Abstract
Background:
Multiple sclerosis (MS) is a chronic disease that may require decades of ongoing treatment. Therefore, the long-term safety and efficacy of disease-modifying therapies is an important consideration.
Methods:
The LONGTERMS study evaluated the safety and efficacy of fingolimod in patients with relapsing MS (RMS) with up to 14 years of exposure. This phase IIIb, open-label extension study included patients aged ⩾ 18 years with confirmed RMS diagnosis who completed previous phase II/III/IIIb core/extension studies of fingolimod. Patients received fingolimod 0.5 mg orally once daily; safety and efficacy (clinical and magnetic resonance imaging) were the main outcomes.
Results:
Of 4086 patients from the core studies who entered LONGTERMS, 3480 (85.2%) completed the study. The median age (range) was 38 (17–65) years and median fingolimod exposure was 944.5 (range 75–4777) days. Overall, 85.5% of patients experienced at least one adverse event (AE); most common AEs (⩾10%) were viral upper respiratory tract infection (17.3%), headache (13.3%), hypertension (11.0%) and lymphopenia (10.7%). Among patients with serious AEs (12.6%), basal cell carcinoma and MS relapse (0.9% each) were most frequently reported. The aggregate annualized relapse rate decreased from 0.22 (in years 0–2) to 0.17 (years 0–10); 45.5% of patients remained relapse free after 10 years. At year 10, 63.2% of patients were free from 6-month confirmed disability worsening.
Conclusion:
This long-term observational study of patients treated for up to 14 years with fingolimod confirmed its established safety profile with no new safety concerns. Patients with RMS receiving fingolimod had sustained low levels of disease activity and progression.
Trial Registration:
ClinicalTrials.gov identifier: NCT01201356.
Keywords: fingolimod, relapsing multiple sclerosis, long-term observational study
Introduction
Multiple sclerosis (MS) is an inflammatory and neurodegenerative central nervous system disease with pathological features that include demyelination, gliosis, and neuroaxonal injury.1–4 MS is a chronic disease, which implies that patients starting therapy should expect ongoing lifelong treatment to control disease activity and reduce the risk of disability accrual. However, data on the safety and efficacy of disease-modifying therapies (DMTs) beyond 5–7 years of therapy are limited, leaving an important knowledge gap in a patient population that may have to undergo several decades or more of therapy.
Fingolimod (FTY720, Gilenya®), a sphingosine 1-phosphate receptor (S1PR) modulator, was the first oral DMT approved for the treatment of relapsing MS (RMS),5–7 after efficacy and safety were demonstrated in pivotal phase III clinical trials against placebo (FREEDOMS,8 FREEDOMS II9) and interferon (IFN) beta-1a (TRANSFORMS).10 First reports from extension studies11,12 and from the use of fingolimod in real-world settings across different geographical regions13–19 were consistent with the results of the pivotal studies. However, as for other DMTs, longer-term data on safety and efficacy of fingolimod are needed to optimize treatment strategies for MS disease management in routine clinical practice. The LONGTERMS study was conducted to collect safety, tolerability, efficacy, and patient-reported outcome data during long-term treatment (up to 14 years) with fingolimod 0.5 mg in patients who had participated in the fingolimod clinical development program.
Methods
Study design
LONGTERMS was a single-arm, phase IIIb, open-label extension study conducted at 469 study centers in 39 countries between 13 September 2010 and 28 March 2017. Patients who completed the phase II (starting in 2003) and phase III/IIIb core studies of fingolimod (FREEDOMS, FREEDOMS II and TRANSFORMS)8–10 and their extension studies,20–24 and for whom fingolimod was not available outside of a clinical study, were offered enrollment into the open-label LONGTERMS study (see eFigure 1, supplementary appendix for patient flow). Regardless of their original treatment (fingolimod 1.25 mg or 0.5 mg once daily, or placebo/active comparator) during the ‘feeder’ studies, in LONGTERMS, all patients were treated with oral fingolimod 0.5 mg once daily.
Patients were allowed to proceed to commercial fingolimod during LONGTERMS; in such cases, they underwent an end-of-study (EoS) visit and were exempted from further follow-up visits; being considered as LONGTERMS completers. Investigators evaluated commercial fingolimod eligibility in their respective countries (i.e. reimbursement for commercial fingolimod) for all remaining active study participants by June 2016. All patients who discontinued fingolimod or did not continue with commercial fingolimod after exiting the study were requested to attend a follow-up visit 3 and 6 months after the last dose of fingolimod.
Patients without access to commercial fingolimod had the option to continue in an ongoing extension study for approximately 2 additional years up to November 2018.
Patients
Participants were aged ⩾ 18 years, diagnosed with RMS, and met the required eligibility criteria for the previous core/extension studies as described in the respective study protocols.8–10,20–24 Key exclusion criteria for the LONGTERMS study were premature permanent discontinuation from any previous fingolimod study due to adverse events (AEs), serious AEs (SAEs), laboratory abnormality, or other conditions; chronic immune system disorder other than MS requiring immunosuppressive therapy; active systemic bacterial, viral or fungal infections; and serious cardiovascular or pulmonary conditions during a previous fingolimod study.
The study was conducted in accordance with the International Conference on Harmonization Guidelines25 for Good Clinical Practice and the Declaration of Helsinki.26 The protocol and all amendments were approved by each site’s institutional review board or independent ethics committee, and all patients provided written informed consent before any study assessments.
Study outcomes and assessments
Safety outcomes
Assessments of safety and tolerability of fingolimod (primary outcome measure) included the monitoring of AEs and SAEs, and assessment of their severity and relationship to the study drug. Any clinically significant abnormalities in physical/neurological examination, hematology, electrocardiogram, and blood chemistry parameters were reported. Ophthalmic examinations, skin assessments and pulmonary function tests were performed if clinically indicated. The proportion of patients with AEs, SAEs, AEs leading to treatment discontinuation and AEs of special interest (i.e. specific to fingolimod) were reported.
Clinical outcomes
Clinical assessments (secondary outcome measures) included annualized relapse rate (ARR); annual percentage of patients with at least one relapse; proportion of patients free from relapses; change in Expanded Disability Status Scale (EDSS) score; time to 6-month confirmed disability progression (6m-CDP); and proportion of patients not reaching EDSS milestones ⩾ 4.0, ⩾6.0 and ⩾7.0. Assessments were performed at baseline, and at months 1, 3, 6, 9, 12, 18, and every 6 months thereafter until study completion/follow-up visits. EDSS scores were determined every 6 months.
Imaging
Magnetic resonance imaging (MRI) metrics included annualized rate of new or newly enlarging T2 lesions; percent brain volume change; annualized rate of brain atrophy (ARBA); and total volumes of T2 lesions and T1 hypointense lesions. These measures were required only for patients from phase II/III fingolimod trials who had MRI assessments in these prior trials and were performed at study completion or discontinuation.
Neurofilament light chain levels
Blood levels of neurofilament light chain (NfL), a key structural component of neurons and axons, and specific marker of neuroaxonal damage, were measured using the single-molecule array (SIMOATM) immunoassay27 at one central laboratory (University Hospital, Basel, Switzerland) that was kept blinded to the clinical and paraclinical data or sequence of sampling. According to sample availability from the pivotal studies, peripheral blood samples were also collected at the EoS visits.
Statistical analyses
Unless otherwise specified, baseline was defined as the last available measurement in the feeder studies before the first dose of fingolimod was administered in LONGTERMS. All patients entering LONGTERMS who received at least one dose of fingolimod in any of the studies were included in the analysis. Baseline demographics, MRI baseline characteristics and MS disease history were summarized using appropriate descriptive statistics with no inferential analysis. Two treatment groups were analyzed separately: the ‘any dose’ group (i.e. patients who received any dose of fingolimod as per the original randomization in feeder studies and received fingolimod 0.5 mg in LONGTERMS) and the ‘0.5 mg’ group (i.e. patients who received fingolimod 0.5 mg in earlier core/extension studies and continued with the same dose in LONGTERMS). Details on the statistical models used are included below the figures.
The safety population included all patients who entered LONGTERMS and received at least one dose of fingolimod. Safety and tolerability data were summarized by frequency of AEs, SAEs, discontinuations due to AEs, and incidence of clinically notable laboratory abnormalities. The incidence rates (IRs) of AEs and SAEs per 100 patient-years were calculated to adjust for treatment exposure. Confirmed and unconfirmed relapse rates were summarized separately by yearly intervals from baseline.
Results
Baseline demographics and patient characteristics
Patient demographics and baseline characteristics at the time of enrollment in the core/extension feeder studies are shown in eTable 1. These include baseline data of the overall population (i.e. patients treated with fingolimod ‘any dose’; n* = 4086), as well as the subpopulation of patients who only received the 0.5 mg dose (n** = 3168) further divided into patients having received treatment for 5 or more years (n** = 895) and patients having been exposed to fingolimod for less than 5 years (n** = 2273). For the overall population, the median age was 38 (17–65) years, 71% of participants were women, and 95% were White. The mean duration of MS since first symptoms was 8.7 years [standard deviation (SD) 6.72] and mean duration since diagnosis 5.9 years (SD 5.56). The mean duration of exposure to fingolimod prior to receiving the initial dose in the LONGTERMS study was 1.7 years (SD 1.78). At time of recruitment to the core feeder studies 1313 (32%) were treatment naïve.
Patient disposition
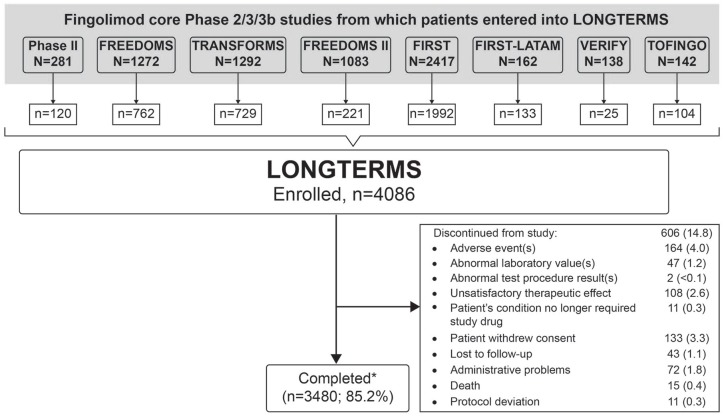
Of the patients eligible from the core/extension feeder studies, 4086 enrolled in LONGTERMS and 3480 (85.2%) completed the study (Figure 1). A total of 35.9% of completers continued on commercially available fingolimod after participating in LONGTERMS. Of the total number of completers at years 5 (80.4%) and 10 (96.3%), 43.4% and 44.3% switched to commercially available fingolimod, respectively.
Figure 1.
Patient disposition.
*Completed per protocol.
N, patients randomized/enrolled in core studies; n, patients entered into LONGTERMS study.
Safety
Fingolimod exposure
During LONGTERMS, the median patient exposure to fingolimod was 944.5 (range 75–4777) days, with 1709 (41.8%) patients being exposed for 5 years or more, 1005 (24.6%) for 8 years or more and 152 (3.7%) for 10 years or more. Although data were available for 13 years and over, continuous declines in the number of observations over the course of this long-term study were evident. A lower number of patients contributed to the results after year 7 of the long-term treatment and observation. Overall exposure to fingolimod from the first dose in the feeder studies until the end of the LONGTERMS study was 17,311 patient-years.
Discontinuation of treatment
In the safety population, a total of 223 (5.5%) patients discontinued fingolimod due to AEs, with the most frequent causes being lymphopenia (n = 17, 0.4%), hepatic enzyme increases (n = 10, 0.2%), and basal cell carcinoma (n = 10, 0.2%).
Adverse events and serious adverse events
Table 1 summarizes the IRs and percentages of the most common AEs and SAEs observed in the fingolimod ‘any dose’ group over the entire period of the study.
Table 1.
Incidence rates of the most common adverse events and serious adverse events (safety set).
| Events | Total n = 4083 n* (%; IR) |
|---|---|
| Adverse events (⩾1.0 per 100 patient-years) | |
| Viral upper respiratory tract infection | 706 (17.3; 4.22) |
| Headache | 541 (13.3; 3.28) |
| Hypertension | 448 (11.0; 2.68) |
| Lymphopenia | 438 (10.7; 2.58) |
| Fatigue | 400 (9.8; 2.34) |
| Back pain | 370 (9.1; 2.15) |
| Upper respiratory tract infection | 353 (8.6; 2.01) |
| Urinary tract infection | 351 (8.6; 1.99) |
| Depression | 345 (8.4; 2.00) |
| Hypercholesterolemia | 314 (7.7; 1.84) |
| Arthralgia | 288 (7.1; 1.65) |
| Influenza | 265 (6.5; 1.49) |
| Insomnia | 230 (5.6; 1.30) |
| Pain in extremity | 225 (5.5 1.28) |
| Bronchitis | 219 (5.4; 1.22) |
| Melanocytic nevus | 209 (5.1; 1.18) |
| Cough | 204 (5.0; 1.14) |
| Anxiety | 202 (4.9; 1.14) |
| Sinusitis | 202 (4.9; 1.13) |
| Blood cholesterol increased | 187 (4.6; 1.05) |
| Oral herpes | 185 (4.5; 1.03) |
| Lymphocyte count decreased | 183 (4.5; 1.02) |
| Cardiac disorders | 188 (4.6; 1.05) |
| Palpitation | 46 (1.1; 0.25) |
| Tachycardia/first-degree atrioventricular block | 21 (0.5; 0.11) |
| Serious adverse events (⩾0.04 per 100 patient-years) | |
| Basal cell carcinoma | 36 (0.9; 0.2) |
| Multiple sclerosis relapse | 36 (0.9; 0.2) |
| Pneumonia | 15 (0.4; 0.08) |
| Urinary tract infection | 12 (0.3; 0.07) |
| Cardiac disorders | |
| Atrial fibrillation | 6 (0.1; 0.03) |
| Myocardial infarction | 4 (0.1; 0.02) |
| Cholelithiasis | 10 (0.2; 0.05) |
| Appendicitis | 10 (0.2; 0.05) |
| Herpes zoster | 9 (0.2; 0.05) |
| Squamous cell carcinoma | 9 (0.2; 0.05) |
| Depression | 10 (0.2; 0.05) |
| Intervertebral disc protrusion | 8 (0.2; 0.04) |
| Breast cancer | 8 (0.2; 0.04) |
| Epilepsy | 8 (0.2; 0.04) |
| Abortion spontaneous | 8 (0.2; 0.04) |
| Cellulitis | 7 (0.2; 0.04) |
| Ankle fracture | 7 (0.2; 0.04) |
| Back pain | 7 (0.2; 0.04) |
| Osteoarthritis | 7 (0.2; 0.04) |
| Uterine leiomyoma | 7 (0.2; 0.04) |
| Cervical dysplasia | 7 (0.2; 0.04) |
IR, incidence rate; n*, number of patients who experienced at least one adverse event.
Adverse events. Overall, 85.5% of patients reported at least one AE during the study [mild (60%), moderate (27%), severe (3%)]. The most common AEs were viral upper respiratory tract infection (17.3%; IR 4.22), headache (13.3%; IR 3.28), hypertension (11.0%; IR 2.68) and lymphopenia (10.7%; IR 2.58). Four patients had extremely low lymphopenia (⩽0.05 × 109/l), observed as singles event during the study. Cardiac abnormalities were noted in 4.6% of patients with the most frequent being palpitations (1.1%), tachycardia (0.5%) and first-degree atrioventricular block (0.5%).
In total, 51.9% (IR 17.06) of patients reported AEs related to infections and infestations; these included viral infection of the upper respiratory tract [n = 706 (IR 4.22)], nonspecified upper respiratory tract infection [353 (IR 2.01)], urinary tract infection [351 (IR 1.99)], influenza [265 (IR 1.49)], bronchitis [219 (IR 1.22)], sinusitis [202 (IR 1.13)], and oral herpes [185 (IR 1.03)]. Of these, the AEs were suspected to be related to fingolimod in 19.9% of patients; 0.7% of patients discontinued the study drug due to infection- and infestation-related AEs. Herpes zoster was reported in 2.4% of patients.
Serious adverse events. Overall, 12.6% of patients experienced at least one SAE during the study. The most common SAEs were basal cell carcinoma and MS relapse (both 0.9%), pneumonia (0.08%) and urinary tract infection (0.3%). Cryptococcal meningitis was reported in one patient (<0.1%; diagnosis remained unconfirmed and patient record incomplete due to patient being lost to follow up). Serious cardiac events occurred in 0.6% of patients, with atrial fibrillation evident in 0.1% of patients and myocardial infarction noted in less than 0.1% of patients (Table 1). A similar proportion of patients (2.6% each) reported SAEs related to infections and infestations, and neoplasms, the latter of which included cysts and polyps as well as benign, malignant, and unspecified neoplasms.
A total of 17 (0.4%) deaths occurred during the long-term extension study; 12 cases were not suspected (cardiopulmonary failure; road traffic accident; subarachnoid hemorrhage; general disorders; metastatic colon cancer; pancreatic carcinoma; small-cell lung cancer; coma; ischemic stroke; thrombotic stroke; adjustment disorder with depressed mood; and acute respiratory distress syndrome) and 4 were suspected to be related to fingolimod treatment by the investigator (nervous system disorder resulting in an altered state of consciousness; suicide; myocardial infarction; and multiorgan dysfunction syndrome); a cause for 1 death remains unknown despite multiple attempts to follow up with the investigator.
Evolution of annual frequencies of AEs and SAEs up to 14 years. Safety data were collected up to 14 years; however, by year 11, AEs substantially declined. Frequencies of AEs and SAEs by yearly interval are shown in Table 2. At least one AE was reported for 75.3% of the patient population, most commonly diarrhea, fatigue, and influenza, all of which occurred at higher frequency in year 1 but subsequently decreased over the course of the study. A clear trend of decreased incidences in headache and lymphopenia were observed from year 1 to year 14. Leukopenia, palpitations, vision blurred, sinusitis, and oral herpes AEs were reported with low frequencies across the study yearly intervals. Urinary tract infection AEs were reported at slightly higher frequency from year 1 to year 4 with gradual decrease observed from year 5 onward. No new- or late-appearing rare AEs emerged during this long-term exposure to fingolimod.
Table 2.
Adverse events and serious adverse events of interest by yearly interval (safety set).
| Fingolimod ‘any dose’ group
(n* = 4086) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preferred term | Year 1 n** = 4086 n (%) | Year 2 n** =2871 n (%) | Year 3 n** = 2252 n (%) | Year 4 n** = 1997 n (%) | Year 5 n** = 1887 n (%) | Year 6 n** = 1767 n (%) | Year 7 n** = 1591 n (%) | Year 8 n** = 1469 n (%) | Year 9 n** = 1138 n (%) | Year 10 n** = 765 n (%) | Year 11 n** = 169 n (%) | Year 12 n** = 105 n (%) | Year 13 n** = 93 n (%) | Total n* = 4086 n (%) |
| Total AEs | 2503 (61.3) | 1195 (41.6) | 920 (40.9) | 685 (34.3) | 634 (33.6) | 491 (27.8) | 412 (25.9) | 349 (23.8) | 234 (20.6) | 103 (13.5) | 29 (17.2) | 25 (23.8) | 15 (16.1) | 3075 (75.3) |
| Lymphopenia | 315 (7.7) | 141 (4.9) | 159 (7.1) | 72 (3.6) | 45 (2.4) | 30 (1.7) | 18 (1.1) | 27 (1.8) | 14 (1.2) | 6 (0.8) | 1 (0.6) | 1 (1.0) | 1 (1.1) | 652 (16.0) |
| Leukopenia | 78 (1.9) | 43 (1.5) | 36 (1.6) | 14 (0.7) | 19 (1.0) | 8 (0.5) | 5 (0.3) | 7 (0.5) | 4 (0.4) | 1 (0.1) | 0 | 0 | 0 | 165 (4.0) |
| Neutropenia | 23 (0.6) | 6 (0.2) | 11 (0.5) | 3 (0.2) | 5 (0.3) | 2 (0.1) | 1 (<0.1) | 2 (0.1) | 0 | 0 | 0 | 0 | 0 | 48 (1.2) |
| Palpitations | 61 (1.5) | 23 (0.8) | 9 (0.4) | 4 (0.2) | 5 (0.3) | 2 (0.1) | 5 (0.3) | 4 (0.3) | 3 (0.3) | 0 | 0 | 0 | 0 | 110 (2.7) |
| Bradycardia | 44 (1.1) | 4 (0.1) | 6 (0.3) | 0 | 1 (<0.1) | 1 (<0.1) | 0 | 0 | 2 (0.2) | 0 | 0 | 0 | 0 | 57 (1.4) |
| Angina pectoris | 10 (0.2) | 2 (<0.1) | 2 (<0.1) | 3 (0.2) | 3 (0.2) | 3 (0.2) | 2 (0.1) | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 23 (0.6) |
| Vision blurred | 55 (1.3) | 20 (0.7) | 14 (0.6) | 8 (0.4) | 10 (0.5) | 7 (0.4) | 7 (0.4) | 9 (0.6) | 3 (0.3) | 2 (0.3) | 0 | 1 (1.0) | 0 | 120 (2.9) |
| Macular edema | 0 | 0 | 0 | 0 | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<0.1) |
| Diarrhea | 253 (6.2) | 82 (2.9) | 58 (2.6) | 38 (1.9) | 26 (1.4) | 25 (1.4) | 22 (1.4) | 17 (1.2) | 6 (0.5) | 4 (0.5) | 0 | 1 (1.0) | 0 | 442 (10.8) |
| Fatigue | 329 (8.1) | 84 (2.9) | 61 (2.7) | 40 (2.0) | 44 (2.3) | 33 (1.9) | 24 (1.5) | 20 (1.4) | 12 (1.1) | 5 (0.7) | 2 (1.2) | 2 (1.9) | 0 | 561 (13.7) |
| Influenza | 258 (6.3) | 108 (3.8) | 91 (4.0) | 57 (2.9) | 70 (3.7) | 51 (2.9) | 34 (2.1) | 42 (2.9) | 20 (1.8) | 13 (1.7) | 3 (1.8) | 4 (3.8) | 2 (2.2) | 562 (13.8) |
| Urinary tract infection | 202 (4.9) | 133 (4.6) | 96 (4.3) | 79 (4.0) | 71 (3.8) | 58 (3.3) | 57 (3.6) | 54 (3.7) | 38 (3.3) | 17 (2.2) | 8 (4.7) | 4 (3.8) | 1 (1.1) | 519 (12.7) |
| Bronchitis | 154 (3.8) | 91 (3.2) | 68 (3.0) | 52 (2.6) | 56 (3.0) | 36 (2.0) | 47 (3.0) | 28 (1.9) | 19 (1.7) | 3 (0.4) | 2 (1.2) | 0 | 1 (1.1) | 427 (10.5) |
| Sinusitis | 158 (3.9) | 91 (3.2) | 57 (2.5) | 44 (2.2) | 46 (2.4) | 45 (2.5) | 22 (1.4) | 22 (1.5) | 19 (1.7) | 12 (1.6) | 2 (1.2) | 3 (2.9) | 1 (1.1) | 374 (9.2) |
| Oral herpes | 150 (3.7) | 85 (3.0) | 72 (3.2) | 53 (2.7) | 45 (2.4) | 38 (2.2) | 27 (1.7) | 25 (1.7) | 14 (1.2) | 7 (0.9) | 1 (0.6) | 1 (1.0) | 1 (1.1) | 325 (8.0) |
| Herpes zoster | 42 (1.0) | 27 (0.9) | 15 (0.7) | 17 (0.9) | 13 (0.7) | 16 (0.9) | 17 (1.1) | 8 (0.5) | 10 (0.9) | 4 (0.5) | 1 (0.6) | 1 (1.0) | 0 | 160 (3.9) |
| ALT increased | 175 (4.3) | 82 (2.9) | 49 (2.2) | 29 (1.5) | 24 (1.3) | 18 (1.0) | 10 (0.6) | 9 (0.6) | 7 (0.6) | 2 (0.3) | 0 | 0 | 0 | 298 (7.3) |
| GGT increased | 114 (2.8) | 59 (2.1) | 38 (1.7) | 30 (1.5) | 17 (0.9) | 9 (0.5) | 9 (0.6) | 9 (0.6) | 6 (0.5) | 5 (0.7) | 0 | 1 (1.0) | 0 | 226 (5.5) |
| Hepatic enzyme increased | 73 (1.8) | 26 (0.9) | 22 (1.0) | 12 (0.6) | 12 (0.6) | 8 (0.5) | 5 (0.3) | 2 (0.1) | 2 (0.2) | 1 (0.1) | 0 | 0 | 1 (1.1) | 140 (3.4) |
| Blood TG increased | 39 (1.0) | 20 (0.7) | 9 (0.4) | 10 (0.5) | 10 (0.5) | 6 (0.3) | 6 (0.4) | 8 (0.5) | 1 (<0.1) | 1 (0.1) | 0 | 0 | 0 | 98 (2.4) |
| AST increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 | 0 | 0 | 78 (1.9) |
| Basal cell carcinoma | 5 (0.1) | 3 (0.1) | 5 (0.2) | 6 (0.3) | 5 (0.3) | 8 (0.5) | 14 (0.9) | 5 (0.3) | 10 (0.9) | 4 (0.5) | 1 (0.6) | 0 | 0 | 62 (1.5) |
| Headache | 690 (16.9) | 189 (6.6) | 128 (5.7) | 83 (4.2) | 66 (3.5) | 57 (3.2) | 36 (2.3) | 26 (1.8) | 19 (1.7) | 6 (0.8) | 4 (2.4) | 2 (1.9) | 0 | 983 (24.1) |
| Hypertension | 215 (5.3) | 89 (3.1) | 64 (2.8) | 50 (2.5) | 40 (2.1) | 31 (1.8) | 31 (1.9) | 23 (1.6) | 15 (1.3) | 5 (0.7) | 1 (0.6) | 3 (2.9) | 0 | 495 (12.1) |
| Total SAEs | 43 (1.1) | 16 (0.6) | 16 (0.7) | 9 (0.5) | 17 (0.9) | 13 (0.7) | 15 (0.9) | 9 (0.6) | 0 | 0 | 0 | 0 | 0 | 138 (3.4) |
| Lymphopenia | 1 (<0.1) | 0 | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (<0.1) |
| Bradycardia | 15 (0.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 (0.4) |
| Angina pectoris | 0 | 1 (<0.1) | 1 (<0.1) | 0 | 1 (<0.1) | 0 | 2 (0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 4 (<0.1) |
| Fatigue | 1 (<0.1) | 0 | 0 | 0 | 0 | 2 (0.1) | 0 | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 4 (<0.1) |
| Urinary tract infection | 3 (<0.1) | 2 (<0.1) | 4 (0.2) | 0 | 1 (<0.1) | 3 (0.2) | 1 (<0.1) | 2 (0.1) | 1 (<0.1) | 0 | 0 | 0 | 0 | 16 (0.4) |
| Herpes zoster | 2 (<0.1) | 2 (<0.1) | 1 (<0.1) | 0 | 2 (0.1) | 2 (0.1) | 3 (0.2) | 0 | 0 | 1 (0.1) | 0 | 0 | 0 | 13 (0.3) |
| Influenza | 1 (<0.1) | 0 | 1 (<0.1) | 1 (<0.1) | 1 (<0.1) | 0 | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 5 (0.1) |
| Back pain | 4 (<0.1) | 2 (<0.1) | 1 (<0.1) | 1 (<0.1) | 1 (<0.1) | 2 (0.1) | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 12 (0.3) |
| Basal cell carcinoma | 4 (<0.1) | 2 (<0.1) | 5 (0.2) | 5 (0.3) | 5 (0.3) | 3 (0.2) | 7 (0.4) | 4 (0.3) | 2 (0.2) | 1 (0.1) | 0 | 0 | 0 | 38 (0.9) |
| Headache | 1 (<0.1) | 1 (<0.1) | 0 | 0 | 2 (0.1) | 0 | 0 | 2 (0.1) | 0 | 0 | 0 | 0 | 0 | 6 (0.1) |
| Hypertension | 1 (<0.1) | 1 (<0.1) | 0 | 1 (<0.1) | 0 | 1 (<0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (<0.1) |
n*, total number of patients; n**, number of active patients (i.e. those who did not discontinue permanently from study treatment) with at least 1 day of exposure in the year interval being reported.
All SAEs with onset on or after the first dose date of fingolimod among all studies are included.
AE, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; SAE, serious adverse events; TG, triglycerides.
With respect to SAEs, 3.4% of patients reported at least one event over the 14-year period, with frequencies of SAEs decreasing gradually to 10 years and none reported from year 11 onward. Bradycardia-related SAEs were evident in year 1 (0.4%) and absent thereafter. Lymphopenia was observed in <0.1% of patients in years 1 and 3, and no cases were reported from year 4 onward. Urinary tract infection SAEs only occurred at very low frequencies (<0.1–0.2%) between years 1 and 9 and were absent thereafter. Likewise, influenza and back pain only occurred at very low frequencies (<0.1%) between years 1 and 7. The frequency of herpes zoster-related SAEs increased slightly from year 1 (<0.1%) to year 7 (0.2%); thereafter, only one case was reported in year 10. Serious AEs related to basal cell carcinoma were reported at a low frequency (⩽0.3%) between years 1 and 6, followed by a slight increase (0.4%) in year 7 that reduced to 0.1% by year 10.
Efficacy
Annualized relapse rates
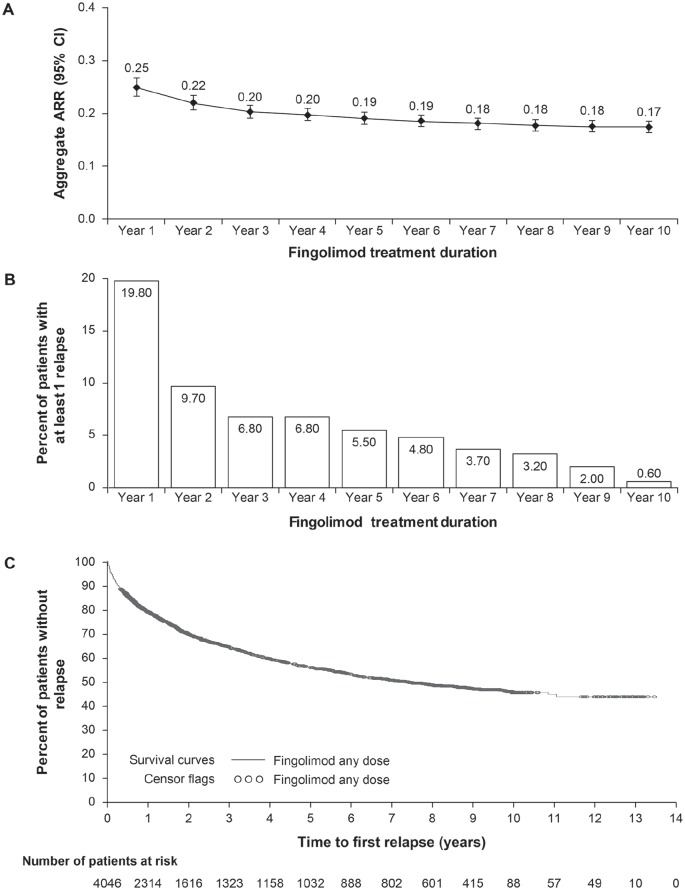
Aggregate ARRs fell from 0.22 at year 2 to 0.17 at year 10, and a low aggregate ARR between years 5 and 10 was sustained in the ‘any dose’ fingolimod treatment group throughout the extension study [Figure 2(a)]. With continuous fingolimod treatment, the annual percentage of patients with a minimum of one relapse decreased from 9.7% in year 2 to 5.5% in year 5 and 0.6% at year 10 [Figure 2(b)]. By year 10, 45.5% of fingolimod-treated patients remained relapse free [Figure 2(c)]. Reductions in relapse rates in the fingolimod ‘0.5 mg’ treatment group followed a similar trend to year 10 as those observed in the fingolimod ‘any dose’ group.
Figure 2.
Study relapse rates.
(a) Aggregate annualized relapse ratesa; (b) annual percentage of patients with at least one relapseb; (c) proportion of patients free from relapses.
aARR estimate and two-sided 95% CI are from a negative binomial regression model, adjusted for number of relapses in the previous 2 years before enrollment in core study and EDSS score at first dose of fingolimod baseline. Log time in study is the offset variable.
bAnnual percentage of patients with at least one relapse included both confirmed and unconfirmed relapses (n = 4046). Confirmed relapses were defined as ⩾0.5-point increase in the EDSS score/1-point in two functional systems (FS) of the EDSS or 2 points in one FS (excluding bowel/bladder/cerebral FS); unconfirmed relapses were those without EDSS confirmation.
ARR, annualized relapse rate; CI, confidence interval; EDSS, Expanded Disability Status Scale.
Disability outcomes
Mean EDSS scores increased slightly from baseline with continuous exposure to fingolimod. In the fingolimod ‘any dose’ group, mean changes from baseline in EDSS scores were −0.01 and 0.40 at years 2 and 10, respectively. Stable EDSS scores from baseline to year 10 were observed in 59.1% of the fingolimod ‘any dose’ group and 72.1% of the fingolimod ‘0.5 mg’ group; at EoS, stable EDSS scores were observed in 63.4% and 67.0%, respectively. Improvements in EDSS scores from baseline to year 10 were observed in 12.8% and 7.0% of the fingolimod ‘any dose’ and ‘0.5 mg’ groups, respectively.
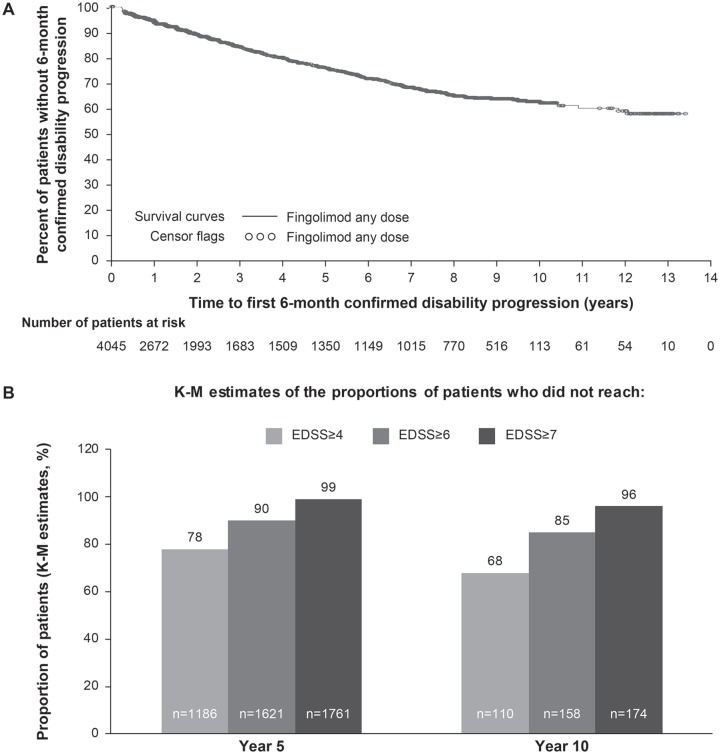
Approximately 63.2% and 68.1% patients from the fingolimod ‘any dose’ and ‘0.5 mg’ groups, respectively, remained free from 6m-CDP at year 10 [Figure 3(a)]. By the end of year 10, the majority of patients did not reach the EDSS milestones of ⩾4.0 (67.7%), ⩾6.0 (84.8%), and ⩾7.0 [96%; Figure 3(b)].
Figure 3.
Disability status of patients over the study period.
(a) Proportion of patients free from 6-month confirmed disability progression; (b) proportion of patients not reaching EDSS of ⩾4.0, ⩾6.0, or ⩾7.0.
EDSS, Expanded Disability Status Scale; K-M, Kaplan–Meier.
MRI outcomes (lesion activity)
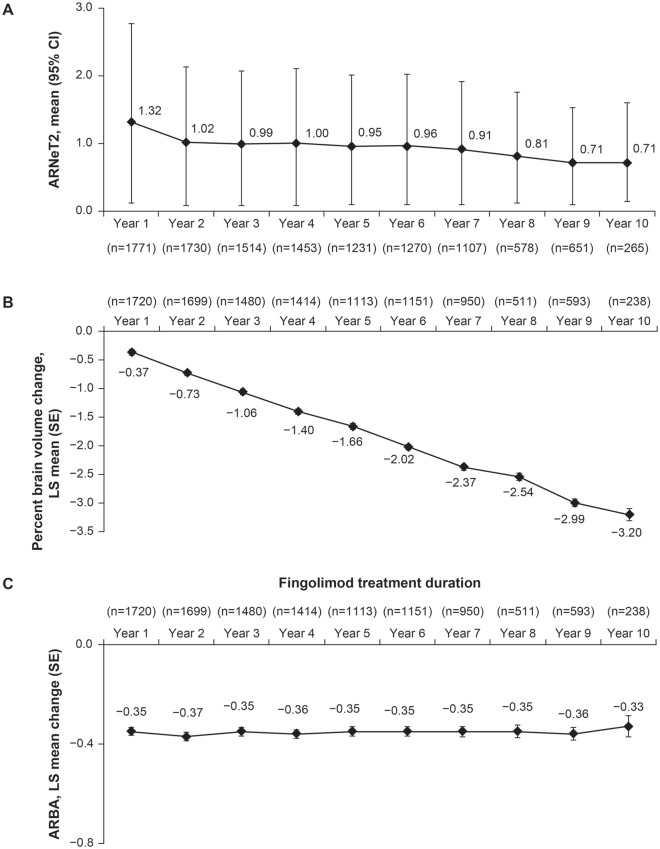
Annualized rates of new/newly enlarging T2 lesions decreased gradually over the course of the study from 1.02 at year 2 to 0.71 at year 10 in the fingolimod ‘any dose’ treatment group [Figure 4(a)]. The mean number of T1 hypointense lesions increased from baseline to year 2, after which they remained stable. At the EoS visit, mean change from baseline in T1 hypointense lesion volume was +800.6 mm3. The mean total volume of T2 lesions increased at years 3 and 4 compared with baseline, and remained stable at later visits, with the mean volume change from baseline being 1588.5 mm3 at the EoS visit. At year 10, the mean decrease in brain volume compared with baseline was 3.2% [Figure 4(b)]. As assessed by ARBA, changes in brain volume remained stable over the duration of the study [Figure 4(c)].
Figure 4.
Physiological parameters versus time.
(a) Annualized rates of new/newly enlarging T2 lesions by timea; (b) cumulative percent brain volume change by timeb; (c) annualized percent brain volume change by timec.
aAnnualized rate of new/newly enlarging T2 lesions estimate is from a negative binomial regression model adjusted for volume of T2 lesions at first dose of fingolimod baseline. Log time in study is the offset variable.
bA mixed-effects model with repeated measures and autoregressive within-subject covariance structure of first order was used with visit, normalized brain volume at core baseline, T2 lesion volume at first dose of fingolimod baseline, and Gd-T1 count at first dose of fingolimod baseline as fixed effects, and individual patient as a random effect. Kenward and Rogers’s adjustment for the degrees of freedom was applied.
cA mixed-effects model with repeated measures and compound symmetry covariance structure was used with visit, baseline normalized brain volume, T2 lesion volume and Gd-T1 count as fixed effects and individual patient as a random effect. Kenward and Rogers’s adjustment for the degrees of freedom was applied.
ARBA, annualized rate of brain atrophy; ARNeT2, annualized rates of new/newly enlarging T2 lesions; CI, confidence interval; Gd-T1, gadolinium-T1 lesion; LS, least squares; SE, standard error.
NfL biomarker outcomes
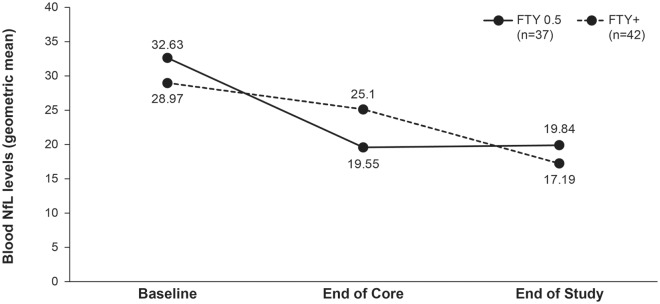
Only a minority of patients had available samples at baseline of the feeder studies, end of the core studies and at EoS. In these patients, geometric mean plasma NfL levels in the ‘0.5 mg’ treatment group were significantly reduced during fingolimod treatment compared with the baseline of the feeder studies by 40.7% (19.54 pg/ml, p < 0.0001) at month 12, 42.9% (19.74 pg/ml, p = 0.0002) at month 24, 40.7% (19.55 pg/ml, p < 0.0001) at the end of the core study, and 39.2% (19.84 pg/ml, p = 0.0002) at EoS (Figure 5).
Figure 5.
Blood NfL levels before starting fingolimod in feeder studies, at LONGTERMS baseline and at end of LONGTERMS study.
FTY, fingolomod; NfL, neurofilament light chain.
Discussion
LONGTERMS, an open-label, phase IIIb extension study, provides further information regarding the long-term safety profile and sustained efficacy of fingolimod. Results indicated that fingolimod treatment for up to 14 years had a well-characterized, manageable safety profile, with no new or unexpected safety concerns. The low percentage of patients who discontinued due to AEs in LONGTERMS (5.5%), suggests good long-term tolerability, although it might be argued that patients with AEs had already discontinued in the course of the feeder studies.
The overall pattern of AEs or SAEs reported with long-term fingolimod treatment was similar to those reported in the feeder studies,8–12 and from real-world studies.13–19 In line with those previous studies, the most commonly reported AEs were headache, lymphopenia, and influenza, all of which showed a clear decreasing trend from year 1 to year 11 in LONGTERMS. In addition to selective drop out in the feeder studies, such a decrease in reported AEs could be due to reduced reporting by the investigators or patients who had adapted to recurring similar AEs.
The reduction in circulating lymphocytes is a known pharmacodynamic effect of fingolimod and is hypothesized to be the main mechanism by which fingolimod exerts its therapeutic effect in MS. Therefore, lymphopenia was not mandated to be reported by investigators in the study protocol and the analysis was based on an absolute laboratory count. Lymphopenia was typically not associated with any signs or symptom but was defined by a low lymphocyte count (<200 cells/mm3) being detected. No extremely low lymphocyte counts (<50 cells/mm3) were reported from year 4 onwards in LONGTERMS, and there was no correlation between low lymphocyte count and rate of infections.
The incidence of herpes infections reported here is in line with observations from a safety analysis of several clinical studies (including patients exposed to fingolimod over 7 years),28 antibody status to varicella zoster virus should be checked before starting fingolimod, and vigilance should be maintained regarding zoster occurrence.28 In the postmarketing setting, cases of cryptococcal meningitis have been reported after approximately 2–3 years of treatment, although an exact relationship with the duration of treatment is unknown. Other fingolimod-specific AEs, as observed in the mentioned safety analysis,28 include transient, mostly asymptomatic reductions in heart rate, increase in blood pressure, macular edema, and liver enzyme elevations. The effect on heart rate is likely to be mediated by the modulation of S1PRs in atrial myocytes, and in sinus and atrioventricular (AV) nodal cells is limited due to internalization of the receptors.29 This was confirmed in LONGTERMS, in which no case of bradycardia was reported from the second year onwards and no patients had reported transient bradycardia post reintroduction of fingolimod after temporary interruption. Hypertension, potentially related to the role of S1PR in regulating the endothelial barrier,30 also steadily decreased from year 1 to year 11. Only two cases of macular edema were reported over the entire study period; one case may have been related to the reintroduction of fingolimod after interruption while the other case was not related to fingolimod. This is in line with previous reports suggesting that macular edema is not associated with long-term use of fingolimod and resolves after drug discontinuation.8–12,28,31
The mechanism responsible for liver enzyme elevations known to occur with fingolimod is unclear. In this study, the number of cases appeared to decrease steadily over time, with elevations of aspartate amino transferase above normal in 0.3% of participants at year 2 and 0.2% at EoS. Similarly, raised alanine aminotransferase levels occurred in 2.6% of participants at year 2 and 1.8% at EoS. Again, over a long treatment duration, mild abnormalities of laboratory results were not considered clinically significant by the investigators.
As with any immunomodulatory agent, the effect of fingolimod on the immune system might confer an increased risk of malignancy, which in the current study was experienced by 1.6% of fingolimod-treated patients. Whereas hematological or solid organ malignancies were rare, skin malignancies, basal cell carcinoma and squamous cell carcinoma were more common. This is consistent with the slightly higher incidence of basal cell carcinoma observed at baseline in LONGTERMS and in completed studies with fingolimod, and should continue to be evaluated in the postmarketing setting.28 However, it was reassuring that the incidence of malignancies did not appear to increase with increasing drug exposure.
Clinical and radiological outcomes, including a reduction in relapse rates and annualized rates of new/newly enlarging T2 lesions, over a decade of fingolimod treatment compare favorably with those reported in studies with shorter duration of follow up.8–12 This is reflected in the high proportion of patients who retained an EDSS score below 3, approximately two thirds not reaching EDSS 4.0, and a similar proportion remaining free from 6m-CDP at year 10. Fingolimod was the first treatment to demonstrate a beneficial effect on brain volume loss (BVL) in phase III studies compared with placebo and IFN beta-1a.8–12 In this extension study, the annual BVL observed in the core ‘feeder studies’ that was significantly reduced compared with the placebo arm was maintained across the entire LONGTERMS study period. Together with the cumulative rate of 3.78% in 11 years, these findings further confirm that slowing of BVL persists with rates close to those of normal aging (0.1–0.3% per year in healthy people32) and supports a potential tissue-protective effect of fingolimod, which is further substantiated by the significant and sustained decrease in NfL observed in a subgroup of patients.
A factor limiting the interpretation of these long-term results is the bias that could result from selective dropout of patients experiencing a lack of efficacy or AEs (attrition bias).33 The post hoc comparison of baseline and available follow-up characteristics of those patients who remained in the study for more than 5 years compared with patients who stayed for a shorter duration indicated that, when adjusted for time on study at time of discontinuation, disease activity, including relapses and MRI outcomes, was somewhat higher in ‘early completers’ (i.e. those that left the study before 5 years) than ‘late completers’ (i.e. those that left the study after ⩾5 years). Some of these differences may be attributed to different baseline disease characteristics (i.e. higher disease activity and longer duration of disease in those who withdrew from the study earlier). It should be noted, however, that more than a third of patients completed the study earlier due to fingolimod becoming commercially available and therefore independent of any safety/efficacy reason. Indeed, AEs and lack of efficacy were given as reasons for discontinuation in only 3.2% and 1.8% of early completers, respectively, and 4.1% and 3.5% for patients who stayed in the study for a least 5 years. In addition, as with all open-label studies, the lack of a placebo control limits conclusions regarding safety.
In conclusion, these results suggest that continuous administration of fingolimod for 10 years or more in patients with RMS is associated with sustained benefits for control of disease activity and disability progression without emerging safety concerns.
Supplemental Material
Supplemental material, LONGTERMS_supplementary_ for Extended treatment with fingolimod for relapsing multiple sclerosis: the 14-year LONGTERMS study results by Jeffrey A. Cohen, Nadia Tenenbaum, Alit Bhatt, Ying Zhang and Ludwig Kappos in Therapeutic Advances in Neurological Disorders
Acknowledgments
All authors critically reviewed each stage in the development of this manuscript and approved the final version for submission.
Footnotes
Funding: Medical funding was provided by Novartis Pharma AG, Basel, Switzerland and medical writing support was provided by Ashwini Patil (Novartis Healthcare Pvt. Ltd., Hyderabad, India), and Ian Wright (Novartis Irl Ltd., Dublin, Ireland). The final responsibility of the content lies with the authors.
Conflict of interest statement: Jeffrey Cohen reports personal compensation for consulting for Convelo, Population Council; speaking for Mylan; and serves as an Editor of Multiple Sclerosis Journal.
Ludwig Kappos’s institution (University Hospital Basel) has received in the last 3 years and used exclusively for research support: steering committee, advisory board, and consultancy fees (Actelion, Addex, Bayer HealthCare, Biogen Idec, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB, and Xenoport); speaker fees (Bayer HealthCare, Biogen Idec, Merck, Novartis, Sanofi, and Teva); support of educational activities (Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen Idec, European Union, Innoswiss, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation).
Nadia Tenenbaum, Alit Bhatt and Ying Zhang are employees of Novartis.
ORCID iD: Alit Bhatt  https://orcid.org/0000-0001-9245-9772
https://orcid.org/0000-0001-9245-9772
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jeffrey A. Cohen, Mellen Center for Multiple Sclerosis Treatment and Research, Cleveland Clinic Foundation, 9500 Euclid Avenue/U10, Cleveland, OH 44195, USA.
Nadia Tenenbaum, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Alit Bhatt, Novartis Healthcare Pvt. Ltd., Hyderabad, India.
Ying Zhang, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Ludwig Kappos, Departments of Medicine, Clinical Research, Biomedicine and Biomedical Engineering, University Hospital and University of Basel, Basel, Switzerland.
References
- 1. De Stefano N, Tomic D, Radue EW, et al. Effect of fingolimod on diffuse brain tissue damage in relapsing-remitting multiple sclerosis patients. Mult Scler Relat Disord 2016; 7: 98–101. [DOI] [PubMed] [Google Scholar]
- 2. Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol 2005; 23: 683–747. [DOI] [PubMed] [Google Scholar]
- 3. Sospedra M, Martin R. Immunology of multiple sclerosis. Semin Neurol 2016; 36: 115–127. [DOI] [PubMed] [Google Scholar]
- 4. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. [DOI] [PubMed] [Google Scholar]
- 5. Kumar A, Sahni JN, Stangos AN, et al. Effectiveness of ranibizumab for neovascular age-related macular degeneration using clinician-determined retreatment strategy. Br J Ophthalmol 2011; 95: 530–533. [DOI] [PubMed] [Google Scholar]
- 6. La Mantia L, Tramacere I, Firwana B, et al. Fingolimod for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 2016; 4: CD009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singer BA. Initiating oral fingolimod treatment in patients with multiple sclerosis. Ther Adv Neurol Dis 2013; 6: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 9. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 10. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 11. Cohen JA, Khatri B, Barkhof F, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiat 2016; 87: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappos L, O’Connor P, Radue EW, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology 2015; 84: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alsop J, Medin J, Cornelissen C, et al. Two studies in one: a propensity-score-matched comparison of fingolimod versus interferons and glatiramer acetate using real-world data from the independent German studies, PANGAEA and PEARL. PLoS One 2017; 12: e0173353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox E, Edwards K, Burch G, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, evaluate patient outcomes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord 2014; 3: 607–619. [DOI] [PubMed] [Google Scholar]
- 15. Izquierdo G, Damas F, Paramo MD, et al. The real-world effectiveness and safety of fingolimod in relapsing-remitting multiple sclerosis patients: an observational study. PLoS One 2017; 12: e0176174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laroni A, Brogi D, Morra VB, et al. Safety and tolerability of fingolimod in patients with relapsing-remitting multiple sclerosis: results of an open-label clinical trial in Italy. Neurolog Sci 2017; 38: 53–59. [DOI] [PubMed] [Google Scholar]
- 17. Rasenack M, Rychen J, Andelova M, et al. Efficacy and safety of fingolimod in an unselected patient population. PLoS One 2016; 11: e0146190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ticha V, Kodym R, Pocikova Z, et al. Real-world outcomes in fingolimod-treated patients with multiple sclerosis in the Czech Republic: results from the 12-month GOLEMS study. Clin Drug Invest 2017; 37: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziemssen T, Albrecht H, Haas J, et al. 36 months PANGAEA: a 5-year non-interventional study of safety, efficacy and pharmacoeconomic data for fingolimod patients in daily clinical practice. Value Health 2015; 18: A749. [Google Scholar]
- 20. Gold R, Comi G, Palace J, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol 2014; 261: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355: 1124–1140. [DOI] [PubMed] [Google Scholar]
- 22. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015; 84: 872–879. [DOI] [PubMed] [Google Scholar]
- 23. Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 2015; 85: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ordonez-Boschetti L, Rey R, Cruz A, et al. Safety and tolerability of fingolimod in Latin American patients with relapsing-remitting multiple sclerosis: the open-label FIRST LATAM study. Adv Ther 2015; 32: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 26. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kappos L, Cohen J, Collins W, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Mult Scler Relat Disord 2014; 3: 494–504. [DOI] [PubMed] [Google Scholar]
- 29. Koyrakh L, Roman MI, Brinkmann V, et al. The heart rate decrease caused by acute FTY720 administration is mediated by the G protein-gated potassium channel I. Am J Transplant 2005; 5: 529–536. [DOI] [PubMed] [Google Scholar]
- 30. Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev Drug Discov 2010; 9: 883–897. [DOI] [PubMed] [Google Scholar]
- 31. Zarbin MA, Jampol LM, Jager RD, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology 2013; 120: 1432–1439. [DOI] [PubMed] [Google Scholar]
- 32. De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147–156. [DOI] [PubMed] [Google Scholar]
- 33. Sormani MP, Bruzzi P. Can we measure long-term treatment effects in multiple sclerosis? Nature Rev Neurol 2015; 11: 176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, LONGTERMS_supplementary_ for Extended treatment with fingolimod for relapsing multiple sclerosis: the 14-year LONGTERMS study results by Jeffrey A. Cohen, Nadia Tenenbaum, Alit Bhatt, Ying Zhang and Ludwig Kappos in Therapeutic Advances in Neurological Disorders