Key Points
Question
What are the benefits and risks of continuing aspirin in addition to P2Y12 receptor inhibition with ticagrelor among patients with acute coronary syndrome between 1 month and 12 months after percutaneous coronary intervention?
Findings
In this nonprespecified, post hoc analysis of the GLOBAL LEADERS randomized clinical trial, beyond 1 month after percutaneous coronary intervention in acute coronary syndrome, aspirin was associated with increased bleeding risk and appeared not to add to the benefit of ticagrelor on ischemic events.
Meaning
The findings of this hypothesis-generating analysis pave the way for further trials evaluating aspirin-free antiplatelet strategies after percutaneous coronary intervention.
Abstract
Importance
The role of aspirin as part of antiplatelet regimens in acute coronary syndromes (ACS) needs to be clarified in the context of newer potent P2Y12 antagonists.
Objective
To evaluate the benefit and risks of aspirin in addition to ticagrelor among patients with ACS beyond 1 month after percutaneous coronary intervention (PCI).
Design, Setting, and Participants
This is a nonprespecified, post hoc analysis of GLOBAL LEADERS, a randomized, open-label superiority trial comparing 2 antiplatelet treatment strategies after PCI. The trial included 130 secondary/tertiary care hospitals in different countries, with 15 991 unselected patients with stable coronary artery disease or ACS undergoing PCI. Patients had outpatient visits at 1, 3, 6, 12, 18, and 24 months after index procedure.
Interventions
The experimental group received aspirin plus ticagrelor for 1 month followed by 23-month ticagrelor monotherapy; the reference group received aspirin plus either clopidogrel (stable coronary artery disease) or ticagrelor (ACS) for 12 months, followed by 12-month aspirin monotherapy. In this analysis, we examined the clinical outcomes occurring between 31 days and 365 days after randomization, specifically in patients with ACS who, within this time frame, were assigned to receive either ticagrelor alone or ticagrelor and aspirin.
Main Outcomes and Measures
The primary outcome was the composite of all-cause death or new Q-wave myocardial infarction.
Results
Of 15 968 participants, there were 7487 patients with ACS enrolled; 3750 patients were assigned to the experimental group and 3737 patients to the reference group. Between 31 and 365 days after randomization, the primary outcome occurred in 55 patients (1.5%) in the experimental group and in 75 patients (2.0%) in the reference group (hazard ratio [HR], 0.73; 95% CI, 0.51-1.03; P = .07); investigator-reported Bleeding Academic Research Consortium–defined bleeding type 3 or 5 occurred in 28 patients (0.8%) in the experimental group and in 54 patients (1.5%) in the reference arm (HR, 0.52; 95% CI, 0.33-0.81; P = .004).
Conclusions and Relevance
Between 1 month and 12 months after PCI in ACS, aspirin was associated with increased bleeding risk and appeared not to add to the benefit of ticagrelor on ischemic events. These findings should be interpreted as exploratory and hypothesis generating; however, they pave the way for further trials evaluating aspirin-free antiplatelet strategies after PCI.
Trial Registration
ClinicalTrials.gov identifier: NCT01813435.
This secondary analysis of the GLOBAL LEADERS randomized clinical trial evaluates the benefit and risks of aspirin in addition to ticagrelor among patients with acute coronary syndrome beyond 1 month after percutaneous coronary intervention.
Introduction
Ticagrelor in combination with aspirin more effectively reduced the rates of the composite end point of cardiovascular death, myocardial infarction (MI), and stroke compared with clopidogrel with aspirin among patients presenting with acute coronary syndromes (ACS) in the Study of Platelet Inhibition and Patient Outcomes (PLATO) trial.1,2 Subgroup analyses revealed an interaction between treatment benefit and geographic region, with a suggestion of lower ticagrelor benefit in North American patients.3 Although this interaction may have been a chance finding, it was potentially attributable to an interaction between ticagrelor and higher maintenance doses of aspirin (≥300 mg).3 The latter were routinely used in North America, and the lower benefit of ticagrelor appeared to mirror the use of higher doses of aspirin in other geographical regions.3
Several experimental and small-scale clinical studies tried to address this hypothesis further.3,4,5,6,7 Nevertheless, the effect of aspirin and ticagrelor coadministration compared with ticagrelor monotherapy has, to our knowledge, not yet been explored in a large-scale patient cohort; the role of aspirin as part of antiplatelet regimens, including more potent P2Y12 antagonists, needs to be further clarified.4
In the randomized GLOBAL LEADERS trial,5 ticagrelor with aspirin for 1 month followed by ticagrelor alone for 23 months was not significantly superior to 12 months of standard dual antiplatelet therapy followed by 12 months of aspirin alone in the prevention of the primary end point of all-cause death or new Q-wave MI 2 years after percutaneous coronary intervention (PCI) in an all-comer (stable coronary artery disease or ACS) population.
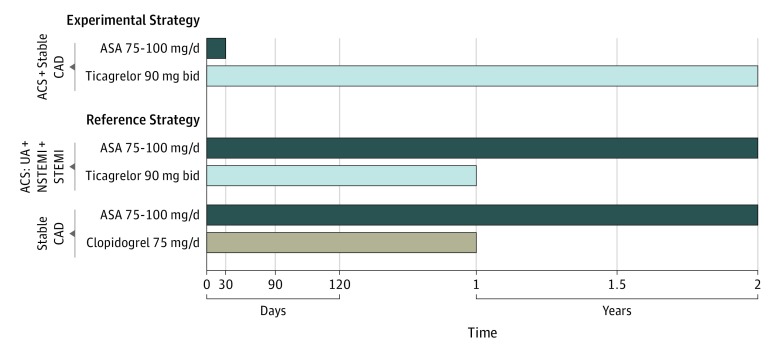
Importantly, the study design and randomization scheme provided the unique opportunity to explore the benefit of continuing aspirin in addition to ticagrelor in patients with ACS beyond 1 month following PCI in a large-scale randomized population.4,6 In this analysis, we examined the clinical outcomes specifically in patients with ACS who, according to the study protocol, between 31 and 365 days after randomization received either ticagrelor alone or ticagrelor and aspirin at a dose of 75 mg to 100 mg daily (Figure 1).
Figure 1. Study Design, Patient Population, and Randomized Treatment in the GLOBAL LEADERS Trial.
In this investigation, we evaluated the clinical outcomes specifically in patients with acute coronary syndrome (ACS) who, according to the study protocol, between 31 and 365 days after randomization were to receive either ticagrelor alone or ticagrelor and aspirin (ASA) at a dose of 75 mg to 100 mg daily. CAD indicates coronary artery disease; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina.
Methods
The GLOBAL LEADERS trial was a randomized, open-label superiority trial conducted at 130 sites in 18 countries.5 The formal trial protocols are in Supplement 1. The study design and protocol have been previously described in detail6 and are presented in the eAppendix in Supplement 2. Patients undergoing PCI with a biolimus A9-eluting stent for stable coronary artery disease or ACS were randomized to the experimental treatment group receiving 75 mg to 100 mg of aspirin daily plus 90 mg of ticagrelor twice daily for 1 month, followed by 23 months of ticagrelor monotherapy or standard DAPT with 75 mg to 100 mg of aspirin daily plus either 75 mg of clopidogrel daily (for patients with stable coronary artery disease) or 90 mg of ticagrelor twice daily (for patients with ACS) for 12 months, followed by 12-month aspirin monotherapy. Randomization was concealed, stratified by center and clinical presentation (stable coronary artery disease vs ACS), and blocked, with randomly varied block sizes of 2 and 4.6 The GLOBAL LEADERS study was approved by the institutional review board at each participating center. All patients provided written informed consent. The study complied with the Declaration of Helsinki and Good Clinical Practices.
The primary outcome was a composite of all-cause mortality or new nonfatal, centrally adjudicated Q-wave MI at 2 years (eAppendix in Supplement 2). The key secondary safety outcome was site-reported bleeding assessed according to the Bleeding Academic Research Consortium (BARC) criteria (type 3 or 5). Secondary site–reported clinical outcomes included all-cause death, cardiovascular death, stroke, site-reported MI (according to the Third Universal Definition), revascularization including target vessel revascularization (TVR), definite stent thrombosis, and the composite end point of death, MI, stroke, or BARC type 3 or 5 bleeding at 2 years. In addition, we evaluated the rates of the Academic Research Consortium 2–defined patient-oriented composite end points (POCE: all-cause mortality, any stroke, MI, or revascularization) and net-adverse clinical events (NACE: POCE or BARC type 3 or 5 bleeding).7,8
Sample size considerations, the primary end point analyses, and the results of formal interaction testing on treatment-by-clinical presentation interaction (ACS vs stable CAD subgroups) with regard to the primary end point and BARC 3 or 5 type bleeding events up to 2 years have been described previously.5,6 In this investigation, analyses of events occurring between the 2 prespecified landmark points in the GLOBAL LEADERS design, 31 days and 365 days of follow-up, were performed in patients with ACS who were alive at 31 days and did not encounter events of the specific type nor were censored prior to this landmark. Analyses were conducted following the intention-to-treat definition using the Mantel-Cox log-rank method up to the point when the first event occurred. Hazard ratios (HR) with 95% confidence intervals were reported. In addition, we performed sensitivity analyses among (1) patients with ACS who remained adherent to the randomized treatment, as assessed at each of the prespecified follow-up visits (at 1 month, 30 days, 90 days, 180 days, and 365 days), and (2) among patients with ACS who underwent complex PCI, defined according to the previously described definitions.9,10
Analyses were performed with SPSS, version 25 (IBM Corp). A 2-sided P value of less than .05 was considered statistically significant. No adjustments were made for multiple comparisons, and therefore all presented results should be viewed as only hypothesis generating.
Results
Of 15 968 participants randomized between July 1, 2013, and November 9, 2015, there were 7487 patients with ACS; 3750 patients had been assigned to the experimental group and 3737 patients to the reference group. Baseline characteristics were well balanced between groups (Table 1; eTable 1 in Supplement 2).
Table 1. Baseline Clinical Characteristics.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Reference (n = 3737) | Experimental (n = 3750) | ||
| Age >75 y | 548 (14.7) | 558 (14.9) | .79 |
| Female | 854 (22.9) | 870 (23.2) | .72 |
| Acute coronary syndrome type | |||
| Unstable angina | 1018 (27.2) | 1004 (26.8) | .75 |
| NSTEMI | 1689 (45.2) | 1684 (44.9) | |
| STEMI | 1030 (27.6) | 1062 (28.3) | |
| Geographic area | |||
| West Europe | 2825 (75.6) | 2879 (76.8) | .48 |
| East Europe | 793 (21.2) | 755 (20.1) | |
| Rest of the world | 119 (3.2) | 116 (3.1) | |
| Diabetes | 795 (21.3) | 809 (21.6) | .74 |
| Insulin-dependent diabetes | 243 (6.5) | 208 (5.6) | .09 |
| Hypertension | 2523 (67.9) | 2560 (68.6) | .48 |
| Hypercholesterolemia | 2211 (62.0) | 2178 (60.8) | .33 |
| Previous stroke more than 30 d ago | 94 (2.5) | 81 (2.2) | .31 |
| Previous myocardial infarction | 695 (18.6) | 685 (18.3) | .72 |
| Previous percutaneous coronary intervention | 872 (23.4) | 854 (22.8) | .55 |
| Previous coronary artery bypass grafting | 145 (3.9) | 130 (3.5) | .34 |
| Peripheral vascular disease | 196 (5.3) | 191 (5.1) | .77 |
| Chronic obstructive pulmonary disease | 177 (4.8) | 174 (4.7) | .85 |
| Previous major bleeding | 24 (0.6) | 24 (0.6) | .99 |
| Current smoking | 1255 (33.6) | 1288 (34.3) | .49 |
| Impaired renal functiona | 467 (12.5) | 500 (13.4) | .27 |
Abbreviations: NSTEMI, non–ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Estimated glomerular filtration rate of creatinine clearance of less than 60 mL/min per 1.73 m2 based on the Modification of Diet in Renal Disease Formula.
Thirty days after PCI, the rates of the primary outcome (22 patients [0.6%] vs 28 patients [0.7%]; HR, 0.78; 95% CI, 0.45-1.37; P = .39) and key safety outcome of BARC type 3 or 5 bleeding (29 patients [0.8%] vs 34 patients [0.9%]; HR, 0.85; 95% CI, 0.52-1.40; P = .52) did not differ significantly between the experimental and the reference arm (eTable 2 in Supplement).
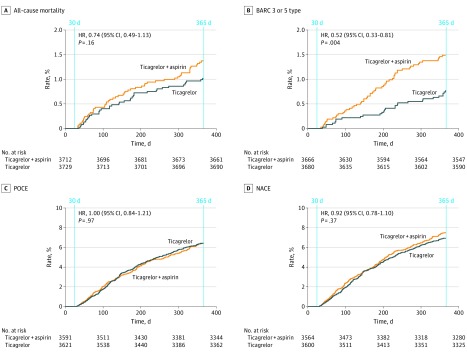
Between 31 days and 365 days after randomization, the primary outcome occurred in 55 patients (1.5%) and 75 patients (2.0%) in the experimental and reference groups, respectively (HR, 0.73; 95% CI, 0.51-1.03; P = .07); BARC type 3 or 5 bleeding occurred in 28 patients (0.8%) in the experimental arm and in 54 patients (1.5%) in the reference arm (HR, 0.52; 95% CI, 0.33-0.81; P = .004) (Table 2, Figure 2; eFigures 1 and 2 in Supplement 2). No differences in the rates of all-cause death, any stroke (hemorrhagic or ischemic), any MI, revascularizations, including TVR, definite stent thrombosis, POCE, or NACE, were observed between 31 days and 365 days in both treatment groups.
Table 2. Clinical Events In Patients With Acute Coronary Syndrome Between 31 Days and 365 Daysa,b.
| Event | No. of Events/No. of Patients at Risk (%) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Reference | Experimental | |||
| ASA Plus Ticagrelor | Ticagrelor Alone | |||
| Primary outcome: all-cause death or new Q-wave MI | 75/3708 (2.0) | 55/3728 (1.5) | 0.73 (0.51-1.03) | .07 |
| Key safety outcome: BARC 3 or 5 bleeding | 54/3666 (1.5) | 28/3680 (0.8) | 0.52 (0.33-0.81) | .004 |
| BARC 3 bleeding | 53/3666 (1.5) | 27/3680 (0.7) | 0.51 (0.32-0.80) | .003 |
| BARC 3a bleeding | 25/3683 (0.7) | 13/3690 (0.4) | 0.52 (0.27-1.01) | .06 |
| BARC 3b bleeding | 24/3681 (0.7) | 9/3693 (0.2) | 0.37 (0.17-0.81) | .01 |
| BARC 3c bleeding | 6/3690 (0.27) | 7/3702 (0.19) | 1.16 (0.39-3.46) | .79 |
| BARC 5 bleeding | 4/3695 (0.11) | 2/3703 (0.05) | 0.50 (0.09-2.73) | .41 |
| BARC 5a bleeding | 2/3695 (0.05) | 1/3703 (0.03) | 0.50 (0.05-5.51) | .57 |
| BARC 5b bleeding | 2/3695 (0.05) | 1/3703 (0.03) | 0.50 (0.05-5.51) | .57 |
| All-cause death | 51/3712 (1.4) | 38/3729 (1.0) | 0.74 (0.49-1.13) | .16 |
| New Q-wave MI | 25/3708 (0.7) | 17/3728 (0.5) | 0.68 (0.37-1.25) | .21 |
| Strokec | 14/3683 (0.4) | 17/3697 (0.5) | 1.21 (0.60-2.46) | .59 |
| Ischemic | 12/3685 (0.3) | 13/3697 (0.4) | 1.08 (0.49-2.37) | .84 |
| Hemorrhagic | 2/3694 (0.1) | 3/3703 (0.1) | 1.50 (0.25-8.96) | .66 |
| Undetermined | 0/3694 | 1/3703 (0.03) | NA | NA |
| MI (site-reported) | 52/3660 (1.4) | 50/3660 (1.4) | 0.96 (0.65-1.42) | .85 |
| Revascularization | 172/3615 (4.8) | 181/3642 (5.0) | 1.05 (0.85-1.29) | .66 |
| TVR | 96/3644 (2.6) | 84/3664 (2.3) | 0.87 (0.65-1.17) | .36 |
| Definite ST | 6/3679 (0.2) | 7/3679 (0.2) | 1.17 (0.39-3.47) | .78 |
| Composite end points | ||||
| Death, new Q-wave MI, stroke | 85/3680 (2.3) | 67/3697 (1.8) | 0.79 (0.57-1.08) | .14 |
| Cardiac death, MI, stroke | 81/3648 (2.2) | 80/3654 (2.2) | 0.99 (0.73-1.35) | .94 |
| POCE | 229/3591 (6.4) | 231/3621 (6.4) | 1.00 (0.84-1.21) | .97 |
| Death, MI, stroke, or BARC 3/5 bleeding | 148/3622 (4.1) | 117/3633 (3.2) | 0.79 (0.62-1.00) | .05 |
| NACE | 266/3564 (7.5) | 248/3600 (6.9) | 0.92 (0.78-1.10) | .36 |
Abbreviations: BARC, Bleeding Academic Research Consortium; HR, hazard ratio; NACE, net adverse clinical events; MI, myocardial infarction; NA, not applicable; POCE, patient-oriented composite end points; ST, stent thrombosis; TVR, target vessel revascularization.
The primary outcome was a composite of all-cause mortality or nonfatal, centrally adjudicated, new Q-wave MI. Patient-oriented composite end point included all-cause mortality, any stroke, MI, or revascularization, whereas NACE comprised POCE or BARC 3 or 5 type bleeding. P values were for the log-rank test.
In these time frames, experimental regimen is ticagrelor monotherapy and reference regimen is ticagrelor plus aspirin.
Not including transient ischemic attack.
Figure 2. Clinical Events in Acute Coronary Syndrome Patients Between 31 Days and 365 Daysa.
All-cause mortality (A), Bleeding Academic Research Consortium (BARC)–defined bleeding type 3 or 5 (B), patient-oriented composite end points (POCE) (C), and net-adverse clinical events (NACE) (D). Patient-oriented composite endpoint (POCE) included all-cause mortality or any stroke, myocardial infarction, or revascularization, whereas net-adverse clinical events (NACE) comprised POCE and BARC type 3 or 5 bleeding. Patients who were alive at 31 days of follow-up and did not encounter event of the specific type nor were censored prior to the landmark of 30 days have been included in this analysis. HR indicates hazard ratio.
aIn these time frames, according to the study protocol, the experimental regimen is ticagrelor monotherapy and the reference regimen is ticagrelor plus aspirin.
The composite end point of all-cause death, stroke, site-reported MI, and BARC type 3 or 5 bleeding occurred in 117 patients (3.2%) in the experimental arm and in 148 patients (4.1%) in the reference arm (HR, 0.79; 95% CI, 0.62-1.00; P = .05). The additional bleeding outcomes occurring between 31 days and 365 days have been presented in eTable 3 in Supplement 2 and Figure 3. The cumulative 1-year event rates have been presented in eTable 4 in Supplement 2. At 1 year after PCI, the rates of the primary outcome (77 patients [2.1%] vs 103 patients [2.8%]; HR, 0.74; 95% CI, 0.55-1.00; P = .05) did not differ significantly between the 2 groups; the rates of key safety outcome of BARC type 3 or 5 bleeding (57 patients [1.5%] vs 88 patients [2.4%]; HR, 0.64; 95% CI, 0.46-0.90; P = .009) were lower in the experimental group compared with the reference group.
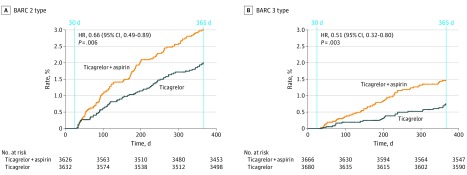
Figure 3. Bleeding Academic Research Consortium (BARC)–Defined Bleeding Events Type 2 (A) and Type 3 (B) Between 31 and 365 Days in Patients With Acute Coronary Syndromea.
aIn these time frames, according to the study protocol, the experimental regimen is ticagrelor monotherapy and the reference regimen is ticagrelor plus aspirin.
HR indicates hazard ratio.
The adherence to the randomized treatment in the experimental vs the reference arm was 97.0% vs 95.1%, 86.9% vs 87.7%, and 84.1% vs 85.2% at 1, 6, and 12 months, respectively. A sensitivity analysis in patients who adhered to the randomized treatment, as assessed at each follow-up visit between discharge and 365 days, indicated lower rates of site-reported MI, any revascularizations, TVR, POCE, NACE, and the composite end point of cardiac death, site-reported MI, and stroke in the experimental arm compared with the reference arm, which was associated with consistently lower, although not statistically different, rates of BARC 3 type bleeding (eTable 5 in Supplement 2). A sensitivity analysis in patients with ACS who underwent complex PCI (n = 2221) showed lower rates of the primary outcome, all-cause death, BARC 3 and BARC 3 or 5 type bleeding, and the composite end point of cardiac death, site-reported MI, and stroke between 31 days and 365 days in the experimental vs the reference group (eTables 6-8 and eFigure 3 in Supplement 2).
Discussion
For the first time to our knowledge, the role of aspirin combined with the potent P2Y12 inhibitor ticagrelor has been specifically evaluated in a large ACS trial population undergoing PCI. The salient findings from this post hoc landmark analysis can be summarized as follows:
There was no evidence of a benefit to continue aspirin in addition to ticagrelor between 1 month and 1 year after PCI in patients with ACS with respect to the rates of all-cause death or new Q-wave MI.
Continuation of aspirin, in addition to ticagrelor, was associated with a significant increase in the key safety end point of BARC type 3 or 5 bleeding.
These findings need to be interpreted cautiously because the presented analyses were not prespecified and the trial did not meet its primary end point.5,6 The observed event rates in GLOBAL LEADERS were lower than anticipated; all presently reported relative risk differences need also to be viewed in the light of small absolute differences in event rates.5,6 Notwithstanding this limitation, the observation of the absence of significant difference in the rates of the primary end point of all-cause death or new Q-wave MI beyond 1 month after PCI in patients with ACS receiving solely ticagrelor is noteworthy because this challenges the additive effect of combining aspirin with ticagrelor for secondary prevention of ischemic events after the initial 30 days following PCI. Putatively, based on numerically lower rates of the primary end point that in our study consisted of 2 ischemic components, one may suspect that the adverse interaction between ticagrelor and aspirin, previously suggested for higher doses of aspirin (> 150 mg), may be also present when aspirin is administered at lower doses (75 mg-100 mg).3,11
Aspirin is widely used in secondary prevention of atherosclerotic disease; however, there is some geographical variation in the dose used, varying from 50 mg to more than 300 mg.3 Importantly, the number of prospective studies addressing the association of aspirin dose with clinical outcome in patients with ACS is still limited.11 The considered magnitude of aspirin benefit for patients with ACS and its very low cost has resulted in all other antiplatelet agents being tested on top of aspirin, with the assumption of additive effects of both medications. Nevertheless, in light of the central role of the P2Y12 signaling pathway on platelet activation and amplification processes, potent P2Y12 blockade can also lead to downregulation of other markers of platelet reactivity, including arachidonic acid-induced and collagen-induced aggregation.4,12,13
Biologically, it has been hypothesized that in the presence of potent P2Y12 inhibitors, aspirin adds little additional inhibition of platelet aggregation mediated by thromboxane A2, while potentially having detrimental effects resulting from inhibition of the formation of prostaglandins such as prostanglandin I2 (prostacyclin).14,15 Prostanglandin I2 inhibits platelet aggregation and induces vasodilation; thus, theoretically, more profound suppression of PGI2 formation as a function of dose-dependent inhibition of cyclooxygenase-2 by high doses of aspirin could attenuate the antithrombotic effect of aspirin and potentially predispose to thrombosis. Such a phenomenon has been previously demonstrated for selective cyclooxygenase-2 inhibitors and other nonsteroidal anti-inflammatory drugs.14,15 Yet aspirin still yields additive inhibition of arachidonic acid-induced and collagen-induced platelet aggregation, even in the presence of potent P2Y12 inhibition.11,16 Moreover, investigations have shown that high-dose aspirin does not interfere with the pharmacokinetic or pharmacodynamic effects of ticagrelor, and aspirin dosing does not modulate pharmacodynamic markers of P2Y12 signaling irrespective of the degree of P2Y12 receptor blockade.12,13 Therefore, the question remains as to whether the additive value of aspirin mediated through inhibition of TXA2-mediated platelet aggregation is offset by the inhibition of prostacyclin synthesis in vivo, which is generally not accurately assessed by laboratory studies.11,12,13 Consequently, the clinical data from prospective studies of aspirin in combination therapy have been eagerly awaited,11 underscoring the incremental value of this report from a large randomized study to the understanding of ticagrelor and aspirin interaction among patients with ACS.
Previously, the CURRENT OASIS 7 trial17 compared different dose ranges of aspirin on clinical outcomes, but this was always in a combination with a P2Y12 antagonist, clopidogrel. Of note, neither the primary composite end point of cardiovascular mortality, MI, or stroke (HR, 0.97; 95% CI, 0.86-1.09; P = .61) nor major bleeding events (HR, 0.99; 95% CI, 0.84-1.17; P = .90) were identified to be different in patients treated with higher or lower doses of aspirin. However, patients receiving higher doses of aspirin had a higher rate of gastrointestinal bleeding (0.4% vs 0.2%; P = .04).17
In the CAPRIE study,18 there were fewer ischemic events observed in patients treated with a less potent P2Y12 receptor antagonist, clopidogrel, than in patients receiving aspirin, and a lower rate of gastrointestinal bleeding was found in clopidogrel group, likely owing to aspirin’s unfavorable local gastrointestinal bleeding effect. In that context, one may suspect even better efficacy and safety of ticagrelor monotherapy given its superior potency and specificity in P2Y12 receptor inhibition.
Between 31 days and 365 days after randomization, similar rates (2.2% vs 2.2%) of the composite end points of site-reported cardiovascular mortality, MI, or stroke were observed in both treatment groups among patients with ACS from the GLOBAL LEADERS trial (HR, 0.99; 95% CI, 0.73-1.35). However, these rates were lower than the rates of the composite end point of cardiovascular mortality, MI, or stroke occurring between 31 days and 365 days in either ticagrelor or clopidogrel arm of the PLATO study1 (5.3% vs 6.6%, respectively; HR, 0.80; 95% CI, 0.70-0.91; P = .001).1 This may be related to the lower risk of patient clinical profile in the GLOBAL LEADERS study compared with the PLATO study patients.1
Notably, this analysis indicated an excess in bleeding risk associated with aspirin use beyond 1 month and up to 1 year after PCI. Such increase in bleeding risk needs also to be considered with its negative effect on treatment adherence, potentially leading to higher rates of ischemic events and mortality. Nevertheless, this relevant observation needs to be confirmed in dedicated clinical trials before any change to clinical practice can be recommended.19 The ongoing TWILIGHT trial19 is addressing a relatively similar hypothesis to GLOBAL LEADERS, with aspirin withdrawal on a background of ticagrelor at 3 months after PCI in high-risk patients defined according to prespecified clinical and anatomical criteria. In contrast to the GLOBAL LEADERS study, TWILIGHT has selected bleeding BARC 2, 3, or 5 type as the composite primary end point for the superiority analysis and the composite secondary end point of ischemic events (all-cause death, nonfatal MI, or stroke) for the noninferiority analysis.
Whereas sole use of ticagrelor following 1 month after PCI in the ACS setting appears a promising strategy for improving net clinical benefit, it also cannot be excluded that new aspirin formulations reportedly associated with predictable antiplatelet efficacy and improved gastrointestinal safety may result in improved clinical performance compared with traditionally used enteric-coated aspirin formulations.20,21
Limitations
This report has to be viewed in light of the following limitations: it is a post hoc exploratory additional landmark analysis, not prespecified in the GLOBAL LEADERS study protocol. The GLOBAL LEADERS trial was neutral in the primary end point analysis in the overall population, and the presented secondary analysis, as in the parent trial, was not powered to detect between-group differences in clinical outcomes.
Because the goal of this investigation was to explore the synergies between aspirin and ticagrelor (with a minimized confounding effect of other antiplatelet P2Y12 antagonists), we selected only the patients with ACS because the patients presenting with stable CAD largely received clopidogrel as a reference treatment in this study.5 The formal subgroup analysis among patients with ACS vs patients with stable CAD, with interaction tests for the primary end point and BARC 3 or 5 type bleeding, has been described previously.5 While the analysis of the event rates occurring between 31 days and 365 days enables comparisons in 2 groups receiving homogenous antiplatelet regimens (ticagrelor alone or ticagrelor and aspirin), landmark analyses at 30 days affect the balance established by randomization. Nevertheless, no significant differences were found in the baseline characteristics between treatment groups among the selected patients that were included in the landmark analysis.
All the presented findings must be interpreted strictly as hypothesis generating. Event rates in both arms were low compared with the PLATO study and so it is uncertain whether ticagrelor monotherapy will provide sufficient efficacy in patients at high risk of ischemic events. Nevertheless, the exploratory sensitivity analyses performed in patients who adhered to the randomized treatment and in patients who underwent complex PCI procedures indicated consistent results in these subsets. The prespecified subanalysis on treatment-by-clinical-presentation (ACS vs stable CAD) interaction in the overall study cohort, with formal interaction tests for treatment effects on 2-year secondary clinical outcomes, will be presented and discussed in a stand-alone manuscript.
Finally, investigator reporting was used without central adjudication for secondary outcomes.6 Therefore, bias and random event misclassification cannot be excluded. This limitation should be considered in particular when interpreting bleeding event rates. However, the trial was monitored for event definition consistency and event underreporting, with as many as 7 onsite monitoring visits done at individual sites and one-fifth of events verified based on the source documentation. Use of site-reported end points is a valid method in clinical research, especially involving large cohorts and well-defined and restricted categories within a classification (eg, BARC-defined bleeding type 3 to 5 as compared with type 1 and 2) are expected to provide higher concordance among sites and a central clinical event adjudication committee, as well as higher reproducibility.
Conclusions
Between 1 month and 12 months after PCI in ACS, aspirin was associated with increased bleeding risk and appeared not to add to the benefit of ticagrelor in the prevention of ischemic outcomes. These findings should be interpreted as exploratory and hypothesis generating. However, they pave the way for further trials evaluating aspirin-free antiplatelet strategies after PCI.
Trial protocol.
eAppendix.
eTable 1. Baseline Characteristics of Patients Included in the Landmark Analysis at 30 Days (Events Between 31 and 365 Days)a
eTable 2. Clinical Events Between 0 and 30 days in Acute Coronary Syndrome Patientsa
eTable 3. Additional Bleeding Endpoints Between 31 and 365 Daysa in Acute Coronary Syndrome Patients
eTable 4. Cumulative Rate of One-Year Clinical Outcomes in Acute Coronary Syndrome Subgroup
eTable 5. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Adhered to the Randomized Treatmentb (Sensitivity Analysis)
eTable 6. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b (Sensitivity Analysis)
eTable 7. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b and Adhered to the Randomized Treatment at Each Follow-Up Visit up to 365 Daysc (Sensitivity Analysis)
eTable 8. Exploratory Analysis of One-Year Clinical Outcomes in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)a
eFigure 1. Bleeding Academic Research Consortium (BARC)-Defined Bleeding Type ≥ 2 and All-Cause Mortality Between 31 and 365 Daysa in ACS Patients
eFigure 2. Exploratory Analyses of Clinical Outcomes Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b (Sensitivity Analysis)
References:
- 1.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Harrington RA, James S, et al. ; PLATelet inhibition and patient Outcomes Investigators . Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283-293. doi: 10.1016/S0140-6736(09)62191-7 [DOI] [PubMed] [Google Scholar]
- 3.Mahaffey KW, Wojdyla DM, Carroll K, et al. ; PLATO Investigators . Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124(5):544-554. doi: 10.1161/CIRCULATIONAHA.111.047498 [DOI] [PubMed] [Google Scholar]
- 4.Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018;15(8):480-496. doi: 10.1038/s41569-018-0049-1 [DOI] [PubMed] [Google Scholar]
- 5.Vranckx P, Valgimigli M, Jüni P, et al. ; GLOBAL LEADERS Investigators . Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392(10151):940-949. doi: 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 6.Vranckx P, Valgimigli M, Windecker S, et al. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. EuroIntervention. 2016;12(10):1239-1245. doi: 10.4244/EIJY15M11_07 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Garcia HM, McFadden EP, Farb A, et al. ; Academic Research Consortium . Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018;137(24):2635-2650. doi: 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 8.Serruys PW, Tomaniak M, Chichareon P, et al. Patient-oriented composite endpoints and net adverse clinical events with ticagrelor monotherapy following percutaneous coronary intervention: Insights from the randomized GLOBAL LEADERS trial. EuroIntervention. 2019;EIJ-D-19-00202. doi: 10.4244/EIJ-D-19-00202 [DOI] [PubMed] [Google Scholar]
- 9.Giustino G, Chieffo A, Palmerini T, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. 2016;68(17):1851-1864. doi: 10.1016/j.jacc.2016.07.760 [DOI] [PubMed] [Google Scholar]
- 10.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 11.Thomas MR, Storey RF. Impact of aspirin dosing on the effects of P2Y12 inhibition in patients with acute coronary syndromes. J Cardiovasc Transl Res. 2014;7(1):19-28. doi: 10.1007/s12265-013-9524-6 [DOI] [PubMed] [Google Scholar]
- 12.Teng R, Maya J, Butler K. Evaluation of the pharmacokinetics and pharmacodynamics of ticagrelor co-administered with aspirin in healthy volunteers. Platelets. 2013;24(8):615-624. doi: 10.3109/09537104.2012.748185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tello-Montoliu A, Thano E, Rollini F, et al. Impact of aspirin dose on adenosine diphosphate-mediated platelet activities. Results of an in vitro pilot investigation. Thromb Haemost. 2013;110(4):777-784. doi: 10.1160/TH13-05-0400 [DOI] [PubMed] [Google Scholar]
- 14.Warner TD, Armstrong PC, Curzen NP, Mitchell JA. Dual antiplatelet therapy in cardiovascular disease: does aspirin increase clinical risk in the presence of potent P2Y12 receptor antagonists? Heart. 2010;96(21):1693-1694. doi: 10.1136/hrt.2010.205724 [DOI] [PubMed] [Google Scholar]
- 15.van Giezen JJ, Sidaway J, Glaves P, Kirk I, Björkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012;17(2):164-172. doi: 10.1177/1074248411410883 [DOI] [PubMed] [Google Scholar]
- 16.Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110(4):925-934. doi: 10.1046/j.1365-2141.2000.02208.x [DOI] [PubMed] [Google Scholar]
- 17.Mehta SR, Tanguay JF, Eikelboom JW, et al. ; CURRENT-OASIS 7 trial investigators . Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233-1243. doi: 10.1016/S0140-6736(10)61088-4 [DOI] [PubMed] [Google Scholar]
- 18.Committee CS; CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329-1339. doi: 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 19.Baber U, Dangas G, Cohen DJ, et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am Heart J. 2016;182:125-134. doi: 10.1016/j.ahj.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Parker WAE, Orme RC, Hanson J, et al. Very-low-dose twice-daily aspirin maintains platelet inhibition and improves haemostasis during dual-antiplatelet therapy for acute coronary syndrome. Platelets. 2019;30(2):148-157. doi: 10.1080/09537104.2019.1572880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt DL, Grosser T, Dong JF, et al. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2017;69(6):603-612. doi: 10.1016/j.jacc.2016.11.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eAppendix.
eTable 1. Baseline Characteristics of Patients Included in the Landmark Analysis at 30 Days (Events Between 31 and 365 Days)a
eTable 2. Clinical Events Between 0 and 30 days in Acute Coronary Syndrome Patientsa
eTable 3. Additional Bleeding Endpoints Between 31 and 365 Daysa in Acute Coronary Syndrome Patients
eTable 4. Cumulative Rate of One-Year Clinical Outcomes in Acute Coronary Syndrome Subgroup
eTable 5. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Adhered to the Randomized Treatmentb (Sensitivity Analysis)
eTable 6. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b (Sensitivity Analysis)
eTable 7. Exploratory Analysis of Clinical Events Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b and Adhered to the Randomized Treatment at Each Follow-Up Visit up to 365 Daysc (Sensitivity Analysis)
eTable 8. Exploratory Analysis of One-Year Clinical Outcomes in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)a
eFigure 1. Bleeding Academic Research Consortium (BARC)-Defined Bleeding Type ≥ 2 and All-Cause Mortality Between 31 and 365 Daysa in ACS Patients
eFigure 2. Exploratory Analyses of Clinical Outcomes Between 31 and 365 Daysa in Acute Coronary Syndrome Patients Who Underwent Complex Percutaneous Coronary Intervention (PCI)b (Sensitivity Analysis)