarthritic rats.
In situ hybridization. Deeply anesthetized animals (chloral hydrate; 400 mg/kg, i.p.), were perfused transcardially with 100 ml of saline (0.9% NaCl) supplemented with 0.1% sodium nitrite, followed by 600 ml of 4% paraformaldehyde in PBS, at room temperature. Spinal cord and L1–L6 dorsal root ganglia were removed, post-fixed for 2 hr in the same solution at 4°C, cryoprotected in 10% sucrose–PBS, frozen, and stored at −80°C until used. Ten micrometer cryostat sections were fixed for 1 hr with 4% paraformaldehyde in PBS at 4°C, rinsed in PBS, and dehydrated through a graded series of ethanol concentrations (30–100%). Slides with tissue sections were incubated in the presence of a cRNA probe of latency-associated transcripts (LATs) labeled with digoxygenin-11-UTP according to the instructions of the manufacturer (Promega, Madison, WI). This probe (188–621 bases PCR amplified segment of HSV-1 LAT sequence; Wagner et al., 1988) allows the detection of all viral particles, independent of their ability to express the PA transgene. Hybridization was performed overnight at 65°C in 1× SSC, 50% formamide, 10% dextran sulfate, 1 mg/ml rRNA, and 1× Denhardt's solution. On the following day, sections were washed twice with 1× SSC, 50% formamide, and 0.1% Tween 20, at 65°C, and twice with 100 mm maleic acid, 150 mm NaCl, and 1% Tween 20, at room temperature. The digoxygenin-labeled hybrids were detected with an alkaline phosphatase-conjugated anti-digoxygenin antibody following the instructions of the manufacturer (Roche Products, Hertforshire, UK). PA mRNA was detected by hybridizing tissue sections with ∼3.3 × 106 cpm/μl antisense [35S]cRNA (corresponding to the entire coding region of the rat preproenkephalin A cDNA) as described in detail previously (Pohl et al., 1994).
Quantitative RT-PCR. Total RNA, extracted as described above, was quantified using as reference a scale of total RNA prepared on cesium chloride gradient and estimated from the optical density at 260 nm. RT-PCR was performed, as described previously (Antunes-Bras et al., 1998), with 0.5 μg of each RNA sample in the presence of various amounts (0.5–16 fg) of internal synthetic standard prepared according to the PCR MIMIC construction kit (Clontech, Cambridge, UK). This 241 bp standard fragment, flanked with PA sequences (21 bp), was amplified with the same set of rat PA-specific primers as the cDNA. Reverse-transcribed RNA was amplified with 30 cycles (96, 58, and 72°C; 1 min each) according to the Access RT-PCR system instructions (Promega) using 40 pmol of primers in a mixture containing 10 mm of each dNTP, 25 mm MgSO4, 2.5 U of avian myeloblastosis virus reverse transcriptase, 2.5 U of thermus flavus DNA polymerase, and 6.5 U of RNase inhibitor (RNasin) in 1× reaction buffer. The RT-PCR products were electrophoresed on 1.2% ethidium bromide-stained agarose gel and quantified with the gel analyzer GDS 5000 (Ultra-Violet Products Ltd., Cambridge, UK).
Immunohistochemistry. Animals were anesthetized and perfused as described in the protocol for in situ hybridization. Segments of sciatic nerve, L4–L5 dorsal roots, and L4—L5 DRG were dissected out and cryoprotected in 10% sucrose (24 hr, 4°C). Fifteen micrometer cryostat sections were preincubated (30 min, room temperature) in PBS containing 0.3% Triton X-100 and 3% normal donkey serum (Jackson ImmunoResearch) and then incubated overnight at 4°C in the same buffer supplemented with a monoclonal anti-ME antibody (1:1000; Valbiotech). After washing in PBS, sections were incubated for 1 hr with rhodamine (Cy3)-conjugated anti-mouse Ig (1:800; Interchim, Montlucon, France), rinsed in PBS, mounted in fluoromount-G (CliniSciences, Montrouge, France), and examined using a Leica (Nussloch, Germany) confocal microscope. Both labeled and unlabeled neurons were counted in every fourth sections of a total of ∼30 sections for each DRG (L4–L5, unilaterally), in three animals per group. A total of 712–852 neurons were analyzed in each group.
Statistical analyses. All data, including those from behavioral experiments, were subjected to the unpaired Student'st test. The performances of rats after versus before treatment with opioid receptor antagonists were compared using the paired Student's t test. The Kolmogorov–Smirnov test was used for statistical analysis of the data derived from clinical evaluation of the joint lesions. When p > 0.05, the corresponding difference was considered to be not significant.
RESULTS
Morphological aspects and osseous lesions of hindpaws in HSVLatEnk-infected polyarthritic rats
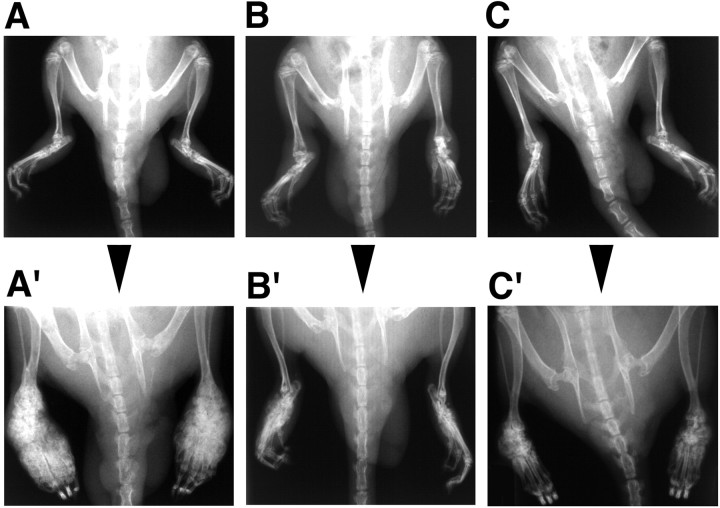
In agreement with previous reports (Pearson, 1956; De Castro Costa et al., 1981), soft tissue swelling and increased joint diameter of hindpaws were apparent in nearly 100% of animals 2 weeks after polyarthritis induction (bilateral hindpaw joint diameter in control polyarthritic rats, 13.3 ± 1.2 mm, mean ± SEM,n = 18; versus 9.0 ± 0.1 mm in healthy rats,n = 8; p < 0.01). In control polyarthritic rats, these signs of inflammation worsened for the following weeks, with larger hindpaw swelling and joint diameter (17.4 ± 1.0 mm; n = 8; p < 0.02) at the end of the fifth week. In contrast, in paired HSVLatEnk-infected polyarthritic rats, the hindpaw joint diameter did not enlarge from the second to the fifth week (13.1 ± 1.0 mm; n = 8) after polyarthritis induction. Just before infection by either HSVLatβ-gal or HSVLatEnk, i.e., 2 weeks after polyarthritis induction, radiogram analysis yielded scores of hindpaw joint lesions ranging from 6 to 10 (Fig.2A–C). Three weeks later, marked lesions, including extensive erosion of bone extremities, narrowing or disappearance of joint spaces, calcification, and new bone proliferation, were observed in control animals (13 ≤ lesion scores ≤ 16) (Fig. 2A′). Radiogram analysis of HSVLatEnk-infected rats (Fig. 2B′,C′) revealed that the mean scores of their hindpaw joint lesions were significantly lower (p < 0.001;n = 10) than those in control polyarthritic rats (Fig.2A′). Despite inter-individual variability in polyarthritis development, 6 of the 10 HSVLatEnk-infected rats examined presented only medium lesions of the hindpaw joints (lesions scores ≤ 9) (Fig. 2B′). The other four HSVLatEnk-infected animals had extensive lesions (9 < lesions scores ≤ 11) (Fig. 2C′), which never reached the severity of joint destruction regularly observed in control polyarthritic rats. Treatment with naloxone methiodide (3 mg · kg−1 · d−1) for 3 d did not affect the radiological lesion scores in HSVLatEnk-infected polyarthritic rats (data not shown).
Fig. 2.
Presence and severity of hindpaw joint lesions in control (representing both sham-infected or HSVLatβ-gal-infected polyarthritic rats) (A′) and HSVLatEnk-infected (B′, C′) polyarthritic rats. Individual evolution of polyarthritis-associated joint destruction was assessed by comparing radiograms made 2 weeks after polyarthritis induction (A, B, C), i.e., just before infection with either HSVLatβ-gal or HSVLatEnk, and then 3 weeks later (A′, B′, C′). Osseous lesions of tarsus and metatarsus toe were evaluated bilaterally in both groups of rats (n = 8–10) using a four-degree rating scale. Radiographs were examined by an expert observer, without knowledge of the treatments. The Kolmogorov–Smirnov test was used for statistical analysis of the data. Six of 10 HSVLatEnk-infected rats presented mild lesions (B′), and the remaining four animals had more extensive lesions (C′), which were, however, less important than those of control polyarthritic rats (A′).
Effects of HSVLatEnk infection on pain-related behavior in polyarthritic rats
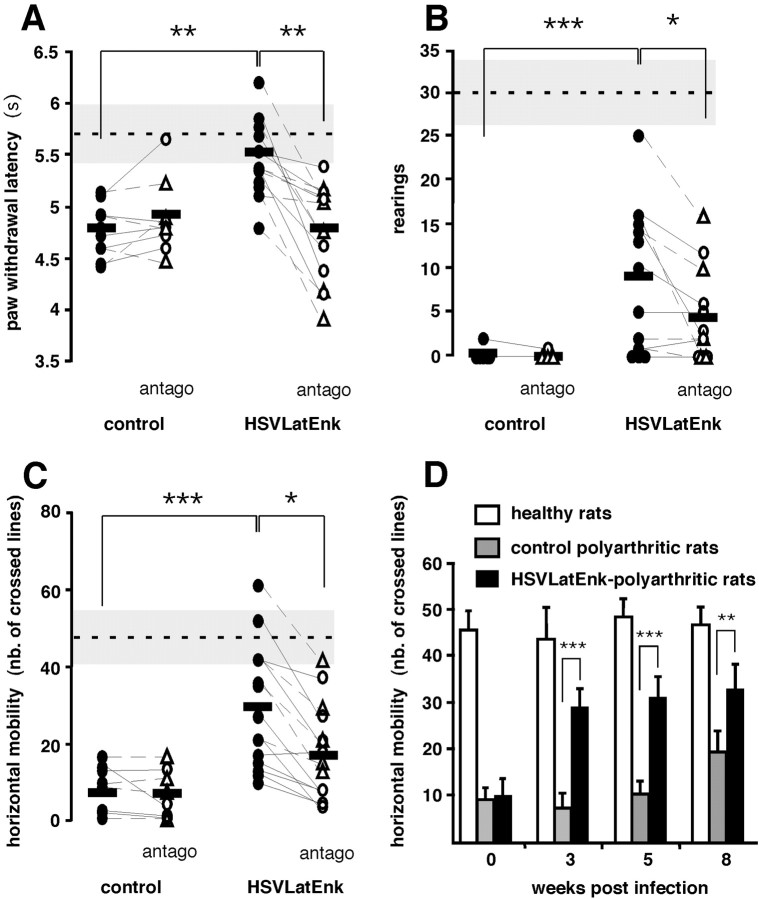
Compared with healthy rats (latency of foot withdrawal to radiant heat, 5.7 ± 0.3 sec; mean ± SEM; n = 7), polyarthritic control rats had slightly reduced latency 5 weeks after polyarthritis induction (4.8 ± 0.2 sec; p < 0.02; n = 15). At the same time (i.e., 3 weeks after injection), the paw withdrawal latencies of HSVLatEnk-infected animals were significantly higher than those of control polyarthritic rats (5.5 ± 0.2 sec; p < 0.001; n = 15) (Fig. 2A). Measurement of spontaneous locomotor activity of healthy rats indicated that they performed 48 ± 7 (mean ± SEM; n = 6) crosses of the lines and 30 ± 4 (mean ± SEM; n = 6) rearings for the 7 min recording period. Five weeks after induction of the disease, both the horizontal locomotor activity (7 ± 2 crosses; mean ± SEM; n = 11) and rearings (0.2 ± 0.1; mean ± SEM; n = 11) were dramatically reduced in control polyarthritic rats (Fig.3B,C). Compared with the latter animals, paired HSVLatEnk-infected rats showed a remarkable improvement in both rearings (by eightfold;p < 0.001; n = 12) (Fig.3B) and horizontal displacements (by 4.2-fold;p < 0.001; n = 15) (Fig.3C) 3 weeks after infection. This amelioration persisted during the whole observation period, i.e., for at least 8 weeks after HSVLatEnk infection (Fig. 3D).
Fig. 3.
HSVLatEnk-infected polyarthritic rats exhibited reduced thermal hyperalgesia and improved spontaneous locomotor activity. Response (paw withdrawal) latencies (A) of controls (n = 9) and HSVLatEnk-infected (n = 15) polyarthritic rats to radiant heating (intensity 7; Ugo Basile) were measured 3 weeks after infection. Rearings (B) and horizontal locomotor activity (C) of control (sham- or HSVLatβ-gal-infected) (n = 12) and HSVLatEnk-infected (n = 15) polyarthritic rats in a red-lighted open field were video monitored and assessed every minute during a 7 min period. Animals were then implanted subcutaneously for 3 d with an Alzet osmotic minipump delivering 3 mg · kg−1 · d−1 of either naloxone (○) or naloxone methiodide (▵) (antago), and thermal hyperalgesia and locomotor activity were assessed. Performances of normal healthy rats are indicated by the horizontal dashed line and gray band in the three behavioral tests (mean ± SEM;n = 6–7). *p < 0.05; **p < 0.01; ***p < 0.001 for control versus HSVLatEnk-infected polyarthritic rats, two-tailed unpaired t test; and for untreated versus naloxone–naloxone methiodide-treated animals, two-tailed pairedt test. Long-term improvement of functional ability (D) in HSVLatEnk-infected polyarthritic rats was evaluated by comparing the locomotor activity in normal healthy rats (n = 5) and in both control (n= 8) and HSVLatEnk-infected (n = 10) polyarthritic rats 3, 5, and 8 weeks after infection. **p < 0.01; ***p < 0.001 for control versus HSVLatEnk-infected rats; two-tailed unpaired ttest.
Behaviors were not significantly changed in both control and HSVLatEnk-infected polyarthritic rats implanted with saline-containing minipumps. Similarly, naloxone or naloxone methiodide, administered at the dose of 3 mg · kg−1 · d−1for 3 d, were without any significant effect in control polyarthritic rats. In contrast, both compounds reversed with a similar efficacy both the antihyperalgesic response (p< 0.001; n = 15) (Fig. 3A) and the improved locomotor activity (p < 0.05; n= 12–15) (Fig. 3B,C) in HSVLatEnk-infected animals.
Localization and quantification of HSVLatEnk-mediated ME overexpression.
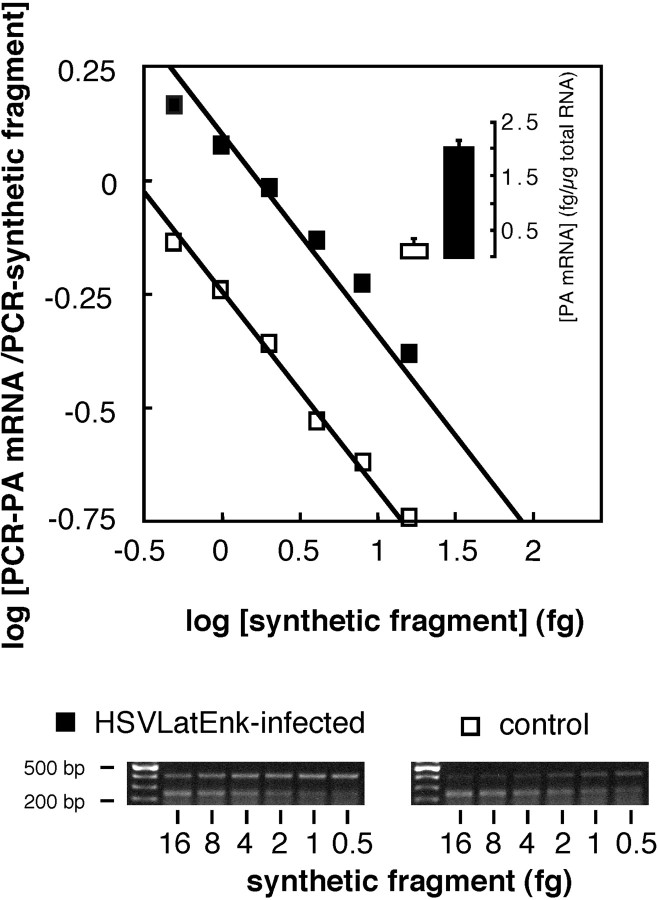
In situ hybridization histochemistry with a digoxygenin-labeled complementary probe of HSV LATs showed that, after the peripheral infection of hindpaws in polyarthritic rats, viral vectors were restricted to L4–L5 DRG (Fig.4A). This indicated rapid entry in latency and no spread of vectors to adjacent DRG or the spinal cord (Fig. 4B,C, respectively). In situ hybridization with [35S]-labeled rat PA complementary probe showed that only ∼2–3% of neurons express PA mRNA in L4–L5 DRG of healthy rats (Fig. 4D). In control polyarthritic rats, no or only scarce PA mRNA-expressing cells were detected in lumbar L4–L5 DRG (Fig. 4E). In contrast, peripheral inoculation of polyarthritic rats with HSVLatEnk led to a significant increase in the number of PA mRNA-expressing neurons in L4–L5 DRG (Fig. 4F), reaching ∼12% of the total neuron population. Quantitative RT-PCR allowed the demonstration that PA mRNA levels in L4–L5 DRG of HSVLatEnk-infected rats were approximately eightfold higher (p < 0.001; n= 5) than in control polyarthritic animals (Fig.5). In addition, MELM concentrations in L4–L5 DRG were also significantly higher (+40%) in HSVLatEnk-infected rats than in paired control polyarthritic animals (71.9 ± 7.1 vs 49.5 ± 5.2 pg/mg protein, respectively; means ± SEM;n = 8 in each group; p < 0.01). On the other hand, measurement of both substance P and CGRP concentrations in L4–L5 DRG of control polyarthritic rats (1.6 ± 0.2 and 8.3 ± 0.2 ng/mg protein, respectively; means ± SEM;n = 8) showed comparable levels with those in HSVLatEnk-infected rats (1.4 ± 0.1 and 8.1 ± 0.4 ng/mg protein; n = 8; respectively).
Fig. 4.
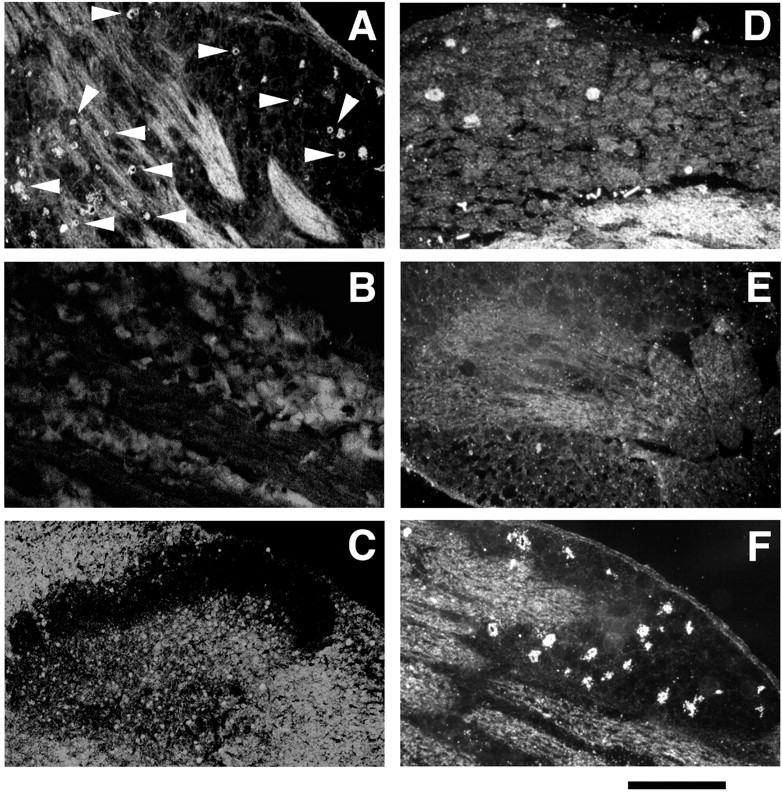
LATs and PA mRNA expression were detected by in situ hybridization histochemistry. Incubation of 10 μm sections of L4–L5 DRG with digoxygenin-labeled LAT cRNA revealed numerous LAT-expressing neurons (arrowheads) in HSVLatEnk-infected polyarthritic rats (A). In contrast, no LAT-expressing nerve somas could be detected in adjacent L1–L3 DRG (B) or in the spinal cord of the lumbar region (C), suggesting no spread of the vectors to these territories. Every fourth section of L4–L5 DRG from normal healthy rats (D), control (E), or HSVLatEnk-infected polyarthritic rats (F) was incubated with [35S]PA cRNA, and then both unlabeled and labeled neurons were counted (in a total of 30 sections in each group). Approximately 2% of neurons in L4–L5 DRG of healthy rats were labeled with [35S]PA cRNA (D). In contrast, no PA mRNA-expressing neurons were detected in L4–L5 DRG of control (vehicle- or HSVLatβ-gal-infected) polyarthritic rats (E). Infection with HSVLatEnk led to the expression of PA mRNA in ∼12% (110 of a total of 955 neuronal somas) of neurons in L4–L5 DRG (F). The photomicrographs are representative of three to four animals per group, examined 5 weeks after polyarthritis induction (i.e., 3 weeks after vehicle, HSVLatβ-gal, or HSVLatEnk infection). Scale bar:A, B, D–F, 250 μm;C, 390 μm.
Fig. 5.
Quantitative RT-PCR measurement of PA mRNA levels in L4–L5 DRG of control (■) or HSVLatEnk-infected (▪) polyarthritic rats. Total RNA was extracted from L4–L5 DRG in both groups of animals (n = 5 for each group) 5 weeks after polyarthritis induction. Five hundred nanograms of total RNA were reverse-transcribed in the presence of six different dilutions of synthetic fragment and amplified for 30 cycles. Measurement of optical density of PCR amplification products of PA mRNA (402 bp) or of the synthetic fragment (241 bp) allowed the drawing of the plot, as described previously (Antunes-Bras et al., 1998). Representative gel analyses of PCR products are shown.
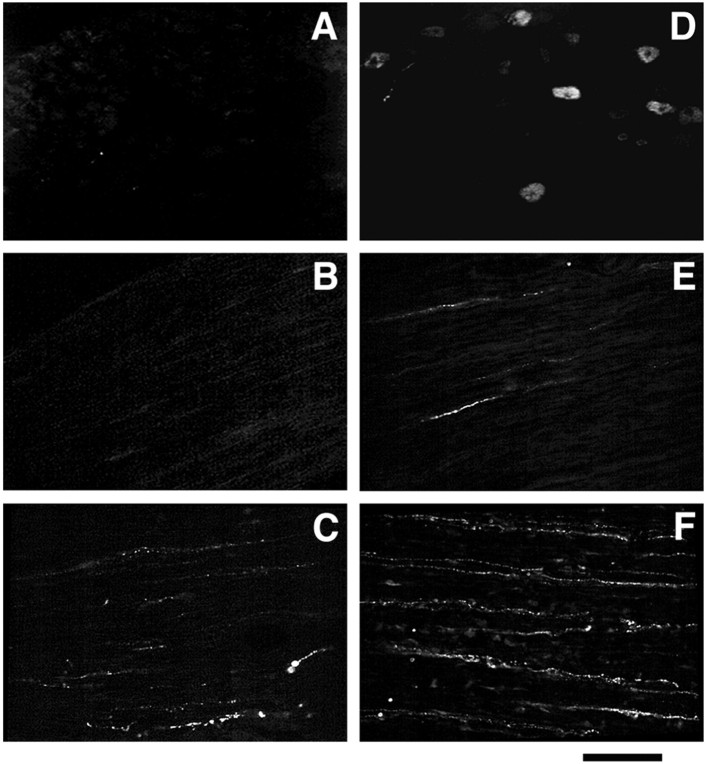
Immunofluorescence investigations showed that no ME-immunoreactive neurons could be detected in L4–L5 DRG and corresponding dorsal roots of untreated polyarthritic rats (Fig.6A,B). Furthermore, neuronal processes stained for MELM were only rarely detected in peripheral outputs of L4–L5 DRG in these animals (Fig.6C). Infection of rats with HSVLatEnk resulted in the appearance of numerous positively stained neuronal cell bodies in L4–L5 DRG (Fig. 6D). Approximately 20% of MELM-positively labeled cell bodies costained for CGRP-like material or substance P-like material (data not shown). No or only scarce MELM-positively labeled nerve fibers were visualized in dorsal roots of infected animals (Fig. 6E). In contrast, a relatively dense bundle of neuronal processes containing MELM was observed in the peripheral output of L4–L5 DRG in HSVLatEnk-infected polyarthritic rats (Fig. 6F).
Fig. 6.
Immunofluorescent detection of Met-enkephalin-like material. Fifteen micrometer sections were stained with monoclonal anti-ME antibody (Valbiotech). No ME staining was detected in DRG neuronal cell bodies (A) and dorsal roots (B) of untreated polyarthritic rats. Only few neuronal processes stained for ME could be visualized in sciatic nerves of these animals (C). In HSVLatEnk-infected polyarthritic rats, numerous ME-stained neuronal soma were present in L4–L5 DRG (D). Scarce neuronal processes were stained in dorsal roots (E), whereas numerous nerve fibers positively labeled for ME were present in sciatic nerves (F) of HSVLatEnk-infected rats. Scale bar:A, D, 250 μm; B,C, E, F, 70 μm.
DISCUSSION
Together with pain, erosive inflammation of the joints and new bone proliferation represent prominent symptoms of polyarthritis in both humans and animal models of the disease. This report shows that PA overexpression in lumbar DRG neurons of polyarthritic rats induces mainly peripherally mediated antihyperalgesic effects and reduces disease-related functional disability in these animals. Furthermore, our studies also demonstrate the beneficial effect of PA-derived peptides on hindpaw joint lesions because overproduction of these opioids limited the progression of bone erosion and periostitis.
HSV type 1-derived vectors have been successfully used for transgene delivery into sensory neurons, and we showed recently their potency to drive PA expression in rat DRG neurons (Davar et al., 1994;Antunes-Bras et al., 1998, 2001; Goins et al., 1999; Wilson et al., 1999). In our previous studies, peripheral infection of healthy rats with replication-competent vectors resulted in vector expression limited to L4–L5 DRG (Antunes-Bras et al., 1998); however, possibly because of the peculiar immune status of polyarthritic rats, we found in a first series of experiments that, in some of these animals, recombinant vectors could spread from the DRG to the spinal cord. This observation lead us to generate conditional defective TK-deleted HSV vectors, severely impaired for the acute replication in ganglionic neurons (Coen et al., 1989). To ensure long-term and relatively high levels of PA-derived peptides synthesis, we placed the rat PA cDNA sequence under the control of Lat-LTR promoter whose in vivo activity is well documented (Lokensgard et al., 1994;Antunes-Bras et al., 1998). To detect all viral vectors (even those that eventually did not synthesize transgene-derived mRNA), we performed in situ hybridization histochemistry not only with an ME mRNA complementary probe but also with a probe complementary to viral latency-associated transcripts. The results obtained with both probes clearly showed that TK-negative recombinants remained restricted to L4–L5 DRG of polyarthritic rats and did not spread to adjacent ganglia or the spinal cord, as expected for this vector with reduced replication in neurons (Coen et al., 1989). Despite the presence of numerous neurons synthesizing MELM in L4–L5 DRG 3 weeks after HSVLatEnk inoculation, only few nerve fibers endowed with MELM were visualized in L4–L5 dorsal roots. In contrast, numerous neuronal profiles containing MELM were present in sciatic nerves of HSVLatEnk-infected polyarthritic rats. These results are consistent with our previous studies in healthy rats showing that, in PA overexpressing animals (as in controls), MELM is preferentially transported to the peripheral terminals of primary afferents from which it can be released by electrical stimulation of the sciatic nerve (Antunes-Bras et al., 2001). Together, these data suggest that, in HSVLatEnk-infected polyarthritic rats, overproduced MELM was also mainly transported to the peripheral side of primary afferent fibers and presumably released.
The concentrations of substance P and CGRP in DRG of polyarthritic rats, which are increased compared those in control healthy rats (Kar et al., 1991; Smith et al., 1992), were not modified in animals infected with either HSVLatβ-gal or HSVLatEnk, suggesting that neither the infection per se nor the ME overexpression affects the DRG content in these major neuropeptides synthesized in sensory ganglia. However, opioids are known to modulate the substance P and CGRP release from primary afferent neurons (Mauborgne et al., 1987; Pohl et al., 1989). Further investigations are thus needed to examine whether their release, which is altered in polyarthritic rats (Cesselin et al., 1999), could be modified in HSVLatEnk-infected rats, notably at the periphery, at which both peptides exert proinflammatory effects.
An important finding is the reduction of hindpaw diameter associated with reduced progression of joint lesions in HSVLatEnk-infected polyarthritic rats. Interestingly, radiological examination of hindpaws revealed reduced osseous lesions and bone proliferation, particularly at the tarsus and metatarsus joints, i.e., at the paw levels at which primary afferents infected with HSVLatEnk project. Immunomodulatory action of opioids is well documented (for review, see Stefano et al., 1996). The existence of a dense network of sensory nerves innervating the periosteum, the bone marrow, and the osteochondral junction (Bjurholm et al., 1988; Hill and Elde, 1991; Hukkanen et al., 1992), as well as the presence of opioid binding sites on osteoblasts and chondrocytes (Castano et al., 1991), support the idea of a direct action of peripherally released opioids on these tissues. Our data are consistent with previous reports suggesting that opioids can modulate inflammation and attenuate joint damage in polyarthritic rats (Levine et al., 1986; Walker et al., 1996; Binder and Walker, 1998).
Overproduction of PA-derived opioid peptides in sensory nerves also resulted in an antihyperalgesic activity. In particular, HSVLatEnk-infected rats exhibited an increased latency of foot withdrawal elicited by noxious radiant heat. However, an acute painful stimulus does not actually reflect the dimension of persistent, spontaneous pain. This is particularly true in polyarthritic rats in which the most prominent behavioral change is a marked reduction of locomotion as a result of intense pain and joints alterations. Indeed, horizontal and vertical mobility in polyarthritic rats were severely reduced (rearings being almost absent), and measurement of locomotor activity provides a sensitive evaluation of “functional disability” in these animals (Larsen and Arnt, 1985; Cain et al., 1997; Lindner et al., 1999). Of special interest in this study was the impressive amelioration of the mobility of HSVLatEnk-infected polyarthritic rats that recovered up to nearly 70% of that of healthy rats. With regard to pain-related behavior, our data are in accordance with the recently reported antihyperalgesic effects of overexpressed PA or β-endorphin at the spinal level in animals with experimentally induced pain (Finegold et al., 1999; Wilson et al., 1999).
The involvement of central and/or peripheral opioid receptors in both the antihyperalgesic response and the improved mobility of HSVLatEnk-infected polyarthritic rats was assessed using naloxone, a centrally and peripherally acting opioid receptor antagonist, and naloxone methiodide, an antagonist acting exclusively at the periphery. Taking into account that PA overexpression in sensory neurons of HSVLatEnk-infected rats is a continuous, long-lasting (i.e., for several weeks) process, we reasoned that prolonged delivery of opioid receptor antagonists should be a valuable approach to inhibit the effects of overproduced ME. Both naloxone and naloxone methiodide, without significant effect in control polyarthritic rats, reversed with a similar efficacy both the antihyperalgesic response and the improved mobility in HSVLatEnk-infected polyarthritic rats. This finding is in line with previous observations showing that naloxone [but at a markedly higher dose (50 mg/kg, i.p.) than that used here] antagonized the antihyperalgesic responses to capsaicin and dimethylsulfoxide as a result of overexpression of human PA in recombinant virus-infected mice (Wilson et al., 1999). In addition, the fact that naloxone methiodide was as efficient as naloxone to suppress the antihyperalgesic response further supports the idea that peripheral opioid receptors play a key role in opioid-induced reduction of inflammatory pain (Stein and Yassouridis, 1997; Binder and Walker, 1998) and related disability in polyarthritic rats infected with HSVLatEnk. However, because sparse MELM-containing nerve fibers were observed in dorsal roots of HSVLatEnk-infected rats, it cannot be excluded that spinal opioid receptors also contributed to the antihyperalgesic effects of PA overexpression in DRG neurons.
Although HSV latency is an almost exclusive feature of nervous tissue (Steiner and Kennedy, 1995), the possibility of latent infection of non-neuronal cells at the site of HSVLatEnk inoculation could not be ruled out. This possibility could account for the mainly peripherally mediated affects observed in our study and, to some extent, also in that of Wilson et al. (1999). However, these authors found that intrathecal administration of naloxone reversed the antihyperalgesic effect in mice infected with proenkephalin encoding vector, thereby suggesting that primary afferents represented the major supply of overproduced opioid peptides. Our previous results also support this idea. Indeed, we found in normal HSVLatEnk-infected rats that MELM was released not only at the dorsal horn of the spinal cord by direct electrical stimulation of dorsal roots but also and to a greater extent at the periphery after electrical stimulation of the sciatic nerve (Antunes-Bras et al., 2001). These results suggest that increased peripheral MELM overflow triggered by stimulation of the sciatic nerve in fact involved peripheral terminals of primary afferents rather than some non-neuronal cells.
Naloxone methiodide treatment did not affect the radiological lesion score in HSVLatEnk-infected polyarthritic rats, indicating that, under such conditions, a 3 d blockade of peripheral opioid receptors was probably too short to reverse the inhibitory action of PA overexpression on the progression of the disease.
In any case, polyarthritis-associated disability in rats appears to be the consequence of both chronic pain and mechanical affection of joints. Accordingly, the long-term amelioration in polyarthritic rats infected with HSVLatEnk probably reflects a synergic action of PA-derived peptides on both aspects of the disease. Gene therapy for rheumatoid arthritis is a rapidly growing field, and several relevant approaches are currently tested in laboratory models of arthritis (Junker and Böhnlein, 1999). Thus, association of proenkephalin A (and perhaps of other opioid peptides precursors) gene with other candidate genes in dicistronic vectors might open novel perspectives for the management of both pain and osseous lesions that characterize this disease.
Footnotes
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Institut UPSA de la Douleur, and Bristol-Myers Squibb Foundation (Unrestricted Biomedical Research Grant). We are grateful to Drs. A. Bogdan, E. Borrelli, and D. Le Bars for critical reading of this manuscript and helpful discussions. We thank Dr. Begon (Department of Radiology,École Nationale Vétérinaire, Maisons-Alfort, France) for her assistance with radiographs.
Correspondence should be addressed to Michel Pohl, Institut National de la Santé et de la Recherche Médicale U288, Faculté de Médecine Pitié-Salpêtrière, 91 boulevard de l'Hôpital, 75634 Paris Cedex 13, France. E-mail:pohl@ext.jussieu.fr.
REFERENCES
- 1.Antunes-Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F, Pohl M. Herpes simplex virus1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299–1303. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- 2.Antunes-Bras JM, Becker C, Bourgoin S, Lombard MC, Cesselin F, Hamon M, Pohl M. Met-enkephalin is preferentially transported to the peripheral processes of primary afferent fibres in both normal and proenkephalin A overexpressing rats. Neuroscience. 2001;103:1073–1083. doi: 10.1016/s0306-4522(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 3.Binder W, Walker JS. Effect of the peripherally selective kappa-opioid agonist, asimadoline, on adjuvant arthritis. Br J Pharmacol. 1998;124:647–654. doi: 10.1038/sj.bjp.0701874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjurholm A, Kreibergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–171. doi: 10.1016/0196-9781(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 5.Cain CK, Francis JM, Plone MA, Emerich DF, Lindner MD. Pain-related disability and effects of chronic morphine in the adjuvant-induced arthritis model of chronic pain. Physiol Behav. 1997;62:199–205. doi: 10.1016/s0031-9384(97)00158-3. [DOI] [PubMed] [Google Scholar]
- 6.Calvino B, Crepon-Bernard MO, Le Bars D. Parallel clinical and behavioural studies of adjuvant-induced arthritis in the rat: possible relationship with “chronic pain.”. Behav Brain Res. 1987;24:11–29. doi: 10.1016/0166-4328(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 7.Carlton SM, Coggeshall RE. Immunohistochemical localization of enkephalin in peripheral sensory axons in the rat. Neurosci Lett. 1997;221:121–124. doi: 10.1016/s0304-3940(96)13304-8. [DOI] [PubMed] [Google Scholar]
- 8.Castano MT, Freire-Garabal M, Giraldez M, Nunez NJ, Belmonte A, Couceiro J, Jorge J. Autoradiographic evidence of 125I-beta-endorphin binding sites in the articular cartilage of the rat. Life Sci. 1991;49:103–105. doi: 10.1016/0024-3205(91)90601-7. [DOI] [PubMed] [Google Scholar]
- 9.Cesselin F, Montastruc JL, Gros C, Bourgoin S, Hamon M. Met-enkephalin levels and opiate receptors in the spinal cord of chronic suffering rats. Brain Res. 1980;191:289–293. doi: 10.1016/0006-8993(80)90335-2. [DOI] [PubMed] [Google Scholar]
- 10.Cesselin F, Benoliel JJ, Bourgoin S, Collin E, Pohl M, Hamon M. Spinal mechanisms of opioid analgesia. In: Stein C, editor. Opioids in pain control: basic and clinical aspects. Cambridge UP; Cambridge, UK: 1999. pp. 70–95. [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Coen DM, Kosz-Vnenchak MN, Jacobson JG, Leib DA, Bogard CL, Schaffer PA, Tyler KL, Knipe DM. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colpaert FC. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain. 1987;28:201–222. doi: 10.1016/0304-3959(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 14.Davar G, Kramer MF, Garber D, Roca AL, Andersen JK, Bebrin W, Coen DM, Kosz-Vnenchak M, Knipe DM, Breakefield XO, Isacson O. Comparative efficacy of expression of genes delivered to mouse sensory neurons with herpes virus vectors. J Comp Neurol. 1994;339:3–11. doi: 10.1002/cne.903390103. [DOI] [PubMed] [Google Scholar]
- 15.De Castro Costa M, De Sutter P, Gybels J, Van Hees J. Adjuvant-induced arthritis in rats: a possible animal model of chronic pain. Pain. 1981;10:173–185. doi: 10.1016/0304-3959(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 16.El Hassan AM, Lindgren JU, Hultenby K, Bergstrom J, Adem A. Methionine-enkephalin in bone and joint tissues. J Bone Miner Res. 1998;13:88–95. doi: 10.1359/jbmr.1998.13.1.88. [DOI] [PubMed] [Google Scholar]
- 17.Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther. 1999;10:1251–1257. doi: 10.1089/10430349950018238. [DOI] [PubMed] [Google Scholar]
- 18.Galbraith JA, Mrosko BJ, Myers RR. A system to measure thermal nociception. J Neurosci Methods. 1993;49:63–68. doi: 10.1016/0165-0270(93)90109-5. [DOI] [PubMed] [Google Scholar]
- 19.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goins WF, Lee KA, Cavalcoli JD, O'Malley ME, DeKosky ST, Fink DJ, Glorioso JC. Herpes simplex virus type 1 vector-mediated expression of nerve growth factor protects dorsal root ganglion neurons from peroxide toxicity. J Virol. 1999;73:519–532. doi: 10.1128/jvi.73.1.519-532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouret C, Mocquet G, Raynaud G. Use of Freund's adjuvant arthritis test in anti-inflammatory drug screening in the rat: value of animal selection and preparation at the breeding center. Lab Animal Sci. 1976;26:281–287. [PubMed] [Google Scholar]
- 22.Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 23.Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 24.Houghton AK, Valdez JG, Westlund KN. Peripheral morphine administration blocks the development of hyperalgesia and allodynia after bone damage in the rat. Anesthesiology. 1998;89:190–201. doi: 10.1097/00000542-199807000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Hukkanen M, Konttinen YT, Rees RG, Santavirta S, Terenghi G, Polak JM. Distribution of nerve endings and sensory neuropeptides in rat synovium, meniscus and bone. Int J Tissue React. 1992;14:1–10. [PubMed] [Google Scholar]
- 26.Junker U, Böhnlein E. Gene therapy for arthritis 1996–1999. Exp Opin Ther Patents. 1999;9:1491–1498. [Google Scholar]
- 27.Kar S, Gibson SJ, Rees RG, Jura WG, Brewerton DA, Polak JM. Increased calcitonin gene-related peptide (CGRP), substance P, and enkephalin immunoreactivities in dorsal spinal cord and loss of CGRP-immunoreactive motoneurons in arthritic rats depend on intact peripheral nerve supply. J Mol Neurosci. 1991;3:7–18. doi: 10.1007/BF02896844. [DOI] [PubMed] [Google Scholar]
- 28.Larsen JJ, Arnt J. Reduction in locomotor activity of arthritic rats as parameter for chronic pain: effect of morphine, acetylsalicylic acid and citalopram. Acta Pharmacol Toxicol. 1985;57:345–351. doi: 10.1111/j.1600-0773.1985.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 29.Levine JD, Dardick SJ, Roizen MF, Helms C, Basbaum AI. Contribution of sensory afferent and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindner MD, Plone MA, Francis JM, Cain CK. Chronic morphine reduces pain-related disability in a rodent model of chronic, inflammatory pain. Exp Clin Psychopharmacol. 1999;7:187–197. doi: 10.1037//1064-1297.7.3.187. [DOI] [PubMed] [Google Scholar]
- 31.Lokensgard JR, Bloom DC, Dobson AT, Feldman LT. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauborgne A, Lutz O, Legrand JC, Hamon M, Cesselin F. Opposite effects of μ and ∂ opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem. 1987;48:529–537. doi: 10.1111/j.1471-4159.1987.tb04125.x. [DOI] [PubMed] [Google Scholar]
- 33.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvant. Proc Soc Exp Biol. 1956;91:95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 34.Pohl M, Lombard MC, Bourgoin S, Carayon A, Benoliel JJ, Mauborgne A, Besson JM, Hamon M, Cesselin F. Opioid control of the in vitro release of calcitonin gene-related peptide from primary afferent fibres projecting in the rat cervical cord. Neuropeptides. 1989;14:151–159. doi: 10.1016/0143-4179(89)90039-5. [DOI] [PubMed] [Google Scholar]
- 35.Pohl M, Benoliel JJ, Bourgoin S, Lombard MC, Mauborgne A, Taquet H, Carayon A, Besson MJ, Cesselin F, Hamon M. Regional distribution of calcitonin gene-related peptide-, substance P-, cholecystokinin-, met5-enkephalin-, and dynorphin A(1–8)-like materials in the spinal cord and dorsal root ganglia of adult rats: effects of dorsal rhizotomy and neonatal capsaicin. J Neurochem. 1990;55:1122–1130. doi: 10.1111/j.1471-4159.1990.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 36.Pohl M, Collin E, Bourgoin S, Conrath M, Benoliel JJ, Nevo I, Hamon M, Giraud P, Cesselin F. Expression of preproenkephalin A gene and presence of met-enkephalin in dorsal root ganglia of the adult rat. J Neurochem. 1994;63:1226–1234. doi: 10.1046/j.1471-4159.1994.63041226.x. [DOI] [PubMed] [Google Scholar]
- 37.Pohl M, Ballet S, Collin E, Mauborgne A, Bourgoin S, Benoliel JJ, Hamon M, Cesselin F. Enkephalinergic and dynorphinergic neurons in the spinal cord and dorsal root ganglia of the polyarthritic rat: in vivo release and cDNA hybridization studies. Brain Res. 1997;749:18–28. doi: 10.1016/s0006-8993(96)01161-4. [DOI] [PubMed] [Google Scholar]
- 38.Schäfer M, Carter L, Stein C. Interleukin 1β and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci USA. 1994;91:4219–4223. doi: 10.1073/pnas.91.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith GD, Harmar AJ, McQueen DS, Seckl JR. Increase in substance P and CGRP, but not somatostatin content of innervating dorsal root ganglia in adjuvant monoarthritis in the rat. Neurosci Lett. 1992;137:257–260. doi: 10.1016/0304-3940(92)90417-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith RL, Geller AI, Ecudero KW, Wilcox CL. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefano GB, Scharrer B, Smith EM, Hughes TK, Jr, Magazine HI, Bilfinger TV, Hartman AR, Fricchione GL, Liu Y, Makman MH. Opioid and opiate immunoregulatory processes. Crit Rev Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- 42.Stein C, Yassouridis A. Peripheral morphine analgesia. Pain. 1997;71:119–121. [PubMed] [Google Scholar]
- 43.Steiner I, Kennedy PG. Herpes simplex virus latent infection in the nervous system. J Neurovirol. 1995;1:19–29. doi: 10.3109/13550289509111007. [DOI] [PubMed] [Google Scholar]
- 44.Wagner EK, Devi-Rao GB, Feldman LT, Dobson AT, Zhang YK, Flangan WM, Stevens JG. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988;62:1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker JS, Chandler AK, Wilson JL, Binder W, Day RO. Effect of mu-opioids morphine and buprenorphine on the development of adjuvant arthritis in rats. Inflamm Res. 1996;45:557–563. doi: 10.1007/BF02342227. [DOI] [PubMed] [Google Scholar]
- 46.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci USA. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshikawa K, Williams C, Sabol SL. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]