Abstract
Multi-walled carbon nanotubes with excellent electrical properties and high aspect ratios can reduce the high field strength required to kill cancer cells in vitro with nanosecond pulsed electric fields. For the first time, this article systematically and comprehensively evaluates the effects of various parameters of nanosecond pulsed electric fields combined with multi-walled carbon nanotubes on cell viability. The effects of field strength, E (2-10 kV/cm); pulse width, τ (100-500 ns); and pulse number, N (5-260) on the viability of A375 human skin cancer cells in the presence of multi-walled carbon nanotubes are studied using the Cell Counting Kit 8 assay. Based on a logistic model, the relationship between cell viability and various parameters is obtained using 1-dimensional nonlinear fitting. The results show a sigmoid-type variation in cell viability with field strength, pulse width, or pulse number. Multivariate scaling analysis shows that the relationship between cell viability and the pulse energy density σE2τN can be described as a sigmoid type. The introduction of multi-walled carbon nanotubes does not affect the above rules but significantly enhances the killing effect of nanosecond pulsed electric fields, which could effectively improve the electrical safety of nanosecond pulsed electric fields for the treatment of tumors.
Keywords: multi-walled carbon nanotubes, nanosecond pulsed electric fields, cell viability, various parameters, logistic model
Introduction
Nanosecond pulsed electric fields (nsPEFs) play an important role in the treatment of cancer. Without the involvement of toxic chemotherapeutic agents, tumor tissues can be shrunk or even eliminated by inducing apoptosis, avoiding the side effects of inflammation, ulcers, and drugs.1,2 However, it is necessary to apply high-intensity nsPEFs into tumor tissues with a pair of electrodes during experiment. An excessive field intensity will easily cause surface discharge of the targeted tissue, which will also affect the progress of the treatment process and the reliability of the treatment equipment.3,4
In recent years, the application potential of carbon nanotubes (CNTs) with excellent electrical properties for bioelectrical effects has attracted increasing attention. Carbon nanotubes are 1-dimensional quantum materials with special structures (nanometer-scale radial dimension and micron-scale axial length).5 Because CNTs have the same lamellar structure of graphite, CNTs have high conductivity. CNTs with high aspect ratio and excellent electrical conductivity can enhance the local field strength.6 Some scholars have applied this characteristic of CNTs to the research on pulsed electric field (PEF) treatment of tumors.
Stacey et al7 introduced multi-walled carbon nanotubes (MWCNTs) into nsPEFs (50 kV/cm, 300 ns, 8 pulses) to kill the pancreatic cancer cell line PANC1 and used trypan blue to evaluate cell viability. Compared with the condition without MWCNTs, the introduction of MWCNTs reduced the cell viability by 2.3 times. It was proven that the unique electrical properties of MWCNTs were synergistic with the PEFs in killing tumor cells. Raffa et al8 added MWCNTs to cell suspensions during PEF treatment (45-55 V/cm, 40 ms, 1 Hz, 500 pulses) of HN9 and CRFK cells to promote cell permeabilization, thereby enhancing cellular uptake of the drug macromolecules. In place of drug macromolecules, trypan blue was used to evaluate cell permeability. The experimental results showed that MWCNTs increased the cell permeability from 4% to 80%. Based on this experiment, it was proposed that the dielectric response of MWCNTs under PEF treatment might be the reason for enhanced electroporation (EP). Wang et al9 treated breast cancer cells with PEFs (50 V/cm, 40 ms, 5 Hz, 500 pulses) in the presence of MWCNTs. The membrane permeability was immediately increased to 38.62%, which was 2.77 times higher than that without MWCNTs. In addition, irreversible EP was observed. After 24 hours of pulse treatment, only 39.23% of the cells survived, whereas 87% of the cells survived in the absence of MWCNTs. Liu et al10 used the finite element method to simulate the interactions between single CNTs and the cell membrane under PEF treatment based on the dielectric electrophoresis theory. The results showed that the dielectrophoretic force induced at the tips of CNTs under the electric field could lead to rapid mechanical deformation of the cell membrane, thereby enhancing cell EP. All of the above mentioned studies have proven the feasibility of using CNTs to enhance the tumor cell-killing effect of PEFs from different perspectives.
However, up to now, related studies have verified the role of CNTs in enhancing the killing effect of PEFs on cancer cells only via a single parameter and at a few levels. There is no systematic and comprehensive study showing the effect of multiple nsPEF parameters combined with CNTs on cell viability. To provide reference data for selecting nsPEF parameters to treat tumors in the presence of CNTs, it is necessary to study how the introduction of CNTs affects nsPEF effects. In this article, to explore the toxicity of MWCNTs, we will systematically study the qualitative rules and quantitative function relationship between cell viability and various parameters, such as field strength, pulse width, and number of pulses, in the presence and absence of MWCNTs. We can analyze the effects of the introduction of MWCNTs on the dose effect, with the hope of providing a reference for subsequent research that introduces MWCNTs into the treatment of tissues.
Materials and Methods
Cell Culture
The human skin cancer cell line A375 is purchased from Chinese Academy of Science (Chongqing, China) and cultured in Dulbecco modified Eagle medium (DMEM, Gibco, Waltham, Massachusetts, USA) with 10% fetal bovine serum (Gibco) and 1% penicillin and streptomycin (Gibco). Cells are maintained in a saturated humidity incubator with 5% CO2 at 37°C and are passaged once every 1 to 2 days.
Determination of Toxicity of MWCNTs
Multi-walled carbon nanotubes are purchased from the Chinese Academy of Sciences Chengdu Organic Chemistry Co, Ltd (TNM2, Chengdu, China). The outer diameter and length of the MWCNTs are approximately 8 to 15 nm and 50 μm, respectively. The microstructure of MWCNTs under a transmission electron microscope is shown in Figure 1. To obtain more homogeneously dispersed MWCNTs solution, the dispersion of MWCNTs in the medium must be improved by a specific dispersing device and dispersion process before the experiment. According to the manual provided by the manufacturer, a dispersed MWCNTs solution is obtained by sonication and centrifugation, and MWCNTs are dispersed in deionized water with low viscosity characteristics. Using different volumes of MWCNT dispersion in fresh medium, the final concentration of MWCNTs is adjusted to 0, 50, 100, 200, 300, and 400 μg/mL. A375 cells in the logarithmic growth phase are digested with 0.25% Trypsin-EDTA (Gibco) and centrifuged for 5 minutes (800 rpm). The supernatant is then discarded, and the cells are resuspended in the above medium containing different concentrations of MWCNTs. The cell concentration is adjusted to 1 × 106 cells/mL. The above cell suspension was kept at room temperature for 1 hour, then washed twice with phosphate-buffered saline (PBS) and resuspended in fresh medium containing no MWCNTs (cell density: 1 × 106 cells/mL), and 200 μL of the cell suspension was seeded in a 96-well plate immediately. After 8 hours, cell viability was measured by the Cell Counting Kit 8 (CCK-8) assay. The control group is equivalent to an experimental group with an MWCNT concentration of 0 μg/mL.
Figure 1.
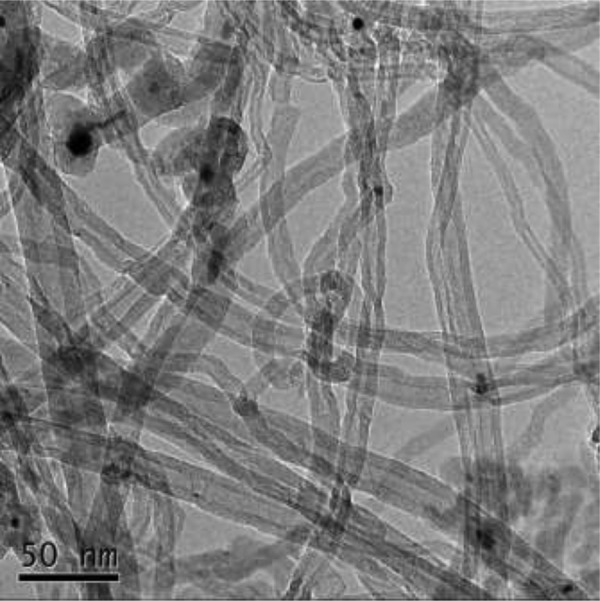
Microstructure diagram of MWCNTs. A transmission electron microscopy image of the MWCNTs is shown; scale bar = 50 nm. MWCNTs indicates multi-walled carbon nanotubes.
Assessment of Cell Viability
A CCK-8 (Dojindo, Kyushu Island, Japan) cell proliferation assay is used to assess the cell viability. After the 96-well plate containing the cells is cultured in the incubator for 8 hours, in order to exclude the effect of the reduction reaction between MWCNTs and CCK-8 on the experimental results, the supernatant in the well is slowly aspirated using a pipette and the plate is washed twice with PBS. After washing twice with PBS, a mixed solution of 110 μL of medium and CCK-8 (10:1 ratio of medium to CCK-8) is added again, and the plate is incubated for 1.5 hours. After 1.5 hours, cell viability is assayed by a microplate reader (Epoch2, BioTek, Winooski, Vermont, USA) at a wavelength of 450 nm, and the entire test process is completed in 1 to 2 minutes. In this assay, CCK-8 is reduced by dehydrogenase in the mitochondria of cells to the highly water-soluble orange-yellow formazan product. All data are normalized with respect to the control group and the blank group (contains only CCK-8 solution and medium) to assess cell viability. The formula of cell viability is defined as:
| 1 |
Where Ae, Ac, and Ab represent the absorbance of the experimental group, the control group, and the blank group, respectively.
PEF System
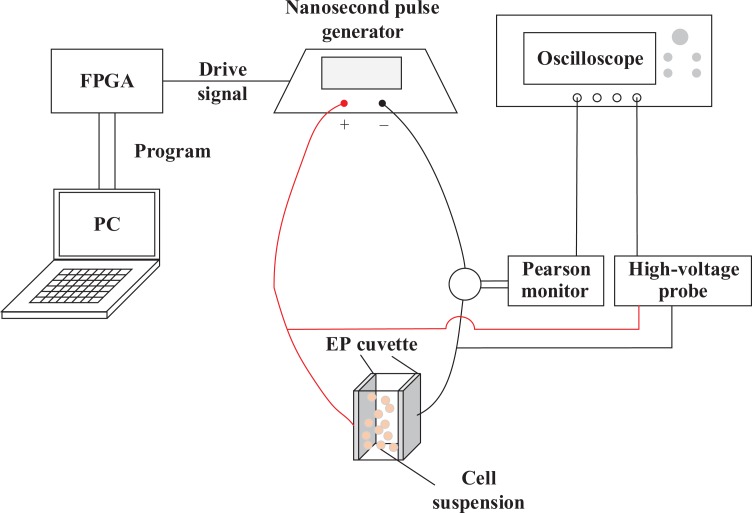
The experimental device comprises a homemade nanosecond pulse generator based on the topology of a half-bridge modular multilevel converter,11 a field-programmable gate array (FPGA) module (AX301, Altera, San Jose, California, USA), a personal computer (PC), an oscilloscope (WavePro 7 Zi-A, Teledyne LeCroy), a high-voltage probe (PPE5KV, Teledyne LeCroy, New York, USA), the Pearson Current Monitor (2877, Pearson Electronics, Palo Alto, California, USA), and an EP cuvette (2 mm gap, BTX, Holliston, Massachusetts, USA).
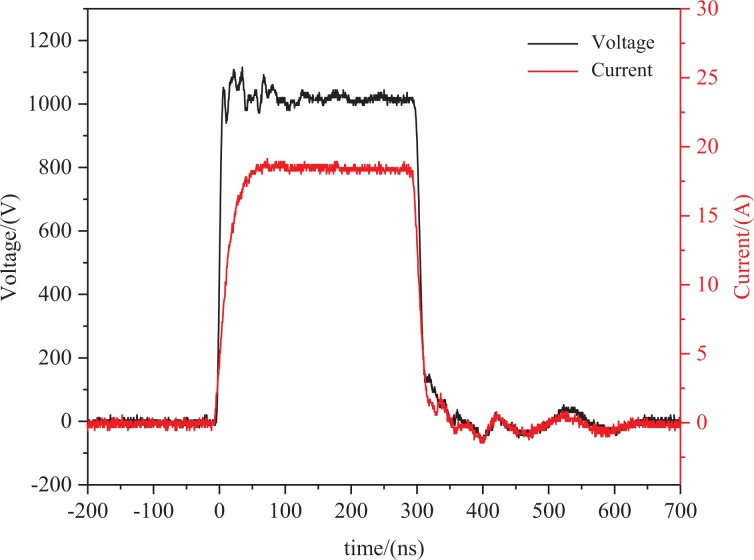
A schematic diagram of the experimental platform is shown in Figure 2. The PC is used to program the FPGA, which outputs the drive signal to the nanosecond pulse generator. The generator output is connected to the EP cuvette containing the cell suspension to be treated by nsPEFs. Simultaneously, both the voltage across the cell suspension and the current flowing through the cell suspension are collected via the oscilloscope. The current is converted to voltage by the Pearson Current Monitor. The pulsed voltage and current waveforms are shown in Figure 3 with an amplitude of 1 kV and a pulse width of 300 ns.
Figure 2.
Experimental PEF system. The drive signal from FPGA programmed by PC was output to nsPEF generator, and the output pulsed voltage from nsPEF generator was added to EP cuvette containing cell suspension. EP indicates electroporation; FPGA, field-programmable gate array; nsPEF, nanosecond pulsed electric field; PC, personal computer; PEF, pulsed electric field.
Figure 3.
Typical waveforms in the experiment. When the output voltage of nsPEF generator was 1000 V, the voltage (black) across the cell suspension and current (red) flowing through the cell suspension were shown. nsPEF indicates nanosecond pulsed electric field.
PEF Protocols
Table 1 shows the pulse parameters used in the experiment. The pulse frequency is fixed at 1 Hz, and the MWCNTs concentration is fixed at the maximum safe dose. For the field strength E and pulse width τ, 5 levels are selected. And for the number of pulses N, 7 levels are selected. When exploring the influence of field strength on cell viability, the pulse width and the number of pulses are fixed at 300 ns and 100, respectively; when exploring the influence of pulse width on cell viability, the field strength and number of pulses are fixed at 6 kV/cm and 100, respectively; when exploring the influence of pulse number on cell viability, the field strength and pulse width are fixed at 6 kV/cm and 300 ns, respectively.
Table 1.
Settings of the Experimental Parameters.
| Parameters | Level | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| E/(kV·cm−1) | 2 | 4 | 6 | 8 | 10 | / | / |
| τ/(ns) | 100 | 200 | 300 | 400 | 500 | / | / |
| N | 5 | 10 | 15 | 50 | 100 | 170 | 260 |
To determine how the addition of MWCNTs changes the effect of nsPEF treatment on cells, each group of experiments is performed with MWCNTs and without MWCNTs. For each EP protocol, after resuspending the cells in fresh DMEM in the presence or in the absence of MWCNTs, 70 μL of cell suspension is placed in the EP cuvette and then subjected to the corresponding nsPEF treatment immediately. For the control groups, cell suspensions with the same volume without MWCNTs and with MWCNTs are placed in the EP cuvette but are not subjected to nsPEF treatment. The only difference in the experimental operation process between the control group and the treated group is whether the group is subjected to nsPEF; the other operations are identical. After the pulse treatment, the cell suspensions are seeded in a 96-well plate, and the plate is cultured in the incubator for 8 hours. After incubation for 8 hours, CCK-8 is added to the plate and cultured for 1.5 hours. Then, a microplate reader is used to measure the cell viability of each experimental group.
Statistical Analysis
Statistical analysis of the data is performed using OriginPro software (OriginLab, Northampton, Massachusetts, USA). Values are expressed as the mean ± standard deviation, and statistical significance between experiments is assessed by 1-way analysis of variance (ANOVA), with values of P < .05 considered significant. It can look for violations of the assumptions for 1-way ANOVA by the premise that the data follow a normal distribution and the variance is homogeneous. If the data do not obey the assumption of normality or the assumption of homogeneity of variance, then there may be abnormal data. In this case, 1-way ANOVA was performed using software, and it can get the box graph of the data. At this time, the box graph could identify the abnormal data and the abnormal data would be removed from the original data.
Results
Toxicity of MWCNTs
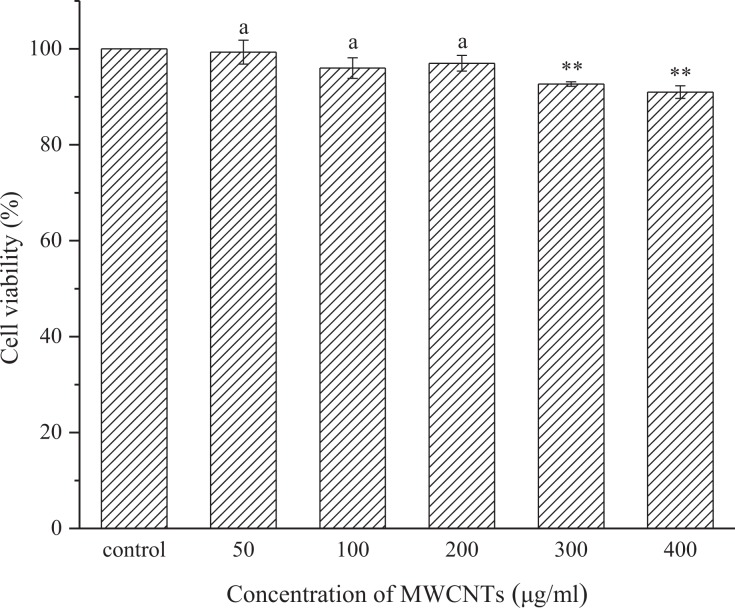
To introduce MWCNTs into research on the use of nsPEFs to kill cancer cells, the toxicity of the introduced MWCNTs must be considered. To determine a reasonable concentration of MWCNTs that would be minimally toxic, A375 cells are grown in the presence of various concentrations of MWCNTs. Figure 4 shows that when the concentration of MWCNTs is 300 μg/mL, the cell viability is reduced to 92% and further decreases as the MWCNT concentration is increased. There is a significant difference between the control and the MWCNT-exposed cells when the concentration is ≥300 μg/mL (P < .05). In contrast, when the MWCNT concentration is ≤200 μg/mL, there is almost no change in cell viability, indicating that the MWCNTs have no effect on cell viability in this concentration range, and there is no statistically significant difference between the control and experimental groups (P > .05). Based on these results, the concentration of MWCNTs is set to 200 μg/mL in the subsequent nanosecond pulse treatments of A375 cells.
Figure 4.
Effects of different concentrations of MWCNTs on cell viability. Cell viability was analyzed by the CCK-8 assay after incubating the cells with MWCNTs-containing medium for 8 hours. The data are presented as the mean ± SD (n = 4), n represents the number of independent experiments, and there were 3 replicates for each independent experiment. **(P < .01) and a(P > .05) compared with the control group. CCK-8 indicates Cell Counting Kit 8; MWCNTs, multi-walled carbon nanotubes; SD, standard deviation.
Relationship Between Cell Viability and Different Pulse Parameters
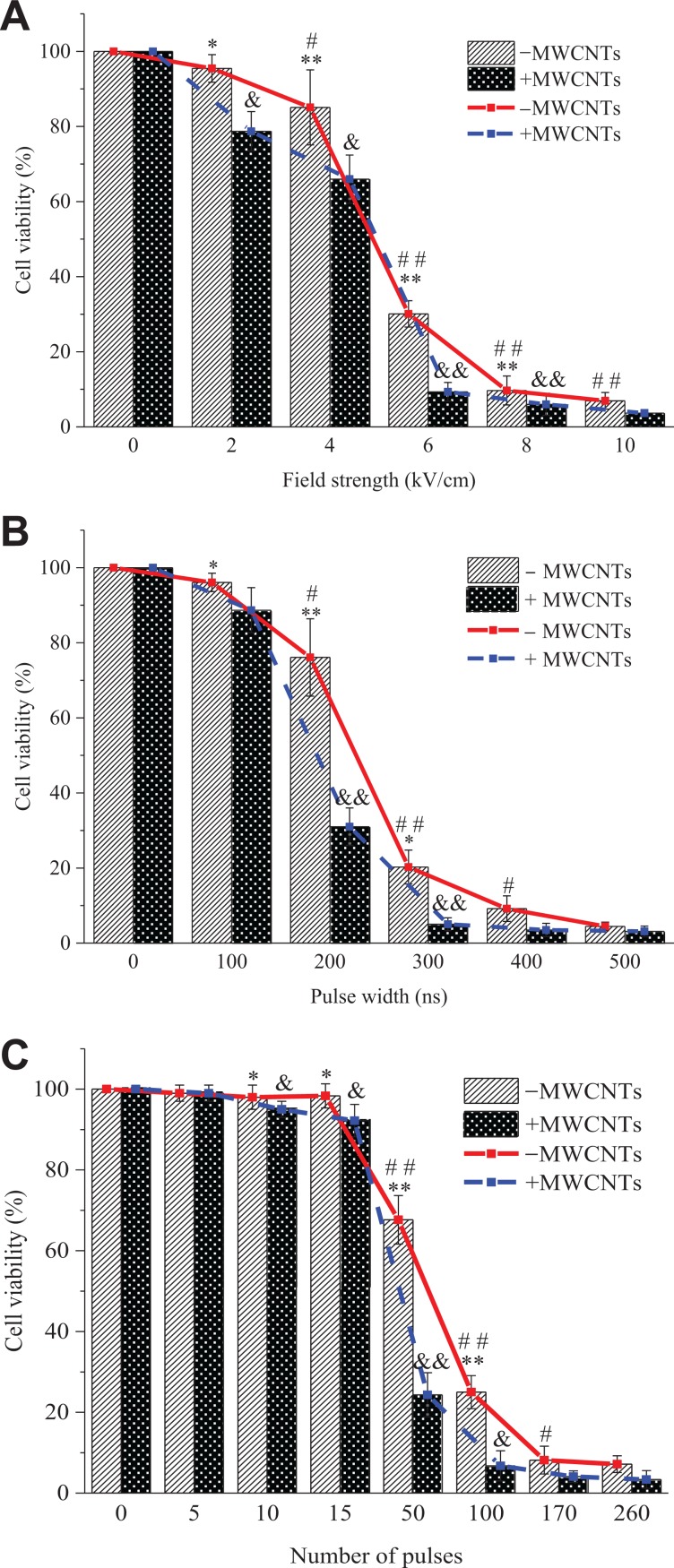
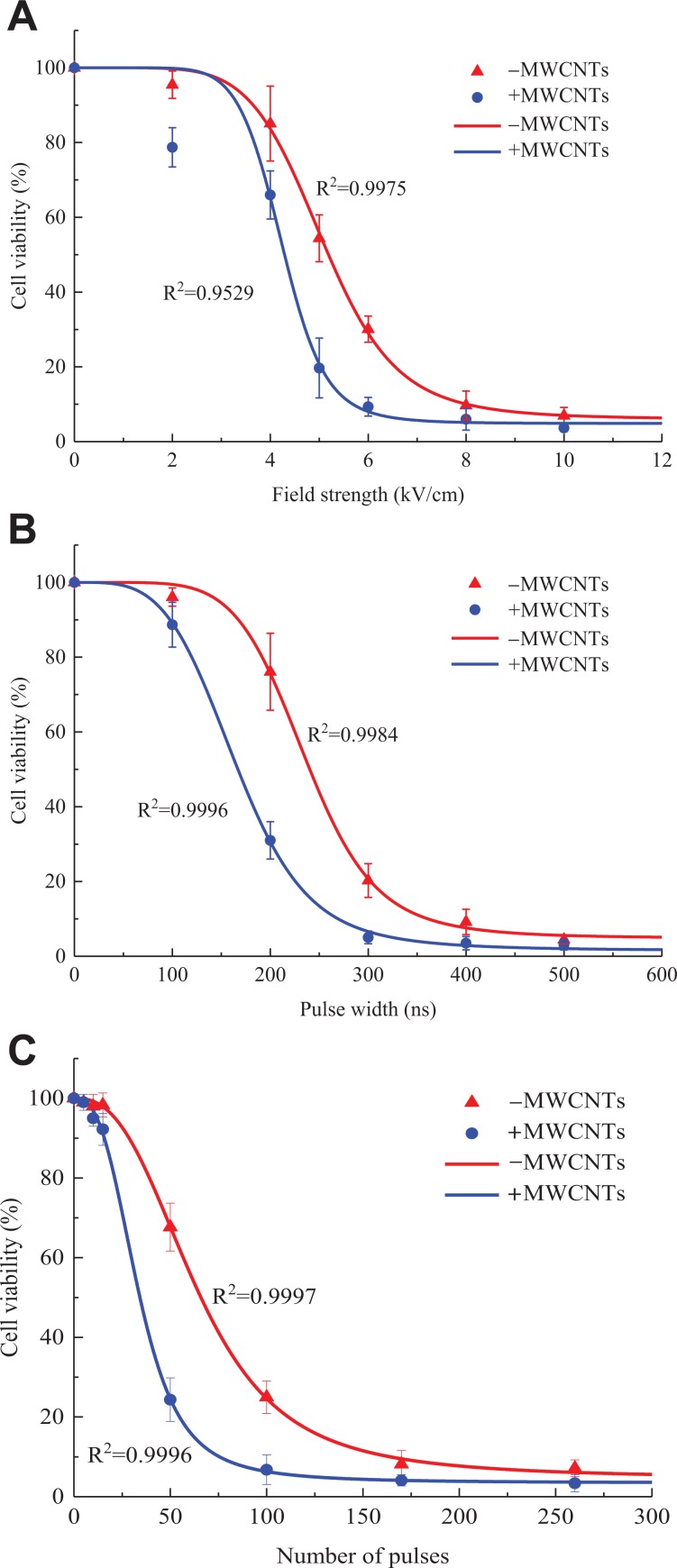
The concentration of MWCNTs selected for the nanosecond pulse treatment of A375 cells is the maximum nontoxic value, which is, 200 μg/mL. With this concentration, the effects of the addition of MWCNTs on nsPEF-induced changes in cells are studied by changing the field strength, the pulse width, and the number of pulses; and a fitting formula is established between cell viability and a single parameter. Figure 5 shows the effect of the above 3 single parameters on cell viability. In the legend, “−MWCNTs” and “+MWCNTs” refer to “without MWCNTs” and “with MWCNTs”, respectively.
Figure 5.
Cell viability dependence on the various parameters. Cell viability was detected using the CCK-8 assay after 8 hours of combined treatment of nsPEF and MWCNTs. A, Field strength, (B) pulse width, (C) number of pulses. −MWCNTs: cell suspension alone, +MWCNTs: cell suspension containing safe concentration of MWCNTs. The data are presented as the mean ± SD (n = 4), n represents the number of independent experiments, and there were 3 replicates for each independent experiment. Significant differences between the presence of MWCNTs and the absence of MWCNTs at each pulse dose are indicated by asterisk (*P < .05, **P < .01). # or ##: Statistically different from previous column in the absence of MWCNTs (#P < .05, ##P < .01). & or &&: Statistically different from previous column in the presence of MWCNTs (&P < .05, &&P < .01). CCK-8 indicates Cell Counting Kit 8; MWCNTs, multi-walled carbon nanotubes; nsPEF, nanosecond pulsed electric field; SD, standard deviation.
Compared with the condition without MWCNTs, the addition of MWCNTs significantly reduces the cell viability but does not change the relationship between cell viability and each parameter. Figure 5A shows that when the pulse width is 300 ns and the number of pulses is 100, the cell viability exhibits a sigmoid-type (S-type) attenuation as the field strength increases. Figure 5B shows that when the field strength is 6 kV/cm and the number of pulses is 100, the cell viability exhibits an S-type attenuation as the pulse width increases. Figure 5C shows that when the strength is 6 kV/cm and the pulse width is 300 ns, the cell viability shows an S-type decay as the number of pulses increases.
To predict the relationship between cell viability and various factors, a logistic regression model is used to fit the experimental data to obtain a formula for cell viability.
The logistic regression model has become nearly the most commonly used analytical method in epidemiology and medicine, especially for predicting the probability of a disease occurring based on risk factors. In a study of microbial thermal inactivation, Cole et al12 proposed a logistic model for the S-type trend of the bacterial survival rate decreasing with the heat treatment time, and the model was also suitable for exponential curve fitting. The relevant formula is defined as:
| 2 |
where t is the independent variable; S is the observed biological effect; the parameters α and ω represent the common logarithm of the upper asymptote (t = 0) and the lower asymptote (t → ∞) of the curve, respectively; δ denotes the maximum slope; and t0 represents the horizontal axis position of the maximum slope.
The results in Figure 5 show almost no change in cell viability at the beginning of the experiments, which is close to 100; that is, the upper asymptote of the curve is 100. Therefore, the value of parameter α can be determined as:
| 3 |
We can modify the logistic model as follows:
| 4 |
Based on the logistic model, we use a 1-dimensional nonlinear fitting method to determine optimal values for the 3 parameters and the goodness of fit, R2, by MATLAB 2016a. Then, the functional relationship between cell viability and various parameters can be obtained, and the fitting curve and the values of R2 are shown in Figure 6; R2 is used to evaluate the fitting accuracy between the predicted model and the experimental values; R2 is between 0 and 1, and values closer to 1 indicate a better fit. The optimal values of the model parameters corresponding to the fitting curves under each pulse variable are shown in Table 2.
Figure 6.
Fit curve relating cell viability to various parameters. A logistic regression model is used to fit the experimental data from Figure 5 to obtain the formula between cell viability and various parameters. A, Field strength, (B) pulse width, (C) number of pulses. The data are presented as the mean ± SD (n = 4), n represents the number of independent experiments, and there were 3 replicates for each independent experiment; R2 between 0 and 1 is used to evaluate the fitting accuracy between the predicted model and the experimental data. SD indicates standard deviation.
Table 2.
Calculated Optimal Parameter Values of the Logistic Model Under Each Pulse Variable.
| Logistic Model Parameters | ω | δ | t 0 |
|---|---|---|---|
| Field strength | |||
| −MWCNTs | 0.7802 | −4.252 | 0.7952 |
| +MWCNTs | 0.6878 | −6.378 | 0.695 |
| Pulse width | |||
| −MWCNTs | 0.6879 | −4.408 | 2.471 |
| +MWCNTs | 0.1773 | −3.983 | 2.408 |
| Number of pulses | |||
| −MWCNTs | 0.651 | −0.8501 | 4.683 |
| +MWCNTs | 0.5392 | −0.98 | 4.036 |
Abbreviation: MWCNTs, multi-walled carbon nanotubes.
The R2 values of each curve indicate that the experimental data are well fitted by the logistic model. The fitting results show that the field strength, pulse width, or number of pulses must exceed a threshold to affect the cell viability. That is, the cell viability is almost constant at low doses, and when the dose is above a certain threshold, the cell viability begins to decrease sharply and then begins to saturate after decreasing to a certain extent.
Scaling Relationship Between Cell Viability and Pulse Energy Density
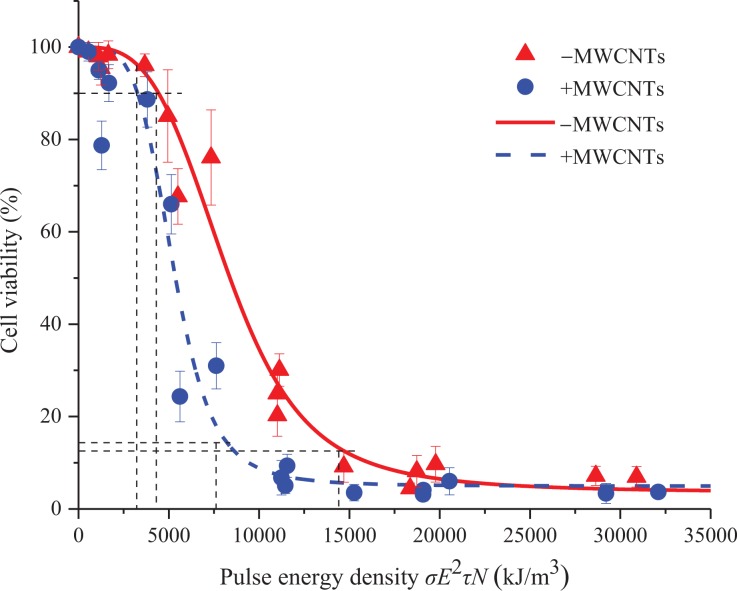
To further analyze the combined effects of 3 parameters, the field strength E, the pulse width τ, and the pulse number N, on cell viability after introducing MWCNTs, we use the multiparameter variable method to quantitatively analyze the cell viability trend for the 3 parameters. It is assumed that cell viability S is related to the injected pulse energy density σE2τN (σ is the conductivity of the suspension):
| 5 |
where S is a function of the pulse-injection energy density σE2τN. The conductivity of the suspension is estimated by the voltage across the suspension and current flowing through the suspension, and the conductivities of the suspension in the presence and absence of MWCNTs are 1.06 and 1.03 S/m, respectively. Taking σE2τN as the abscissa and cell viability as the ordinate, we plot the results in Figure 7. The results show that regardless of the presence or absence of MWCNTs, the cell viability as a whole follows the S-type law for changes in the pulse-injection energy density. When the pulse-injection energy density does not reach the required threshold, the viability is not substantially affected; when the pulse-injection energy density exceeds the threshold value, the cell viability begins to decrease sharply and finally stabilizes at a certain saturation value.
Figure 7.
The relationship between cell viability and the pulse energy density σE2τN. Experimental data and fitted lines are displayed simultaneously in the figure. A logistic regression model is used to fit the experimental data to obtain the formula between cell viability and σE2τN. The conductivities of the cell suspension in the presence and absence of MWCNTs are 1.06 and 1.03 S/m, respectively. The data are presented as the mean ± SD (n = 4), n represents the number of independent experiments, and there were 3 replicates for each independent experiment. The starting threshold energy (the position at which the curve is 10% lower than the upper asymptote value) and saturation energy value (the position at which the curve is 10% greater than the lower asymptote value) of the S-shaped fit curve are indicated by dashed lines. MWCNTs indicates multi-walled carbon nanotubes; SD, standard deviation.
Based on the logistic model, the fitting curves are shown in Figure 7. Table 3 shows the optimal parameter values and R2.
Table 3.
Calculated Optimal Parameter Values and R2 of the Logistic Model.
| Parameters | Without MWCNTs | With MWCNTs |
|---|---|---|
| ω | 0.5493 | 0.6946 |
| δ | −1.187 | −1.39 |
| t0 | 9.444 | 8.872 |
| R 2 | 0.9812 | 0.9547 |
Abbreviation: MWCNTs, multi-walled carbon nanotubes.
The parameter ω in the logistic model has a physical meaning which is used to determine the saturation values of cell viability in the absence and presence of MWCNTs, which are 100.5493 = 3.54 and 100.6946 = 4.95, respectively. The threshold values for a decrease in cell viability are both approximately 90 (the position at which the curve is 10% lower than the upper asymptote value), and the saturation values are approximately 13 and 14.5 (the position at which the curve is 10% greater than the lower asymptote value).13 The pulse-injection energy density required for the cell viability to reach the threshold in the absence and presence of MWCNTs is 4338 and 3205 kJ/cm3, respectively, and the pulse-injection energy density required to reach the saturation value is 14156 and 7548 kJ/cm3, respectively. This result means that the introduction of MWCNTs can reduce the threshold energy and saturation energy required for killing cells.
Discussion
Toxicity Effects of Different Concentrations of MWCNTs on Cell Viability
Toxicity experiments with different concentrations of MWCNTs show that the cytotoxicity induced by MWCNTs is dose dependent, that is, the higher the concentration of MWCNTs is, the stronger the toxicity. Notably, in addition to containing MWCNTs, MWCNT dispersions contain a small amount of the residual catalyst and nonionic surfactant (dispersant) used to produce nanotubes.7,14 Nonionic surfactants are mainly used to solve the agglomeration problem of CNTs. Studies have shown that the toxicity of MWCNTs is mainly caused by residual catalysts and dispersants and that higher concentrations of these compounds lead to stronger effects on cell viability, which is consistent with the results of our experiments.15,16
In addition to residual catalysts and nonionic surfactants, MWCNTs are also toxic to cells. Multi-walled carbon nanotubes have a tendency to aggregate into the lipid bilayer of the cell membrane and pass through the cell membrane due to the mobility of lipid bilayers. Additionally, high concentrations of MWCNTs can disrupt the integrity of the cell membrane, leading to cell rupture and death.17
In this article, only the toxicity of MWCNTs to isolated cancer cells was studied; however, if MWCNTs need to be introduced into the field of treating tumors, the safety of MWCNTs to tissues must be considered, which is the important work to be done in the next step of this article. The next step is to comprehensively evaluate the potential of MWCNTs combined with nsPEF in the treatment of tumors from the biodistribution, metabolism, and effects on major organs of MWCNTs in vivo.
Relationship Between Cell Viability and Various Pulse Parameters
Under normal physiological conditions, cells can prevent the passage of macromolecular substances due to the selective permeability of their membranes, thereby maintaining the balance between the internal and external environments of the cell. However, when a cell is exposed to an electric field, charge will accumulate on both sides of the membrane and increase the transmembrane potential. When the transmembrane potential exceeds the dielectric strength of the cell membrane, micropores are generated on the cell membrane.18 The production of micropores will break the essential ionic balance across the cell membrane, thereby affecting the normal growth of the cell. For spherical cells, the threshold of the field strength required for irreversible EP can be estimated as19:
| 6 |
where Δϕ is the threshold transmembrane potential, d is the diameter of the cell, and θ is the angle between the direction of normal at the target position of the cell membrane and the direction of the electric field E.
In addition to the applied voltage needed to reach the threshold value, whether EP can occur depends on the pulse duration.20 If the duration of the applied pulse field is too short, the charge distribution on both sides of the cell membrane will return to the initial state after the end of the pulse and will thus not lead to the EP effect. The shortest pulse duration (time constant) required for a spherical cell to reach a threshold transmembrane potential is21:
| 7 |
where ρ1 and ρ2 are the resistivities of the external cell medium and cytoplasm, respectively, and C is the equivalent capacitance of the cell membrane.
In summary, according to the principle of EP, it is known that the occurrence of EP requires not only the applied voltage exceeds a certain threshold but also that the pulse width reaches the charging time constant of the cell membrane. This situation explains why nanosecond pulse processing has almost no effect on cell viability with or without MWCNTs when the field strength or pulse width is too low in Figure 6, so the cell viability exhibits S-type changes with field strength or pulse width.
It can be found that when the number of pulses is lower, nsPEF has no effect on cell viability (P > .05), that is, the killing effect of nsPEF on cells has a threshold effect. Gianulis et al22 compared nsPEF cytotoxicity for human cell lines of cancerous (IMR-32, Hep G2, HT-1080, and HPAF-II) and noncancerous origin (BJ and MRC-5) under strictly controlled and identical conditions. Although each cell line has a different sensitivity to nsPEF, for all tested cell types, a change in S-type between cell survival and number of pulses is observed. That is, the killing effect of nsPEF on cells has a threshold effect, which is consistent with the experimental results obtained in this article.
Research based on YO-PRO-1 and propidium emission experiments shows that a greater number of pulses maintains a longer cell perforation state caused by the PEFs.23 In other words, the exchange of substances inside and outside the cell is more complete. Additionally, it might be easier to destroy the balanced environment between the inside and outside of the cell, resulting in cell death.
In this article, the functional relation between cell viability and the pulse energy density σE2τN is established, allowing analysis of the combined effects of multiple parameters on cell viability. Based on the hypothesis that the intensity of bioelectric effects is determined by the charge transferred through the cellular membrane above the threshold for EP, Schoenbach et al24 established the scaling law between the bioelectrical effects of a cell under nsPEF and 3 pulse parameters, such as field strength, pulse width, and number of pulses.
For a single nsPEF, when the pulse width is larger than the dielectric relaxation time of the cytoplasm and less than the charging time constant of the cell membrane (about 75 ns), the cell bioelectrical effects scale with Eτ. Additionally, as the pulse width increases, under the action of a single nsPEF, the bioelectric effects are expected to scale with the pulse energy density E2τ, rather than the electrical impulse Eτ, which is consistent with the dose–response relation obtained in this article.
For multiple pulses, if the field strength and pulse width are not far above the values that define the onset of EP, the bioelectric effects caused by nsPEF scale with N0.5, which can be explained by the random walk statistical model. When multiple pulses are applied, the position of cells with respect to the direction of the applied electric field changes randomly between pulses, analogous to the random walk statistical model, and the influence of the statistical motion of cells between pulses on bioelectric effects can be expressed as N0.5. However, in this article, cell viability scales with N rather than N0.5. In fact, as the electric field and pulse width increase, the permeabilized area of the cell will expand from the poles to the equator, and the effect of random motion of the cell on the bioelectrical effects is not significant. The equivalent effect is that the cell only has minor rotation between pulses. According to the theory, if there is only minor cell rotation between pulses, the effect of multiple pulses will approach a scaling law with a linear dependence on N. The simulation results prove that the perforation area of the cell membrane will obviously expand outward from the poles based on the nsPEF parameters in this study25, which might be the reason why cell viability scales with N in this study.
In addition, the scaling law deduced by Schoenbach et al is applicable not only to membrane permeabilization but also to secondary bioelectric effects (such as apoptosis and cell viability) caused by membrane permeabilization,26 which is in line with the application range of this article.
Mechanism of MWCNTs Enhancing the Killing Effect of NsPEFs
Carbon nanotubes are 1-dimensional quantum materials with special structures (nanoscale in the radial dimension, micron-scale in the axial dimension, and almost all ends of the CNTs are sealed). Carbon nanotubes have unique electrical properties and exhibit good metallicity (conductivity of approximately 104 S·cm−1). A previous study showed that their current-carrying capacity is 1000 times higher than that of copper wires, making them a 3-dimensional conductive matrix in the vicinity of cancer cells.27 When single CNT with a 1-dimensional structure is inserted into a uniform electric field region with an amplitude of E0, the field strength-enhancing effect is generated at the tip of the CNT via the “lightning rod” effect. The degree of electric field enhancement at the tip of the CNT can be estimated by the following equation28:
| 8 |
where Etip is the electric field strength at the tip of CNT, and β is a constant. L and D represent the length and outer diameter of CNT, respectively. The high aspect ratio (L/D) of CNT explains why CNT can concentrate the field well and promote EP.
In addition, it has been reported that CNTs in electromagnetic fields could also cause mechanical damage by direct physical contact with the cell membrane.8,29 Carbon nanotubes will randomly adsorb around the cell membrane due to their large specific surface area and strong surface electrostatic interaction before the electric field is applied, and some of CNTs will also bind to cell membrane phospholipids.8 At this time, the presence of CNTs has no effect on cell morphology. However, when the electric field is applied, the CNTs will rotate in the electromagnetic fields due to polarization until the CNTs are finally rotated in the same direction as the applied electric field. During the rotation of the CNTs, the dielectrophoretic force induced at the tips of the CNTs will cause deformation and perforation of the cell membrane due to mechanical action.29
In summary, the local field enhancement and physical destruction ability of CNTs can effectively enhance the killing effect of nsPEFs on cancer cells.
At present, the combination of nsPEFs and MWCNTs has not been applied to in vivo research. However, based on the results of the cell experiments in this article and the biological effect of MWCNTs, it is foreseeable that the combination of nsPEF and MWCNTs has a high potential for application in cancer treatment. In vivo experiments show that functionally modified MWCNTs after acupuncture or intravenous injection have the effect of targeting cancer cells, which can be highly enriched in tumor tissues. Furthermore, the near-infrared fluorescence characteristics of MWCNTs can achieve nondestructive imaging observation of tumor tissues. By combining nsPEFs with MWCNTs, which have targeted recognition and autofluorescence imaging properties, electric field energy can be more accurately delivered to tumors under the premise of improving the electrical safety of nsPEFs, and it is also convenient to study the action mechanism of nsPEFs combined with MWCNTs on in vivo tissues, which is of great significance in the field of clinical medicine.
Conclusion
This article is the first to investigate the effect of multiple nsPEF parameters on the viability of in vitro A375 cells treated with nsPEFs combined with MWCNTs applied at a safe concentration, as measured in this study. With increasing field strength, pulse width, or pulse number, the cell viability increases. Field strength, pulse width, or pulse number must exceed a threshold to affect cell viability. Additionally, the effect of each pulse parameter on cell viability becomes saturated. The relationship between cell viability and pulse energy density, σE2τN, is S-type. The introduction of MWCNTs with good electrical properties and a 1-dimensional structure does not affect the above rules but can significantly enhance the ability of nsPEFs to kill cancer cells. And MWCNTs have the potential to improve the electrical safety of nsPEFs for the treatment of tumors, which is of great significance in promoting the process of nsPEFs treatment of tumors.
Abbreviations
- ANOVA
analysis of variance
- CCK-8
Cell Counting Kit 8
- CNTs
carbon nanotubes
- DMEM
Dulbecco modified Eagle medium
- EP
electroporation
- FPGA
field-programmable gate array
- MWCNTs
multi-walled carbon nanotubes
- nsPEFs
nanosecond pulsed electric fields
- PBS
phosphate-buffered saline
- PC
personal computer
- PEF
pulsed electric field
- S-type
sigmoid-type
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Natural Science Foundation of China (51477022),the Natural Science Foundation Project of CQ CSTC (cstc2016jcyjA0500), and the National “111” Project of China (B08036).
ORCID iDs: Yan Mi  https://orcid.org/0000-0001-7554-422X
https://orcid.org/0000-0001-7554-422X
References
- 1. Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6(6):385–393. [DOI] [PubMed] [Google Scholar]
- 2. Buescher ES, Schoenbach KH. Effects of submicrosecond, high intensity pulsed electric fields on living cells intracellular electromanipulation. IEEE Trans Dielectr Electr Insul. 2003;10(5):788–794. [Google Scholar]
- 3. Chen X, Swanson RJ, Kolb JF, Nuccitelli R, Schoenbach KH. Histopathology of normal skin and melanomas after nanosecond pulsed electric field treatment. Melanoma Res. 2009;19(6):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mi Y, Xu J, Tang X, et al. Scaling relationship of in vivo muscle contraction strength of rabbits exposed to high-frequency nanosecond pulse bursts. Technol Cancer Res Treat. 2018;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58. [Google Scholar]
- 6. Ba L, Shu J, Lu Z. Probing local electric field distribution of nanotube arrays using electrostatic force microscopy. J Appl Phys. 2003;93(12):9977–9982. [Google Scholar]
- 7. Stacey M, Osgood C, Kalluri BS, Cao W, Elsayed-Ali H, Abdel-Fattah T. Nanosecond pulse electrical fields used in conjunction with multi-wall carbon nanotubes as a potential tumor treatment. Biomed Mater. 2011;6(1):70–76. [DOI] [PubMed] [Google Scholar]
- 8. Raffa V, Ciofani G, Vittorio O, Pensabene V, Cuschieri A. Carbon nanotube-enhanced cell electropermeabilisation. Bioelectrochemistry. 2010;79(1):136–141. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Liu D, Zhou R, Wang Z, Cuschieri A. Tumour cell membrane poration and ablation by pulsed low-intensity electric field with carbon nanotubes. Int J Mol Sci. 2015;16(4):6890–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu D, Wang L, Wang L, Cuschieri A. FEM analysis of carbon nanotube induced cell poration under electric field. biomedical engineering and informatics Proceedings of the 3rd Annual International Conference of the IEEE 2010; October 16, 2010; Yantai: IEEE; 1:182–186. doi: 10.1109/BMEI.2010.5639585. [Google Scholar]
- 11. Mi Y, Wan H, Bian C, et al. An MMC-based modular unipolar/bipolar high-voltage nanosecond pulse generator with adjustable rise/fall time. IEEE Trans Dielectr Electr Insul. 2019; 26(2): 515–522. [Google Scholar]
- 12. Cole DMB, Davies KW, Munro G, et al. A vitalistic model to describe the thermal inactivation of listeria monocytogenes. J Ind Microbiol, 1993;12(3):232–239. [Google Scholar]
- 13. Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015;62(1):4–20. [DOI] [PubMed] [Google Scholar]
- 14. Jose RC, Miguel CD, Ren Z, et al. Enhanced introduction of gold nanoparticles into vital acidothiobacillus ferrooxidans by carbon nanotube-based microwave electroporation. Nano Letters. 2004;4(5):985–988. [Google Scholar]
- 15. Hussain MA. On the cytotoxicity of carbon nanotubes. Current Science. 2009;96(5):664–673. [Google Scholar]
- 16. Kostarelos K. The long and short of carbon nanotube toxicity. Nat Biotechnol. 2008;26(7):774–776. [DOI] [PubMed] [Google Scholar]
- 17. Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharm. 2008; 232(2): 244–251. [DOI] [PubMed] [Google Scholar]
- 18. Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerget. 1996;41(2):135–160. [Google Scholar]
- 19. Neumann E, Sowers AE, Jordan CA. Electroporation and Electrofusion in Cell Biology. New York, NY: Springer Press; 1989. [Google Scholar]
- 20. Shahini M, Yeow JT. Carbon nanotubes for voltage reduction and throughput enhancement of electrical cell lysis on a lab-on-a-chip. Nanotechnology. 2011;22(32):325–330. [DOI] [PubMed] [Google Scholar]
- 21. Schoenbach KH, Stark RH, Deng J, Aly RE. Biological/medical pulsed electric field treatments. Power modulator symposium Proceedings of the twenty-fourth Annual International Conference of the IEEE 2000; June 26, 2000; Norfolk: IEEE; 1:17–22. doi: 10.1109/MODSYM.2000.896160. [Google Scholar]
- 22. Gianulis E C, Labib C, Saulis G, et al. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell Mol Life Sci. 2017;74(9):1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pakhomov A G, Gianulis E, Vernier P T, Semenov I, Xiao S, Pakhomova ON. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochimica et Biophysica Acta. 2015;1848(4):958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoenbach K H, Joshi R P, Beebe S J, et al. A scaling law for membrane permeabilization with nanopulses. IEEE Trans. Dielectr. Electr. Insul. 2009; 16(5): 1224–1235. [Google Scholar]
- 25. Mi Y, Xu J, Yao C, et al. Electroporation modeling of a single cell exposed to high-frequency nanosecond pulse bursts. IEEE Trans Dielectr Electr Insul. 2019;26(2):461–468. [Google Scholar]
- 26. Schoenbach K H, Hargrave B, Joshi R P, et al. Bioelectric effects of intense nanosecond pulses. IEEE Trans Dielectr Electr Insul. 2007;14(5):1088–1109. [Google Scholar]
- 27. Macdonald RA, Voge CM, Kariolis M, Stegemann JP. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008;4(6):1583–1592. [DOI] [PubMed] [Google Scholar]
- 28. Yantzi JD, Yeow JTW. Carbon nanotube enhanced pulsed electric field electroporation for biomedical applications. Mechatronics and automation Proceedings of the Annual International Conference of the IEEE 2005; July 29, 2005; Niagara Falls: IEEE; 4:1872–1877. doi: 10.1109/ICMA.2005.1626847. [Google Scholar]
- 29. Liu D, Wang L, Wang Z, Cuschieri A. Magnetoporation and magnetolysis of cancer cells via carbon nanotubes induced by rotating magnetic fields. Nano Lett. 2012;12(10):5117–5121. [DOI] [PubMed] [Google Scholar]