Abstract
In vertebrate neuromuscular junctions, ATP is stored at the motor nerve terminals and is co-released with acetylcholine during neural stimulation. Here, we provide several lines of evidence that the synaptic ATP can act as a synapse-organizing factor to induce the expression of acetylcholinesterase (AChE) and acetylcholine receptor (AChR) in muscles, mediated by a metabotropic ATP receptor subtype, the P2Y1 receptor. The activation of the P2Y1receptor by adenine nucleotides stimulated the accumulation of inositol phosphates and intracellular Ca2+ mobilization in cultured chick myotubes. P2Y1 receptor mRNA in chicken muscle is very abundant before hatching and again increases in the adult. The P2Y1 receptor protein is shown to be restricted to the neuromuscular junctions and colocalized with AChRs in adult muscle (chicken, Xenopus, and rat) but not in the chick embryo. In chicks after hatching, this P2Y1 localization develops over ∼3 weeks. Denervation or crush of the motor nerve (in chicken or rat) caused up to 90% decrease in the muscle P2Y1 transcript, which was restored on regeneration, whereas the AChR mRNA greatly increased. Last, mRNAs encoding the AChE catalytic subunit and the AChR α-subunit were induced when the P2Y1 receptors were activated by specific agonists or by overexpression of P2Y1 receptors in cultured myotubes; those agonists likewise induced the activity in the myotubes of promoter–reporter gene constructs for those subunits, actions that were blocked by a P2Y1-specific antagonist. These results provide evidence for a novel function of ATP in regulating the gene expression of those two postsynaptic effectors.
Keywords: ATP receptors, P2Y1 receptor, neuromuscular junction, trophic factors, acetylcholine receptor gene regulation, acetylcholinesterase gene regulation, chick muscle
In vertebrate neuromuscular junctions, acetylcholine receptors (AChRs) and collagen-tailed acetylcholinesterase (AChE) become localized and densely clustered, although the mechanisms and interactions (Duclert and Changeux, 1995;Krejci et al., 1999; Sanes and Lichtman, 1999) that are involved are different. For AChR in muscle, also, biosynthesis is regulated by nerve-derived factors, which include calcitonin gene-related peptide (CGRP) (New and Mudge, 1986; Fontaine et al., 1987), ascorbic acid (Horovitz et al., 1989), and neuregulin (Sandrock et al., 1997). Simultaneously, the nerve evokes muscle cell electrical activity that suppresses AChR synthesis in the extrasynaptic regions (Duclert and Changeux, 1995); hence, focalization of AChRs at the synapse occurs.
ATP is an additional potential trophic factor for endplates. ATP is co-stored in and constantly co-released quantally with acetylcholine (in a ratio of ∼1:6) from the nerve terminals in vertebrate skeletal muscles (Silinsky and Redman, 1996) or electroplaques (Israel and Dunant, 1998). The action of released ATP would be confined locally by its subsequent hydrolysis to adenosine by ectonucleotidases (Redman and Silinsky, 1994; Zimmermann, 1999). Receptors for extracellular ATP (P2 receptors) would become activated if present at or near the junctions. P2 receptors are nucleotide receptors, either ionotropic (P2X) or G-protein coupled (P2Y) (North and Barnard, 1997; Ralevic and Burnstock, 1998). Nine P2Y subtypes (of varying nucleotide agonist specificities) in mammals and birds are known by cloning and expression, although species ortholog are not always clear (Barnard and Simon, 2001).
Indeed, earlier studies have suggested that ATP could exert effects on neuromuscular transmission. In skeletal muscles, exogenous ATP can potentiate responses to acetylcholine (Ewald, 1976; Akasu et al., 1981;Lu and Smith, 1991). In Xenopus embryonic nerve–muscle cocultures, focal application of ATP increased spontaneous synaptic currents, whereas on myocytes it potentiated the response to acetylcholine (Fu et al., 1997). These ATP-induced effects were blocked by general antagonists for P2 receptors and by protein kinase C (PKC) inhibitors and were not caused by adenosine receptors (Lu and Smith, 1991; Fu et al., 1997). Pharmacological and patch-clamp evidence on those responses to ATP on chick and mammalian muscle cells (Häggblad and Heilbronn, 1988; Lu and Smith, 1991; Fu et al., 1997; Henning, 1997) generally correspond to the now-known P2Y class of receptors. However, ion channels directly opened by ATP are also detectable on embryonic muscle cells from chicken (Kolb and Wakelam, 1983; Thomas et al., 1991) and mammals (for review, see Henning, 1997). Possible trophic effects in muscles of ATP mediated by either P2Y or P2X receptors now merit consideration.
Here we have examined actions of nucleotides on skeletal muscle cells in terms of current molecular knowledge of P2 receptors. In chick myotubes, the signaling responses showed a major contribution of the P2Y1 receptor subtype. In chicken and rat muscle, the expression and development of P2Y1 receptors and their localization in relation to the neuromuscular junctions were demonstrated. Further results support a novel function of synaptic ATP and its activation of P2Y1 receptors in the regulation of gene expression of postsynaptic AChR and AChE.
MATERIALS AND METHODS
Materials. Cell culture media, fetal calf or horse sera, and other cell culture reagents were from Life Technologies (Grand Island, NY). UTP and UDP were ultra-pure grade from Amersham Pharmacia Biotech (Little Chalfont, UK). Other P2Y receptor agonists and antagonists were the purest grades available from either Research Biochemicals International (Natick, MA) or Sigma (St. Louis, MO). These included adenosine 3′-5′-bismonophosphate (sodium salt; A3P5P), ATP (disodium salt), ADP (sodium salt), α,β-methyleneadenosine 5′-triphosphate (lithium salt; αβ-meATP), 2-methylthioadenosine 5′-triphosphate (tetrasodium salt; 2-MeSATP), 2-methylthioadenosine diphosphate (trisodium salt; 2-MeSADP), pyridoxal-phosphate-6-azophenyl-2′ 4′-disulfonic acid (tetrasodium salt; PPADS), Reactive blue 2 (RB-2), and suramin. The commercial antibodies were purchased from Sigma or Cappel (Durham, NC), and tetramethylrhodamine-conjugated-α-bungarotoxin (TMR-BuTX) was from Molecular Probes (Eugene, OR). Radiochemicals were from Amersham Pharmacia Biotech. Apyrase (Grade VII) was also from Sigma, and other reagents of unspecified source were analytical grades.
Purity of nucleotides. ADP, 2-MeSADP, and UDP stock solutions (1 mm) were preincubated with 20 U/ml yeast hexokinase (Roche Biochemicals, Lewes, UK) in Buffer A (2.5 mm MgCl2/50 mm HEPES, pH 7.3) containing 25 mm glucose at 37°C for 30 min (90 min for UDP) to remove all contaminating triphosphates (Boyer et al., 1996). ATP and 2-MeSATP stock solutions (1 mm) were pretreated in Buffer A with 20 U/ml creatine phosphokinase (CPK; Sigma) and 10 mm creatine phosphate (CP; Sigma) at room temperature for 90 min to remove all contaminating diphosphates, as described and validated elsewhere (Simon et al., 2001). UTP (10 mm) was treated similarly but for 3 hr and with CP at 50 mm, because of its higherKm and lowerVmax. During the incubations for the inositol phosphate assays and for the stimulation of myotube expression of AChE/AChR or promoter activity, when triphosphate agonists were used CPK was also present throughout at 2 U/ml, CP at 5 mm, and Mg2+ at 2.5 mm. When diphosphate agonists were used, hexokinase was present at 2 U/ml, glucose at 5 mm, and Mg2+ at 2.5 mm. Those media used alone in the control incubations in each case had no effect. For the longer incubations with an agonist, renewals at intervals of the agonist and that medium were also made, as stated.
Animals. Pectoral muscles of New Hampshire chickens at the stated ages were collected immediately after chickens were killed, frozen in liquid nitrogen, and stored at −80°C for total RNA extraction. For reversible denervation, post-hatch day (P) 18 chicks or adult rats (∼300 gm) were fully anesthetized with isoflurane, and ∼5 mm of the left sciatic nerve was removed using aseptic surgical technique (Ip et al., 1996). Gastrocnemius muscle of the operated side was collected immediately after the animal was killed at the indicated time points after the surgery, frozen in liquid nitrogen, and stored at −80°C. Nerve crush was performed with a prechilled fine forceps on the left sciatic nerve in the upper thigh of chicks or rats, and the gastrocnemius muscles were collected similarly. All procedures conformed to the Guidelines by Animal Research Panel of Hong Kong University of Science and Technology for the use and care of laboratory animals in research.
Cell cultures. Primary chick myotube cultures were prepared from hindlimb muscles dissected from 11-d-old chick embryos as described previously (Fischbach, 1972; modified by Tsim et al., 1992). The muscle cells were cultured in Eagle's MEM supplemented with 10% heat-inactivated horse serum, 2% (v/v) chick embryo extract, 1 mml-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cultures were then incubated at 37°C in a water-saturated 5% CO2atmosphere. Myotubes were treated with a mitotic inhibitor (10 μm cytosine arabinoside) at day 3 after plating and used on day 4. COS-7 cells were cultured in 100 mm culture dishes in DMEM with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. Because skeletal muscle cell cultures can release some ATP into the medium and can also convert it there to ADP, and because over longer periods these agents may give some desensitization of P2Y receptors, they were pretreated with apyrase (2 U/ml) for 1 hr or when loading with [3H]myoinositol (24 hr) to eliminate all such free nucleotides, before a gentle wash and drug application in apyrase-free medium.
Inositol phosphate and cAMP accumulation assays. Inositol-free DMEM (750 μl), containing 10% horse serum and 2.5 μCi/ml [3H]myoinositol and apyrase (2 U/ml) to prevent desensitization by released nucleotides, was added to cultured myotubes and incubated 24 hr. The labeled media were subsequently replaced by 1 ml of inositol phosphate assay medium (DMEM buffered with 20 mm HEPES, pH 7.5) with apyrase also present at 0.2 U/ml and further incubated 1 hr to reduce basal inositol phosphate level. The media were then replaced by fresh inositol phosphate assay medium, apyrase-free but with the usual inositol phosphatase inhibitor LiCl (20 mm) present, and incubated for a further 10 min. After one gentle replacement by the same medium, the appropriate agonists were applied in a final volume of 1 ml, and the cells were incubated at 37°C for 30 min. In these assays the medium also contained the protective enzyme mixture as noted above. Reactions were then stopped by the addition of 750 μl of ice-cold 20 mm formic acid and held at 4°C for 1 hr. The total [3H]inositol phosphates were separated from other labeled inositol species by sequential ion-exchange chromatography as described previously (Tsu et al., 1995). cAMP assays were performed as described previously (Choi et al., 1998). Briefly, 4-d-old myotube cultures were prelabeled for 16–20 hr with [2-3H]adenine (1 μCi/ml) and treated with agonist. The reaction was stopped by addition of 5% trichloroacetic acid containing 1 mm ATP, and the [3H]cAMP fraction was isolated via Dowex and alumina columns. The [3H]cAMP accumulation was expressed as a percentage of that present (basal) before nucleotide addition.
Calcium imaging. Myotubes were grown at 37°C on 25-mm-diameter coverslips, incubated with apyrase (2 U/ml, 1 hr), and gently washed with culture medium. The calcium-indicator dye, calcium green-1 conjugated to 10 kDa dextran (Molecular Probes), together with heparin (5 mg/ml) where stated, was loaded into them by electroporation (1.1 kV/cm electroporation intensity, 2 msec pulse width, and 10 pulses per train) (Chang, 1997). During the recovery period, apyrase (2 U/ml) was again present. After 2–3 hr incubation, each coverslip was washed and transferred in culture medium to a temperature (37°C)-controlled microincubation chamber mounted on the stage of aZeiss Axiovert 35 microscope. With excitation at 488 nm, the calcium green-1 fluorescence images were recorded at 529 nm using a digital imaging system with a cooled CCD camera (MicroMax, Princeton Instruments, Trenton, NJ) and MetaMorph Image-processing software v3.0 (Universal Imaging, Hollis, NH), and ligand was applied as stated. The calibration curve relating calcium concentration to the fluorescence intensity was determined and used according to a previously described method (Kao, 1994).
Microphysiometry. Functional assays using microphysiometry were performed according to the methods of Pitchford et al. (1995). Briefly, 5-d-old cultured chick myotubes in capsules were transferred to microphysiometer sensor chambers (Cytosensor; Molecular Devices, Sunnyvale, CA) at 37°C and equilibrated by perfusion for 2 hr in running medium (DMEM supplemented with 0.1% bovine serum albumin, adjusted with NaOH to pH 7.38). When stable acidification rates were established thus, cells were exposed for 6 min to various agents in the running medium at a flow rate of 100 μl/min. The acidification rates were measured at 2 min intervals. The pH changes were converted by the sensor computer program to H+ ion output from the cells.
Northern blots. Total RNA was prepared from the tissues or cells using the LiCl method (Sambrook et al., 1989), and the RNA samples were electrophoresed through a 1% formaldehyde gel. RNA concentration and purity were determined by UV spectrometry, and a nominal loading of 30 μg per lane was used. After electrophoretic separation, RNA bands were transferred to a charged nylon membrane and were UV cross-linked. Blots were hybridized in turn with the following probes (from within, or spanning all, the coding sequence): ∼1.5 kb chick P2Y1 receptor cDNA (Webb et al., 1993), ∼0.6 kb from the chick AChE catalytic subunit cDNA (Tsim et al., 1992), ∼1.2 kb from the chick AChR α-subunit cDNA (Pun and Tsim, 1997), ∼0.5 kb from the rat P2Y1 receptor cDNA (Tokuyama et al., 1995) (between bases 930 and 1390), and ∼1.9 kb rat AChR α-subunit (Ip et al., 1996). Probes were labeled with [α-32P]dCTP; the hybridization was performed at 42°C overnight in 40% deionized formamide, 5× Denhardt's solution, 0.5% SDS, 5× SSC, 10% dextran sulfate, and 0.1 mg/ml denatured salmon sperm DNA. After hybridization, filters were washed twice with 2× SSC with 0.1% SDS at room temperature for 30 min each, and then twice with 0.1× SSC with 0.1% SDS at 55°C for 30 min each (Sambrook et al., 1989). The filters were then exposed to x-ray film with double intensifying screens at −80°C to suitable intensity. Quantitation was made for samples run on the same gel. On bands scanned on an image analyzer, the relative densities were determined within the linear region of a calibration curve constructed with parallel samples containing 32P microscale standards. Ribosomal RNA was stained with ethidium bromide, and the 28S band was quantitated by a parallel method to allow correction for the loading in each lane.
Reporter gene constructs and transfections. The cDNA (∼2.2 kb) encompassing the human AChE promoter (Ben Aziz-Aloya et al., 1993) (a gift from H. Soreq, Hebrew University of Jerusalem) and the ∼930 bp chick AChR α-subunit promoter region excised from a pnlacZ plasmid (Sanes et al., 1991) (a gift from J. Sanes, Washington University) were subcloned into a pGL3 vector that contained a luciferase gene downstream (Promega, Madison, WI). This resulted in the following reporter gene expression vectors: pAChE-Luc for the human AChE promoter and pAChRα-Luc for the chick AChR α-subunit promoter. The full-length cDNA encoding the chicken P2Y1receptor in a mammalian expression vector, as described previously (Webb et al., 1993; Simon et al., 1995), was cotransfected where stated. Myoblasts from 11 d chick embryos were cultured for 2 d and then transfected transiently with the purified plasmids (2 μg per well in 12-well plates) by standard calcium precipitation (Pun and Tsim, 1997), and they were allowed to fuse as myotubes. COS-7 cells were transfected by DEAE Dextran method to express the chick P2Y1 receptor and cultured in their medium (see above) a further 48 hr before use. The transfection efficiency was determined in test cases in which cells were cotransfected with β-galactosidase cDNA in the same vector, followed by enzymatic staining. The transfection efficiency in myoblasts was consistently from ∼15 to 20%, whereas COS-7 cells showed a transfection efficiency of >40%.
Western blotting for AChE. Cultured myotubes were collected and homogenized in 10 mm HEPES, pH 7.5, 0.5% Triton X-100, 5 mm EDTA, 5 mm EGTA, 1 mg/ml bacitracin, and 1m NaCl and centrifuged at 12,000 ×g for 5 min. The supernatant was used for both enzymatic assay and immunoblotting. In immunoblotting, the sample was denatured at 100°C for 5 min in SDS sample buffer, pH 7.5, containing 1% SDS and 1% dithiothreitol. The proteins were then separated on a 7.5% SDS-polyacrylamide gel and electroblotted onto nitrocellulose filters for 16 hr (Tsim et al., 1997). The blot was incubated (1 hr, 37°C) with 5% nonfat dried milk as blocking agent in 20 mm Tris/137 mm NaCl/0.1% Tween 20, pH 7.6. After washing in the latter medium alone, purified monoclonal antibody ACB-1 to the chick AChE catalytic subunit (Tsim et al., 1988) (5–10 μg/ml) or anti-α-tubulin antibody (1:5000) was applied, followed by peroxidase-conjugated goat anti-mouse Ig antibody [with procedures as in Tsim et al. (1988)]. The immunoreactive band present in each case was visualized by chemiluminescence according to the ECL protocol (Amersham Pharmacia Biotech) under strictly standardized conditions. The labeling intensities of the protein band from the control and from the agonist-stimulated samples, run in the same gel, were compared by densitometry as for Northern blots in the nonsaturating range of calibration curves constructed with serial dilutions of parallel samples.
Polyclonal antibody against chicken P2Y1 receptor and labeling of receptors in muscles. A cDNA encoding the thioredoxin protein in the prokaryotic expression vector pET32 (Novagen, Madison, WI) was tagged in-frame at its 5′ end with nucleotides 1143–1417 of the chicken P2Y1 receptor cDNA sequence as described and numbered in Webb et al. (1993), corresponding to its final C-terminal residues 320–362. That sequence is beyond the last accepted transmembrane domain and has no homology with any other P2Y receptor. This plasmid was used to transform Escherichia coli strain BL21 (Lys S) to obtain overexpression of the fusion protein. The latter was purified using the Novagen protocol, and rabbits were each immunized three times with it. Antibodies were purified by protein-A affinity chromatography (High Trap column, Amersham Pharmacia Biotech). The polyclonal antibody was used at ∼20 μg/ml in immunohistochemistry on muscle sections (20 μm), with fluorescein-5-isothiocyanate-conjugated goat anti-rabbit Ig second antibody, by methods described elsewhere for other antibodies (Tsim et al., 1997). The sections were double labeled for P2Y1 receptor thus and for AChR with 10−8m TMR-BuTX (Tsim et al., 1997). Staining was viewed under a 20–40× objective alternately with phase-contrast and fluorescence optics, the latter using excitation at 555 or 488 nm and emission at 580 or 515 nm for rhodamine or fluorescein, respectively. To measure percentage colocalization in chick muscle, ∼50 fields were selected at random, and all sites therein that were clearly labeled with either stain and not coinciding with the other stain were taken as not colocalized and compared with the total number of discrete sites seen for both stains. This exaggerates somewhat the colocalization at early stages (up to approximately hatching), where some of the noncoincident staining was too diffuse to assign, but the diffuse staining had essentially disappeared after day 4.
Other assays. AChE activity was determined by the Ellman spectroscopic method in a medium containing 0.1 mm tetraisopropylpyro-phosphoramide to inhibit chick butyrylcholinesterase (Tsim et al., 1988). For luciferase assay, the cultures were washed in PBS and resuspended in 0.2 ml lysis buffer (0.1 M potassium phosphate, pH 7.8/0.2% Triton X-100/1 mm dithiothreitol). The lysates were then centrifuged at 15,800 × g for 2 min, and the supernatants were used to assay the luciferase activity according to the manufacturer's instructions (Tropix, Bedford, MA). The reaction was quantified on the Tropix TR717 microplate luminometer. Protein concentrations were measured throughout by the method of Bradford (1976).
RESULTS
Pharmacological properties of P2Y receptors in chick skeletal muscle cells
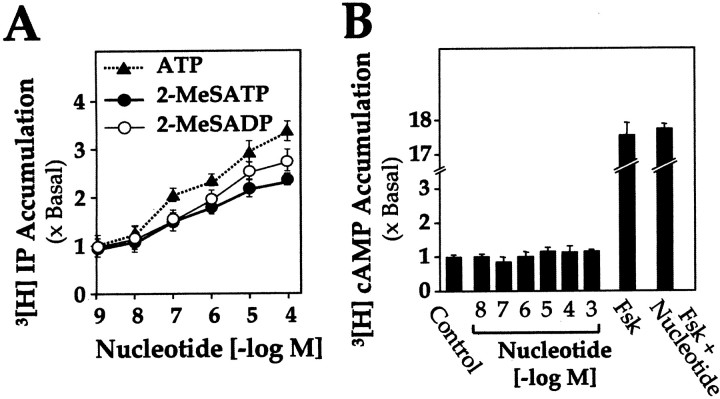
The effects of various agonists or antagonists of P2Y receptors on second messenger responses were tested in cultured chick myotubes. Included as selective agonists in these and later experiments were 2-MeSATP and 2-MeSADP: the former selects P2Y1(Filippov et al., 2000) among all known P2Y receptors, and the latter selects P2Y1 among all known P2Y and P2X receptors [the one exception being P2Y12, a platelet receptor unknown in muscle cells and linked to adenylate cyclase inhibition (Barnard and Simon, 2001)], a transduction absent here (Fig.1B). Chick myotubes were previously known (Häggblad and Heilbronn, 1988) to show an increase in inositol phosphates when treated with ATP, but the P2Y receptors were then unknown and impure ATP was in use. Here, application of purified ATP or 2-MeSATP or 2-MeSADP increased significantly the accumulation of inositol phosphates in a dose-dependent manner (Fig. 1A). Under the same assay conditions, application of those agents up to 1 mm showed no change in the intracellular cAMP level (Fig. 1B). Stimulation by forskolin served as a positive control and showed a ∼17-fold increase in cAMP level; none of the nucleotide treatments used inhibited that forskolin response (Fig. 1B).
Fig. 1.
Pharmacological properties of P2Y receptors in chick muscle cells. Myotubes cultured for 5 d were pretreated with apyrase, washed, and treated with the indicated concentrations of agonist (or with control medium). Values are expressed as the ratio of the stimulated to the basal level. A, ATP or 2-MeSATP or 2-MeSADP induces the accumulation of inositol phosphates (IP). B, ATP or 2-MeSATP or 2-MeSADP does not change the level of intracellular cAMP. The values for all three nucleotides, applied separately, fell within the SEM bars shown. Forskolin (Fsk) at 50 μm served as a positive control and also to show that none of these agents decreased a stimulated level of cAMP. In both A andB, data are mean ± SEM values for four independent experiments, each with triplicate samples.
For comparison, COS-7 cells transiently expressing the recombinant P2Y1 receptor were treated under the same conditions. The line of COS-7 cells used contains endogenous receptors that behave similarly to P2Y2 receptors, and these untransfected cells responded to ATP and UTP but not to the P2Y1 agonists 2-MeSADP or 2-MeSATP (Fig.2). In the COS-7 cells heterologously expressing the P2Y1 receptor, the intracellular level of inositol phosphates was increased by the application of 2-MeSATP, 2-MeSADP, or ATP, as in myotubes (Fig. 2). Under these conditions adenosine was inactive, showing that no fraction of the response to ATP in the myotubes (Fig. 2) is due its degradation to adenosine and subsequent activation of adenosine receptors. The P2Y1-specific antagonist A3P5P (Boyer et al., 1996), as well as the wide-range P2 antagonists PPADS, RB2, and suramin, were each able to block completely the accumulation of 2-MeSADP-induced inositol phosphates in the myotubes, as with the recombinant P2Y1 receptor (Fig. 2). UTP, which has negligible activity at the chicken P2Y1receptor, is active on the myotubes (Fig. 2). Overall, the patterns of second messenger responsiveness and of agonist and antagonist selectivity on the chick muscle cells could be accounted for by a predominant P2Y1 receptor subtype together with a smaller contribution from one or more other receptors activated by UTP and ATP. This would also account for the biphasic form of the ATP concentration–response curve and for the greater response to ATP than to 2-MeSADP or 2-MeSATP (Fig. 1A).
Fig. 2.
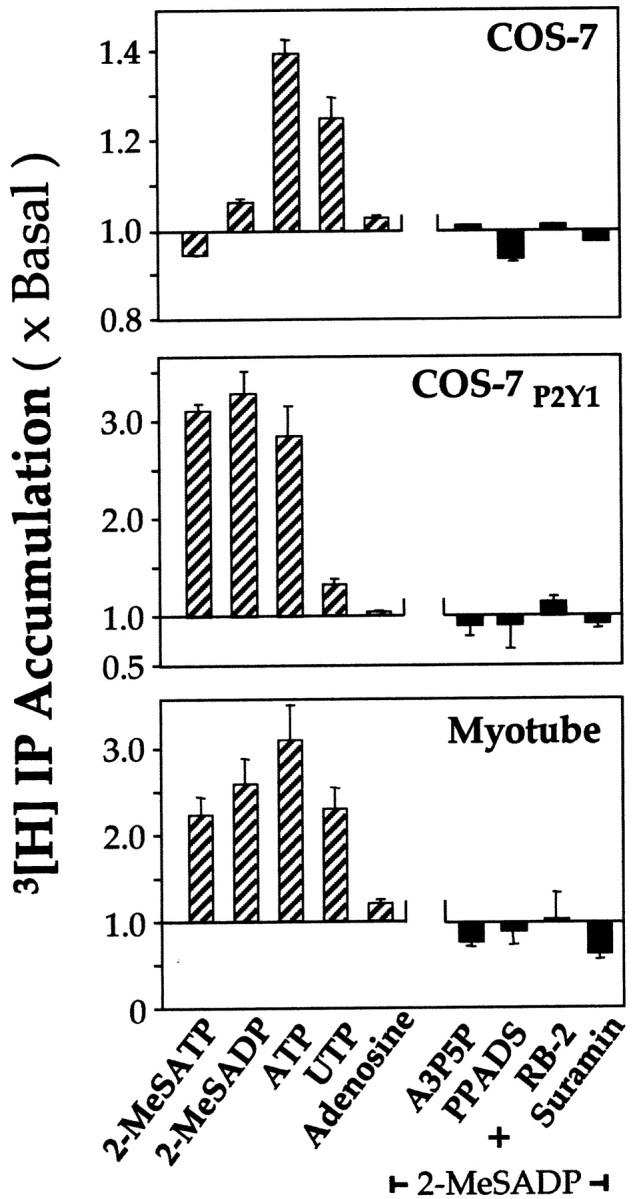
Stimulation of inositol phosphates (IP) accumulation in chick muscle cells or in COS-7 cells expressing the chicken P2Y1 receptor. The results were obtained and expressed as in Figure 1A(n = 4). Left-hand sets ofbars: 2-MeSATP (50 μm), 2-MeSADP (50 μm), ATP (50 μm), UTP (50 μm), and adenosine (50 μm) were tested as agonists on myotubes and, for comparison, on COS-7 cells expressing the chicken P2Y1 receptor (COS-7P2Y1) cells. Non-transfected COS-7 cells (top panel) show negligible responses to these drugs, except to ATP and UTP, attributable to endogenous P2Y2-like receptors; these COS-7 cells contain no P2Y1 receptors. Antagonists tested (right-hand set of bars) were A3P5P (50 μm), PPADS (50 μm), RB-2 (5 μm), and suramin (100 μm); each was applied together with 2-MeSADP (50 μm).
The additional P2Y receptor subtype(s) could not be identified at present, because only the P2Y1, P2Y3, and P2Y5 receptors have been cloned from chicken. The chicken P2Y3receptor (which is highly sensitive to UDP) is not expressed in chick muscle (Webb et al., 1996), nor is the P2Y5receptor [which has recently been shown to give functional responses to ATP in heterologous expression (King and Townsend-Nicholson, 2000)]. The total absence of the mRNA of either of these in the myotubes was confirmed by Northern blotting (data not shown). The P2Y4 receptor of the rat (Webb et al., 1998) and the P2Y2 receptor as cloned from several mammals (Lustig et al., 1993; Parr et al., 1995) are activated by UTP and ATP and insensitive to 2-MeSATP or 2-MeSADP; the presence of a chick ortholog of either (or both) would account for the additional component seen here.
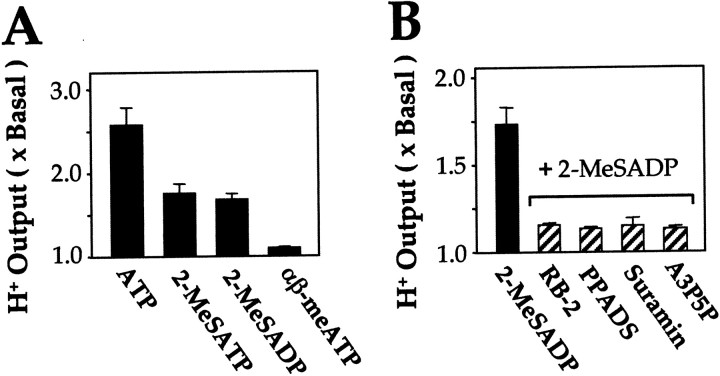
Functional analysis was also performed using microphysiometry, which sensitively detects a change of extracellular pH during agonist application. In chick myotubes, ATP, 2-MeSATP, and 2-MeSADP each induced a significant change in the H+output, but not αβ-meATP (Fig. 3). The 2-MeSADP-induced activation was blocked by the P2 antagonists RB-2, PPADS, and suramin and by the P2Y1-selective antagonist A3P5P (Fig. 3). These agonist and antagonist selectivities again reflect the pharmacological characteristics of a P2Y1 receptor in chick myotubes.
Fig. 3.
Extracellular output of H+ ions from chick myotubes is activated by P2Y1 agonists and blocked by P2Y antagonists. A, Myotubes, treated as for Figure 1, were exposed for 6 min to the agonists shown (50 μm, superfused at 100 μl/min). B, Treatment with 50 μm 2-MeSADP, alone or (right-hand set of bars) plus the antagonists shown (concentrations as in Fig. 2). The H+ output from the cell to the medium, which induced a change of pH, was determined by the sensor. Values are expressed as the ratio to the basal level (i.e., where no drug was present in the perfusing medium). Data are mean ± SEM values for four independent experiments, each with triplicate samples.
ATP-induced calcium mobilization
Mobilization of Ca2+ from cytosolic stores during activation by ATP and ADP is a well established consequence of P2Y1 receptor activation in other cell types. Chick myotubes in culture were preloaded with calcium green-1, and the mobilization of cytosolic Ca2+ during agonist stimulation was monitored by the induced fluorescence in real time in the fluorescence microscope. The cytosolic Ca2+ sharply increased across the myotube in <30 sec after the application of ATP (Fig. 4A). The time course of the Ca2+ signal is plotted in Figure 4B for three P2Y1receptor agonists. The calculated mean Ca2+ concentration changed from ∼0.09 μm in the resting state to ∼0.5 μm in the stimulated state. The general P2 antagonist, suramin, blocked the 2-MeSADP-induced cytosolic Ca2+ accumulation; it was also completely blocked by preloading the cells with heparin, an antagonist of inositol trisphosphate receptors (Wu et al., 2000). In addition, it was unchanged when the myotubes were cultured in Ca2+-free medium (Fig.4B). Hence, the source of the induced accumulation was confirmed to be the intracellular stores.
Fig. 4.
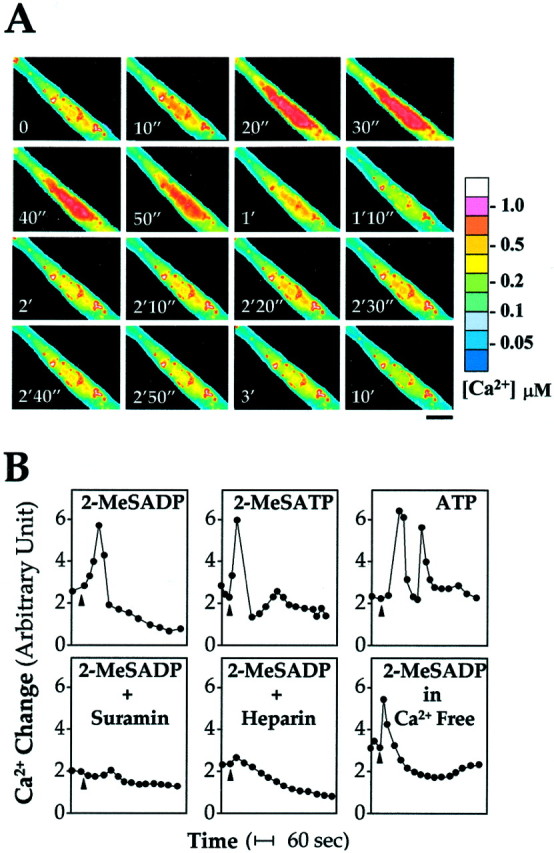
Intracellular Ca2+ mobilization in response to P2Y1 receptor agonists. A, Calcium green-1 fluorescence images of myotubes, captured at the times shown after 2-MeSADP (100 μm) application at 37°C. Calcium concentration (on the right) was calibrated from a standard curve. Scale bar, 20 μm. B, Responses obtained as shown in A were digitally integrated to compare on a relative scale of their time courses. ATP (100 μm) or 2-MeSADP or 2-MeSATP was applied at zero time (arrowhead). Co-applied suramin (100 μm) completely blocks the induced mobilization, as does preloaded heparin (5 mg/ml). The response is not affected by removal of Ca2+ from the medium. One representative experiment is shown, the results being completely replicated in three independent experiments.
It was found reproducibly that a doublet of Ca2+ waves was induced by ATP, in contrast to the single Ca2+ transient seen with 2-MeSADP and 2-MeSATP (Fig. 4B). Such multiple Ca2+ responses have been seen with ATP and UTP (but not with ADP) in rat hepatocytes, where both P2Y1 and P2Y2 receptors were shown to be expressed endogenously (Dixon et al., 2000).
Regulation of P2Y1 receptor mRNA synthesis in muscle and spinal cord
A single transcript (∼3.2 kb) for the P2Y1receptor was found in Northern blots of chick muscle and spinal cord RNA. This was true for embryonic day (E) 18 post-hatch and adult muscles (Fig. 5A), expanding the original finding of Webb et al. (1993). In E7 spinal cord, this P2Y1 mRNA was below the detectable level. The transcript level increased significantly from E10 until hatching. Post-hatch it was initially lower (per micrograms of total RNA present) but increased considerably up to approximately day 11 and remained equally high in the adult (Fig. 5C). Chick skeletal muscle showed a roughly similar profile to the spinal cord, but expression there was reproducibly highest in the adult (Fig. 5B). When normalized for the amount of RNA loaded onto the gel, there was (at all stages) approximately threefold higher expression of P2Y1 receptor mRNA in muscle than in the spinal cord.
Fig. 5.
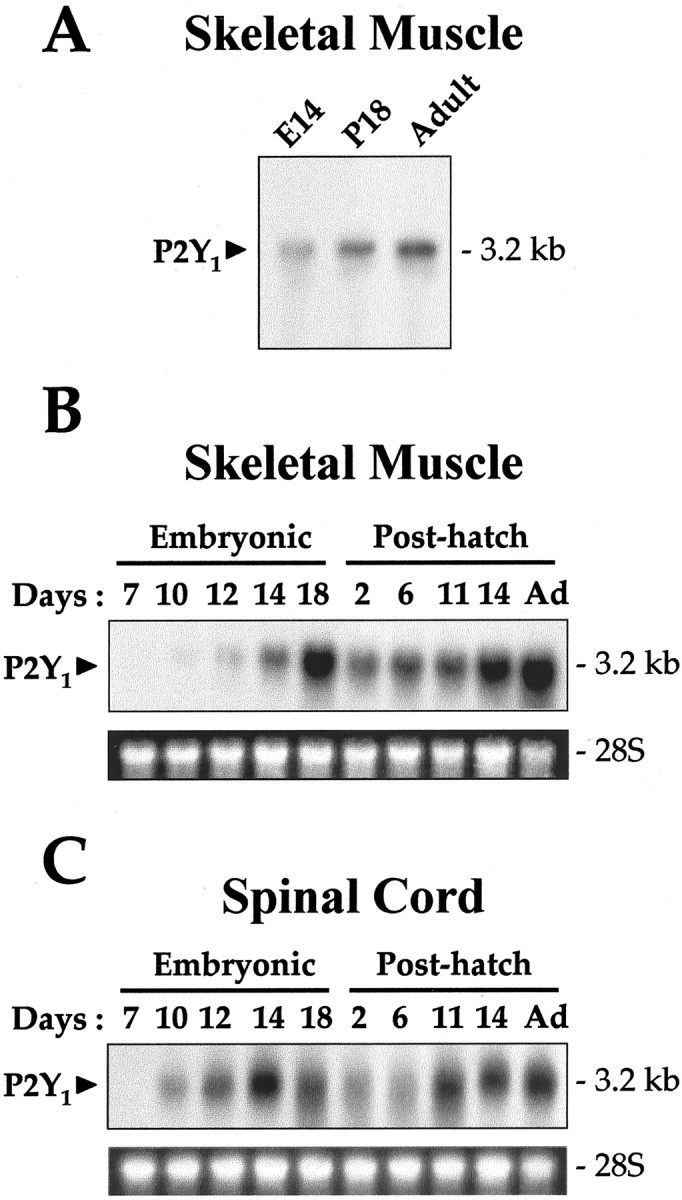
Changes in P2Y1 receptor mRNA levels in chick spinal cord and muscle during development. A, A low exposure of the blot to show a single P2Y1 receptor transcript at ∼3.2 kb is detectable in chicken muscle, from 14 d embryo (E14) or 18 d post-hatch (P18) or adult. Expression of the P2Y1receptor transcript was examined in chick gastrocnemius muscle (B) and spinal cord (C) from embryonic day 7 to adult (Ad; 6 months) as indicated. Ribosomal RNA loading markers (28S) are shown.
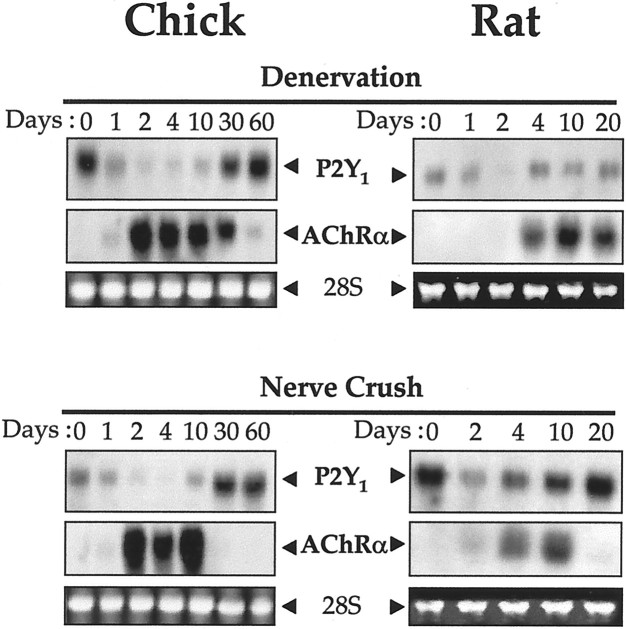
The level of P2Y1 receptor transcript was examined after denervation and subsequent nerve regeneration. In denervated gastrocnemius muscle of the 18-d-old chick, the level of P2Y1 receptor transcript was decreased ∼10-fold at day 2 after denervation (Fig. 6). This expression remained very low until ∼30 d after denervation, when reinnervation begins (Ip et al., 1996) in the system used. This restored the P2Y1 receptor mRNA expression thereafter (Fig. 6). To assess the effectiveness of the denervation procedure, the levels of AChR α-subunit transcript at ∼3.2 kb were also determined, and as is general in vertebrate skeletal muscles during denervation, this AChR subunit was greatly increased, here by >20-fold after 2 d (Fig. 6). This effect and the decline seen at ∼30 d after denervation for AChR α-subunit mRNA expression were similar to the previously reported findings for this muscle system (Ip et al., 1996). The same procedures were applied to the adult rat gastrocnemius muscle, with similar results (Fig. 6). In the rat a transcript was revealed at ∼3.6 kb, corresponding to the known rat P2Y1 receptor mRNA. The return of the P2Y1 receptor mRNA in the reinnervation phase began in the rat muscle before the AChR mRNA declined again. An alternative temporary interruption of the motor nerve influence, reversible nerve crush, was also used, in both the chick and the adult rat. The expression of the P2Y1 mRNA was decreased by the nerve crush (when assessed by densitometry) to a similar extent as before, by 80–90% in both species. The P2Y1 mRNA returned to the normal level in the regeneration phase, and this again occurred faster in the adult rat (Fig. 6). The effectiveness of neural interruption, and of the neural regeneration, was likewise confirmed in each crush case by the upregulation and later downregulation of the AChR α-subunit (Fig.6).
Fig. 6.
The transcript encoding the P2Y1receptor is downregulated in muscles after reversible nerve denervation or nerve crush. Gastrocnemius muscles of P18 chicks (left panels) or of adult rats (right panels) were treated as indicated. P2Y1 receptor mRNA (at ∼3.2 kb for chick and ∼3.6 kb for rat) is shown in Northern blots at the indicated days after operation. The AChR α-subunit (at ∼3.2 kb) in both chick and rat muscles served as an internal marker for effective nerve denervation or crush: that mRNA is greatly upregulated after denervation or nerve crush but declines to the original low level as reinnervation proceeds after the longer periods.
Localization of P2Y1 receptor protein at the neuromuscular junction
The localization of P2Y1 receptor protein in skeletal muscle was determined using a polyclonal antibody directed against the C-terminal portion of the chicken P2Y1 receptor. The antibody recognized a protein band with an apparent molecular mass of ∼50 kDa from cell membranes heterologously expressing the chicken P2Y1receptor (data not shown). The full characterization of this antibody will be published elsewhere. For control, the immunofluorescence observed with this antibody on each type of muscle section studied was completely blocked by the chicken P2Y1 C-terminal peptide that had been used as the immunogen (data not shown). The P2Y1 immunoreactivity was colocalized with the binding of tetramethyl-rhodamine-conjugated α-bungarotoxin, indicating the restricted localization of P2Y1receptor at the adult neuromuscular junctions (Fig.7A). That location of P2Y1 receptor was revealed not only in chicken muscle, but it was also colocalized with AChR in adult rat andXenopus muscles (Fig. 7A). Cross-reactivity was expected because the chicken sequence used as immunogen has 86% identity to that region in the rat P2Y1receptor.
Fig. 7.
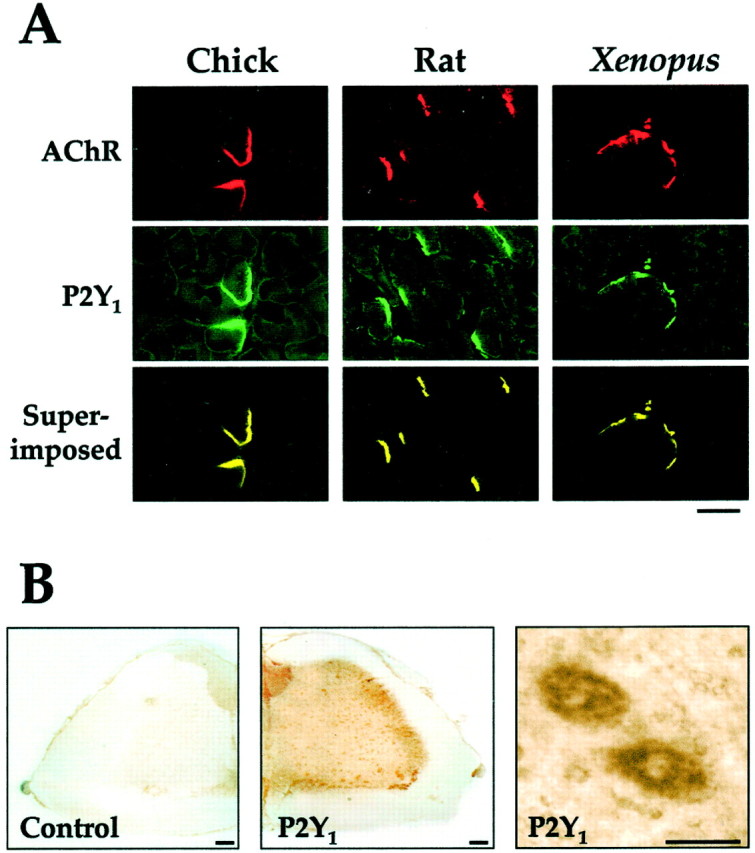
The localizations of P2Y1 receptor in muscles and motor neurons. A, Chick, rat, orXenopus muscle section (20 μm) was used. For each, the same field is shown stained by the anti-P2Y1-receptor antibody (green) or for AChR (red) by TMR-BuTX (10 nm) or superimposed (yellow). Controls are noted in Results. Scale bar, 20 μm. B, Spinal cord from adult chicken. Peroxidase-conjugated secondary antibody was used here to reveal (brown) the P2Y1 receptor sites in the ventral horn. In the control, the antibody was pretreated with an excess of the recombinant P2Y1 receptor antigen. A high-power magnification is shown for two adjacent positive cells seen in the stained low-power field. Scale bars, 500 μm (low power); 100 μm (high power).
The extent of colocalization was also analyzed during the development of chick muscles. From E10 to E19, there was no significant colocalization of P2Y1 receptors with the AChRs. However, a high degree of colocalization of P2Y1receptors with AChRs in chick muscles was revealed during later stages of development, from P20 to adulthood (Fig.8). A very weak staining by anti-P2Y1 receptor antibody could still be observed in some extra-junctional areas.
Fig. 8.
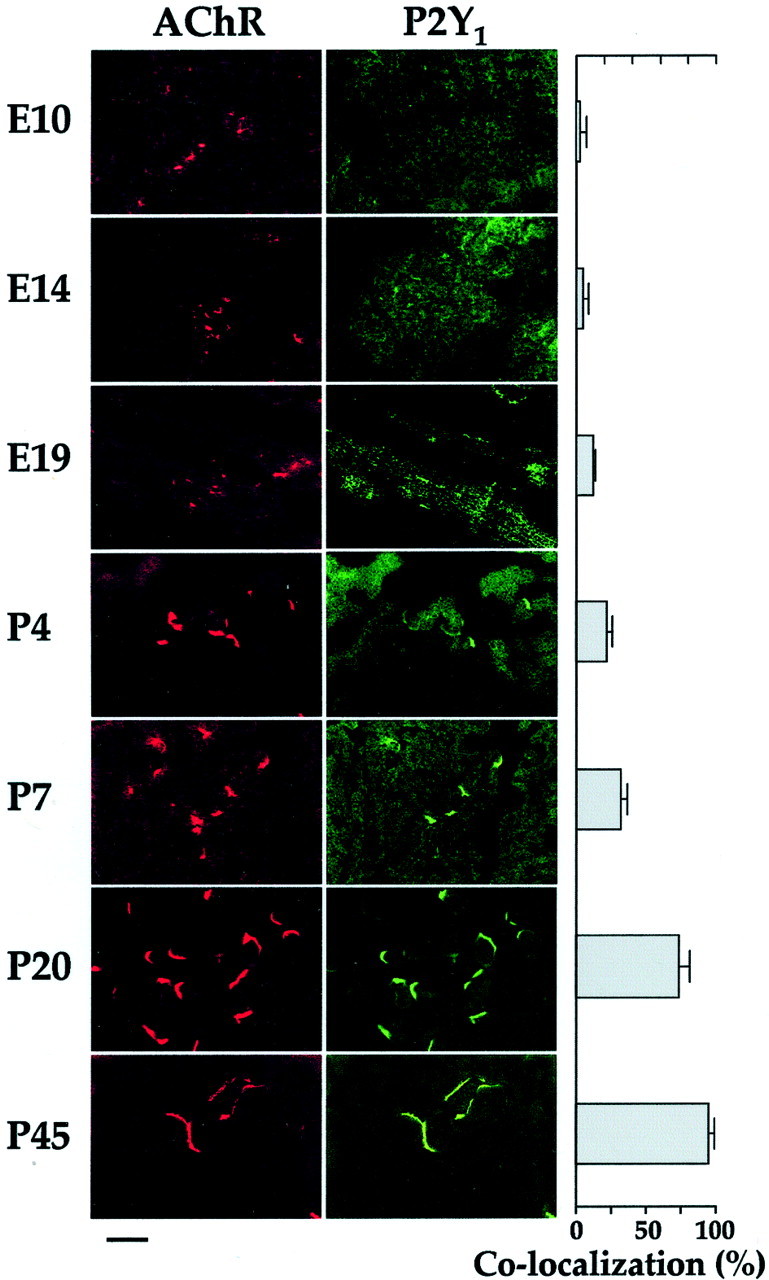
Colocalization of P2Y1 receptors and AChRs in chick muscle during development. Sections are double stained as in Figure 7. Chick pectoral muscle was used from embryonic day 10 (E10) to post-hatch day 45 (P45). The percentage of colocalization between P2Y1 receptors and AChRs was determined manually (see Materials and Methods) after revealing AChR staining first, and then shifting the red fluorescence filter to green for the detection of P2Y1 receptor staining. Data are mean ± SEM values, from counts over five or six fields from each of 10 sections from four animals. Scale bar, 10 μm.
Because an appreciable concentration of the P2Y1receptor mRNA was found in the developing and adult spinal cord (Fig.5B), the question arose as to whether it is located in the motor neurons there. If so, does it produce the protein for axonal transport to their terminals in the muscles, as a presynaptic receptor, which might contribute to the junctional concentration seen in Figures7 and 8. When sections of chick spinal cord were stained with the anti-P2Y1 antibody, motor neurons (having large nuclei and cell bodies) at the ventral horn of the cord were identified and seen to be strongly stained (Fig. 7B). Hence, at least a significant fraction of the P2Y1 receptor translated in the motor neurons is present in the cell bodies, being partly in the region of the somatic cell membranes and partly cytoplasmic. This therefore leaves open the possibility that some is transported to become a presynaptic receptor at the terminals in the muscles. Testing this would require higher resolution, in a future electron microscope immunolocalization study when an antibody resistant to the procedures involved is obtained.
Activation or overexpression of P2Y1 receptors induces AChR and AChE expression
A potential regulatory function of P2Y1receptors, to induce the expression of AChE and AChR, was explored in cultured myotubes. After the addition to the culture medium of 2-MeSADP (50 μm), the expression of transcripts encoding the AChR α-subunit rose to a maximum of 5.7-fold the level in the ligand-free control culture; for the mRNA of the AChE catalytic subunit (∼4.8 and ∼6.0 kb isoforms), a similar stimulation occurred (Fig.9A). A lag phase of ∼8 hr was seen with both, and a plateau was reached by 48 hr. The lag is consistent with activation of mRNA syntheses. The results were the same with 100 μm 2-MeSADP and similar at 10 μm except that the level of stimulation was then lower (data not shown). ATP, ADP, and 2-MeSATP, applied for 24 hr, all gave statistically significant elevations of these mRNAs (Fig.9B). The time course with 2-MeSATP was also tested and found to give results for each mRNA that were indistinguishable from those in Figure 9A (data not shown). Protection of the nucleotides from metabolism (see Materials and Methods) was applied throughout this period. The AChE protein levels were also determined during these incubations, using an AChE-specific antibody (Tsim et al., 1988). The content of the AChE catalytic subunit (apparent molecular mass ∼105 kDa) was increased up to 1.6-fold the control level by the application of ATP or 2-MeSATP, but not at all by adenosine (Fig. 9C). The level of the control protein α-tubulin (∼55 kDa) was not affected by the ligands (Fig. 9C). Suramin (100 μm) was able to block completely this ATP-induced AChE protein synthesis. Myotubes secrete some of their AChE into the medium (Rotundo, 1988; Choi et al., 1998), but all of the ATP-mediated increase in AChE protein here was within the cells, the level in the medium being unaltered (data not shown).
Fig. 9.
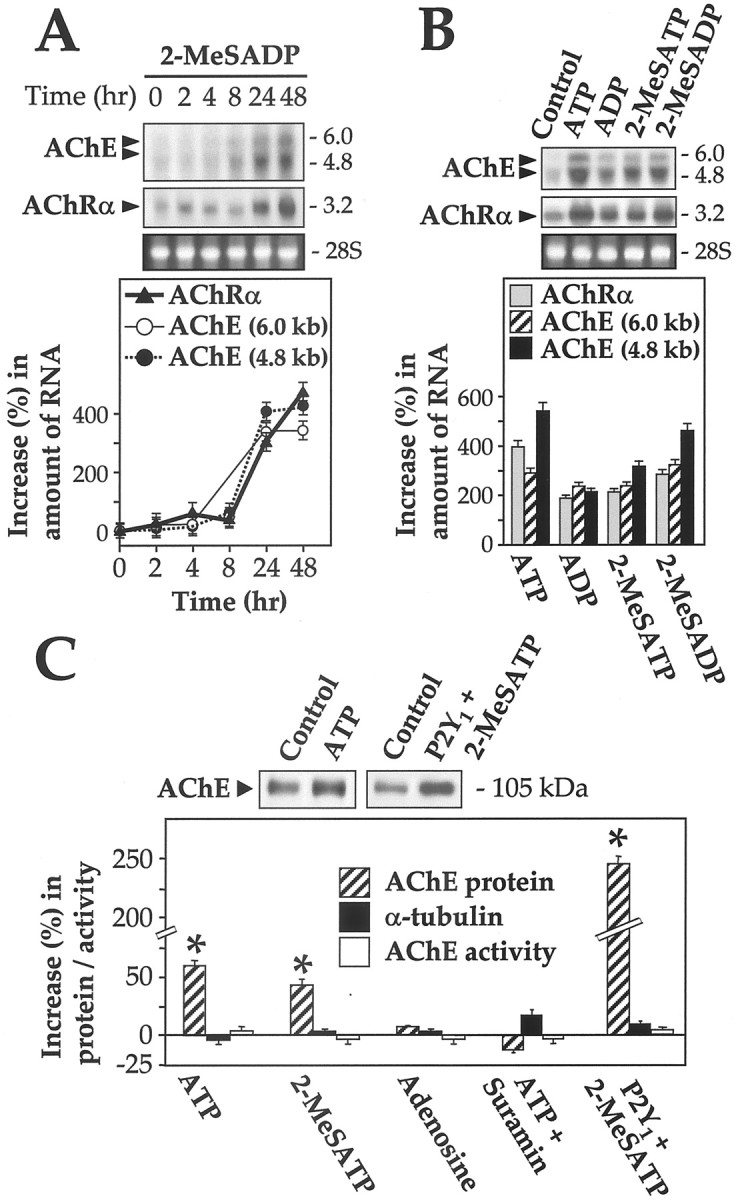
P2Y1 receptor agonists stimulate the expression of AChE catalytic subunit and AChR α-subunit. Agonists alone or together with antagonists were applied onto cultured myotubes (with 3 changes of medium and the appropriate ligand-regenerating enzyme system present, during the incubation at 37°C). A, The induction of transcripts encoding AChE (∼4.8 and ∼6.0 kb) and AChR α-subunit (∼3.2 kb) by 50 μm 2-MeSADP is shown as a function of time, and the quantitation from such blots is shown by densitometry. Total RNA applied per lane was 30 μg. Values are expressed as the percentage of the increase in each mRNA, above the control level, which is measured in samples from myotubes incubated in parallel without ligand and run in an adjacent lane. B, Transcripts of AChE and AChR α-subunit are increased by ATP, ADP, 2-MeSADP, and 2-MeSATP applications (each 50 μm, 24 hr treatment). Total RNA applied per lane was 30 μg. The bottom panel inB shows the quantitation from such blots by densitometry. Values are expressed as in A.C, After drug treatments of myotubes as above, or after ATP plus suramin (100 μm), total protein was extracted into SDS medium at 100°C and loaded (20 μg per lane) for Western blotting of AChE catalytic subunit (∼105 kDa) as shown in thetop panel (only representative lanes are shown there for clarity). This procedure was also applied (last lane) to myotubes that were supertransfected with cDNA encoding the chicken P2Y1 receptor (1 μg total DNA per well) and exposed to 50 μm 2-MeSATP as above. The stimulation by ATP or 2-MeSATP of AChE protein formation in these cells was quantitated on the blots by densitometry. Samples of the same ligand-treated cell cultures were extracted and assayed for AChE enzyme activity. Values are expressed as in A, for either the AChE enzymatic activity or the AChE protein content. As a marker of sample protein, α-tubulin protein (∼55 kDa) was detected by its antibody in the same gel lane in each case and quantitated similarly. Its amount was approximately the same in all samples, and it was unaffected by the ligands (data not shown). The same analyses were also performed on the myotubes that were supertransfected with a vector encoding the P2Y1 receptor cDNA (last set), with the control level being that in cells transfected with the vector alone and without ligand added. Changes from the control level are significant at p< 0.01 (asterisk) by t test. All data are mean ± SEM values from four independent experiments.
A more dramatic induction of AChE protein synthesis was found when the recombinant chicken P2Y1 receptor was overexpressed in the myotubes and again activated as before by 50 μm 2-MeSATP; the increase in the P2Y1 receptor content stimulated the production of AChE protein to severalfold the control level of cells transfected with the empty vector or the level in untransfected cells stimulated likewise by the agonist (Fig.9C). However, although the AChE protein level was increased by ATP treatment and further by P2Y1 receptor overexpression, the AChE enzymatic activity remained unchanged (Fig.9C). This behavior is parallel to that which we have found for AChE (Choi et al., 1996, 1998) in muscle cells exposed to CGRP, another regulatory factor released from the motor nerve terminals. CGRP was known previously to increase the expression of AChR subunits in cultured muscle cells (New and Mudge, 1986; Fontaine et al., 1987; Moss et al., 1991). When myotubes are stimulated by CGRP, AChE mRNA and protein increase as here, but the enzymatic activity does not (Choi et al., 1998).
To confirm that these stimulations arise directly from ATP-induced gene activation, the promoters of the human AChE catalytic subunit and the chicken AChR α-subunit in a vector were tagged downstream with the luciferase reporter gene, giving the pAChE-Luc and pAChRα-Luc constructs. When transfected into myotubes, the selective agonist 2-MeSATP induced the promoter activities in a dose-dependent manner (Fig. 10A). The extent and concentration-dependence of the 2-MeSATP activation were identical for the AChE and the AChR gene promoters (Fig.10A). The conclusion that this intracellular effect on the two specific gene promoters is mediated through the P2Y1 receptor on the cell membrane was further tested by using the aforementioned P2Y1receptor-specific antagonist, A3P5P. A3P5P (50 μm) gave a significant block of the action of either ATP or 2-MeSATP. The general P2 antagonist, suramin (50 μm), was also effective in blockade (Fig.10B). Furthermore, when either pAChE-Luc or pAChRα-Luc was cotransfected as above with cDNA encoding the chicken P2Y1 receptor and stimulated (with either 2-MeSATP or 2-MeSADP), a dramatic increase in luciferase activity was produced. This increase in luciferase activity was doubled (to reach approximately eightfold the basal level, for pAChE-Luc) when the P2Y1-plasmid concentration was increased from 1 to 2 μg per well (Fig. 10C). In this case, the effect on the AChE gene promoter was greater. Again, both AChE and AChR α-subunit gene activation induced by P2Y1receptor overexpression were blocked by suramin in a dose-dependent manner (Fig. 10C).
Fig. 10.
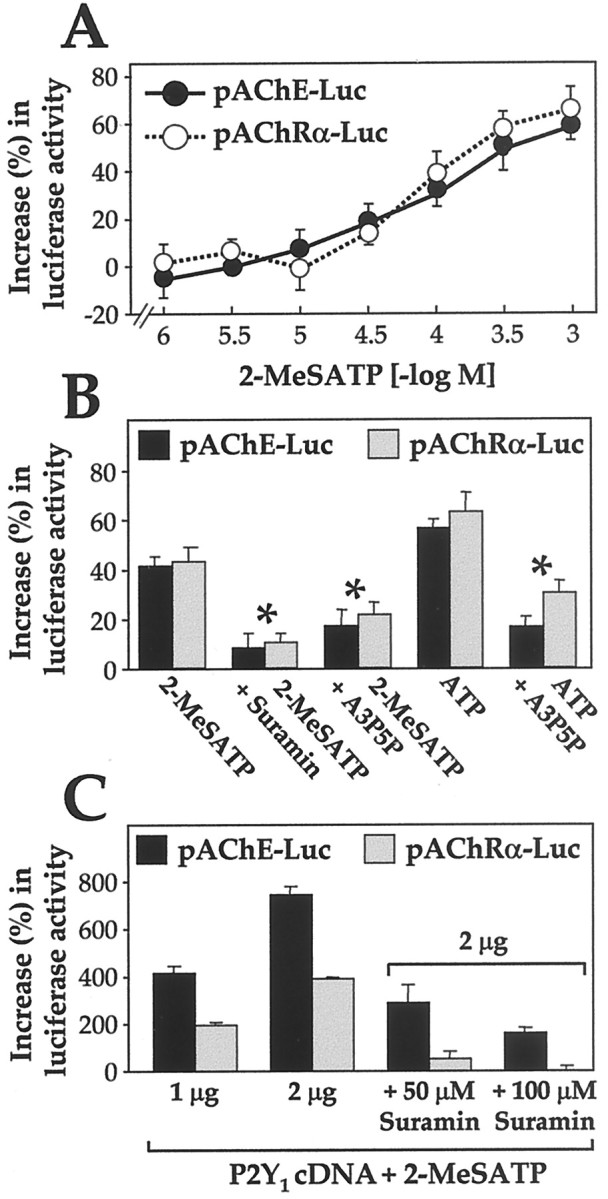
Promoters of the AChE catalytic subunit and AChR α-subunit genes are activated by ATP and 2-MeSATP. A, Two days after transfection of myotubes (in 12-well plates) with pAChE-Luc or pAChRα-Luc, 2-MeSATP concentrations were applied onto the myotubes for 24 hr in the protective conditions used for Figure 9. The luciferase activity was finally assayed in a lysate of the cells. Values are expressed as the percentage of the increase in activity, above the control level measured in samples from transfected myotubes incubated in parallel without ligand. B, Chick myotubes transfected with pAChE-Luc or pAChRα-Luc were treated with a P2Y1 receptor agonist (100 μm) and analyzed, and the results are expressed as in A. The agonist was present alone or with an antagonist, A3P5P (50 μm) or suramin (50 μm). UDP or UTP (50 μm) had no effect (data not shown). The antagonists reduced significantly (p < 0.01 by t test;asterisk) the effect of agonists. C, A vector encoding the P2Y1 receptor cDNA was cotransfected (1 or 2 μg DNA per well) with pAChE-Luc or pAChRα-Luc and after 2 d incubation and a wash was incubated as above with 2-MeSATP (50 μm). The activity is expressed relative to the control level of cells in which the P2Y1-cDNA vector was replaced by the vector alone. Induction of each gene expression is seen, which is approximately doubled when the cDNA concentration applied is doubled (first 2 sets). 2-MeSADP (50 μm) incubation of these P2Y1-transfected cells gave essentially the same values as with 2-MeSATP (data not shown). In the blocking experiment also shown (last 3 sets), 2 μg P2Y1-vector per well DNA was transfected, and the incubation with 2-MeSATP (50 μm) was made without (control) or with suramin (50 or 100 μm). All the data are mean ± SEM values for four independent experiments.
DISCUSSION
The P2Y1 receptor in skeletal muscle
P2Y1 is clearly an abundant receptor of skeletal muscle. The P2Y1 receptor mRNA was already known to be strongly expressed in chicken (Webb et al., 1993) and human (Ayyanathan et al., 1996) adult skeletal muscles; this mRNA was also noted (Meyer et al., 1999a) in chick embryo limb buds. Here we demonstrate the mRNA (Fig. 5) and protein (Figs. 7, 8) of the P2Y1 receptor in adult rat and amphibian muscles, and in chicken muscle cells at all stages from E10 to the adult. A functionally significant finding is that in developed muscle these P2Y1 receptors are localized to the neuromuscular junctions (Figs. 7, 8). This could provide a molecular basis for the postsynaptic potentiating effects of the ATP that is co-released with acetylcholine (see introductory remarks), effects that in avian and mammalian developed muscle show characteristics of a second messenger system (Henning, 1997). We could not distinguish in the light microscope between postsynaptic and presynaptic sites for the P2Y1 receptors, but we deduce that at least many of these are postsynaptic, because (1) here these receptors were also found in active form on myotubes in the absence of neurons; (2) although at neuromuscular junctions a presynaptic effect of ATP on acetylcholine release is known, this can be primarily accounted for by its hydrolysis by nucleotidases to adenosine, that effect being abolished by A1 receptor antagonists and mimicked by adenosine (Redman and Silinsky, 1994; Fu et al., 1997; Henning, 1997).
A role in the expression and maintenance of junctional AChR and AChE
Our results suggest that an important function of extracellular ATP and its P2Y1 receptor on skeletal muscle is to participate in the control of postsynaptic gene expression of AChE and AChR. Thus, application of P2Y1-selective agonists such as 2-MeSADP on myotubes induced the expression of the AChE catalytic subunit and of the AChR α-subunit. This was in parallel with the P2Y1 receptor-mediated stimulation of inositol phosphate formation and of Ca2+ mobilization from intracellular stores, as well as a newly detected P2Y effect, the output of H+ ions (Fig. 3). All of those responses were blocked by a P2Y1-specific antagonist. Second, introduction of an excess of the P2Y1 receptor greatly increased the expression of AChE protein. Third, the promoters of both the AChE and the AChRα genes exhibit positive control by P2Y1 receptor activation in a dose-dependent manner. Fourth, that latter effect was magnified, dose dependently, by overexpression of the recombinant chicken P2Y1receptor.
P2Y1 receptor expression rises strongly at the late embryonic period of neuromuscular junction formation and is highest in adult muscle, and the protein is concentrated at those junctions in adult life (Figs. 5, 7, 8). The neural release of ATP onto a muscle cell at the synapse is likewise continuous throughout post-embryonic life. Similarly, the synthesis in muscles of the junctional AChR and AChE remains high in the adult (Duclert and Changeux, 1995; Krejci et al., 1999). The evidence just summarized on the expression of AChE and AChR suggests, therefore, a trophic function of the extracellular ATP/P2Y1 receptor system in the maintenance of those two components of the junction. Such an action would have to be integrated with concurrent regulation by other nerve-derived agents, e.g., CGRP and neuregulin (see introductory remarks). The expression in the multinucleated mature muscle fibers of synaptic AChR and of the heteromeric synaptic form (“A12”) of AChE is compartmentalized to the subsynaptic nuclei (Sanes et al., 1991; Tsim et al., 1992; Jasmin et al., 1993; Duclert and Changeux, 1995; Krejci et al., 1999), implying that intracellular signals from the membrane to those nuclei (Schaeffer et al., 1998) mediate the maintenance of those synaptic forms. Furthermore, specific isoforms of PKC [a kinase family established to be activable in cells by the P2Y1 receptor (Sellers et al., 2001)] are also localized under the neuromuscular junctions (Hilgenberg et al., 1996). In support of such a maintenance role of the muscle P2Y1 receptor, we can refer to the significant observation of O'Malley et al. (1997) that continuous application of ATP stabilizes the rapidly degraded newly synthesized fraction of AChRs in rat myotubes.
Activities of nucleotides on the muscle cells attributable to the P2Y1 receptor
Certain other P2Y subtypes acting likewise might also be present in the innervated muscle, as is known by PCR detection in adult mammalian muscle (Webb et al., 1998) but unexplored in the chicken because of limited sequence information there. However, the P2Y1 selectivity, discussed above, of agonists and an antagonist, A3P5P, which are effective in all of the gene activation effects, plus the lack of gene effects with UTP or UDP, minimized any contribution from other subtypes, whereas the anti-P2Y1 antibody and the effects of P2Y1 overexpression provided positive identification.
Nevertheless, some features of the inositol phosphate and H+ ion responses (Figs. 2, 3) showed that P2Y receptor(s) other than P2Y1 is indeed present on the myotubes. The response in this case to UTP, together with the higher activity of ATP relative to 2-MeSATP and 2-MeSADP, can be explained by a lesser contribution from a P2Y2and/or P2Y4 receptor, as noted in Results. The above-mentioned ligand specificity criteria also mean that there is little or no contribution here from the P2X ion channel ATP receptors, which have been detected (see introductory remarks) on chick myotubes. Furthermore, channel responsiveness to ATP disappears in chick embryonic muscles by E17 (Wells et al., 1995), and in parallel the P2X receptors, earlier prominent (by antibody staining), decline (Meyer et al., 1999b; Bo et al., 2000). In rat embryonic muscles, likewise, the P2X receptors are embryonic and all are lost by P14 (Ryten et al., 2001). This is the converse of the chick muscle P2Y1 receptor expression (Fig. 5). The muscle P2Y1 receptor develops later (Fig. 5) and, alone of the nucleotide receptors known in avian and mammalian skeletal muscles, will be available to transduce long-term trophic maintenance functions of ATP.
Denervation effects and relation to AChE and AChR expression
The rapid loss found after denervation or nerve crush, in both chicken and rat muscle, of the P2Y1 receptor transcript is a parallel to the loss of the specific synaptic (collagen-tailed) AChE isoform in chicken (Silman et al., 1979) or mammalian muscle (Weinberg and Hall, 1979; Lai et al., 1986) and its mRNAs (Michel et al., 1994), which occurs after mature muscles are denervated. Both P2Y1 receptor and synaptic AChE also are restored after reinnervation. The P2Y1disappearance could be attributable to loss of a trophic action of ATP, or to loss of another nerve-derived regulator such as CGRP if that can act on P2Y1 receptor synthesis as it does (Choi et al., 1998) (and see above) on the synaptic isoform of AChE. ATP and CGRP are thus seen to have a trophic effect in common: CGRP induces (in cultured myotubes) the upregulation of AChE protein but in an inactive form (Choi et al., 1998), as ATP did (Fig. 9) here. Pools of inactive and active AChE are known to occur in native muscles (Chatel et al., 1994). The reason is unclear, but this is not unique, and a reserve of incompletely folded protein has been suggested, because heat shock (which can release chaperone activity) recovered some AChE activity in chick myotubes (Eichler and Silman, 1995). However, the high proportion of AChE in the inactive pool, produced in myotubes during stimulation by ATP as here or by CGRP (Choi et al., 1998), may be caused by the lack of motor innervation, because ∼80% of AChE protein made normally in chick myotubes does not mature to the active form (Rotundo, 1988). Factor(s) for AChE maturation may be more available in mature innervated muscle.
Although denervation obviously removes the synaptic ATP influence, the muscle AChRα expression, in an apparent paradox here, is increased greatly. This is, however, a long-known consequence in vertebrates, which is attributable to an independent and much stronger opposing effect of denervation, i.e., the liberation from the action potential-mediated suppression of AChR synthesis in all the extrasynaptic myonuclei (Duclert and Changeux, 1995). This is an overwhelming effect: the content of AChR mRNAs in the muscle can increase up to 100-fold after denervation (Moscoso et al., 1995). For synaptic AChE and for P2Y1 receptors, there is no such de-repression of extra-junctional synthesis, so the loss of neural influence becomes evident. A working hypothesis, to be tested, is that ATP is in the same category as neuregulin (Moscoso et al., 1995;Sandrock et al., 1997; Schaeffer et al., 1998) and CGRP (Duclert and Changeux, 1995), which act via their postsynaptic receptors to activate factors locally to enhance transcription of AChR genes specifically in the adjacent subsynaptic nuclei.
Footnotes
This work was supported by grants from the Research Grants Council of Hong Kong [Hong Kong University of Science and Technology (HKUST) 6099/98M, 6112/00M, and 2/99C] and Biotechnology Research Institute at HKUST (K.W.K.T.) and from the Wellcome Trust (E.A.B.). We thank Drs. Kathy Luo, Donald Chang, and Fanny Ip from HKUST for discussion and assistance with this work, and Dr. J. Sanes of Washington University (St. Louis, MO) and Prof. H. Soreq of The Hebrew University of Jerusalem (Jerusalem, Israel) for providing AChR α-subunit and AChE promoter DNAs.
R.C.Y.C. and M.L.S.M. contributed equally to this study.
Correspondence should be addressed to Dr. Karl W. K. Tsim, Department of Biology, The Hong Kong University of Science and Technology, Clear Water Bay Road, Hong Kong, China. E-mail:botsim@ust.hk.
REFERENCES
- 1.Akasu T, Hirai K, Koketsu K. Increase of acetylcholine receptor sensitivity by adenosine triphosphate: a novel action of ATP on ACh-sensitivity. Br J Pharmacol. 1981;74:505–507. doi: 10.1111/j.1476-5381.1981.tb09997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyanathan K, Webb TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Simon J. An elusive receptor finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol Sci. 2001;22:388–391. doi: 10.1016/s0165-6147(00)01759-4. [DOI] [PubMed] [Google Scholar]
- 4.Ben Aziz-Aloya R, Seidman S, Timberg R, Sternfeld M, Zakut H, Soreq H. Expression of a human acetylcholinesterase promoter-reporter construct in developing neuromuscular junctions of Xenopus embryos. Proc Natl Acad Sci USA. 1993;90:2471–2475. doi: 10.1073/pnas.90.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bo X, Schoepfer R, Burnstock G. Molecular cloning and characterization of a novel ATP P2X receptor subtype from embryonic chick skeletal muscle. J Biol Chem. 2000;275:14401–14407. doi: 10.1074/jbc.275.19.14401. [DOI] [PubMed] [Google Scholar]
- 6.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang DC. Experimental strategies in efficient transfection of mammalian cells: electroporation. Electroporation Methods Mol Biol. 1997;62:307–318. doi: 10.1385/0-89603-480-1:307. [DOI] [PubMed] [Google Scholar]
- 9.Chatel JM, Eichler J, Vallette FM, Bon S, Massoulié J, Grassi J. Two-site immunoradiometric assay of chicken acetylcholinesterase: active and inactive molecular forms in brain and muscle. J Neurochem. 1994;63:1111–1118. doi: 10.1046/j.1471-4159.1994.63031111.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi RCY, Leung PWY, Dong TTX, Wan DCC, Tsim KWK. Calcitonin gene-related peptide increases the expression of acetylcholinesterase in cultured chick myotubes. Neurosci Lett. 1996;217:165–168. [PubMed] [Google Scholar]
- 11.Choi RCY, Yung LY, Dong TTX, Wan DCC, Wong YH, Tsim KWK. The calcitonin gene-related peptide-induced acetylcholinesterase synthesis in cultured chick myotubes is mediated by cyclic AMP. J Neurochem. 1998;71:152–160. doi: 10.1046/j.1471-4159.1998.71010152.x. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JC, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duclert A, Changeux JP. Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol Rev. 1995;75:339–368. doi: 10.1152/physrev.1995.75.2.339. [DOI] [PubMed] [Google Scholar]
- 14.Eichler J, Silman I. The activity of an endoplasmic reticulum-localized pool of acetylcholinesterase is modulated by heat shock. J Biol Chem. 1995;270:4466–4472. doi: 10.1074/jbc.270.9.4466. [DOI] [PubMed] [Google Scholar]
- 15.Ewald DA. Potentiation of postjunctional cholinergic sensitivity of rat diaphragm muscle by high-energy-phosphate adenine nucleotides. J Membr Biol. 1976;29:47–65. doi: 10.1007/BF01868951. [DOI] [PubMed] [Google Scholar]
- 16.Filippov AK, Brown DA, Barnard EA. The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br J Pharmacol. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischbach GD. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972;28:407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine B, Klarsfeld A, Changeux JP. Calcitonin gene-related peptide and muscle activity regulate acetylcholine receptor α-subunit mRNA levels by distinct intracellular pathways. J Cell Biol. 1987;105:1337–1342. doi: 10.1083/jcb.105.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu WM, Chan YH, Lee KF, Liou JC. Regulation of quantal transmitter secretion by ATP and protein kinase at developing neuromuscular synapses. Eur J Neurosci. 1997;9:676–685. doi: 10.1111/j.1460-9568.1997.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 20.Häggblad J, Heilbronn E. P2-receptor-stimulated phosphoinositide turnover in chick myotubes. FEBS Lett. 1988;235:133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- 21.Henning RH. Purinoceptors in neuromuscular transmission. Pharmacol Ther. 1997;74:115–128. doi: 10.1016/s0163-7258(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 22.Hilgenberg L, Yearwood S, Milstein S, Miles K. Neural influence on protein kinase C isoform expression in skeletal muscle. J Neurosci. 1996;16:4994–5003. doi: 10.1523/JNEUROSCI.16-16-04994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horovitz O, Spitsberg V, Salpeter MM. Regulation of acetylcholine receptor synthesis at the level of translation in rat primary muscle cells. J Cell Biol. 1989;108:1817–1822. doi: 10.1083/jcb.108.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip FCF, Fu AKY, Tsim KWK, Ip NY. Differential expression of ciliary neurotrophic factor receptor in skeletal muscle of chick and rat after nerve injury. J Neurochem. 1996;67:1607–1612. doi: 10.1046/j.1471-4159.1996.67041607.x. [DOI] [PubMed] [Google Scholar]
- 25.Israel M, Dunant Y. Acetylcholine release and the cholinergic genomic locus. Mol Neurobiol. 1998;16:1–20. doi: 10.1007/BF02740600. [DOI] [PubMed] [Google Scholar]
- 26.Jasmin BJ, Lee RK, Rotundo RL. Compartmentalization of acetylcholinesterase mRNA and enzyme at the vertebrate neuromuscular junction. Neuron. 1993;11:467–477. doi: 10.1016/0896-6273(93)90151-g. [DOI] [PubMed] [Google Scholar]
- 27.Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 28.King BF, Townsend-Nicholson A. Recombinant P2Y receptors: the UCL experience. J Auton Nerv Syst. 2000;81:164–170. doi: 10.1016/s0165-1838(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 29.Kolb HA, Wakelam MJ. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983;303:621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- 30.Krejci E, Legay C, Thomine S, Sketelj J, Massoulié J. Differences in expression of acetylcholinesterase and collagen Q control the distribution and oligomerization of the collagen-tailed forms in fast and slow muscles. J Neurosci. 1999;19:10672–10679. doi: 10.1523/JNEUROSCI.19-24-10672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai J, Jedrzejczyk J, Pizzey JA, Green D, Barnard EA. Neural control of the forms of acetylcholinesterase in slow mammalian muscles. Nature. 1986;321:72–74. doi: 10.1038/321072a0. [DOI] [PubMed] [Google Scholar]
- 32.Lu Z, Smith DO. Adenosine 5′triphosphate increases acetylcholine channel opening frequency in rat skeletal muscle. J Physiol (Lond) 1991;436:45–56. doi: 10.1113/jphysiol.1991.sp018538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999a;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Meyer MP, Gröschel-Stewart U, Robson T, Burnstock G. Expression of two ATP-gated ion channels, P2X5 and P2X6, in developing chick skeletal muscle. Dev Dyn. 1999b;216:442–449. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<442::AID-DVDY12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Michel RN, Vu CQ, Tetzlaff W, Jasmin BJ. Neural regulation of acetylcholinesterase mRNAs at mammalian neuromuscular synapses. J Cell Biol. 1994;127:1061–1069. doi: 10.1083/jcb.127.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscoso LM, Chu GC, Gautam M, Noakes PG, Merlie JP, Sanes JR. Synapse-associated expression of an acetylcholine receptor-inducing protein, ARIA/heregulin, and its putative receptors, ErbB2 and ErbB3, in developing mammalian muscle. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- 38.Moss SJ, Harkness PC, Mason IJ, Barnard EA, Mudge AW. Evidence that CGRP and cAMP increase transcription of AChR alpha-subunit gene, but not of other subunit genes. J Mol Neurosci. 1991;3:101–108. doi: 10.1007/BF02885531. [DOI] [PubMed] [Google Scholar]
- 39.New HV, Mudge AW. Calcitonin gene-related peptide regulates muscle acetylcholine receptor synthesis. Nature. 1986;323:809–811. doi: 10.1038/323809a0. [DOI] [PubMed] [Google Scholar]
- 40.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 41.O'Malley JP, Moore CP, Salpeter MM. Stabilization of acetylcholine receptors by exogenous ATP and its reversal by cAMP and calcium. J Cell Biol. 1997;138:159–165. doi: 10.1083/jcb.138.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacology. Proc Natl Acad Sci USA. 1995;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitchford S, De Moor K, Glaeser BS. Nerve growth factor stimulates rapid metabolic responses in PC12 cells. Am J Physiol. 1995;268:936–943. doi: 10.1152/ajpcell.1995.268.4.C936. [DOI] [PubMed] [Google Scholar]
- 44.Pun S, Tsim KWK. Anti-sense agrin cDNA transfection blocks neuroblastoma cell-induced acetylcholine receptor aggregation when co-cultured with myotubes. Mol Cell Neurosci. 1997;10:87–99. doi: 10.1006/mcne.1997.0637. [DOI] [PubMed] [Google Scholar]
- 45.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 46.Redman RS, Silinsky EM. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol (Lond) 1994;477:117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotundo RL. Biogenesis of acetylcholinesterase molecular forms in muscle. Evidence for a rapidly turning over, catalytically inactive precursor pool. J Biol Chem. 1988;263:19398–19406. [PubMed] [Google Scholar]
- 48.Ryten M, Hoebertz A, Burnstock G. Sequential expression of three receptor subtypes of extracellular ATP in developing rat skeletal muscle. Dev Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, Ed 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 50.Sandrock AW, Jr, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 51.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 52.Sanes JR, Johnson YR, Kotzbauer PT, Mudd J, Hanley T, Martinou JC, Merlie JP. Selective expression of an acetylcholine receptor-lac Z transgene in synaptic nuclei of adult muscle fibers. Development. 1991;113:1181–1191. doi: 10.1242/dev.113.4.1181. [DOI] [PubMed] [Google Scholar]
- 53.Schaeffer L, Duclert N, Huchet-Dymanus M, Changeux JP. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 1998;17:3078–3090. doi: 10.1093/emboj/17.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PPA, Barnard EA. Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem. 2001;276:16379–16390. doi: 10.1074/jbc.M006617200. [DOI] [PubMed] [Google Scholar]
- 55.Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J Physiol (Lond) 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silman I, di Giamberardino L, Lyles L, Couraud JY, Barnard EA. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979;280:160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- 57.Simon J, Webb TE, King BF, Burnstock G, Barnard EA. Characterization of a recombinant P2Y purinoceptor. Eur J Pharmacol. 1995;291:281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 58.Simon J, Vigne P, Eklund KM, Michel AD, Frelin C, Barnard EA. Activity of adenosine diphosphates and triphosphates on a P2YT receptor in brain capillary endothelial cells. Br J Pharmacol. 2001;132:173–182. doi: 10.1038/sj.bjp.0703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas SA, Zawisa MJ, Lin X, Hume RI. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. Br J Pharmacol. 1991;103:1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokuyama Y, Hara M, Jones EMC, Fan Z, Bell GI. Cloning of rat and mouse P2Y receptors. Biochem Biophys Res Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- 61.Tsim KWK, Randall WR, Barnard EA. An asymmetric form of muscle acetylcholinesterase contains three subunits types and two enzymatic activities in one molecule. Proc Natl Acad Sci USA. 1988;85:1262–1266. doi: 10.1073/pnas.85.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsim KWK, Greenberg I, Rimer M, Randall WR, Salpeter MM. Transcripts for AChR and AChE show distribution differences in cultured chick muscle cells. J Cell Biol. 1992;118:1201–1212. doi: 10.1083/jcb.118.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsim KWK, Choi RCY, Dong TTX, Wan DCC. A globular, not asymmetric, form of AChE is expressed in chick motor neurons: down-regulation towards maturity and after denervation. J Neurochem. 1997;68:479–487. doi: 10.1046/j.1471-4159.1997.68020479.x. [DOI] [PubMed] [Google Scholar]
- 64.Tsu RC, Chan JS, Wong YH. Regulation of multiple effectors by the cloned delta-opioid receptor: stimulation of phospholipase C and type II adenylyl cyclase. J Neurochem. 1995;64:2700–2707. doi: 10.1046/j.1471-4159.1995.64062700.x. [DOI] [PubMed] [Google Scholar]
- 65.Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 66.Webb TE, Henderson D, King BF, Wang S, Simon J, Bateson AN, Burnstock G, Barnard EA. A novel G protein-coupled P2 purinoceptor (P2Y3) activated preferentially by nucleoside. Mol Pharmacol. 1996;50:258–265. [PubMed] [Google Scholar]
- 67.Webb TE, Henderson DJ, Roberts JA, Barnard EA. Molecular cloning and characterization of the rat P2Y4 receptor. J Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg CB, Hall ZW. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979;68:631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- 69.Wells DG, Zawisa MJ, Hume RI. Changes in responsiveness to extracellular ATP in chick skeletal muscle during development and upon denervation. Dev Biol. 1995;172:585–590. doi: 10.1006/dbio.1995.8062. [DOI] [PubMed] [Google Scholar]
- 70.Wu J, Kamimura N, Takeo T, Suga S, Wakui M, Maruyama T, Mikoshiba K. 2-Aminoethoxydiphenyl borate modulates kinetics of intracellular Ca2+ signals mediated by inositol 1,4,5-trisphosphate-sensitive Ca2+ stores in single pancreatic acinar cells of mouse. Mol Pharmacol. 2000;58:1368–1374. doi: 10.1124/mol.58.6.1368. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann H. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends Pharmacol Sci. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]