Abstract
GABAA receptors are the major inhibitory transmitter receptors in the CNS. Recombinant GABAA receptors composed of α1β3γ2 subunits have been demonstrated to assemble as pentamers consisting of two α1, two β3, and one γ2 subunit. Using truncated and chimeric α1subunits, we identified the α1(80–100) sequence as a major binding site for γ2 subunits. In addition, we demonstrated its direct interaction with γ2(91–104), a sequence that previously has been identified to form the contact to α1 subunits. The observation that the amino acid residues α1P96 and α1H101, which can be photolabeled by [3H]flunitrazepam, are located within or adjacent to the α1(80–100) sequence, indicates that the benzodiazepine binding site of GABAA receptors is located close to this intersubunit contact. The observation that α1(80–100) interacts with γ2 but not with β3 subunits indicates the existence of an additional β3 binding site on α1 subunits. The preferred alternate use of the γ2 and β3binding sites in two different α1 subunits of the same receptor ensures the incorporation of only a single γ2subunit and thus, determines subunit stoichiometry of α1β3γ2 receptors. Distinct binding sites and their alternate use can therefore explain how subunits of hetero-oligomeric transmembrane proteins assemble into a defined protein complex.
Keywords: GABAA receptor, assembly, subunit interface, structure, subunit stoichiometry, benzodiazepine binding pocket
Members of the ligand-gated ion channel family, such as the nicotinic acetylcholine receptor (nAChR), the GABAA receptor, the glycine receptor, or the 5-HT3 receptor, are heteromeric proteins composed of five subunits (Bertrand and Changeux, 1995). The subunits of these proteins are cotranslationally inserted into the membrane, lumen, or both, of the endoplasmic reticulum, after which the subunits fold and oligomerize (Verrall and Hall, 1992; Green and Claudio, 1993; Connolly et al., 1996a; Griffon et al., 1999). During these folding and oligomerization events, each subunit must recognize its neighbors by specific high-affinity interactions. To achieve the correct order of subunits around the pore, in addition selective discriminations must be made between different subunits. So far, little is known about the molecular structures involved in these mechanisms.
GABAA receptors are the major inhibitory neurotransmitter receptors in the CNS. These receptors are chloride ion channels that can be opened by GABA (Macdonald and Olsen, 1994) and are the site of action of various pharmacologically and clinically important drugs, such as benzodiazepines, barbiturates, steroids, anesthetics, and convulsants. These drugs modulate GABA-induced chloride ion flux by interacting with separate and distinct allosteric binding sites (Sieghart, 1995).
GABAA receptors are hetero-oligomeric proteins consisting of five subunits (Nayeem et al., 1994; Tretter et al., 1997). So far at least 19 GABAA receptor subunits belonging to several subunit classes (six α, three β, three γ, one δ, one ε, one π, one θ, and three ρ) have been identified in mammalian brain (Barnard et al., 1998; Sieghart et al., 1999). In situ hybridization and immunocytochemical studies indicate a distinct but overlapping temporal and regional expression of these subunits. The finding that multiple receptor subunits are expressed within single neurons (Fritschy et al., 1992; Pirker et al., 2000) raises the possibility for the formation of an extremely large variety of GABAA receptor subtypes. However, not all receptors that can be formed theoretically are formed in the cells (Sieghart et al., 1999). Thus, GABAA receptor heterogeneity is limited by the temporal and spatial pattern of subunit expression and by the selective oligomerization mediated by receptor assembly.
Recently, the subunit stoichiometry and arrangement of recombinant α1β3γ2GABAA receptors have been determined (Tretter et al., 1997). In this receptor only a single γ2subunit is present and is situated between an α1 and a β3 subunit. In addition, the amino acid sequence γ2(91–104) was identified to form the binding site to α1subunits (Klausberger et al., 2000).
In the present study truncated and chimeric α1 subunits were used to identify the α1 sequence mediating assembly with γ2 subunits. It was demonstrated that the sequence α1(80–100) directly interacts with γ2(91–104) and forms part of the α1–γ2 interface. The observation that α1(80–100) mediates assembly with γ2 but not with β3subunits suggests the existence of an additional binding site for β3 subunits. The preferred alternate use of the γ2 and β3 binding sites in different α1 subunits of the same receptor indicates that the α1–γ2 intersubunit contact controls assembly and subunit stoichiometry of GABAA receptors.
MATERIALS AND METHODS
Antibodies. The antibodies anti-peptide α1(1–9), anti-peptide β3(1–13), anti-peptide γ2(319–366), and anti-peptide γ2(1–33) were generated and affinity purified as described previously (Tretter et al., 1997; Jechlinger et al., 1998;Klausberger et al., 2000).
Generation of cDNA constructs. For the generation of recombinant receptors, α1, β3, and γ2 subunits of GABAA receptors from rat brain were cloned and subcloned into pCDM8 expression vectors (Invitrogen, San Diego, CA) as described previously (Tretter et al., 1997). Truncated subunits were constructed by PCR amplification using the full-length subunit as a template. The PCR primers contained EcoRI andHindIII restriction sites, which were used to clone the fragments into pCDNAIAmp vectors (Invitrogen). The truncated subunits were confirmed by sequencing. Chimeras were constructed using the “gene splicing by overlap extension” technique (Horton, 1993) and were cloned into pCDNAIAmp vectors using theEcoRI and HindIII restriction sites of the primers.
Culture and transfection of human embryonic kidney 293 cells. Transformed human embryonic kidney (HEK 293) cells (CRL 1573; American Type Culture Collection, Rockville, MD) were cultured as described in Tretter et al. (1997). We transfected 3 × 106 cells with 20 μg of subunit cDNA for single subunit transfection using the calcium phosphate precipitation method (Chen and Okayama, 1988). After cotransfection with two different subunits, for each subunit 10 μg of cDNA was used. When cells were cotransfected with three different subunits, 7 μg of cDNA was used per subunit. A total of ∼20 μg of cDNA per transfection and a cDNA ratio of 1:1:1 seemed to be optimal for the expression of GABAA receptors under the conditions used, as judged by receptor binding studies in cells transfected with α1, β3, and γ2 subunits. Changing the subunit ratio by doubling the amount of a single subunit at the cost of other subunits did not significantly change the number of [3H]Ro 15–1788 binding sites detected.
The cells were then harvested 36 hr after transfection. At this time point the number of [3H]Ro 15–1788 binding sites formed per milligram of protein was at its maximum for cells transfected with α1, β3, and γ2 subunits. Results obtained, however, did not change when cells were harvested 34–48 hr after transfection. In addition, judged by Western blot analysis, expression levels of full-length, truncated, or chimeric subunits were comparable (see Figs. 1, 6) at all harvesting times.
Fig. 1.
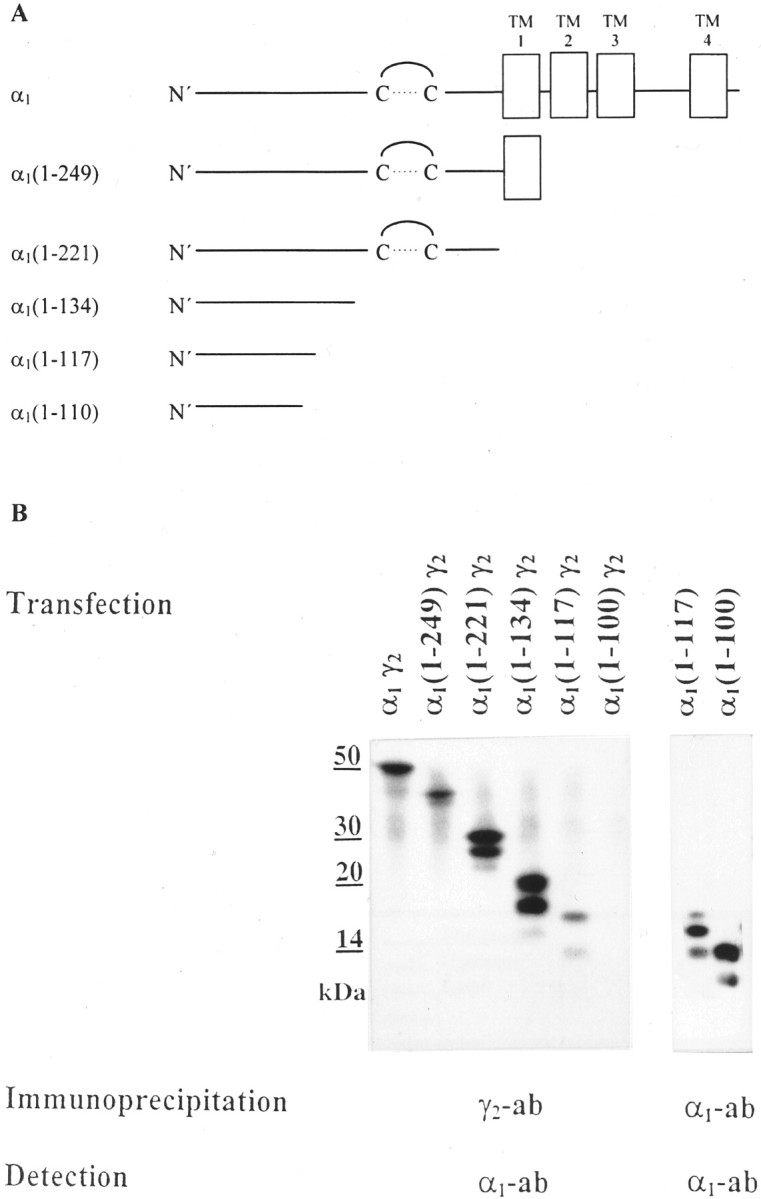
Coimmunoprecipitation of truncated α1 with full-length γ2 subunits.A, Schematic drawing of the α1 subunit and of C-terminally truncated α1 constructs. The α1 subunit consists of the N-terminal extracellular domain with the typical cysteine loop, of four transmembrane domains (TM1–4), and the large cytoplasmic loop between TM3 and TM4. The sequences of the C-terminally truncated α1 constructs are indicated by the amino acid numbers given inparentheses. A 1 represents the first amino acid of the mature subunit. B, HEK cells were transfected with truncated α1 constructs together with full-length γ2 subunits, as indicated. Cell extracts were immunoprecipitated with γ2(319–366) antibodies. α1 fragments coprecipitated were identified by SDS-PAGE and Western blot analysis using digoxygenized α1(1–9) antibodies (lanes 1–6). In control experiments (lanes 7, 8), truncated α1(1–117) and α1(1–100) constructs were transfected separately into HEK cells, and the fragments formed were precipitated with α1(1–9) antibodies and subjected to SDS-PAGE and Western blot analysis using digoxygenized α1(1–9) antibodies. The protein fragments formed from these constructs [apparent molecular mass: 14, 16, and 18 kDa for α1(1–117) and apparent molecular mass: 12 and 14 kDa for α1(1–100)] were expressed to a similar extent. Interestingly, the relative abundance of the unglycosylated, monoglycosylated, and diglycosylated α1(1–117) fragments differed when these fragments were expressed in the absence or presence of γ2 subunits, possibly suggesting that γ2 subunits preferentially assemble with fully glycosylated α1(1–117) fragments. All experiments were performed three times with comparable results.
Fig. 6.
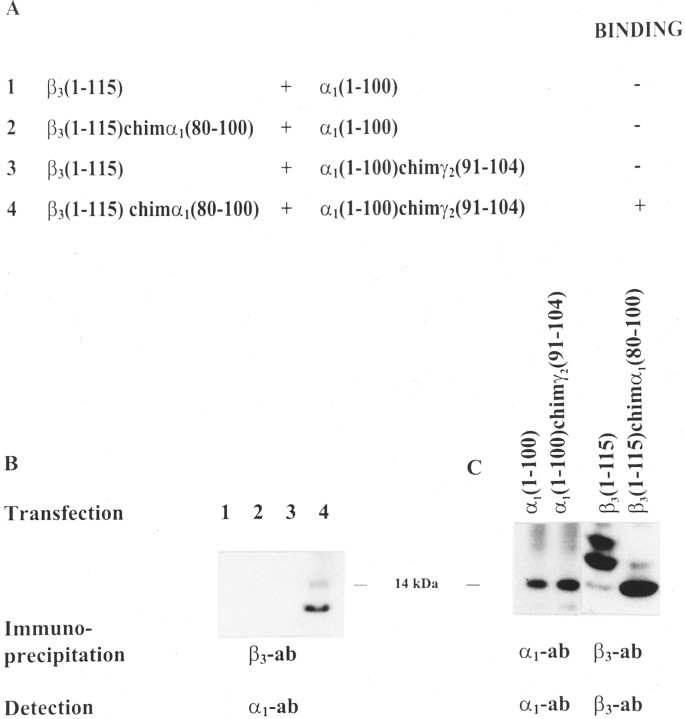
Amino acid sequence α1(80–100) directly binds to γ2(91–104). A, B, HEK cells were cotransfected with the constructs as indicated. Cell extracts were immunoprecipitated with β3(1–13) antibodies, and the precipitate was subjected to SDS-PAGE and Western Blot analysis using digoxygenized α1(1–9) antibodies.A, Schematic representation of the experiment. + indicates binding, and − indicates absence of binding between the cotransfected constructs. B, Western blots demonstrating binding between constructs under condition “4”. C, Western blots demonstrating the extent of expression of the indicated constructs on single transfection into HEK cells. All experiments were performed three times with similar results.
Purification and immunoprecipitation of complete, truncated, and chimeric subunits. The culture medium was removed from transfected HEK 293 cells, and cells from four culture dishes were extracted with 800 μl of a Lubrol extraction buffer (1% Lubrol PX, 0.18% phosphatidylcholine, 150 mm NaCl, 5 mm EDTA, 50 mm Tris-HCl, pH 7.4, containing 0.3 mm PMSF, 1 mm benzamidine, and 100 μg/ml bacitracin) for 8 hr at 4°C. The extract was centrifuged for 40 min at 150,000 ×g at 4°C, and the clear supernatant was incubated overnight at 4°C under gentle shaking with 15 μg antibodies directed against the full-length subunit. After addition of Immunoprecipitin (Life Technologies, Gaithersburg, MD; for preparation, see Tretter et al., 1997) and 0.5% nonfat dry milk powder and shaking for additional 3 hr at 4°C, the precipitate was washed three times with a low-salt buffer for immunoprecipitation (IP low buffer) (50 mm Tris-HCl, 0.5% Triton X-100, 150 mm NaCl, and 1 mm EDTA, pH 8.0). The precipitated proteins were dissolved in sample buffer [108 mm Tris-sulfate, pH 8.2, 10 mm EDTA, 25% (w/v) glycerol, 2% SDS, and 3% dithiothreitol]. SDS-PAGE and Western blot analysis with digoxygenized antibodies was performed as described in Tretter et al. (1997).
All truncated or chimeric constructs used in this study could be expressed to a comparable extent after single transfection into HEK cells. After cotransfection of different constructs, however, the stability of fragments that could not bind stably to each other was reduced. This might have been caused by proteolytic degradation because of an unstable or unproductive interaction of the fragments. In all control experiments the extent of expression of fragments was therefore determined in singly transfected HEK cells.
Immunoprecipitation of receptors expressed on the cell surface. The culture medium was removed from HEK 293 cells transfected with cDNA (21 μg per 3 × 106 cells) of GABAAreceptor subunits (cDNA ratio 1:1:1), and the cells were washed once with PBS (in mm: 2.7 KCl, 1.5 KH2PO4, 140 NaCl, and 4.3 Na2HPO4, pH 7.3). Cells were then detached from the culture dishes by incubating with 2.5 ml of 5 mm EDTA in PBS for 5 min at room temperature. The resulting cell suspension was diluted in 6.5 ml of cold DMEM and centrifuged for 5 min at 1000 × g. The pellet from two dishes was incubated with 30 μg of α1(1–9) antibodies in 3 ml of the same medium for 30 min at 37°C. Cells were again pelleted, and free antibodies were removed by washing twice with 10 ml of PBS buffer. Then receptors were extracted with IP low buffer containing 1% Triton X-100 for 1 hr under gentle shaking. Cell debris was removed by centrifugation (30 min; 150,000 × g; 4°C). After addition of Immunoprecipitin and 0.5% nonfat dry milk powder and shaking for 3 hr at 4°C, the precipitate was centrifuged for 10 min at 10,000 × g and washed three times with IP low buffer. The precipitated proteins were dissolved in sample buffer and subjected to SDS-PAGE and Western blot analysis using digoxygenized antibodies. Secondary antibodies (anti-digoxygenin-AP, Fab fragments; Roche Diagnostics GmbH, Mannheim, Germany) were visualized by the reaction of alkaline phosphatase with CSPD (Tropix, Bedford, MA). Protein bands were quantified by densitometry of Kodak X-Omat S films with the Docu Gel 2000i gel documentation system using restriction fragment length polymorphism scan software (MWG Biotech, Ebersberg, Germany). The linear range of the detection system was established by determining the antibody response to a range of antigen concentrations after immunoblotting. The experimental conditions were designed such that immunoreactivities obtained in the assay were within this linear range, thus permitting a direct comparison of the amount of antigen applied per gel lane between samples. Different exposures of the same membrane were used to ensure that the measured signal was in the linear range of the x-ray film.
To verify that only receptors on the cell surface were labeled by the antibodies, parallel samples were incubated with antibodies directed against the intracellular loop of GABAA receptor subunits (data not shown). These antibodies could not precipitate any GABAA receptor subunits under the conditions used. A possible redistribution of the antibodies during the extraction procedure could be excluded by an experiment performed analogous to that described in Klausberger et al. (2000).
Immunofluorescence. HEK cells were fixed with 2% paraformaldehyde in PBS 30–35 hr after transfection, followed by a 10 min wash in 50 mm NH4Cl in PBS. Washes between incubation steps were performed in PBS. For detection of intracellular receptors, cells were permeabilized with 0.1% Triton X-100 for 5 min. Blocking was performed in 5% bovine serum albumin (BSA) in PBS for 10 min, followed by an incubation with primary antibody in 1% BSA in PBS. Primary antibodies were detected with goat anti-rabbit IgG(H+L) bodipy FL (Molecular Probes, Eugene, OR) in 1% BSA in PBS. Labeling was visualized using a Zeiss Axiovert 135 M microscope attached to a confocal laser system (Carl Zeiss LSM 410, BRD), equipped with an argon laser and a helium–neon laser and suitable filter sets. To verify that labeling of cells without permeabilization was restricted to the cell surface, parallel samples were stained with antibodies directed against the intracellular loop of GABAA receptor subunits (data not shown). These antibodies detected GABAAreceptor subunits only after permeabilization of transfected cells.
Electrophysiological investigations. HEK cells were cotransfected with GABAA receptor subunits together with pEGFP-N1 (Clontech, Palo Alto, CA) as a transfection marker. Electrophysiologic recordings were performed at room temperature 1–2 d after transfection using the perforated patch technique (Rae et al., 1991). GABA and ZnCl2 were applied using a DAD-12 superfusion system (Adams and List Associates Ltd., Westbury, NY). Extracellular solution contained (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES, pH 7.4. The pipette solution contained (in mm): 140 KCl, 11 EGTA, 1 CaCl2, 1 MgCl2, 10 HEPES, and 0.2 amphotericin B, pH 7.2. The cells were clamped at −60 mV, and currents were filtered at 1 kHz, recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) and analyzed with Clampfit software (Axon Instruments).
RESULTS
Truncated α1 constructs are able to assemble with full-length γ2 subunits
In the present study C-terminally truncated α1 subunits (Fig.1A) were cloned, and it was investigated which of these fragments could assemble with full-length γ2 subunits. For this, HEK cells were cotransfected with γ2 subunits and either full-length or truncated α1 subunits. Expressed subunits were extracted from these cells and were immunoprecipitated with γ2(319–366) antibodies. The precipitate was subjected to SDS-PAGE and Western blot analysis using digoxygenized α1(1–9) antibodies. As shown in Figure1B, full-length α1 subunits (protein band of 51 kDa), as well as the fragments α1(1–249) (two bands of 39 and 41 kDa), α1(1–221) (three bands of 26, 28, 30 kDa), α1(1–134) (three bands of 17, 19, 21 kDa), and α1(1–117) [three bands of 14, 16 (very weak), and 18 kDa] could be coimmunoprecipitated with full-length γ2 subunits from appropriately transfected HEK cells. Because all α1 fragments investigated contained two glycosylation sites, the three bands presumably represented unglycosylated, partially, and fully glycosylated fragments. The observation that only one or two protein bands could be observed for the full-length α1 subunit or the α1(1–249) construct might indicate that these subunits predominantly occur in the double-glycosylated or double- and mono-glycosylated state, respectively. This conclusion is supported by the apparent molecular mass of these proteins that amounted to 51 kDa or 41 kDa for the full-length α1 subunit or the α1(1–249) construct, respectively, although the ungycosylated mass of these proteins can be calculated to be 47 or 37 kDa. Alternatively, the differentially glycosylated protein bands with higher molecular mass might not have been resolved by the 15% polyacrylamide gel used in this investigation.
Binding between γ2 subunits and these fragments seemed to be the result of a specific assembly process because after cotransfection of HEK cells with full-length γ2subunits and the fragment α1(1–221), high-affinity binding sites for the benzodiazepine [3H]Ro15–1788 were formed. These sites are assumed to be located at the interface of α1 and γ2 subunits in GABAA receptors (Sigel and Buhr, 1997). The number of [3H]Ro15–1788 binding sites formed (16.8 ± 2.3 fmol/mg protein) was comparable with that observed after transfection of HEK cells with full-length α1 and γ2 subunits (17.6 ± 1.2 fmol/mg protein) but was significantly smaller (p < 0.001; unpaired Student's ttest) than that of HEK cells transfected with α1, β3, and γ2 subunits in parallel experiments (874 ± 19 fmol/mg of protein). Data given are mean values ± SEM from three different experiments performed in triplicate.
In contrast to the protein fragments formed from α1(1–117), the fragments formed from α1(1–100) could not be coprecipitated with γ2 subunits from appropriately cotransfected HEK cells (Fig. 1B), although the extent of expression of these fragments (12 and 14 kDa) was similar to that of the fragments derived from the α1(1–117) construct (Fig. 1B, lanes 7, 8). The inability of γ2(319–366) antibodies to coprecipitate the fragment α1(1–100) confirmed previous conclusions (Jechlinger et al., 1998) that these antibodies did not cross-react with α1 subunits. These results indicate that the amino acid sequence of the α1 subunit that is responsible for binding to γ2 subunits is located in the N-terminal 117 amino acids of the α1 subunit.
Amino acid sequence α1(80–100) mediates binding to γ2 subunits
To identify this contact site, it was investigated which α1 amino acid sequence could induce binding to γ2 subunits after incorporation into a fragment that originally could not bind to these subunits. The fragment β3(1–115) seemed to be suitable for this purpose because it is homologous to α1(1–117) but could not be coprecipitated with γ2subunits (or β3 subunits) after coexpression in HEK cells (Fig.2). To incorporate binding sites of the α1 subunit, several chimeras were constructed by replacing the C-terminal part of the β3(1–115) fragment with the corresponding α1 sequences (Fig. 2). These chimeras were transfected into HEK cells together with full-length γ2 subunits. Expressed subunits were precipitated from cell extracts with γ2(319–366) antibodies. The precipitate was subjected to SDS-PAGE, and the proteins were detected with digoxygenized β3(1–13) antibodies in Western blots. The actual expression of the chimeras was confirmed by precipitation and detection with β3(1–13) antibodies (data not shown).
Fig. 2.
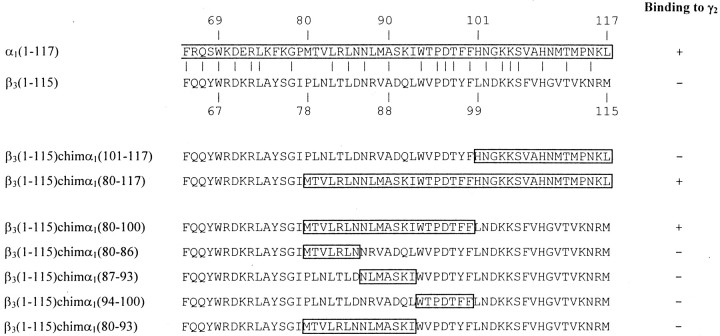
α1(80–100) forms the contact site to γ2 subunits. C-terminal sequences of the fragments α1(1–117), β3(1–115), and of different chimeras are shown. Amino acid sequences of the α1subunit are boxed. HEK cells were cotransfected with these constructs together with γ2 subunits, and a possible coimmunoprecipitation was investigated, as described in Results. + indicates binding, and − indicates absence of binding between these constructs and full-length γ2subunits. The experiments were performed three times with similar results.
In β3(1–115)chimα1(101–117) the 17 C-terminal amino acids of the β3(1–115) fragment were replaced by amino acids 101–117 of the α1 subunit. As indicated in Figure 2, this chimera could not be coprecipitated with full-length γ2 subunits from appropriately cotransfected HEK cells, demonstrating the specificity of the γ2(319–366) antibodies used and indicating that amino acids α1(101–117) are not able to induce binding to γ2 subunits. In β3(1–115)chimα1(80–117), the amino acid sequence β3(78–115) was replaced by α1(80–117). This construct was able to bind to full-length γ2 subunits (Fig.2), but not to full-length β3 subunits (data not shown). Because amino acids α1(101–117) were not sufficient to induce binding to γ2subunits as discussed above, this indicated that amino acids 80–100 of the α1 subunit are important for binding to γ2 subunits. To directly confirm this conclusion, the construct β3(1–115)chimα1(80–100) was generated (Fig. 2), in which amino acids α1(80–100) were incorporated into β3(1–115), replacing amino acids β3(78–98). As expected, this chimera was able to bind to γ2 subunits.
To investigate which part of the α1(80–100) sequence is responsible for binding to γ2subunits, four additional chimeras were constructed. In β3(1–115)chimα1(80–86), amino acids β3(78–84) were replaced by the amino acids α1(80–86), in β3(1–115)chimα1(87–93) the sequence β3(85–91) was replaced by α1(87–93), in β3(1–115)chimα1(94–100) the sequence β3(92–98) was replaced by amino acids α1(94–100), and in β3(1–115)chimα1(80–93) the sequence β3(78–91) was replaced by α1(80–93) in the β3(1–115) fragment. None of these chimeras was able to bind to γ2 subunits. These results indicate that the whole α1(80–100) sequence is necessary for binding to γ2 subunits.
The sequence α1(80–100) is important for the assembly of GABAA receptors composed of α1β3γ2 subunits
To investigate the importance of the α1(80–100) sequence not only for the assembly of truncated subunits and dimers, but also for assembly of full-length subunits and pentameric receptors, a full-length α1 chimera (α1*) was constructed in which the sequence α1(79–100) was replaced by the sequence β3(77–98). The additional exchange of the amino acid 79 of the α1 subunit in α1* was necessary to avoid the generation of two adjacent prolines that could have destroyed the conformation of the resulting chimera (Fig. 2). In control experiments, it was demonstrated that the extent of expression of the α1* chimera was similar to that of the α1 subunit in HEK cells (data not shown).
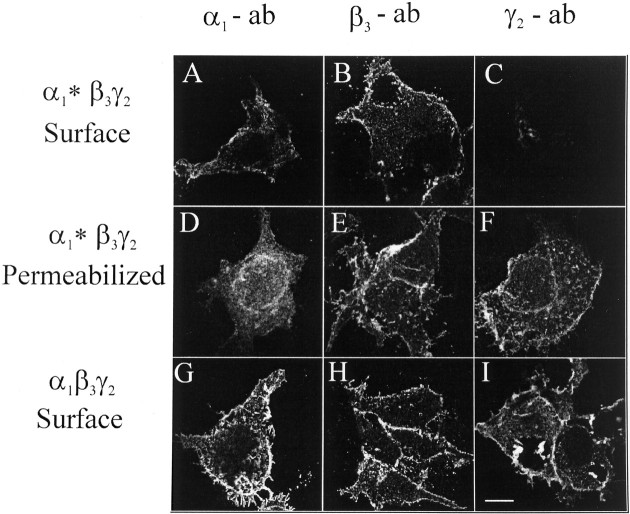
HEK cells were then cotransfected with α1*, β3, and γ2 subunits and subunits expressed were investigated by immunofluorescence and confocal laser microscopy. As shown in Figure 3, α1* (Fig. 3A) and β3 subunits (Fig. 3B) could be easily detected on the surface of intact cells, but for the γ2 subunit only a weak labeling was observed (Fig. 3C), although the labeling of the γ2 subunit in permeabilized cells (Fig.3F) was comparable with that of α1 (Fig. 3D) and β3 (Fig. 3E) subunits. In HEK cells cotransfected with α1, β3, and γ2 subunits, all three subunits could be detected on the cell surface (Fig.3G–I). Because previous results have indicated that α1 subunits alone in contrast to α1β3 subunit combinations do not form receptors that are incorporated into the plasma membrane to a significant extent, these results suggested that α1* predominantly formed receptors with β3 subunits that are expressed on the cell surface, but the ability to form receptors containing γ2 subunits was significantly reduced.
Fig. 3.
Immunofluorescence of HEK cells cotransfected with GABAA receptor subunits. HEK cells were cotransfected with α1*, β3, and γ2subunits (A–F) or with α1, β3, and γ2 subunits(G–I). Immunofluorescence was performed using α1(1–9) antibodies (A, D, G), β3(1–13) antibodies (B, E, H), or γ2(1–33) antibodies (C, F, I) on the cell surface (A–C, G–I) or in permeabilized cells(D–F) by confocal laser microscopy (single sections). Scale bar, 10 μm. The experiment was performed four times with similar results.
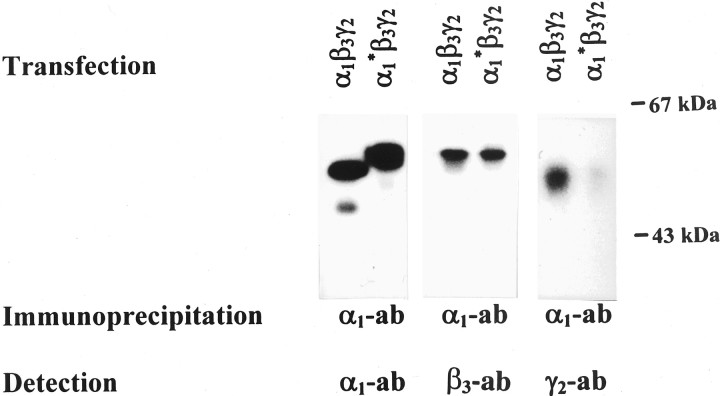
To quantify this phenomenon, HEK cells were cotransfected with α1, β3, and γ2 subunits or with α1*, β3, and γ2 subunits. GABAAreceptors expressed on the surface of the cells were labeled by an incubation of intact cells with α1(1–9) antibodies. Antibody labeled receptors were extracted and precipitated by addition of Immunoprecipitin. The precipitate was subjected to SDS-PAGE and Western blot analysis using digoxygenized α1(1–9) antibodies (Fig.4). In contrast to α1 subunits (51 kDa, the weak 46 kDa band presumably represents a degradation product), the protein band of α1* exhibited an apparent molecular mass of 53 kDa because of an additional glycosylated asparagine at position 80 of the newly introduced β3 subunit insert. The protein bands were quantified, and results obtained indicated that α1* and α1 subunits were expressed to a similar extent on the surface of transfected cells. Then, the Western blot was stripped and analyzed using digoxygenized β3(1–13) antibodies (Fig. 4). Finally, blots were again stripped and were probed with γ2(1–33) antibodies. Whereas similar amounts of β3 subunits (54 kDa) were coprecipitated with α1 subunits from α1β3γ2or α1*β3γ2transfected cells, the amount of γ2 subunits (49 kDa) coprecipitated with α1* subunits was only 32 ± 3% (mean ± SEM, n = 3; from three different transfections) of that coprecipitated with α1 subunits. Similar results were obtained when the order of detection of subunits was changed and Western blots were first probed with γ2(1–33) antibodies and after stripping were re-analyzed with α1(1–9) or β3(1–13) antibodies. These results indicate that α1* was able to form receptors with β3 subunits, but that the ability to form receptors containing γ2 subunits was reduced by 68%.
Fig. 4.
Western blot analysis of GABAAreceptors labeled on the surface of HEK cells. HEK cells were cotransfected with α1, β3, and γ2 subunits or with α1*, β3, and γ2 subunits. GABAA receptors expressed on the cell surface were immunolabeled by adding α1(1–9) antibodies to intact cells, and were then extracted, immunoprecipitated, and analyzed by SDS-PAGE and Western blots using digoxygenized α1(1–9), β3(1–13), or γ2(1–33) antibodies.
Properties of GABAA receptors composed of α1*β3γ2 or α1*β3 subunits
To investigate the properties of the receptors formed, HEK cells cotransfected with α1*, β3, and γ2 subunits were subjected to patch-clamp analysis, and whole-cell recordings were compared with those from cells transfected with α1, β3, and γ2 subunits. GABA exhibited an apparent EC50 of 68 ± 10 μm (mean ± SEM; n = 11 cells from different plates; total of four transfections) (Fig. 5E) in HEK cells transfected with α1*, β3, and γ2 subunits and elicited a maximal current of 713 ± 170 pA at a GABA concentration of 1000 μm (Fig. 5A). In contrast, GABA exhibited an EC50 of 7.7 ± 2.3 μm (mean ± SEM; n= 8 cells from different plates; total of four transfections) (Fig.5E) in HEK cells transfected with α1, β3, and γ2 subunits and elicited a maximal current of 2988 ± 469 pA at a concentration of 300 μm (Fig. 5B). These data not only indicated that GABA exhibited a 10-fold reduced potency for activating α1*β3γ2receptors, but also that the maximal current of cells transfected with α1*β3γ2subunits was only ∼24% of that transfected with α1β3γ2subunits.
Fig. 5.
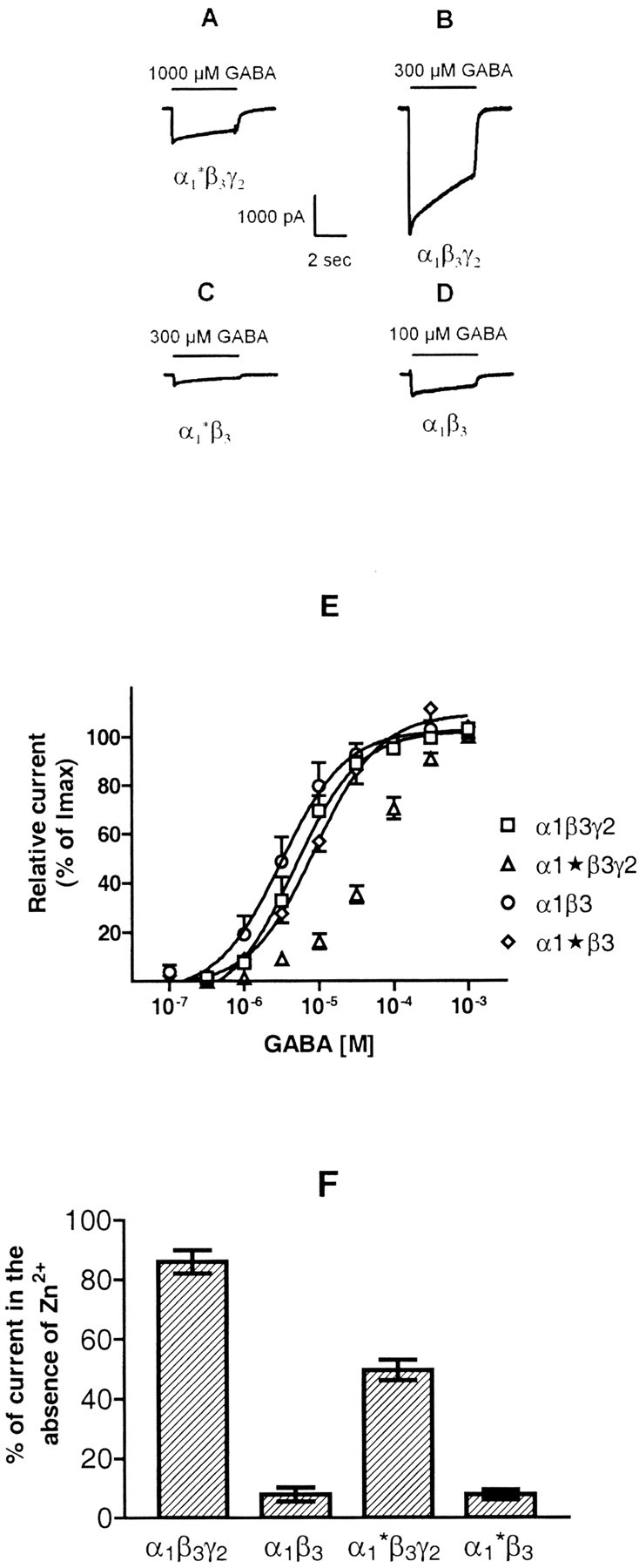
Functional properties of GABAAreceptors containing the α1* subunit.A—D, Whole-cell recordings from HEK cells cotransfected with α1*, β3, and γ2, or α1, β3, and γ2, α1* and β3, or α1 and β3subunits after application of GABA concentrations producing maximal currents. Shown are single experiments that were reproduced with similar results 8–11 times in different cells. E,Relative currents induced by various GABA concentrations in cells transfected with subunit combinations as indicated. Data shown are mean values ± SEM of relative currents from 8–11 individual dose–response curves obtained from different cells derived from a total of four transfections. F, Bar graph showing the effect of 100 μm Zn2+ on currents activated by 100 μm GABA. HEK cells were transfected, as indicated. Height of bars indicates fraction of control current remaining in the presence of Zn2+, measured 1.5 sec after application was started; error bars indicate ± SEM (n = 6–8).
Because surface expression studies indicated a significant formation of α1*β3 receptors in α1*β3γ2transfected cells, the properties of receptors in α1*β3 transfected cells were also investigated. Although homo-oligomeric receptors composed of β3 subunits could also have been formed under the conditions used, they would not have contributed to the GABA evoked current because these receptors apparently are not gated by GABA (Connolly et al., 1996b). Using various GABA concentrations, it was demonstrated that GABA exhibited an EC50 of 10.5 ± 2.1 μm (mean ± SEM; n= 8 cells from different plates; total of four transfections) (Fig.5E) in HEK cells transfected with α1* and β3 subunits and elicited a maximal current of 270 ± 63 pA at a concentration of 300 μm (Fig. 5C). In contrast, GABA exhibited an EC50 of 3.0 ± 1.2 μm (mean ± SEM; n = 9 cells from different plates; total of three transfections) (Fig.5E) in cells transfected with α1 and β3 subunits and elicited a maximal current of 426 ± 146 pA at a concentration of 100 μm(Fig. 5D). These data supported the conclusion that the α1* construct was able to form functional receptors with β3 subunits. The potency of GABA for activating α1*β3receptors, however, was significantly (p < 0.05; Student's t test) reduced compared with receptors composed of α1β3subunits. Similarly, the maximal currents elicited by GABA in α1*β3 transfected cells were significantly smaller than those in α1β3 transfected cells (p < 0.05).
Although α1*β3receptors significantly contribute to receptors formed in α1*β3γ2transfected HEK cells, as indicated by surface expression studies, because of the low maximum currents observed in α1*β3 receptors (Fig.5C), these receptors overall have a comparatively small contribution to currents elicited in α1*β3γ2transfected cells (Fig. 5A) that is apparent only as a slightly increased range of GABA concentrations that are able to elicit currents in these cells (Fig. 5E). Thus, most of the current elicited in the cells investigated was produced by α1*β3γ2receptors. The low apparent potency of GABA to activate currents in these cells as well as the increased dose range of GABA for stimulation of currents clearly indicated the formation of α1*β3γ2receptors in addition to α1*β3 receptors.
This conclusion was supported by investigating the effects of 100 μm Zn2+ on whole-cell currents stimulated by 100 μm GABA. In agreement with previous results (Draguhn et al., 1990; Gingrich and Burkat, 1998), currents mediated by the wild-type α1β3γ2receptors were only weakly reduced (86 ± 4% of control;n = 6; total of four transfections), whereas currents mediated by α1β3receptor were reduced to 7 ± 3% (n = 6; total of three transfections) (Fig. 5F) in the presence of Zn2+. For HEK cells transfected with α1*, β3, and γ2 subunits, currents mediated by 100 μm GABA were reduced to 50 ± 4% (n = 7; total of four transfections), and for cells transfected with α1* and β3 subunits, GABA-mediated currents were reduced to 8 ± 2% (n = 8; total of four transfections) in the presence of 100 μmZn2+ (Fig. 5F). Because α1β3 and α1*β3 receptors exhibit a comparable Zn2+ sensitivity, it is reasonable to assume that the Zn2+sensitivity of α1*β3γ2and α1β3γ2receptors was also comparable. The 36% increase in Zn2+ sensitivity of α1*β3γ2-transfected cells therefore indicated that ∼40% of the α1*β3γ2current was mediated by the additionally formed α1*β3 receptors. Combined with the observation that the main conductance level of αβ receptors (15–18 pS) is only half of that of αβγ receptors (∼30 pS; Hevers and Lüddens, 1998), and assuming that the same holds true for α1*β3and α1*β3γ2receptors, a ratio of α1*β3:α1*β3γ2receptors of 80:60 can be calculated, indicating that α1*β3γ2receptors represented 43% of receptors formed in these cells. Given the many assumptions that had to be made in the course of this calculation, this percentage is in good agreement with the data from the immunoprecipitation experiments shown in Figure 4.
Amino acid sequence α1(80–100) binds to the γ2(91–104) sequence
Recently it has been demonstrated that incorporation of the amino acid sequence γ2(91–104) into the fragment α1(1–100), that per se could not bind to α1 subunits, resulted in the chimera α1(1–100)chimγ2(91–104) that was able to bind to α1 subunits. From this it was concluded that the amino acid sequence γ2(91–104) forms the contact site to α1 subunits (Klausberger et al., 2000). It therefore seemed interesting to investigate whether the γ2(91–104) sequence directly interacts with the α1(80–100) sequence.
To clarify this question, it first was investigated whether the α1(1–100) fragment and the fragment β3(1–115), which was used to identify the α1(80–100) contact site (Fig. 2), could bind to each other. For this, fragments β3(1–115) and α1(1–100) were cotransfected into HEK cells. The extract of HEK cells expressing β3(1–115) and α1(1–100) fragments was then immunoprecipitated with β3(1–13) antibodies, and the precipitate was subjected to SDS-PAGE and Western blot analysis using digoxygenized α1(1–9) antibodies. As shown in Figure 6, A andB, α1(1–100) fragments were not coprecipitated by β3(1–13) antibodies, confirming the absence of cross-reactivity of these antibodies with the α1(1–100) fragments and indicating that β3(1–115) could not bind to α1(1–100) fragments. Similarly, the construct β3(1–115)chimα1(80–100), which contains the putative binding site for γ2subunits, was unable to bind to α1(1–100) fragments after cotransfection into HEK cells (Fig.6A,B). In the reverse experiment, the construct α1(1–100)chimγ2(91–104), containing the binding site for α1 subunits, could also not bind to the β3(1–115) fragment. Only when the α1(80–100) sequence was incorporated into the β3(1–115) fragment and the γ2(91–104) sequence was incorporated into the α1(1–100) fragment, the resulting chimeras could bind to each other (Fig. 6A,B). These results indicate that the α1(80–100) and the γ2(91–104) sequences can directly bind to each other.
In control experiments (Fig. 6C) it was demonstrated that each of the constructs used in this experiment was expressed to a similar extent after single transfection into HEK cells. Constructs α1(1–100) and α1(1–100)chimγ2(91–104) each contained a single glycosylation site and thus, gave rise to two fragments: a weakly labeled of 12 kDa and a strongly labeled of 14 kDa. Construct β3(1–115) contained two glycosylation sites and thus, formed three fragments, two strongly labeled of 16 and 18 kDa and a weakly labeled fragment of 14 kDa. Construct β3(1–115)chimα1(80–100) contained only one glycosylation site and formed a strongly labeled protein band of 14 and weakly labeled band of 16 kDa.
Interestingly, predominantly the unglycosylated α1(1–100)chimγ2(91–104) fragment of 12 kDa seemed to assemble with β3(1–115)chimα1(80–100) on cotransfection of these fragments into HEK cells, although the glycosylated fragment of 14 kDa was the predominant one expressed after single transfection of HEK cells (Figs. 1B,6C). This suggests that assembly of subunit fragments already starts when subunits are not fully glycosylated. This conclusion is supported by previous observations (Klausberger et al., 2000, 2001) as well as by observations with other constructs (Fig.1B).
DISCUSSION
Amino acid sequence α1(80–100) forms the binding site to γ2 but not to β3 subunits
The present study demonstrated that the N-terminal extracellular domain of the α1 subunit [α1(1–221)] could bind to full-length γ2 subunits after coexpression in HEK cells, as indicated by coimmunoprecipitation with subunit-specific antibodies. Binding between α1(1–221) and γ2 subunits represented a specific assembly process, as indicated by the formation of specific [3H]Ro15–1788 binding sites that are assumed to be formed on the interface of α1 and γ2 subunits of GABAAreceptors. These results are consistent with previous studies indicating that N-terminal sequences of GABAAreceptor (Hackam et al., 1997; Klausberger et al., 2000) or K+ channel (Shen et al., 1993) subunits can assemble with full-length subunits.
A subsequent reduction in the size of the truncated subunit indicated that the α1(1–117), but not the α1(1–100) construct was still able to bind to γ2 subunits. The respective binding site was then identified by incorporating various α1sequences into the β3(1–115) fragment. This fragment is homologous to α1(1–117) but in contrast to the latter construct could not bind to γ2 subunits after coexpression in HEK cells. The incorporation of the sequence α1(80–100) into the β3(1–115) fragment was sufficient to induce binding to γ2 but not to β3 subunits, suggesting that the α1 binding sites for γ2and β3 subunits are different.
The observation that the α1(1–100) fragment was unable to bind to γ2 subunits although it contained the α1(80–100) sequence is consistent with previous results indicating that γ2(1–113) was the smallest fragment that could bind to α1 subunits, although the respective binding site was identified to be formed by the γ2(91–104) sequence (Klausberger et al., 2000). The additional length of the fragments presumably is required for stabilizing the conformation of the actual binding sites located in a more N-terminal position.
In other experiments a chimeric α1 subunit (α1*) was constructed in which the α1(79–100) sequence was replaced by the homologous β3(77–98) sequence. Chimera α1* was then coexpressed with β3 and γ2 subunits in HEK cells. Confocal immunofluorescence microscopy, whole-cell patch-clamp experiments, as well as immunolabeling and quantification of receptors on the cell surface indicated a 60–70% reduction in receptors containing α1*, β3, and γ2 subunits, although the level of expression of α1* subunits and its extent of assembly with β3subunits was unimpaired. These results confirmed the importance of the α1(80–100) sequence for assembly with γ2 but not with β3subunits. The remaining formation of α1*β3γ2receptors can be explained by the existence of additional binding sites between α1 and γ2subunits that partially can compensate for the absence of the α1(80–100) sequence in α1* subunits.
Amino acid sequences α1(80–100) and γ2(91–104) form part of the α–γ interface and are located close to the benzodiazepine binding site of GABAAreceptors
Recently it was demonstrated that the sequence γ2(91–104) forms the contact site to α1 subunits (Klausberger et al., 2000). To investigate whether the sequences α1(80–100) and γ2(91–104) directly interact with each other, these sequences were incorporated into GABAA receptor fragments β3(1–115) and α1(1–100), respectively, which could not bind to each other. The observation that α1(80–100) had to be incorporated into β3(1–115) and γ2(91–104) into α1(1–100) to induce coprecipitation of the fragments indicated that the α1(80–100) and the γ2(91–104) sequences can directly bind to each other and thus, form part of the α1–γ2 subunit interface. This is the first time that interacting sequences from two different subunits could be identified in this receptor superfamily. It is possible, however that subunits rearrange during assembly (Mitra et al., 2001). Whether the identified sequences also form part of the final intersubunit contact, thus, will have to be determined by further studies. Interestingly, two amino acid residues (L90 and S92) identified previously as important for homo-oligomeric assembly of glycine receptor α1 subunits (Griffon et al., 1999) are located in a region homologous to α1(80–100), but their identity is different in GABAA receptors. In addition, L90 as well as a further amino acid residue (P79) that also is important for binding between glycine receptor subunits, are located in a region homologous to γ2(91–104). This indicates that GABAA receptors, glycine receptors, and possibly also nAChRs (Kreienkamp et al., 1995) use homologous regions with receptor-specific assembly signals for forming intersubunit contacts.
Interestingly, the amino acid H101 of the α1subunit that can be photolabeled by [3H]flunitrazepam (Smith and Olsen, 2000) and seems to be involved in the formation of the benzodiazepine binding pocket (Sigel and Buhr, 1997) is located immediately adjacent to the α1(80–100) sequence forming the intersubunit contacts to γ2 subunits. In addition, P96 of the α1 subunit, which also can be photolabeled by [3H]flunitrazepam (Smith and Olsen, 2000), contributes to the α1–γ2 subunit interface. This indicates that the benzodiazepine binding site of GABAA receptors is located close to the intersubunit contact between α1 and γ2 subunits.
Other amino acid residues possibly contributing to the benzodiazepine binding site are α1Y159, α1G200, α1S204, α1T206, α1Y209, γ2M130, and γ2F77 (Sigel and Buhr, 1997). Although located outside the α1(80–100) and γ2(91–104) sequences, all these residues are embedded in domains with a hydrophobicity comparable with that of these sequences (data not shown). These residues might thus be involved in the formation of other parts of this interface. In any case, all residues forming the benzodiazepine binding site must be in spatial proximity to α1H101.
Implications for GABAA receptor assembly and subunit stoichiometry
The present observation that the α1(80–100) sequence binds to γ2 but not to β3subunits suggests the existence of at least three distinct subunit binding sites on α1 subunits: a (+) and a (−) site for binding of β3 subunits and an additional (✣) site for binding of γ2subunits (Fig. 7). Whereas the (+) and the (✣) sites are located at the same side and are possibly situated closely together, the (−) site is located at the other side of the subunit. The recently identified sequence α1(58–67), which mediates binding to β3 subunits (Taylor et al., 2000), seems to form part of the (−) site because one of its residues (α1F64; Smith and Olsen, 1994) contributes to the GABA binding site assumed to be located at the α1–β3 interface of GABAA receptors (Fig. 7).
Fig. 7.
Stoichiometry and subunit arrangement of the recombinant α1β3γ2GABAA receptor (Tretter et al., 1997). A mirror image arrangement is equally possible. +, ✣, and − indicate the clockwise and counterclockwise binding sites of a subunit, respectively. BZ or GABA indicate site of interaction of benzodiazepines or GABA with GABAA receptors, respectively.
Interestingly, one α1 subunit of GABAA receptors uses the (✣) site for binding to a γ2 subunit, whereas the other one uses the (+) site for a β3 subunit (Fig. 7). Previous studies have indicated that cells transfected with α1, β3, and γ2 subunits predominantly form pentameric receptors composed of 2α1, 2β3, and one γ2subunit, whereas cells transfected with α1 and β3 subunits form tetramers and pentamers (Tretter et al., 1997). This delayed formation of subunit pentamers indicates that a γ2 subunit can be more easily accommodated into an α1β3 tetramer than an additional β3 subunit. A preferential use of the α1(✣) site under these conditions ensures the incorporation of a γ2 subunit into the receptor. Because the alternate use of the α1(+) and the α1(✣) site in the two α1 subunits of GABAA receptors seems to be sterically or energetically favored, binding to the α1(+) site of the second α1 subunit should be preferred when a γ2 subunit is already present in an assembly intermediate. In this case the preferred use of the α1(+) site prevents the incorporation of a second γ2 subunit into the receptor. This conclusion is supported by most of the experimental data available, suggesting that there is only one γ2 subunit in GABAA receptors (for review, see Sieghart et al., 1999) and indicates that the α1–γ2 intersubunit contact controls subunit assembly and stoichiometry of GABAA receptors.
Footnotes
This work was supported by Grant P12637-Med of the Austrian Science Fund.
Correspondence should be addressed to W. Sieghart, Brain Research Institute, Division of Biochemistry and Molecular Biology, University of Vienna, Spitalgasse 4, A-1090 Vienna, Austria. E-mail:Werner.Sieghart@univie.ac.at.
T. Klausberger's present address: Medical Research Council Anatomical Neuropharmacology Unit, Oxford University, Oxford OX1 3TH, UK.
REFERENCES
- 1.Barnard EA, Skolnick P, Olsen RW, Möhler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 2.Bertrand D, Changeux JP. Nicotinic receptor: an allosteric protein specialized for intercellular communication. Semin Neurosci. 1995;7:75–90. [Google Scholar]
- 3.Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 4.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric γ-aminobutyric acid type A receptors. J Biol Chem. 1996a;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Connolly CN, Wooltorton JRA, Smart TG, Moss SJ. Subcellular localization of γ-aminobutyric acid type A receptors is determined by receptor β subunits. Proc Natl Acad Sci USA. 1996b;93:9899–9904. doi: 10.1073/pnas.93.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakman B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- 7.Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A γ-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingrich KJ, Burkat PM. Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the γ-subunit. J Physiol (Lond) 1998;506:609–625. doi: 10.1111/j.1469-7793.1998.609bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green WN, Claudio T. Acetylcholine receptor assembly: subunit folding and oligomerization occur sequentially. Cell. 1993;74:57–69. doi: 10.1016/0092-8674(93)90294-z. [DOI] [PubMed] [Google Scholar]
- 10.Griffon N, Büttner C, Nicke A, Kuhse J, Schmalzing G, Betz H. Molecular determinants of glycine receptor subunit assembly. EMBO J. 1999;18:4711–4721. doi: 10.1093/emboj/18.17.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackam AS, Wang TL, Guggino WB, Cutting GR. The N-terminal domain of human GABA receptor ρ1 subunits contains signals for homooligomeric and heterooligomeric interaction. J Biol Chem. 1997;272:13750–13757. doi: 10.1074/jbc.272.21.13750. [DOI] [PubMed] [Google Scholar]
- 12.Hevers W, Lüddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 13.Horton RM. In vitro recombination and mutagenesis of DNA. In: White BA, editor. PCR protocols. Humana; Totowa, NJ: 1993. pp. 251–261. [DOI] [PubMed] [Google Scholar]
- 14.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of heterooligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klausberger T, Fuchs K, Mayer B, Ehya N, Sieghart W. GABAA receptor assembly: identification and structure of γ2 sequences forming the intersubunit contacts with α1 and β3 subunits. J Biol Chem. 2000;275:8921–8928. doi: 10.1074/jbc.275.12.8921. [DOI] [PubMed] [Google Scholar]
- 16.Klausberger T, Ehya N, Fuchs K, Fuchs T, Ebert V, Sarto I, Sieghart W. Detection and binding properties of GABAA receptor assembly intermediates. J Biol Chem. 2001;276:16024–16032. doi: 10.1074/jbc.M009508200. [DOI] [PubMed] [Google Scholar]
- 17.Kreienkamp HJ, Maeda RK, Sine SM, Taylor P. Intersubunit contacts governing assembly of mammalian nicotinic acetylcholine receptor. Neuron. 1995;14:635–644. doi: 10.1016/0896-6273(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 19.Mitra M, Wanamaker CP, Green WN. Rearrangement of nicotinic receptor α subunits during formation of the ligand binding sites. J Neurosci. 2001;21:3000–3008. doi: 10.1523/JNEUROSCI.21-09-03000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayeem N, Green TP, Martin IL, Barnard EA. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J Neurochem. 1994;62:815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- 21.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 22.Rae J, Cooper K, Gate P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 23.Shen NV, Chen X, Boyer MM, Pfaffinger PJ. Deletion analysis of K+ channel assembly. Neuron. 1993;11:67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- 24.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 25.Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Höger H, Adamiker D. Structure and subunit composition of GABAA receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 27.Smith GB, Olsen RW. Identification of a [3H]muscimol photoaffinity substrate in the bovine γ-aminobutyric acidA receptor α-subunit. J Biol Chem. 1994;269:20380–20387. [PubMed] [Google Scholar]
- 28.Smith GB, Olsen RW. Deduction of amino acid residues in the GABAA receptor α subunits photoaffinity labeled with the benzodiazepine flunitrazepam. Neuropharmacology. 2000;39:55–64. doi: 10.1016/s0028-3908(99)00104-5. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PM, Connolly CN, Kittler JT, Gorrie GH, Hosie A, Smart TG, Moss SJ. Identification of residues within GABAA receptor α subunits that mediate specific assembly with receptor β subunits. J Neurosci. 2000;20:1297–1306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verrall S, Hall ZW. The N-terminal domains of acetylcholine receptor subunits contain recognition signals for the initial steps of receptor assembly. Cell. 1992;68:23–31. doi: 10.1016/0092-8674(92)90203-o. [DOI] [PubMed] [Google Scholar]