This case series study characterizes the composition of lipoaspirates and their wound-healing properties in patients receiving autologous fat injections during head and neck surgical procedures.
Key Points
Question
What is the composition of lipoaspirates used for lipotransfer in the head and neck, and what is their potential for wound healing in vitro?
Findings
In this case series study of lipoaspirate samples obtained from 15 patients, adipose-derived mesenchymal stem cells were found in high purity and were able to multidifferentiate. The adipose-derived stem/stromal cells and their supernatants had proliferation- and immune-modulating properties in vitro.
Meaning
The findings suggest that lipoaspirates contain a concentration of adipose-derived stem/stromal cells that can be used for regenerative head and neck surgical procedures.
Abstract
Importance
Adipose-derived mesenchymal stem cells (ASCs) have been used commonly in regenerative medicine and increasingly for head and neck surgical procedures. Lipoaspiration with centrifugation is purported to be a mild method for the extraction of ASCs used for autologous transplants to restore tissue defects or induce wound healing. The content of ASCs, their paracrine potential, and cellular potential in wound healing have not been explored for this method to our knowledge.
Objective
To evaluate the characteristics of lipoaspirates used in reconstructive head and neck surgical procedures with respect to wound healing.
Design, Setting, and Participants
This case series study included 15 patients who received autologous fat injections in the head and neck during surgical procedures at a tertiary referral center. The study was performed from October 2017 to November 2018, and data were analyzed from October 2017 to February 2019.
Main Outcomes and Measures
Excessive material of lipoaspirates from subcutaneous abdominal fatty tissue was examined. Cellular composition was analyzed using immunohistochemistry (IHC) and flow cytometry, and functionality was assessed through adipose, osteous, and chondral differentiation in vitro. Supernatants were tested for paracrine ASC functions in fibroblast wound-healing assays. Enzyme-linked immunosorbent assay measurement of tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF), stromal-derived factor 1α (SDF-1α), and transforming growth factor β3 (TGF-β3) was performed.
Results
Among the 15 study patients (8 [53.3%] male; mean [SD] age at the time of surgery, 63.0 [2.8] years), the stromal vascular fraction (mean [SE], 53.3% [4.2%]) represented the largest fraction within the native lipoaspirates. The cultivated cells were positive for CD73 (mean [SE], 99.90% [0.07%]), CD90 (99.40% [0.32%]), and CD105 (88.54% [2.74%]); negative for CD34 (2.70% [0.45%]) and CD45 (1.74% [0.28%]) in flow cytometry; and negative for CD14 (10.56 [2.81] per 300 IHC score) and HLA-DR (6.89 [2.97] per 300 IHC score) in IHC staining; they differentiated into osteoblasts, adipocytes, and chondrocytes. The cultivated cells showed high expression of CD44 (mean [SE], 99.78% [0.08%]) and CD273 (82.56% [5.83%]). The supernatants were negative for TNF (not detectable) and SDF-1α (not detectable) and were positive for VEGF (mean [SE], 526.74 [149.84] pg/mL for explant supernatants; 528.26 [131.79] pg/106 per day for cell culture supernatants) and TGF-β3 (mean [SE], 22.79 [3.49] pg/mL for explant supernatants; 7.97 [3.15] pg/106 per day for cell culture supernatants). Compared with control (25% or 50% mesenchymal stem cell medium), fibroblasts treated with ASC supernatant healed the scratch-induced wound faster (mean [SE]: control, 1.000 [0.160]; explant supernatant, 1.369 [0.070]; and passage 6 supernatant, 1.492 [0.094]).
Conclusions and Relevance
The cells fulfilled the international accepted criteria for mesenchymal stem cells. The lipoaspirates contained ASCs that had the potential to multidifferentiate with proliferative and immune-modulating properties. The cytokine profile of the isolated ASCs had wound healing–promoting features. Lipoaspirates may have a regenerative potential and an application in head and neck surgery.
Level of Evidence
NA.
Introduction
For more than a century, fat grafts have been used in plastic surgery.1 In reconstructive and aesthetic procedures, autologous adipose tissue is frequently used as a filler material.2,3,4 Although volume decreases over time owing to the resorption of the transplanted material, the regenerative wound-healing aspect of the autologous adipose grafts after radiotherapy and in other wound-healing disorders is a major topic in clinical and experimental medicine.5,6,7 The underlying mechanisms including the actual cellular composition of grafts, however, remain not yet fully examined. Mesenchymal stem cells are nonhematopoietic, pluripotent progenitor cells.8 These cells were first isolated from the bone marrow in 1970.9 Later, cells with similar properties were shown to reside in various tissues and organs, such as adipose tissue, umbilical cord matrix, synovial membrane, and dental tissues.10,11 A major advantage of the isolation of mesenchymal stem cells from adipose tissue is the uncomplicated acquisition of cells by liposuction or resection from subcutaneous fat tissue.12 Fat-derived mesenchymal stem cells are termed adipose-derived mesenchymal stem cells (ASCs) and can be expanded from liposuction material.13,14 In 2006, the International Society for Cellular Treatment (ISCT) proposed minimal criteria for mesenchymal stem cells: the cells should adhere to plastic under standard culture conditions; be highly pure for the specific markers CD73, CD90, and CD105; and be negative for CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR. Furthermore, ASCs must possess multilineage differentiation ability into adipocytes, osteocytes, and chondrocytes.8 Except for the ISCT criteria markers, the following markers were chosen for the present study: CD44 as a general stem cell marker,11 CD273 as an immune checkpoint marker,15 CD31 as an endothelial marker,16 CD36 as an adipocyte marker,17 and epithelial cell adhesion molecule (EpCAM) as a marker for epithelial differentiation.18
Several studies have shown that ASCs promote wound healing in vitro19 and in animal models for vocal cord injury and impaired wound healing.20,21 These characteristics are largely associated with soluble growth factors and cytokines secreted by ASCs, such as vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF), stromal-derived factor 1α (SDF-1α), and transforming growth factor β3 (TGF-β3), which modulate various aspects of the wound-healing process,22 including neovascularization, inflammation, homing of stromal cells, and the inhibition of fibrosis.
In contrast to fat transfer procedures (eg, breast augmentation) in plastic surgery using large amounts of lipoaspirates, only small volumes of lipoaspirates are required in head and neck surgical procedures. The volumes of lipoaspirate that are needed for procedures like vocal fold augmentation typically amount to a few milliliters.12 This information highlights the need for new minimally invasive fat harvesting techniques. However, important factors for the clinical outcome include the amount and viability of ASCs as well as their secretory activity.
In this study, we aimed to characterize lipoaspirate samples acquired from 15 patients using a new atraumatic device for liposuction and injection. The present study focused on ASC content and wound-healing properties of the isolated cells. We assessed multilineage differentiation and migration capacities.
Methods
This experimental study included samples from patients who underwent elective surgeries for autologous fat grafting to the head and neck area at a tertiary referral center. All samples were obtained after written informed consent during routine surgical procedures, and the study was approved by the Ethikkommission des Klinikums der Universität München ethics committee.
Patient samples were obtained from the abdominal fat tissue. A thin liposuction cannula (Spiggle & Theis) was inserted through a small incision to harvest approximately 15 mL of lipoaspirate material. Lipoaspirates then underwent centrifugation, and only the stromal-vascular fraction (SVF) was transferred to 1-mL syringes for further injection. Explant cultivation and isolation of ASCs were performed as described previously.23
Immunohistochemistry and Fluorescent Staining of Lipoaspirates
For immunofluorescence staining, slides were incubated for 1 hour at room temperature in darkness with Alexa Fluor 594 goat anti-mouse IgG (H+L) conjugate (Molecular Probes Inc) and mounted with ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (Invitrogen; Thermo Fisher Scientific). Confocal microscopy images were recorded with a TCS-SP5 system (Leica Microsystems).
Immunophenotyping of ASCs
To assess the immunophenotype of the ASCs, cell culture passage 2 and passage 6 ASCs were trypsinized, washed in flow cytometry buffer, and incubated with conjugated antibodies or the respective isotype control antibodies for 1 hour at 4 °C in darkness. Cells were then washed 3 times and resuspended in 500 μL flow cytometry buffer. Fluorescence was analyzed by flow cytometry in a CYTOflex instrument (Beckman Coulter).
Differentiation Assay
For adipogenic and osteogenic differentiation, passage 2 ASCs were seeded in 6-well plates (2 × 105 cells per well) and cultured in mesenchymal stem cell medium (MSCM) for 2 days to reach confluency. The medium was then replaced with the respective differentiation medium. Cells were cultivated for 2 weeks, and medium was changed 3 times per week.
Adipogenic differentiation was detected by Oil Red O lipid staining (Abcam). Osteogenesis was detected by Alizarin S staining (Morphisto).
For chondrogenic differentiation, 2 × 104 passage 2 ASCs per well were seeded in 96-well ultra-low attachment spheroid microplates (Corning Inc) and were cultured in MSCM for 2 days, after which the medium was changed to differentiation medium. Cells were cultivated for 4 weeks, and the medium was changed 3 times per week. The resulting spheroids were then placed in disposable vinyl specimen molds, embedded with Tissue-Tek optimum cutting temperature compound (Sakura Finetek), snap frozen in liquid nitrogen, cut, and mounted onto SuperFrost microscope slides (Thermo Fisher Scientific). For assessment of chondrogenic differentiation, these slides were stained with 1% Alcian blue (pH, 1; Morphisto).
Enzyme-Linked Immunosorbent Assay
Conditioned media from explants, passage 2 ASCs, and passage 6 ASCs were tested using enzyme-linked immunosorbent assay kits for TNF-α (Invitrogen; Thermo Fisher Scientific), VEGF (Invitrogen; Thermo Fisher Scientific), human TGF-β3 (Abnova), and human SDF-1α secretion (Promocell) according to the manufacturer’s protocol.
Scratch Assay
Normal human dermal fibroblasts were cultured in 12-well plates for 5 days until they reached 90% confluency. A scratch was inflicted in the monolayer using a sterile 200-μL micropipette tip. Cells were washed 2 times with prewarmed phosphate-buffered saline to remove cell debris and then given serum-free Dulbecco modified Eagle medium containing 25% or 50% explant supernatant or cell culture supernatant; 25% or 50% MSCM served as control. Reference points were created on the bottom of the plates with a marker pen, and photographs were taken using a digital camera and light field microscope at 0 hours and 24 hours. Wound borders ware manually tracked and measured.24
Statistical Analysis
All statistical analyses were performed using GraphPad Prism, version 8.0 (GraphPad Software). Statistical significance of differences between 2 groups was calculated with paired or unpaired t tests. Statistical significance of differences between the control and more than 1 group was calculated using 1-way analysis of variance followed by the Dunnet multiple comparison test. Statistical significance of differences between different time points within the same sample was calculated using repeated measures 1-way analysis of variance followed by the Dunnet multiple comparison test. P < .05 was considered to be statistically significant. All tests were 2-tailed.
Results
Among the 15 patients included in this study who underwent elective surgical procedures for autologous fat grafting to the head and neck area; 8 (53.3%) were male; mean (SD) age at the time of the surgical procedure was 63.0 (2.8) years; and mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 27.2 (1.1). A total of 8 patients (53.3%) had a history of cancer, and 10 (66.7%) received vocal fold augmentation for treatment of dysphonia (eTable 1 in the Supplement).
Isolation of ASCs
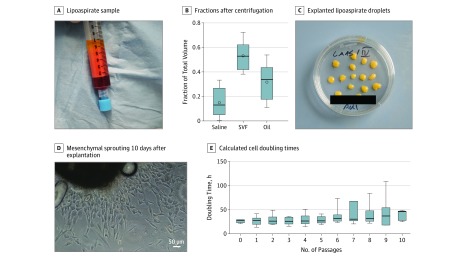
After centrifugation of the samples, 3 distinct phases were recovered from lipoaspirates: a saline fraction, an SVF, and an oily fraction (Figure 1A). The SVF represented the largest fraction within the native lipoaspirates (mean [SD] saline fraction, 15.0% [4.3%]; SVF, 53.3% [4.2%]; and oily fraction, 31.8% [5.3%]) (Figure 1A).
Figure 1. Explant Culture and Isolation.
A, Lipoaspirate from independent samples (n = 8) after centrifugation, with clear separation between oil, stromal vascular fraction, and saline fraction. B, Graph depicts the mean (SE) volume distribution of 8 lipoaspirate fractions. Box plots show the 25th to 75th percentiles, with whiskers indicating minimum and maximum values, horizontal lines in the boxes indicating medians, and circles indicating means. C, Representative tissue culture dish with explanted lipoaspirate droplets. D, Cells with a predominantly mesenchymal phenotype sprouting from 1 representative explanted lipoaspirate 10 days after explantation (original magnification ×100). E, Mean (SE) doubling times calculated from cell counting with viability staining from explant cultures (n = 6 independent samples). Box plots show the 25th to 75th percentiles, with whiskers indicating minimum and maximum values and horizontal lines in the boxes indicating medians. Oil indicates oil from dissolving lipocytes due to centrifugation; and SVF, stromal-vascular fraction.
To isolate potential ASCs from lipoaspirates, we transferred fat droplets onto cell culture plates in the presence of MSCM (Figure 1B). This resulted in the outgrowth of cells with mesenchymal phenotype (Figure 1C). Cultivation of isolated cells showed cell doubling times ranging from 24.7 hours to 28.9 hours in passages 0 to 5. Starting from passage 6, there were higher cell doubling times in later passages (repeated measures analysis of variance: mean [SE] for passage 0, 26.39 [1.81] hours; passage 1, 26.70 [3.83] hours; or passage 2, 28.81 [3.36] hours vs passage 6, 28.81 [3.36] hours; P ≥ .23) (Figure 1D). Viability remained stable above 92%.
Marker Expression of Lipoaspirates
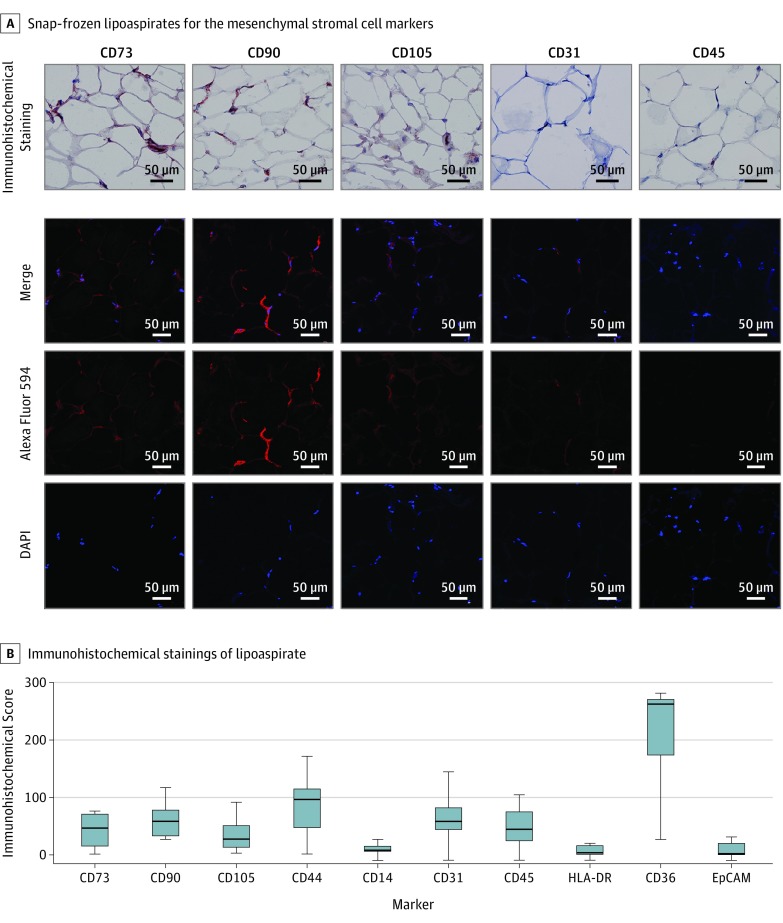
Figure 2 shows representative immunohistochemistry (IHC) stainings of CD73, CD90, CD105, CD31, and CD45 (Figure 2A) as well as immunofluorescence staining and laser scanning confocal imaging of CD73, CD90, CD105, CD31, and CD45 (Figure 2B).
Figure 2. Immunohistochemical Staining of Lipoaspirates.
A, Immunohistochemical (brown; top) and hemalaun (blue; top) staining and confocal imaging of snap frozen lipoaspirate samples for the mesenchymal stromal cell markers CD73, CD90, and CD105; endothelial cell marker CD31; and leukocyte common antigen CD45 (original magnification ×200). The 4′,6-diamidino-2-phenylindole (DAPI) signal is shown in blue and Alexa Flour 594 signal in red. B, Immunohistochemical scores for stained lipoaspirates (n = 9) for the indicated markers. Box plots show the 25th to 75th percentiles, with whiskers indicating minimum and maximum values and horizontal lines in the boxes indicating medians. EpCAM indicates epithelial cell adhesion molecule.
Owing to the heterogeneous cell composition, a limited set of markers showed homogenously low or high expression. To quantify and compare expression levels, we applied IHC scores to all staining images. The IHC scores represent the product of the percentages of positive cells and the staining intensity score (negative indicates 0; low, 1; intermediate, 2; or strong, 3 [range, 0-300]). Among markers defined within the ISCT criteria, CD14 and HLA-DR showed homogenously low expression with mean IHC scores of 10.6 (range, 0-26.7) for CD14 and 6.9 (range, 0-20.3) for HLA-DR. Of the additional markers, EpCAM as a marker of epithelial differentiation showed low expression with a mean IHC score of 8.9 (range, 0-31.7). The protein CD36 (fatty acid translocase) as a marker of adipocytes had strong expression in most of the lipoaspirates with 2 lower outliers, resulting in a mean IHC score of 220.7 (range, 27.0-281.7). In all other ISCT criteria and additional markers, we observed heterogenous expression and intermediate mean IHC scores (Figure 2C).
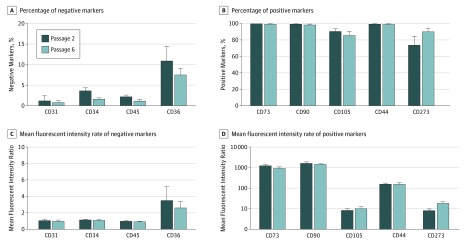
Cell surface expression of markers was assessed by flow cytometry analysis of isolated cells from the early passage (passage 2) or late passage (passage 6). We were able to distinguish positive and negative markers, both regarding mean fluorescence intensity ratios and in the fraction of marker-positive cells compared with isotype control. Representative histograms of each marker in passages 2 and 6 are shown in the eFigure in the Supplement. Of the ISCT criteria markers, almost all cells were positive for CD73 (passage 2: mean [SE], 100% [0] vs passage 6: mean [SE], 99.80% [0.13%]), and CD90 (99.88% [0.04%] vs 98.93% [0.59%]) (Figure 3C), with high expression levels for both proteins (ie, mean [SE] fluorescent intensity ratios of 1292.27 [121.69] for passage 2 and 947.25 [3.23] for passage 6 for CD73, and 1692.58 [193.22] for passage 2 and 1469.13 [103.55] for passage 6 for CD90) (Figure 3B). Most cells expressed CD105 in passages 2 (mean [SE] cells, 91.04% [2.72%]) and 6 (mean [SE] cells, 86.04% [4.81%]). For the negative ISCT criteria markers CD34 and CD45, we found only a small proportion of marker-positive cells and a significant decrease of positive cells in the late passage compared with the early passage (CD34: mean [SE], 3.71% [0.65%] for passage 2 vs 1.68% [0.23%] for passage 6; CD45: mean [SE], 2.26% [0.33%] for passage 2 vs 1.22% [0.36%] for passage 6; P < .05 for both comparisons).
Figure 3. Flow Cytometry of Adipose Tissue–Derived Isolated Explant Cells for 6 Independent Samples.
A and B, Percentages of positive populations of passages 2 and 6 adipose-derived mesenchymal stem cells (ASCs) were compared for the expression of the indicated markers. C and D, Mean (SE) fluorescence intensity ratios of passages 2 and 6 ASCs were compared for the expression of the indicated markers.
aP ≤ .05.
Of the additional markers, almost all cells were positive for CD44 in passage 2 (mean [SE], 99.86% [0.04%]) and passage 6 (99.71% [0.15%]) and negative for CD31 (mean [SE], 1.28% [1.16%]) in passage 2 and (0.89% [0.33%]) passage 6. CD36 was expressed at low levels in a minor subpopulation of cells and showed a decrease toward the late passage, with a high spread between the different patient samples (10.98% [3.50%] for passage 2 and 7.58 [1.54%] for passage 6). Most of the cells were CD273 positive. We found both an increase in the fraction of CD273-positive cells (74.36% [10.41%] for passage 2 and 90.76% [3.77%] for passage 6) (Figure 3C) and a significantly higher mean fluorescent intensity ratio (8.34 [1.44] for passage 2 vs 18.77 [0.04] for passage 6; P < .05 by paired t test) (Figure 3B). Marker expression of all conducted experiments of this section is summarized in eTable 2 in the Supplement.
Multipotency of ASCs
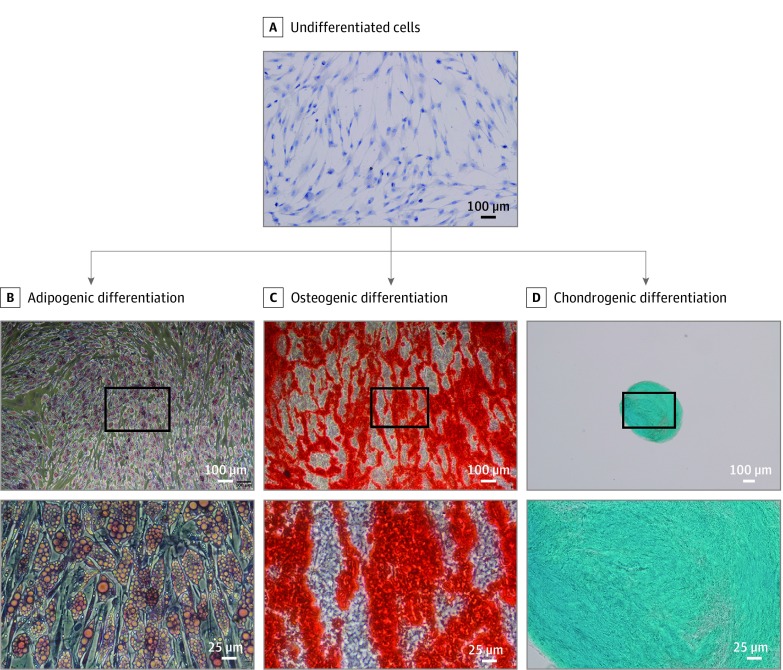
After adipogenic differentiation of undifferentiated ASCs (representative photograph in Figure 4A), accumulation of lipid vesicles was confirmed by Oil Red O staining (Figure 4B). Osteogenic differentiation of ASCs was visualized through Alizarin S staining of extracellular calcium deposition (Figure 4C), whereas chondrogenic differentiation was verified through Alcian blue staining (Figure 4D). In summary, differentiation of ASCs in all 3 lineages was successful among all ASC explant cultures (n = 6) (Figure 4).
Figure 4. Multilineage Differentiation Potential In Vitro of ASCs Into Adipocytes, Osteocytes, and Chondrocytes.
A, Undifferentiated passage 2 adipose-derived mesenchymal stem cells (ASCs) grown on glass slides and stained with Mayer hematoxylin counterstain. One representative image from 3 samples is shown (original magnification ×100). B, Lipid vesicle accumulation visualized by Oil Red O staining in adipogenic differentiated ASCs after 2 weeks of incubation with adipogenic differentiation medium (original magnification ×50 and ×200). C, Alizarin S staining of extracellular calcium deposits in osteogenic differentiated ASCs after 2 weeks of incubation with osteogenic differentiation medium (original magnification ×50 and ×200). D, Alcian blue staining of cartilage extracellular matrix in ASCs cultured as 3-dimensional spheroids after 4 weeks of incubation with chondrogenic differentiation medium (original magnification ×40 and ×100). Representative images of 6 independent experiments are shown. The area of higher magnification is indicated with the black boxes in the upper images of lower magnification.
ASC-Derived Soluble Factors and Fibroblast Migration
We used conditioned supernatants of ASCs to stimulate fibroblast migration in vitro in wound-healing assays. In the 50% supernatant groups (1:2 dilution of the respective explant or passage 6 supernatant), migration of primary human fibroblasts was significantly higher in both supernatant groups compared with control (explant supernatant: mean [SE] normalized to control, 1.369 [0.070] vs control: 1.000 [0.160]; P = .03; passage 6 supernatant: 1.492 [0.094] vs control: 1.000 [0.160]; P = .004 by analysis of variance followed by the Dunnet multiple comparison test) (Figure 5A and B).
Figure 5. Exocrine Functions of Explant Culture and Isolated Cells.
A, Representative examples of wound areas at 0 and 24 hours for control cells and after incubation with explant supernatants or cell culture supernatants collected at passage 6; 25% and 50% supernatant concentrations were used. Representative images of 3 experiments are shown (original magnification ×50). B, Cell migration date extrapolated from wound-healing assay was compared. C, Enzyme-linked immunosorbent assay results for vascular endothelial growth factor (VEGF), transforming growth factor β3 (TGF-β3), stromal-derived factor 1α (SDF-1α), and tumor necrosis factor (TNF) in explant and ASC culture supernatants collected in passages 1 and 6.
aP ≤ .05, by 1-way analysis of variance and the Dunnet multiple comparison test (n = 3).
bP ≤ .01, by 1-way analysis of variance and the Dunnet multiple comparison test (n = 3).
cResults below the assay range.
In the 25% supernatant groups (1:4 dilution of the respective explant or passage 6 supernatant), we found no changes between groups regarding the refilled area of the gap (explant supernatant: mean [SE] normalized to control, 0.810 [0.101] vs control: 1.000 [0.155]; P = .38; passage 6 supernatant: 0.917 [0.111] vs control: 1.000 [0.155]; P = .85 by analysis of variance followed by the Dunnet multiple comparison test) (Figure 5A and B).
The VEGF and TGF-β3 were secreted by ASCs in the explant and the passage 1 and passage 6 cell supernatants (VEGF in explant supernatants: mean [SE], 526.74 [149.84] pg/mL; VEGF in passage 1 cell culture supernatants: 388.26 [281.30] pg per 106 cells per day; VEGF in passage 6 cell culture supernatant: 621.60 [663.38] pg per 106 cells per day; TGF-β3 in explant supernatants: 22.79 [3.49] pg/mL; TGF-β3 in passage 1 cell culture supernatants: 7.81 [11.82] pg per 106 cells per day; TGF-β3 in passage 6 cell culture supernatants: 8.07 [10.58] pg per 106 cells per day); SDF-1α and TNF levels remained below the detection limit of the assay.
Discussion
Compared with other techniques, lipoaspiration is a minimally invasive procedure. Because of the small amount of filling material that is needed for local augmentation in the head and neck area, a small-diameter needle device is sufficient and can help to minimize donor site pain or complications from wound-healing disorders, as well as hematoma or seroma.25 Regarding the content of viable ASCs and their regenerative potential, different methods of liposuction, such as vacuum-assisted, water-assisted, and ultrasonography-assisted liposuction, have been shown to be equivalent to surgical resection approaches.14,25,26,27 In accordance with the literature, we also found high SVF and low saline fraction in our centrifuged lipoaspirates.
For the detection and characterization of ASCs, we performed both IHC analysis of native lipoaspirate frozen sections and flow cytometry analysis of short-term primary cell cultures. Thereby, we were able to examine isolated cells for the ISCT criteria in a cell mixture environment of the centrifuged lipoaspirate and in early passages of primary cell culture.8 Purity of ASC content and the quality of identification was superior in the flow cytometry data of the cell culture compared with the IHC data from the frozen sections.28 This finding was probably because primary lipoaspirates contain many different cells, such as endothelial cells, fibroblasts, and white blood cells.28 Nevertheless, the negative markers CD14 and HLA-DR within the ISCT criteria showed a homogenously low IHC score that we were able to interpret as negative. In contrast, many of the positive markers within the ISCT criteria showed low and heterogenous stainings, whereas flow cytometry of the cell culture single cells showed the expected high fraction of positive cells. As postulated in ISCT criteria,8 adipogenic, osteogenic, and chondrogenic differentiation of ASCs was successful for all samples analyzed. Therefore, we concluded that the centrifuged lipoaspirates were enriched with viable ASCs of high purity.
Of the additional markers that we chose to characterize ASCs, panepithelial marker EpCAM was negative, which is expected in lipoaspirates as an ideally epithelium-free tissue.18 The CD31 cells were negative in the flow cytometry experiments but had a weak IHC score in the lipoaspirates, which might reflect smaller CD31 positive small vessels and microvessels within the lipoaspirate samples.29 Recently, it was shown that endothelial differentiation of ASCs was associated with improved survival and neovascularization of fat transplants.30 Thus, the temporary presence of CD31-positive cells that we observed in some of the lipoaspirates might reveal them to be beneficial for wound healing as well. Although CD36 cells had the highest IHC scores observed in our experiments, CD36 cells were mostly negative in the flow cytometry experiments of the cell cultures. The decrease in the CD36-positive cell fraction in the late passages might reflect a purification process toward barely differentiated ASCs, whereas the lipoaspirates still contained many adipose progenitor cells and adipocytes. In addition, CD44 cells showed strong positivity on ASCs. A possible explanation is that CD44 cells aid wound healing through their influence on hyaluronic metabolism and facilitate possible paracrine functions of ASCs by the presentation of cytokines.31 Of most interest, we found a distinctive fraction of CD273-positive cells within isolated ASCs that increased in the later passages. This high positivity of CD273/PD-L2 cells within ASCs might reflect beneficial cellular associations with wound healing that are mediated by immune modulation and especially inhibitory effects on T effector cells.32 These associations could diminish over time, as has recently been shown after 10 serial culture passages in bone marrow–derived mesenchymal stem cells.33
To assess paracrine functions, we cultivated fibroblasts with diluted supernatants of the explant cultures and late passage cell cultures. Highly concentrated supernatants were able to increase fibroblast cell migration. In addition, we tested for different secreted growth factors and cytokines, which reflected different aspects of the wound-healing process, in the supernatants of explant cultures and early and late passage cell cultures.22
Explant cultivation has been described as an efficient method to harvest mesenchymal stem cells from fatty tissue that does not involve external proteolysis or strong physical shear stress to isolate stem cells.23 Therefore, we used this method to preserve cell-bound molecules and the intercellular microenvironment for further experiments. Nevertheless, there was a risk of other cell types remaining in later passages of cell culture that might have led to differences in the supernatants consecutively. We hypothesized that the ASCs were mainly associated with the observed paracrine functions, instead of other cell types of the SVF. Our data showed a lack of significant differences for fibroblast cell migration in the wound-healing assay and in the concentrations of secreted growth factors and cytokines between explant cultures and early and late passage cell cultures.
In recent years, different groups have shown paracrine effects of the SVF and ASCs. The SDF-1α has been associated with supporting wound healing in vitro and in vivo and is secreted by and supports the homing and survival of ASCs.34,35,36,37 Although we did not find SDF-1α in our supernatants, the highly concentrated supernatants (50%) promoted fibroblast migration in vitro for wound-healing assays. Therefore, alternative secreted factors were also able to mediate the paracrine functions of the SVF and ASC supernatants. Of the other secreted factors assessed in our study, VEGF and TGF-β3 were recovered from ASC supernatants. Although both factors are not likely to induce the increased migration of the fibroblasts, they might play pivotal roles in increasing the wound-healing capacities of the SVF and ASC supernatants. The VEGF is able to improve nutritive supply through neovascularization, including lymphangiogenesis, and aid in ASC survival38,39,40; TGF-β3 was shown to mediate fibrochondrogenic differentiation in ASCs and, thereby, might beneficially influence tissue turnover.41,42 However, the SVF and ASC supernatants were negative for TNF, which was expected because the ability of ASCs to decrease the secretion of inflammatory cytokines, such as IL-1β, IL-6, and TNF, has been demonstrated.43
Limitations
This study has limitations. The functional in vitro assays can only display surrogates for wound healing in vivo and have limited predictive value in wound-healing settings such as vocal fold augmentation. Because of technical challenges, we were not able to assess exact proportions of ASCs within the lipoaspirates.
Conclusions
Cells isolated from lipoaspirates comprised substantial amounts of ASCs that have potential to differentiate in all 3 lineages described for ASCs. As an additional cellular function, the isolated ASCs might have proliferation- and immune-modulating properties. As paracrine functions, the SVF and ASC supernatants had anti-inflammatory, neoangiogenic, and wound-healing features that promote wound healing in vitro. Our findings appear to provide insight into the successful clinical outcomes of fatty transplantation at the cellular level and support the potential of lipoaspirates in regenerative head and neck surgical procedures.
eFigure. Cell Counts and Fluorescence Intensity
eTable 1. General Patient Characteristics
eTable 2. Marker Expression Summary
References
- 1.Bui P, Lepage C. Benefits of volumetric to facial rejuvenation. Part 1: fat grafting [in French]. Ann Chir Plast Esthet. 2017;62(5):532-549. doi: 10.1016/j.anplas.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 2.Delay E, Savu T, Atanasiu M. Lipomodeling in breast reconstruction [in French]. Ann Chir Plast Esthet. 2018;63(5-6):505-515. doi: 10.1016/j.anplas.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 3.Krastev TK, Beugels J, Hommes J, Piatkowski A, Mathijssen I, van der Hulst R. Efficacy and safety of autologous fat transfer in facial reconstructive surgery: a systematic review and meta-analysis. JAMA Facial Plast Surg. 2018;20(5):351-360. doi: 10.1001/jamafacial.2018.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashir MM, Sohail M, Bashir A, et al. Outcome of conventional adipose tissue grafting for contour deformities of face and role of ex vivo expanded adipose tissue-derived stem cells in treatment of such deformities. J Craniofac Surg. 2018;29(5):1143-1147. doi: 10.1097/SCS.0000000000004367 [DOI] [PubMed] [Google Scholar]
- 5.Hespe GE, Albornoz CR, Mehrara BJ, Kraus D, Matros E. Pharyngocutaneous fistula closure using autologous fat grafting. Eplasty. 2013;13:e23. [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman JM, Long J, Gugatschka M, et al. Stem cell approaches for vocal fold regeneration. Laryngoscope. 2016;126(8):1865-1870. doi: 10.1002/lary.25820 [DOI] [PubMed] [Google Scholar]
- 7.Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409-1422. doi: 10.1097/01.prs.0000256047.47909.71 [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393-403. doi: 10.1111/j.1365-2184.1970.tb00347.x [DOI] [PubMed] [Google Scholar]
- 10.Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93(1):19-31. doi: 10.1002/cyto.a.23242 [DOI] [PubMed] [Google Scholar]
- 11.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—current trends and future prospective. Biosci Rep. 2015;35(2):e00191. doi: 10.1042/BSR20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muschter D, Geyer F, Bauer R, Ettl T, Schreml S, Haubner F. A comparison of cell survival and heat shock protein expression after radiation in normal dermal fibroblasts, microvascular endothelial cells, and different head and neck squamous carcinoma cell lines. Clin Oral Investig. 2018;22(6):2251-2262. doi: 10.1007/s00784-017-2323-8 [DOI] [PubMed] [Google Scholar]
- 13.Di Taranto G, Cicione C, Visconti G, et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy. 2015;17(8):1076-1089. doi: 10.1016/j.jcyt.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Duscher D, Atashroo D, Maan ZN, et al. Ultrasound-assisted liposuction does not compromise the regenerative potential of adipose-derived stem cells. Stem Cells Transl Med. 2016;5(2):248-257. doi: 10.5966/sctm.2015-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290(1):72-79. doi: 10.1016/j.cellimm.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 16.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial PECAM-1 and its function in vascular physiology and atherogenic pathology. Exp Mol Pathol. 2016;100(3):409-415. doi: 10.1016/j.yexmp.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Volat F, Sandhow L, et al. CD36 Is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem Cells. 2017;35(7):1799-1814. doi: 10.1002/stem.2635 [DOI] [PubMed] [Google Scholar]
- 18.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl). 1999;77(10):699-712. doi: 10.1007/s001099900038 [DOI] [PubMed] [Google Scholar]
- 19.Haubner F, Leyh M, Ohmann E, Pohl F, Prantl L, Gassner HG. Effects of external radiation in a co-culture model of endothelial cells and adipose-derived stem cells. Radiat Oncol. 2013;8(66):66. doi: 10.1186/1748-717X-8-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng M, Huang Y, Zhang H. Application of stems cells in wound healing–an update. Wound Repair Regen. 2014;22(2):151-160. doi: 10.1111/wrr.12152 [DOI] [PubMed] [Google Scholar]
- 21.Hong SJ, Lee SH, Jin SM, et al. Vocal fold wound healing after injection of human adipose-derived stem cells in a rabbit model. Acta Otolaryngol. 2011;131(11):1198-1204. doi: 10.3109/00016489.2011.599816 [DOI] [PubMed] [Google Scholar]
- 22.Guillén MI, Platas J, Pérez Del Caz MD, Mirabet V, Alcaraz MJ. Paracrine anti-inflammatory effects of adipose tissue-derived mesenchymal stem cells in human monocytes. Front Physiol. 2018;9:661. doi: 10.3389/fphys.2018.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendijani F. Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50(2). doi: 10.1111/cpr.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreml S, Babilas P, Fruth S, et al. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11(7):947-957. doi: 10.3109/14653240903204322 [DOI] [PubMed] [Google Scholar]
- 26.Duscher D, Maan ZN, Luan A, et al. Ultrasound-assisted liposuction provides a source for functional adipose-derived stromal cells. Cytotherapy. 2017;19(12):1491-1500. doi: 10.1016/j.jcyt.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP. Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int J Mol Sci. 2018;19(8):E2200. doi: 10.3390/ijms19082200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145. doi: 10.1186/s13287-017-0598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178(2):449-460. doi: 10.1084/jem.178.2.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris WM, Plastini M, Kappy N, et al. Endothelial differentiated adipose-derived stem cells improvement of survival and neovascularization in fat transplantation. Aesthet Surg J. 2019;39(2):220-232. doi: 10.1093/asj/sjy130 [DOI] [PubMed] [Google Scholar]
- 31.Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5(18). doi: 10.3389/fcell.2017.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moravcikova E, Meyer EM, Corselli M, Donnenberg VS, Donnenberg AD. Proteomic profiling of native unpassaged and culture-expanded mesenchymal stromal cells (MSC). Cytometry A. 2018;93(9):894-904. doi: 10.1002/cyto.a.23574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chae DS, Han S, Son M, Kim SW. Stromal vascular fraction shows robust wound healing through high chemotactic and epithelialization property. Cytotherapy. 2017;19(4):543-554. doi: 10.1016/j.jcyt.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Guo Y, Chen F, Liu J, Jin P. Stromal cell-derived factor-1 promotes human adipose tissue-derived stem cell survival and chronic wound healing. Exp Ther Med. 2016;12(1):45-50. doi: 10.3892/etm.2016.3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, Ji FK, Wang JH, Nan H, Liu DL. Stromal cell-derived factor 1 promoted migration of adipose-derived stem cells to the wounded area in traumatic rats. Biochem Biophys Res Commun. 2015;467(1):140-145. doi: 10.1016/j.bbrc.2015.09.097 [DOI] [PubMed] [Google Scholar]
- 37.Stuermer EK, Lipenksy A, Thamm O, et al. The role of SDF-1 in homing of human adipose-derived stem cells. Wound Repair Regen. 2015;23(1):82-89. doi: 10.1111/wrr.12248 [DOI] [PubMed] [Google Scholar]
- 38.Liu GS, Peshavariya HM, Higuchi M, Chan EC, Dusting GJ, Jiang F. Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine. J Tissue Eng Regen Med. 2016;10(3):E167-E176. doi: 10.1002/term.1796 [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Wang H, Cao J, Ye C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell Physiol Biochem. 2018;49(1):160-171. doi: 10.1159/000492851 [DOI] [PubMed] [Google Scholar]
- 40.Song SY, Chung HM, Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10(11):1529-1537. doi: 10.1517/14712598.2010.522987 [DOI] [PubMed] [Google Scholar]
- 41.Sasaki H, Rothrauff BB, Alexander PG, et al. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and TGF-β3. Am J Sports Med. 2018;46(10):2402-2413. doi: 10.1177/0363546518782973 [DOI] [PubMed] [Google Scholar]
- 42.Sun Q, Zhang L, Xu T, et al. Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo. Biotech Histochem. 2018;93(3):168-176. doi: 10.1080/10520295.2017.1401663 [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Wang Y, Liu Y, Zeng H, Xu H, Lian F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing [published online October 13, 2018]. J Cell Biochem.doi: 10.1002/jcb.27530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Cell Counts and Fluorescence Intensity
eTable 1. General Patient Characteristics
eTable 2. Marker Expression Summary