This cohort study assesses whether the addition of frailty measures to traditional comorbidity-based risk-adjustment models improved prediction of outcomes in patients with acute myocardial infarction, heart failure, and pneumonia.
Key Points
Question
Does the addition of frailty to traditional comorbidity-based risk-adjustment models improve the prediction of 30-day mortality and readmission for these conditions?
Findings
In this cohort study of 785 127 participants, frailty as determined by an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision claims-based frailty score was associated with a higher risk of 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia hospitalizations. When added to traditional comorbidities typically used in risk-adjustment models for these conditions, this claims-based frailty score significantly improved prediction of 30-day outcomes.
Meaning
Unless frailty is adequately captured in risk-adjustment metrics, it is possible that hospitals that care for a higher proportion of frail patients are disproportionately financially penalized for worse outcomes owing to unrecognized comorbidities among the patients they care for, rather than quality of care delivered.
Abstract
Importance
The addition of a claims-based frailty metric to traditional comorbidity-based risk-adjustment models for acute myocardial infarction (AMI), heart failure (HF), and pneumonia improves the prediction of 30-day mortality and readmission. This may have important implications for hospitals that tend to care for frail populations and participate in Centers for Medicare & Medicaid Services value-based payment programs, which use these risk-adjusted metrics to determine reimbursement.
Objective
To determine whether the addition of frailty measures to traditional comorbidity-based risk-adjustment models improved prediction of outcomes for patients with AMI, HF, and pneumonia.
Design, Setting, and Participants
A nationwide cohort study included Medicare fee-for-service beneficiaries 65 years and older in the United States between January 1 and December 1, 2016. Analysis began August 2018.
Main Outcomes and Measures
Rates of mortality within 30 days of admission and 30 days of discharge, as well as 30-day readmission rates by frailty group. We evaluated the incremental effect of adding the Hospital Frailty Risk Score (HFRS) to current comorbidity-based risk-adjustment models for 30-day outcomes across all conditions.
Results
For 785 127 participants, there were 166 200 hospitalizations [21.2%] for AMI, 348 619 [44.4%] for HF, and 270 308 [34.4%] for pneumonia. The mean (SD) age at the time of hospitalization was 79.2 (8.9) years; 656 315 (83.6%) were white and 402 639 (51.3%) were women. The mean (SD) HFRS was 7.3 (7.4) for patients with AMI, 10.8 (8.3) for patients with HF, and 8.2 (5.7) for patients with pneumonia. Among patients hospitalized for AMI, an HFRS more than 15 (compared with an HFRS <5) was associated with a higher risk of 30-day postadmission mortality (adjusted odds ratio [aOR], 3.6; 95% CI, 3.4-3.8), 30-day postdischarge mortality (aOR, 4.0; 95% CI, 3.7-4.3), and 30-day readmission (aOR, 3.0; 95% CI, 2.9-3.1) after multivariable adjustment for age, sex, race, and comorbidities. Similar patterns were observed for patients hospitalized with HF (30-day postadmission mortality: aOR, 3.5; 95% CI, 3.4-3.7; 30-day postdischarge mortality: aOR, 3.5; 95% CI, 3.3-3.6; and 30-day readmission: aOR, 2.9; 95% CI, 2.8-3.0) and among patients with pneumonia (30-day postadmission mortality: aOR, 2.5; 95% CI, 2.3-2.6; 30-day postdischarge mortality: aOR, 3.0; 95% CI, 2.9-3.2; and 30-day readmission: aOR, 2.8; 95% CI, 2.7-2.9). The addition of HFRS to traditional comorbidity-based risk-prediction models improved discrimination to predict outcomes for all 3 conditions.
Conclusions and Relevance
Among Medicare fee-for-service beneficiaries, frailty as measured by the HFRS was associated with mortality and readmissions among patients hospitalized for AMI, HF, or pneumonia. The addition of HFRS to traditional comorbidity-based risk-prediction models improved the prediction of outcomes for all 3 conditions.
Introduction
Among Medicare fee-for-service beneficiaries, acute myocardial infarction (AMI), heart failure (HF), and pneumonia are among the top causes of hospitalization.1 In addition, 1 in 5 Medicare patients hospitalized for these conditions is readmitted to a hospital within 30 days of discharge.2 As a result, the Centers for Medicare & Medicaid Services (CMS) has increasingly focused policy efforts on improving care for these conditions by publicly reporting hospital-level mortality and readmission rates.3,4,5,6 In addition, these measures have been incorporated into value-based programs, including the mandatory Hospital Value-Based Purchasing program, which financially rewards or penalizes hospitals based on their relative performance on 30-day risk-adjusted mortality rates for AMI, HF, and pneumonia,7 and the Hospital Readmissions Reduction Program, which financially penalizes hospitals with higher-than-expected 30-day risk-adjusted readmission rates.8
Using hospital-level readmission and mortality rates as measures of care quality requires accurate risk adjustment to account for differences in patient populations among hospitals. However, current risk-adjustment models used by the Hospital Value-Based Purchasing program and Hospital Readmissions Reduction Program do not account for frailty, an important marker of patient complexity that contributes to the risk of adverse outcomes. Frailty has been shown to modify the treatment effect of multiple high-risk interventions and independently predicts adverse outcomes beyond traditional comorbidity measures in several populations.9,10 In addition, frailty is associated with significant health care use, with frail elderly adults responsible for nearly half of all preventable Medicare spending.11 Whether the addition of a claims-based frailty metric to traditional comorbidity-based risk-adjustment models for AMI, HF, and pneumonia improves the prediction of 30-day mortality and readmission rates is unknown and may have important implications for hospitals that participate in CMS value-based programs and tend to care for frail populations.
Therefore, in this study, we aimed to address 2 questions. First, is patient frailty, as identified by administrative claims, associated with adverse outcomes for Medicare beneficiaries hospitalized with AMI, HF, and pneumonia? Second, does the addition of frailty to traditional comorbidity-based risk-adjustment models improve the prediction of 30-day mortality and readmission for these conditions?
Methods
Study Cohort and Clinical Comorbidities
We used the CMS Medicare Provider Analysis and Review files to identify all Medicare fee-for-service beneficiaries 65 years and older who were hospitalized at acute care hospitals between January 1, 2016, and December 1, 2016, with a principal discharge diagnosis of AMI, HF, or pneumonia (eTable 1 in the Supplement). The Medicare Provider Analysis and Review files include a 100% sample of administrative billing claims for inpatient hospitalizations for fee-for-service beneficiaries. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center with a waiver of informed consent for retrospective data analysis.
Study cohorts were identified using codes from the International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM).12,13,14,15,16 We excluded hospitalizations missing a date of admission or discharge and linked transfers from the index hospitalizations to other acute care hospitals to avoid double counting of single episodes of care. For patients with multiple hospitalizations within the period, 1 index hospitalization was randomly selected for each condition.17 To ensure consistent ascertainment of patients, we excluded patients who were not enrolled in Medicare fee-for-service for at least 3 months before the index hospitalization and 1 month after discharge for alive patients. Patients leaving against medical advice were excluded. Patients defined as being admitted for AMI and then discharged on the same day were excluded because it is unlikely these were clinically significant AMIs.
Baseline comorbidities were ascertained using secondary diagnosis codes that were coded as present on admission during the index hospitalization, as well as from all principal and secondary diagnosis codes from all hospitalizations in the 3-month period preceding the date of index hospitalization (eTable 2 in the Supplement). The race of all beneficiaries was categorized as white, black, or other (ie, Asian, Hispanic, North American Native, other, and unknown).
Assessment of Frailty
The primary predictor of interest was frailty, as assessed by the Hospital Frailty Risk Score (HFRS).18 This score was developed and validated in a large cohort of British adults 75 years or older, based on clustering of diagnoses associated with 30-day mortality, long hospital stay (>10 days in hospital), and emergency readmission within 30 days of discharge.18 It has been externally validated in elderly patients from Canada, where it was found to independently predict long hospital length of stay, 30-day readmission, and 1-year mortality.19 For each patient, we calculated the HFRS based on 1 or more of 109 ICD-10-CM secondary diagnosis codes that were coded as present on admission during the index hospitalization and from all principal and secondary diagnosis codes from any hospitalization within the prior 3 months (eTable 3 in the Supplement). Individuals were categorized into 3 frailty risk groups (low [<5], intermediate [5-15], and high risk [>15]) according to their calculated HFRS, based on previously validated cut points.18
Outcomes
The primary outcome of the study was all-cause mortality within 30 days of the date of admission (30-day postadmission rate), obtained by cross-referencing vital status data in the 2016 Medicare Master Beneficiary Summary File. We also evaluated long length of stay, defined as more than 10 days in hospital.18Among patients discharged alive, we examined rates of all-cause mortality within 30 days (30-day postdischarge mortality) and readmission within 30 days (30-day readmission).
Statistical Analysis
Continuous variables are presented as means and SDs, and categorical variables are presented as counts and percentages. We compared all outcomes among HFRS risk categories using the Pearson χ2 or analysis of variance tests as appropriate. We constructed multivariable logistic regression models, adjusted for age, sex, race, and comorbidities, to assess the independent association between levels of frailty (as a categorical measure) and mortality outcomes. We fit a similar model to evaluate the association between frailty levels and readmission, adjusted for patient characteristics as described earlier. We also conducted sensitivity analyses to assess the association of frailty as a continuous variable with outcomes. Race was also included as a variable owing to its known association with mortality for each condition.20
The extent to which the inclusion of frailty improved each model’s discrimination of 30-day outcomes was assessed by comparing the concordance statistics (C statistics) of models including and not including HFRS, using the DeLong test.21 The integrated discrimination improvement metric was also estimated to assess the improvement in discrimination of augmented models.22 Finally, restricted cubic spline regression models with 7 knots were used to display the association between HFRS and 30-day postadmission mortality, 30-day postdischarge mortality, and 30-day readmission rates, adjusted for age, sex, race, and comorbidities.23 As an HFRS of 5 has previously been considered the cutoff value for identifying frail patients, we selected this value as the reference population for restricted cubic spline plots.18 All statistical analyses were performed in Stata, version 15.0 (StataCorp) and SAS, version 9.4 (SAS Institute) using a 2-tailed P value of less than .05 to define statistical significance. Analysis began August 2018.
Results
Overall Results
A total of 785 127 hospitalizations (166 200 AMI hospitalizations [21.2%], 348 619 HF hospitalizations [44.4%], and 270 308 pneumonia hospitalizations [34.4%]) were included in analysis. The mean (SD) age of the patients in this analysis was 77.4 (8.7) years for individuals with AMI hospitalizations, 80.1 (9.0) years for individuals with HF hospitalizations, and 79.2 (8.9) for individuals with pneumonia hospitalizations. Women accounted for 44.5% (n = 73 959) of the admissions for AMI, 52.7% (n = 183 722) of the admissions for HF, and 53.6% (n = 144 895) of the admissions for pneumonia. Overall, 83.6% (n = 656 315) of patients hospitalized for each target condition were white. Further information regarding demographics and clinical comorbidities for each condition are shown in Table 1.
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Acute Myocardial Infarction (n = 166 200) | Heart Failure (n = 348 619) | Pneumonia (n = 270 308) | |
| Age, mean (SD), y | 77.4 (8.7) | 80.1 (9.0) | 79.2 (8.9) |
| Male | 92 249 (55.5) | 164 830 (47.3) | 125 409 (46.4) |
| Race | |||
| White | 142 486 (85.7) | 284 450 (81.6) | 229 379 (84.9) |
| Black | 13 489 (8.1) | 43 781 (12.6) | 23 412 (8.7) |
| Othera | 10 225 (6.2) | 20 388 (5.8) | 17 517 (6.5) |
| History of myocardial infarction | 25 666 (15.4) | 55 277 (15.9) | 21 527 (8.0) |
| History of coronary artery bypass graft | 22 350 (13.4) | 64 532 (18.5) | 23 900 (8.8) |
| Valvular heart disease | 33 917 (20.4) | 117 057 (33.6) | 28 277 (10.5) |
| Hypertension | 141 602 (85.2) | 298 356 (85.6) | 208 489 (77.1) |
| Peripheral vascular disease | 35 971 (21.6) | 106 154 (30.4) | 36 773 (13.6) |
| Cerebrovascular disease | 20 616 (12.4) | 42 415 (12.2) | 19 432 (7.2) |
| Chronic obstructive pulmonary disease | 47 715 (28.7) | 167 426 (48.0) | 137 384 (50.8) |
| Diabetes mellitus | 62 482 (37.6) | 151 235 (43.4) | 80 764 (29.9) |
| Obesity | 22 613 (13.6) | 63 875 (18.3) | 27 264 (10.1) |
| Liver disease | 4700 (2.8) | 10 950 (3.1) | 6708 (2.5) |
| Renal failure | 50 540 (30.4) | 164 109 (47.1) | 70 408 (26.0) |
| Iron deficiency anemia | 6553 (3.9) | 25 029 (7.2) | 12 949 (4.8) |
| Rheumatoid disease | 6226 (3.7) | 15 808 (4.5) | 13 255 (4.9) |
| Peptic ulcer disease | 3304 (2.0) | 9355 (2.7) | 3519 (1.3) |
| Dementia | 20 805 (12.5) | 56 833 (16.3) | 48 742 (18.0) |
| Depression | 14 800 (8.9) | 39 891 (11.4) | 36 211 (13.4) |
| Cancer | 9737 (5.9) | 28 076 (8.1) | 34 646 (12.8) |
| Substance abuse | 4577 (2.8) | 9029 (2.6) | 7251 (2.7) |
| Acquired immunodeficiency syndrome | 92 (0.1) | 185 (0.1) | 231 (0.1) |
| Hospital Frailty Risk Score, mean (SD) | 7.3 (7.4) | 10.8 (8.3) | 8.2 (5.7) |
| Hospital Frailty Risk Score categories | |||
| Low risk (<5) | 81 988 (49.3) | 96 183 (27.5) | 90 258 (33.4) |
| Intermediate risk (5-15) | 61 154 (36.8) | 165 310 (47.4) | 149 084 (55.2) |
| High risk (>15) | 23 058 (13.9) | 87 126 (25.0) | 30 966 (11.5) |
Other includes Asian, Hispanic, North American Native, other, and unknown.
Hospital Frailty Risk Score
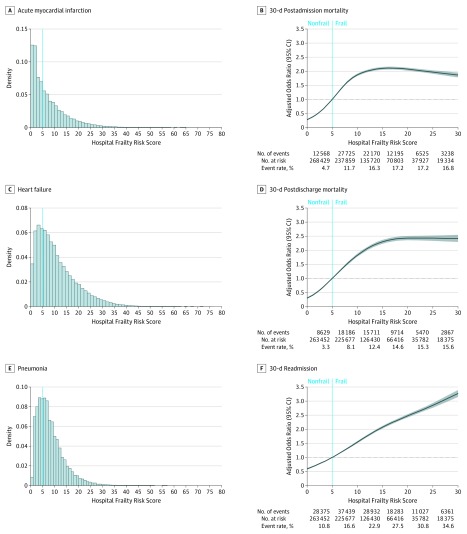
The HFRS ranged from 0 to 80 with a mean (SD) HFRS of 7.3 (7.4) for patients with AMI, 10.8 (8.3) for patients with HF, and 8.2 (5.7) for patients with pneumonia (Figure A, C, and E). Hospitalizations among individuals with the highest level of frailty (HFRS, >15) comprised 23 058 AMI hospitalizations (13.9%), 87 126 HF hospitalizations (25.0%), and 30 966 pneumonia hospitalizations (11.5%) (Table 1).
Figure. Distribution of the HFRS Among the Study Population and the Association of the HFRS with 30-Day Outcomes for AMI, HF, and Pneumonia.
Histograms showing the distribution of the Hospital Frailty Risk Score (HFRS) among patients with acute myocardial infarction (AMI) (A), heart failure (HF) (C), and pneumonia (E). The dotted line indicates the cutoff score for frailty, where patients with a score of less than 5 are considered not frail and those with a score of more than 5 are considered frail. Association of the HFRS (5 is reference standard) with 30-day postadmission mortality (B), 30-day postdischarge mortality (D), and 30-day readmission (F) among the combined cohort of patients hospitalized for AMI, HF, and pneumonia. The spline curves are truncated at a frailty score of 30.
Outcomes
Patients with higher frailty scores had higher observed rates of 30-day postadmission mortality, 30-day postdischarge mortality, and 30-day readmission for all 3 conditions studied. While long length-of-stay rates among patients with an HFRS less than 5 were 2.6% (n = 2149), 1.8% (n = 1712), and 2.4% (n = 2136), among patients with an HFRS more than 15, the rates were 19.5% (n = 4492), 13.0% (n = 11 357), and 16.2% (n = 5008) in AMI, HF, and pneumonia cohorts, respectively (Table 2). Among patients hospitalized for AMI, after adjustment for age, sex, race, and comorbidities, an HFRS more than 15 (compared with an HFRS <5), was associated with a higher risk of 30-day postadmission mortality (adjusted odds ratio [aOR], 3.6; 95% CI, 3.4-3.8; P < .001), 30-day postdischarge mortality (aOR, 4.0; 95% CI, 3.7-4.3; P < .001), and 30-day readmission (aOR, 3.0; 95% CI, 2.9-3.1; P < .001). Similar patterns were observed for patients hospitalized with HF (30-day postadmission mortality: aOR, 3.5; 95% CI, 3.4-3.7; P < .001; 30-day postdischarge mortality: aOR, 3.5; 95% CI, 3.3-3.6; P < .001; and 30-day readmission: aOR, 2.9; 95% CI, 2.8-3.0; P < .001) and among patients with pneumonia (30-day postadmission mortality: aOR, 2.4; 95% CI, 2.3-2.6; P < .001; 30-day postdischarge mortality: aOR, 3.0; 95% CI, 2.9-3.2; P < .001; and 30-day readmission: aOR, 2.8; 95% CI, 2.7-2.9; P < .001). These findings remained consistent when frailty was evaluated on a continuous scale (Table 3).
Table 2. Outcomes of the Study Population According to Hospital Frailty Risk Score Categories.
| Characteristic | Hospital Frailty Risk Score, No. (%) | P Value | ||
|---|---|---|---|---|
| Low Risk (<5) | Intermediate Risk (5-15) | High Risk (>15) | ||
| Acute myocardial infarction, total No. | 81 988 | 61 154 | 23 058 | NA |
| Long length of stay (>10 d) | 2149 (2.6) | 8288 (13.6) | 4492 (19.5) | <.001 |
| Observed 30-d postadmission mortality | 3464 (4.2) | 11 019 (18.0) | 4567 (19.8) | <.001 |
| Observed 30-d postdischarge mortalitya | 1670 (2.1) | 5184 (9.5) | 3198 (15.1) | <.001 |
| Observed 30-d readmissiona | 8323 (10.4) | 11 735 (21.5) | 6919 (32.8) | <.001 |
| Heart failure, total No. | 96 183 | 165 310 | 87 126 | NA |
| Long length of stay (>10 d) | 1712 (1.8) | 12 644 (7.7) | 11 357 (13.0) | <.001 |
| Observed 30-d postadmission mortality | 4181 (4.4) | 20 674 (12.5) | 14 236 (16.3) | <.001 |
| Observed 30-d postdischarge mortalitya | 3440 (3.6) | 15 782 (10.0) | 11 983 (14.5) | <.001 |
| Observed 30-d readmissiona | 11 176 (11.8) | 29 707 (18.8) | 26 240 (31.7) | <.001 |
| Pneumonia, total No. | 90 258 | 149 084 | 30 966 | NA |
| Long length of stay (>10 d) | 2136 (2.4) | 12 156 (8.2) | 5008 (16.2) | <.001 |
| Observed 30-d postadmission mortality | 4923 (5.5) | 18 568 (12.5) | 5085 (16.4) | <.001 |
| Observed 30-d postdischarge mortalitya | 3519 (4.0) | 13 193 (9.3) | 4592 (15.7) | <.001 |
| Observed 30-d readmissiona | 8876 (10.1) | 25 420 (18.0) | 7791 (26.7) | <.001 |
Abbreviation: NA, not applicable.
The number at risk for these outcomes was counted as patients who were alive at discharge.
Table 3. Multivariable Logistic Regression Analyses Results.
| Characteristic | Acute Myocardial Infarction | Heart Failure | Pneumonia | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| 30-d Postadmission mortalitya | ||||||
| HFRS (continuous) | 1.039 (1.037-1.042) | <.001 | 1.035 (1.034-1.037) | <.001 | 1.049 (1.046-1.051) | <.001 |
| HFRS categories | ||||||
| Low risk (<5) | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Intermediate risk (5-15) | 3.313 (3.126-3.511) | 2.800 (2.703-2.901) | 2.081 (2.013-2.152) | |||
| High risk (>15) | 3.593 (3.440-3.753) | 3.537 (3.398-3.682) | 2.445 (2.334-2.562) | |||
| 30-d Postdischarge mortalitya | ||||||
| HFRS (continuous) | 1.043 (1.040-1.046) | <.001 | 1.037 (1.035-1.039) | <.001 | 1.060 (1.057-1.062) | <.001 |
| HFRS categories | ||||||
| Low risk (<5) | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Intermediate risk (5-15) | 3.147 (2.963-3.341) | 2.535 (2.438-2.635) | 2.040 (1.962-2.122) | |||
| High risk (>15) | 3.984 (3.700-4.290) | 3.475 (3.325-3.640) | 3.032 (2.878-3.195) | |||
| 30-d Readmissiona | ||||||
| HFRS (continuous) | 1.056 (1.054-1.058) | <.001 | 1.054 (1.053-1.055) | <.001 | 1.061 (1.059-1.064) | <.001 |
| HFRS categories | ||||||
| Low risk (<5) | 1 [Reference] | <.001 | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Intermediate risk (5-15) | 1.902 (1.840-1.967) | 1.569 (1.531-1.608) | 1.814 (1.766-1.863) | |||
| High risk (>15) | 2.983 (2.850-3.123) | 2.909 (2.827-2.994) | 2.822 (2.713-2.935) | |||
Abbreviation: HFRS, Hospital Frailty Risk Score.
Models adjusted for age, sex, race, and comorbidities.
Improvement in Risk Adjustment
Addition of the HFRS to risk-adjustment models significantly improved model discrimination of each outcome for all target conditions (Table 4). After adjustment for age, sex, race, and comorbidities, the risk of each outcome (Figure B, 30-day postadmission mortality; Figure D, 30-day postdischarge mortality; Figure F, 30-day readmission) increased with an increasing HFRS.
Table 4. Discrimination of the Models and the Improvement of Performance After Adding Hospital Frailty Risk Score on Prediction of Outcomes.
| Characteristic | C Statistic (95% CI) | DeLong P Value | IDI | IDI P Value | |
|---|---|---|---|---|---|
| Without Hospital Frailty Risk Score | With Hospital Frailty Risk Score | ||||
| 30-d Postadmission mortality | |||||
| Acute myocardial infarction | 0.73 (0.72-0.74) | 0.76 (0.75-0.76) | <.001 | 0.0226 | <.001 |
| Heart failure | 0.67 (0.66-0.67) | 0.70 (0.69-0.70) | <.001 | 0.0121 | <.001 |
| Pneumonia | 0.70 (0.69-0.71) | 0.73 (0.72-0.73) | <.001 | 0.0178 | <.001 |
| 30-d Postdischarge mortality | |||||
| Acute myocardial infarction | 0.76 (0.75-0.76) | 0.78 (0.77-0.79) | <.001 | 0.0136 | <.001 |
| Heart failure | 0.68 (0.67-0.68) | 0.70 (0.69-0.71) | <.001 | 0.0096 | <.001 |
| Pneumonia | 0.69 (0.68-0.70) | 0.71 (0.70-0.72) | <.001 | 0.0088 | <.001 |
| 30-d readmission | |||||
| Acute myocardial infarction | 0.65 (0.64-0.65) | 0.68 (0.68-0.69) | <.001 | 0.0172 | <.001 |
| Heart failure | 0.61 (0.60-0.61) | 0.64 (0.64-0.65) | <.001 | 0.0184 | <.001 |
| Pneumonia | 0.60 (0.59-0.61) | 0.63 (0.62-0.64) | <.001 | 0.0116 | <.001 |
Abbreviation: IDI, Integrated discrimination improvement.
Discussion
In this study of US Medicare fee-for-service beneficiaries, frailty as determined by an ICD-10 claims-based frailty score (the HFRS) was associated with a higher risk of 30-day outcomes for AMI, HF, and pneumonia hospitalizations. Nearly 15% of AMI, 25% of HF, and 12% of pneumonia hospitalizations were for individuals at the highest level of frailty. The addition of this claims-based frailty score improved the prediction of 30-day outcomes when added to traditional comorbidities typically used in risk adjustment for these conditions. These findings may also have implications for ongoing evaluations of hospital performance in the United States and suggest that the absence of frailty in most current risk-adjustment models may place hospitals that care for a substantial number of frail patients at a disadvantage under programs that compare hospital performance.
As the Medicare population ages, understanding the relationship between frailty, a syndrome involving multisystem impairment in functional recovery, and outcomes24 is increasingly important to accurately predict health care use and adverse outcomes.25 The addition of frailty to risk models is also important to ensure adequate risk adjustment. While several claims-based methods exist to measure frailty, these have been mostly based on ICD-9-CM claims and may not comprehensively quantify frailty across all patients owing to a limited number of available codes.26,27 Since the transition to ICD-10-CM on October 1, 2015, which contains nearly 5-fold (from 14 000 to 70 000) the number of available claims,28 the increased granularity of claims data now permits a more comprehensive assessment of conditions associated with frailty and allows a more detailed longitudinal record of how frailty influences risk. The HFRS is a claims-based frailty index that uses ICD-10-CM diagnostic codes and has been both internally and externally validated using administrative data from different countries. This score was validated against the Fried Phenotype and the Rockwood Frailty Index, 2 clinical frailty scales that are widely used but require more time and resources for data collection.18 Prior studies have demonstrated that HFRS is predictive of outcomes including mortality, readmission, and prolonged length of stay among older individuals (≥75 years) from the United Kingdom and Canada and after in-hospital cardiac arrest in populations in Australia.18,19,29 In this study, nearly 20% of patients were categorized in the high frailty risk category of the HFRS. Thus, the HFRS may identify hospitalized patients at higher risk for short-term health care use and allow for better-targeted strategies, such as more intensive follow-up or postacute care service use, during the vulnerable postdischarge period to improve outcomes such as mortality and readmission.
Notably, CMS does not currently include frailty in risk-adjustment models for AMI, HF, and pneumonia hospitalizations among Medicare beneficiaries. The magnitude of improvement as assessed by changes in the C statistic was modest but statistically significant with the inclusion of the HFRS and was more robust as measured by the integrated discrimination improvement. Future studies should examine whether adding frailty to current risk models could meaningfully improve these risk models and alter the assessment of hospital performance.30 This would have important implications for current value-based reimbursement initiatives, including the Hospital Value-Based Purchasing program and the Hospital Readmissions Reduction Program, each of which uses 30-day mortality and 30-day readmission measures to evaluate performance. Unless frailty is adequately captured in risk-adjustment metrics, it is possible that hospitals that care for a higher proportion of patients with frailty are disproportionately financially penalized for worse outcomes owing to unrecognized comorbidities among the patients they care for, rather than quality of care delivered.
Limitations
Our study has a few limitations. First, administrative codes may not capture the severity of a given condition or its alteration postprocedure. Second, our analysis was limited to Medicare fee-for-service beneficiaries and may therefore have limited generalizability outside of this population. Third, as the HFRS was developed to identify clusters of health care use, it may not be useful to identify phenotypic frailty, and the degree to which phenotypic frailty confers an increased risk of health care use above that of comorbidities alone is unknown.
Conclusions
Among Medicare fee-for-service beneficiaries, frailty as measured by the HFRS was strongly associated with short-term mortality and readmissions among patients hospitalized for AMI, HF, or pneumonia. The addition of HFRS to traditional comorbidity-based risk-prediction models significantly improved prediction of adverse outcomes for all 3 conditions. Further research is needed to understand whether the addition of frailty as measured by the HFRS to current CMS risk-adjustment models affects which hospitals are financially rewarded or penalized under current value-based programs.
eTable 1. Codes to identify hospitalizations
eTable 2. Codes to identify comorbidities
eTable 3. List of ICD-10 codes, prevalence and number of points for each to create the Hospital Frailty Risk Score in acute myocardial infarction, chronic heart failure and pneumonia patients
References
- 1.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355-363. doi: 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Normand S-LT, Wang Y, et al. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med. 2010;362(12):1110-1118. doi: 10.1056/NEJMsa0907130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. doi: 10.1001/jama.2013.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai NR, Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PLoS One. 2016;11(10):e0160492. doi: 10.1371/journal.pone.0160492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318(3):270-278. doi: 10.1001/jama.2017.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan AM, Krinsky S, Maurer KA, Dimick JB. Changes in hospital quality associated with hospital value-based purchasing. N Engl J Med. 2017;376(24):2358-2366. doi: 10.1056/NEJMsa1613412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. doi: 10.1161/CIRCULATIONAHA.114.010270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Op Het Veld LPM, Ament BHL, van Rossum E, et al. Can resources moderate the impact of levels of frailty on adverse outcomes among (pre-) frail older people? a longitudinal study. BMC Geriatr. 2017;17(1):185. doi: 10.1186/s12877-017-0583-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundi H, Valsdottir LR, Popma JJ, et al. Impact of a claims-based frailty indicator on the prediction of long-term mortality after transcatheter aortic valve replacement in Medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2018;11(10):e005048. doi: 10.1161/CIRCOUTCOMES.118.005048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueroa JF, Joynt Maddox KE, Beaulieu N, Wild RC, Jha AK. Concentration of potentially preventable spending among high-cost Medicare subpopulations: an observational study. Ann Intern Med. 2017;167(10):706-713. doi: 10.7326/M17-0767 [DOI] [PubMed] [Google Scholar]
- 12.Gobbens RJ, van Assen MA. Frailty and its prediction of disability and health care utilization: the added value of interviews and physical measures following a self-report questionnaire. Arch Gerontol Geriatr. 2012;55(2):369-379. doi: 10.1016/j.archger.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 13.Sutton M, Nikolova S, Boaden R, Lester H, McDonald R, Roland M. Reduced mortality with hospital pay for performance in England. N Engl J Med. 2012;367(19):1821-1828. doi: 10.1056/NEJMsa1114951 [DOI] [PubMed] [Google Scholar]
- 14.Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345-353. doi: 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 15.Bosco-Lévy P, Duret S, Picard F, et al. Diagnostic accuracy of the International Classification of Diseases, Tenth Revision, codes of heart failure in an administrative database. Pharmacoepidemiol Drug Saf. 2019;28(2):194-200. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 17.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf. 2019;28(4):284-288. doi: 10.1136/bmjqs-2018-008661 [DOI] [PubMed] [Google Scholar]
- 20.Downing NS, Wang C, Gupta A, et al. Association of racial and socioeconomic disparities with outcomes among patients hospitalized with acute myocardial infarction, heart failure, and pneumonia: an analysis of within- and between-hospital variation. JAMA Netw Open. 2018;1(5):e182044. doi: 10.1001/jamanetworkopen.2018.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198-1202. doi: 10.1093/jnci/80.15.1198 [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 25.Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73(1):19. doi: 10.1186/s13690-015-0068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980-987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59-66. doi: 10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Centers for Disease Control and Prevention International Classification of Diseases, (ICD-10-CM/PCS) transition—background. https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm. Accessed August 15, 2019.
- 29.Smith RJ, Reid DA, Santamaria JD. Frailty is associated with reduced prospect of discharge home after in-hospital cardiac arrest. Intern Med J. 2019;49(8):978-985. doi: 10.1111/imj.14159 [DOI] [PubMed] [Google Scholar]
- 30.Johnston KJ, Wen H, Hockenberry JM, Joynt Maddox KE. Association between patient cognitive and functional status and Medicare total annual cost of care: implications for value-based payment. JAMA Intern Med. 2018;178(11):1489-1497. doi: 10.1001/jamainternmed.2018.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes to identify hospitalizations
eTable 2. Codes to identify comorbidities
eTable 3. List of ICD-10 codes, prevalence and number of points for each to create the Hospital Frailty Risk Score in acute myocardial infarction, chronic heart failure and pneumonia patients