Abstract
Background:
Folfirinox (FFX) and gemcitabine/nab-paclitaxel (GN) are both standard first-line treatments in patients with metastatic pancreatic cancer (mPC). However, data comparing these two chemotherapeutic regimens and their sequential use remain scarce.
Methods:
Data from two independent cohorts enrolling patients treated with FFX (n = 107) or GN (n = 109) were retrospectively pooled. Primary endpoint was overall survival (OS). Progression-free survival (PFS) was the secondary endpoint. A propensity score based on age, gender, performance status (PS), and presence of liver metastases was used to make groups comparable.
Results:
In the whole study population, OS was significantly higher in FFX (14 months; 95% CI: 10–21) than in GN groups (9 months; 95% CI: 8–12) before (p = 0.008) and after (p = 0.021) adjusting for age, number of metastatic sites, liver metastases, peritoneal carcinomatosis and CA19.9 level at baseline. PFS tends to be higher in FFX (6 months) than GN groups (5 months; p = 0.053). After matching (n = 49/group), patients were comparable for all baseline characteristics including PS. In the matched population, there was a trend toward greater OS in patients treated with FFX (HR = 0.67; p = 0.097). However, survival in each group was not solely a result of the first-line regimen. The proportion of patients who were fit for GN after FFX failure (FFX–GN sequence) was higher (46.9%) than the reverse sequence (20.4%; p = 0.01), which suggests a higher feasibility for the FFX–GN sequence. Corresponding median OS were 19 months versus 9.5 months, respectively (p = 0.094).
Conclusion:
This study shows greater OS with FFX than with GN in patients with mPC. GN after FFX failure appears more feasible than the reverse sequence.
Keywords: Folfirinox, gemcitabine/nab-paclitaxel, metastatic, pancreatic adenocarcinoma, survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is still associated with poor prognosis, even if significant therapeutic improvements have been observed during the last decade. The folfirinox (FFX) triplet therapy (fluoropyrimidin, oxaliplatin and irinotecan) improves overall survival (OS) compared with gemcitabine and became the standard first-line chemotherapy in 2010 for fit patients with metastatic PDAC (mPDAC). In the PRODIGE4/ACCORD11 trial, median OS was 11.8 months with FFX versus 6.8 months with gemcitabine (p < 0.001).1 Two years later, the phase III trial MPACT showed improvement in OS with the combination gemcitabine/nab-paclitaxel (GN) (8.5 months) compared with gemcitabine alone (6.7 months; p < 0.001).2 Populations of these two pivotal trials were not comparable since patients in the MPACT trial were older and more of them had performance status-2 (PS-2). However, patients with PS-0 (Karnosfky 90–100) were also more common in the MPACT trial than in the PRODIGE trial (58.0% versus 37.4%, respectively), and fewer patients had peritoneal carcinomatosis (4.0% versus 19.4%, respectively).
To date, no randomized clinical trials have been performed to compare these two chemotherapy regimens in the metastatic setting.3 Hence, both FFX and GN are standard first-line therapy for patients with metastatic PDAC. However, safety profiles differ somewhat between FFX and GN. Both induce neuropathy, but patients have more haematologic toxicities with FFX. Comparisons of efficacy and safety profiles between FFX and GN are likely to be useful. There is little data on FFX in patients aged more than 75 years and in those with PS-2. Hence, GN is preferred for these patients. However, nab-paclitaxel is more than 10 times the price of FFX, which limits its prescription in many countries, such as France and the UK. Given all these points, it would be difficult to perform a randomized clinical trial comparing these two chemotherapy regimens in the metastatic setting. The number of patients needed to enrol to demonstrate the noninferiority of GN compared with FFX would be substantial.
Some retrospective studies have already provided preliminary data, but with controversial results.4–14 Limitations of these studies were: inclusion of patients with heterogeneous disease populations (metastatic, locally advanced, recurrence after resection) and lack of comparability between patients in each group (FFX and GN), especially in terms of age and PS. In the absence of prospective randomized controlled trials we propose in the present study to compare survival outcomes of patients treated by first-line FFX or GN before and after matching the two groups with a propensity score.
Methods
Population
Data were prospectively and retrospectively collected from patients’ electronic charts, in agreement with the French authority Commission nationale de l’informatique et des libertés, and in accordance with the Declaration of Helsinki. This study was approved by the local ethical committee (IRBN632017/CHUSTE).
Patients in the GN group came from a previous national multicentric study which enrolled prospective patients with mPDAC consecutively treated with GN as first-line chemotherapy between June 2015 and June 2018. The primary objective was to describe the efficacy and safety of GN as first-line chemotherapy in clinical practice and to describe second-line regimens after GN. Results have not been published but were presented at the 2018 French National Congress of Hepatogastroenterology and Digestive Oncology (‘Journées Francophones d’Hépato-gastroentérologie et d’Oncologie Digestive’ 2018; Abst. P.285).15
For the FFX group the same data collection was performed for all patients with mPDAC treated between 2011 and 2018 in two French centres (University Hospital of Saint-Etienne and European Georges-Pompidou’s Hospital, Paris). In both groups, second-line therapy was left to the clinician’s discretion and according to the patient’s PS. There was no exclusion criterion.
Chemotherapy regimens
FFX is a triplet chemotherapy associating oxaliplatin (85 mg/m2), irinotecan (150–180 mg/m2), leucovorin (400 mg/m2) and 5 fluorouracil (5FU) (bolus: 0–400 mg/m2; intravenous infusion: 2400 mg/m2 for 46–48 h). GN is a doublet chemotherapy combining gemcitabine (1000 mg/m2) with nab-paclitaxel (125 mg/m2), administered weekly for 3 consecutive weeks.
Chemotherapy-related toxicities were managed by dosage reduction (by 20–25%) of one or more drugs according to the clinician’s discretion: the bolus of 5FU was often dropped at baseline or decreased/stopped later due to haematologic toxicity and/or fatigue. GN was very exceptionally switched to an every 2 weeks regimen. UGT1A1 was not used for irinotecan dose adaptation. Chemotherapy-related toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE Version 4.0).
Statistical analyses
The primary objective was to compare the OS obtained with FFX and GN before and after matching the two groups with a propensity score based on age, gender, PS and presence of liver metastasis. The secondary objective was PFS. OS was defined as the time from the start of chemotherapy to the date of death or last follow up. PFS was defined as the time from the start of chemotherapy to the date of disease progression, death or last follow up. Disease progression was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Patients alive without progression were censored at the date of last follow up.
Survivals were estimated using the Kaplan–Meier method and compared with the log-rank test. Prognostic factors were identified using Cox proportional hazards regression by univariate analyses performed in the whole study population and the matched population. Multivariate analyses were performed only in the whole study population. The factors included in multivariate analyses were variables associated with a p value < 0.1 in univariate analyses, in addition to age, PS, carcinomatosis, liver metastases and CA19.9 level at baseline. Qualitative variables were reported as numbers and percentages, and compared by using chi-squared or Fisher’s exact test, as appropriate. Percentages of ordinal variables were compared using the Cochrane–Armitage test. Quantitative variables were reported as median value with their interquartile ranges from 25% to 75%, and compared using the Mann–Whitney Wilcoxon test. Some of those were additionally reported as mean value and corresponding standard deviation (SD).
A propensity score was computed for each patient, based on age, gender, PS and presence of liver metastasis. As previously described,16 a matching 1:1 using the nearest-neighbour distance of the propensity score (calliper: 0.1) was carried out to select two groups of patients with balanced characteristics. Qualitative (McNemar’s test) and quantitative data (pairwise Wilcoxon’s test) were used adequately to compare these two matched groups.
All statistical analyses were performed using R, version 3.2.2 (R project, Auckland, New Zealand). Propensity score generation and matching were performed by using the R package MatchIt.17
Results
Whole study population
For the GN group, 109 patients treated with mPDAC between June 2015 and June 2018 were included. A total of 107 patients treated with FFX between 2011 and 2018 were included in the FFX group.
In Table 1, all patients characteristics and disease are reported for FFX and GN groups. Patients were different regarding mean age (62 versus 68 years, respectively; p < 0.001), performance status (PS-2: 4.7% versus 29.4%; p < 0.001) and median bilirubin level [8.7 µmol/l (5–15) versus 12 µmol/l (7–18); p = 0.003]. The number of alcohol consumers was lower in the FFX group (6.5%) compared with the GN group (14.7%; p = 0.09). The proportion of men was numerically higher in the FFX group (59.8% versus 49.5%; p = 0.17). However, there was no difference regarding primary tumour location, number of metastatic sites and serum albumin levels. Even if the median CA19.9 level at baseline appeared numerically superior in the FFX group (2951 µg/l; IQR: 322–11,560) compared with the GN group (1229 µg/l; IQR: 130–12,000), value distribution was not statistically different between the two groups (p = 0.38). The median number of folfirinox courses was 6 (IQR: 3–11). The corresponding number of GN courses was 10 (IQR: 5–18).
Table 1.
Characteristics of the population study and tumour before and after matching on propensity score.
| Whole study population |
Matched population |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 216) | Folfirinox (n = 107) | Gemcitabine/nab-paclitaxel (n = 109) | p value | Total (n = 98) | Folfirinox (n = 49) | Gemcitabine/nab-paclitaxel (n = 49) | Adjusted p value | |
| Females | 98 (45.4%) | 43 (40.2%) | 55 (50.5%) | 0.17 | 47 (48%) | 25 (51%) | 22 (44.9%) | 0.71* |
| Age, mean (±SD) | 64.9 (±9.6) | 61.8 (±8.5) | 68.1 (±9.6) | <0.001 | 64.3 (±9.8) | 63.9 (±9.1) | 64.8 (±10.6) | 0.46** |
| Age, median (IQR) | 66 (58–72) | 63 (55–69) | 70 (62–75) | <0.001 | 66 (58–71) | 67 (58– 71) | 66 (59–71) | 0.15** |
| Diabetes | 38 (17.6%) | 16 (15%) | 22 (19.8%) | 0.41 | 18 (18.4%) | 12 (24.5%) | 7 (14.3%) | 0.36* |
| Alcohol addiction | 23 (10.7%) | 7 (6.5%) | 16 (14.7%) | 0.09 | 7 (7.1%) | 2 (4.1%) | 6 (12.2%) | 0.29* |
| Performance status at baseline | <0.001 | 1** | ||||||
| 0 | 47 (21.8%) | 30 (28%) | 17 (15.6%) | 26 (26.5%) | 13 (26.5%) | 13 (26.5%) | ||
| 1 | 115 (53.2%) | 71 (66.4%) | 44 (40.4%) | 62 (63.3%) | 31 (63.3%) | 31 (63.3%) | ||
| 2 | 37 (17.1%) | 5 (4.7%) | 32 (29.4%) | 10 (10.2%) | 5 (10.2%) | 5 (10.2%) | ||
| Tumour location | 0.79 | 0.69* | ||||||
| Head | 98 (45.4%) | 47 (43.9%) | 51 (46.8%) | 43 (43.9%) | 21 (42.9%) | 22 (44.9%) | ||
| Body | 54 (25%) | 26 (24.3%) | 28 (25.7%) | 29 (29.6%) | 14 (28.6%) | 15 (30.6%) | ||
| Tail | 64 (29.6%) | 34 (31.8%) | 30 (27.5%) | 26 (26.5%) | 14 (28.6%) | 12 (24.5%) | ||
| Number of metastatic sites | 0.56 | 0.51** | ||||||
| 1 | 1 (0.5%) | 1 (0.9%) | 0 (0%) | 61 (62.2%) | 28 (57.1%) | 33 (67.3%) | ||
| 2 | 142 (65.7%) | 64 (59.8%) | 78 (71.6%) | 32 (32.7%) | 19 (38.8%) | 13 (26.5%) | ||
| 3 | 58 (26.9%) | 36 (33.6%) | 22 (20.2%) | 5 (5.1%) | 2 (4.1%) | 3 (6.1%) | ||
| Peritoneal carcinomatosis | 63 (29.2%) | 33 (30.8%) | 30 (27.5%) | 0.70 | 30 (30.6%) | 16 (32.7%) | 14 (28.6%) | 0.84* |
| Liver metastases | 159 (73.6%) | 81 (75.7%) | 78 (71.6%) | 0.59 | 78 (79.6%) | 39 (79.6%) | 39 (79.6%) | 1* |
| Lung metastases | 39 (18.1%) | 17 (15.9%) | 22 (20.2%) | 0.52 | 16 (16.3%) | 7 (14.3%) | 9 (18.4%) | 0.79* |
| Lymph node metastases | 41 (19%) | 23 (21.5%) | 18 (16.5%) | 0.45 | 14 (14.3%) | 9 (18.4%) | 5 (10.2%) | 0.34* |
| Bone metastases | 8 (3.7%) | 4 (3.7%) | 4 (3.7%) | 1 | 3 (3.1%) | 2 (4.1%) | 1 (2%) | 1* |
| CA19.9, median (IQR) | 1471 (184.2–11,820) | 2951 (322–11,560) | 1229 (130–12,000) | 0.38 | 1670 (340–18,600) | 2938 (583.8–20,000) | 1148 (76.2–13690) | 0.37** |
| Albumin, mean (SD) | 34.7 (±6.4) | 34.4 (±6.5) | 34.9 (±6.4) | 0.65 | 34.6 (±7.1) | 34.1 (±6.2) | 34.9 (±7.7) | 0.49** |
| Albumin, median (IQR) | 35.7 (31.4–39.1) | 34.5 (31–38) | 36 (31.6–39.2) | 0.54 | 36 (31.3– 39.3) | 33.8 (30.9–38) | 36.1 (32.1–39.9) | 0.46** |
| Bilirubin, median (IQR) | 10.2 (6–15.1) | 8.7 (5–15) | 12 (7–18) | 0.003 | 10 (5.3–15) | 8 (5–15) | 12 (6–18) | 0.17** |
| Chemotherapy regimen | ||||||||
| Median duration months (IQR) | – | 3.2 months (1.4–8.3) | 3.2 months (1.2–8.6) | – | – | 4.0 months (1.8–8.3) | 3.5 months (2.1–6.6) | – |
| Median number of courses of chemotherapy | – | 5FU: 6 (3–12) irinotecan: 6 (3–11) oxaliplatin: 5 (3–9) |
GEM: 10 (5–18) Nab-P: 8 (4–15) |
– | – | 5FU: 8 (4–15) irinotecan: 6 (4–11) oxaliplatin: 6 (4–9) |
GEM: 10 (5–20) Nab-P: 8 (4–18) |
– |
McNemar test; **Wilcoxon signed-rank.
5FU, 5 fluorouracil; CT, chemotherapy; GEM, gemcitabine; Nab-P, nab-paclitaxel.
Survivals in the whole study population
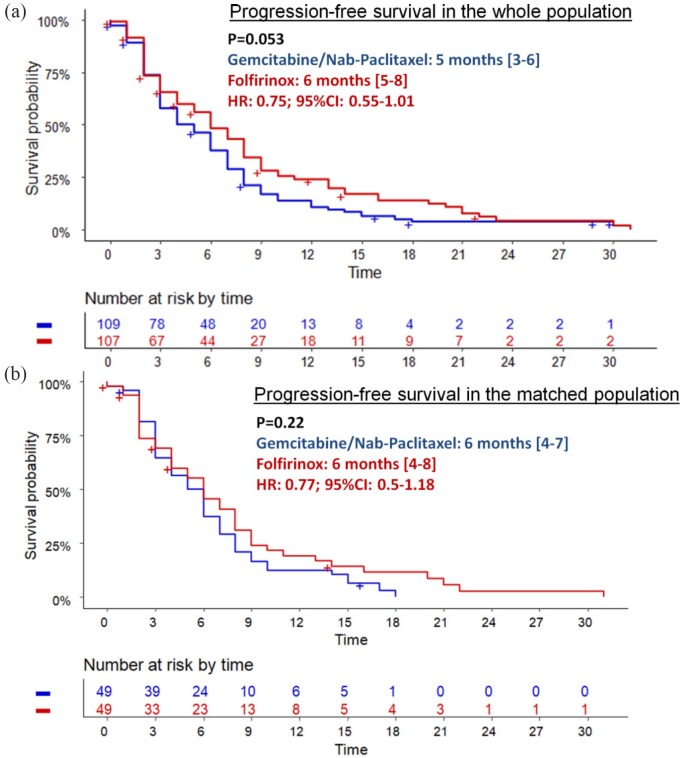
During the follow-up period, 81 (75.7%) and 99 (90.8%) patients experienced disease progression in the FFX and GN groups, respectively. PFS was better with FFX (median: 6.0 months; 95% CI: 5.0–8.0) compared with GN (median: 5.0 months; 95% CI: 3.0–6.0), but statistical significance was not reached (HR = 0.75; 95% CI: 0.55–1.01; p = 0.053) (Figure 1a). Proportions of patients who underwent second-line therapy were statistically higher in the FFX group (72.0%) versus the GN group (57.8%; p = 0.042) (Table 2). The regimen and numbers of subsequent therapies are reported in Table 2. The sequence FFX followed by GN (FFX–GN) was feasible in a higher proportion (43.0%) than the reverse sequence (GN–FFX) (12.8%; p < 0.001).
Figure 1.
Progression-free survival in whole (a) and matched (b) populations according the chemotherapy regimen.
CI, confidence interval; HR, hazard ratio.
Table 2.
Treatments beyond the first-line chemotherapy.
| Whole study population |
Matched population |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 216) | Folfirinox (n = 107) | Gemcitabine/ nab-paclitaxel (n = 109) | p value | Total (n = 98) | Folfirinox (n = 49) | Gemcitabine/nab-paclitaxel (n = 49) | Adjusted p value | |
| Second-line chemotherapy | 140 (64.8%) | 77 (72%) | 63 (57.8%) | 0.042 | 68 (69.4%) | 35 (71.4%) | 33 (67.3%) | 0.82 |
| LV5FU2 | 1 | 0 | 1 | – | 1 | 0 | 1 | – |
| 5FU-cisplatin | 4 | 2 | 2 | – | 3 | 1 | 2 | – |
| Folfox | 26 | 0 | 26 | – | 11 | 0 | 11 | – |
| Folfiri | 12 | 0 | 12 | – | 6 | 0 | 6 | – |
| Gemzar | 24 | 24 | 0 | – | 7 | 7 | 0 | – |
| Gemox | 1 | 0 | 1 | – | 0 | 0 | 0 | – |
| Folfiri3 | 12 | 5 | 7 | – | 7 | 4 | 3 | – |
| Folfirinox | 14 | 0 | 14 | – | 10 | 0 | 10 | – |
| Gemcitabine/ nab-paclitaxel | 46 | 46 | 0 | – | 23 | 23 | 0 | – |
| Sequence folfirinox–gemcitabine/nab-paclitaxel versus reverse sequence | 60 (27.8%) | 46 (43%) | 14 (12.8%) | <0.001 | 33 (33.7%) | 23 (46.9%) | 10 (20.4%) | 0.009* |
| Third-line chemotherapy | 42 (19.4%) | 24 (22.4%) | 18 (16.5%) | 0.35 | 21 (21.4%) | 12 (24.5%) | 9 (18.4%) | 0.62* |
McNemar test.
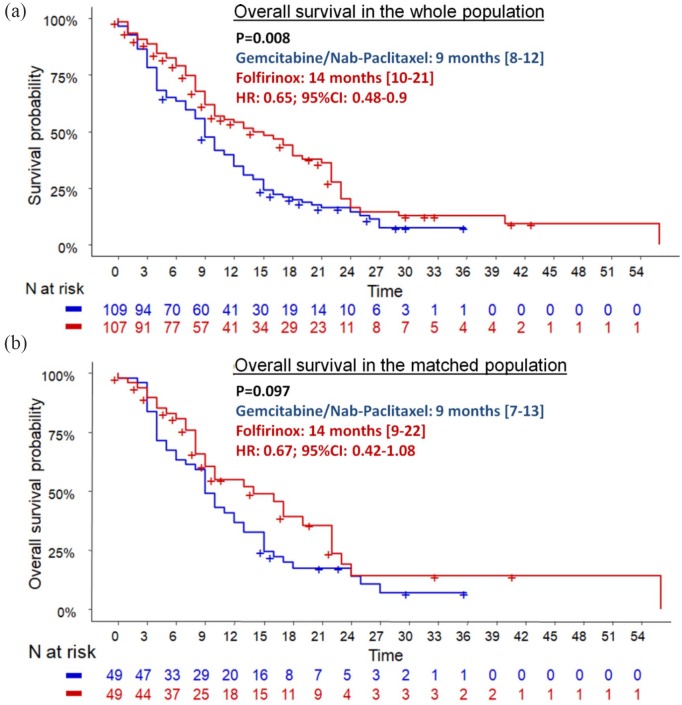
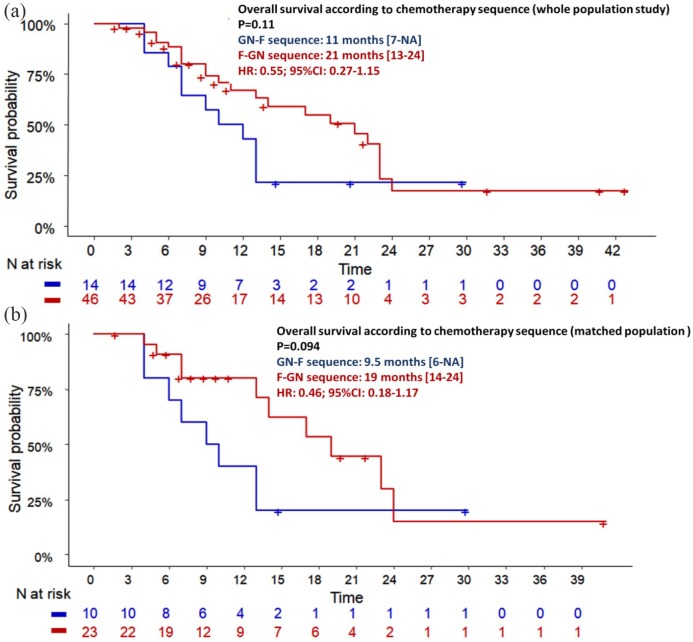
During the follow-up period, 69 (64.5%) and 93 (85.3%) patients died in the FFX and GN groups, respectively. OS was statistically better in the FFX group (median: 14.0 months; 95% CI: 10.0–21.0) compared with the GN group (median: 9.0 months; 95% CI: 8.0–12.0), leading to a reduction in mortality rates by 35% (HR = 0.65; 95% CI: 0.48–0.90; p = 0.008) (Figure 2a). After adjustment for age, PS, CA19.9 level, presence of liver metastases, peritoneal carcinomatosis and number of metastatic sites, FFX was still independently associated with OS (HR = 0.62: 95% CI: 0.41–0.93; p = 0.021). In addition, CA19.9 level (p = 0.045), liver metastasis (HR = 2.28; 95% CI: 1.31–3.97; p = 0.004) and peritoneal carcinomatosis (HR = 1.7; 95% CI: 1.05–2.74; p = 0.031) were independent prognosis factors (Table 3). Patients who underwent the FFX–GN sequence (FFX as first-line and GN as second-line therapy) tended to have a better OS (21 months) than patients who underwent the reverse sequence (11 months; p = 0.11) (Figure 3a).
Figure 2.
Overall survival in folfirinox and gemcitabine/nab-paclitaxel groups before and after matching on propensity score.
CI, confidence interval; HR, hazard ratio.
Table 3.
Univariate and multivariate analyses for overall survival in the whole study population.
| Variables | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|
| p value | HR (95% CI) | p value | HR (95% CI) | |
| Men | 0.42 | 1.14 (0.83–1.55) | ||
| Age | 0.26 | 1.01 (0.99–1.03) | 0.67 | 1 (0.98–1.02) |
| Diabetes | 0.56 | 0.55 (0.07–4.07) | ||
| Alcohol addiction | 0.75 | 0.72 (0.1–5.42) | ||
| Performance status | ||||
| 2 versus 0–1 | 0.07 | 1.56 (0.96–2.56) | ||
| 0 versus 1–2 | 0.06 | 1.47 (0.98–2.22) | 0.7 | 0.92 (0.59–1.42) |
| Tumour location | ||||
| Head (ref) | 1 | 1 | ||
| Body | 0.42 | 0.85 (0.57–1.27) | ||
| Tail | 0.79 | 1.05 (0.74–1.5) | ||
| Number of metastatic sites | 0.045 | 1.25 (1.01–1.55) | 0.51 | 1.1 (0.83–1.47) |
| Peritoneal carcinomatosis | 0.08 | 1.36 (0.97–1.9) | 0.031 | 1.7 (1.05–2.74) |
| Liver metastases | 0.009 | 1.64 (1.13–2.39) | 0.004 | 2.28 (1.31–3.97) |
| Pulmonary metastases | 0.25 | 0.79 (0.53–1.18) | ||
| Lymph node metastases | 0.62 | 0.91 (0.61–1.34) | ||
| Bone metastases | 0.66 | 1.18 (0.55–2.53) | ||
| CA19.9 at baseline | 0.026 | 1 (1–1) | 0.045 | 1 (1–1) |
| Albumin | 0.08 | 0.97 (0.95–1) | ||
| Bilirubin | 0.13 | 1 (1–1.01) | ||
| Folfirinox versus gemcitabine/nab-paclitaxel | 0.008 | 0.65 (0.48–0.90) | 0.021 | 0.62 (0.41– 0.93) |
CI, confidence interval; HR, hazard ratio.
Figure 3.
Overall survival according the sequence folfirinox followed by gemcitabine/nab-paclitaxel versus the reverse sequence in the whole and matched population.
CI, confidence interval; HR, hazard ratio.
Matched population
After matching, the two groups (n = 49/group) were comparable for all characteristics (Table 1), including mean age (63.9 years ± 9.1 versus 64.8 years ± 10.6; p = 0.46), baseline PS (PS-0/1/2: 26.5%/63.3%/10.2%), median bilirubin levels [8 µmol/l (5–15) versus 12 µmol/l (6–18); p = 0.13] and median propensity scores (Supplementary Figure 1). Almost half of the population was male (52%). The tumour was located in the pancreatic head in 43% and there was only one metastatic site in about two-thirds (62.2%) of cases, including the liver (79.6%). Median albumin was 36 g/l (IQR: 31.3–39.3).
During the follow-up period, 42 (85.7%) and 46 (93.9%) patients experienced disease progression in the FFX and GN groups, respectively. PFS was higher in the FFX versus GN group (HR = 0.77 95% CI: 0.5–1.18; p = 0.22) (Figure 1b). Rates of patients who underwent second-line chemotherapy were not statistically different between the two groups: 71.4% versus 67.3% (p = 0.82). However, the rate of patients who underwent the sequence FFX–GN was higher (46.9%) than for the reverse sequence (20.4%; p = 0.009).
During the follow-up period, 31 (63.3%) and 42 (85.7%) patients died in the FFX and GN groups, respectively. A nonsignificant trend toward better OS was observed in patients treated with FFX (14.0 months; 95% CI: 9.0–22.0) compared with the GN group (9.0 months; 95% CI: 7.0–13.0; HR = 0.67; 95% CI: 0.42–1.08; p log-rank = 0.097) (Figure 2b). Moreover, median OS was higher in patients who underwent the FFX–GN sequence (n = 23) than those who underwent the reverse sequence (n = 10): 19 months versus 9.5 months (HR = 0.46; 95% CI: 0.18–1.17; p = 0.094) (Figure 3b). Only CA19.9 level was significantly correlated with mortality (p = 0.002). Patients with PS-2 had poorer prognosis than those with PS-0 (HR = 2.22; p = 0.06) (Table 4).
Table 4.
Univariate analyses for overall survival in the matched population.
| Univariate analyses |
||
|---|---|---|
| Variables | p value | HR (95% CI) |
| Men | 0.19 | 1.36 (0.86–2.16) |
| Age | 0.56 | 1.01 (0.98–1.03) |
| Performance status | ||
| 1 versus 0 | 0.89 | 1.04 (0.61–1.76) |
| 2 versus 0 | 0.06 | 2.22 (0.97–5.1) |
| Tumour location | ||
| Head (ref) | 1 | 1 |
| Body | 0.07 | 0.59 (0.34–1.05) |
| Tail | 0.85 | 0.95 (0.55–1.65) |
| Number of metastatic sites >1 | 0.65 | 1.13 (0.67–1.89) |
| Peritoneal carcinomatosis | 0.76 | 1.08 (0.66–1.79) |
| Liver metastases | 0.07 | 1.76 (0.95–3.28) |
| Pulmonary metastases | 0.2 | 0.65 (0.33–1.26) |
| Lymph node metastases | 0.5 | 1.25 (0.66–2.38) |
| Bone metastases | 0.85 | 1.12 (0.35–3.57) |
| CA19.9 at baseline | 0.002 | 1 (1–1) |
| Albumin | 0.4 | 0.98 (0.95–1.02) |
| Bilirubin | 0.43 | 1 (1–1.01) |
| Folfirinox versus gemcitabine/nab-paclitaxel | 0.099 | 0.67 (0.42–1.08) |
CI, confidence interval; HR, hazard ratio.
Discussion
The present study reports survival outcomes with FFX (14 months) and GN (9 months) that are in accordance with respective pivotal phase III trials.1,2 In the whole study population, characteristics of patients, especially age and PS, were statistically different between FFX and GN groups, which correspond to real life situations and reflect the criteria on which decisions are usually made for recognizing patients fit for FFX rather than GN, or vice versa. This is a major point to underline because PS and age have been clearly recognized as important predictors of survival in metastatic pancreatic cancer.18 However, after adjustment for age, PS, number of metastatic sites, liver metastases, carcinomatosis and CA19.9 level, FFX was still independently associated with improved OS (HR = 0.62; 95% CI: 0.41–0.93; p log-rank = 0.021). Moreover, and interestingly, we have provided for the first time a matched-pair comparison between patients treated with FFX or GN, to minimize potential bias in the selection of patients. For that, we used a propensity score based on age, gender, PS and liver metastases, which allowed for a balance of characteristics at baseline between FFX and GN groups. However, this method reduced the size of the population (n = 49/group) and so the statistical power of the analysis, probably explaining why we showed only a trend to improvement of OS with FFX compared with GN in the matched analyses (HR = 0.67; 95% CI: 0.42–1.08; p log-rank = 0.097).
To our knowledge, the present study is the first to compare FFX with GN as first-line chemotherapy in mPDAC by using a propensity score method to make groups comparable. In previous retrospective studies, survival results have been conflicting. Several retrospective studies included locally advanced tumours in addition to mPDAC.11–14 However, in 2009 a consensus statement on pancreatic cancer treatment recommended studying separately patients with locally advanced tumours and metastatic disease.19 Four studies compared survival between FFX and GN in the metastatic setting only5,6,8,9 (Supplementary Table 1). Three of these showed no difference in terms of PFS and OS.5,8,9 Only one Korean large retrospective study showed better OS with GN (11.4 months versus 9.6 months; p = 0.002) without statistical difference in terms of PFS (median: 6.8 months versus 5.1 months, respectively). Disease progression was the most common reason for treatment discontinuation (74.5% versus 67.4%, respectively), and rates of second-line chemotherapy were significantly higher in FFX (80.1%) than GN groups (67.2%, p = 0.02) in this study. However, only 2/117 (1.7%) patients underwent GN after FFX failure. Indeed, most of the patients were treated with gemcitabine only as second-line therapy. In contrast, after GN failure, a majority of patients underwent oxaliplatin-based doublet chemotherapy, suggesting the crucial role of the therapeutic sequence in OS in mPDAC. In our study, there was a higher rate of second-line therapy after FFX failure (72%) than after GN (57.8%; p = 0.042). However, this difference fades after matching the study population: 71.4% versus 67.3% (p = 0.82). Despite this, an intensive second-line therapy such as the combination of GN remained more frequently used after FFX failure (46.9%) than the reverse sequence (FFX after GN failure: 20.4%; p < 0.01) in the matched population, which may explain improved OS observed in the FFX group. This is a major point to consider because it suggests that survival in each group is likely not solely the result of the initial regimen in our study, and that FFX first-line therapy followed by GN as second-line therapy is the most feasible sequence in clinical practice in patients able to tolerate this, although neither of these two therapeutic sequences have been validated or recommended. The feasibility of this sequence was already demonstrated in a French prospective study.20 In the present study, patients who underwent this sequence (n = 46) tended to have better OS (21 months) than patients who underwent the reverse sequence (n = 14; 11 months; p = 0.11) in the whole study population. These outcomes were confirmed in the matched population: 19 months versus 9.5 months (p = 0.094). Only a randomized clinical trial could confirm these results, which are only hypothesis-generating; however, there is no industrial support to conduct this trial.
Similarly, PFS seemed better with FFX than GN in the whole study population (6 months versus 5 months; p log-rank = 0.053). A larger population is likely needed to demonstrate such a small difference with statistical significance. Hence, the present study was not designed to compare PFS in the whole and matched population. Despite the fact that it is commonly admitted that PFS reflects the effectiveness of an oncological treatment, OS is recommended as the primary endpoint for phase II and phase III clinical trials for pancreatic cancer because of the relatively short survival time in the metastatic setting.19
Another aspect which should be taken into account in the selection of the first-line chemotherapy regimen is the cost of treatment. Indeed, in systems such as that used in France, nab-paclitaxel is not yet reimbursed and is more expensive than FFX even when adding the cost of a GCSF, often combined with FFX for the prevention of febrile neutropenia. The design of the present study was not appropriate to evaluate the economic aspects of these treatments, though others have done so.21–23 In addition, rates and modalities of reimbursement of these chemotherapy regimens can vary considerably across countries.
The main limit of our study is its retrospective nature and potential confounders in the whole study population. However, rates of missing data are low and propensity-score-based analyses should theoretically reduce these biases, except maybe the bias related to the centre effect or bias related to dose of frequency modifications of chemotherapy regimens. Indeed, the FFX population is bicentric while the GN population is multicentric (n = 11). However, the inclusion of centres as a covariate in the propensity score would lead to significantly reduced population size (<20 patients/group) and so the statistical power of the analysis. Randomized clinical trials comparing these two chemotherapy regimens are currently being completed, but only in the locally advanced setting24–26 or for resectable tumours.27,28 To the best of the authors’ knowledge, there has been no clinical trial comparing these two chemotherapy regimens in mPDAC. Our study hence provides new interesting data supporting the current daily practice in France, and could serve to provide a statistical hypothesis for design of further randomized clinical trials comparing these two chemotherapy regimens as first-line treatment in mPDAC.
Another limit of our study is the lack of sufficiently accurate data regarding chemotherapy-related toxicity. Indeed, we have estimated that the retrospective nature of the study is a substantial limit for such an evaluation. Even so, we reported these data in Supplementary Figure 2. Despite grade 3–4 toxicity rates appearing lower than in pivotal randomized clinical trials, there was no major difference between the two groups except for fatigue being more frequently associated with FFX (19%) than with GN (1.1%; p < 0.001).
In conclusion, our study supports the superiority of FFX over GN in terms of OS in patients with mPDAC, maybe because it allows using GN as second-line therapy more frequently than the reverse sequence. However, further randomized clinical trials are needed to confirm these data.
Supplementary Material
Footnotes
Author contributions: NW performed data collection, drafting the manuscript, interpretation of data, conception of the study, study supervision, statistical analyses. AS, ALP, SP, DB, IT, NL, VH, CL, JD, ATB and BLR performed data collection. DT performed data collection, interpretation of data and critical review of the manuscript. DP and JMP performed interpretation of data and critical review of the manuscript. JT performed conception of the study, study supervision, interpretation of data and critical review of the manuscript.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval and consent to participate: This study was approved by the local ethical committee (IRBN632017/CHUSTE). Patients were informed before being enrolled in this study by general information made available in a poster in departments of the university hospital. Comité d’Ethique du CHU de Saint-Etienne; Commission recherche de Terre d’éthique; comite.ethique@chu-st-etienne.fr; Dr Pascale Vassal; pascale.vassal@chu-st-etienne.fr; Institutional Review Board: IORG0007394.
Data availability: The data that support the findings of this study are available from Nicolas Williet, but restrictions apply regarding their availability, as they were used under licence for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of Julien Taieb and the AGEO (‘Association des Gastroentérologues et Oncologues’).
ORCID iD: Nicolas Williet  https://orcid.org/0000-0002-7296-5464
https://orcid.org/0000-0002-7296-5464
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nicolas Williet, Hepatogastroenterology Department, University Hospital of Saint-Etienne, Avenue Albert Raimond, Saint-Etienne 42270, France; EA 7425 HESPER, Health Services and Performance Research, Claude Bernard Lyon 1 University, Lyon, France.
Angélique Saint, Department of Medical Oncology, Antoine Lacassagne Center, Nice, France.
Anne-Laure Pointet, Department of Gastroenterology and Gastro-intestinal Oncology, Hôpital Européen Georges-Pompidou, APHP, Paris Descartes University, Sorbonne Paris Cité, Paris, France.
David Tougeron, Department of Gastroenterology, Poitiers University Hospital, Poitiers, France.
Simon Pernot, Department of GI Oncology, Hôpital Européen Georges-Pompidou, APHP, Paris Descartes University, Sorbonne Paris Cité, Paris, France.
Astrid Pozet, Methodology and Quality of Life in Oncology Unit (INSERM UMR 1098), University Hospital of Besançon, Besançon, France.
Dominique Bechade, Department of Medical oncology, Bergonié Institut, Bordeaux, France.
Isabelle Trouilloud, Department of Medical Oncology, Saint-Antoine Hospital, Paris, France.
Nelson Lourenco, Gastroenterology Unit, Saint-Louis Teaching Hospital, Paris, France.
Vincent Hautefeuille, Department of Gastroenterology, Amiens-Picardie University Hospital, Amiens, France.
Christophe Locher, Department of Gastroenterology, CH Meaux, Meaux, France.
Jérome Desrame, Department of Oncology, Hôpital Privé Jean Mermoz, Lyon, France.
Pascal Artru, Department of Oncology, Hôpital Privé Jean Mermoz, Lyon, France.
Anne Thirot Bidault, Department of Gastroenterology, Hôpital Kremlin Bicêtre, Le Kremlin-Bicêtre, Paris, France.
Bertrand Le Roy, Department of Digestive and Hepatobiliary Surgery, University Hospital of Saint-Etienne, Saint-Priest-en-Jarez, France.
Denis Pezet, Department of Digestive and Hepatobiliary Surgery, University Hospital of Clermont-Ferrand, Clermont-Ferrand, France.
Jean-Marc Phelip, Department of Hepatogastroenterology, University Hospital of Saint-Etienne, Saint-Etienne, France; EA 7425 HESPER, Health Services and Performance Research, Claude Bernard Lyon 1 University, Lyon, France.
Julien Taieb, Department of Gastroenterology and Gastro-intestinal Oncology, Hôpital Européen Georges-Pompidou, APHP, Paris Descartes University, Sorbonne Paris Cité, Paris, France.
References
- 1. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 2. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ClinicalTrials.gov. Search of: metastatic AND nab-paclitaxel | Pancreatic Cancer | Phase 3 – List Results [Internet], https://clinicaltrials.gov (accessed 10 November 2018).
- 4. Peixoto RD, Ho M, Renouf DJ, et al. Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol 2017; 40: 507–511. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Camateros P, Cheung WY. A real-world comparison of FOLFIRINOX, gemcitabine plus nab-paclitaxel, and gemcitabine in advanced pancreatic cancers. J Gastrointest Cancer. Epub ahead of print 16 November 2017. DOI: 10.1007/s12029-017-0028-5. [DOI] [PubMed] [Google Scholar]
- 6. Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 7. Tahara J, Shimizu K, Otsuka N, et al. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 2018; 82: 245–250. [DOI] [PubMed] [Google Scholar]
- 8. Kim S, Signorovitch JE, Yang H, et al. Comparative effectiveness of nab-paclitaxel plus gemcitabine vs FOLFIRINOX in metastatic pancreatic cancer: a retrospective nationwide chart review in the United States. Adv Ther. Epub ahead of print 12 September 2018. DOI: 10.1007/s12325-018-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cartwright TH, Parisi M, Espirito JL, et al. Clinical outcomes with first-line chemotherapy in a large retrospective study of patients with metastatic pancreatic cancer treated in a US community oncology setting. Drugs: Real World Outcomes 2018; 5: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegewisch-Becker S, Aldaoud A, Wolf T, et al. Results from the prospective German TPK clinical cohort study: treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer 2019; 144: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Javed MA, Beyer G, Le N, et al. Impact of intensified chemotherapy in metastatic pancreatic ductal adenocarcinoma (PDAC) in clinical routine in Europe. Pancreatology 2019; 19: 97–104. [DOI] [PubMed] [Google Scholar]
- 12. Papneja N, Zaidi A, Chalchal H, et al. Comparisons of outcomes of real-world patients with advanced pancreatic cancer treated with FOLFIRINOX versus gemcitabine and nab-paclitaxel: a population-based cohort study. Pancreas. Epub ahead of print 7 June 2019. DOI: 10.1097/MPA.0000000000001340. [DOI] [PubMed] [Google Scholar]
- 13. Rochefort P, Lardy-Cleaud A, Sarabi M, et al. Long-term survivors in metastatic pancreatic ductal adenocarcinoma: a retrospective and matched pair analysis. Oncologist. Epub ahead of print 4 June 2019. DOI: 10.1634/theoncologist.2018-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gbolahan OB, Tong Y, Sehdev A, et al. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer 2019; 19: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pointet AL, Tougeron D, Pernot S, et al. JFHOD 2018. P.285 – nab-paclitaxel plus gemcitabine en première ligne de cancers pancréatiques avancés chez 138 patients : résultats d’une étude AGEO en conditions ‘de vie réelle’, https://www.snfge.org/content/nab-paclitaxel-plus-gemcitabine-en-premiere-ligne-de-cancers-pancreatiques-avances-chez-138 (accessed 18 December 2018).
- 16. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho DE, Imai K, King G, et al. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42: 1–28. [Google Scholar]
- 18. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015; 20: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 2009; 27: 5660–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015; 113: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gharaibeh M, McBride A, Bootman JL, et al. Economic evaluation for the US of nab-paclitaxel plus gemcitabine versus FOLFIRINOX versus gemcitabine in the treatment of metastatic pancreas cancer. J Med Econ 2017; 20: 345–352. [DOI] [PubMed] [Google Scholar]
- 22. McBride A, Bonafede M, Cai Q, et al. Comparison of treatment patterns and economic outcomes among metastatic pancreatic cancer patients initiated on nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Expert Rev Clin Pharmacol 2017; 10: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 23. Gharaibeh M, McBride A, Alberts DS, et al. Economic evaluation for the UK of systemic chemotherapies as first-line treatment of metastatic pancreatic cancer. Pharmacoeconomics 2018; 36: 1333–1343. [DOI] [PubMed] [Google Scholar]
- 24. Mizusawa J, Fukutomi A, Katayama H, et al. Protocol digest of randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer: Japan clinical oncology group study (JCOG1407). Pancreatology 2018; 18: 841–845. [DOI] [PubMed] [Google Scholar]
- 25. ClinicalTrials.gov. Folfirinox or gemcitabine-nab paclitaxel followed by stereotactic body radiotherapy for locally advanced pancreatic cancer [Internet], https://clinicaltrials.gov/ct2/show/NCT03600623 (2018, accessed 18 December 2018).
- 26. ClinicalTrials.gov. A dose escalation trial of SBRT after induction chemotherapy for locally advanced pancreatic cancer [Internet], https://clinicaltrials.gov/ct2/show/NCT02873598 (2016, accessed 18 December 2018).
- 27. ClinicalTrials.gov. Phase II study of preoperative FOLFIRINOX versus gemcitabine/nab-paclitaxel in patients with resectable pancreatic cancer [Internet], https://clinicaltrials.gov/ct2/show/NCT02243007 (2014, accessed 18 December 2018).
- 28. ClinicalTrials.gov. Neoadjuvant FOLFIRINOX or nab-paclitaxel with gemcitabine for borderline resectable pancreatic cancer [Internet], https://clinicaltrials.gov/ct2/show/NCT02717091 (2016, accessed 18 December 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.