Abstract
Background:
Poor habits can worsen gastroesophageal reflux disease (GERD) and reduce treatment efficacy. Few large-scale studies have examined lifestyle influences, particularly eating habits, on GERD in China, and research related to eating quickly, hyperphagia, and eating hot foods is quite limited. The aim of this study was to evaluate the relationship between GERD pathogenesis and lifestyle factors to produce useful information for the development of a clinical reference guide through a national multicenter survey in China.
Methods:
Symptom and lifestyle/habit questionnaires included 19 items were designed. The questionnaire results were subjected to correlation analysis relative to GERD symptom onset. A standard proton pump inhibitor (PPI) was advised to correct patients with unhealthful lifestyle habits.
Results:
A total of 1518 subjects (832 GERD, 686 non-GERD) enrolled from six Chinese hospitals completed symptom and lifestyle/habit questionnaires. The top lifestyle factors related to GERD were fast eating, eating beyond fullness, and preference for spicy food. Univariate analysis showed that 21 factors, including male gender, a supra-normal body mass index (BMI), smoking, drinking alcohol, fast eating, eating beyond fullness, eating very hot foods, and drinking soup, among others, were associated with GERD (p < 0.05). Logistic multivariate regression analysis revealed the following risk factors for GERD [with odds ratios (ORs)]: fast eating (4.058), eating beyond fullness (2.849), wearing girdles or corsets (2.187), eating very hot foods (1.811), high BMI (1.805), lying down soon after eating (1.544), and smoking (1.521). Adjuvant lifestyle interventions improved outcomes over medication alone (z = –8.578, p < 0.001 Mann–Whitney rank sum test).
Conclusions:
Lifestyle interventions can improve medication efficacy in GERD patients. Numerous habits, including fast eating, eating beyond fullness, and eating very hot foods, were associated with GERD pathogenesis. The present results may be useful as a reference for preventive education and treatment.
Keywords: dietary habits, gastroesophageal reflux disease (GERD), hyperphagia, life style, therapeutics
Introduction
Gastroesophageal reflux disease (GERD) is characterized by abnormal gastric reflux into the esophagus at least once a week leading to heartburn and acid regurgitation.1 It is a common disease globally, with increasing prevalence, and, consequently, greater burden on healthcare systems.2,3 The prevalence rates of GERD in Western countries, where it is most prevalent, have been reported to be 10–20%.4 Meanwhile, recent modernization of living standards, and the accompanying lifestyle changes and acceleration of the pace of life, have led to an increasing prevalence of symptomatic GERD in China, which reached 3.8% in 2016.5 Because of repeated treatment and prolonged healing, GERD is associated with reduced health-related quality of life,6 substantial costs for patients,7 and increased risk of esophageal adenocarcinoma.8
GERD has been reported to be alleviated, or even cured, with a combination of lifestyle interventions and medication.9 Moreover, poor lifestyle habits can worsen GERD and reduce treatment efficacy.10 A consensus of GERD treatment strategy has yet to be established due to the lack of a unified view of GERD-promoting behavior. For example, opposing effects of coffee or caffeine on GERD have been reported,11,12 questions remain about the potential relationship between esophageal acid exposure and meal times,13,14 as well as about whether GERD symptoms are related to body mass index (BMI).15,16 Because it is difficult for patients who lack awareness of what constitutes a high-risk lifestyle to correct unhealthful habits after the emergence of red-flag symptoms before irreversible damage has been done, such patients tend to have poor drug treatment outcomes.
Lifestyle changes for GERD recommended by the American College of Gastroenterology, in 2013, and the Chinese Medical Association Digestive Diseases Branch, in 2014, include weight loss, head-of-bed elevation, cessation of smoking and frequent alcohol use, avoidance of meals 2–3 h before bedtime, and reduced intake of coffee, chocolate, spices, acidic foods, and high-fat foods. The lifestyle interventions mentioned in the guide, however, are quite limited.
GERD may progress from reflux esophagitis to Barrett’s esophagus (precancerous esophageal adenocarcinoma lesions), and, ultimately, to esophageal adenocarcinoma, a gravely serious outcome of GERD comorbidity with esophageal injury syndrome. In our prior investigation of 103 patients with esophageal cancer, we found that many patients with esophageal cancer had habits such as eating quickly, eating until very full, and consuming very hot foods, that are not addressed in GERD lifestyle intervention therapy guidance.
Few large-scale studies have examined lifestyle influences on GERD in China, and research related to eating quickly, eating beyond fullness, and eating hot foods, particularly, is quite limited. In the present study, we sought to examine which of these habits may be responsible for GERD pathogenesis. Toward this aim, we analyzed lifestyle questionnaires from GERD patients from six hospitals in China relative to GERD onset and aggravation.
Materials and methods
Participants
From August 2015 to August 2017, patients with upper gastrointestinal symptoms attending digestive clinics at six hospitals (the Third Xiangya Hospital of Central South University, the General Hospital of Chinese People’s Liberation Army, the People’s Hospital of Wuhan University, the Second Affiliated Hospital of Nanjing Medical University, the People’s Hospital of Jilin Province, and Army General Hospital) were invited to complete a questionnaire. This study was approved by the Third Xiangya Hospital Ethics Committee of Central South University (approval number 2018-S384). Written informed consent forms were obtained from all participants before questionnaire disbursement. All of our GERD group patients’ symptoms were treated with a standard proton pump inhibitor (PPI). All of these patients were advised to correct bad lifestyle habits.
Inclusion criteria
The GERD case group inclusion criteria could be met in two ways: presentation with typical clinical GERD manifestations (i.e. nausea, acid regurgitation, and heartburn), a total score ⩾12 on the reflux diagnostic questionnaire used for initial GERD diagnosis, and a positive PPI test; or endoscopic/imaging demonstration of esophageal disease, an erosive esophagus inflammation diagnosis, or a Barrett’s esophagus diagnosis. Patients meeting either one of these two criteria sets were eligible for enrollment.
The control group inclusion criteria sets were as follows: an absence of GERD-typical symptoms (nausea, acid reflux, heartburn, and substernal pain), a total score <12 on the reflux diagnostic questionnaire used for initial GERD diagnosis; and endoscopic- or imaging-based ruling out of the a forementioned esophageal disease manifestations. To be selected as controls, subjects were required to meet both criteria sets.
Exclusion criteria
The exclusion criteria for the GERD case group were gastrointestinal related organic lesions (including esophageal hiatal hernia); surgery within 1 year before being diagnosed with GERD; diagnosis with diffuse esophageal fistula or achalasia, and suspected malignancy; inability to complete 2 weeks of treatment and follow up; major mental illness or communication disorder; and serious comorbidity. The exclusion criteria for the control group were diagnosis with a disease or major mental illness.
Study design
Outpatients completed questionnaires independently. We conducted diagnostic PPI treatments in all enrolled patients. GERD patients were selected according to clinical symptom score, endoscopy findings, and PPI test results. The relationships between GERD incidence and habits were analyzed.
Questionnaires
The survey included four Simplified Chinese Questionnaires: a demographic questionnaire (name, gender, age, occupation, height, weight, contact information); a reflux disease questionnaire (RDQ) to assess typical GERD symptoms; a query for gastroscope/imaging findings; and a lifestyle questionnaire.
RDQ structure and scoring
The RDQ was used to collect detailed information about GERD symptoms, including reflux, acid regurgitation, heartburn, and substernal pain in the past 4 weeks,1 as well as information regarding symptom frequency and severity, classified according to intrinsic scoring criteria.17 Prior studies have demonstrated the utility of the RDQ in GERD diagnosis.17,18 In the RDQ, frequency of the symptoms of nausea, acid regurgitation, heartburn, and substernal pain were graded as: never = 0 points; <1 day/week = 1 point; 1 day/week = 2 points; 2–3 day/week = 3 points; 4–5 day/week = 4 points, and 6–7 day/week = 5 points. Severity of these symptom categories were graded as follows: not present = 0 points; not obvious/subtle = 1 point; mild (degree of symptoms intermediate between 1 point and 3 points) = 2 points; severe enough to sometimes affect daily life and requires medication occasionally = 3 points; moderately severe (degree of symptoms intermediate between 3 points and 5 points); very severe, affecting daily life markedly and requiring medication regularly = 5 points. RDQ frequency and severity subscores each ranged from 0 (none of the symptoms experienced) to 20 (maximal frequency or severity of all four categories), with a maximal combined score of 40 (sum of frequency and severity scores) and higher scores indicating a more severe presentation. The common RDQ screening GERD cut-off of 12 points was adopted.
Assessment of lifestyle and eating habits
The lifestyle questionnaire included 19 items assessing the following habits: smoking, alcohol drinking, fast eating, eating beyond fullness, lying down soon after eating, eating shortly before bedtime, difficulty with defecation, sleep difficulties, feeling stress continually, wearing girdles or corsets, and consumption of very hot substances, strong teas, and coffee, as well as preferences for drinking soup, spicy foods, high-fat foods, acidic foods, sweets, and hard/solid foods. A regular smoker was defined as a person who smokes ⩾1 cigarette/day, for 6 months continuously or cumulatively, in accordance with World Health Organization (WHO) standards. A drinker was defined as a person with a daily alcohol consumption level of >25 g for men or >15 g for women, for 6 months continuously or cumulatively, in accordance with the recommendations of the Chinese Ministry of Health. Dietary habits were assessed with 11 items (defining clarifications in parentheses): fast eating (<10 min per meal and chewing <10 times per bite); eating beyond fullness (continuing to eat beyond a sensation of fullness until unable to eat any more); eating too-hot foods (>60°C); preference for drinking soup; preference for spicy foods; preference for high-fat food (e.g. chocolates, fried foods, animal offal); preference for acidic foods (e.g. citrus fruits and acidic drinks); preference for sweets (e.g. cream, cake, chocolate); preference for hard foods (e.g. walnuts, peanuts); preference for strong teas (>3 g of tea); and preference for coffee drinking. Lifestyle habits were assessed with the following six items: lying down soon after eating (<30 min); eating just before bedtime (within 2 h); difficulty defecating (inability or time consuming); sleep difficulties (insufficient sleep sleepwalking, night terrors, nightmares, etc.); anxiety (e.g. irritability, panic); and wearing girdles or corsets. Preference was defined as engaging in the habit >3 day/week, continuously or cumulatively for 6 months.
PPI test
Participants received a standard diagnostic oral PPI test in which they took esomeprazole (20 mg) or lansoprazole (30 mg) enteric-coated tablets twice per day for 2 weeks. No other drugs were taken during the treatment. Subsequently, patients were followed up in an outpatient clinic or telephone appointment, and completed the RDQ again. The PPI test was considered positive (supporting a GERD diagnosis) if the symptom score was reduced by >80% versus pretreatment.
Follow up and efficacy
We performed regular telephone follow up (half-month, 1 month, 3 months, and 6 months after commencing treatment). At each follow up, the patients recompleted the RDQ and were asked about whether they had made favorable lifestyle changes. The half-month follow-up results were used to judge PPI test results. Patients who followed all recommended habit changes formed the observation subgroup; those who followed some or none of the recommendations formed the lifestyle control subgroup. We compared pretreatment versus 6-month follow-up RDQ scores. If the total score decreased by >80%, 50–80%, or <50%, the intervention was considered substantially effective, moderately effective, or invalid, respectively. The total effective rate was the sum of the effective and moderately effective rates.
Statistical analysis
Quantitative data were compared across groups with t-tests. The Wald test was used for univariate analysis. Significant factors from the univariate analysis were included in a multivariate logistic regression analysis (method: Enter). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression. Our sample was derived from the grade/frequency table data of two-independent samples, so the relationship between outcomes and habit revision was analyzed by variance and the Mann–Whitney rank sum test. p < 0.05 was considered significant. SPSS 20.0 were used for the analysis.
Results
Characteristics of study subjects
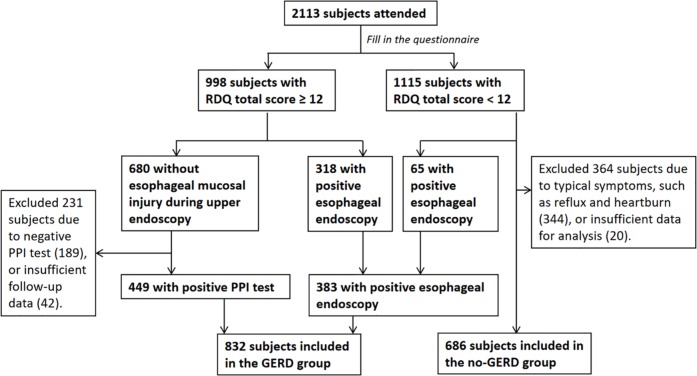
Enrollment and group designation are summarized in Figure 1. The size, gender ratio, and age characteristics of the GERD case group and non-GERD control group are reported in Table 1. The ages of patients in both groups have a normal distribution.
Figure 1.
Study recruitment flowchart.
Of 2113 potential participants, 832 met the GERD case group criteria. Among them, 449 had an RDQ score ⩾12 and a positive PPI test, and 383 had a positive esophageal endoscopy. The non-GERD group included 686 patients with RDQ scores <12 and negative esophageal endoscopy. Patients with data analysis difficulties due to data loss or incomplete data were excluded.
GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; RDQ, reflux disease questionnaire.
Table 1.
Baseline demographic characteristics of GERD and non-GERD groups.
| Baseline characteristics | GERD (n = 832) |
Non-GERD (n = 686) |
|---|---|---|
| Age, years | ||
| Mean (standard deviation) | 48.51 (13.22) | 47.45 (14.86) |
| Range | 17–86 | 15–84 |
| Gender, n (%) | ||
| Male | 455 (54.69) | 302 (44.02) |
| Female | 377 (45.31) | 384 (55.98) |
GERD, gastroesophageal reflux disease.
GERD-correlated factors
The composition ratio analysis results for GERD-correlated factors are reported in Table 2. Eating too fast and eating beyond fullness were habits shared by majority of the GERD patient group. Eating foods hotter than 60°C was also common.
Table 2.
Number and composition ratio of lifestyle habit factors by group.
| Lifestyle factors | GERD |
Non-GERD |
||
|---|---|---|---|---|
| n | Composition ratio, % | n | Composition ratio, % | |
| Fast eating | 663 | 79.7 | 244 | 35.6 |
| Eating beyond fullness | 568 | 68.3 | 190 | 27.7 |
| Preference for spicy foods | 509 | 61.2 | 28 | 42.0 |
| Preference for soup | 437 | 52.5 | 312 | 45.5 |
| Preference for sweets | 435 | 52.3 | 270 | 39.4 |
| Chronic stress | 431 | 51.8 | 280 | 40.8 |
| Preference for high-fat foods | 418 | 50.2 | 170 | 24.8 |
| Eating too-hot food | 371 | 44.6 | 148 | 21.6 |
| Sleep difficulty | 334 | 40.1 | 218 | 31.8 |
| Lying down soon after eating | 316 | 38.0 | 144 | 21.0 |
| Preference for hard foods | 294 | 35.3 | 112 | 16.3 |
| Smoking | 289 | 34.7 | 122 | 17.8 |
| Preferring spicy food | 287 | 34.5 | 124 | 18.1 |
| Drinking alcohol | 235 | 28.2 | 98 | 14.3 |
| Drinking strong tea | 232 | 27.9 | 82 | 12.0 |
| Eating just before bedtime | 209 | 25.1 | 132 | 19.2 |
| Difficulty with defecation | 170 | 20.4 | 92 | 13.4 |
| Wearing girdles or corsets | 156 | 18.8 | 40 | 5.8 |
| Drinking coffee | 113 | 13.6 | 36 | 5.2 |
GERD, gastroesophageal reflux disease.
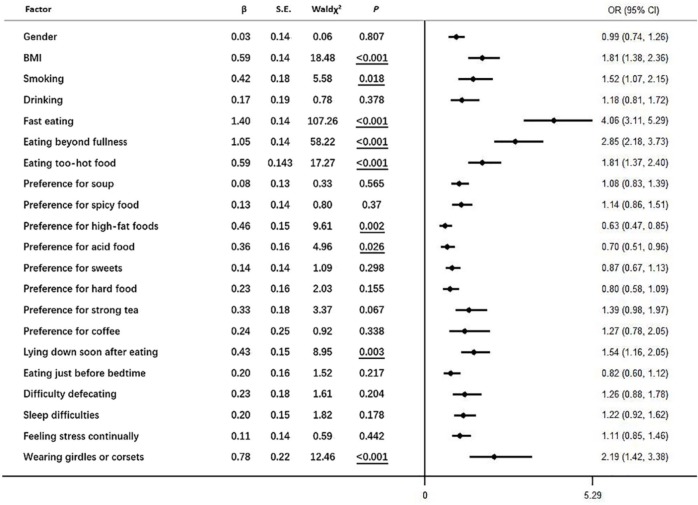
Univariate analysis with Chi-square tests indicated that the demographic factors of being male and having a BMI >24 (Table 3) as well as 18 lifestyle habits (smoking, drinking, fast eating, eating beyond fullness, eating food too hot, preference for drinking soup, preference for spicy foods, preference for high-fat foods, preference for acidic foods, preference for sweets, preference for hard foods, preference for strong tea, preference for coffee, lying down soon after eating, eating just before bedtime, difficulty with defecation, sleep difficulties, feeling stress continually, and wearing girdles or corsets; Table 4) were associated with the presence of GERD symptoms. As reported in Figure 2, logistic regression analysis of risk factors reported being related to GERD in the univariate analysis implicated the following habits as risk factors (more to less robust): fast eating, eating beyond fullness, wearing girdles or corsets, eating food too hot, BMI above normal, lying down soon after eating, and smoking.
Table 3.
Univariate analysis of GERD-related demographic risk factors.
| Factor | Number in group |
χ 2 | p | |
|---|---|---|---|---|
| GERD | Non-GERD | |||
| Gender | 17.11 | <0.001 | ||
| Female | 455 | 302 | ||
| Male | 377 | 384 | ||
| Age | 1.11 | 0.290 | ||
| <60 years (young/middle-aged adult) | 631 | 536 | ||
| ⩾60 years (elderly) | 201 | 150 | ||
| BMI | 35.15 | <0.001 | ||
| ⩽23.9 (normal) | 477 | 494 | ||
| ⩾24 (overweight/obese) | 355 | 192 | ||
Highly significant p values are shown in bold.
GERD, gastroesophageal reflux disease.
Table 4.
Univariate analysis of lifestyle risk factors for GERD.
| Lifestyle factor | Number in group |
|||||
|---|---|---|---|---|---|---|
| GERD |
Non-GERD |
χ 2 | p | |||
| No | Yes | No | Yes | |||
| Smoking | 543 | 289 | 564 | 122 | 54.72 | <0.001 |
| Drinking | 597 | 235 | 588 | 98 | 42.79 | <0.001*** |
| Fast eating | 169 | 663 | 442 | 244 | 304.31 | <0.001*** |
| Eating beyond fullness | 264 | 568 | 496 | 190 | 247.57 | <0.001*** |
| Eating too-hot food | 461 | 371 | 538 | 148 | 88.53 | <0.001*** |
| Preference for soup | 395 | 437 | 374 | 312 | 7.46 | 0.006** |
| Preference for spicy food | 322 | 509 | 398 | 288 | 56.79 | <0.001*** |
| Preference for high-fat foods | 412 | 418 | 516 | 170 | 105.19 | <0.001*** |
| Preference for acid food | 544 | 287 | 562 | 124 | 52.38 | <0.001*** |
| Preference for sweets | 396 | 435 | 416 | 270 | 26.31 | <0.001*** |
| Preference for hard food | 537 | 294 | 574 | 112 | 70.43 | <0.001*** |
| Preference for strong tea | 600 | 232 | 604 | 604 | 58.17 | <0.001*** |
| Preference for coffee | 719 | 113 | 650 | 36 | 29.5 | <0.001*** |
| Lying down soon after eating | 516 | 316 | 542 | 144 | 51.39 | <0.001*** |
| Eating just before bedtime | 23 | 209 | 554 | 132 | 7.46 | 0.006** |
| Difficulty defecating | 662 | 170 | 594 | 2 | 12.98 | <0.001*** |
| Sleep difficulties | 498 | 334 | 468 | 218 | 11.37 | 0.001** |
| Feeling stress continually | 401 | 431 | 406 | 280 | 18.23 | <0.001*** |
| Wearing girdles or corsets | 676 | 156 | 646 | 40 | 55.81 | <0.001*** |
Significance levels: *p < 0.05, **p < 0 .01, ***p < 0 .001.
GERD, gastroesophageal reflux disease.
Figure 2.
Multivariate analysis of GERD-related risk factors. Underline indicates p < 0.05.
GERD, gastroesophageal reflux disease.
Treatment efficacy
Of 832 patients, 699 completed the 6-month follow up, yielding a successful follow-up rate of 84.01%; 133 people were lost to follow up, primarily due to personal factors and contact information changes. All patients with GERD were treated with standardized drug therapy (PPI test) and guided to correct bad habits. Of the 699 patients in the GERD group who were followed up successfully for 6 months, 326 (46.6%) experienced significant efficiency and 332 (44.5%) experienced marginal efficacy, yielding a total effective rate of 658/699 (94.1%). The lifestyle observation subgroup (patients who heeded all lifestyle recommendations) included 464 GERD patients, a majority of whom (264/464; 56.9%) had substantial treatment efficacy. Many patients in the observation subgroup (192/464; 41.4%) had moderate treatment efficacy, while relatively few (8/464; 1.7%) had invalid treatment outcomes. The fully followed-up lifestyle control subgroup (patients who maintained some bad habits), included 235 GERD patients, only about a quarter of whom (62/235; 26.4%) had substantial treatment efficacy. A majority of the control subgroup patients (140/235; 59.6%) had moderate treatment efficacy, while the remainder (33/235; 14.0%) had invalid treatment outcomes. Mann–Whitney rank sum test results indicated that the lifestyle observation subgroup patients had better relief after 6 months than those in the control subgroup, with both subgroups receiving medication (Mann–Whitney U = 35276.000, Z = –8.578, p < 0.001). The average ranks of the observation and control subgroups were 308.53 and 431.89, respectively.
Discussion
In the present study, we demonstrated that the top lifestyle elements favoring GERD were fast eating, eating beyond fullness, and preference for spicy food. Logistic multiple regression analysis implicated fast eating, eating beyond fullness, wearing girdles or corsets, eating too-hot foods, a BMI >24, lying down soon after eating, and smoking as contributors to GERD symptoms. Mann–Whitney rank sum tests indicated that medication was more effective for GERD symptom alleviation when combined with lifestyle interventions, which is consistent with the results of several studies involving lifestyle interventions in GERD. For instance, Eivind Ness-Jensen’s studies suggested that weight loss and tobacco smoking cessation could be great recommendations, and avoiding late evening meals decreased time of supine acid exposure.19 The American College of Gastroenterology (ACG) guideline (2013) also recommended weight loss for GERD patients with overweight or recent weight gain.20 Concerning dietary patterns, predominantly Mediterranean (frequent consumption of composite/traditional dishes, fresh fruit and vegetables, olive oil, and fish) was reported as having a beneficial effect in the occurrence of GERD versus largely non-Mediterranean (frequent consumption of red meat, fried food, sweets, and junk/fast food).21 However, few studies on dietary habits in the literature including eating, eating beyond fullness and eating too-hot foods, which are subjective indicators needing objective definition and a large number of RCTs. Lower GERD incidence in females may be relevant to estrogen inactivating inflammatory cells, thereby delaying GERD progression.22 Although GERD incidence has been reported to raise with age,23 GERD prevalence did not differ between our young/middle-aged adults and elderly adults.
GERD appears to have a multifactorial etiology,24 and it has been supposed that poor dietary habits and lifestyle factors may induce or aggravate GERD symptoms. The recent growth in GERD among young adult and middle-aged Chinese people is thought to have something to do with dietary changes, acceleration in the pace of work expected, and chronic stress. Epidemiological studies have pointed to an association between a high BMI and GERD.25 GERD in overweight people may be relevant to gastric overfilling, which can loosen the lower esophageal sphincter (LES) and cause hiatal hernia.
Our findings that GERD symptoms were interlocked with cigarette smoking, drinking of alcohol, consumption of spicy, fatty, acidic, sweet, and hard foods are consistent with physiological studies26–28 showing reduced esophageal pressure, accelerated gastric peristalsis, augmented secretion, delayed mucosal nerve-stimulated gastric emptying, augmented esophageal acid exposure, and aggrandized inflammation in relation to these food habits. Our discoveries of a connection between strong tea drinking and GERD stay in alignment with some other studies involving Chinese subjects,29 though others have failed to find drinking of strong tea to be a significant risk factor for GERD.30 Theophylline, a major component of tea, can ease LES31 and alleviate visceral discomfort.32 The inconsistency of results with respect to tea drinking may be related to differences in tea type, production/processing, and additives, as well as cultural differences related to tea consumption.
Keeping in step with the present study’s insights into the link between coffee drinking and GERD symptoms, the results of a double-blind crossover study pointed to an association between coffee intake and GERD reflux.33 Conversely, the results of a single-oval twin study suggested that coffee may reduce the occurrence of GERD.34 A meta-analysis did not confirm a relationship between coffee intake and GERD.35 It is our view that coffee may increase gastric acid secretion by encouraging gastrin excretion, thereby promoting transient LES looseness, which may contribute to the development of GERD by delaying gastric emptying.36
The most striking findings in our research were related to three factors: fast eating, eating beyond fullness, and eating very hot foods. Research on these three factors is quite limited at present. Regarding the reasons that these behaviors may induce or aggravate the onset of GERD, it may be that swallowing large, rough boli subjected to limited chewing due to fast eating can do damage to the esophageal mucous membranes. Meanwhile, eating too fast also leads to the consumption of very large volumes, which can produce gastric pressure while irritating hydrochloric acid in gastric juice production and lessening LES rigidity and gastrointestinal motility. Because the gastric emptying rate is limited physiologically, mechanical and physiological factors induce an extended delay in the gastric emptying of a large food volume and may increase the risk of gastric contents spilling into the esophagus, promoting or aggravating the occurrence of GERD. Moreover, eating excessive amounts leads to overfullness, and, in the long term, obesity, which combines with multiple risk factors to accelerate disease progression. Very hot foods can destroy the esophageal mucosal defence directly. Our findings of an association between a preference for soup and GERD may reflect the combined effects of eating quickly and eating very hot food. There is also a danger that people may add soup to a meal that was already calorically sufficient, leading to weight gain, which itself favors GERD.
The linkage of GERD with difficulty defecating and wearing tight girdles or corsets may be related to abdominal pressure. Refraining from lying down or going to bed shortly after eating and sleeping with the head elevated are key lifestyle interventions for GERD. The interconnection between sleep duration and GERD appears to be bidirectional,37 and sleep can lead to esophageal hyperalgesia.38 Hence, GERD patients will be prone to have difficulty falling and staying asleep if they are suffering from frequent reflux symptoms, and poor sleep can promote reflux, forming a vicious circle. GERD symptoms have been linked with psychosocial characteristics, such as obsessive-compulsive, interpersonal sensitivity, and phobias.39
In the treatment, we found that compared with patients without lifestyle interventions, GERD patients with lifestyle interventions had a significantly higher remission rate of symptoms under the same PPI treatment conditions. In another study, a prospective population-based cohort study demonstrated that decrease in BMI was dose-dependence linked to a reduction of gastroesophageal reflux symptoms, but also an increased chance of losing reflux symptoms with PPI treatment in the general population (OR 1.98, 95% CI 1.45–2.72 with >3.5 units decrease in BMI and no or less than weekly medication compared with OR 3.96, 95% CI 2.03–7.65 with >3.5 units decrease in BMI and at least weekly medication),40 which is consistent with the findings of our research. A HUNT study reported that there was only an association between tobacco smoking cessation and gastroesophageal reflux symptoms status among individuals with severe reflux symptoms (adjusted OR 1.78; 95% CI: 1.07–2.97) of normal BMI (OR 5.67; 95% CI: 1.36–23.64) using anti-reflux medication at least weekly rather than other individuals with gastroesophageal reflux symptoms,41 suggesting that lifestyle interventions lead to a superposition effect. Drugs may temporarily inhibit acid reflux, but the effects of bad habits on the disease are persistent and serious, so that the accumulation of bad living habits reduces the efficiency of drug treatment in GERD patients, and even accelerates the progress of the disease, as said ‘A spark can start a prairie fire’. To sum up, the efficiency of treatment can be significantly improved with the combination of medication and lifestyle interventions.
In conclusion, risk factors for GERD include fast eating, eating beyond fullness, wearing girdles or corsets, eating too-hot food, a BMI above the normal range, lying down soon after eating, and smoking. Lifestyle changes that address these factors, especially fast eating, eating beyond fullness, and eating too-hot food, which are rarely studied or discussed despite being common, may improve GERD management and treatment outcomes. It is important to gain a holistic impression of symptoms and their impact on quality of life because GERD diagnoses may be missed in patients who are suffering from symptoms in the absence of endoscopic signs of disease.
Acknowledgments
The authors acknowledge Dr. Xiao Shi from the Third Xiangya Hospital, Central South University, China for revising the manuscript.
Footnotes
Author contributions: LZY wrote the paper; PY corrected and revised the text; GSW, SYT, GMH, LZQ, and YJ collected and analyzed data; FW designed the research, contributed to survey design, and conducted statistical analysis of the data. All authors contributed intellectually to the writing process and approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The present study was supported by the National Key Research and Development Program of China (No. 2016YFC1201800), the National Natural Science Foundation of China (No. 81670509), and the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (No. 20180304).
Conflict of interest statement: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ling-Zhi Yuan  https://orcid.org/0000-0001-5004-494X
https://orcid.org/0000-0001-5004-494X
Contributor Information
Ling-Zhi Yuan, Department of Gastroenterology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China; Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Central South University, Changsha, China.
Ping Yi, Department of Gastroenterology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China; Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Central South University, Changsha, China.
Gang-Shi Wang, Department of Gastroenterology, The General Hospital of Chinese People’s Liberation Army, Beijing, China.
Shi-Yun Tan, Department of Gastroenterology, The People’s Hospital of Wuhan University, Hubei, China.
Guang-Ming Huang, Department of Gastroenterology, The Second Affiliated Hospital of Nanjing Medical University, Jiangsu, China.
Ling-Zhi Qi, Department of Gastroenterology, The People’s Hospital of Jilin Province, Changchun, China.
Yan Jia, Department of Gastroenterology, Army General Hospital, Beijing, China.
Fen Wang, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, 138 Tongzipo Road, Changsha 410013, Hunan, China; Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Central South University, Changsha, China.
References
- 1. Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101: 1900–1920, 1943. [DOI] [PubMed] [Google Scholar]
- 2. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018; 67: 430–440. [DOI] [PubMed] [Google Scholar]
- 3. Tack J, Becher A, Mulligan C, et al. Systematic review: the burden of disruptive gastro-oesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther 2012; 35: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 4. El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan VP, Wong BC, Wong WM, et al. Gastroesophageal reflux disease: cross-sectional study demonstrating rising prevalence in a Chinese population. J Clin Gastroenterol 2016; 50: e1–e7. [DOI] [PubMed] [Google Scholar]
- 6. Lee SW, Lee TY, Lien HC, et al. Correlation between symptom severity and health-related life quality of a population with gastroesophageal reflux disease. Gastroenterology Res 2017; 10: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang R, Zou D, Ma X, et al. Impact of gastroesophageal reflux disease on daily life: the systematic investigation of gastrointestinal diseases in China (SILC) epidemiological study. Health Qual Life Outcomes 2010; 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010; 32: 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vemulapalli R. Diet and lifestyle modifications in the management of gastroesophageal reflux disease. Nutr Clin Pract 2008; 23: 293–298. [DOI] [PubMed] [Google Scholar]
- 10. Chen M, Xiong L, Chen H, et al. Prevalence, risk factors and impact of gastroesophageal reflux disease symptoms: a population-based study in South China. Scand J Gastroenterol 2005; 40: 759–767. [DOI] [PubMed] [Google Scholar]
- 11. Thomas FB, Steinbaugh JT, Fromkes JJ, et al. Inhibitory effect of coffee on lower esophageal sphincter pressure. Gastroenterology 1980; 79: 1262–1266. [PubMed] [Google Scholar]
- 12. Kim J, Oh SW, Myung SK, et al. Association between coffee intake and gastroesophageal reflux disease: a meta-analysis. Dis Esophagus 2014; 27: 311–317. [DOI] [PubMed] [Google Scholar]
- 13. Orr WC, Harnish MJ. Sleep-related gastro-oesophageal reflux: provocation with a late evening meal and treatment with acid suppression. Aliment Pharmacol Ther 1998; 12: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 14. Duroux P, Bauerfeind P, Emde C, et al. Early dinner reduces nocturnal gastric acidity. Gut 1989; 30: 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madisch A, Weihs C, Schlaud M, et al. The body-mass-index (BMI) has no impact on the frequency of typical reflux symptoms — Results of a nationwide telephone-based informing campaign in Germany. Zentralbl Chir 2002; 127: 1064–1067. [DOI] [PubMed] [Google Scholar]
- 16. Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol 2006; 101: 2619–2628. [DOI] [PubMed] [Google Scholar]
- 17. Value of reflux diagnostic questionnaire in the diagnosis of gastroesophageal reflux disease. Chin J Dig Dis 2004; 5: 51–55. [DOI] [PubMed] [Google Scholar]
- 18. Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009; 30: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 19. Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016; 14: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108: 308–328, 329. [DOI] [PubMed] [Google Scholar]
- 21. Mone I, Kraja B, Bregu A, et al. Adherence to a predominantly Mediterranean diet decreases the risk of gastroesophageal reflux disease: a cross-sectional study in a South Eastern European population. Dis Esophagus 2016; 29: 794–800. [DOI] [PubMed] [Google Scholar]
- 22. Asanuma K, Iijima K, Shimosegawa T. Gender difference in gastro-esophageal reflux diseases. World J Gastroenterol 2016; 22: 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005; 54: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil 2015; 27: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 25. Hallan A, Bomme M, Hveem K, et al. Risk factors on the development of new-onset gastroesophageal reflux symptoms. A population-based prospective cohort study: the HUNT study. Am J Gastroenterol 2015; 110: 393–400, 401. [DOI] [PubMed] [Google Scholar]
- 26. Ness-Jensen E, Lagergren J. Tobacco smoking, alcohol consumption and gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2017; 31: 501–508. [DOI] [PubMed] [Google Scholar]
- 27. Shapiro M, Green C, Bautista JM, et al. Assessment of dietary nutrients that influence perception of intra-oesophageal acid reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007; 25: 93–101. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez-Stanley S, Collings KL, Robinson M, et al. The effects of capsaicin on reflux, gastric emptying and dyspepsia. Aliment Pharmacol Ther 2000; 14: 129–134. [DOI] [PubMed] [Google Scholar]
- 29. Chang CH, Wu CP, Wang JD, et al. Alcohol and tea consumption are associated with asymptomatic erosive esophagitis in Taiwanese men. PLoS One 2017; 12: e0173230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nilsson M, Johnsen R, Ye W, et al. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut 2004; 53: 1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berquist WE, Rachelefsky GS, Kadden M, et al. Effect of theophylline on gastroesophageal reflux in normal adults. J Allergy Clin Immunol 1981; 67: 407–411. [DOI] [PubMed] [Google Scholar]
- 32. Rao SS, Mudipalli RS, Mujica V, et al. An open-label trial of theophylline for functional chest pain. Dig Dis Sci 2002; 47: 2763–2768. [DOI] [PubMed] [Google Scholar]
- 33. Gudjonsson H, McAuliffe TL, Kaye MD. The effect of coffee and tea upon lower esophageal sphincteric function. Laeknabladid 1995; 81: 484–488. [PubMed] [Google Scholar]
- 34. Zheng Z, Nordenstedt H, Pedersen NL, et al. Lifestyle factors and risk for symptomatic gastroesophageal reflux in monozygotic twins. Gastroenterology 2007; 132: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim J, Oh SW, Myung SK, et al. Association between coffee intake and gastroesophageal reflux disease: a meta-analysis. Dis Esophagus 2014; 27: 311–317. [DOI] [PubMed] [Google Scholar]
- 36. Wendl B, Pfeiffer A, Pehl C, et al. Effect of decaffeination of coffee or tea on gastro-oesophageal reflux. Aliment Pharmacol Ther 1994; 8: 283–287. [DOI] [PubMed] [Google Scholar]
- 37. Lindam A, Ness-Jensen E, Jansson C, et al. Gastroesophageal reflux and sleep disturbances: a bidirectional association in a population-based cohort study, the HUNT study. Sleep 2016; 39: 1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schey R, Dickman R, Parthasarathy S, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology 2007; 133: 1787–1795. [DOI] [PubMed] [Google Scholar]
- 39. Nunez-Rodriguez MH, Miranda SA. Psychological factors in gastroesophageal reflux disease measured by SCL-90-R questionnaire. Dig Dis Sci 2008; 53: 3071–3075. [DOI] [PubMed] [Google Scholar]
- 40. Ness-Jensen E, Lindam A, Lagergren J, et al. Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2013; 108: 376–382. [DOI] [PubMed] [Google Scholar]
- 41. Ness-Jensen E, Lindam A, Lagergren J, et al. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2014; 109: 171–177. [DOI] [PubMed] [Google Scholar]