Key Points
Question
What individual and institutional factors are associated with radiation dose variation in lung cancer screening computed tomographic (CT) scans?
Findings
Despite guidelines from the American College of Radiology on recommended CT radiation dose levels, this cohort study of 12 529 patients undergoing lung cancer screening CT scans at 72 institutions found wide dose variation across US institutions performing CT scans. Institutions with protocol creation limited to lead radiologists or medical physicists and collaborating internal medical physicists had lower doses, whereas institutions where any radiologists could establish protocols had higher doses.
Meaning
Dose optimization practices for CT radiation need to be tailored to specific practice types and different organizational structures to be more effective.
This study identifies factors associated with radiation dose variation occuring during computed tomography screening scans for lung cancer.
Abstract
Importance
The American College of Radiology (ACR) has recognized the importance of minimizing radiation doses used for lung cancer screening (LCS) computed tomography (CT). However, without standard protocols, doses could still be unnecessarily high, reducing screening margin of benefit.
Objective
To characterize LCS CT radiation doses and identify factors explaining variation.
Design, Setting, and Participants
We prospectively collected LCS examination dose metrics, from 2016 to 2017, at US institutions in the University of California, San Francisco International Dose Registry. Institution-level factors were collected through baseline survey. Mixed-effects linear and logistic regression models were estimated using forward variable selection. Results are presented as percentage excess dose and odds ratios (ORs) with 95% confidence intervals (CIs). The analysis was conducted between 2018 and 2019.
Main Outcomes and Measures
Log-transformed measures of (1) mean volume CT dose index (CTDIvol, mGy), reflecting the average radiation dose per slice; (2) mean effective dose (ED, mSv), reflecting the total dose received and estimated future cancer risk; (3) proportion of CT scans using radiation doses above ACR benchmarks (CTDIvol >3 mGy, ED >1 mSv); and (4) proportion of CT scans using radiation doses above 75th percentile of registry doses (CTDIvol >2.7 mGy, ED >1.4 mSv).
Results
Data were collected for 12 529 patients undergoing LCS CT scans performed at 72 institutions. Overall, 7232 participants (58%) were men, and the median age was 65 years (interquartile range [IQR], 60-70). Of 72 institutions, 15 (21%) had median CTDIvol and 47 (65%) had median ED above ACR guidelines. Institutions allowing any radiologists to establish protocols had 44% higher mean CTDIvol (mean dose difference [MDD], 44%; 95% CI, 19%-69%) and 27% higher mean ED (MDD, 27%; 95% CI, 5%-50%) vs those limiting who established protocols. Institutions allowing any radiologist to establish protocols had higher odds of examinations exceeding ACR CTDIvol guidelines (OR, 12.0; 95% CI, 2.0-71.4), and 75th percentile of registry CTDIvol (OR, 19.0; 95% CI, 1.9-186.7) or ED (OR, 8.5; 95% CI, 1.7-42.9). Having lead radiologists establish protocols resulted in lower odds of doses exceeding ACR ED guidelines (OR, 0.01; 95% CI, 0.001-0.1). Employing external vs internal medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1; 95% CI, 1.8-20.8). Having medical physicists establish protocols was associated with decreased odds of exceeding 75th percentile of registry CTDIvol (OR, 0.09; 95% CI, 0.01-0.59). Institutions reporting protocol updates as needed had 27% higher mean CTDIvol (MDD, 27%; 95% CI, 8%-45%).
Conclusions and Relevance
Facilities varied in LCS CT radiation dose distributions. Institutions limiting protocol creation to lead radiologists and having internal medical physicists had lower doses.
Introduction
Few explicit standards exist for the radiation doses to use for computed tomographic (CT) scans. Several organizations including the American College of Radiology (ACR) promote performing CT scans using the “as low as reasonably achievable” principle for radiation doses. However, the lack of more specific guidelines and established standards for the numerous types of CT examinations results in dose variation within and across institutions.1,2,3,4,5 Institutional decisions, such as those about the use of multiphase scanning, and choices about technical parameters can result in large differences in the radiation doses that patients receive.3,6 Further, little information has been reported on institution-level factors that could influence CT doses.
One protocol receiving increased focus is low-dose CT for lung cancer screening (LCS). Lung cancer screening must balance the potential for earlier cancer detection through screening with concerns about false-positive results, false-negative results, invasive workups, and increased cancer risk from CT radiation exposure.1,7,8,9,10 Lung cancer screening is beneficial when low-dose techniques are used but not when higher CT doses—similar to those used for routine chest CT scans—are used, because radiation from higher doses may cause almost as many cancers as are detected early by screening.11
As part of the Centers for Medicare and Medicaid Services (CMS) requirements for LCS reimbursement, institutions must use low-dose techniques and participate in a dose registry. The ACR recommends that LCS scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 mSv or lower.12,13,14,15,16 Although variation in LCS doses is reported,17,18 the proportion of patients receiving appropriately low-dose examinations is unknown. Further, to our knowledge, no study has identified factors associated with optimum low-dose performance.
This study identified factors associated with CT dose variation among institutions participating in a large CT radiation-dose registry. We assessed how often patients received appropriate low-dose LCS examinations according to ACR guidelines and identified institution-level factors associated with high CT radiation doses. Identifying institution-level factors will help facilities performing CT scans avoid unnecessary variation in LCS CT doses.
Methods
UCSF International CT Dose Registry and Partnership for Dose Trial
In 2015, an international CT radiation dose registry was established at the University of California, San Francisco (UCSF), to collect data on radiation doses for CT examinations of consecutive patients from 151 institutions in the United States and 7 other countries. All institutions participating in the registry use the same radiation dose-monitoring software (Radimetrics, Bayer), completed data use agreements, and received institutional review board approval, enabling data sharing in the registry using HIPAA-compliant tools. Written informed consent was waived by the institutional review board at UCSF because all data used were deidentified. For the Partnership for Dose trial, a National Institutes of Health–funded, pragmatic, randomized comparative effectiveness study on approaches to optimizing radiation doses for routine head, chest, abdomen, and combined chest and abdomen CT scans, we surveyed institutions prior to trial start about characteristics including how they perform and oversee CT. Data from the organizational survey and the dose registry were combined to assess associations between institutional characteristics and radiation dose.
Inclusion/Exclusion
Only US institutions that performed a minimum of 24 LCS scans during the study period (2016-2017) and returned a completed survey were included (n = 72). Thus, institutions outside the United States (n = 15) and institutions that did not perform a minimum of 24 LCS scans (n = 64) were excluded. Although LCS is recommended for patients aged 55 to 80 years with risk factors for cancer (eg, ≥30 pack-year smoking history and current smoking or quitting <15 years ago),19 our analyses included patients aged 40 years or older who were provided a lung cancer screening examination—despite the fact that some individuals might not have been age-eligible for the scan. We could not determine patients’ smoking history, which is an eligibility requirement for insurance coverage of LCS scans. Non-US institutions were excluded because their CT LCS scans are not subject to the ACR LCS guidelines.
Organizational Survey Predictors and Scan Covariates
All institutions eligible for the study completed the organizational survey, which was required to participate in the Partnership for Dose trial. The survey asked about structural and organizational aspects of the institution’s CT imaging workflow that might be associated with radiation dose, including type of facility and role of individuals who establish and modify CT protocols, which are the instructions that technologists use to program CT scanners. Protocols vary by reasons for scans and by institution. The survey also asked (1) the type of institution—academic/teaching hospital, trauma center (level 1, 2, or 3), public hospital, community hospital, private hospital, acute care facility, primary care institution, pediatric hospital, tertiary referral hospital, or outpatient imaging institution [not mutually exclusive]; (2) if a medical physicist was involved in creating protocols and if so, if the physicist was employed by the organization on staff or external (ie, vendor or contractor not directly employed by the facility conducting CT scans); (3) individual(s) who established or altered protocols for the institution (manufacturer, organizational leadership, organization-level medical physicist, site-level medical physicist, radiology site, lead radiologists [the leader among the radiologists within radiology facility], any individual radiologists, head technologist, technologists performing examinations, or other individuals [not mutually exclusive]); (4) frequency at which protocols were updated (as needed, less than yearly, yearly, or more than yearly); and (5) if protocols were locked, meaning unchangeable after being established (yes/no). With the exception of the frequency of protocols updates question, survey questions were formatted so respondents could “select all that apply.” As a result, responses to any of these questions were not mutually exclusive. All 151 institutions in the registry completed the organizational survey.
We also considered the patient-level factors of age, sex, and chest diameter (the proxy for patient size) as potential covariates in the analyses.
Outcomes
We evaluated 2 measures of dose, CTDIvol and ED. Measurement of CTDIvol reflects the average radiation dose output per standardized volume, typically described as dose per slice in mGy. The ED is the total dose output of the scanner (dose per slice × length scanned or total number of slices) weighted by organ sensitivity in anatomic scan region to represent the future risk of cancer from this exposure in mSv. Choices made by technologists performing scans directly influence both CTDIvol and ED. Although LCS should include only single CT scans, multiple CT scans may be performed, as is done for diagnostic CT. Multiple scans would be reflected only in ED, not CTDIvol measures because average dose per slice does not vary by number of scans.
The ACR and CMS guidelines for LCS specify doses of 3.0 mGy or less for CTDIvol and 1 mSv or less for ED for a standard patient defined as 170 cm and 70 kg with a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.3. Because these doses are about 15% to 50% lower than doses used for standard chest CT imaging,20,21 LCS scans are described as low-dose CT. Doses for LCS CT vary by patient size: patients who are larger or smaller than the standard size receive doses slightly above or below guideline thresholds. We accounted for dose variation by size by adjusting doses using average chest diameters measured on CT images. We calculated the 75th percentile of the distribution of radiation doses among the study sample of eligible institutions and defined doses above this percentile as high dose. To account for the nonnormal nature of the CTDIvol and ED measures, these variables were log-transformed for incorporation into linear models.
As a sensitivity analysis to better account for size-specific CTDIvol estimates, we analyzed an additional radiation dose metric, size-specific dose estimate (SSDE), which is defined as the patient dose estimate taking into consideration corrections based on the size of the patient.22
Statistical Analysis
We assessed facility-level distributions of CTDIvol and ED for LCS scans using boxplots, adjusted for chest diameter by standardizing facility-level doses by median facility-level chest diameter. We used mixed-effects linear regression models to evaluate factors associated with adjusted CT dose levels.23 We included facility-level and machine-level random effects to account for correlation among examinations performed on the same machine or at the same facility. Variables included in models were selected using forward selection with final model only including factors with a P < .05. After models were selected, coefficients were exponentiated to calculate excess percentage of dose, with corresponding 95% confidence intervals (CIs). Mixed-effects logistic regression with forward selection was used to evaluate associations between institutional factors and having doses above ACR guidelines and the 75th percentile benchmark. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Corporation). The analysis was carried out between 2018 and 2019.
Results
Data were for 12 529 patients who underwent LCS CT scans performed at 72 institutions (Table 1). Overall, 7232 participants (58%) were men, and the median age was 65 years (interquartile range [IQR], 60-70). The mean (SD) CTDIvol adjusted for patient size was 2.4 (2.0) mGy and the mean (SD) ED for LCS adjusted for chest diameter was 1.2 (1.1) mSv. Unadjusted mean (SD) values were the same.
Table 1. Characteristics of All Low-Dose CT Lung Cancer Screening Scans.
| Characteristic | Lung Cancer Screening |
|---|---|
| Scans (mean/facility), no. | 12 529 (174) |
| Age, median (IQR), y | 65 (60-70) |
| Sex, % male | 58 |
| CTDIvol, mGy | |
| Unadjusted for patient diameter | |
| Mean (SD) | 2.4 (2.0) |
| Median (IQR) | 2.1 (1.5-2.7) |
| Adjusted for patient diameter | |
| Mean (SD) | 2.4 (2.0) |
| Median (IQR) | 2.0 (1.5-2.7) |
| Effective Dose, mSv | |
| Unadjusted for patient chest diameter | |
| Mean (SD) | 1.2 (1.1) |
| Median (IQR) | 1.0 (0.7-1.4) |
| Adjusted for patient chest diameter | |
| Mean (SD) | 1.2 (1.1) |
| Median (IQR) | 1.0 (0.7-1.4) |
Abbreviations: CT, computed tomography; CTDIvol, volume computed tomography dose index; IQR, interquartile range.
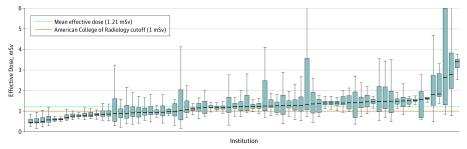
Distributions of adjusted CTDIvol and ED are in Figure 1 and Figure 2. We found 15 institutions (21%) with a median adjusted CTDIvol value higher than the ACR guideline of 3 mGy (overall median, 2.0 mGy; IQR, 1.5-2.7) and 47 (65%) with a median adjusted ED higher than the ACR guideline of 1 mSv (overall median, 1.0 mSv; IQR, 0.7-1.4). Of 12 529 CT scans, 2296 (18%) had a CTDIvol higher than guidelines and 6303 (50%) had an ED higher than ACR guidelines. The results did not appreciably change when we used unadjusted values to characterize the number of institutions and patients whose doses exceeded guidelines.
Figure 1. Volume Computed Tomography Dose Index for Lung Cancer Screening Scans by Facility, Adjusted for Chest Diameter.
Institutions are characterized by box plots. The black line indicates the median, the blue boxes indicate the 25th to 75th percentiles, and the error bars indicate the lower and upper extremes corresponding to values within 1.5 times the interquartile range (Q3-Q1) below and above Q1 and Q3, respectively.
Figure 2. Effective Dose for Lung Cancer Screening Scans by Facility, Adjusted for Chest Diameter.
Institutions are characterized by box plots. The black line indicates the median, the blue boxes indicate the 25th to 75th percentiles, and the error bars indicate the lower and upper extremes corresponding to values within 1.5 times the interquartile range (Q3-Q1) below and above Q1 and Q3, respectively.
Institutional responses to the organizational survey are in eTable 1 in the Supplement. Of 72 institutions, 42 (58%) reported serving as outpatient imaging facilities, with lead radiologists ([63] 88%) or a head technologist ([35] 49%) establishing scan protocols. Technologists performing examinations were the most likely to alter scan protocols ([24] 33%) compared with other personnel. The most common method of reviewing protocols was reported as “as needed” ([34] 47%). Most institutions ([42] 58%) locked their protocols after they were established.
Factors Associated With CTDIvol
Predictors of CTDIvol levels by CT scan type are listed in Table 2. Doses and likelihood of exceeding benchmarks increased with patient size and age and doses were higher among women, although differences were not large.
Table 2. Patient and Facility Factors Associated With CTDIvola.
| Variable | Difference From Mean Dose, % (95% CI) | High Dose, Odds Ratio (95% CI) | |
|---|---|---|---|
| Compared With ACR Guidelines | Compared With 75th Percentile Benchmark From the Registry | ||
| Age | NA | 0.99 (0.98 to 0.996) | NA |
| Female sex | 5 (4 to 6) | 1.31 (1.13 to 1.53) | 1.36 (1.17 to 1.59) |
| Patient chest diameter, cm | 0.6 (0.55 to 0.59) | 1.05 (1.04 to 1.05) | 1.055 (1.052 to 1.058) |
| Medical physicist type | |||
| External consultant | NA | 6.1 (1.8 to 20.8) | NA |
| Who establishes protocols | |||
| Medical physicist | NA | NA | 0.09 (0.01 to 0.59) |
| Any individual radiologists | 44 (19 to 69) | 12.0 (2.0 to 71.4)b | 19.0 (1.9 to 186.7)b |
| Who alters protocols | NA | NA | NA |
| Lead radiologists | −27 (−52 to −2) | NA | NA |
| How frequently are protocols updated? | |||
| As needed | 27 (8 to 45) | NA | NA |
Abbreviations: ACR, American College of Radiology; CTDIvol, volume computed tomography dose index; NA, not applicable.
Only factors with P < .05 are included in final forward selection model.
Confidence intervals are unstable owing to small sample sizes within institutions.
When the medical physicist was external instead of being on staff at the institution, doses were higher (OR for exceeding ACR benchmarks, 6.1; 95% CI, 1.8-20.8). For institutions reporting that any radiologist could establish protocols, doses were also higher (44% higher mean dose; 95% CI, 19%-69%; OR for a study exceeding ACR guidelines, 12.0; 95% CI, 2.0-71.4; OR for exceeding 75th percentile benchmark, 19.0; 95% CI, 1.9-186.7). Having any type of medical physicist (OR, 0.09; 95% CI, 0.01-0.59) establish the LCS CT protocol was associated with decreased odds of exceeding the 75th percentile benchmark. Institutions reporting that lead radiologists altered protocols had a 27% lower CTDIvol (95% CI, −52% to −2%). Institutions that updated protocols as needed had higher average doses (27% higher dose; 95% CI, 8%-45%).
In a sensitivity analysis examining SSDE results compared with CTDIvol findings, the mean (SD) SSDE was 3.0 (2.3) mGy. When measuring predictors of SSDE levels, our findings were consistent with those in the CTDIvol analysis (eTable 2 in the Supplement).
Factors Associated With ED
Significant ED predictors are in Table 3 and are similar to CTDIvol predictors. Sex and chest diameter were significant ED predictors with higher doses among men and those with increased chest diameter. Having any individual radiologist-establish protocols was associated with higher doses (27% excess effective dose; 95% CI, 5%-50%; OR for exceeding 75th percentile benchmark, 8.5; 95% CI, 1.7-42.9). Having the lead radiologist (OR, 0.01; 95% CI, 0.001-0.12) or technologist performing examinations (OR, 0.07; 95% CI, 0.01-0.56) responsible for establishing protocols was associated with lower odds of exceeding ACR effective dose guidelines. Having any type of medical physicist (OR, 0.17; 95% CI, 0.05-0.54) establish the LCS CT protocol was associated with decreased odds of exceeding the 75th percentile benchmark. Institutions that updated protocols “as needed” had higher average doses (17% higher dose, 95% CI, 0.01%-34%) and higher odds of exceeding the ACR guidelines (OR, 5.9; 95% CI, 1.3-27.9).
Table 3. Patient and Facility Factors Associated With Effective Dosea.
| Variable | Difference From, Mean Dose, % (95% CI) | High Dose, Odds Ratio (95% CI) | |
|---|---|---|---|
| Compared With ACR Guidelines | Compared With 75th Percentile Benchmark From the Registry | ||
| Female sex | −2% (−4% to −1) | 0.73 (0.64 to 0.83) | |
| Patient diameter, per cm | 0.5 (0.5 to 0.6) | 1.05 (1.05 to 1.06) | 1.038 (1.036 to 1.041) |
| Who establishes protocols | |||
| Medical physicist | NA | NA | 0.17 (0.05 to 0.54) |
| Any individual radiologists | 27 (5 to 50) | NA | 8.5 (1.7 to 42.9)b |
| Lead radiologists | NA | 0.01 (0.001 to 0.12)b | NA |
| Technologist performing exams | NA | 0.07 (0.01 to 0.56) | NA |
| How frequently are protocols updated? | |||
| As needed | 17 (0.01 to 34) | 5.9 (1.3 to 27.9) | NA |
Abbreviations: ACR, American College of Radiology; NA, not applicable.
Only factors with P < .05 are included in final forward selection model.
Confidence intervals are unstable owing to small sample sizes within institutions.
Discussion
Overall, we found wide variation in the distribution of LCS CT doses across facilities participating in our study, despite defined ACR guidelines. We found that 65% of participating institutions had median EDs for LCS scans that exceeded ACR guidelines, with a significant number of patients receiving doses above benchmarks created to ensure low radiation-dosage examinations. The ED reflects doses used for imaging and can indicate future cancer risk resulting from these studies. Over half of the patients received doses above ACR targets after accounting for patient size, measured using chest diameter. Furthermore, 1047 (8%) of 12 529 scans were performed on individuals who were outside the recommended age range of 55 to 80 years. If LCS CT scans are not performed using low-dose techniques, potential screening benefits and margins of benefits over risks are reduced.18 Although the risk of radiation-induced cancer and resultant risk of mortality is low compared with the benefits of LCS using low-dose techniques, the risk of radiation-induced cancer rises in parallel with doses used.
Our findings of odds of higher CTDIvol dose among women was notable given its contradiction to current evidence that women tend to receive lower radiation doses owing to the larger average chest diameter in men and concerns about greater radiosensitivity of breast tissue.24 However, our effective dose findings showed odds of a lower dose among women which aligned with findings from another study using data from this registry.25 One possible explanation for this is decision making on the part of the technologist to avoid multiphase scans and scans with longer scan lengths among women of reproductive age, which could increase the CTDIvol, but would minimize the effective dose by using a smaller scan length.
We identified several institution-level factors associated with using doses higher than needed. The magnitude of associations was as high as 44%, and the identified factors increased the odds of a high-dose examination by as much as 19-fold or decreased odds by as much as 100-fold. The factors that were most predictive of high doses included allowing individual radiologists to establish protocols, having an external rather than an internal staff medical physicist, and updating protocols as needed instead of yearly. Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols.
The inclusion of any individual radiologists in protocol establishment was associated with markedly higher odds of increased radiation dose. A potential cause of this finding could be the lack of training on dose optimization and motivation to change in some radiologists.26,27,28,29,30 Specifically, radiologists may not believe CT radiation risk is particularly concerning, may prefer that people not involved in reading scans alter protocols, or prefer the image quality available in higher-dose diagnostic CT scans rather than the lower quality of LCS.30 Ways to improve dose levels in institutions where individual radiologists establish protocols may include ensuring that radiologists are aware of the current CT scan guidelines and the potential for harmful effects related to dose, particularly for standardized protocols such as LCS, limiting the use of multiphase LCS scans, and providing feedback to radiologists on the doses they use, which is currently not standard practice.
Having an on-staff medical physicist was associated with an institution having significantly decreased odds of scans with high CTDIvol compared with institutions with medical physicists who are outside consultants or employed by a CT manufacturer. Similarly, odds of CTDIvol or ED exceeding the 75th percentile benchmarks were lower when any medical physicist, internal or external to the institution, was involved in establishing protocols. Given that medical physicists are trained to focus on safe, effective application of radiation in medical imaging, having a medical physicist on staff at an institution and actively involved in CT protocol development may help radiologists better manage CT radiation doses31; our data supported this possibility. On-staff medical physicists may provide quality control more closely or more frequently. External medical physicists may focus more on phantom studies and less on reviewing doses for examination subtypes such as LCS. Furthermore, because medical physicists are responsible for monitoring a practice’s doses against national benchmarks, having a medical physicist onsite instead of contracted for annual or less frequent visits may better ensure that institutions maintain appropriate radiation dose levels.31
Involving technologists who perform examinations in the protocol establishment process was also associated with lower odds of EDs higher than ACR guidelines. Importantly, no association was found with CTDIvol levels, meaning that in general, technologists who established protocols used shorter scan lengths or used single rather than multiple CT scans more often. Technologists may be less sensitive to image quality than radiologists because technologists do not interpret scans.
Being able to modify protocols can lead to lowering doses at some institutions, but can also lead to higher doses.32 Our results indicated that the type of personnel involved in establishing protocols may have a profound impact on CT radiation doses delivered. Allowing any individual radiologist to adjust protocols tended to result in higher doses, whereas having lead radiologists manage protocol adjustment may lower doses. Given the clear guidelines for low-dose LCS, having fewer individuals involved in scan protocols may avoid unnecessary variation in radiation doses. Future studies should further investigate the effects of limiting personnel involved in protocol development on CT scan radiation dose levels.
Strengths and Limitations
Our study has several strengths, including using data from the largest trial of CT scan radiation doses to date, using data from a wide variety of types of institutions performing CT scans, and including random effects to account for institution-level and scanner-level variation. Analyses also adjusted for key, individual-level scan factors, such as sex and patient chest diameter, which can slightly affect resultant radiation doses.
Our study had several limitations. Because we measured only LCS scans and resultant radiation doses, we did not follow individuals longitudinally to assess the relationships among CT dose and lung cancer detection or resultant effects on reducing mortality. These data would be informative for learning how exposures affect long-term outcomes. Our survey relied on self-reporting by leaders at participating facilities. These leaders were responsible for providing responses that represented practices throughout their institution but could be biased and reflect aspirational goals rather than current practice. To ensure that responses were representative of their facility, leaders were asked to contact institutional medical physicists, technologists, and radiologists. Data were collected during a trial to optimize CT doses. However, the trial was not focused on LCS and we did not see changes in LCS during the trial.
We identified LCS CT by finding scans that were indicated as LCS in their protocol name or study description. Some scans might have been misclassified as LCS scans, which could lead to misrepresentation of radiation dose distributions within an institution. However, we thoroughly reviewed our sorting methodology and performed sensitivity analyses to account for potential misclassification, and our findings were robust to these concerns. Our statistical analyses measured institution-level factors while clustering at the facility and machine levels; thus, some estimates from mixed models have wide standard errors owing to small sample sizes for some institutions and machines. Our study was conducted during the time of the pragmatic trial being conducted using some of the facilities included in this study sample. Although it is possible that the effects of the intervention in the trial might have impacted the results of this analysis, we believe this effect to be minor. Lastly, we did not determine if patients were appropriate candidates for LCS based on risk factors such as smoking history.
Conclusions
Among institutions performing low-dose CT scans for LCS, a significant proportion of institutions and patients exceeded guideline-recommended dose levels. Institutional characteristics, such as allowing any individual radiologists to establish CT scan protocols and updating protocols as necessary rather than annually or at other fixed times were associated with likelihood of higher radiation doses than other institutions. Conversely, having on-staff medical physicists, lead radiologists, technologists performing examinations, or any medical physicists responsible for establishing protocols was associated with lower radiation doses. These findings indicated that dose-optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines.
eTable 1. Institutional Characteristics
eTable 2. Patient and Facility Factors Associated with SSDE
References
- 1.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086. doi: 10.1001/archinternmed.2009.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith-Bindman R, Moghadassi M, Wilson N, et al. Radiation doses in consecutive CT examinations from five University of California Medical Centers. Radiology. 2015;277(1):134-141. doi: 10.1148/radiol.2015142728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith-Bindman R, Wang Y, Yellen-Nelson TR, et al. Predictors of CT radiation dose and their effect on patient care: a comprehensive analysis using automated data. Radiology. 2017;282(1):182-193. doi: 10.1148/radiol.2016151391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. doi: 10.1148/radiol.14131601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demb J, Chu P, Nelson T, et al. Optimizing radiation doses for computed tomography across institutions: dose auditing and best practices. JAMA Intern Med. 2017;177(6):810-817. doi: 10.1001/jamainternmed.2017.0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen A, Hughes K, Fahey N, Caldwell B, Wang CH, Park S. Wide variation in radiation exposure during computerized tomography. Urology. 2016;95:47-53. doi: 10.1016/j.urology.2016.05.036 [DOI] [PubMed] [Google Scholar]
- 7.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418-2429. doi: 10.1001/jama.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrington de González A, Kim KP, Knudsen AB, et al. Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol. 2011;196(4):816-823. doi: 10.2214/AJR.10.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 11.Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290. doi: 10.2214/AJR.12.9720 [DOI] [PubMed] [Google Scholar]
- 12.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157(8):571-573. doi: 10.7326/0003-4819-157-8-201210160-00524 [DOI] [PubMed] [Google Scholar]
- 13.Mathieu KB, Ai H, Fox PS, et al. Radiation dose reduction for CT lung cancer screening using ASIR and MBIR: a phantom study. J Appl Clin Med Phys. 2014;15(2):4515. doi: 10.1120/jacmp.v15i2.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oguchi K, Sone S, Kiyono K, et al. Optimal tube current for lung cancer screening with low-dose spiral CT. Acta Radiol. 2000;41(4):352-356. doi: 10.1080/028418500127345451 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt BT, Hupfer M, Saltybaeva N, Kolditz D, Kalender WA. Dose optimization for computed tomography localizer radiographs for low-dose lung computed tomography examinations. Invest Radiol. 2017;52(2):81-86. doi: 10.1097/RLI.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 16.Kazerooni E, Leung A, McNitt-Gray M, Munden R ACR–STR Practice Parameter for the Performance and Reporting of Lung Cancer Screening Thoracic Computed Tomography (CT).2014. https://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/CT. Accessed September 14, 2017. [DOI] [PubMed]
- 17.Lung Cancer Screening CT Protocols. Version 4.0. Alexandria, VA; 2016. https://www.aapm.org/pubs/CTProtocols/documents/LungCancerScreeningCT.pdf. Accessed January 25, 2018.
- 18.Cody DD, Kim H-J, Cagnon CH, et al. Normalized CT dose index of the CT scanners used in the National Lung Screening Trial. AJR Am J Roentgenol. 2010;194(6):1539-1546. doi: 10.2214/AJR.09.3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan VA, Kalra MK, Rehani M, Digumarthy SR. Lung cancer screening: computed tomography radiation and protocols. J Thorac Imaging. 2015;30(5):283-289. doi: 10.1097/RTI.0000000000000150 [DOI] [PubMed] [Google Scholar]
- 20.Moyer VA; U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 21.Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=274. Accessed November 13, 2014.
- 22.Jacobs CD, Jafari ME. Early results of lung cancer screening and radiation dose assessment by low-dose ct at a community hospital. Clin Lung Cancer. 2017;18(5):e327-e331. doi: 10.1016/j.cllc.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Boone JM, Strauss KJ, Cody DD, et al. Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations. College Park; 2011. https://www.aapm.org/pubs/reports/RPT_204.pdf. Accessed August 19, 2019.
- 24.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. Stat Methodol. 2011;73(3):273-282. doi: 10.1111/j.1467-9868.2011.00771.x [DOI] [Google Scholar]
- 25.Boice JD Jr, Monson RR. Breast cancer in women after repeated fluoroscopic examinations of the chest. J Natl Cancer Inst. 1977;59(3):823-832. doi: 10.1093/jnci/59.3.823 [DOI] [PubMed] [Google Scholar]
- 26.Smith-Bindman R, Wang Y, Chu P, et al. International variation in radiation dose for computed tomography examinations: prospective cohort study. BMJ. 2019;364:k4931. doi: 10.1136/bmj.k4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolicchi F, Faggioni L, Bastiani L, et al. Optimizing the balance between radiation dose and image quality in pediatric head CT: findings before and after intensive radiologic staff training. AJR Am J Roentgenol. 2014;202(6):1309-1315. doi: 10.2214/AJR.13.11741 [DOI] [PubMed] [Google Scholar]
- 28.Lee CI, Haims AH, Monico EP, Brink JA, Forman HP. Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology. 2004;231(2):393-398. doi: 10.1148/radiol.2312030767 [DOI] [PubMed] [Google Scholar]
- 29.Divrik Gökçe S, Gökçe E, Coşkun M. Radiology residents’ awareness about ionizing radiation doses in imaging studies and their cancer risk during radiological examinations. Korean J Radiol. 2012;13(2):202-209. doi: 10.3348/kjr.2012.13.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee RKL, Chu WCW, Graham CA, Rainer TH, Ahuja AT. Knowledge of radiation exposure in common radiological investigations: a comparison between radiologists and non-radiologists. Emerg Med J. 2012;29(4):306-308. doi: 10.1136/emermed-2011-200481 [DOI] [PubMed] [Google Scholar]
- 31.Hara AK, Wellnitz CV, Paden RG, Pavlicek W, Sahani DV. Reducing body CT radiation dose: beyond just changing the numbers. AJR Am J Roentgenol. 2013;201(1):33-40. doi: 10.2214/AJR.13.10556 [DOI] [PubMed] [Google Scholar]
- 32.McCollough CH. The role of the medical physicist in managing radiation dose and communicating risk in CT. AJR Am J Roentgenol. 2016;206(6):1241-1244. doi: 10.2214/AJR.15.15651 [DOI] [PubMed] [Google Scholar]
- 33.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47(1):27-40. doi: 10.1016/j.rcl.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Institutional Characteristics
eTable 2. Patient and Facility Factors Associated with SSDE