Key Points
Question
What is the rate of a stroke defined by diffusion restriction detected on magnetic resonance imaging scans among patients with low-risk suspected transient ischemic attack or minor stroke presentations?
Findings
This cohort study of 1028 patients with low-risk transient focal neurologic events found a 13.5% rate of acute ischemic stroke detected on magnetic resonance imaging scans; the final diagnosis was revised in 30.0% of patients after brain magnetic resonance imaging. The clinical risk of recurrent clinical stroke at 1 year in this low-risk population was confirmed to be low, at 0.7%.
Meaning
There is a higher than expected rate of true ischemia in patients with low-risk suspected transient ischemic attack and minor stroke; magnetic resonance imaging is required for diagnosis because clinical assessment could not reliably identify the correct diagnosis.
Abstract
Importance
Early treatment of patients with transient ischemic attack (TIA) reduces the risk of stroke. However, many patients present with symptoms that have an uncertain diagnosis. Patients with motor, speech, or prolonged symptoms are at the highest risk for recurrent stroke and the most likely to undergo comprehensive investigations. Lower-risk patients are much more likely to be cursorily investigated.
Objective
To establish the frequency of acute infarct defined by diffusion restriction detected on diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) scan (DWI positive).
Design, Setting, and Participants
The Diagnosis of Uncertain-Origin Benign Transient Neurological Symptoms (DOUBT) study was a prospective, observational, international, multicenter cohort study of 1028 patients with low-risk transient or minor symptoms referred to neurology within 8 days of symptom onset. Patients were enrolled between June 1, 2010, and October 31, 2016. Included patients were 40 years or older and had experienced nonmotor or nonspeech minor focal neurologic events of any duration or motor or speech symptoms of short duration (≤5 minutes), with no previous stroke.
Exposures
Patients underwent a detailed neurologic assessment prior to undergoing a brain MRI within 8 days of symptom onset.
Main Outcomes and Measures
The primary outcome was restricted diffusion on a brain MRI scan (acute stroke).
Results
A total of 1028 patients (522 women and 506 men; mean [SD] age, 63.0 [11.6] years) were enrolled. A total of 139 patients (13.5%) had an acute stroke as defined by diffusion restriction detected on MRI scans (DWI positive). The final diagnosis was revised in 308 patients (30.0%) after undergoing brain MRI. There were 7 (0.7%) recurrent strokes at 1 year. A DWI-positive brain MRI scan was associated with an increased risk of recurrent stroke (relative risk, 6.4; 95% CI, 2.4-16.8) at 1 year. Absence of a DWI-positive lesion on a brain MRI scan had a 99.8% negative predictive value for recurrent stroke. Factors associated with MRI evidence of stroke in multivariable modeling were older age (odds ratio [OR], 1.02; 95% CI, 1.00-1.04), male sex (OR, 2.03; 95% CI, 1.39-2.96), motor or speech symptoms (OR, 2.12; 95% CI, 1.37-3.29), ongoing symptoms at assessment (OR, 1.97; 95% CI, 1.29-3.02), no prior identical symptomatic event (OR, 1.87; 95% CI, 1.12-3.11), and abnormal results of initial neurologic examination (OR, 1.71; 95% CI, 1.11-2.65).
Conclusions and Relevance
This study suggested that patients with transient ischemic attack and symptoms traditionally considered low risk carry a substantive risk of acute stroke as defined by diffusion restriction (DWI positive) on a brain MRI scan. Early MRI is required to make a definitive diagnosis.
This cohort study examines the frequency of acute infarction defined by diffusion restriction detected on magnetic resonance imaging scans among patients with low-risk, mild, or transient focal neurologic symptoms.
Introduction
The clinical problem of patients with acute but transient or minor neurologic symptoms who present to the emergency department, acute care clinic, or office is first to determine if the diagnosis is truly due to brain ischemia and then to risk stratify. The problem is exactly akin to patients who present with chest pain. Ischemia is the most important diagnosis to exclude because of the threat of progression to infarction. Among the half million North Americans who had a transient ischemic attack (TIA) or minor stroke annually, 10% to 17% will experience an early recurrent stroke within 90 days.1,2 Alternate diagnoses (stroke mimics) comprise at least 50% of these presentations and generally have more benign prognoses.3,4 Research has shown that, even with a final diagnosis of TIA or minor stroke, the extent of investigations and treatment varies widely, suggesting that in a patient with an uncertain diagnosis, this variation may be even wider.5
The diagnosis of TIA or minor stroke is clinical, based almost exclusively on the patient’s history and there is poor diagnostic agreement even among experts.6 Transient ischemic attack can be treated aggressively, resulting in a low risk of stroke.7,8,9 Motor or speech symptoms have a known association with a higher risk for recurrent stroke.10 However, half of all patients presenting with transient or mild neurologic deficits have low-risk symptoms and an uncertain diagnosis or prognosis.3 A nonischemic final diagnosis is more common among patients with TIA-like clinical presentations without high-risk features.11 Patients with lower-risk clinical presentations have been underrepresented in past studies and were excluded from most modern stroke prevention clinical trials.12
Patients with evidence of ischemia on brain diffusion-weighted magnetic resonance imaging (MRI) scans, or evidence of a symptom relevant arterial occlusion or stenosis on neurovascular imaging scans, definitively have an ischemic cause and are at highest risk of early recurrent stroke.13,14,15,16,17 We sought to establish the frequency of acute infarction defined by diffusion restriction detected on MRI scans among patients with mild focal neurologic, but low-risk, symptoms. We hypothesized that MRI evidence of brain ischemia confirming the diagnosis would be low and that clinical features could determine the presence of brain ischemia.
Methods
The Diagnosis of Uncertain-Origin Benign Transient Neurological Symptoms (DOUBT) study was a multicenter, international, prospective, observational cohort study involving 9 sites from Canada (6), Czech Republic (1), and Australia (2). Inclusion criteria were the following: (1) possible minor stroke or TIA and referral to a stroke neurologist for a focal neurologic event that included either nonmotor or nonspeech symptoms of any duration, or motor or speech symptoms of short duration (≤5 minutes) (in previous studies, event duration of ≥10 minutes was associated with a greater risk of recurrent stroke,10 so we deliberately chose a 5-minute threshold to identify a low-risk cohort18); (2) a National Institutes of Health Stroke Scale score of 3 or lower if symptoms were persistent; (3) aged 40 years or older; and (4) brain MRI within 8 days of symptom onset (MRI was always completed after the clinical assessment). Exclusion criteria were the following: (1) focal motor or speech symptoms lasting more than 5 minutes; (2) symptoms of isolated monocular vision loss; (3) prior clinical stroke; (4) modified Rankin Score of 2 or greater, indicating dependence for activities of daily living; (5) serious comorbid illness with an estimated life expectancy of less than 1 year; (6) contraindication to MRI; or (7) the examining neurologist concluded that the diagnostic criteria were definitively met for an alternative cause (eg, a subdural hemorrhage). All patients provided written informed consent. This study was approved by the local institutional ethics boards at each site (Calgary, Alberta, Canada [Foothills Hospital], Conjoint Health Research Ethics Board; Sherbrooke [CHUS], Comité d’éthique de la recherche du CIUSSS de l’Estrie–CHUS; North Sydney, Human Research Ethics Committee Northern Sydney Local Health District; Melbourne, Melbourne Health Human Research Ethics Committee; Toronto, Ontario, Canada [Sunnybrook], Research Ethics Board, Sunnybrook Health Sciences Centre; Vancouver, British Columbia, Canada, University of British Columbia Clinical Research Ethics Board; London, Ontario, Canada, Western Research Ethics Board, The Office of Human Research Ethics; Brno, Czech Republic, St. Anne’s University Hospital Ethical Committee; Quebec City, Quebec, Canada [Laval University] Comité d’éthique de la researche du CHU de Québec, Université de Laval; Victoria, British Colombia, Canada, Vancouver Island Clinical Research Ethics Board; and Sherbrooke, Quebec, Canada [Charles-LeMoyne Hospital], Comité d’éthique de la researche du CISSS de la Montérégie-Centre).
Participants were enrolled as soon as possible after their neurologic event, but no later than 8 days after symptom onset, and prior to undergoing MRI. Imaging was performed within 8 days to ensure capture of small restricted diffusion lesions. All participants were examined by stroke neurologists. Features of the medical history were self-reported. For each participant, a detailed standardized questionnaire describing the nature of the event was completed by the neurologist, the neurologic examination results were rated as normal or not, and a provisional diagnosis was recorded, all prior to the MRI. Participants were asked if they thought that their symptoms had completely resolved or were ongoing. After the MRI was completed and any further investigations were completed, a final diagnosis for the event was recorded. Transient ischemic attack was defined clinically as a sudden loss of focal brain or ocular function of presumed vascular origin with complete symptom resolution within 24 hours (even if the MRI scans showed evidence of a diffusion-weighted imaging [DWI]–positive lesion).19
Follow-up at 1 year from symptom onset was completed by telephone to assess for recurrent strokes or death. All patients who had recurrent strokes were interviewed in person and had their medical records reviewed to confirm the outcome event. Transient ischemic attack and myocardial infarction outcome events were self-reported. Images were then re-reviewed with clinical knowledge to assess the possible association of imaging lesion location with reported symptoms and signs.
Imaging
Brain MRI was conducted on clinically available MR machines at 1.5 T or 3.0 T using standard local clinical protocols, but minimally with an axial DWI sequence. Images were centrally reviewed for acute infarction by a neuroradiologist without knowledge of the patients’ clinical symptoms.
Statistical Analysis
The proportion of participants with acute stroke detected on brain MRI scans (ie, DWI positive) was the primary outcome. Secondary outcomes were recurrent ischemic stroke, death, myocardial infarction, and TIA at 1 year. The composite outcome of stroke, myocardial infarction, and death was also reported. Fisher exact test for comparison of proportions was used to assess for the primary outcome; P < .05 was considered statistically significant, and all tests were 2-sided. To assess if clinical factors were associated with the primary outcome, demographic and event data were assessed as univariable factors associated with the primary outcome. Backward, manual, stepwise elimination was used to develop a parsimonious multivariable model, assessing main effects only. Variables were entered into the model if they were significant at the P < .10 level in the univariable analysis. The model was developed from the perspective of what was known clinically at the time of neurologic assessment. This model was assessed for its discriminative value using receiver operator curve characteristics.
At the time of study design, one study described a 23% rate of DWI-positive lesions in patients with TIA without motor and speech symptoms.20 We conservatively based our sample size estimation on a 10% rate of DWI-positive lesions and concluded that a sample size of 1000 participants would provide clinically relevant precision with a 95% CI width of 8.2% to 12.0%. Statistical analyses were completed by 2 of us (S.B.C. and M.D.H.) using Stata, version 15 (StataCorp). Missing data were not imputed. There was no adjustment for multiplicity.
Results
Between June 1, 2010, and October 31, 2016, 1028 participants were prospectively enrolled from an outpatient clinic setting (732 [71.2%]) and the remainder from the emergency department. Median time from symptom onset to neurologic assessment was 50 hours (interquartile range [IQR], 15-106 hours). Median time from symptom onset to MRI was 102 hours (interquartile range, 53-144 hours) (eFigure 1 in the Supplement). The mean (SD) age was 63.0 (11.6) years. Median National Institutes of Health Stroke Scale score was 0 (range, 0-3). Most patients (656 [63.8%]) reported that all symptoms related to the event had resolved at the time of assessment. Among those with resolved symptoms, the median symptom duration was 120 minutes (interquartile range, 15-360 minutes). Participant characteristics are shown in Table 1.
Table 1. Baseline Patient Characteristics.
| Variable | No. (%) (N = 1028) |
|---|---|
| Medical history | |
| Age, median (IQR), y | 63.0 (54.1-71.5) |
| Hypertension | 480 (46.7) |
| Type 1 or 2 diabetes | 140 (13.6) |
| Current smoker | 105 (10.2) |
| Ischemic heart disease | 87 (8.5) |
| Atrial fibrillation | 51 (5.0) |
| Hyperlipidemia | 342 (33.3) |
| Taking aspirin prior to event | 347 (33.8) |
| Taking statin prior to event | 285 (27.7) |
| Taking antihypertensive medication prior to event | 419 (40.8) |
| No known vascular risk factors | 352 (34.2) |
| Historical and clinical features of the transient neurologic event | |
| Any migraine history | 256 (24.9) |
| History of migraine with aura | 80 (7.8) |
| Any reported stress by patient | 158 (15.4) |
| Any motor or speech symptoms | 218 (21.2) |
| Isolated sensory symptoms | 482 (46.9) |
| Symptom duration <5 min | 122 (11.9) |
| Progression of first to last symptoms >10 min | 179 (17.4) |
| Ongoing symptoms at time of assessment | 370 (36.0) |
| Previous identical event | 238 (23.2) |
| Initial neurologic examination results normal | 740 (72.0) |
Abbreviation: IQR, interquartile range.
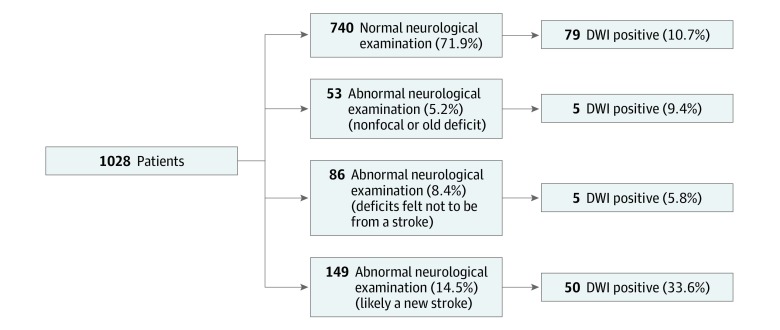
Acute infarcts (ie, DWI-positive lesions detected on brain MRI scans) were observed in 139 participants (13.5%; 95% CI, 11.5%-15.8%). Among these, 92 participants (8.9%) had single lesions and 47 (4.6%) had multiple lesions. Those with acute infarcts were older than participants without acute infarcts (mean age, 65.9 years vs 62.7 years; P = .003). In univariable analysis, age greater than 60 years (relative risk [RR], 1.94; 95% CI, 1.36-2.78), male sex (RR, 1.73; 95% CI, 1.25-2.38), motor or speech symptoms (RR, 1.45; 95% CI, 1.03-2.03), and ongoing symptoms at the time of assessment (RR, 1.91; 95% CI, 1.41-2.60) were associated with acute infarcts (Table 2). The Figure shows the breakdown of patients and their MRI status depending on whether results of the neurologic examination were normal or not. eFigures 2 and 3 in the Supplement show the MRI status according to patient-determined complete symptom resolution. More important, there was a similar event rate to that in the overall population in the setting of normal neurologic examination results (79 of 740 [10.7%]). Only 50 of 149 patients (33.6%) for whom the treating physician thought that the patient had a deficit consistent with a stroke actually had evidence of ischemia on MRI scans. Among patients who reported completed symptom resolution, there was no difference in the rate of DWI positivity based on quartiles of symptom duration (quartile 1, 24 of 159 [15.1%]; quartile 2, 11 of 157 [7.0%]; quartile 3, 13 of 157 [8.3%]; and quartile 4, 19 of 183 [10.4%]). Any history of migraine (RR, 0.66; 95% CI, 0.44-0.995), a prior identical event (RR, 0.56; 95% CI, 0.36-0.88), and normal results of a neurologic examination at initial assessment (RR, 0.51; 0.38-0.70) were associated with a lower risk of acute infarct detected on MRI scans. Slow migration of symptoms and duration less than 5 minutes were not discriminatory. In 135 of 137 patients (98.5%) the acute infarct was rated as definitely or possibly associated with symptoms.
Table 2. Univariable Analysis of DWI-Positive Imaging.
| DWI Positive (Single or Multiple Lesions) | Risk Factor, No./Total No. (%) | RR (95% CI) | |
|---|---|---|---|
| Present | Absent | ||
| Medical history | |||
| Age >60 y | 103/612 (16.8) | 36/416 (8.7) | 1.94 (1.36-2.78) |
| Female sex | 52/522 (10.0) | 87/506 (17.2) | 0.58 (0.42-0.80) |
| Hypertension | 68/480 (14.2) | 71/548 (13.0) | 1.09 (0.80-1.49) |
| Type 1 or 2 diabetes | 25/140 (17.9) | 114/888 (12.8) | 1.39 (0.94-2.06) |
| Current smoker | 17/105 (16.2) | 122/923 (13.2) | 1.25 (0.77-1.95) |
| Ischemic heart disease | 11/87 (12.6) | 128/941 (13.6) | 0.93 (0.52-1.65) |
| Atrial fibrillation | 6/51 (11.8) | 133/977 (13.6) | 0.86 (0.40-1.86) |
| Hyperlipidemia | 46/342 (13.5) | 93/686 (13.6) | 0.99 (0.71-1.38) |
| Taking aspirin prior to event | 48/347 (13.8) | 91/681 (13.4) | 1.04 (0.75-1.43) |
| Taking statin prior to event | 35/285 (12.3) | 104/743 (14.0) | 0.88 (0.61-1.25) |
| Taking antihypertensive medication prior to event | 59/419 (14.1) | 80/609 (13.1) | 1.07 (0.78-1.47) |
| No known vascular risk factors | 48/352 (13.6) | 91/676 (13.5) | 1.01 (0.73-1.40) |
| Any history of migraine | 25/256 (9.8) | 114/772 (14.8) | 0.66 (0.44-0.995) |
| History of migraine with aura | 10/80 (12.5) | 129/948 (13.6) | 0.92 (0.50-1.68) |
| Any self-reported stress | 14/158 (8.9) | 125/870 (14.4) | 0.62 (0.36-1.04) |
| Any motor or speech symptoms | 39/218 (17.9) | 100/810 (12.3) | 1.45 (1.03-2.03) |
| Isolated sensory symptoms | 61/482 (12.7) | 78/546 (14.3) | 0.89 (0.65-1.21) |
| Symptom duration <5 min | 19/122 (15.6) | 120/906 (13.2) | 1.18 (0.75-1.84) |
| Progression of first to last symptoms >10 min | 21/179 (11.7) | 118/849 (13.9) | 0.84 (0.55-1.30) |
| Ongoing symptoms at time of assessment | 72/370 (19.5) | 67/658 (10.2) | 1.91 (1.41-2.60) |
| Previous identical event | 20/238 (8.4) | 119/790 (15.1) | 0.56 (0.36-0.88) |
| Initial neurologic examination results normal | 79/740 (10.7) | 60/288 (20.8) | 0.51 (0.38-0.70) |
Abbreviations: DWI, diffusion-weighted imaging; RR, relative risk.
Figure. Association Between Neurologist’s Assessment of Results of the Neurologic Examination and Magnetic Resonance Imaging (MRI).
This assessment was completed prior to the MRI scan. The presence of neurologic findings and suspicion of a stroke was associated with having a diffusion-weighted imaging (DWI)–positive lesion detected on an MRI scan. However, a substantial portion of patients with normal examination results or abnormal examination results with another explanation also had a DWI-positive lesion detected on an MRI scan.
In multivariable modeling, age (odds ratio [OR], 1.02; 95% CI, 1.00-1.04), male sex (OR, 2.03; 95% CI, 1.39-2.96), and 4 clinical features (motor or speech symptoms [OR, 2.12; 95% CI, 1.37-3.29], ongoing symptoms at assessment [OR, 1.97; 95% CI, 1.29-3.02], abnormal results of initial neurologic examination [OR, 1.71; 95% CI, 1.11-2.65], and no prior identical symptomatic event [OR, 1.87; 95% CI, 1.12-3.11]) remained important factors associated with acute infarction (Table 3). However, using this model, the discriminative value (C statistic = 0.6999; 95% CI, 0.65-0.74) is inadequate to reliably determine whether or not there will be a DWI-positive lesion detected on an MRI scan in an individual. At the ideal threshold, maximizing sensitivity and specificity, this model results in correct classification (DWI negative vs positive) of individuals 64.1% of the time.
Table 3. Multivariable Analysis of Variables Associated With DWI-Positive Lesion Detected on MRI Scana.
| Variable | OR (95% CI) |
|---|---|
| Age (per year) | 1.02 (1.00-1.04) |
| Male sex | 2.03 (1.39-2.96) |
| Any motor or speech symptoms | 2.12 (1.37-3.29) |
| Ongoing symptoms | 1.97 (1.29-3.02) |
| Abnormal results of initial neurologic examination | 1.71 (1.11-2.65) |
| No prior identical symptomatic event | 1.87 (1.12-3.11) |
Abbreviations: DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; OR, odds ratio.
This model shows the effect size of independently important clinical variables. However, using this model, the discriminative value (C statistic = 0.6999; 95% CI, 0.65-0.74) is inadequate to reliably determine whether there will be a DWI-positive lesion detected on an MRI scan for an individual. At the ideal threshold, maximizing sensitivity and specificity, this model results in correct classification (DWI negative vs positive) of individuals 64.1% of the time.
The initial clinical diagnosis before MRI was ischemia (minor stroke, probable or possible TIA) in 532 of 1028 participants (51.8%) (eFigure 4 in the Supplement). Among those with a provisional diagnosis of ischemia, 20.7% (110 of 532) had DWI-positive lesions. Stroke mimic diagnoses included migrainous phenomena, epilepsy, anxiety, peripheral vestibulopathy, somatoform disorder, bizarre spell, or other (eFigure 4 in the Supplement). Among participants with a provisional diagnosis of stroke mimic, 5.8% (29 of 496) had a DWI-positive lesion. Of those with a DWI-positive lesion, 29 of 139 participants (20.9%) were incorrectly diagnosed prior to the MRI scan as having a stroke mimic or other nonischemic event. The final diagnosis after all investigations (including MRI) were completed was minor stroke or TIA in 382 individuals (37.2%). The final diagnosis (ischemic vs nonischemic cause) was unchanged after all investigations in 720 individuals (70.0%), revised from ischemic to nonischemic in 79 (7.7%), and revised from nonischemic to ischemic in 229 (22.3%). There was no difference in diagnostic revision by sex (P = .63).
One-year follow-up was 96.8% complete (995 of 1028). There were 7 recurrent strokes (0.7%) at 1 year—1 participant who had early symptom progression and 6 recurrent ischemic strokes. Two recurrent ischemic events (1 progression and 1 recurrence) occurred in the first 48 hours from symptom onset. The remainder occurred more than 1 month from symptom onset. The presence of a DWI-positive lesion was associated with an increased risk of recurrent stroke (RR, 6.4; 95% CI, 2.4-16.8). The presence of multiple DWI-positive lesions was associated with an increased risk of recurrent stroke compared with either a single lesion or no lesion (2 of 47 [4.3%] vs 2 of 92 [2.2%] vs 2 of 889 [0.2%]; P = .002). Other secondary outcomes are shown in Table 4. Nine participants (0.9%) died during the 1-year follow-up period, of whom 3 participants (0.3%) died in the first 90 days. The causes of death were intracerebral hemorrhage (1), glioblastoma multiforme (1), congestive heart failure (1), prostate cancer (1), multiple myeloma (1), multiple trauma (1), respiratory failure (1), and unknown (2).
Table 4. Brain MRI and 1-Year Clinical Outcome Rates.
| Outcome | Patients, No. (%) (N = 1028) |
|---|---|
| Primary outcome | |
| Stroke on MRI results (DWI positive) | 139 (13.5) |
| Secondary outcomes | |
| Recurrent ischemic stroke | 7 (0.7) |
| Death | 9 (0.9) |
| Myocardial infarction | 4 (0.4) |
| Transient ischemic attack | 9 (0.9) |
| Composite of ischemic stroke, MI, or death | 20 (1.9) |
Abbreviations: DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Discussion
The rate of radiologically confirmed acute stroke using early MRI was 13.5% among participants with suspected low-risk TIA or minor stroke, defined as focal, mild, nonmotor, nonspeech symptoms or very short duration motor or speech symptoms. The implication of this finding is that if the diagnosis of TIA or minor stroke is considered, even with low-risk features, early MRI must be completed within 1 week to make a definitive diagnosis of ischemic stroke. A simple short-sequence brain MRI (eg, axial DWI, fluid-attenuated inversion recovery, or gradient recalled echo) is typically adequate.
A total of 30.0% of patients in this population had a change in their final clinical diagnosis, partially based on findings from urgent MRI. Clinical and demographic factors including age, sex, and symptom types and duration were associated with the presence of brain ischemia (DWI-positive lesions). The risk of recurrent stroke was low and only 2 participants worsened in the first 48 hours. More important, the risk of recurrence can be stratified by MRI findings. Multiple DWI-positive lesions were associated with greater recurrence compared with solitary lesions, which in turn were associated with a higher risk of recurrence than no DWI-positive lesions, confirming results from Amarenco et al.7 The corollary is that normal MRI scans are reassuring in this population and have a high negative predictive value (99.8%) for future stroke.
A key finding is that a detailed history and neurologic examination were only partially helpful and did not obviate the need for a brain MRI. In adjusted analysis, only age, sex, lateralizing focal motor or focal speech symptoms, the presence of ongoing symptoms at the time of clinical evaluation, abnormal results of neurologic examination, and the absence of a prior identical event were associated with the primary outcome. However, neither persisting symptoms nor abnormal results of a neurologic examination were sufficiently discriminatory to identify a stroke; many participants with these clinical features still had stroke mimic diagnoses. Although we observed a greater prevalence of migraine than is seen in the general population,21 this finding is expected because of the population studied and was not relevant in adjusted analysis. More importantly, classical clinical aphorisms thought to suggest a benign diagnosis, including a slow progression of symptoms (defined here as an evolution from first to last body part involved lasting more than 10 minutes), a history of “stress” or very short symptom duration (<5 minutes) were not substantiated. Cortical spreading depression, which commonly results in a progression of symptoms, may be triggered by ischemia,22 and should therefore not necessarily be used as a clue to a nonischemic diagnosis. Clinical features associated with acute infarction were not adequately discriminatory to remove the need for MRI in most patients but will remain helpful in situations where MRI is not performed or not available within the first 8 days. Patients in whom a stroke is identified will then require further investigation including vascular imaging for carotid artery stenosis and assessment for a cardiac source of embolus.
We confirm that clinically defined low-risk TIA or minor stroke presentations are associated with a very low risk of recurrent stroke in the first year. The caveat is that patients with confirmed ischemia in our study were all treated with preventive measures according to current guidelines. This low risk of recurrence is dependent both on a nonischemic diagnosis and the use of preventive therapies in those with true ischemia. The failure to make a correct diagnosis of stroke results in a missed opportunity for stroke prevention and the incorrect diagnosis of stroke results in use of therapy that has the capacity to harm. Long-term antiplatelet therapy with aspirin in the healthy elderly is harmful.23 Clinical judgment will continue to be required to decide which patients with DWI-negative MRI findings require preventive treatment.
Limitations
Our study has several limitations. The results apply to a study population in which participants were all referred to and evaluated by a stroke neurologist with an initial suspicion of brain ischemia as a potential diagnosis and all underwent an MRI within 8 days of symptom onset. Our results do not necessarily apply to patients assessed differently or at later time points. The median time to MRI was 4 days in our study, which is longer than assessment times in similar studies of patients with high-risk TIA or mild stroke.7 Most recurrent ischemic events in patients with high-risk TIA or mild stroke occur within the first 24 to 72 hours after symptom onset. It is plausible that a proportion of eligible participants were excluded owing to development of early recurrent infarcts by day 4, creating bias by selection. We did not include vascular imaging, which, when performed acutely, might identify more patients at risk for recurrent events.17 The low risk of long-term recurrent stroke may be an underestimate owing to the potential for telephone follow-up to miss outcome events. However, the risk of missing outcome events is low, as all patients with stroke events were seen prospectively by the neurology team and all admissions to the hospital were reviewed. Further aggressive secondary preventive measures in the population with positive MRI findings may underestimate recurrent stroke rates as in routine clinical practice, when patients receive a misdiagnosis of nonvascular conditions, secondary stroke prevention treatments are likely not implemented. However, the details of secondary stroke prevention treatments after diagnosis were not collected. Transient ischemic attack and myocardial infarction outcomes were also self-reported and not confirmed by medical record review.
Conclusions
We found that 13.5% of participants aged 40 years or older with transient or minor persistent nonmotor or speech neurologic symptoms or 5 minutes or less of motor or speech symptoms, who were referred to stroke neurologists with a possible diagnosis of TIA or minor stroke, had evidence of an acute stroke on neuroimaging. The final diagnosis was revised after brain MRI for 30.0% of patients. Because clinical features are not adequately discriminatory to obviate the need for MRI, a fast-head protocol MRI should be completed in similar patients within the first week after onset of symptoms.
eFigure 1. Onset to MR Time Distribution
eFigure 2. Association Between Evidence of Ischemia on MR Imaging and Persisting or Completely Resolved Symptoms
eFigure 3. Association Between Symptom Resolution Among Participants With a Normal Neurologic Examination
eFigure 4. Pre-MRI Clinical Diagnosis Stratified by MRI Result
References
- 1.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901-2906. doi: 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 2.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167(22):2417-2422. doi: 10.1001/archinte.167.22.2417 [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366(9479):29-36. doi: 10.1016/S0140-6736(05)66702-5 [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26(6):630-635. doi: 10.1159/000166839 [DOI] [PubMed] [Google Scholar]
- 5.Bravata DM, Myers LJ, Arling G, et al. Quality of care for veterans with transient ischemic attack and minor stroke. JAMA Neurol. 2018;75(4):419-427. doi: 10.1001/jamaneurol.2017.4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle J, Mlynash M, Lee K, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41(7):1367-1370. doi: 10.1161/STROKEAHA.109.577650 [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Lavallée PC, Labreuche J, et al. ; TIAregistry.org Investigators . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542. doi: 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Giles MF, Chandratheva A, et al. ; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study . Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison [published correction appears in Lancet. 2008;371(9610):386]. Lancet. 2007;370(9596):1432-1442. doi: 10.1016/S0140-6736(07)61448-2 [DOI] [PubMed] [Google Scholar]
- 9.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960. doi: 10.1016/S1474-4422(07)70248-X [DOI] [PubMed] [Google Scholar]
- 10.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292. doi: 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 11.Tarnutzer AA, Lee SH, Robinson KA, Wang Z, Edlow JA, Newman-Toker DE. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: a meta-analysis. Neurology. 2017;88(15):1468-1477. doi: 10.1212/WNL.0000000000003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston SC, Easton JD, Farrant M, et al. ; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators . Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AYX, Coutts SB. Role of brain and vessel imaging for the evaluation of transient ischemic attack and minor stroke. Stroke. 2018;49(7):1791-1795. doi: 10.1161/STROKEAHA.118.016618 [DOI] [PubMed] [Google Scholar]
- 14.Ay H, Arsava EM, Johnston SC, et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. Stroke. 2009;40(1):181-186. doi: 10.1161/STROKEAHA.108.521476 [DOI] [PubMed] [Google Scholar]
- 15.Coutts SB, Simon JE, Eliasziw M, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. 2005;57(6):848-854. doi: 10.1002/ana.20497 [DOI] [PubMed] [Google Scholar]
- 16.Brazzelli M, Shuler K, Quayyum Z, et al. Clinical and imaging services for TIA and minor stroke: results of two surveys of practice across the UK. BMJ Open. 2013;3(8):e003359. doi: 10.1136/bmjopen-2013-003359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutts SB, Modi J, Patel SK, Demchuk AM, Goyal M, Hill MD; Calgary Stroke Program . CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke. 2012;43(4):1013-1017. doi: 10.1161/STROKEAHA.111.637421 [DOI] [PubMed] [Google Scholar]
- 18.Jeerakathil T, Shuaib A, Majumdar SR, et al. ; ASPIRE Investigators . The Alberta Stroke Prevention in TIAs and mild strokes (ASPIRE) intervention: rationale and design for evaluating the implementation of a province-wide TIA triaging system. Int J Stroke. 2014;9(SA100)(Suppl A100):135-143. doi: 10.1111/j.1747-4949.2012.00881.x [DOI] [PubMed] [Google Scholar]
- 19.WHO MONICA Project Principal Investigators The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41(2):105-114. doi: 10.1016/0895-4356(88)90084-4 [DOI] [PubMed] [Google Scholar]
- 20.Calvet D, Touzé E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke. 2009;40(1):187-192. doi: 10.1161/STROKEAHA.108.515817 [DOI] [PubMed] [Google Scholar]
- 21.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21-34. doi: 10.1111/head.12482 [DOI] [PubMed] [Google Scholar]
- 22.Waters MJ, Cheong E, Jannes J, Kleinig T. Ischaemic stroke may symptomatically manifest as migraine aura. J Clin Neurosci. 2018;55:62-64. doi: 10.1016/j.jocn.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 23.McNeil JJ, Wolfe R, Woods RL, et al. ; ASPREE Investigator Group . Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Onset to MR Time Distribution
eFigure 2. Association Between Evidence of Ischemia on MR Imaging and Persisting or Completely Resolved Symptoms
eFigure 3. Association Between Symptom Resolution Among Participants With a Normal Neurologic Examination
eFigure 4. Pre-MRI Clinical Diagnosis Stratified by MRI Result