Abstract
Background:
Few studies have evaluated the prognostic value of the integrated model consisting of gross tumor volume of lymph nodes (GTVnd) and pretreatment plasma Epstein–Barr virus DNA (pre-EBV DNA) in nasopharyngeal carcinoma (NPC) patients.
Methods:
A well-established big-data intelligence platform with 10,126 NPC patients was used for a retrospective review. A total of 1500 cases with cervical nodal metastases but without distant metastases were randomly assigned to a training (n = 503) or test condition (n = 997) for analyses. The cut-off point for the GTVnd derived from the receiver operating characteristic (ROC) curve was combined with the published cut-off point for pre-EBV DNA to develop an integrated model by which patients were classified into four groups.
Results:
Both GTVnd and pre-EBV DNA were independent prognostic factors. Regardless of whether patients received induction chemotherapy (IC), the 5-year distant metastasis-free survival (DMFS) (69.5%) and overall survival (OS) (68.4%) were significantly worse in those with both a GTVnd >20 ml and pre-EBV DNA >2000 copies/ml (all p-values < 0.001). In patients with IC, all others had better 5-year DMFS and OS; in patients without IC, those with either a GTVnd >20 ml or pre-EBV DNA >2000 copies/ml had the medium 5-year DMFS and OS, while patients with neither of them had the best.
Conclusions:
The integrated GTVnd and pre-EBV DNA model not only predicted DMFS and OS in NPC patients effectively, but was an indicator of timely adjustment of therapeutic strategies for NPC patients, especially those completing IC.
Keywords: distant metastasis-free survival, gross tumor volume of lymph nodes, nasopharyngeal carcinoma, overall survival, pretreatment plasma Epstein–Barr virus DNA, prognostic model
Introduction
Nasopharyngeal carcinoma (NPC) is a unique head and neck cancer with an unbalanced ethnic and geographic distribution, with 80% of global cases occurring in Asian countries.1 Radiotherapy (RT) is the standard treatment option for nonmetastatic NPC, and with intensity-modulated radiation therapy (IMRT), a milestone development in RT technology, local-regional control of NPC has improved considerably with 5-year local and regional recurrence-free survival rates of 83–92% and 83–97%, respectively. Currently, distant metastasis after standard treatment has become the main reason for failure in this disease.2
The tumor–node–metastasis (TNM) staging system is the key determinant for risk stratification for treatment decisions and prognostic predictions of distant metastasis.3 However, among patients with the same TNM stage receiving similar treatments, clinical outcomes, especially the development of distant metastasis, vary widely.4 Hence, the current anatomical-based staging system is not sufficient to predict which patients will develop distant metastasis. Extensive efforts have been undertaken to search for better predictors.
Larger tumors have been found to be related to a large number of clonogenic tumor cells and other adverse factors, including tumor hypoxia.5,6 Accordingly, the tumor volume of metastatic cervical lymph nodes (CLNs) represents a significant independent prognostic factor in the treatment of most malignant tumors, and has been used to predict the prognosis of patients with lung cancer, oropharyngeal carcinoma and other malignancies.7,8 Chen and colleagues reported that NPC patients with a gross tumor volume of lymph nodes (GTVnd) larger than 37.5 ml had the poorest 5-year distant metastasis-free survival (DMFS).9 A recent study by Lu and colleagues10 showed that the GTVnd may better reflect the status of lymph node metastasis than the conventional N category, and was a significant factor affecting distant metastasis in NPC patients.
Apart from GTVnd, pretreatment plasma Epstein–Barr virus DNA copy number (pre-EBV DNA) has been demonstrated to be an accurate and reliable predictive value for NPC metastasis.11,12 Thus, it has been suggested as a supplement for the TNM staging system.13,14 Patients with higher pre-EBV DNA copy numbers seem to be at greater risk of developing distant metastasis and in need of more drastic treatments.
Although GTVnd and pre-EBV DNA represent promising predictors for distant metastatic failure in NPC during the IMRT era; other predictors with the potential to be more effective have yet to be explored. There is limited evidence for incorporating GTVnd with pre-EBV DNA in an integrated model; therefore, the model’s effectiveness and reliability warrant further studies. As induction chemotherapy (IC) is now a routine treatment for NPC patients, there is a need to determine whether the integrated GTVnd and pre-EBV DNA model is an effective predictor of survival outcomes in patients with and without IC. Based on this premise, we conducted a big-data intelligence platform-based retrospective study of patients with NPC treated with IMRT, with the aim of developing and evaluating an integrated GTVnd and pre-EBV DNA prognostic model.
Materials and methods
Data extraction
The NPC-specific database from the well-established big-data intelligence platform at Sun Yat-sen University Cancer Center (SYSUCC) was used to identify NPC patients diagnosed between April 2009 and December 2015. Patients’ demographic, diagnostic, and therapeutic information was obtained. A detailed description of the intelligence platform at SYSUCC is presented in our previously published study.15 Briefly, it is a novel ‘big data’ research system, which enables real-time data to be organized, integrated, and updated automatically from numerous clinical and business systems.
Patient characteristics
We reviewed data from 10,126 patients retrospectively in a big-data intelligence platform, and 2021 were enrolled in the study in accordance with the following inclusion criteria: histologically proven NPC; 2017 AJCC N1-N3 category; no evidence of distant metastasis when diagnosed; receiving IMRT; and no prior history of malignancy. Of them, 521 were excluded because of no cervical lymph node metastasis or lack of pre-EBV DNA. Data from 1500 patients were included in the final analysis. The patients’ characteristics are shown in Table 1. The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (No. YB2019-76). As this was a retrospective analysis of routine data, we requested, and were granted, a waiver of individual informed consent from the ethics committee. Patient records/information was anonymized and deidentified prior to analysis.
Table 1.
Characteristics of the patients with nasopharyngeal carcinoma (n = 1500).
| Patients characteristics | No of patients (%) |
|---|---|
| Age | |
| Median | 44.4 years |
| Range | 7–78 years |
| Gender | |
| Male | 1138 (75.9) |
| Female | 362 (24.1) |
| Histology | |
| WHO type I | 11 (0.7) |
| WHO type II | 51 (3.4) |
| WHO type III | 1438 (95.9) |
| T category* | |
| T1 | 208 (13.9) |
| T2 | 265 (17.7) |
| T3 | 734 (48.9) |
| T4 | 293 (19.5) |
| N category* | |
| N1 | 797 (53.1) |
| N2 | 445 (29.7) |
| N3 | 258 (17.2) |
| Clinical stage* | |
| II | 253 (16.9) |
| III | 737 (49.1) |
| IVa | 510 (34.0) |
| Chemotherapy | |
| Yes | 1436 (95.7) |
| No | 64 (4.3) |
WHO, World Health Organization.
According to the 8th American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) of the staging system.
Clinical staging
All patients completed a pretreatment evaluation, including a complete patient history, physical examination, hematology and biochemistry profiles, magnetic resonance imaging (MRI) of the neck and nasopharynx, chest radiography, abdominal sonography, and a whole-body bone scan using single-photon emission computed tomography (SPECT) or 18F-Fluorodeoxyglucose positron emission tomography and CT (PET-CT). All patients were restaged according to the 8th staging system of the American Joint Committee on Cancer (AJCC).1 All MRI materials and clinical records were reviewed to minimize heterogeneity in the restaging process. Two radiologists specializing in head and neck cancers independently evaluated all of the scans; disagreements were resolved by consensus.
Measurement of the volume of metastatic cervical lymph nodes
Patients were immobilized in a supine position using a thermoplastic mask. After administration of intravenous contrast material, 3-mm CT slices, depicting the area of the head to 2 cm below the sternoclavicular joint were captured. All the images were transferred to the CORVUSR inverse IMRT-planning system, in which the metastatic cervical lymph nodes (CLNs) were outlined manually by a radiation oncologist according to the pretreatment MRI image, and then verified by another radiation oncologist who specialized in NPC treatment. The diagnosis of metastatic CLN cancer was based on MRI. The diagnostic criteria were derived from the radiologic criteria for cervical lymph node metastasis of head and neck cancer published by Michiel and colleagues.16 This work is well-recognized and the criteria have been used in many published studies.17,18 Among patients receiving IC, all CLNs that were diagnosed before treatment were outlined, while their volume was delineated based on the residual size after IC. The GTVnd was calculated using the planning system by the summation-of-area technique, which multiplies the entire area by the image reconstruction interval of 3 mm.
Quantification of plasma EBV DNA
Before treatment, peripheral venous blood (3 ml) was collected from each patient, placed in an EDTA tube and centrifuged at 3000 × g for 5 min. Total plasma DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Fluorescence polymerase chain reaction (PCR) was performed using an EBV PCR quantitative diagnostic kit (Da-An Genetic Diagnostic Center, Guangzhou, China) targeting the BamHI-W region of the EBV genome. Data were analyzed using Applied Biosystems 7300 SDS software (Beijing, China). Undetectable EBV DNA was defined as 0 copies/ml.
Radiotherapy
Target volume was delineated according to our institutional treatment protocol, which is consistent with the International Commission on Radiation Units and Measurements in Reports 50 and 62. Clinical target volumes (CTVs) were individually delineated on the basis of the tumor invasion pattern.19 The prescribed dose was 68–72 Gy to the planning target volume (PTV) of the GTV, 60 Gy to the PTV of the CTV-1 (i.e. high-risk regions), and 54 Gy to the PTV of the CTV-2 (i.e. low-risk and neck nodal regions). Treatment was delivered once daily, over five fractions per week. All targets were treated simultaneously using the simultaneous integrated boost (SIB) technique.
Chemotherapy
RT alone or concurrent chemoradiotherapy (CCRT) ± IC or adjuvant chemotherapy (AC) was recommended for Stage II patients, and CCRT ± IC or AC for Stage III to IVa NPC, as defined by the 8th edition of the AJCC staging system.1 Of all the patients, 1436 (95.7%) received chemotherapy, 932 (62.1%) received IC and 1259 (83.9%) received CCRT. IC consisted of cisplatin with 5-fluorouracil or taxanes delivered every 3 weeks for two or three cycles. CCRT consisted of cisplatin delivered weekly or on weeks 1, 4, and 7 of RT.
Patient follow up and statistical analysis
The duration of follow up was measured from the 1st day of therapy to either the day of death or the day of the last examination. Patients were seen every 3 months during the first 2 years, and every 6 months thereafter until death. Endpoints (time to the first defining event) included DMFS, overall survival (OS), disease-free survival (DFS), and local-regional relapse-free survival (LRRFS).
SPSS version 25.0 (IBM Corp, Armonk, NY, USA) was used for all statistical analyses. For patient assignments to groups, random numbers from 0 to 1 were generated by SPSS for each patient and then sorted from small to large. Using an expected ratio of 1:2 for the training group and test group,20 patients whose random number was not more than 0.33 were assigned to the training condition. As the random number had an accuracy of 2 decimal points, several patients with the number 0.33 were all assigned to the training group. Finally, 1500 patients were assigned to the training (n = 503) or the testing condition (n = 997) for analyses. The receiver operating characteristics (ROC) curve analysis was used to determine the cut-off point for the GTVnd. Chi-square or a nonparametric test was used to compare clinical characteristics. Time-to-event data were described using Kaplan–Meier curves and survival differences were compared using the log-rank test. The multivariable Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Host factors (age and gender), tumor factors (T and N categories, GTVnd and pre-EBV DNA) and treatment factors (chemotherapy) were included as covariates in all analyses. All statistical tests were two-tailed; a p-value < 0.05 was considered statistically significant.
Results
Patient outcomes
The median duration of follow up for the entire patient cohort was 65 months (range = 3.4– 99.5 months). Of the 1500 patients analyzed, 17.5% (262) developed distant metastases, 59 of the bone, 58 of the lung, 38 of the liver, and 20 of other sites, such as the mediastinal lymph nodes, parotid and parotid lymph nodes, and 87 in multiple sites; 20.8% (312) died and 12.2% (183) developed local or regional failure. The 5-year DMFS, OS, DFS, and LRRFS rates for the entire cohort were 83.1%, 81.9%, 73.3%, and 88.3%, respectively.
Characteristics and analysis of the GTVnd and pre-EBV DNA as prognostic factors
The median GTVnd was 22.6 ml (range 0.5–264.8 ml) and the median concentration of pre-EBV DNA was 7.8 × 103 copies/ml (range 0–68,700 × 103 copies/ml). Multivariable analysis indicated that both the GTVnd and pre-EBV DNA were independent and significant negative prognostic factors for DMFS and OS (Table 2).
Table 2.
Multivariable analysis of prognostic factors in patients with nasopharyngeal carcinoma (n = 1500).
| Distant metastasis-free
survival |
Overall survival |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| GTVnd | 1.012 (1.008–1.015) | <0.001 | 1.010 (1.007–1.013) | <0.001 |
| pre-EBV DNA | 2.079 (1.498–2.887) | <0.001 | 1.783 (1.339–2.373) | <0.001 |
| T category* | 1.375 (1.038–1.821) | 0.027 | 1.334 (1.038–1.744) | 0.025 |
| N category* | 1.872 (1.430–2.449) | <0.001 | 1.751 (1.372–2.233) | <0.001 |
| Gender | 1.541 (1.098–2.164) | 0.012 | NS | |
| Age | NS | 1.575 (1.258–1.972) | <0.001 | |
GTVnd, gross tumor volume of cervical lymph nodes; NS, no significant difference; Pre-EBV DNA, pretreatment plasma or pretreatment serum Epstein–Barr virus DNA.
According to the 8th American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system. The following parameters were included in the Cox proportional hazards model by backward elimination: age (>45 versus ⩽45 years), gender (male versus female), T category (T3–4 versus T1–2), N category (N2–3 versus N1), use of chemotherapy (with versus without), pre-EBV DNA (>2000 versus ⩽2000 copies/ml) and GTVnd as a continuous variable.
Identification of the GTVnd cut-off point for patients in the training condition
The GTVnd cut-off point was identified in the training condition through the ROC curve. The points were 20.2 ml for DMFS [sensitivity = 56.8%, specificity = 68.0%; AUC (area under the ROC) = 0.651, p < 0.001] and 20.1 ml for OS (sensitivity = 55.6%, specificity = 68.3%; AUC = 0.657, p < 0.001), respectively. Therefore, a uniform cut-off point of 20 ml (>20 ml versus ⩽20 ml) was selected to stratify patients in the test condition and all the other patients into high and low GTVnd groups for survival analysis. A uniform cut-off point of 2000 copies/ml for pre-EBV DNA was retested in the training condition.13 Both of these cut-off points were independent and significant negative prognostic factors for DMFS and OS (Supplementary Table 1).
Prognostic value of the GTVnd and pre-EBV DNA cut-off points for patients in the test condition and for all patients
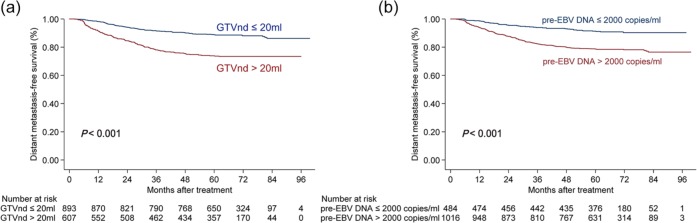
In the test condition, the 5-year DMFS, OS, and DFS rates for NPC patients with a GTVnd ⩽20 ml and >20 ml were 86.6% versus 73.7%, 85.6% versus 72.0% and 76.1% versus 64.5%, respectively (all p-values < 0.05). Of all patients in the study, the 5-year DMFS (Figure 1a), OS, and DFS rates for those with a GTVnd ⩽20 ml and >20 ml were 88.9% versus 73.9%, 88.2% versus 72.4% and 79.4% versus 64.0%, respectively (all p-values < 0.001).
Figure 1.
Stratified analysis using the gross tumor volume of lymph nodes (GTVnd) (a) and pretreatment plasma Epstein–Barr virus DNA copy numbers (pre-EBV DNA) for the prognostic prediction (b) of distant metastasis-free survival in all patients.
GTVnd, gross tumor volume of lymph nodes.
The 5-year DMFS, OS, and DFS rates of patients with ⩽2000 and >2000 copies/ml in the test condition were 91.4% versus 76.5%, 88.1% versus 76.1%, and 80.3% versus 67.0%, respectively (all p-values < 0.05). In all patients, the 5-year DMFS (Figure 1b), OS and DFS rates of patients with a pre-EBV DNA ⩽2000 and >2000 copies/ml were 91.3% versus 78.7%, 88.8% versus 78.4%, and 82.0% versus 69.0%, respectively (all p-values < 0.001). Overall, patients with a GTVnd >20 ml or pre-EBV DNA ⩽2000 copies/ml had poorer DMFS, OS, and DFS.
Integrated model of the GTVnd and pre-EBV DNA
To address the question of whether the GTVnd plus pre-EBV DNA would be a more effective prognostic tool than either one alone, patients were classified into four groups as follows: Group 1 (GTVnd ⩽20 ml and pre-EBV DNA ⩽2000 copies/ml), Group 2 (GTVnd ⩽20 ml and pre-EBV DNA >2000 copies/ml), Group 3 (GTVnd >20 ml and pre-EBV DNA ⩽2000 copies/ml), and Group 4 (GTVnd >20 ml and pre-EBV DNA >2000 copies/ml). ROC curves were used to compare the prognostic validity. The AUCs for DMFS in all 1500 patients were 0.606 for the GTVnd and 0.594 for pre-EBV DNA, respectively, and rose to 0.659 when these two factors were incorporated (all p-values < 0.05, Figure 2).
Figure 2.
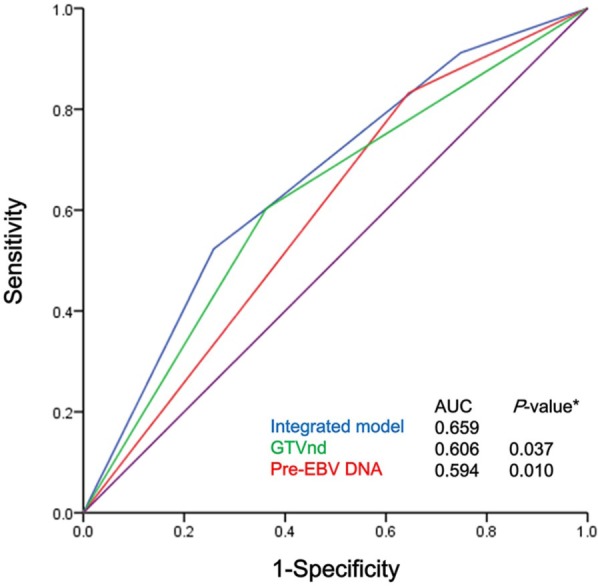
ROC curves for the cut-off points for the GTVnd (>20 ml versus ⩽20 ml) and pre-EBV DNA (>2000 copies/ml versus ⩽ 2000 copies/ml) and the integrated model for all NPC patients (n = 1500).
*Compared with the integrated model.
GTVnd, gross tumor volume of lymph nodes; pre-EBV, pretreatment plasma Epstein–Barr virus DNA; ROC, receiver operator characteristic.
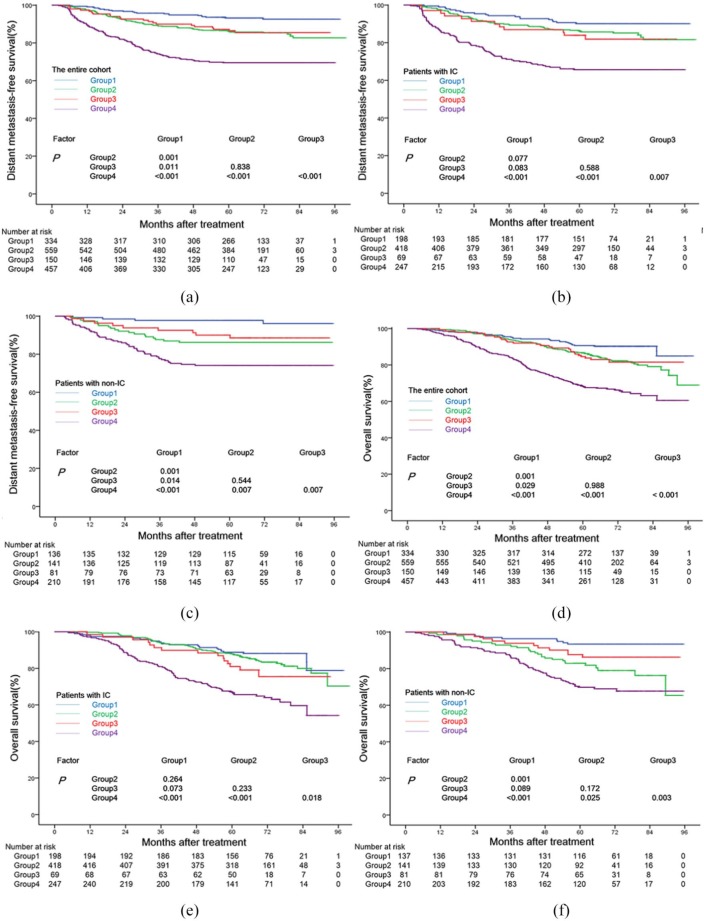
The prognostic significance of the integrated model for all patients
The 5-year DMFS rates of the patients in Groups 1–4 were 93.2%, 86.3%, 87.2%, and 69.5%, respectively. An analysis of the OS showed a similar outcome, with 5-year OS rates of 90.7%, 86.6%, 84.6%, and 68.4%, respectively. Patients in Group 4 had the lowest 5-year DMFS (Figure 3a) and OS (Figure 3d) rates among the four groups (all p-values < 0.01), whereas patients in Group 1 had the highest rates (all p-values < 0.05). No significant difference was found in the 5-year DMFS or OS rates between Group 2 and Group 3 (p = 0.421 and p = 0.483). Patients in Group 4 had the lowest 5-year DFS rate (59.2%) among the four groups (all p-values < 0.01), and patients in Group 1 (83.5%) had a significantly higher 5-year DFS rate than patients in Group 2 (77.0%) (p < 0.001), but both groups shared a similar 5-year DFS rate with the patients in Group 3 (78.6%) (Supplementary Figure S1a). When comparing the 5-year LRRFS rates between the four groups, we found that patients in Group 4 had the lowest rate (83.0%) (p < 0.01), but found no significant difference between the other three groups (91.0%, 88.5% and 89.6%, respectively) (Supplementary Figure S1b).
Figure 3.
Stratified analysis using the integrated model of the GTVnd and pre-EBV DNA copy numbers for the prognostic prediction of DMFS and OS in all patients (a, d), and patients with (b, e) or without (c, f) induction chemotherapy. Group 1 (patients with GTVnd ⩽20 ml and pre-EBV DNA ⩽2000 copies/ml), Group 2 (patients with GTVnd ⩽20 ml and pre-EBV DNA >2000 copies/ml), Group 3 (patients with GTVnd >20 ml and pre-EBV DNA ⩽2000 copies/ml), and Group 4 (patients with GTVnd >20 ml and pre-EBV DNA >2000 copies/ml).
DMFS, distant metastasis-free survival; GTVnd, gross tumor volume of lymph nodes; pre-EBV, pretreatment plasma Epstein–Barr virus DNA; OS, overall survival.
The prognostic significance of the integrated model for patients with and without IC (IC cohort and non-IC cohort)
As the addition of IC to CCRT has been found to improve distant control, and, thus, survival in NPC,21 we further investigated the prognostic value of the integrated model among patients stratified by whether or not they received IC.
In the IC cohort, the 5-year DMFS (Figure 3b), OS (Figure 3e), DFS (Supplementary Figure S2a), and LRRFS (Supplementary Figure S2b) rates for patients in Groups 1 to 3 were 90.1% versus 86.3% versus 83.9%, 88.8% versus 87.9% versus 81.0%, 82.2% versus 77.1% versus 73.8% and 92.7% versus 88.1% versus 87.8%, respectively (all p-values > 0.05). Group 4 had the lowest 5-year DMFS (Figure 3b), OS (Figure 3e), and DFS (Supplementary Figure S2a) rates (65.7%, 67.1%, and 57.9%, respectively, all p-values < 0.05). The 5-year LRRFS rate of patients in Group 1 to Group 4 were 92.7%, 88.1%, 87.8% and 82.4%, respectively, and a significant difference was found only between Group 1 and Group 4. (Supplementary Figure S2b).
In the non-IC cohort, patients in Group 1 and Group 4 had the highest and lowest 5-year DMFS rates, respectively, (97.8% and 74.1%, all p-values < 0.05), while patients in Groups 2 and 3 had similar intermediate 5-year DMFS rates (86.2% versus 90.0%, p > 0.05) (Figure 3c). Additionally, patients in Group 4 had the lowest 5-year OS (Figure 3f) and DFS (Supplementary Figure S3a) rates (69.8% and 60.8%, respectively) among the four groups (all p-values < 0.05). Patients in Group 1 had higher 5-year OS and DFS rates than patients in Group 2 (OS: 93.4% versus 82.9%, DFS: 85.3% versus 76.5%; all p-values < 0.05), but both of their 5-year OS and DFS rates were similar to those of Group 3 (all p-values > 0.05). Finally, the four groups had similar 5-year LRRFS rates (88.8%, 89.5%, 91.2%, and 83.7%; all p-values > 0.05, Supplementary Figure S3b).
Multivariable analysis indicated that both a large GTVnd and high pre-EBV DNA were independent and significant negative prognostic factors for DMFS and OS in patients with IC and for DMFS in patients without IC (non-IC), while only pre-EBV DNA independently and significantly predicted poor OS in patients without IC (Table 3).
Table 3.
Multivariable analysis of the prognostic factors for distant metastasis-free survival and overall survival of patients with and without induction chemotherapy.
| Endpoints | Variable | HR (95% CI) | p-value |
|---|---|---|---|
| Patients with IC | |||
| Distant metastasis-free survival | GTVnd | 2.084 (1.537–2.827) | <0.001 |
| pre-EBV DNA | 1.710 (1.157–2.527) | 0.007 | |
| Gender | 1.553 (1.029–2.344) | 0.036 | |
| N category* | 2.104 (1.503–2.946) | <0.001 | |
| Overall survival | GTVnd | 2.152 (1.611–2.873) | <0.001 |
| pre-EBV DNA | 1.423 (1.003–2.020) | 0.048 | |
| Age | 1.515 (1.143–2.008) | 0.004 | |
| N category* | 1.796 (1.315–2.452) | <0.001 | |
| Patients with non-IC | |||
| Distant metastasis-free survival | GTVnd | 2.323 (1.430–3.771) | 0.001 |
| pre-EBV DNA | 3.113 (1.711–5.665) | <0.001 | |
| T category* | 1.897 (1.167–3.084) | 0.001 | |
| Overall survival | GTVnd | NS | |
| pre-EBV DNA | 2.731 (1.671–4.465) | <0.001 | |
| Age | 1.575 (1.204–2.564) | 0.003 | |
| T category* | 1.787 (1.168–2.734) | 0.007 | |
| N category* | 1.693 (1.129–2.539) | 0.011 | |
GTVnd, gross tumor volume of cervical lymph node; IC, induction chemotherapy; NS, no significant difference; Pre-EBV DNA, pretreatment plasma pretreatment serum Epstein–Barr virus DNA.
The following parameters were also included in the Cox proportional hazards model by backward elimination: age (>45 versus ⩽45 years), gender (male versus female), T category (T3–4 versus T1–2), N category (N2–3 versus N1), use of chemotherapy (with versus without), pre-EBV DNA (>2000 versus ⩽2000 copies/ml) and GTVnd (>20 versus ⩽20 ml.
Discussion
Local-regional control in the management of NPC has improved significantly in recent decades because of the revolutionary development of IMRT. In contrast, distant metastasis still threatens NPC patients who have received standard treatment. Therefore, it is reasonable and necessary to search for an effective prognostic factor for distant metastasis in IMRT era.
In addition to TNM stage, GTVnd and pre-EBV DNA have been recognized as important prognostic factors in NPC patients.9,10,14,22 In this research, by evaluating a training cohort of 503 cases and examining 1500 cases, we found that patients with a GTVnd larger than 20 ml had poor survival. We retested the cut-off point of pre-EBV DNA (2000 copies/ml) in 1500 cases, and confirmed its effectiveness in distinguishing patients with different treatment outcomes. To further identify patients at high risk for distant metastasis, we integrated the GTVnd and the pre-EBV DNA, and found an enhanced prognostic value of the integrated model. To our knowledge, this is the largest single-institutional study to date that has examined correlations between integrated model and treatment outcomes in patients with NPC.
A recent study by Yao and colleagues reported that, among N1-patients, those with a larger GTVnd and higher pre-EBV DNA had poorer survival outcomes than the other patients.23 In a study of 180 NPC patients by Lu and colleagues, those with both a large GTVnd and high pre-EBV DNA had poorer 5-year OS. In the current study, the integrated model of GTVnd and pre-EBV DNA effectively classified all patients into three risk groups. Patients with both (Group 4), either (Group 2 and Group 3), and neither (Group 1) larger GTVnd nor higher pre-EBV DNA, had the poorest, intermediate, and most improved 5-year DMFS and OS, respectively. Although patients with low pre-EBV DNA and a large GTVnd had comparable 5-year OS rates to those with low pre-EBV DNA and a smaller GTVnd in the study by Lu and colleagues, which was inconsistent with our findings due to their different cut-off points, the integrated model showed an improved capacity to discriminate patients at high risk. In contrast to our study, Yao and colleagues examined N1 patients only, the study by Lu and colleagues included N0 patients whose GTVnd = 0, whereas our study included all patients except those without cervical lymph node metastasis. As more patients are included, the newly generated cut-off points for the GTVnd and the retested cut-off points for pre-EBV DNA in our study should be more convincing and practicable. Overall, our study is the first to support the integrated GTVnd and pre-EBV DNA model as a predictor of prognosis using big-data analysis.
The addition of IC to CCRT has been found to improve distant control and prognosis,21,24,25 and has been recommended for local advanced NPC. However, within the cohorts with and without IC, the prognosis of individual patients still varies. Thus, we further verified the usefulness of our integrated model in patients with and without IC. Among the patients who did not receive IC, the current model showed the capacity for prognostic prediction as it classified patients into three groups based on the risk of distant metastasis: low-risk (Group 1), moderate-risk (Group 2 and 3), and high-risk (Group 4). However, the integrated model classified patients receiving IC into only two risk groups: low-risk (Group 1, Group 2, and Group 3) and high-risk (Group 4), which was unexpected but reasonable. In patients receiving IC, the GTVnd was the volume of residual metastatic lymph nodes after IC, indicating the tumor load and chemosensitivity of the tumor. We intriguingly found that patients whose GTVnd was not greater than 20 ml after IC had similar outcomes regardless of their pre-EBV DNA (Group 1 versus Group 2, all p-values > 0.05), which is consistent with the fact that tumor response to IC had been identified as an independent prognostic factor in NPC.26,27 Group 2 patients whose tumors were sensitive to chemotherapy benefited from IC with reduced distant and local-regional failure. However, differences were found between patients with a large GTVnd, but high or low pre-EBV DNA (Group 3 versus Group 4). Patients with a large GTVnd but low pre-EBV DNA (Group 3) had outcomes similar to patients in Group 1. This was possible because low pre-EBV DNA indicates a low risk of developing distant failure, and, therefore, the synergistic effect of chemoradiotherapy was sufficient for these patients in spite of the large GTVnd. Nevertheless, patients with a larger GTVnd and higher pre-EBV DNA (Group 4) had the poorest outcomes because of the large tumor burden and treatment insensitivity.
Based on its effective survival prediction, our current model provided a way for exploring appropriate treatment for NPC patients, especially timely interventions and therapeutic strategies for patients after IC. Consistent with the conclusion of a recent investigation by Zhao and colleagues that reduction of the target volume and radiation dose to post-IC tumor volume was reasonable,28 our study showed that adequate CCRT is sufficient for patients at low risk after IC. In contrast, we recommend therapy that is more aggressive for patients at high risk in order to reduce the incidence of distant metastasis. Similarly, patients who did not receive IC but were at medium and high risk need more treatment after CCRT. Recent studies have found that patients who received a higher cumulative dose of concurrent cisplatin (>200 mg/m2) had better OS.29 Twu and colleagues retrospectively analyzed 85 patients with persistent detectable EBV DNA after radiation, and found that patients who received AC consisting of oral tegafur-uracil (2 capsules twice daily) for 12 months showed a significant reduction in distant failure.30 Thus, patients at high risk might benefit from more aggressive CCRT or AC. Furthermore, current clinical trials (NCT02958111, NCT03427827) by our research group evaluating the effects of adjuvant capecitabine chemotherapy and adjuvant anti-PD-1 antibody in NPC indicate might further improve the prognosis for these patients in the near future.
With the largest sample size to date, this single-institutional study demonstrated the prognostic value and clinical applicability of the integrated GTVnd and pre-EBV DNA in NPC model. However, the limitations of our study should be noted. First, it is a retrospective study. Multi-center prospective studies are still needed to verify the prognostic value of this model in the future. Second, other predictors, such as T category, N category, the gross tumor volume of the primary tumor, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) using PET-CT, are needed to achieve greater precision in prognostic predictions and corresponding treatment. Effective nomograms consisting of the above factors should be established and validated in prospective multicenter cohort studies in the future.
Conclusion
The integrated GTVnd and the pre-EBV DNA model predicts patient outcomes more effectively, classifies patients with and without IC into different risk groups, and may aid in the development of individualized treatment strategies to improve the treatment outcomes of NPC patients, especially after IC.
Supplemental Material
Supplemental material, Supplementary_Figure_S1 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplementary_Figure_S2 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplementary_Figure_S3 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplementary_table_S1 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Jun-Yan Li, Cheng-Long Huang and Wei-Jie Luo contributed equally.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by grants from the Natural Science Foundation of Guangdong Province (No. 2017A030312003), the National Natural Science Foundation of China (No. 81802707), the Health and Medical Collaborative Innovation Project of Guangzhou City, China (201803040003), the Innovation Team Development Plan of the Ministry of Education (No. IRT_17R110), the Planned Science and Technology Project of Guangdong Province (2019B020230002) and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B14035)
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jun-Yan Li, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Cheng-Long Huang, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Wei-Jie Luo, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Yuan Zhang, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Ling-Long Tang, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Hao Peng, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Ying Sun, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, People’s Republic of China.
Yu-Pei Chen, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People’s Republic of China.
Jun Ma, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People’s Republic of China.
References
- 1. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. Ca Cancer J Clin 2017; 67: 93–99. [DOI] [PubMed] [Google Scholar]
- 2. Shu-Zhen L, Wen-Fei L, Lei C, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol 2011; 80: 661–668. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Li WF, Mao YP, et al. Establishment of an integrated model incorporating standardised uptake value and N-classification for predicting metastasis in nasopharyngeal carcinoma. Oncotarget 2016; 7: 13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 2004; 101: 300–306. [DOI] [PubMed] [Google Scholar]
- 5. Johnson CR, Thames HD, Huang DT, et al. The tumor volume and clonogen number relationship: Tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol 1995; 33: 281–287. [DOI] [PubMed] [Google Scholar]
- 6. Lartigau E, Ridant AML, Lambin P, et al. Oxygenation of head and neck tumors. Cancer 1993; 71: 2319–2325. [DOI] [PubMed] [Google Scholar]
- 7. Dieleman EM, Uitterhoeve AL, van Hoek MW, et al. Concurrent daily cisplatin and high-dose radiation therapy in patients with stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2018; 102: 543–551. [DOI] [PubMed] [Google Scholar]
- 8. Chao KC, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol 2004; 59: 43–50. [DOI] [PubMed] [Google Scholar]
- 9. Chen FP, Zhou GQ, Qi ZY, et al. Prognostic value of cervical nodal tumor volume in nasopharyngeal carcinoma: analysis of 1230 patients with positive cervical nodal metastasis. PLoS One 2017; 12: e0176995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu L, Li J, Zhao C, et al. Prognostic efficacy of combining tumor volume with Epstein-Barr virus DNA in patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma. Oral Oncol 2016; 60: 18–24. [DOI] [PubMed] [Google Scholar]
- 11. Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. New Engl J Med 2004; 350: 2461–2470. [DOI] [PubMed] [Google Scholar]
- 12. Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F] fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013; 31: 2861–2869. [DOI] [PubMed] [Google Scholar]
- 13. Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer 2019; 125: 79–89. [DOI] [PubMed] [Google Scholar]
- 14. Lee VHF, Kwong DLW, Leung TW, et al. The addition of pretreatment plasma Epstein-Barr virus DNA into the 8th edition of nasopharyngeal cancer TNM stage classification. Int J Cancer 2018; 144: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 15. Lv JW, Chen YP, Huang XD, et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: a large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer 2017; 123: 3540–3549. [DOI] [PubMed] [Google Scholar]
- 16. van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177: 379–384. [DOI] [PubMed] [Google Scholar]
- 17. Li WF, Sun Y, Mao YP, et al. Proposed lymph node staging system using the international consensus guidelines for lymph node levels is predictive for nasopharyngeal carcinoma patients from endemic areas treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 249–256. [DOI] [PubMed] [Google Scholar]
- 18. Tang L, Li L, Mao Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging: prognostic value and staging categories. Cancer 2008; 113: 347–354. [DOI] [PubMed] [Google Scholar]
- 19. Liang SB, Sun Y, Liu LZ, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol 2009; 75: 742–750. [DOI] [PubMed] [Google Scholar]
- 20. Guo R, Sun Y, Yu XL, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012; 104: 294–299. [DOI] [PubMed] [Google Scholar]
- 21. Chen YP, Tang LL, Yang Q, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res 2018; 24: 1824–1833. [DOI] [PubMed] [Google Scholar]
- 22. Leung S, Chan K, Ma B, et al. Plasma Epstein–Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014; 25: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 23. Yao JJ, Zhou GQ, Wang YQ, et al. Prognostic values of the integrated model incorporating the volume of metastatic regional cervical lymph node and pretreatment serum Epstein-Barr virus DNA copy number in predicting distant metastasis in patients with N1 nasopharyngeal carcinoma. Chin J Cancer 2017; 36: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17: 1509–1520. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. Epub ahead of print 1 June 2019. DOI: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- 26. Peng H, Chen L, Li WF, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a randomized phase 3 clinical trial. Cancer 2017; 123: 1643–1652. [DOI] [PubMed] [Google Scholar]
- 27. Peng H, Chen L, Zhang Y, et al. The tumour response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. Sci Rep 2016; 6: 24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao C, Miao JJ, Hua YJ, et al. Locoregional control and mild late toxicity after reducing target volumes and radiation doses in patients with locoregionally advanced nasopharyngeal carcinoma treated with induction chemotherapy (IC) followed by concurrent chemoradiotherapy: 10-year results of a phase 2 study. Int J Radiat Oncol Biol Phys 2019; 104: 836–844. [DOI] [PubMed] [Google Scholar]
- 29. Loong HH, Ma BB, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol 2012; 104: 300–304. [DOI] [PubMed] [Google Scholar]
- 30. Twu CW, Wang WY, Chen CC, et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol 2014; 89: 21–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_S1 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S2 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S3 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_table_S1 for An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein–Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis by Jun-Yan Li, Cheng-Long Huang, Wei-Jie Luo, Yuan Zhang, Ling-Long Tang, Hao Peng, Ying Sun, Yu-Pei Chen and Jun Ma in Therapeutic Advances in Medical Oncology