Abstract
Acute brain ischemia causes changes in several neural networks and related cortico-subcortical excitability, both in the affected area and in the apparently spared contralateral hemisphere. The modulation of these processes through modern techniques of noninvasive brain stimulation, namely repetitive transcranial magnetic stimulation (rTMS), has been proposed as a viable intervention that could promote post-stroke clinical recovery and functional independence. This review provides a comprehensive summary of the current evidence from the literature on the efficacy of rTMS applied to different clinical and rehabilitative aspects of stroke patients. A total of 32 meta-analyses published until July 2019 were selected, focusing on the effects on motor function, manual dexterity, walking and balance, spasticity, dysphagia, aphasia, unilateral neglect, depression, and cognitive function after a stroke. Only conventional rTMS protocols were considered in this review, and meta-analyses focusing on theta burst stimulation only were excluded. Overall, both HF-rTMS and LF-rTMS have been shown to be safe and well-tolerated. In addition, the current literature converges on the positive effect of rTMS in the rehabilitation of all clinical manifestations of stroke, except for spasticity and cognitive impairment, where definitive evidence of efficacy cannot be drawn. However, routine use of a specific paradigm of stimulation cannot be recommended yet due to a significant level of heterogeneity of the studies in terms of protocols to be set and outcome measures that have to be used. Future studies need to preliminarily evaluate the most promising protocols before going on to multicenter studies with large cohorts of patients in order to achieve a definitive translation into daily clinical practice.
Keywords: neuroplasticity, neurorehabilitation, noninvasive brain stimulation, stroke
Introduction
Background
Stroke is a common acute neurovascular disorder that causes disabling long-term limitations to daily living activities. The most common consequence of a stroke is motor deficit of variable degree,1 although nonmotor symptoms are also relevant and often equally disabling.2 To date, to the best of the authors’ knowledge, there is no validated treatment that is able to restore the impaired functions by a complete recovery of the damaged tissue. Indeed, stroke management basically consists of reducing the initial ischemia in the penumbra, preventing future complications, and promoting a functional recovery using physiotherapy, speech therapy, occupational therapy, and other conventional treatments.3,4
Ischemic damage is associated with significant metabolic and electrophysiological changes in cells and neural networks involved in the affected area. From a pure electrophysiological perspective, however, beyond the affected area, there is a local shift in the balance between the inhibition and excitation of both the affected and contralateral hemisphere, consisting of increased excitability and disinhibition (reduced activity of the inhibitory circuits).3,5 In addition, subcortical areas and spinal regions may be altered.3,5 In particular, the role of the uninjured hemisphere seems to be of utmost significance in post-stroke clinical and functional recovery.
Different theoretical models have been proposed to explain the adaptive response of the brain to acute vascular damage. According to the vicariation model, the activity of the unaffected hemisphere contributes to the functional recovery after a stroke through the replacement of the lost functions of the affected areas. The interhemispheric competition model considers the presence of mutual inhibition between the hemispheres, and the damage caused by a stroke disrupts this balance, thus producing a reduced inhibition of the unaffected hemisphere by the affected side. This results in increased inhibition of the affected hemisphere by the unaffected side. More recently, a new model, called bimodal balance recovery, has been proposed.3,5 It introduces the concept of a structural reserve, which describes the extent to which the nondamaged neural pathways contribute to the clinical recovery. The structural reserve determines the prevalence of the interhemispheric imbalance over vicariation. When the structural reserve is high, the interhemispheric competition model can predict the recovery better than the vicariation model, and vice versa.3
Repetitive transcranial magnetic stimulation
One of the proposed interventions to improve stroke recovery, by the induction of neuromodulation phenomena, is based on methods of noninvasive brain stimulation. Among them, transcranial magnetic stimulation (TMS) is a feasible and painless neurophysiological technique widely used for diagnostic, prognostic, research, and, when applied repetitively, therapeutic purposes.6–9 By electromagnetic induction, TMS generates sub or suprathreshold currents in the human cortex in vivo and in real time.10,11
The most common stimulation site is the primary motor cortex (M1), that generates motor evoked potentials (MEPs) recorded from the contralateral muscles through surface electromyography electrodes.11 The intensity of TMS, measured as a percentage of the maximal output of the stimulator, is tailored to each patient based on the motor threshold (MT) of excitability. Resting MT (rMT) is found when the target muscle is at rest, it is defined as the minimal intensity of M1 stimulation required to elicit an electromyography response with a peak-to-peak amplitude > 50 µV in at least 5 out of 10 consecutive trials.11 Alternatively TMS MTAT 2.0 software (http://www.clinicalresearcher.org/software.htm) is a free tool for TMS researchers and practitioners. It provides four adaptive methods based on threshold-tracking algorithms with the parameter estimation by sequential testing, using the maximum-likelihood strategy for estimating MTs. Active MT (aMT) is obtained during a tonic contraction of the target muscle at approximately 20% of the maximal muscular strength.11
The rMT is considered a basic parameter in providing the global excitation state of a central core of M1 neurons.11 Accordingly, rMT is increased by drugs blocking the voltage-gated sodium channels, where the same drugs may not have an effect on the gamma-aminobutyric acid (GABA)-ergic functions. In contrast, rMT is reduced by drugs increasing glutamatergic transmission not mediated by the N-methyl-D-aspartate (NMDA) receptors, suggesting that rMT reflects both neuronal membrane excitability and non-NMDA receptor glutamatergic neurotransmission.12 Finally, the MT increases, being often undetectable, when a substantial portion of M1 or the cortico-spinal tract is damaged (i.e. by stroke or motor neuron disease), and decreases when the motor pathway is hyperexcitable (such as epilepsy).13
Repetitive (rTMS) is a specific stimulation paradigm characterized by the administration of a sequence of consecutive stimuli on the same cortical region, at different frequencies and inter sequence intervals. As known, rTMS can transiently modulate the excitability of the stimulated cortex, with both local and remote effects outlasting the stimulation period. Conventional rTMS modalities include high-frequency (HF-rTMS) stimulation (>1 Hz) and low-frequency (LF-rTMS) stimulation (⩽1 Hz).11 High-frequency stimulation typically increases motor cortex excitability of the stimulated area, whereas low-frequency stimulation usually produces a decrease in excitability.14 The mechanisms by which rTMS modulates the brain are rather complex, although they seem to be related to the phenomena of long-term potentiation (LTP) and long-term depression (LTD).15
When applied after a stroke, rTMS should ideally be able to suppress the so called ‘maladaptive plasticity’16,17 or to enhance the adaptive plasticity during rehabilitation. These goals can be achieved by modulating the local cortical excitability or modifying connectivity within the neuronal networks.10
rTMS in stroke rehabilitation: an overview
According to the latest International Federation of Clinical Neurophysiology (IFCN) guidelines on the therapeutic use of rTMS,10 there is a possible effect of LF-rTMS of the contralesional motor cortex in post-acute motor stroke, and a probable effect in chronic motor stroke. An effect of HF-rTMS on the ipsilesional motor cortex in post-acute and chronic motor stroke is also possible.
The potential role of rTMS in gross motor function recovery after a stroke has been assessed in a recent comprehensive systematic review of 70 studies by Dionisio and colleagues.18 The majority of the publications reviewed report a role of rTMS in improving motor function, although some randomized controlled trials (RCTs) were not able to confirm this result,19–23 as shown by a recent large randomized, sham-controlled, clinical trial of navigated LF-rTMS.24 It has also been suggested that rTMS can specifically improve manual dexterity,10 which is defined as the ability to coordinate the fingers and efficiently manipulate objects, and is of crucial importance for daily living activities.25 Notably, most of the studies focused on motor impairment in the upper limbs, whereas limited data is available on the lower limbs.18 Walking and balance are frequently impaired in stroke patients and significantly affect the quality of life (QoL),26,27 and rTMS might represent a valid aid in the recovery of these functions.28,29 Spasticity is another common complication after a stroke, consisting of a velocity-dependent increase of muscular tone,30 and for which rTMS has been proposed as a rehabilitation tool.31
Dysphagia is highly common in stroke patients, it impairs the global clinical recovery, and predisposes to complications.32 It has been pointed out that rTMS targeting the M1 area representing the muscles involved in swallowing may contribute to the treatment of post-stroke dysphagia.33
Nonmotor deficit is also a relevant post-stroke disability that negatively impacts the QoL. Aphasia is a very common consequence of stroke, affecting approximately 30% of stroke survivors and significantly limiting rehabilitation.34 According to the IFCN guidelines, to date, there is no recommendation for LF-rTMS of the contralesional right inferior frontal gyrus (IFG). Similarly, no recommendation for HF-rTMS or intermittent theta burst stimulation (TBS) of the ipsilesional left IFG or dorsolateral prefrontal cortex (DLPFC) in Broca’s aphasia has been currently approved.10 The same is true for LF-rTMS of the right superior temporal gyrus in Wernicke’s aphasia.10
Neglect is the incapacity to respond to tactile or visual contralateral stimuli that are not caused by a sensory-motor deficit.35 Although hard to treat, rTMS has been proposed as a tool for neglect rehabilitation.36 However, the IFCN guidelines state that currently there is no recommendation for LF-rTMS of the contralesional left posterior parietal cortex, or for HF-rTMS of the ipsilesional right posterior parietal cortex.10 In a recent systematic review, most of the included studies supported the use of TMS for the rehabilitation of aphasia, dysphagia, and neglect, although the heterogeneity of stimulation protocols did not allow definitive conclusions to be drawn.37
Post-stroke depression is a relevant complication of cerebrovascular diseases.38 The role of rTMS in the management of major depressive disorders is well documented,39,40 and currently, rTMS is internationally approved and indicated for the treatment of major depression in adults with antidepressant medication resistance, and in those with a recurrent course of illness, or in cases of moderate-to-severe disease severity.39 In major depression disorders, according to the IFCN guidelines, there is a clear antidepressant effect of HF-rTMS over the left DLPFC, a probable antidepressant effect of LF-rTMS on the right DLPFC, and probably no differential antidepressant effect between right LF-rTMS and left HF-rTMS. Moreover, there is currently no recommendation for bilateral stimulation combining HF-rTMS of the left DLPFC and LF-rTMS of the right DLPFC. The mentioned guidelines also state that the antidepressant effect when stimulating DLPFC is probably additive, and possibly potentiating, to the efficacy of antidepressant drugs.10 However, no specific recommendation currently addresses the use of rTMS in post-stroke depression. Recently, rTMS has been proposed as a treatment option for the late-life depression associated with chronic subcortical ischemic vascular disease, the so called ‘vascular depression’.41–44 Three studies tested rTMS efficacy in vascular depression (one was a follow-up study with citalopram). Although presenting positive findings, further trials should refine clinical and diagnostic criteria to assess its impact on antidepressant efficacy.45
Approximately 25–30% of stroke patients develop an immediate or delayed cognitive impairment or an overt picture of vascular dementia.46 There is evidence of an overall positive effect on cognitive function for both LF-rTMS47 and HF-rTMS,48 supported by studies on experimental models of vascular dementia.49–52 Nonetheless, the few trials examining the effect on stroke-related cognitive deficit produced mixed results.53–56 In particular, two studies found no effect on cognition when stimulating the left DLPFC at 1 Hz and 10 Hz,53,54 whereas a pilot study found a positive effect on the Stroop interference test with HF-rTMS over the left DLPFC in patients with vascular cognitive impairment without dementia.55 However, this finding was not replicated in a follow-up study.56 To summarize, rTMS can induce beneficial effects on specific cognitive domains, although data are limited and their clinical significance needs to be further validated. Major challenges exist in terms of appropriate patient selection and optimization of the stimulation protocols.57
Central post-stroke pain (CPSP) is the pain resulting from an ischemic lesion of the central nervous system.58 It represents a relatively common complication after a stroke, although it is often under-recognized and, therefore, undertreated.59 According to the IFCN guidelines for the use of rTMS in the treatment of neuropathic pain, there is a definite analgesic effect of HF-rTMS of contralateral M1 to the pain side, and LF-rTMS of contralateral M1 to the pain side is probably ineffective. In addition, there is currently no recommendation for cortical targets other than contralateral M1 to the pain side.10 Notably, rTMS might be effective in drug-resistant CPSP patients.58 A recent systematic review that included nine HF-rTMS studies suggested an effect on CPSP relief, but also underlined the insufficient quality of the studies considered.60
Study objective
In this article, we aim to provide an up-to-date overview of the most recent evidence on the efficacy of rTMS in the rehabilitation of stroke patients. Although several studies have been published, a conclusive statement supporting a systematic use of rTMS in the multifaceted clinical aspects of stroke rehabilitation is still lacking.
Methods
Search strategy
A literature review was performed on all the meta-analyses on conventional rTMS protocols in post-stroke rehabilitation studies indexed in PubMed, Cochrane Library, Scopus, and Web of Science, from database inception until 31 July 2019. We focused on the recovery of motor function, manual dexterity, walking and balance, spasticity, dysphagia, aphasia, unilateral neglect, post-stroke depression, vascular depression, cognitive function, and CPSP.
Search queries and results
Pubmed: ((“transcranial magnetic stimulation” [MeSH Terms] OR (“transcranial”[All Fields] AND “magnetic”[All Fields] AND “stimulation” [All Fields]) OR “transcranial magnetic stimulation”[All Fields] OR (“repetitive”[All Fields] AND “transcranial”[All Fields] AND “magnetic”[All Fields] AND “stimulation”[All Fields]) OR “repetitive transcranial magnetic stimulation”[All Fields]) AND (“stroke”[MeSH Terms] OR “stroke”[All Fields])) AND ((Meta-Analysis[ptyp] OR systematic[sb]) AND “humans”[MeSH Terms] AND English[lang]). Results: 59.
Cochrane Database of Systematic Reviews: “transcranial magnetic stimulation stroke in Title Abstract Keyword”. Results: 4.
Scopus: TITLE-ABS-KEY (“repetitive transcranial magnetic stimulation” AND stroke AND meta-analysis) AND DOCTYPE (ar OR re) AND (LIMIT-TO (LANGUAGE, “English”). Results: 46.
Web of Science Core Collection: TOPIC: (“repetitive transcranial magnetic stimulation” AND stroke AND meta-analysis) Refined by: LANGUAGES: (ENGLISH) AND DOCUMENT TYPES: (REVIEW OR ARTICLE) Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI. Results: 30.
Study selection
To be included, a study had to: Use conventional rTMS protocols for post-stroke patients recovery, it is mentioning that studies using other stimulation techniques in addition to rTMS were included only when rTMS data could be independently extracted from the meta-analysis, an exception was made for the studies where data from other techniques (TBS) were pooled in the same meta-analysis but represented only a minority of the total amount of the analyzed studies. Describe a systematic process for searching for and selecting relevant articles. Perform the statistical analysis.
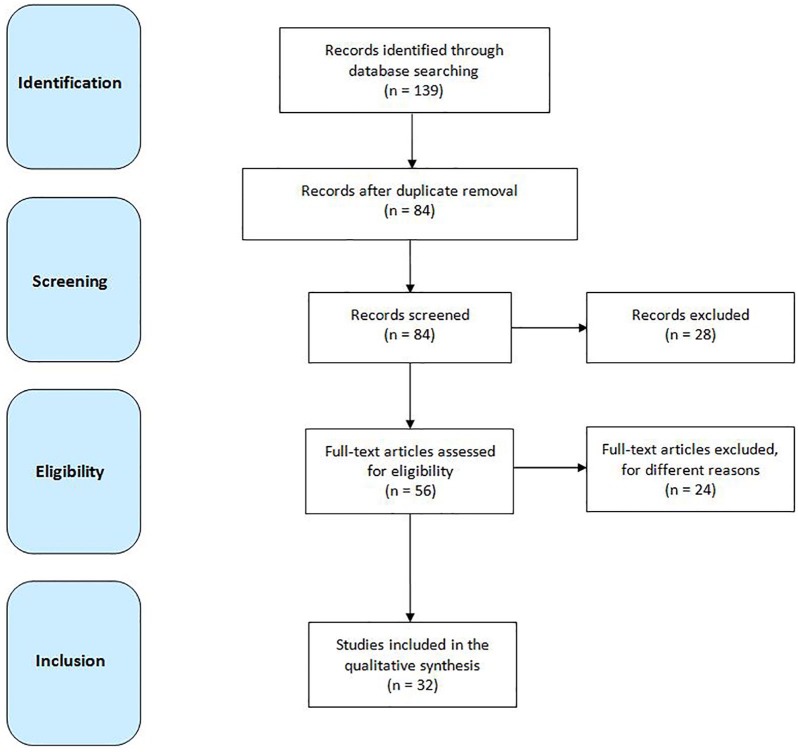
Two independent authors (FF and GL) screened all titles and abstracts of the identified publications. Disagreements were solved by the consensus of a third author (MP). Duplicated entries, retracted publications, studies on other diseases or conditions different from stroke, works on animals, studies without statistical analysis, non-English written papers, publications that are not research studies (i.e. commentaries, letters, editorials, reviews, etc.), and any other study that did not fit within the scope of this review, were excluded. Publications listed in the references were also reviewed in search of more data (Figure 1).
Figure 1.
Flow diagram showing the search strategy, the number of records identified, the excluded articles, and the studies eventually included.61
Data extraction
Two authors (RB and GP) independently extracted the following information from the retrieved meta-analyses: type and number of studies included, stimulation parameters and settings, main findings. Disagreements were solved by a third author (AAG). The relevant data are summarized in Table 1.
Table 1.
Summary of the main characteristics and findings obtained from the meta-analyses of rTMS and stroke rehabilitation.
| Clinical feature | References | Studies included (study design) | Stimulation settings | Main findings |
|---|---|---|---|---|
| Motor function | Xiang et al.62 | 43 RCTs (8 cross-over) | Site: affected M1, unaffected M1, bilateral M1; premotor cortex of the unaffected hemisphere Frequency: 1–25 Hz (7 studies with TBS) Pulses per session: 160–1800 Session number: 1–24 Intensity: 80–130% rMT, 80–120% aMT |
Positive effect of rTMS (particularly at 1 Hz) on limb motor recovery and ADL. HF-rTMS modulated MEP. No difference regarding rMT versus aMT, stimulation intensity or frequencies, number of pulses, stimulation number and sites. More pronounced effects for 1–7 sessions, early stroke (<30 days), and subcortical lesions. |
| Zhang et al.63 | 22 RCTs (3 cross-over) |
Site: contralesional M1 Frequency: 1 Hz Pulses per session: 600–1800 Session number: 1–24 Intensity: 90–120% rMT |
Positive effect on upper limb motor recovery. Short-term efficacy for finger ability, hand strength, and dexterity (in descending order). The long-term effect on finger ability. Enhancing effect of MEPs in the affected hemisphere and suppressing effect in the unaffected hemisphere. Suppressing effect on the rMT of the ispilesional hemisphere and enhancing effect on the rMT of the contralesional hemisphere. | |
| Graef et al.64 | 8 RCTs (1 cross-over) |
Site: unaffected M1, affected M1 Frequency: 1 Hz Pulses per session: 240–1500 Session number: 8–22 Intensity: 90–120% rMT |
No effect for rTMS combined with upper limb training versus upper limb training alone. | |
| Kang et al.65 | 12 RCTs | Site: unaffected M1, affected M1 Frequency: 1–3–10 Hz Pulses per session: 200–1800 Intensity: 90–130% rMT |
Positive effect on limb force production for HF-rTMS on ipsilesional M1 and for LF-rTMS on contralesional M1, regardless of the stroke phase (acute, subacute, chronic). | |
| Hao et al.66 | 19 RCTs | Site: unaffected M1, affected M1 Frequency: 0.5–50 Hz Treatment duration: 1 day–4 weeks |
No effect on the Barthel Index, motor recovery, HDRS, and cognitive function, regardless of stimulation frequencies or disease duration. | |
| Hsu et al.67 | 18 RCTs | Site: unaffected, affected M1, bilateral Frequency: 1–20 Hz (2 cTBS or iTBS) Pulses per session: 160–2000 Treatment duration: 1–10 days Intensity: 80–130% rMT |
Positive effect of on motor function, especially for subcortical strokes. Contralesional LF-rTMS more effective than ipsilesional HF-rTMS. No significant effect on affected side MT. Few adverse events reported. | |
| Tang68 | 9 (5 RCTs, 4 not specified) | Site: unaffected M1, affected M1. Frequency: 1–20 Hz Pulses per session: 100–1600 Treatment duration: 1–10 sessions Intensity: 80–100% rMT |
Positive effect of rTMS on upper limb motor function. Sub-analysis shows a significant effect for acute stroke and LF-rTMS over the unaffected M1. | |
| Manual dexterity | O’ Brien et al.69 | 11 (not clear which studies have been included in the analysis) |
Site: contralateral M1, contralateral dorsal premotor cortex. Frequency: 1–10–20 Hz (1 iTBS – cTBS) Pulses per session: 300–200 Treatment duration: 1–10 sessions Intensity: 80–110% rMT |
Positive effect of rTMS on hand dexterity in mild-to-moderate chronic stroke. |
| Zhang et al.70 | 31 RCTs (8 cross-over) |
Site: ipsilesional M1, contralesional M1 Frequency: 1–10–20 Hz (4 studies with cTBS or iTBS) Pulses per session: 160–2000 Treatment duration: 1–26 sessions Intensity: 80–130% rMT |
Short- and long-term time-dependent improvement. LF-rTMS to the unaffected hemisphere more effective than HF-rTMS to the affected one. Better results in subcortical stroke. Session number-dependent effect (peak after 5 sessions). Few adverse events reported. | |
| Le at al.71 | 8 RCTs (3 cross-over) | Site: unaffected M1, affected M1, unaffected premotor cortex Frequency: 1–25 Hz Pulses per session: 150–2000 Treatment duration: 1–10 days Intensity: 80–130% rMT |
Positive effect on finger motor ability and hand function. No significant changes in neurophysiologic measures (MEPs amplitude and aMT from the paretic side). Few adverse events observed. | |
| Walking and balance | Tung et al.72 | 8 RCTs (7 parallel, 1 cross-over) | Site: affected M1, unaffected M1, left DLPFC, ipsilesional cerebellar hemisphere Frequency: 1–20 Hz Pulses per session: 600–1500 Treatment duration: 5–40 sessions Intensity: 90–130% rMT |
rTMS significantly improved lower limb function, walking speed, lower limb scores at the Fugl-Meyer Assessment scale, and MEPs. No difference regarding the stroke phase and the stimulation frequency. |
| Vaz et al.73 | 3 RCTs | Site: affected hemisphere, unaffected hemisphere Frequency: 1–10 Hz Pulses per session: 600–2000 Treatment duration: 10–30 sessions Intensity: 90% rMT |
rTMS combined with other therapies induced positive effects on gait speed and walking cadence compared with the sham procedure; both excitatory and inhibitory stimulation improved gait speed in acute, subacute, and chronic stroke | |
| Li et al.74 | 9 (5 RCTs, 4 cross-over) | Site: affected M1, unaffected M1, bilateral M1 leg area, trunk motor spot Frequency: 1–50 Hz Pulses per session: 600–2000 Treatment duration: 1–40 sessions Intensity: 90% rMT |
Significant effect on walking speed for ipsilesional HF-rTMS but not for contralesional or bilateral stimulation; no improvement in balance function and motor function. Significant decrease of MEP amplitude from unaffected hemisphere; no effect on MEP amplitude from the affected hemisphere. | |
| Spasticity | McIntyre et al.75 | 10 (1 RCT, 1 cross-over RCT, 8 pre/post studies) | Site: contralesional or bilateral Frequency: LF-rTMS (1 Hz), except for one using ipsilesional HF-rTMS (10 Hz) Session number: 10–15 Pulses per session: 100–2400 Intensity: 90% rMT, apart from 1 (30%) |
Uncontrolled studies: significant improvements in spasticity at the elbow, wrist, and finger flexors. RCT: no significant effect on the wrist. |
| Korzhova et al.76 | 3 (2 RCTs, 1 parallel) | Site: M1 of the unaffected hemisphere Frequency: LF-rTMS (1 Hz) |
No difference between real versus sham stimulation. | |
| Graef et al.64 | 2 RCTs (1 cross-over) | Site: M1 of the unaffected hemisphere Frequency: LF-rTMS (1 Hz) Pulses per session: 240–1500 Session number: 10–14 Intensity: 90% rMT |
No effect for rTMS combined with upper limb training versus upper limb training alone. | |
| Dysphagia | Bath et al.77 | 9 RCTs | Site: affected, unaffected, bilateral sides Frequency: 1–10 Hz Pulses per day: 300–1200 Treatment duration: 5 days–2 weeks |
Positive effect of rTMS on swallowing ability. No effect on case fatality or Penetration Aspiration Scale. |
| Chiang et al.78 | 6 RCTs | Site: affected, unaffected, bilateral side Frequency: 1, 3, 5 Hz Pulses per day: 300–1200 Treatment duration: 5–10 days Intensity: 90–130% rMT |
Positive effect of rTMS on acute and subacute post-stroke dysphagia. rTMS more effective compared with other neuromodulation techniques. No significant adverse events reported. | |
| Liao et al. 201779 | 6 RCTs | Site: affected hemisphere, unaffected hemisphere, bilateral Frequency: 1, 3, 5 Hz Pulses per day: 300–1200 Treatment duration: 1–2 weeks Intensity: 90–130% rMT |
Positive effect on the unaffected hemisphere and bilateral stimulation. HF-rTMS more effective than LF-rTMS. Effect lasting for at least four weeks. | |
| Pisegna et al.80 | 4 RCTs | Site: affected and unaffected hemisphere Frequency: 1, 3, 5 Hz Pulses per session: 250–1200 Treatment duration: 1–10 days Intensity: 90–120% rMT |
Positive effect for stimulation of the unaffected side. | |
| Momosaki et al.81 | 5 RCTs | Site: affected hemisphere, unaffected hemisphere, bilateral Frequency: 1, 3, 5 Hz Treatment duration: 5–10 sessions |
Positive effect of rTMS on dysphagia measured as improvement at the Dysphagia Outcome Severity Scale and Penetration Aspiration Scale | |
| Yang et al.82 | 3 RCTs | Site: affected hemisphere, unaffected hemisphere, bilateral Frequency: 3–5 Hz Pulses per session: 300–500 Treatment duration: 5–10 days Intensity: 90–130% rMT |
Positive effect of rTMS on dysphagia compared with sham stimulation. | |
| Aphasia | Bucur and Papagno83 | 8 (7 RCTs, 1 randomized partial cross-over) |
Site: unaffected hemisphere; both sides Frequency: 1–20 Hz Pulses per session: 600–1800 Treatment duration: 10–15 sessions. Intensity: 80–110% rMT |
Positive effect of rTMS on naming for both subacute and chronic stroke; the effect maintained over time. |
| Shah-Basak et al.84 | 8 (4 between subject, 3 within subject, 1 cross-over) | Site: right PTr, left PTr, right POp, left POp, right IFG, left IFG Frequency: 1, 6, 20 Hz (1 iTBS; 1 HF-rTMS followed by LF-rTMS) Treatment duration: 10–15 days Intensity: 90–110% rMT |
Positive effect on aphasia in subacute and chronic stroke. Subgroup analysis for trial design: statistically significant effect for between and within-subjects designs, no significant effect for cross-over trial. | |
| Otal et al.85 | 6 RCTs | Site: right IFG Frequency: 1 Hz Treatment duration: 10–15 days Intensity: 90% rMT |
Positive effect on aphasia for LF-rTMS over the nonaffected hemisphere. | |
| Li et al.86 | 4 RCTs | Site: right PTr Frequency: 1 Hz Treatment duration: 10–15 days Intensity: 90% rMT |
Positive effect of LF-rTMS on naming but not on repetition and comprehension. No adverse effects reported. | |
| Ren et al.87 | 7 RCTs | Site: right PTr Frequency: 1 Hz Treatment duration: 10–15 days Intensity: 90% rMT |
Positive effect on severity of aphasia, naming, repetition, writing, and comprehension. No adverse effects reported. | |
| Unilateral neglect | Kashiwagi et al.88 |
3 RCTs |
Site: Parietal cortex area 3 and 4 Frequency: 1–10 Hz. Pulses per session: 656–1200 Treatment duration: 10–28 sessions Intensity: 80–90% rMT |
Positive effect compared with sham on overall USN measured with different scales, more evident at 1 Hz but also present at 10 Hz. |
| Fan et al.89 | 6 RCTs | Site: Parietal cortex area 3, 4, and 5 Frequency: 0.5, 1, 10 Hz Pulses per session: 656–1200 Treatment duration: 10–28 sessions Intensity: 80–90% rMT |
Rapid and long-lasting improvement for both LF- and HF-rTMS applied on ipsilesional or contralesional site. More pronounced effect for ipsilesional stimulation and for HF-rTMS. No serious adverse events reported. | |
| Salazar et al.90 | 6 RCTs | Site: Parietal cortex area 3, 4, and 5 Frequency: 0.5, 1, 10 Hz Pulses per session: 656–1200 Treatment duration: 10–28 sessions Intensity: 80–90% rMT |
Positive effect of both LF- and HF-rTMS. | |
| Post-stroke depression and vascular depression | Liu et al.91 | 17 RCTs | Site: left DLPFC Frequency: 10 Hz Treatment duration: 2–12 weeks Intensity: 60–110% rMT |
Positive effect of HF-rTMS on depression measured by the HDRS; significant response and remission rates; positive effect on ADL; positive effect on NIHSS. Significant incidence of headache in the treatment group reported. |
| Shen et al.92 | 22 RCTs | Site: right DLPFC, left DLPFC, bilateral DLPFC, bilateral prefrontal cortex, M1, left temporal-parietal Frequency: 0.2–15 Hz Sequence duration: 4–30 s Number of sequences: 20–30 Pulses per session: 1000–1960 Session number: 2–280 Intensity: 60–110% rMT |
Primary outcome: significant decrease in HDRS. Secondary outcomes: significant effect on clinical response rate. No effect on remission; positive effect on NIHSS and ADL. No clear relationship with the stimulation site and frequency, disease duration, conventional treatment, type of intervention used as control, and total number of sessions. | |
| Hao et al.66 | 2 RCTs (subanalysis) |
Site: bilateral frontal lobes, bilateral prefrontal lobes Frequency: 0.5 Hz. Treatment duration: 1–4 weeks |
No effect on HDRS score. | |
| Cognitive function | Hao et al.66 | 2 RCTs (subanalysis) |
Site: unaffected M1, bilateral frontal lobes Frequency: 0.5–1 Hz. Treatment duration: 2–4 weeks Intensity: 60–100% rMT |
No positive effect on cognitive function. |
| Central post-stroke pain | Leung et al.93 | 5 RCTs (1 parallel, 4 cross-over) | Site: M1 Frequency: HF-rTMS (range 5–20 Hz) Session number: 1–5 Pulses per session: 500–2000 |
Significant analgesic effect of rTMS compared with sham. Greater effect after multiple sessions of stimulation, with a frequency ranging from >1 to ⩽10 Hz. |
ADL, activities of daily living; aMT, active motor threshold; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex; HDRS, Hamilton depression rating scale; HF-rTMS, high-frequency repetitive transcranial magnetic stimulation; IFG, inferior frontal gyrus; iTBS, intermittent theta burst stimulation; LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; M1, primary motor cortex; MEPs, motor evoked potentials; NIHSS, National Institutes of Health stroke scale; POp, pars opercularis; PTr, pars triangularis; RCTs, randomized controlled trials; rMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation; TBS, theta burst stimulation.
Results
A total of 139 results were originally found. Of these, 32 peer reviewed publications were selected according to the above-mentioned inclusion and exclusion criteria (Figure 1). The publication year ranges from 2009 to 2019.
In detail, the results included the following: seven studies for motor function,62–68 three for manual dexterity,69–71 three for walking and balance,72–74 three for spasticity,64,75,76 six for dysphagia,77–82 five for aphasia,83–87 three for unilateral neglect,88–90 three for post-stroke depression and vascular depression,66,91,92 one for cognitive function,66 and one for CPSP.93 The study by Graef and colleagues64 included data on both motor function and spasticity and, therefore, the results were independently considered for both categories. Given that the meta-analyses specifically addressing the treatment of cognitive function were not found, data were extracted from a subanalysis of the study by Hao and coworkers.66 The same study also included a subanalysis on post-stroke depression.66
Motor function
Overall, Tang found a positive effect of rTMS for upper limb motor function, and in particular for LF-rTMS over the unaffected M1 area in acute stroke patients.68 Hsu and colleagues found an overall positive effect of rTMS on motor function, with more pronounced results in patients with subcortical lesions.67
Interestingly, LF-rTMS applied over the unaffected hemisphere appears to be more beneficial than HF-rTMS over the affected hemisphere.67 A meta-analysis focusing on LF-rTMS applied over the contralesional hemisphere also found a positive short- and long-term effects on upper limb motor recovery.63 Nevertheless, Kang and colleagues investigated the effect of rTMS on paretic limb strength during the acute, subacute, and chronic phases of the stroke. Their results show a positive effect in all stroke phases, either for HF-rTMS applied over the ipsilesional M1 or for LF-rTMS over the contralesional hemisphere.65 Recently, Xiang and colleagues reported a positive effect of rTMS (in particular, by using 1 Hz stimulation) on limb motor recovery and activities of daily living (ADL). This effect was more evident for acute and subcortical strokes, as well as after seven sessions of stimulation, whereas it decreased with more prolonged treatments.62
Finally, it is worth mentioning that some meta-analyses failed to prove a significant impact of rTMS in stroke recovery. Graef and colleagues did not observe substantial differences when rTMS was combined with upper limb training versus upper limb training alone.64 Hao and colleagues found no effect of rTMS on the Barthel index score, motor function, Hamilton Depression Rating Scale (HDRS), and cognitive status, regardless of different frequencies of stimulation or stroke duration.66
Overall, there is currently conflicting evidence regarding the efficacy of rTMS in motor recovery. LF-rTMS applied over the unaffected hemisphere seems to be the most promising protocol, although further studies are required.
Manual dexterity
Le and colleagues found a positive effect of rTMS on finger motor ability and hand function.71 A meta-analysis of studies using LF-rTMS, HF-rTMS, and TBS for the recovery of the upper limb found a significant short- and long-term improvement in the outcome measures of motor function. Interestingly, the authors reported the time-dependent effectiveness of rTMS, with a descending order from acute to subacute until the chronic phase of a stroke. Finally, they also suggested a number-dependent effect of rTMS sessions on the manual dexterity recovery, with the most beneficial effect obtained after five sessions.
Regarding the stroke location, rTMS seems to be more effective in those patients with subcortical lesions with respect to other cerebral sites.70 On the other hand, a more recent meta-analysis, focusing exclusively on studies considering manual dexterity as a specific outcome measure after rTMS, shows a significant effect in improving hand dexterity in patients with mild-to-moderate chronic stroke.69
To summarize, the evidence available suggests a positive effect of rTMS in manual dexterity recovery, but the optimal timing of administration remains uncertain.
Walking and balance
A recent meta-analysis including nine rTMS studies (seven HF-rTMS and two LF-rTMS) showed a significant treatment effect on walking speed for ipsilesional HF-rTMS but not for contralesional or bilateral stimulation. In addition, no improvement in balance or motor function was observed.74 Similarly, a further meta-analysis showed a positive effect of rTMS combined with other rehabilitation therapies on gait speed and walking cadence in patients with acute, subacute, and chronic stroke.73 Conversely, Tung and colleagues found a positive effect of rTMS on lower limb motor function, regardless of the stimulation frequency or stroke phase.72
To date, studies specifically addressing the walking and balance recovery are too limited to provide a definitive conclusion, although the effects reported on walking speed are encouraging.
Spasticity
McIntyre and colleagues evaluated the effectiveness of rTMS in improving post-stroke spasticity by taking into consideration the modified Ashworth scale as the main outcome measure.75 All of the studies included used LF-rTMS (1 Hz) to inhibit the contralesional hemisphere,75 apart from one, that used bihemispheric stimulation by combining 1 Hz and 10 Hz.94 Among the 10 uncontrolled studies considered in the meta-analysis, a significant improvement of spasticity at the elbow, wrist, and finger flexors were found. However, the two only RCTs did not show a significant effect for the wrist.75
The same two RCTs31,95 were previously included in a meta-analysis by Graef and colleagues, who did not conclude there was any positive effect on spasticity after rTMS combined with upper limb training versus upper limb training alone.64 Finally, in the meta-analysis of three studies by Korzhova and colleagues, no significant difference between LF-rTMS versus sham stimulation over M1 of the unaffected hemisphere was detected.76
Based on the limited available data, there is no current evidence to support the role of rTMS in the treatment of spasticity.
Dysphagia
Two meta-analyses found an overall positive effect82 and a positive effect for the stimulation of the unaffected side,80 respectively. Momosaki and colleagues observed a positive effect of rTMS on the dysphagia severity rating scale and the penetration aspiration scale.81 Bath and colleagues found an improvement of swallowing but no effect on case fatality or penetration aspiration scale.77
Another recent meta-analysis included six RCTs.45 The result showed a significant improvement in dysphagia, although HF-rTMS seems to be more effective than LF-rTMS. Regarding the stimulation site, an effect for bilateral or contralesional stimulation was found, but not for ipsilesional stimulation. The therapeutic effect lasts for at least 4 weeks after the procedure.79 Finally, rTMS seems to be the most effective, among the other neuromodulation techniques (transcranial direct current stimulation, surface neuromuscular electrical stimulation, and pharyngeal electrical stimulation), for the treatment of acute and subacute post-stroke dysphagia.78
In brief, rTMS seems to be a promising neuromodulation technique for the treatment of post-stroke dysphagia, although the optimal stimulation setting needs to be defined.
Aphasia
A recent meta-analysis including eight studies (one of which used TBS and another one a combination of HF-rTMS and LF-rTMS) found a pooled positive effect on aphasia after treatment in both subacute and chronic stroke patients.84 The efficacy of rTMS on naming in subacute and chronic patients is also supported by Bucur and Papagno, who confirmed that the positive effect was maintained over time.83 In previous work, a positive effect on the accuracy of naming was observed after LF-rTMS over the right IFG.85 Li and colleagues found a positive effect of LF-rTMS on naming but not on repetition and comprehension,86 whereas Ren and colleagues showed an effect on severity of impairment, as well as in naming, repetition, writing, and comprehension.87
In conclusion, the evidence seems to support the role of LF-rTMS over the unaffected side in the recovery of post-stroke aphasia, with more evident beneficial effects on naming.
Unilateral neglect
A recent meta-analysis including six rTMS studies showed an improvement of unilateral neglect outcome measures, with an immediate and long-lasting effect. Both LF-rTMS and HF-rTMS exerted significant results, either applied on the ipsilesional or the contralesional site, although the effect was more pronounced for ipsilesional stimulation and for HF-rTMS.89 Similar results after both excitatory and inhibitory stimulation were found by another meta-analysis that considered the same studies.90 According to the most recent meta-analysis on the same topic, the benefits of rTMS seem to be particularly evident when 1 Hz frequency of stimulation is used.88
Although there are promising results, the efficacy of rTMS in the treatment of neglect remains controversial, particularly in terms of the best stimulation parameters to be used.
Post-stroke depression and vascular depression
In their meta-analysis including two RCTs, Hao and colleagues found no effect on the HDRS score.66 In contrast, a large meta-analysis of 22 RCTs found a significant clinical response after rTMS, as indexed by a significant reduction of HDRS score. However, a clear relationship with the stimulation site and frequency, as well as with the disease duration, the conventional treatment, the type of intervention used as a control, and the total number of sessions, was not found.92 Regarding the stimulation frequency, the efficacy of 10 Hz stimulation over the left DLPFC was supported by a recent meta-analysis including only HF-rTMS studies.91
In short, the evidence seems to support the efficacy of rTMS (particularly HF-rTMS) over the left DLPFC for post-stroke depression. The widely proved efficacy of rTMS for the treatment of major depression disorder encourages further trials.
Cognitive function
In their meta-analysis including two RCTs,96,97 Hao and colleagues found no significant effect on global cognitive functioning indexed by the Mini Mental State Examination score.66 Therefore, data regarding the efficacy of rTMS on cognitive functions is currently lacking.
CPSP
A meta-analysis including five HF-rTMS studies found an analgesic effect in terms of a significant decrease of the score at the pain visual analog scale compared with the sham procedure. The effect was greater with multiple stimulation sessions and within a frequency range of 1 and 10 Hz.93
To date, given the limited number of low-quality studies available, no recommendation can be made regarding the role of rTMS for post-stroke pain treatment.
Discussion
General considerations
The rationale for using rTMS in stroke recovery is based on the neuroplastic effects that it exerts on altered electrophysiological mechanisms including reduced intracortical inhibition and increased transcallosal inhibition of the healthy hemisphere over the damaged side.98 Therefore, the therapeutic approaches with rTMS, in accordance with the interhemispheric competition model, are targeted at the normalization of the imbalance between the affected and the unaffected hemispheres.3,99,100 This can be reached either by delivering HF-rTMS on the ipsilesional hemisphere (to upregulate the level of cortical excitability), or by LF-rTMS to the contralesional hemisphere (thus, downregulating the effect that it exerts on the ipsilesional cortex).98
In this context, the selection of the cortical targets is based on the pathophysiological mechanisms that are known to be involved. Functional magnetic resonance imaging (fMRI) studies have demonstrated the role of both ipsilesional and contralesional M1 areas after a stroke, suggesting a reduction of functional connectivity between the areas related to the severity of motor impairment. On the other hand, stronger functional connectivity between M1 and other brain areas is associated with better motor recovery.101,102 Notably, although conventional fMRI is limited in providing information on cortical locations, which are active during motor movements, sensory stimuli, or cognitive tasks, resting-state fMRI is a recently evolving method from which functional connectivity between distant brain regions is extracted based on low-frequency fluctuations.101 In particular, Grefkes and colleagues used the dynamic causal modeling, which is a novel approach to capture the intrinsic and task-dependent influences that a particular area exerts over the activity of another area, known as ‘effective connectivity’.102 Based on these assumptions, M1 was confirmed to be the most common stimulation site when aiming to treat motor impairment and spasticity.
The same principle applies to dysphagia, although deglutition is physiologically mediated by bilateral innervation, with a prevalence by the dominant hemisphere. In dysphagia patients, the increasing contralesional activity might help recovery.33,80 Nevertheless, hemispheric dominance for swallowing can vary among individuals, it is not necessarily the same for language.
Similarly, in patients with aphasia, fMRI demonstrated hyperactivity of the homologous of the Broca’s area in the right hemisphere. This activity was associated with poor recovery,103,104 thus providing the rationale for an rTMS-mediated suppression of the right hemisphere activity.105
Hemispatial neglect is typically attributed to a lesion of the right hemisphere, especially involving the parietal cortex. In normal patients, each hemisphere is responsible for the attention toward the contralateral space, a mechanism normally balanced by reciprocal interhemispheric inhibition. Following a stroke, the impaired activity of the right hemisphere favors the disinhibition of the contralateral side. Therefore, the increased activity of the left hemisphere shifts the attention to the right space of the patient, thus further increasing the inhibition over the affected side.106 Accordingly, patients with neglect show increased cortical excitability of the left parietal regions.107 In this scenario, inhibitory or excitatory rTMS, applied over the left or right parietal cortex respectively, can modulate the excitability of the regions involved in post-stroke neglect.89,106
In depressed patients, many studies pointed out a hypometabolism/hypoexcitability of the left frontal region and a hypermetabolism/hyperexcitability of the right frontal region.108,109 The DLPFC is easy to access with rTMS, and its crucial involvement in mood/affect regulation and executive functions makes it an ideal target for neuromodulation interventions.10,110
Finally, the rationale for the application of rTMS in the treatment of chronic pain is based on the efficacy of the epidural stimulation of the motor cortex in treating drug-resistant neuropathic pain.111 Repetitive TMS on M1 is capable of modulating pain perception, as demonstrated by experimental models of pain.112 Although the exact mechanisms are not known, an fMRI study in CPSP patients showed that rTMS can influence the activity of the secondary somatosensory cortex, insula, prefrontal cortex, and putamen, suggesting a more widespread modulation of a complex pain network.113
Proposed pathomechanisms
Regardless of the clinical manifestation of the specific neuroanatomical region involved, the human brain typically shows a wide spectrum of innate capacities to react as a dynamic system, in both physiological and pathological conditions, with the final goal to plastically modulate the characteristics of both single cells and neural circuits.114 These phenomena are recognized under the umbrella term of ‘neuroplasticity’, defined as the ability of the brain to reorganize itself, with a long-lasting remodeling of neural communication.115 The recovery of post-stroke motor deficit probably requires long and complex motor learning processes,116 which are mediated at a molecular level by mechanisms of LTP and LTD.117 These basically consist in long-lasting modifications of the synaptic activity, mainly mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptors and by GABAergic transmission.
In this context, rTMS is able to reliably mimic the experimental paradigms inducing LTD and LTP phenomena, thus producing changes in MEPs amplitude that outlast the stimulation application.118 In particular, LF-rTMS inhibits cortical excitability,119 whereas HF-rTMS produces the opposite effect.120 The NMDA-dependency of the long-lasting effects of rTMS is suggested by some neuropharmacological studies: memantine, a well-known NMDA antagonist, blocks the effects of TBS.121
However, rTMS exerts more diffuse effects, including the induction of specific structural changes within the cortex and the modification of functional connections between different and remote areas of the brain, eventually modulating network oscillations.15,118 Furthermore, rTMS can trigger the release of different neuromodulators (such as acetylcholine, dopamine, norepinephrine, and serotonin),122,123 promote the induction of neurotrophic factors,124–126 and modulate the expression of genes such as c-Fos.127,128 Of particular interest, a 10 Hz stimulation of the left DLPFC seems to modulate dopamine release, an effect that is not observed during stimulation of the right DLPFC.129 It is important to note that susceptibility to the neuroplasticity-related modification induced by rTMS might be genetically encoded, thus making the response to treatment customized and possibly predictable.130–132
Finally, the modulation of neural activity induced by rTMS might also result from dynamic changes of the blood flow through specific cerebral regions, including those implicated in cognition and mood regulation.133
Safety and controversies
Overall, based on the data reviewed, rTMS is shown to be safe, painless, and generally well-tolerated, except for a few patients experiencing mild side effects, such as transient headache and anxiety. Nonetheless, before undergoing any rTMS procedure, candidate patients should always be screened according to the safety guidelines134 to rule out possible contraindications (e.g. history of seizures, head trauma, syncope, pacemaker, and medical implants or devices, pregnancy). The risk/benefit ratio of the procedure should be carefully evaluated for each patient.
Regarding efficacy, with the exception of spasticity (in which the evidence of benefit is not conclusive yet) and cognitive impairment (for which data are limited), the current literature seems to agree on a positive effect of rTMS for all the other clinical applications of stroke. However, there is still uncertainty regarding the optimal protocols to follow, in terms of patient characteristic and stimulation settings, as briefly summarized in the following and reported in Table 1.
Stroke location
Zhang,70 Hsu67 and Xiang62 reported a more pronounced effect of rTMS on motor function recovery for subcortical stroke. However, it is still unclear whether rTMS should be set for the specific stroke site or not.
Stroke phase
Shah-Basak84 and Bucur83 reported a positive effect of rTMS on aphasia in subacute and chronic stroke, whereas O’Brien found a positive effect on fine motor function in chronic stroke only.69 According to Zhang, the effectiveness of rTMS on manual dexterity recovery follows a descending order from acute to subacute and chronic phase of stroke.70 On the other hand, Kang described a positive effect on motor function in acute, subacute, and chronic stroke,65 whereas Tang68 and Xiang62 described more pronounced effects for acute stroke compared with chronic stroke, whereas Hao found no effect regardless of the stroke phase.66 Vaz reported efficacy in walking recovery in all stroke patients.73 Still, the optimal timing of intervention remains controversial.
Stroke severity
O’Brien described an effect on fine motor function in mild-to-moderate stroke,69 although data on the correlation between stroke severity and rTMS efficacy are lacking.
Stimulation site
As expected, M1 is the most common site for motor recovery, CPSP, and spasticity, whereas the cortical area representing the muscles involved in deglutition is targeted in post-stroke dysphagia. The DLPFC is the most common target for depression, while the stimulation areas proposed for neglect are parietal cortex areas three, four, and five. In post-stroke aphasia, rTMS is targeted to the pars triangularis, the pars opercularis, and the IFG. The most studied paradigms usually consist of ipsilesional HF-rTMS or contralesional LF-rTMS, although some authors have proposed a combined approach that uses bilateral stimulation. A contralesional HF-rTMS has been also tested in dysphagic patients.79
Stimulation frequency
Leung found a positive effect of HF-rTMS in CPSP, particularly in the frequency range > 1 Hz and ⩽ 10 Hz.93 HF-rTMS over the unaffected side seems to be more effective than LF-rTMS for dysphagia.79 Both HF-rTMS and LF-rTMS seems to be effective in patients with unilateral neglect.88–90 The most used protocol for aphasic patients is LF-rTMS at 1 Hz over the unaffected side. Ipsilesional HF-rTMS appears to be the more effective for gait recovery,74 while LF-rTMS over the unaffected side seems to be more beneficial than HF-rTMS over the affected side for gross motor function recovery. HF-rTMS seems to be the most effective protocol for post-stroke depression. Overall, this bulk of heterogeneity does not allow us to draw a definitive conclusion on the ideal stimulation frequency.
Stimulation intensity
As for the stimulation frequency, there is great variability among the studies for the intensity of stimulation to be used, with values oscillating from below to well above the patient’s rMT. Suprathreshold upper intensities and frequencies are limited according to the safety guidelines.134 Therefore, the most appropriate stimulation intensity is still unclear.
Coil type
The type of coil been used in the studies analyzed was not always clearly stated. Moreover, when reported, significant heterogeneity of coil types was noted. These issues make the comparison of the obtained results rather challenging. To date, therefore, a clear recommendation regarding the type of coil to be used cannot be reached.
Stimulation length
Leng found a greater effect of rTMS on CPSP with repeated stimulations.93 Zhang found a number of dependent effects of rTMS sessions on motor function, with a peak of efficacy after five sessions.70 Similarly, Xiang describes the best effects on motor function after seven sessions.62 However, the number of stimulation sessions and the total length of treatment significantly vary among the studies and, to date, there is no conclusive statement about this feature.
Long-term efficacy
Liao observed that the effect of rTMS on dysphagia persisted for at least 4 weeks.79 A long-lasting positive effect on unilateral neglect was also found by Fan.89 Zhang described a long-term improvement in motor function.70 The effects of stimulation seem to be long-lasting for aphasia.83 Nonetheless, more data is needed to firmly establish the long-term effects of rTMS and to determine the best stimulation parameters to achieve consistent results.
Outcome measures
There is a large heterogeneity of the outcome measures in the studies considered, making the different interventions employed and the expected results difficult to compare. As known, an objective measure of the effectiveness of an intervention is crucial to translate the research results into clinical practice. Therefore, the outcome measures should be selected according to the WHO International Classification of Functioning, Disability, and Health (ICF) in order to ensure comparability, reliability, and validity.135
Concomitant treatment
The difference between outcomes from TMS combined with conventional therapy versus TMS alone has to be addressed. Indeed, many of the studies reviewed do not report specific results on this aspect, and others do not separately consider the effect of the different therapeutic interventions concomitantly performed.
Strengths and limitations
The strength of this review is that it comprehensively summarizes the evidence from a large number of meta-analyses covering the impact of rTMS on the most common consequences of stroke. The main criticism is of the review is that data were extracted from meta-analyses rather than individual studies, although the meta-analyses provide the highest level of evidence. Another limitation is that, in addition to the conventional HF-rTMS and LF-rTMS, other protocols of repetitive stimulation can be set, such as TBS or the quadripulse stimulation, although this goes beyond the main goal of the present review. When a meta-analysis included both conventional rTMS and TBS, the results on rTMS were in most cases independently extracted, although in some cases data from both techniques were pooled. In these circumstances,62,67,69,70,84 the meta-analyses were eventually included given that TBS studies represented only a minority of the total results (Table 1). Finally, although the research methodology was systematic, this article cannot be considered as a systematic review because it provides a description of the studies but not a detailed evaluation of the quality of the studies themselves.
Conclusions and future perspectives
In combination, the evidence from the literature reviewed allows us to state that rTMS is a feasible nonpharmacological tool to assist the neurorehabilitation of different motor and nonmotor clinical manifestations of stroke. Integrated with other conventional rehabilitative modalities, rTMS might synergistically act by further enhancing the clinical recovery and the prognostic perspective of stroke survivors.
However, it is not possible to recommend a particular protocol at present. The significant heterogeneity of the studies currently available, especially in terms of the protocol to be set and outcome measures that have to be used, makes it hard to compare the different interventions employed and the expected results. This leads to a lack of consensus on the best clinical and technical settings to be adopted in order to achieve optimal long-lasting results and to expand the use of rTMS into a large-scale application.
To overcome the previously mentioned limitations, future research should preliminarily evaluate the most promising protocols before going on to multicenter studies with large cohorts of patients in order to achieve a definitive translation into daily clinical practice and a reliable group stratification.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: WP is a member of the Scientific Advisory Board of the Precisis AG, entirely dedicated to epilepsy. FF, GL, AAG, GP, RB, and MP declare that there is no conflict of interest.
ORCID iD: Giuseppe Lanza  https://orcid.org/0000-0002-5659-662X
https://orcid.org/0000-0002-5659-662X
Contributor Information
Francesco Fisicaro, Department of Medical and Surgical Sciences and Advanced Technologies, Section of Neurosciences, University of Catania, Catania, Italy.
Giuseppe Lanza, Department of Surgery and Medical-Surgery Specialties, University of Catania, Via Santa Sofia, 78, Catania, 95125, Italy; Department of Neurology IC, Oasi Research Institute – IRCCS, Troina, Italy.
Alfio Antonio Grasso, Department of Surgery and Medical-Surgery Specialties, University of Catania, Catania, Italy.
Giovanni Pennisi, Department of Surgery and Medical-Surgery Specialties, University of Catania, Catania, Italy.
Rita Bella, Department of Medical and Surgical Sciences and Advanced Technologies, Section of Neurosciences, University of Catania, Catania, Italy.
Walter Paulus, Department of Clinical Neurophysiology, University Medical Center, Georg August University, Göttingen, Germany.
Manuela Pennisi, Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy.
References
- 1. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009; 8: 741–754. [DOI] [PubMed] [Google Scholar]
- 2. Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. [DOI] [PubMed] [Google Scholar]
- 3. Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597–608. [DOI] [PubMed] [Google Scholar]
- 4. Clarke DJ, Forster A. Improving post-stroke recovery: the role of the multidisciplinary health care team. J Multidiscip Healthc 2015; 8: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alia C, Spalletti C, Lai S, et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front Cell Neurosci 2017; 11: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cantone M, Lanza G, Vinciguerra L, et al. Age, Height, and Sex on Motor Evoked Potentials: Translational Data From a Large Italian Cohort in a Clinical Environment. Front Hum Neurosci 2019; 13: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pennisi G, Lanza G, Giuffrida S, et al. Excitability of the motor cortex in de novo patients with celiac disease. PLoS One 2014; 9: e102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bella R, Lanza G, Cantone M, et al. Effect of a Gluten-Free Diet on Cortical Excitability in Adults with Celiac Disease. PLoS One 2015; 10: e0129218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lanza G, Cantone M, Aricò D, et al. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory-motor network in patients with restless legs syndrome. Ther Adv Neurol Disord 2018; 11: 1756286418759973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125: 2150–2206. [DOI] [PubMed] [Google Scholar]
- 11. Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. committee. Clin Neurophysiol 2015; 126: 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol 2015; 126: 1847–1868. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003; 2: 145–156. [DOI] [PubMed] [Google Scholar]
- 14. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006; 117: 2584–2596. [DOI] [PubMed] [Google Scholar]
- 15. Hoogendam JM, Ramakers GMJ, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi N, Izumi SI. Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plast 2012; 2012: 359728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho JS. Motor function-related maladaptive plasticity in stroke: a review. NeuroRehabilitation 2013; 32: 311–316. [DOI] [PubMed] [Google Scholar]
- 18. Dionísio A, Duarte IC, Patrício M, et al. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: a systematic review. J Stroke Cerebrovasc Dis 2018; 27: 1–31. [DOI] [PubMed] [Google Scholar]
- 19. Higgins J, Koski L, Xie H. Combining rTMS and task-oriented training in the rehabilitation of the arm after stroke: a pilot randomized controlled trial. Stroke Res Treat 2013; 2013: 539146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blesneag A, Slăvoacă D, Popa L, et al. Low-frequency rTMS in patients with subacute ischemic stroke: clinical evaluation of short and long-term outcomes and neurophysiological assessment of cortical excitability. J Med Life 2015; 8: 378–387. [PMC free article] [PubMed] [Google Scholar]
- 21. Rose DK, Patten C, McGuirk TE, et al. Does inhibitory repetitive transcranial magnetic stimulation augment functional task practice to improve arm recovery in chronic stroke? Stroke Res Treat 2014; 2014: 305236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil Assoc Acad Physiatr 2007; 86: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seniów J, Bilik M, Leśniak M, et al. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair 2012; 26: 1072–1079. [DOI] [PubMed] [Google Scholar]
- 24. Harvey RL, Edwards D, Dunning K, et al. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018; 49: 2138–2146. [DOI] [PubMed] [Google Scholar]
- 25. Wang YC, Magasi SR, Bohannon RW, et al. Assessing dexterity function: a comparison of two alternatives for the NIH toolbox. J Hand Ther 2011; 24: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van de Port IGL, Kwakkel G, van Wijk I, et al. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke 2006; 37: 167–171. [DOI] [PubMed] [Google Scholar]
- 27. Tyson SF, Hanley M, Chillala J, et al. The relationship between balance, disability, and recovery after stroke: predictive validity of the brunel balance assessment. Neurorehabil Neural Repair 2007; 21: 341–346. [DOI] [PubMed] [Google Scholar]
- 28. Chieffo R, Comi G, Leocani L. Noninvasive neuromodulation in poststroke gait disorders: rationale, feasibility, and state of the art. Neurorehabil Neural Repair 2016; 30: 71–82. [DOI] [PubMed] [Google Scholar]
- 29. Fleming MK, Pavlou M, Newham DJ, et al. Non-invasive brain stimulation for the lower limb after stroke: what do we know so far and what should we be doing next? Disabil Rehabil 2017; 39: 714–720. [DOI] [PubMed] [Google Scholar]
- 30. Thibaut A, Chatelle C, Ziegler E, et al. Spasticity after stroke: physiology, assessment and treatment. Brain Inj 2013; 27: 1093–1105. [DOI] [PubMed] [Google Scholar]
- 31. Barros Galvão SC, Borba Costa, dos Santos R, et al. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil 2014; 95: 222–229. [DOI] [PubMed] [Google Scholar]
- 32. Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil 2000; 79: 170–175. [DOI] [PubMed] [Google Scholar]
- 33. Doeltgen SH, Bradnam LV, Young JA, et al. Transcranial non-invasive brain stimulation in swallowing rehabilitation following stroke—a review of the literature. Physiol Behav 2015; 143: 1–9. [DOI] [PubMed] [Google Scholar]
- 34. Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging 2005; 22: 163–182. [DOI] [PubMed] [Google Scholar]
- 35. Heilman KM, Valenstein E, Watson RT. Neglect and related disorders. Semin Neurol 2000; 20: 463–470. [DOI] [PubMed] [Google Scholar]
- 36. Fasotti L, van Kessel M. Novel insights in the rehabilitation of neglect. Front Hum Neurosci 2013; 7: 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dionísio A, Duarte IC, Patrício M, et al. Transcranial magnetic stimulation as an intervention tool to recover from language, swallowing and attentional deficits after stroke: a systematic review. Cerebrovasc Dis 2018; 46: 178–185. [DOI] [PubMed] [Google Scholar]
- 38. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2016; 173: 221–231. [DOI] [PubMed] [Google Scholar]
- 39. Perera T, George MS, Grammer G, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulat 2016; 9: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cantone M, Bramanti A, Lanza G, et al. Cortical plasticity in depression. ASN Neuro 2017; 9: 1759091417711512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McIntyre A, Thompson S, Burhan A, et al. Repetitive transcranial magnetic stimulation for depression due to cerebrovascular disease: a systematic review. J Stroke Cerebrovasc Dis 2016; 25: 2792–2800. [DOI] [PubMed] [Google Scholar]
- 42. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 43. Krishnan KRR. MRI-defined vascular depression. Am J Psychiatry 1997; 5: 497–501. [DOI] [PubMed] [Google Scholar]
- 44. Coffey CE, Wilkinson WE, Weiner RD, et al. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch Gen Psychiatry 1993; 50: 7–16. [DOI] [PubMed] [Google Scholar]
- 45. Brunoni AR, Benseñor IM, Alves TC, de TF. Therapeutic interventions for vascular depression: a systematic review. Braz J Psychiatry 2011; 33: 400–409. [DOI] [PubMed] [Google Scholar]
- 46. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 2016; 1862: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lage C, Wiles K, Shergill SS, et al. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. J Neural Transm 2016; 123: 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm 2010; 117: 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang N, Xing M, Wang Y, et al. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience 2015; 311: 284–291. [DOI] [PubMed] [Google Scholar]
- 50. Yang HY, Liu Y, Xie JC, et al. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav Brain Res 2015; 281: 149–155. [DOI] [PubMed] [Google Scholar]
- 51. Guo F, Lou J, Han X, et al. Repetitive transcranial magnetic stimulation ameliorates cognitive impairment by enhancing neurogenesis and suppressing apoptosis in the hippocampus in rats with ischemic stroke. Front Physiol 2017; 8: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang XQ, Li L, Huo JT, et al. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res 2018; 13: 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim BR, Kim DY, Chun MH, et al. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil 2010; 89: 362–368. [DOI] [PubMed] [Google Scholar]
- 54. Jorge RE, Robinson RG, Tateno A, et al. Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry 2004; 55: 398–405. [DOI] [PubMed] [Google Scholar]
- 55. Rektorova I, Megova S, Bares M, et al. Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci 2005; 229–230: 157–161. [DOI] [PubMed] [Google Scholar]
- 56. Sedlackova S, Rektorova I, Fanfrdlova Z, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia. J Psychophysiol 2008; 22: 14–19. [Google Scholar]
- 57. Bordet R, Ihl R, Korczyn AD, et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Med 2017; 15: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumar B, Kalita J, Kumar G, et al. Central poststroke pain: a review of pathophysiology and treatment. Anesth Analg 2009; 108: 1645–1657. [DOI] [PubMed] [Google Scholar]
- 59. Jönsson A, Lindgren I, Hallström B, et al. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry 2006; 77: 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen CC, Chuang YF, Huang ACW, et al. The antalgic effects of non-invasive physical modalities on central post-stroke pain: a systematic review. J Phys Ther Sci 2016; 28: 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiang H, Sun J, Tang X, et al. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 847–864. [DOI] [PubMed] [Google Scholar]
- 63. Zhang L, Xing G, Shuai S, et al. Low-frequency repetitive transcranial magnetic stimulation for stroke-induced upper limb motor deficit: a meta-analysis. Neural Plast 2017; 2017: 2758097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Graef P, Dadalt MLR, Rodrigués DAM da S, et al. Transcranial magnetic stimulation combined with upper-limb training for improving function after stroke: a systematic review and meta-analysis. J Neurol Sci 2016; 369: 149–158. [DOI] [PubMed] [Google Scholar]
- 65. Kang N, Summers JJ, Cauraugh JH. Non-invasive brain stimulation improves paretic limb force production: a systematic review and meta-analysis. Brain Stimulat 2016; 9: 662–670. [DOI] [PubMed] [Google Scholar]
- 66. Hao Z, Wang D, Zeng Y, et al. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013; 2013: CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hsu WY, Cheng CH, Liao KK, et al. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke 2012; 43: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 68. Tang IN. The effect of repetitive transcranial magnetic stimulation on upper extremity motor function in stroke patients: a meta-analytical review. J Food Drug Anal 2012; 20: 5. [Google Scholar]
- 69. O’Brien AT, Bertolucci F, Torrealba-Acosta G, et al. Non-invasive brain stimulation for fine motor improvement after stroke: a meta-analysis. Eur J Neurol 2018; 25: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 70. Zhang L, Xing G, Fan Y, et al. Short- and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis. Clin Rehabil 2017; 31: 1137–1153. [DOI] [PubMed] [Google Scholar]
- 71. Le Q, Qu Y, Tao Y, et al. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil 2014; 93: 422–430. [DOI] [PubMed] [Google Scholar]
- 72. Tung YC, Lai CH, Liao CD, et al. Repetitive transcranial magnetic stimulation of lower limb motor function in patients with stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 73. Vaz PG, Salazar AP, da S, Stein C, et al. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: a systematic review and meta-analysis. Top Stroke Rehabil 2019; 26: 201–213. [DOI] [PubMed] [Google Scholar]
- 74. Li Y, Fan J, Yang J, et al. Effects of repetitive transcranial magnetic stimulation on walking and balance function after stroke: a systematic review and meta-analysis. Am J Phys Med Rehabil 2018; 97: 773–781. [DOI] [PubMed] [Google Scholar]
- 75. McIntyre A, Mirkowski M, Thompson S, et al. A systematic review and meta-analysis on the use of repetitive transcranial magnetic stimulation for spasticity poststroke. PM R 2018; 10: 293–302. [DOI] [PubMed] [Google Scholar]
- 76. Korzhova J, Sinitsyn D, Chervyakov A, et al. Transcranial and spinal cord magnetic stimulation in treatment of spasticity: a literature review and meta-analysis. Eur J Phys Rehabil Med 2018; 54: 75–84. [DOI] [PubMed] [Google Scholar]
- 77. Bath PM, Lee HS, Everton LF. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst Rev 2018; 10: CD000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiang CF, Lin MT, Hsiao MY, et al. Comparative efficacy of non-invasive neurostimulation therapies for acute and subacute post-stroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil 2019; 100: 739–750.e4. [DOI] [PubMed] [Google Scholar]
- 79. Liao X, Xing G, Guo Z, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: a systematic review and meta-analysis. Clin Rehabil 2017; 31: 289–298. [DOI] [PubMed] [Google Scholar]
- 80. Pisegna JM, Kaneoka A, Pearson WG, et al. Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol 2016; 127: 956–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Momosaki R, Kinoshita S, Kakuda W, et al. Noninvasive brain stimulation for dysphagia after acquired brain injury: a systematic review. J Med Invest 2016; 63: 153–158. [DOI] [PubMed] [Google Scholar]
- 82. Yang SN, Pyun SB, Kim HJ, et al. Effectiveness of non-invasive brain stimulation in dysphagia subsequent to stroke: a systemic review and meta-analysis. Dysphagia 2015; 30: 383–391. [DOI] [PubMed] [Google Scholar]
- 83. Bucur M, Papagno C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci Biobehav Rev 2019; 102: 264–289. [DOI] [PubMed] [Google Scholar]
- 84. Shah-Basak PP, Wurzman R, Purcell JB, et al. Fields or flows? A comparative meta analysis of transcranial magnetic and direct current stimulation to treat post-stroke aphasia. Restor Neurol Neurosci 2016; 34: 537–558. [DOI] [PubMed] [Google Scholar]
- 85. Otal B, Olma MC, Flöel A, et al. Inhibitory non-invasive brain stimulation to homologous language regions as an adjunct to speech and language therapy in post-stroke aphasia: a meta-analysis. Front Hum Neurosci 2015; 9: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li Y, Qu Y, Yuan M, et al. Low-frequency repetitive transcranial magnetic stimulation for patients with aphasia after stoke: a meta-analysis. J Rehabil Med 2015; 47: 675–681. [DOI] [PubMed] [Google Scholar]
- 87. Ren CL, Zhang GF, Xia N, et al. Effect of low-frequency rTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PLoS One 2014; 9: e102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kashiwagi FT, El Dib R, Gomaa H, et al. Noninvasive brain stimulations for unilateral spatial neglect after stroke: a systematic review and meta-analysis of randomized and nonrandomized controlled trials. Neural Plast 2018; 2018: 1638763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fan J, Li Y, Yang Y, et al. Efficacy of noninvasive brain stimulation on unilateral neglect after stroke: a systematic review and meta-analysis. Am J Phys Med Rehabil 2018; 97: 261–269. [DOI] [PubMed] [Google Scholar]
- 90. Salazar APS, Vaz PG, Marchese RR, et al. Noninvasive brain stimulation improves hemispatial neglect after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil 2018; 99: 355–366.e1. [DOI] [PubMed] [Google Scholar]
- 91. Liu C, Wang M, Liang X, et al. Efficacy and safety of high-frequency repetitive transcranial magnetic stimulation for poststroke depression: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2019; (In press). [DOI] [PubMed] [Google Scholar]
- 92. Shen X, Liu M, Cheng Y, et al. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: a systematic review and meta-analysis of randomized controlled clinical trials. J Affect Disord 2017; 211: 65–74. [DOI] [PubMed] [Google Scholar]
- 93. Leung A, Donohue M, Xu R, et al. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain 2009; 10: 1205–1216. [DOI] [PubMed] [Google Scholar]
- 94. Yamada N, Kakuda W, Kondo T, et al. Bihemispheric repetitive transcranial magnetic stimulation combined with intensive occupational therapy for upper limb hemiparesis after stroke: a preliminary study. Int J Rehabil Res 2013; 36: 323–329. [DOI] [PubMed] [Google Scholar]
- 95. Etoh S, Noma T, Ikeda K, et al. Effects of repetitive trascranial magnetic stimulation on repetitive facilitation exercises of the hemiplegic hand in chronic stroke patients. J Rehabil Med 2013; 45: 843–847. [DOI] [PubMed] [Google Scholar]
- 96. Du DQ, Wu YB. Living ability and cognitive function ameliorated by low frequency repetitive transcranial magnetic stimulation in patients with post-stroke depression: comparison with drug plus psychological treatment. Chinese J Clin Rehabil 2005; 9: 22–23. [Google Scholar]
- 97. Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006; 37: 2115–2122. [DOI] [PubMed] [Google Scholar]
- 98. Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci 2011; 29: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nowak DA, Grefkes C, Ameli M, et al. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair 2009; 23: 641–656. [DOI] [PubMed] [Google Scholar]
- 100. Boddington LJ, Reynolds JNJ. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimulat 2017; 10: 214–222. [DOI] [PubMed] [Google Scholar]
- 101. Park C, Chang WH, Ohn SH, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke J Cereb Circ 2011; 42: 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008; 63: 236–246. [DOI] [PubMed] [Google Scholar]
- 103. Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Arch Phys Med Rehabil 2012; 93: S15–S25. [DOI] [PubMed] [Google Scholar]
- 104. Crosson B, McGregor K, Gopinath KS, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev 2007; 17: 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shah PP, Szaflarski JP, Allendorfer J, et al. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front Hum Neurosci 2013; 7: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mylius V, Ayache SS, Zouari HG, et al. Stroke rehabilitation using noninvasive cortical stimulation: hemispatial neglect. Expert Rev Neurother 2012; 12: 983–991. [DOI] [PubMed] [Google Scholar]
- 107. Koch G, Oliveri M, Cheeran B, et al. Hyperexcitability of parietal-motor functional connections for the intact left-hemisphere in neglect patients. Brain J Neurol 2008; 131: 3147–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kennedy SH, Javanmard M, Vaccarino FJ. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry Rev Can Psychiatr 1997; 42: 467–475. [DOI] [PubMed] [Google Scholar]
- 109. Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med 1995; 25: 247–261. [DOI] [PubMed] [Google Scholar]
- 110. Turriziani P, Smirni D, Zappalà G, et al. Enhancing memory performance with rTMS in healthy subjects and individuals with mild cognitive impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci 2012; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991; 52: 137–139. [DOI] [PubMed] [Google Scholar]
- 112. Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain 2012; 153: 1350–1363. [DOI] [PubMed] [Google Scholar]
- 113. Ohn SH, Chang WH, Park C, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair 2012; 26: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch 2015; 82: 4–13. [DOI] [PubMed] [Google Scholar]
- 115. Butler AJ, Wolf SL. Putting the brain on the map: use of transcranial magnetic stimulation to assess and induce cortical plasticity of upper-extremity movement. Phys Ther 2007; 87: 719–736. [DOI] [PubMed] [Google Scholar]
- 116. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol 2006; 19: 84–90. [DOI] [PubMed] [Google Scholar]
- 117. Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci 1998; 21: 149–186. [DOI] [PubMed] [Google Scholar]
- 118. Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J NeuroEngineering Rehabil 2009; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997; 48: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 120. Pascual-Leone A, Valls-Solé J, Wassermann EM, et al. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain J Neurol 1994; 117: 847–858. [DOI] [PubMed] [Google Scholar]
- 121. Huang YZ, Chen RS, Rothwell JC, et al. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 2007; 118: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 122. Ben-Shachar D, Gazawi H, Riboyad-Levin J, et al. Chronic repetitive transcranial magnetic stimulation alters beta-adrenergic and 5-HT2 receptor characteristics in rat brain. Brain Res 1999; 816: 78–83. [DOI] [PubMed] [Google Scholar]
- 123. Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 2002; 13: 2401–2405. [DOI] [PubMed] [Google Scholar]
- 124. Zhang X, Mei Y, Liu C, et al. Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J Huazhong Univ Sci Technol 2007; 27: 415–418. [DOI] [PubMed] [Google Scholar]
- 125. Brunoni AR, Boggio PS, Fregni F. Can the ‘yin and yang’ BDNF hypothesis be used to predict the effects of rTMS treatment in neuropsychiatry? Med Hypotheses 2008; 71: 279–282. [DOI] [PubMed] [Google Scholar]
- 126. Chervyakov AV, Chernyavsky AYu, Sinitsyn DO, et al. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci 2015; 9: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Aydin-Abidin S, Trippe J, Funke K, et al. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res 2008; 188: 249–261. [DOI] [PubMed] [Google Scholar]
- 128. Ljubisavljevic MR, Javid A, Oommen J, et al. The effects of different repetitive transcranial magnetic stimulation (rTMS) protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injury. PLoS One 2015; 10: e0139892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 2009; 4: e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zanardi R, Magri L, Rossini D, et al. Role of serotonergic gene polymorphisms on response to transcranial magnetic stimulation in depression. Eur Neuropsychopharmacol 2007; 17: 651–657. [DOI] [PubMed] [Google Scholar]
- 131. Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol 2008; 586: 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fedi M, Berkovic SF, Macdonell RAL, et al. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex 2008; 18: 664–669. [DOI] [PubMed] [Google Scholar]
- 133. Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol 2009; 219: 2–13. [DOI] [PubMed] [Google Scholar]
- 134. Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009; 120: 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Salter K, Jutai J, Zettler L, et al. Outcome measures in stroke rehabilitation. In: Teasell R. (eds) Evidence-based review of stroke rehabilitation. London: EBRSR, 2013, p.144. [Google Scholar]