Abstract
Cochlear outer sulcus cells (OSC) and vestibular transitional cells (VTC) are part of the parasensory epithelium in the inner ear and are located in homologous positions between the sensory hair cells and the cation secretory epithelial cells in the cochlea and the vestibular labyrinth. OSC are known to sustain a reabsorptive transepithelial current and to contain an immunoreactivity for P2X2purinergic receptors. This study addresses whether OSC and VTC share functional similarities and extends this hypothesis to the question of whether both cell types contain functional P2X2 receptors. The current density (Isc) was recorded with the vibrating probe technique and was found to be similar in VTC and OSC. Both gadolinium and flufenamic acid reducedIsc in VTC, as reported previously for OSC.Isc was stimulated by extracellular ATP but not by selective agonists of P2Y receptors. Purinergic receptor agonists increased Isc with a potency order of ATP > 2′- and 3′-O-(4-benzoyl-benzoyl)adenosine 5′-triphosphate ≫ α,β-methyleneadenosine 5′-triphosphate in both OSC and VTC. In the presence of suramin (100 μm) or gadolinium (100 μm), the responses of ATP were inhibited significantly in both OSC and VTC. This pharmacological profile is consistent with that of the P2X2receptor. These results demonstrate that VTC participate in vestibular parasensory cation absorption and that both OSC and VTC regulate their parasensory cation flux via P2X2 receptors, which would regulate the endolymphatic concentration of the current-carrying ion species in auditory and vestibular transduction.
Keywords: voltage-sensitive vibrating probe, regulation of transduction, P2X receptor, inner ear, cochlea, vestibular end organ
Auditory and vestibular transduction depend on the balance of secretion and absorption of cations by the epithelial cells bounding the endolymphatic spaces (Marcus, 2001). Potassium is secreted by strial marginal cells in the cochlea and by dark cells in the vestibular labyrinth. There is a quiescent and a stimulus-induced efflux of K+ from the endolymphatic space through cochlear and vestibular hair cells. Variations in the intensity and duration of acoustic and vestibular stimuli would cause fluctuations in endolymph cation composition if there were no regulation of the rates of secretion and/or absorption. Secretion is known to be under the control of several extracellular hormones and factors, including purinergic agonists (Marcus et al., 1997; Marcus and Scofield, 2001). It has been shown recently that the outer sulcus epithelial cells (OSC) in the cochlea provide a parasensory pathway in the cochlea that sustains an apical-to-basal transepithelial cation current (Marcus and Chiba, 1999; Chiba and Marcus, 2000, 2001). The OSC would therefore contribute to the ionic homeostasis of endolymph if they possess signaling pathways that regulate this cation current.
Vestibular transitional cells (VTC) occupy a position in the vestibular labyrinth analogous to that of OSC in the cochlea, lying between the K+ secretory cells and the sensory hair cells. More importantly, functional similarities have been reported at the cellular level. The basolateral membrane of both cell types is dominated by a large K+ conductance that has a similar and unusual pharmacologic profile (Wangemann and Marcus, 1989; Chiba and Marcus, 2001). These similarities suggest that VTC may provide a parasensory pathway in the vestibular labyrinth for cation absorption from endolymph.
P2X2 purinergic receptors are ligand-gated nonselective cation channels that were found by immunohistochemistry to be expressed in OSC (Jarlebark et al., 2000). The expression of these and other purinergic receptors in several cells bordering the endolymphatic space in conjunction with a putative source of agonist and with ectoenzymes for agonist degradation have led to the proposition that the cochlea and vestibular labyrinth use paracrine and/or autocrine purinergic systems to maintain the homeostasis of endolymph (Housley et al., 1999; Marcus and Scofield, 2001).
The present study used the vibrating probe to determine whether VTC are homologous to OSC (i.e., sustain a transepithelial current directed from the apical to the basolateral side) and whether VTC and OSC regulate this current via P2X2 purinergic ligand-gated ion channels. Our results demonstrate that VTC participate in vestibular parasensory cation absorption and that both OSC and VTC regulate their parasensory cation flux via P2X2receptors. This flux would regulate the endolymphatic concentration of the current-carrying ion species in auditory and vestibular transduction.
MATERIALS AND METHODS
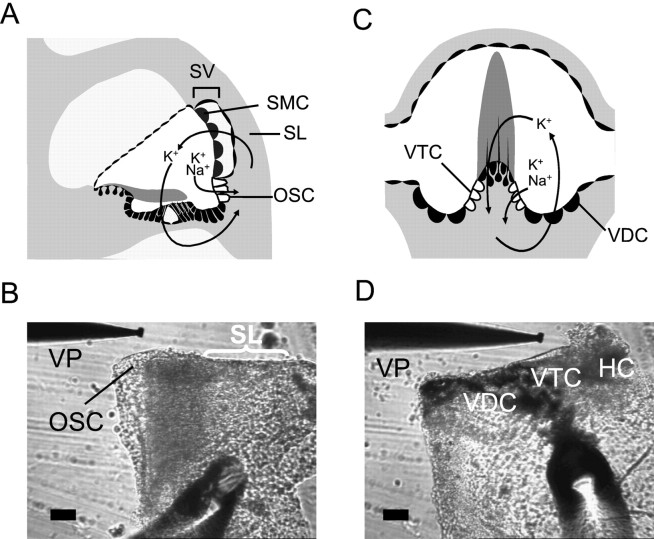
Tissue preparation. Gerbils (4–5 weeks of age) were anesthetized with sodium pentobarbital (50–100 mg/kg, i.p.) and killed under a protocol approved by the Institutional Animal Care and Use Committee of Kansas State University to remove the temporal bones. The methods for dissecting OSC and VTC epithelia have been described previously (Wangemann and Marcus, 1989; Chiba and Marcus, 2000). Briefly, the lateral wall from the upper cochlear turn was isolated and the stria vascularis was removed from spiral ligament to exclude any contribution of the marginal cells to the current density (Isc). The lateral wall was folded with OSC facing outward (Fig.1A,B). Ampullas of the semicircular canals were isolated and a cut was made along the border between VTC and the vestibular hair cells. The tissue was folded with VTC facing outward (Fig. 1C,D). A potent inhibitor of dark cell Isc, bumetanide (10 μm), was added to all bath solutions used for VTC experiments to exclude contaminating contributions from vestibular dark cells (VDC) (Marcus and Shipley, 1994). We confirmed this by observing that Isc reversibly changed its sign from positive to negative when bumetanide was perfused (see Fig. 4D), consistent with its known inhibitory action on the basolateral Na+–2Cl−–K+cotransporter of the vestibular dark cells. Each tissue was mounted in a perfusion chamber on the stage of an inverted microscope (TE-300; Nikon, Tokyo, Japan) and continuously perfused at 37°C at an exchange rate of 1.1 times/sec.
Fig. 1.
Tissue preparation for OSC and VTC.A, C, Schematic illustration showing the location of OSC and VTC in the cochlea and semicircular canal ampulla, respectively.B, D, Prepared tissue for the measurement ofIsc in OSC and VTC, respectively.HC, Vestibular hair cell (damaged); SL, spiral ligament; SMC, strial marginal cell;SV, stria vascularis; VP, vibrating probe. Scale bar, 50 μm. A andC adapted from graphics by P. Wangemann (Wangemann, 1997).
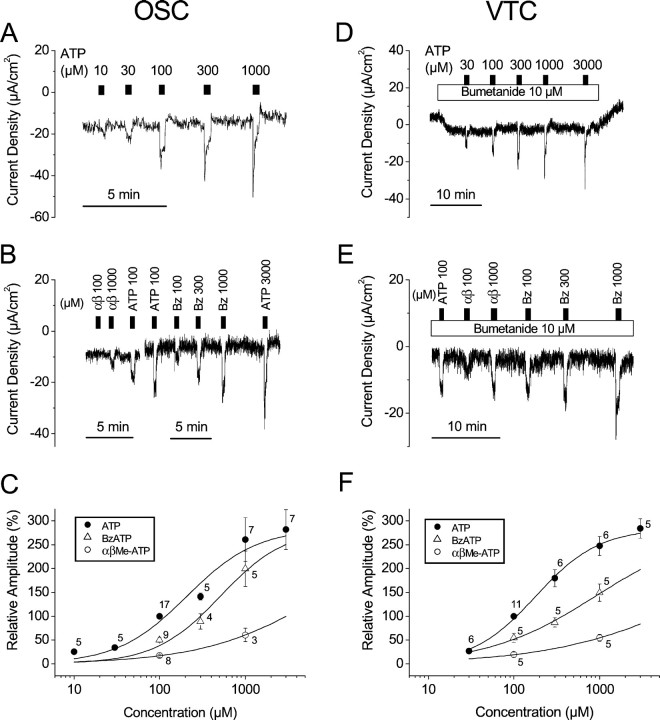
Fig. 4.
Dose–response relationships of ATP analogs in OSC and VTC. A, B, C, OSC; D, E, F, VTC. There is a discontinuity in B that indicates recordings from two different tissues. Numbersin C and F indicate numbers of observations at each concentration. In the dose–response curves (C, F), the data were normalized based on the response to 100 μm ATP. In the experiments on VTC, bumetanide (10 μm) was added to the bath solution to exclude influence from vestibular dark cells. αβ, αβmeATP; Bz, BzATP.
Voltage-sensitive vibrating probe. The vibrating-probe technique was chosen to measure transepithelial currents under short-circuit conditions because of the small extent of the epithelial domains of the OSC and VTC. In the upper turn of the cochlea, the apical membranes of OSC are exposed to endolymph, the luminal fluid, in a band that is two to four cells wide (Spicer and Schulte, 1996); VTC are similarly located in a narrow band between vestibular hair cells and dark cells in the ampulla (Oudar et al., 1988) (Fig. 1). The diameter of the vibrating-probe tip is ∼20 μm and allows detection of voltages in the low nanovolt range; vibration between two positions within the line of current flow yields voltages that correspond to current flow through the resistive physiological saline (Marcus, 1996).
The vibrating probe technique was identical to that described previously (Marcus and Shipley, 1994; Marcus, 1996). Briefly,Isc was monitored by vibrating a platinum–iridium wire microelectrode that was insulated with parlene-C (Micro Electrodes, Gaithersburg, MD) and coated with Pt black on the exposed tip. The vibration was ∼20 μm along both a horizontal (x) and vertical (z) axis. Thex-axis was perpendicular to the face of the epithelium. The probe was positioned 20–30 μm from the apical surface of the epithelium with computer-controlled, stepper-motor manipulators (Applicable Electronics, Forestdale, MA) and specialized probe software (Automated Scanning Electrode Technique version 1.05; Science Wares, East Falmouth, MA). The bath references were 26-gauge Pt-black electrodes. Calibration was performed in physiologic saline (see below) using a glass microelectrode (tip, <1 μm outer diameter) filled with 3 m KCl as a point source of current. The frequencies of vibration were in the range of 200–400 Hz and were well-separated for the two orthogonal directions. The signals from the oscillators driving the probe were also fed to a dual-channel phase-sensitive detector. The asymmetry of the probe design yielded different resonant frequencies for the two directions of vibration. The signals of the X and Z detectors were connected to a 16 bit analog-to-digital converter (CIO-DAS1602/16; ComputerBoards, Mansfield, MA) in a Pentium III, 700 MHz computer. The sampling interval was 0.5 sec, which is the minimum for this software. The electrode was positioned where Isc showed a maximumx value and minimum z value; data are expressed as the vector length of current density and were plotted with Origin software, version 6.1 (OriginLab Software, Northampton, MA).
The output from the vibrating probe depends not only on the specific short-circuit current of the epithelium but also on the position of the probe from the surface of the tissue and the exact geometry of each tissue sample. The current density reported here refers to the flux at the position of the probe and represents only a fraction of the current crossing the epithelium. No changes in the relative position of the probe attributable to swelling or shrinking of the tissue during experimental treatments were observed.
Solutions and chemicals. In all experiments, both sides of the epithelium were perfused with a perilymph-like physiologic saline containing (in mm): 150 NaCl, 3.6 KCl, 1 MgCl2, 0.7 CaCl2, 5 glucose, and 10 HEPES, pH 7.4. ATP (A-9187; Sigma, St. Louis, MO), UTP (U-4630; Sigma), 2′- and 3′-O-(4-benzoyl-benzoyl)adenosine 5′-triphosphate (BzATP) (B-6396; Sigma), α,β-methyleneadenosine 5′-triphosphate (αβmeATP) (M-6517; Sigma), suramin (S-2671; Sigma), and gadolinium chloride (G-7532; Sigma) were directly dissolved in physiologic saline just before use. UDP (U-4125; Sigma) and ADP (A-2754; Sigma) were preincubated for 1.5 hr at room temperature with hexokinase (1 U/ml; H-4502; Sigma) and glucose (5 mm) because the commercial preparations of UDP and ADP may be supplied with a minor component of UTP and ATP (Nicholas et al., 1996). Bumetanide (B-3023; Sigma) and flufenamic acid (F-9005; Sigma) were dissolved in DMSO and then diluted to 0.1% DMSO in the control solution before application. DMSO at this concentration had no effect on the short-circuit current. All purines and pyrimidines used here were applied to the bath only briefly (∼20 sec) to avoid desensitization of the receptors.
Data presentation and statistics. As an internal control, the response to 100 μm ATP was included in each experiment to compare the magnitude of effects among all of the purines and pyrimidines tested in this study. For the analysis, the peakIsc was chosen, but when a peak was not unambiguously defined we used the averaged data for the 5 sec afterIsc reached steady-state and compared these data with the averaged data for the 5 sec before the solution change. Data were expressed as the mean ± SEM (n= number of tissues) of the Isc. Increases or decreases in Isc were considered significant at a level of p < 0.05. A paired t test was used.
RESULTS
Cation absorption by VTC
The Isc from VTC in physiologic saline was −4.2 ± 0.4 μA/cm2(n = 38). Perfusion of Gd3+ (1 mm) or flufenamic acid (100 μm) for 2 min each significantly decreased the Isc by 54 ± 17% (from −3.1 ± 0.6 to −1.1 ± 0.4 μA/cm2, n = 6) and by 52 ± 11% (from −2.8 ± 0.6 to −1.3 ± 0.3 μA/cm2, n = 9), respectively (Fig. 2).
Fig. 2.
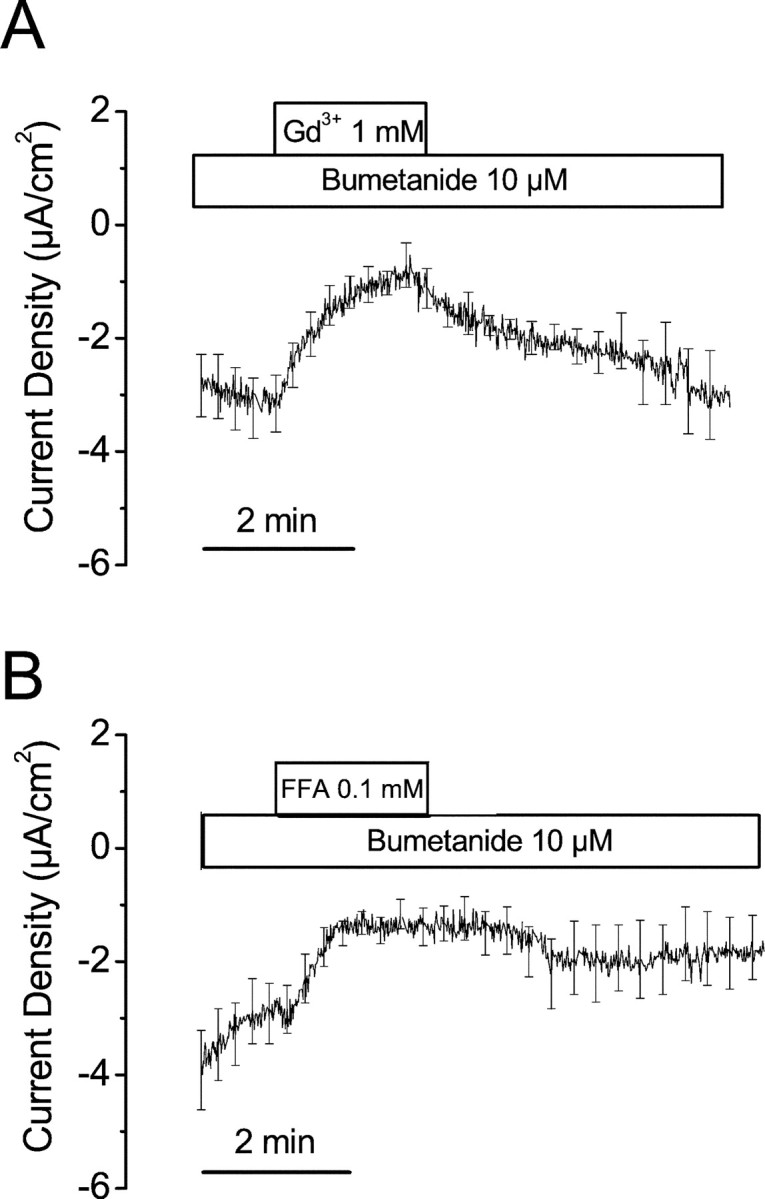
Effects of Gd3+ and flufenamic acid on Isc in VTC. A, Gd3+ (1 mm, n = 6).B, Flufenamic acid (FFA) (0.1 mm, n = 9). Bumetanide (10 μm) was added to the bath solution to exclude influence from vestibular dark cells. The SEM bars are plotted only at intervals for clarity.
Modulation of absorptive cation flux by purinergic agonists: OSC and VTC
Perfusion of ATP (100 μm) increased theIsc from −9.8 ± 1.0 to −26.4 ± 2.0 μA/cm2 in OSC (n = 28) and from −5.1 ± 0.6 to −16.9 ± 1.2 μA/cm2 in VTC (n = 23) (Figs. 3,4, 5). We first tested for mediation of this response by the P2Y family of purinergic receptors by perfusion of agonists for rodent P2Y1 (ADP), P2Y2 (UTP), P2Y4 (UTP), and P2Y6 (UDP). None of these agonists at a concentration of 100 μm changed theIsc of either OSC (Fig. 3A,n = 5 each) or VTC (Fig. 3B,n = 5 each).
Fig. 3.

Comparison of effects of ATP, UTP, UDP, and ADP in OSC and VTC. A, OSC; B, VTC. Each drug was perfused at 100 μm. For the experiments in VTC, bumetanide (10 μm) was added to the bath solution to exclude influence from vestibular dark cells. UDP and ADP were preincubated with hexokinase (1 U/ml) in the presence of glucose for at least 1.5 hr (see Materials and Methods).
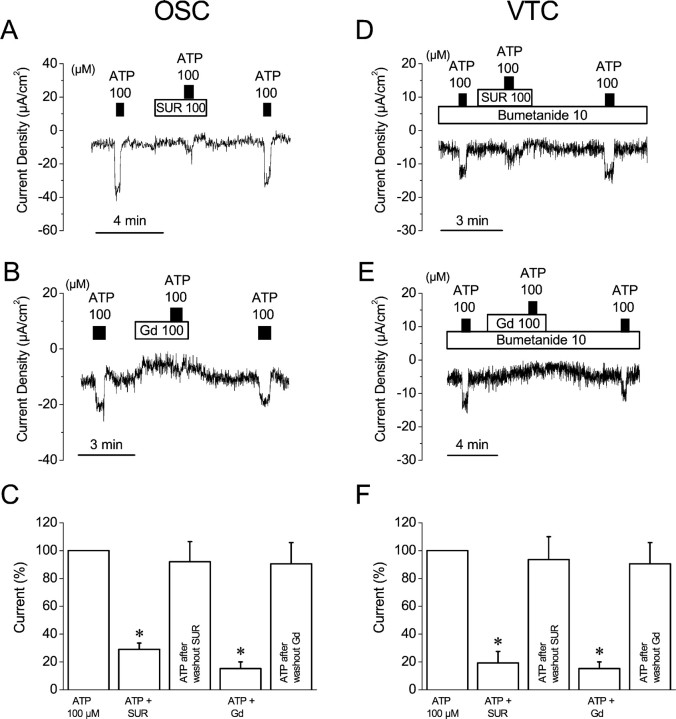
Fig. 5.
Effect of ATP in the presence of suramin or gadolinium on OSC and VTC. A, B, C, OSC; D, E, F, VTC. In the experiments on VTC, bumetanide (10 μm) was added to the bath solution to exclude influence from vestibular dark cells. Significance was tested based on the 100 μm ATP response (C, F).SUR, Suramin; Gd, gadolinium. *p < 0.05.
The subtypes of the P2X family of purinergic receptors are best identified by a comparison of the agonist potency of αβmeATP and BzATP with that of ATP (North and Surprenant, 2000). The results showed an increase in Isc by agonists with a potency order (EC50) of ATP > BzATP ≫ αβmeATP in both OSC and VTC (Fig. 4; Table1). The EC50 values from the dose–response relationship for ATP, BzATP, and αβmeATP were 209, 511, and 7951 μm in OSC, and 180, 897, and 16,434 μm in VTC, respectively.
Table 1.
Effects of ATP, BzATP, and αβmeATP onIsc (μA/cm2)
| ATP (μm) | BzATP (μm) | αβmeATP (μm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 300 | 1000 | 3000 | 100 | 300 | 1000 | 100 | 1000 | |
| OSC | 5.4 ± 1.3 | 10.4 ± 2.6 | 30.1 ± 6.7 | 37.2 ± 4.2 | 44.7 ± 6.8 | 9.5 ± 2.0 | 11.5 ± 1.5 | 24.5 ± 1.7 | 2.7 ± 0.5 | 5.8 ± 1.5 |
| (21.3 ± 4.5) | (21.3 ± 4.5) | (21.3 ± 4.5) | (17.0 ± 3.4) | (17.8 ± 3.7) | (19.3 ± 3.4) | (13.8 ± 2.7) | (13.8 ± 2.7) | (18.5 ± 4.0) | (9.8 ± 1.3) | |
| n = 5 | n = 5 | n = 5 | n = 8 | n = 7 | n = 9 | n = 4 | n = 4 | n = 8 | n = 4 | |
| VTC | 3.8 ± 0.8 | 14.2 ± 2.5 | 18.9 ± 2.4 | 23.6 ± 3.5 | 6.0 ± 1.0 | 9.7 ± 1.8 | 16.7 ± 3.6 | 2.1 ± 0.7 | 6.1 ± 1.2 | |
| (7.9 ± 1.2) | (7.9 ± 1.2) | (7.9 ± 1.2) | (8.4 ± 1.3) | (10.9 ± 1.5) | (10.9 ± 1.5) | (10.9 ± 1.5) | (10.9 ± 1.5) | (10.9 ± 1.5) | ||
| n = 6 | n = 6 | n = 6 | n = 5 | n = 5 | n = 5 | n = 5 | n = 5 | n = 5 | ||
Values were expressed as changes in Isc by application of drugs. Values in parentheses indicate changes inIsc by 100 μm ATP in the same tissues. These data were normalized and are plotted in Figure4C,F.
Suramin, a P2 receptor antagonist, was used to further assist in the identification of the P2X receptor mediating the response to ATP. Application of 100 μm suramin for 1 min resulted in no significant change in Isc for either OSC or VTC. The Isc before and after suramin was −10.6 ± 1.6 and −11.3 ± 1.7 μA/cm2 (n = 5) in OSC and −6.0 ± 1.6 and −6.4 ± 1.8 μA/cm2 (n = 6) in VTC (Fig. 5A,D). In the presence of 100 μm suramin, the stimulation of theIsc by 100 μmATP was inhibited by 71 ± 5% (n = 5) in OSC and 81 ± 8% (n = 6) in VTC compared with the stimulation by ATP in the absence of suramin (Fig. 5A,C,D,F; Table 2).
Table 2.
Effects of ATP in the presence of SUR or Gd onIsc (μA/cm2)
| ATP + SUR | ATP after wash | ATP + Gd | ATP after wash | |
|---|---|---|---|---|
| OSC | 6.2 ± 0.8* | 21.9 ± 5.1 (NS) | 1.4 ± 0.6* | 9.1 ± 0.5 (NS) |
| (23.7 ± 4.5, n = 5) | (23.7 ± 4.5, n = 5) | (11.9 ± 1.7) | (11.9 ± 1.7) | |
| VTC | 1.8 ± 0.6* | 9.7 ± 1.0 (NS) | 1.5 ± 0.5* | 8.4 ± 1.1 (NS) |
| (10.5 ± 1.1, n = 6) | (10.5 ± 1.1, n = 6) | (12.8 ± 1.5, n = 5) | (12.8 ± 1.5, n = 5) |
Values were expressed as changes in Isc by application of drugs. Values in parentheses indicate changes inIsc by the first application of 100 μm ATP in the same tissues. SUR, Suramin; Gd, gadolinium.
p<0.05. Significance was tested based on the changes resulting from the first application of 100 μmATP. NS, Not significant.
Gd3+ is known to inhibit nonselective cation channels of several types, including P2X ligand-gated channels and the nonselective cation channels in the apical membrane of OSC (Marcus and Chiba, 1999; Chiba and Marcus, 2000). Application of Gd3+ (100 μm) for 3–4 min decreased the Isc significantly, by 55 ± 9% (from −7.8 ± 1.7 to −3.2 ± 0.6 μA/cm2, n = 5) in OSC and by 34 ± 3% (from −8.2 ± 0.9 to −5.4 ± 0.7 μA/cm2, n = 5) in VTC (Fig. 5B,E). In the presence of 100 μm Gd3+, the stimulation of the Isc by 100 μm ATP was inhibited by 89 ± 3% (n = 5) in OSC and 85 ± 5% (n = 5) in VTC compared with the stimulation by ATP in the absence of Gd3+ (Fig. 5B,C,E,F; Table2).
DISCUSSION
VTC are homologous to OSC
Previous examinations of a transitional cell epithelium posited a simple barrier function in maintaining ion gradients between endolymph and perilymph (Oudar et al., 1988). In contrast, our finding of a constitutive transepithelial current demonstrates an active role in endolymph homeostasis. The direction ofIsc measured in the control solution was negative in both OSC and VTC (−9.8 and −5.1 μA/cm2, respectively). The former value is similar to previous observations in OSC (Marcus and Chiba, 1999) and is accounted for by the absorption of cations (primarily Na+ in this perilymph-like solution, but primarily K+ under in vivoconditions) through the nonselective cation channels in the apical membrane of OSC (Chiba and Marcus, 2000).
The similar effects of Gd3+ and flufenamic acid on Isc in OSC (Marcus and Chiba, 1999; Chiba and Marcus, 2000) and VTC (Fig. 2) support the notion of a strong homology in function of the two cell types. A minor difference was that there was no transient overshoot ofIsc in VTC from flufenamic acid, which was caused in OSC by the additional activation of BK (large conductance, calcium-dependent) K+channels in the apical membrane (Chiba and Marcus, 2000). This difference in response suggests a lower density of BK channels in VTC than in OSC. Additional evidence for homology between VTC and OSC is the stimulation of Isc by purinergic agonists in both cell types. The fact that suramin did not affect the constitutive Isc but Gd3+ inhibited the current implies that there are two populations of nonselective cation channels, one sensitive and the other insensitive to extracellular ATP in both OSC and VTC.
Functional expression of P2X2 receptor in OSC and VTC
Receptors for purines and pyrimidines have been investigated extensively in various systems and have been well summarized in recent reviews (Ralevic and Burnstock, 1998; North and Surprenant, 2000; Khakh et al., 2001). To date it is evident that there is no single agonist or antagonist that discriminates adequately between families of P2X and P2Y receptors. However, we used a series of strong agonists that together determined the absence of involvement of the P2Y receptor family in the stimulation of the Iscof OSC and VTC.
The P2Y family of purinergic receptors signals cellular events via G-protein-coupled signal pathways. In this study, the possibility of the coupling of P2Y receptors to stimulation ofIsc could be excluded in both OSC and VTC because of the absence of response of Isc to UTP, UDP, and ADP and because of the weaker response to BzATP than to ATP. Five mammalian P2Y receptors, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, have been cloned and are known to be valid members of the P2Y receptor family (Ralevic and Burnstock, 1998). ADP is well known as a potent agonist to P2Y1, UTP is well known as a potent agonist to rodent P2Y2 and P2Y4, UDP is well known as a potent agonist to P2Y6, and BzATP (BzATP > ATP) is well known as a potent agonist to P2Y11 (Ralevic and Burnstock, 1998; Communi et al., 1999). One criterion that is sometimes used to distinguish the involvement of P2X from P2Y receptors is the faster onset of response (within 10 msec) of P2X, which is in contrast to an onset of ∼100 msec in P2Y receptors (Ralevic and Burnstock, 1998). It was not possible in our experiments to perfuse the tissue at rates that were sufficiently high enough to use this criterion.
Our results clearly showed that P2X receptors are functionally expressed in both OSC and VTC and that their activation stimulatesIsc. By exclusion of the P2Y receptors and by previous immunolocalization of P2X2receptors on OSC (Jarlebark et al., 2000), we predicted that the stimulation of Isc by purinergic agonists occurred via a P2X2 receptor. Sensitivity to αβmeATP and inhibition by suramin have been used as important tools to discriminate among the seven recombinant homomeric P2X receptors (Khakh et al., 2001). P2X1 and P2X3 receptors are not likely expressed in OSC and VTC because of the insensitivity ofIsc to αβmeATP shown here, and P2X4 and P2X7 are not likely expressed in OSC and VTC because of the response ofIsc to suramin shown here.
The remaining possibilities are P2X2, P2X5, and P2X6 receptors. The agonist and antagonist profiles for P2X2 are not known to be distinguished clearly from ones for P2X5 (North and Surprenant, 2000). However, the characteristics of the P2X receptor in OSC and VTC are more consistent with those of the P2X2 receptor subtype. First, the reported EC50 of BzATP in the P2X5 receptor was at least 50 times higher than that of ATP, which is at least a decade greater than found here (2.5 times in OSC and 5 times in VTC). In contrast, the EC50 of BzATP in the heterologously expressed P2X2 receptor was three times higher than that of ATP, in accordance with our results. Second, mRNA transcripts for P2X1, P2X5, and P2X6 were not detected in the inner ear, including the cochlea and vestibular end organ, whereas transcripts for P2X2, P2X3, P2X4, and P2X7 were detected by reverse transcriptase-PCR (Brandle et al., 1999). Although our data are most consistent with functional P2X2 receptors, we cannot completely exclude the possibility of the presence of heteromeric P2X receptors in OSC and VTC. P2X2 subunits are known to coassemble with other subunits such as P2X3 (Radford et al., 1997).
Interestingly, there was at least a one decade difference in the EC50 values for ATP between those reported here in gerbil native tissues and those reported elsewhere in the rat recombinant homomeric P2X2 receptor. The EC50 for stimulation ofIsc by ATP was near 200 μm in OSC and VTC, but it was reported to be in the range 1–30 μm in the recombinant homomeric P2X2 receptors of rats (Khakh et al., 2001). Therefore, the EC50 appears to be higher in native tissues than in expression systems, but the reason for this difference is not clear. Possible factors could be (1) the contribution of ectonucleotidase activity in the native tissue (Dunwiddie et al., 1997), (2) possible differences among species or among the cell types, or (3) differences in glycosylation states (Torres et al., 1998).
Ectonucleotidase activity has been found in the cochlea (Vlajkovic et al., 1998a,b) and, if present in our in vitro preparations, might be expected a priori to degrade the agonist to lower concentrations than originally supplied. However, this consideration does not likely apply in this study because our results showed much higher sensitivities to ATP than to the poorly metabolized αβmeATP. Any ectonucleotidase activity present was minimized by the relatively high exchange rate of the perfusion system; problems with enzymatic activity have primarily been noted in static chambers (Khakh et al., 2001).
Our findings of stimulation ofIsc by purinergic agonists are most consistent with an apical membrane location of P2X2. The ATP-insensitive nonselective cation channels found in OSC were located in the apical membrane, as shown by excised patch-clamp recordings, and those channels provided the primary pathway for the transepithelial current from the apical to the basolateral side that resulted in a negativeIsc. P2X receptors are ligand-gated ion channels that are nonselective for cations (North and Surprenant, 2000). Because activation of these channels leads to an increase in the magnitude of the negative Isc, it is highly likely that these receptor channels are also located in the apical membrane.
Physiologic significance
There is accumulating evidence that purinergic agonists such as ATP are used by the cochlea and vestibular labyrinth to regulate transduction processes. Elements of a complete signaling system have been identified; sources of agonists, receptors, and terminating enzymes have all been demonstrated in the cochlea, and functional receptors have been demonstrated in the vestibular labyrinth. A constitutive level of ATP in the perilymph and endolymph of the guinea pig cochlea was reported (Munoz et al., 1995) that increased significantly in the endolymph during noise exposure (Munoz et al., 2001). An increase in agonist during an increase in acoustic stimulation would lead to an increased parasensory flux. This signaling cascade would then serve as a protective mechanism to reduce the flux through the sensory pathway during intense stimulation. Our findings of purinergic stimulation of Isc from VTC suggest that a similar regulatory system is operant in the vestibular labyrinth.
In conclusion, (1) VTC actively absorb cations by cellular mechanisms homologous to OSC rather than merely providing a simple barrier to sustain the high concentration differences of K+ and Na+between endolymph and perilymph; (2) both OSC and VTC serve as parasensory pathways to regulate K+ efflux through sensory hair cells during changes in the level of acoustic and vestibular stimulation; and (3) among the possible roles of extracellular nucleotides, one mechanism of regulation involves purinergic signaling via P2X2 receptors in OSC and VTC, most likely in their apical membranes.
Note added in proof. P2X2receptors have been localized recently in vestibular transitional cells by immunostaining (S. N. Syeda and A. Lysakowski, personal communication).
Footnotes
This work was supported by Research Grant 5R01-DC00212 (D.C.M.) from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Correspondence should be addressed to Daniel C. Marcus, Department of Anatomy and Physiology, Kansas State University, 126 Coles Hall, 1600 Denison Avenue, Manhattan, KS 66506-5802. E-mail: marcus@ksu.edu.
T. Chiba's present address: Department of Otolaryngology, Tohoku University School of Medicine, 1-1 Seiryomachi, 980-77 Sendai, Japan.
REFERENCES
- 1.Brandle U, Zenner HP, Ruppersberg JP. Gene expression of P2X-receptors in the developing inner ear of the rat. Neurosci Lett. 1999;273:105–108. doi: 10.1016/s0304-3940(99)00648-5. [DOI] [PubMed] [Google Scholar]
- 2.Chiba T, Marcus DC. Nonselective cation and BK channels in apical membrane of outer sulcus epithelial cells. J Membr Biol. 2000;174:167–179. doi: 10.1007/s002320001041. [DOI] [PubMed] [Google Scholar]
- 3.Chiba T, Marcus DC. Basolateral K+ conductance establishes driving force for cation absorption by outer sulcus epithelial cells. J Membr Biol. 2001;184:101–112. doi: 10.1007/s00232-001-0079-0. [DOI] [PubMed] [Google Scholar]
- 4.Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarlebark LE, Housley GD, Thorne PR. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. J Comp Neurol. 2000;421:289–301. doi: 10.1002/(sici)1096-9861(20000605)421:3<289::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 9.Marcus DC. Vibrating probes: new technology for investigation of endolymph homeostasis. Keio J Med. 1996;45:301–305. doi: 10.2302/kjm.45.301. [DOI] [PubMed] [Google Scholar]
- 10.Marcus DC. Acoustic transduction. In: Sperelakis N, editor. Cell physiology source book. Academic; San Diego: 2001. pp. 775–794. [Google Scholar]
- 11.Marcus DC, Chiba T. K+ and Na+ absorption by outer sulcus epithelial cells. Hear Res. 1999;134:48–56. doi: 10.1016/s0378-5955(99)00074-x. [DOI] [PubMed] [Google Scholar]
- 12.Marcus DC, Scofield MA. Apical P2Y4 purinergic receptor controls K+ secretion by vestibular dark cell epithelium. Am J Physiol Cell Physiol. 2001;281:C282–C289. doi: 10.1152/ajpcell.2001.281.1.C282. [DOI] [PubMed] [Google Scholar]
- 13.Marcus DC, Shipley AM. Potassium secretion by vestibular dark cell epithelium demonstrated by vibrating probe. Biophys J. 1994;66:1939–1942. doi: 10.1016/S0006-3495(94)80987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus DC, Sunose H, Liu J, Shen Z, Scofield MA. P2U purinergic receptor inhibits apical IsK/KvLQT1 channel via protein kinase C in vestibular dark cells. Am J Physiol. 1997;273:C2022–C2029. doi: 10.1152/ajpcell.1997.273.6.C2022. [DOI] [PubMed] [Google Scholar]
- 15.Munoz DJ, Thorne PR, Housley GD, Billett TE. Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res. 1995;90:119–125. doi: 10.1016/0378-5955(95)00153-5. [DOI] [PubMed] [Google Scholar]
- 16.Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 17.Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- 18.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 19.Oudar O, Ferrary E, Feldmann G. Ultrastructural study of the semicircular canal cells of the frog Rana esculenta. Anat Rec. 1988;220:328–334. doi: 10.1002/ar.1092200316. [DOI] [PubMed] [Google Scholar]
- 20.Radford KM, Virginio C, Surprenant A, North RA, Kawashima E. Baculovirus expression provides direct evidence for heteromeric assembly of P2X2 and P2X3 receptors. J Neurosci. 1997;17:6529–6533. doi: 10.1523/JNEUROSCI.17-17-06529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 22.Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100:80–100. doi: 10.1016/0378-5955(96)00106-2. [DOI] [PubMed] [Google Scholar]
- 23.Torres GE, Egan TM, Voigt MM. N-Linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry. 1998;37:14845–14851. doi: 10.1021/bi981209g. [DOI] [PubMed] [Google Scholar]
- 24.Vlajkovic SM, Thorne PR, Housley GD, Munoz DJ, Kendrick IS. Ecto-nucleotidases terminate purinergic signalling in the cochlear endolymphatic compartment. NeuroReport. 1998a;9:1559–1565. [PubMed] [Google Scholar]
- 25.Vlajkovic SM, Thorne PR, Housley GD, Munoz DJ, Kendrick IS. The pharmacology and kinetics of ecto-nucleotidases in the perilymphatic compartment of the guinea-pig cochlea. Hear Res. 1998b;117:71–80. doi: 10.1016/s0378-5955(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Wangemann P. Kalium-Ionensekretion und Eutstehung des endocochlearen Potentials in der Stria vascularis. HNO. 1997;45:205–209. doi: 10.1007/s001060050105. [DOI] [PubMed] [Google Scholar]
- 27.Wangemann P, Marcus DC. Membrane potential measurements of transitional cells from the crista ampullaris of the gerbil. Effects of barium, quinidine, quinine, tetraethylammonium, cesium, ammonium, thallium, and ouabain. Pflügers Arch. 1989;414:656–662. doi: 10.1007/BF00582132. [DOI] [PubMed] [Google Scholar]