Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) has become prevalent in recent decades, especially in developed countries, and approaches for the prevention and treatment of NAFLD are not clear. The aim of this research was to analyze and summarize randomized controlled trials that investigated the effects of probiotics on NAFLD.
Methods:
Seven databases (PubMed, Embase, the Web of Science, the Cochrane Library, China National Knowledge Infrastructure, Wan Fang Data, and VIP Database) were searched. Then, eligible studies were identified. Finally, proper data extraction, synthesis and analysis were performed by trained researchers.
Results:
Anthropometric parameters: with use of probiotics weight was reduced by 2.31 kg, and body mass index (BMI) was reduced by 1.08 kg/m2. Liver function: probiotic treatment reduced the alanine aminotransferase level by 7.22 U/l, the aspartate aminotransferase level by 7.22 U/l, the alkaline phosphatase level by 25.87 U/l, and the glutamyl transpeptidase level by −5.76 U/l. Lipid profiles: total cholesterol, low-density lipoprotein cholesterol, and triglycerides were significantly decreased after probiotic treatment. Their overall effects (shown as standard mean difference) were −0.73, −0.54, and −0.36, respectively. Plasma glucose: probiotics reduced the plasma glucose level by 4.45 mg/dl and the insulin level by 0.63. Cytokines: probiotic treatment decreased tumor necrosis factor alpha by 0.62 and leptin by 1.14. Degree of liver fat infiltration (DFI): the related risk of probiotics for restoring DFI was 2.47 (95% confidence interval, 1.61–3.81, p < 0.001).
Conclusion:
Probiotic treatment or supplementation is a promising therapeutic method for NAFLD.
Keywords: NAFLD, Probiotics, Anthropometric parameters, Liver function, Lipid profiles, Plasma glucose, Cytokines, Degree of liver fat infiltration, Meta-analysis
Introduction
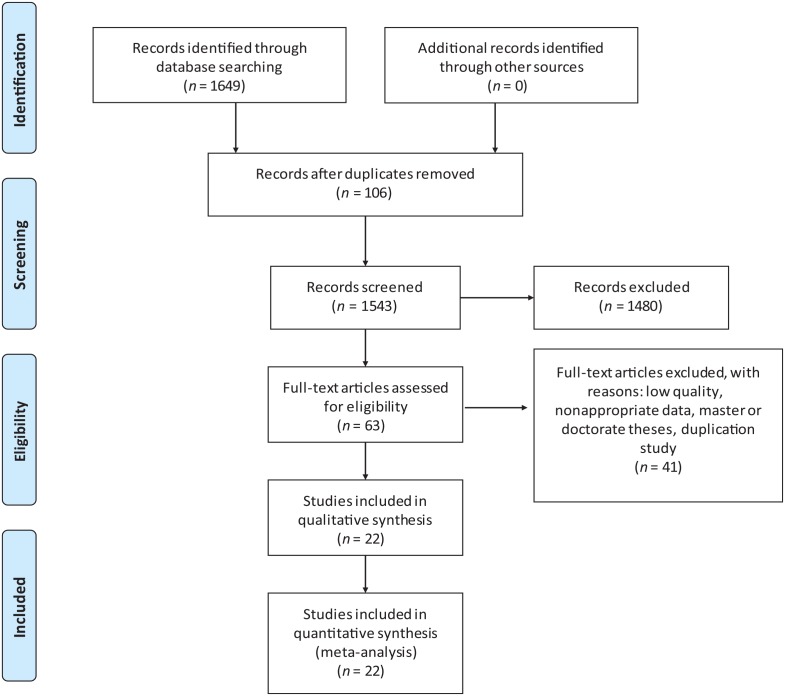
Nonalcoholic fatty liver disease (NAFLD) is a hepatic disease in which an abnormal amount of fat accumulates in hepatic cells. It occurs when lipid metabolism disorders are present. Hence, it usually occurs together with obesity, which is accompanied by dyslipidemia. However, not all obese patients develop NAFLD, and many lean patients exhibit dyslipidemia and NAFLD.1–3 As there is increasing prevalence of fried food in the diet and overnutrition, and as physical labor has become less frequent, the morbidity of dyslipidemia has risen in recent decades, as has that of NAFLD, especially in developed countries. Short-term (3 weeks) hypercaloric diet could induce tenfold greater relative fat accumulation in the liver (27%) than in body weight (2%) and de novo lipogenesis is seen to play a role in the pathogenesis of NAFLD.4 The current consensus is that hypercaloric/western diet is associated with onset of NAFLD.5,6 Many NAFLD models were constituted by hypercaloric/western diet.7–9 Gabbia and colleagues10 found western diet changed hepatic lipid metabolism and the circulation bile acids in rats, which could represent an early marker of NAFLD development. The global prevalence of NAFLD is approximately 25.2%11 and NAFLD causes huge social and economic burdens. It affects approximately 64 million people in the USA and 52 million in Europe and costs US$1613 per patient in the USA and 1163€ per patient in Europe. Moreover, the expected 10-year burden of NAFLD is estimated to increase tenfold in the USA and Europe.2 In China, the prevalence of NAFLD is lower than that in developed countries, but has still reached epidemic proportions in 2013.12 Its prevalence has continued to increase over the last several years. While hepatic cirrhosis, led by chronic viral hepatitis, accounts for the main proportion of liver transplantations, nonalcoholic steatohepatitis (NASH) is the second leading etiology of liver transplantation.13 Given the high prevalence and very large burden of NAFLD, many studies have investigated its prevention and treatment, but the exact pathogenesis and efficient treatment of NAFLD are not fully elucidated. NAFLD is a spectrum disease with multifactorial etiology. The so-called ‘two-hit’ and ‘multiple-hit’ hypotheses both emphasize its multifactorial etiology. The ‘two-hit’ hypothesis was first raised by Day and colleagues14 in 1998 and evolved over time.15 The ‘two-hit’ hypothesis assumes that the ‘second hit’ activates inflammatory cascades and fibrogenesis in the context of obesity, insulin resistance (IR), hepatic steatosis and oxidative stress. NAFLD is advanced by chronic inflammation, caused by complex factors such as inflammatory activity, lipotoxicity, and the recruitment of Kupffer cells. Elena Buzzetti and colleagues16 deemed NASH a ‘multiple-hit’ disease and determined that multiple parallel insults and processes contribute to NAFLD. The multiple-hit hypothesis can more reasonably explain the results from clinical practice, such as ‘lean NAFLD’. ‘Lean NAFLD’ patients have different clinical characteristics (no obesity and no IR) from most NAFLD patients and are not in the setting of obesity or IR. Furthermore, Yilmaz17 performed a review to discuss simple hepatic steatosis and NASH. He believed simple steatosis and NASH to be two independent diseases rather than two stages of NAFLD. NASH can be the initial liver lesion. However, the conclusion requires more clinical trials, animal trials, and liver histological biopsies. It will be very difficult to distinguish whether steatosis causes hepatitis or whether hepatitis causes steatosis because steatosis and hepatitis exist in the same liver, and the early profile of the patient is usually unavailable. The clinician always tries to seek a series of treatments to intervene and cure NAFLD.18–25 Sridharan and colleagues26 summarized the most common treatment for NAFLD and performed a meta-analysis that concluded that elafibranor, gemfibrozil, metadoxine, obeticholic acid, pentoxifylline, pioglitazone, probiotics, telmisartan, vildagliptin, and vitamin E significantly increased the response rate compared with that of standard care. Probiotics are defined as live microbial dietary supplements that benefit the host animal by improving its intestinal microbial balance. In recent years, the effect of probiotics on NAFLD has raised concern, and many clinical randomized controlled trials (RCTs) have been performed to verify the treatment effect. These trials have focused on aminotransferase, alkaline phosphatase (ALP), anthropometric indices, lipid profiles, glycemic profiles, and inflammatory factors. For NAFLD patients, the therapeutic endpoint is not only the improvement of biochemical markers but also the alleviation of fat accumulation in the liver which was not mentioned in recent research. In addition, results and conclusions have not been consistent. Most trials have concluded that probiotics and body mass index (BMI) are efficient and, at least in part, beneficial biochemical criteria. Considering the uncompleted and contradictory results from clinical RCTs, we performed this systematic review and meta-analysis to further discuss the effect of probiotics on NAFLD. We analyzed the changes in anthropometric parameters, liver function, lipid profiles, plasma glucose profiles, degree of liver fat infiltration (DFI) and cytokines to evaluate the treatment efficiency of probiotics for NAFLD. This systematic review with meta-analysis was registered on PROSPERO [www.crd.york.ac.uk, ID: CRD42019128193]. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow chart is presented in Figure 1.
Figure 1.
PRISMA flow diagram.
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
Methods
This systematic review and meta-analysis was conducted according to the PRISMA guidelines27 to guarantee the reliability and integrality of the data and conclusion.
Inclusion and exclusion criteria
The included clinical studies met the following criteria: the clinical trials were RCTs; the participants were NAFLD patients without a limitation in age, sex, or race; the intervention was probiotics; the treatment of the controlled group was the same as that of the intervention group except for the intervention method; the outcomes, including DFI, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (γ-GT), albumin (ALB), ALP, BMI, waist circumference (WC), waist-to-hip ratio (WHR), weight, fat mass (FM), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), glucose (Glu), homeostatic model assessment of IR (HOMA-IR), insulin, C-reactive protein (CRP), leptin, adiponectin (Ad), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α), were associated with NAFLD; and the article was published in a peer-reviewed journal or magazine without language or date limitations. The exclusion criteria included the following: patients with alcoholic fatty liver disease; liver transplantation patients; probiotic transplant patients; conference or abstract; dissertation for a master’s or doctorate degree; outcomes were presented as the mean difference or interquartile range and the original data could not be acquired; the risk of bias of all papers was assessed by the Cochrane Collaboration’s tool;28 and if more than three unclear risks or at least one high risk existed.
Database resource
The English databases searched included PubMed, Embase, the Cochrane Library, and the Web of Science; the Chinese databases searched included the China National Knowledge Infrastructure (CNKI), Wan Fang Data, and VIP Database. The full texts of the searched papers can be acquired by Chongqing Medical University Library or upon request from the authors.
Search strategy
We searched the databases with free terms, subject heading terms and key words. Probiotics were searched with ‘probiotics’, ‘yogurt’, ‘yoghurt’, ‘lactic acid bacterium’, ‘lactobacillus’, ‘Bifidobacterium’, ‘enterococcus’, ‘streptococcus’, ‘saccharomyces’, ‘Lactococcus’, ‘microbiome’, ‘microbiota’, ‘synbiotics’, and ‘prebiotics’; fatty liver was searched with ‘Liver, Fatty’, ‘Steatohepatitis’, ‘Steatohepatitides’, ‘Steatosis of Liver’, ‘Visceral Steatosis’, ‘Steatoses, Visceral’, ‘Steatosis, Visceral’, ‘Visceral Steatoses’, ‘Liver Steatosis’, ‘Liver Steatoses’, ‘Steatoses, Liver’, ‘Steatosis, Liver’, and ‘fatty liver’; and the search strategy for RCTs was guided by the Cochrane Handbook for Systematic Reviews of Interventions (2009) and published strategy studies.29–33 All synonymous words were united with the logic operator ‘OR’; probiotics, fatty liver and RCTs were united with the logic operator ‘AND’. The search fields were limited to title or abstract. A reference search was not performed.
Study selection
Three researchers (Y Tang, WY Zhang, and J Huang) performed the study search and selection. All three researchers were trained before the database search to ensure that they shared the same opinions and were informed of the inclusion and exclusion criteria. WY Zhang and J Huang searched the databases and screened the studies independently. They first screened the studies by title and abstract, and the full texts of the screened studies were then scrutinized for further assessment according to the inclusion and exclusion criteria. Finally, the screened full-texts were compared by Y Tang. Divergence and discrepancies were resolved by negotiation and voting among the three researchers.
Data extraction
The extracted tables were prepared and consisted of six sublists: the liver function table included ALT, AST, ALP, ALB, and γ-GT; the anthropometric parameters table included WC, WHR, BMI, weight, and FM; the plasma Glu table included Glu, insulin, HOMA-IR, and glycated hemoglobin A1c (HbA1c); the plasma lipid table included HDL-C, LDL-C, TG, and TC; the cytokines table included leptin, adiponectin, CRP, TNF-α, and IL-6; and the DFI was used to count the number of patients whose liver fat infiltration was relieved after treatment with probiotics. Before data extraction, the meaning of every parameter was fully explained to the three researchers (Y Tang, WY Zhang, and J Huang) to maintain the same criteria in the process of data extraction. WY Zhang and J Huang extracted the data independently. The extracted data were assessed by Y Tang, and any divergence or discrepancy was discussed among the three researchers. In addition, data extraction was conducted according by each study, not by article (i.e. the data obtained from different articles on the same study were extracted only once; if the data between the two articles differed, the data from the most recent article were adopted).
Data synthesis and risk-of-bias assessment
DFI is expressed as the risk ratio, whereas the other parameters are expressed as the mean difference. I2 statistics were used to assess the magnitude of heterogeneity, and the significance level was set as 0.1. The fixed-effects model was used only when the p value exceeded 0.1 and when the I2 value was less than 50%; otherwise, the random-effects model was used. The sensitivity analysis and subgroup analysis would be performed to define the source of heterogeneity. The risk of bias of each study was assessed by the same three researchers who performed data extraction with the Cochrane Collaboration’s tool. The study was excluded if one high-risk bias or more than three unclear biases existed. Publication bias was assessed by Egger’s test. The statistical procedures were performed by Stata/SE 15.1 (StataCorp LLC Texas, USA). Any referenced statistic adopted a two-sided test, and the significance level was set as 0.05, except for that of the heterogeneity test, which was set as 0.1.
Results
Study selection
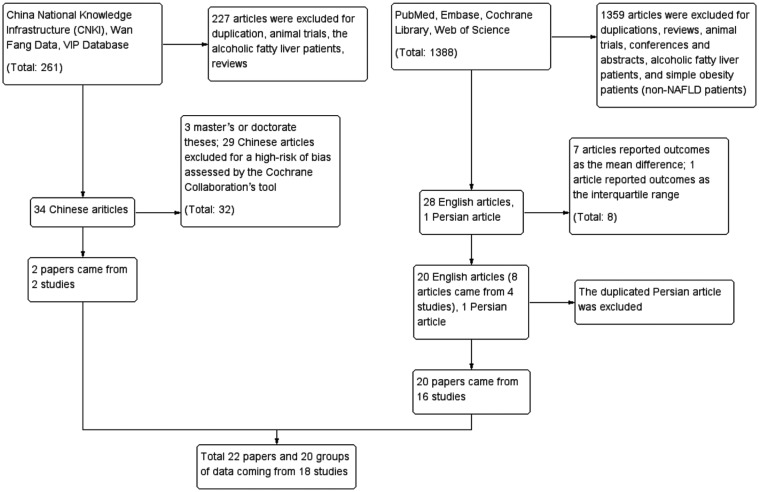
We searched the databases until 8 April 2019. A total of 1649 articles were retrieved, including 261 Chinese articles, 1387 English articles and 1 Persian article. The Persian article34 was the same study performed by Nabavi and colleagues.35,36 A total of 1585 articles were excluded after reading the title and abstract. Reasons for exclusion included duplications, reviews, animal trials, conferences and abstracts, alcoholic fatty liver patients, and simple obesity patients (non-NAFLD patients). There were 64 papers whose full texts were assessed. The 64 papers consisted of 34 Chinese papers, 28 English papers and 1 Persian paper.34 After scrutinizing the full texts, 22 papers were ultimately included in the meta-analysis. The excluded papers included three Chinese master or doctorate theses, 29 low-quality Chinese papers that were assessed by the Cochrane Collaboration’s tool in which a high-risk bias or more than three unclear risk biases existed, seven English papers that reported outcomes as the mean difference,1,37–42 and one English paper43 that reported outcomes as the interquartile range. Asgharian and colleagues,44,45 Ekhlasi and colleagues,46,47 and Javadi and colleagues48,49 published two articles on one study. Nabavi and colleagues34–36 published three articles on one study, and the Persian article34 did not report any different parameters; thus, the Persian article34 was regarded as a duplication. The studies of Javadi and colleagues48 and Ekhlasi and colleagues46,47 consisted of four groups. Every set of two groups was used to investigate the efficiency of probiotics independently; hence, both studies generated two groups of independent data. Finally, the meta-analysis included 22 papers from 18 studies that generated 20 groups of data. The flow chart is displayed in Figure 2.
Figure 2.
Study selection flow.
NAFLD, nonalcoholic fattly liver disease.
Study characteristics and risk-of-bias assessment
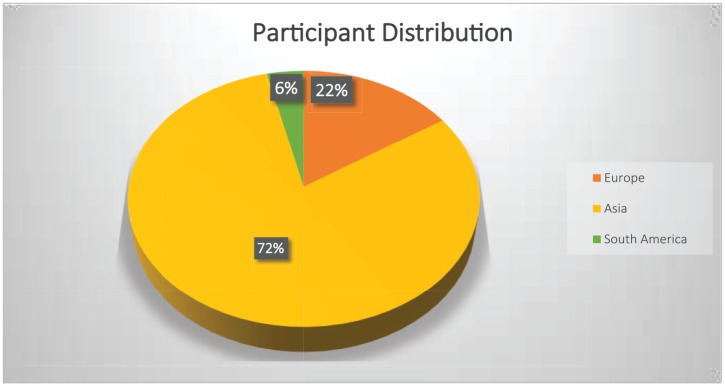
Table 135,36,44–63 summarizes the characteristics of the included studies. All included clinical studies were designed according to the Declaration of Helsinki. They were all approved by the Ethics Committees or Institutional Review Boards they belonged to. The written informed consent was obtained from all participants which was stated in each included study. Each study is noted by the author and publication year. If multiple articles existed for one study, the study is noted by the author and each publication year (e.g. ‘Nabavi_2014_15’ indicates that Nabavi and colleagues published two articles, with one study each in 2014 and 2015). All the included studies were performed between 2011 and 2019 and involved a total of 1356 NAFLD patients. Two studies52,55 investigated children (age 11.8 ± 2.2 years, M/F = 56/52, total 108 NAFLD patients), and 16 studies investigated adults (age 43.9 ± 12.4 years, M/F = 749/499, total 1248 NAFLD patients). The treatment period ranged from 4 weeks to 24 weeks, and the interquartile range of the treatment period was 12 weeks (8–16). The mean treatment period was 13.1 weeks. Multistrain probiotic intervention accounted for 77.8% (14 studies). The efficiency of single-strain probiotics was observed only in 4 studies.56,57,62,63 All the included subjects came from three continents (Europe: 213, Asia: 1093, and South America: 50, Figure 3). All the included studies concluded that probiotics are somewhat beneficial in treating NAFLD, but different investigators had different opinions on the exact benefit of probiotic treatment. The different studies usually observed at least one beneficial parameter, but some studies reported the benefits of probiotics within, but not between, groups.
Table 1.
Characteristics of the included studies.
| Study | Trial type | Duration (weeks) | Probiotics | Country | Age (E; C) | M (F) (E; C) |
Sample size E (C) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Sepideh et al.61 | RCT-DB | 8 | Lactobacillus, Bifidobacterium, Streptococcus | Iran | 42.10 ± 1.99; 47.33 ± 2.53 |
13 (8); 15 (6) |
21 (21) | Glu, insulin, HbA1c, HOMA-IR, TNF-α, IL-6 |
| Shavakhi et al.50 | RCT-DB | 24 | Lactobacillus, Bifidobacterium, Streptococcus | Iran | 41.5 ± 12.7; 38.7 ± 11.9 |
17 (14); 15 (17) |
31 (32) | DFI, BMI, TC, TG, Glu, ALT, AST |
| Ahn et al.51 | RCT-DB | 12 | Lactobacillus, Pediococcus, Bifidobacterium | Korea | 41.7 ± 12.5; 44.7 ± 13.3 |
15 (15); 18 (17) |
30 (35) | WHR |
| Alisi et al.52 | RCT-DB | 16 | Streptococcus, Bifidobacterium, Lactobacillus | Italy | 11 (10,12); 10 (9,12) |
10 (12); 14 (8) |
22 (22) | DFI, BMI, TG, ALT, HOMA-IR |
| Aller et al.59 | RCT-DB | 12 | Lactobacillus, Streptococcus | Spain | 49.4 ± 10.9; 44.3 ± 15.1 |
10 (4); 10 (4) |
14 (14) | WHR, FM, weight, BMI, TC, LDL-C, HDL-C, TG, Glu, insulin, HOMA-IR, TNF-α, IL-6 |
| Asgharian et al.44,45 | RCT-DB | 8 | Lactobacillus, Bifidobacterium, Streptococcus | Iran | 46.57 ± 10.5; 47.78 ± 10.2 |
7 (31); 12 (34) |
38 (36) | DFI, WC, WHR, FM, weight, BMI, ALT, AST, CRP |
| Manzhalii et al.58 | RCT-OL | 12 | Lactobacillus, Bifidobacterium, Streptococcus | Germany | 44.3 ± 1.5; 43.5 ± 1.3 |
11 (27); 16 (21) |
38 (37) | BMI, TC, TG, Glu, ALT, AST, γ-GT |
| Bakhshimoghaddam et al.53 | RCT-OL | 24 | Streptococcus, Lactobacillus, Bifidobacterium | Iran | 39.4 ± 9.88; 41.1 ± 8.5 | 33 (35) versus 17 (17) | 68 (34) | DFI |
| Famouri et al.55 | RCT-TB | 12 | Lactobacillus, Bifidobacterium | Iran | 12.7 ± 2.2; 12.6 ± 1.7 |
14 (18); 18 (14) | 32 (32) | DFI, WC, TC, LDL-C, HDL-C, TG, ALT, AST |
| Ekhlasi et al.46,47 | RCT-DB | 8 | Streptococcus, Lactobacillus, Bifidobacterium | Iran | 44 ± 20 | 48 (12) | 15 (15); 15 (15) | WC, weight, BMI, TC, LDL-C, HDL-C, TG, Glu, insulin, AST, ALT, ALP, HOMA-IR, TNF-α, leptin |
| Javadi et al.48,49 | RCT-DB | 12 | Bifidobacterium, Lactobacillus | Iran | 43.90 ± 9.0; 42.21 ± 9.1 |
17 (3) versus 13 (6); 14 (3) versus 16 (3) | 20 (19); 17 (19) | DFI, WHR, weight, BMI, TC, LDL-C, HDL-C, TG, Glu, insulin, ALT, AST, ALP, γ-GT, ALB, HOMA-IR |
| Dengyi et al.62 | RCT | 12 | Bacillus | China | 45.76 ± 4.25 | 40 (38) | 39 (39) | DFI, TC, HDL-C, LDL-C, TG |
| Malaguarnera et al.57 | RCT-DB | 24 | Bifidobacterium | Italy | 46.9 ± 5.4; 46.7 ± 5.7 | 18 (16); 15 (17) |
34 (32) | BMI, TC, LDL-C, HDL-C, TG, Glu, insulin, ALT, AST, ALB, HOMA-IR, CRP, TNF-α |
| Nabavi et al.35,36 | RCT-DB | 8 | Bifidobacterium, Lactobacillus | Iran | 42.75 ± 8.72; 44.05 ± 8.14 |
19 (17); 18 (18) |
36 (36) | DFI, WC, weight, BMI, TC, LDL-C, HDL-C, TG, Glu, insulin |
| Sayari et al.60 | RCT | 16 | Lactobacillus, Bifidobacterium, Streptococcus | Iran | 42.48 ± 11.41; 43.42 ± 11.65 |
45 (25); 38 (30) |
70 (68) | weight, BMI, TC, LDL-C, HDL-C, TG, Glu, ALT, AST |
| Ferolla et al.56 | RCT | 12 | Lactobacillus | Brazil | 57.3 (25,74) | 12 (38) | 27 (23) | DFI, WC, weight, BMI, TC, LDL-C, HDL-C |
| Behrouz et al.54 | RCT-DB | 12 | Lactobacillus, Bifidobacterium | Iran | 38.46 ± 7.11; 38.43 ± 10.09 |
22 (8) versus 21 (9) | 30 (30) | Weight, BMI, Glu, insulin, HOMA-IR, leptin, Ad |
| Wang et al.63 | RCT | 4 | Bifidobacterium, Bacillus, Enterococcus | China | 42.9 ± 5; 44.3 ± 4.7 |
94 (56); 34 (16) |
150 (50) | DFI, TC, LDL-C, HDL-C, TG, Glu, ALT, AST, TNF-α, Ad |
Ad, adinopectin; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; C, control group; CRP, C-reactive protein; DB, double-blind; DFI, degree of liver fat infiltration; E, experimental group; F, female; FM, fat mass; γ-GT, gamma glutamyl transpeptidase; Glu, glucose; HbA1c, glycated hemoglobin A1c; HDL-C, ; HOMA-IR, homeostatic model assessment of insulin resistance; IL-6, interleukin 6; LDL-C, ; M, male; OL, open label; RCT, randomized controlled trial; TB, triple blind; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor alpha; WC, waist circumference; WHR, waist-to-hip ratio.
Age is expressed as the mean ± SD or the median (interquartile range).
Figure 3.
Participant distribution.
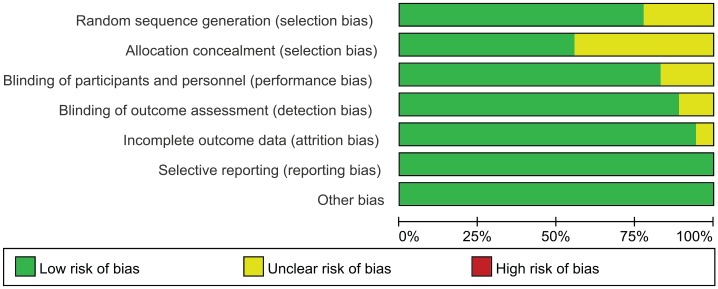
The risk-of-bias assessment is displayed in Figures 4 and 5. According to the Cochrane Collaboration’s tool,28 there were 8 studies (44.5%) in which a bias did not exist; two studies (11.1%) had three unclear biases; four studies (22.2%) had two unclear biases; and four studies (22.2%) had one unclear bias. Nonspecific allocation concealment was the main risk of bias, followed by nonspecific random sequence generation. Nearly all studies reported lost data or patients lost to follow up when necessary.
Figure 4.
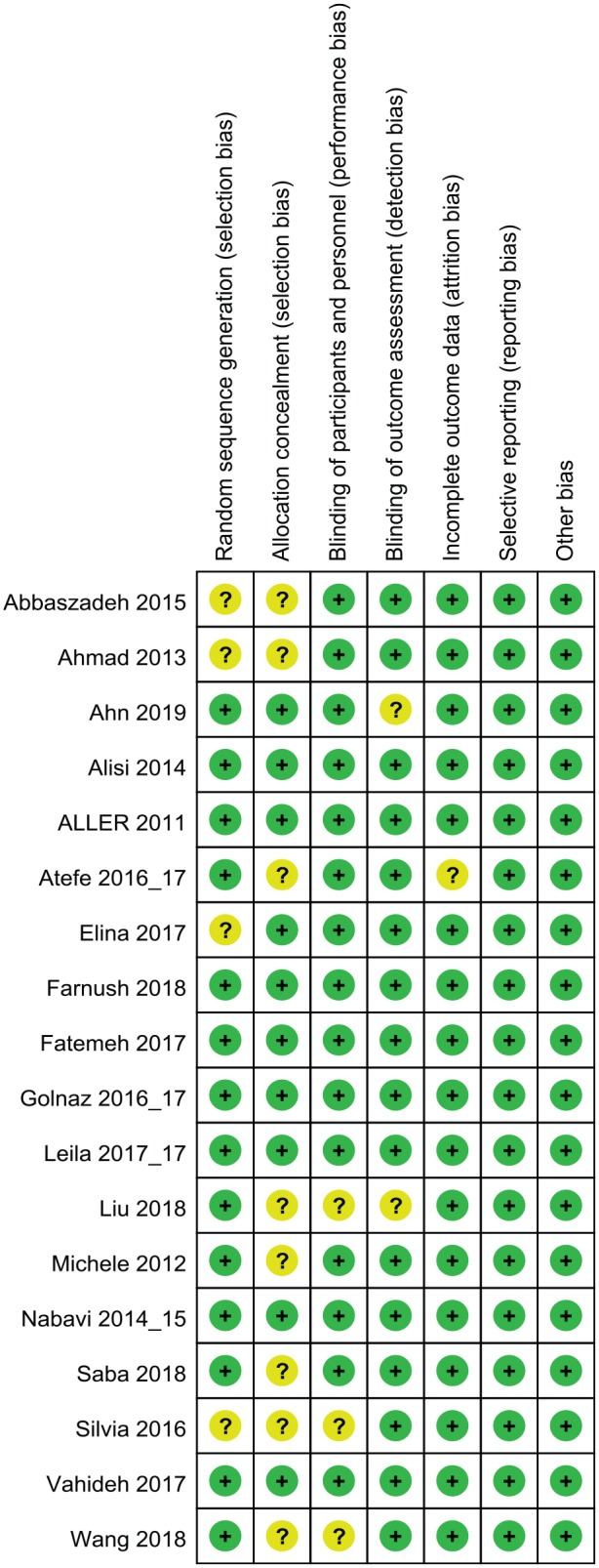
Risk-of-bias graph.
Figure 5.
Risk-of-bias summary.
The effect of probiotics on anthropometric parameters
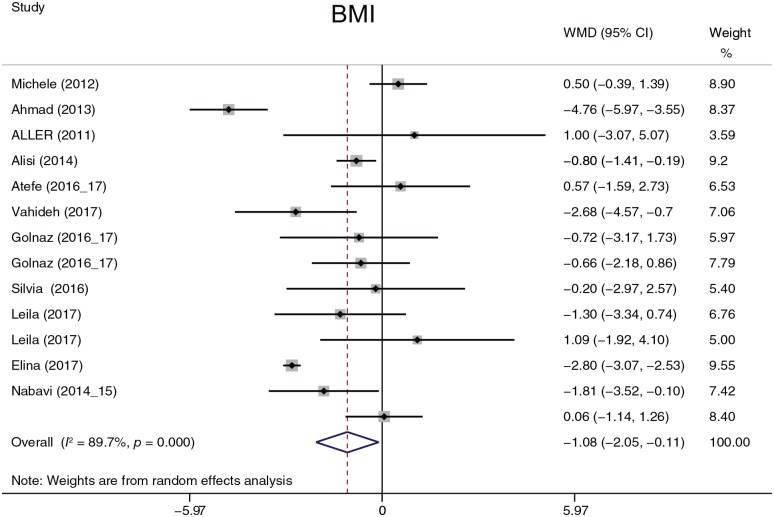
The analyzed anthropometric parameters consisted of WC, WHR, weight (Figure 6), BMI (Figure 7), and FM. FM was analyzed in only two studies. Except for BMI (I2 = 89.7%, p = 0.000), heterogeneity was not detected between studies. BMI was summarized by a random-effects model, and WC, WHR, weight, and FM were summarized by a fixed-effects model. Probiotic treatment reduced BMI by 1.08 [95% confidence interval (CI), −2.05 to −0.11, p = 0.030] and weight by 2.31 kg (95% CI, −4.45 to −0.16, p = 0.035). There were no significant differences in WC, WHR, FM. A subgroup analysis was performed on BMI. When BMI was evaluated by continent, the heterogeneity did not decrease; when evaluated by treatment duration time, the heterogeneity decreased only at 16 weeks (I2 = 36.3%, p = 0.210). A sensitivity analysis was used to find heterogeneity between studies, but the omission of any study did not seriously affect the outcomes.
Figure 6.
Weight.
WMD, weighted mean difference; CI, confidence interval.
Figure 7.
Body mass index.
BMI, body mass index; CI, confidence interval; WMD, weighted mean difference.
The effect of probiotics on liver function
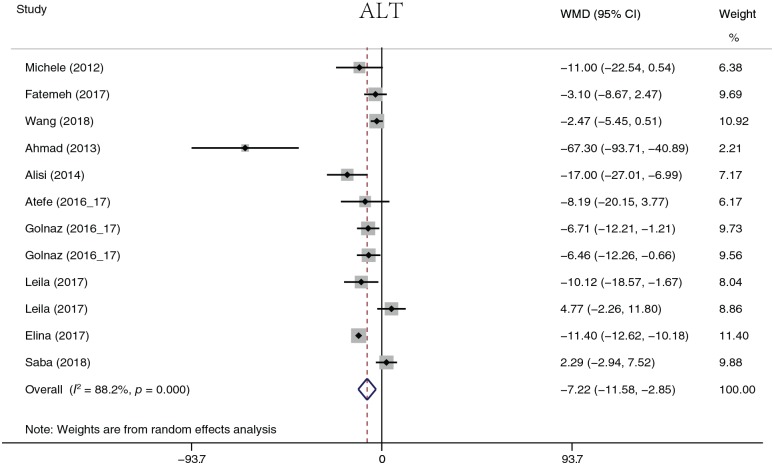
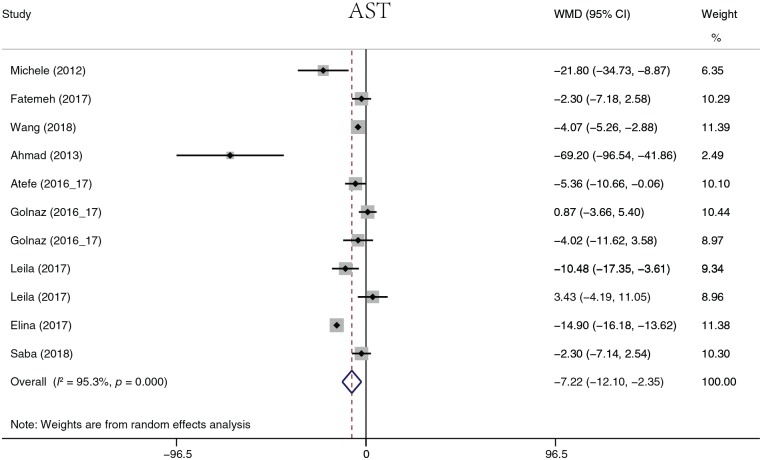
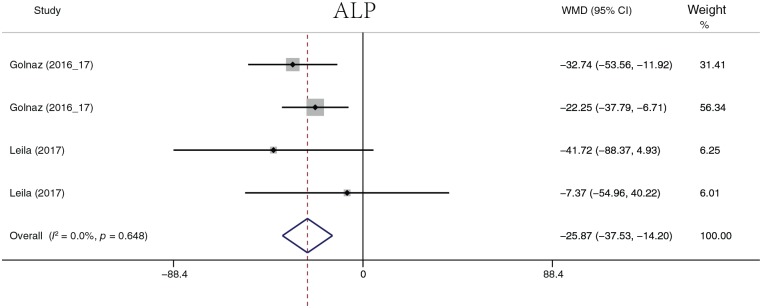
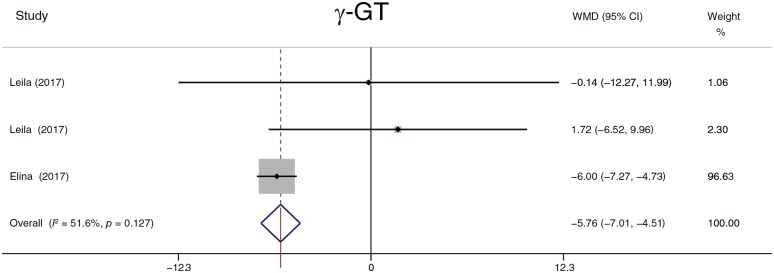
The analyzed liver function parameters included ALT, AST, ALP, ALB, and γ-GT. Probiotic treatment reduced the ALT level (Figure 8) by 7.22 U/l (95% CI, −11.58 to −2.85, p = 0.001), the AST level (Figure 9) by 7.22 U/l (95% CI, 12.10 to −2.35, p = 0.004), the ALP level (Figure 10) by 25.87 U/l (95% CI, −37.53 to −14.20, p < 0.001), and the γ-GT level (Figure 11) by −5.76 U/l (95% CI, −7.01 to −4.51, p < 0.001). It should be noted that ALP was summarized by four groups of data from two studies, and γ-GT was summarized by three groups of data from two studies. There was no significant difference in ALB (p = 0.109). Heterogeneity in AST (I2 = 88.2%, p < 0.001) and ALT (I2 = 95.3%, p < 0.001) levels was detected. A sensitivity analysis was performed on ALT and AST levels to determine heterogeneity between studies. The overall effect did not change significantly after omitting any study. When ALT data were sorted by treatment duration time, heterogeneity (I2 = 0.0%, p = 0.968) was nearly eliminated in the 8-week group, but significant heterogeneity still existed in the other groups. When evaluated by continent, there was no heterogeneity in the Europe group (I2 = 0.0%, p = 0.551), but heterogeneity still existed in the Asia group (I2 = 79%, p < 0.001). The heterogeneity analysis of AST yielded similar results.
Figure 8.
Alanine aminotransferase.
ALT, alanine aminotransferase; CI, confidence interval; WMD, weighted mean difference.
Figure 9.
Aspartate aminotransferase.
AST, aspartate aminotransferase; CI, confidence interval; WMD, weighted mean difference.
Figure 10.
Alkaline phosphatase.
ALP, alkaline phosphatase; CI, confidence interval; WMD, weighted mean difference.
Figure 11.
γ-glutamyl transpeptidase.
γ-GT, γ-glutamyl transpeptidase; CI, confidence interval; WMD, weighted mean difference.
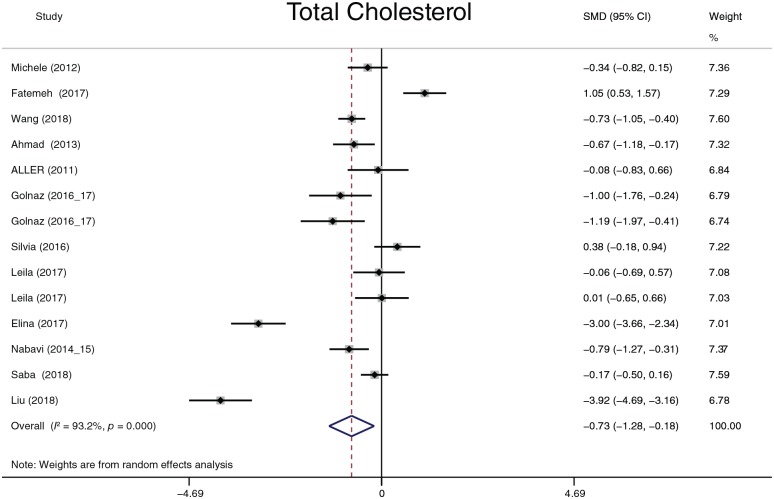
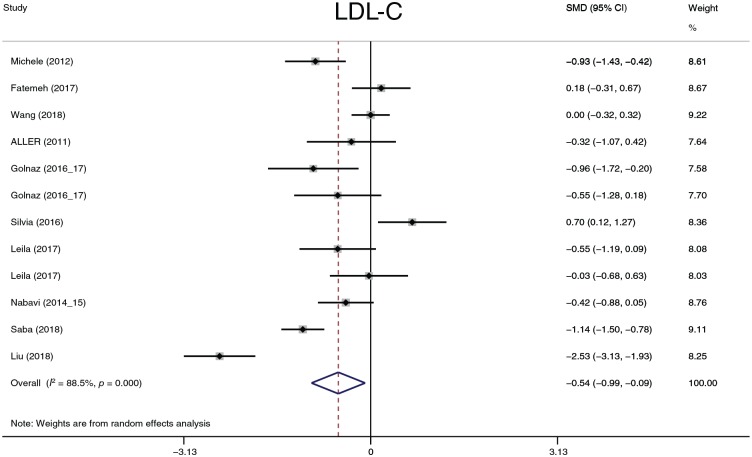
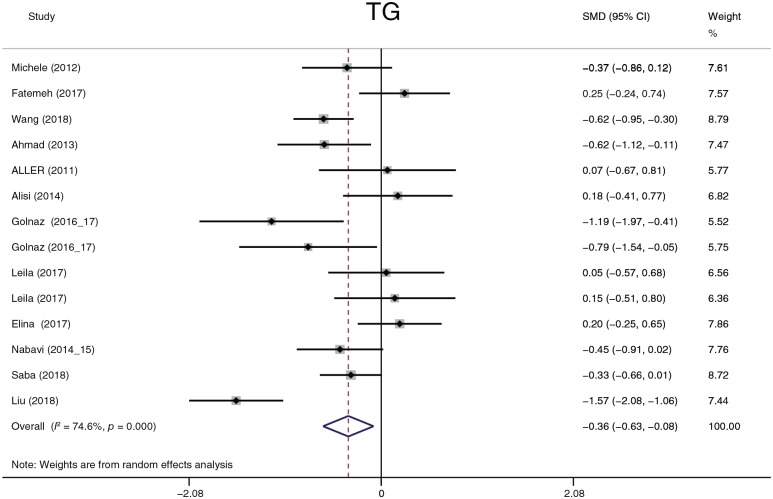
The effect of probiotics on lipid profiles
TC, LDL-C, HDL-C and TG were analyzed. Considering that four studies57,58,62,63 reported results in different dimensions and with different measurement methods from other studies, we adopted the standard mean difference (SMD) to summarize the data. TC, LDL-C and TG (Figures 12–14) significantly decreased after probiotic treatment. Their overall effects (shown as the SMD) were −0.73 (−1.28 to −0.18), p = 0.009; −0.54 (−0.99 to −0.09), p = 0.018; and −0.36 (−0.63 to −0.08), p = 0.011, respectively. Although HDL-C showed an increasing trend (0.43, −0.03 to 0.89, p = 0.0.69), the statistic difference was not significant. Heterogeneity in the four parameters was detected. A sensitivity analysis was conducted to define heterogeneity between studies. There was no significant heterogeneity in TC, HDL-C, or TG. Two studies62,63 exhibited significant heterogeneity in LDL-C compared with other studies. However, after omitting these two studies, the heterogeneity decreased only slightly from 88.5% to 78.8%, and the overall effects still exhibited significant differences. We performed three subgroup analyses according to the dimension, treatment duration, and continent. In all subgroups analyses, the heterogeneity of TC decreased only in the 8-week and 24-week treatment duration times (I2 = 0.0%, p = 0.675 and I2 = 0.0%, p = 0.349, respectively); the heterogeneity of LDL-C decreased only in the 8-week treatment duration time (I2 = 0.0%, p = 0.487); the heterogeneity of HDL-C decreased in the Europe group (I2 = 0.0%, p = 0.710) and in the 8-week treatment duration group (I2 = 10.5%, p = 0.327); and the heterogeneity of TG decreased in the Europe group (I2 = 10.2%, p = 0.342) and in the 8-, 16-, and 24-week treatment duration groups (I2 = 24.8%, p = 0.265; I2 = 53.1%, p = 0.144; and I2 = 0.0%, p = 0.492, respectively). According to the results of the subgroup analyses, the partial heterogeneity came from race and treatment duration. However, these results could not explain all sources of heterogeneity. The dimension or measurement method did not generate heterogeneity in this meta-analysis.
Figure 12.
Total cholesterol.
CI, confidence interval; SMD, standard mean difference.
Figure 13.
Low-density lipoprotein.
CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; SMD, standard mean. difference.
Figure 14.
Triglycerides.
CI, confidence interval; SMD, standard mean; TG, triglycerides.
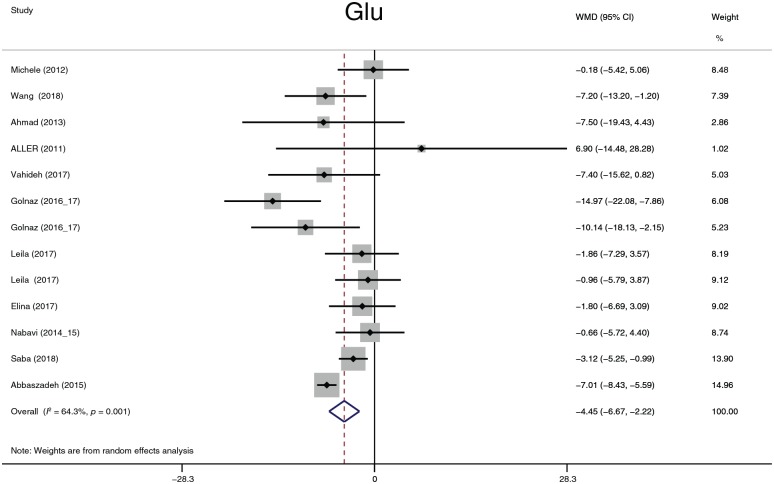
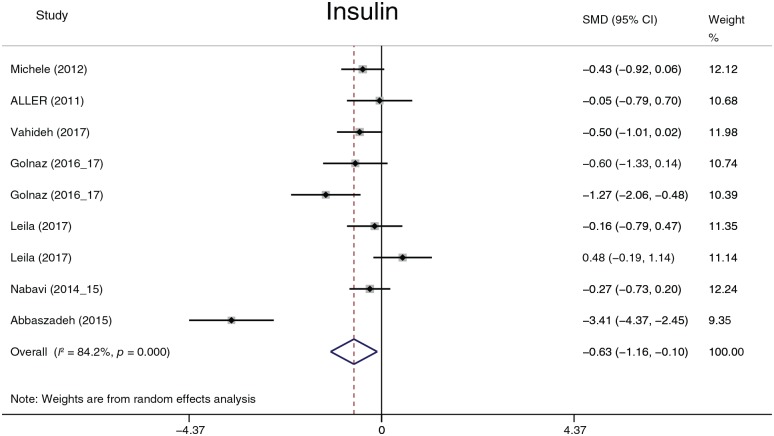
The effects of probiotics on plasma glucose
The parameters of this section included Glu (Figure 15), insulin (Figure 16), and HOMA-IR. Given that the reported insulin and HOMA-IR values exhibit considerable variance, the SMD was used to summarize the data on insulin and HOMA-IR. Probiotics reduced the plasma glucose level by 4.45 mg/dl (95% CI, −6.67 to −2.22, p = 0.001) and the insulin level (−0.63, −1.16 to −0.10, p = 0.02). There was no significant statistic difference in HOMA-IR (p = 0.1). Heterogeneity in Glu, insulin and HOMA-IR was detected. The sensitivity analysis did not reveal significant variation in Glu, but noteworthy heterogeneity existed in the study by Sepideh and colleagues61 compared with other studies. When the study was omitted, the heterogeneity (I2 index) of insulin decreased from 84.2% to 46.6% and that of HOMA-IR decreased from 84.9% to 44.5%. Although the I2 index significantly decreased after omitting the study, the p value in the chi-squared test did not exceed 0.1 (the predefined statistic critical value). Omission of the study did not affect the former statistic conclusion. When the subgroup analysis was performed, the heterogeneity of Glu decreased in the 12-week group (I2 = 0.0%, p = 0.643), the 24-week group (I2 = 17.5%, p = 0.271), and the Europe group (I2 = 0.0%, p = 0.7); the heterogeneity of insulin decreased in the 12-week group (I2 = 42.8%, p = 0.155) and the Europe group (I2 = 0.0%, p = 0.398); and the heterogeneity of HOMA-IR decreased in the 12-week group (I2 = 32%, p = 0.221). In summary, the heterogeneity of Glu was derived from treatment duration and race; the heterogeneity of insulin and HOMA-IR was mainly derived from the study of Sepideh and colleagues61 and partially derived from treatment duration and race. We had intended to analyze Peptide C and HbA1c but there were not enough data to perform the summary.
Figure 15.
Plasma glucose.
CI, confidence interval; Glu, glucose; WMD, weighted mean difference.
Figure 16.
Insulin.
CI, confidence interval; SMD, standard mean.
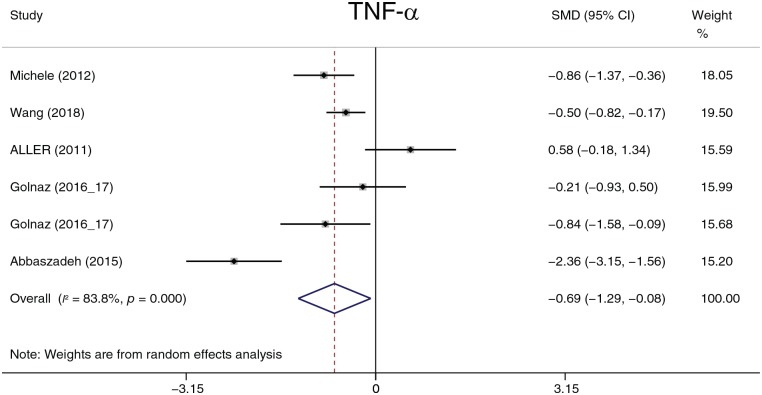
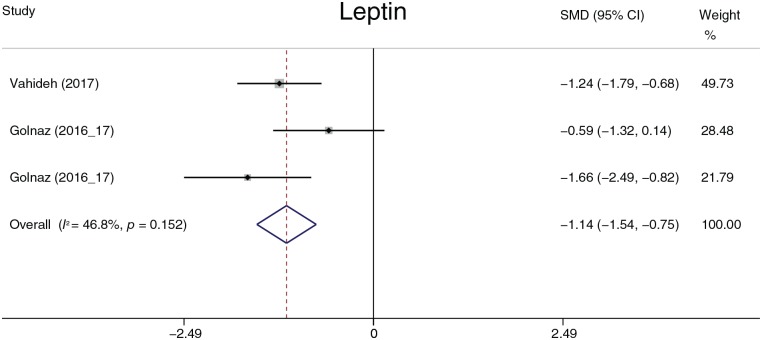
The effect of probiotics on cytokines
This section describes the analysis and summary of five parameters (CRP, TNF-α, IL-6, leptin, and Ad). The data described in this section are lacking; therefore, the results of this section should be interpreted with caution. Probiotic treatment decreased TNF-α (−0.62, −0.84 to −0.40, p = 0.026) and leptin (−1.14, −1.54 to −0.75, p < 0.001) levels (Figures 17, 18). There was no statistically significant difference in CRP, IL-6, or Ad. Except for leptin, heterogeneity was detected in all parameters. Considering the deficient data, we performed sensitivity and subgroup analyses only on TNF-α. Aller and colleagues59 and Sepideh and colleagues61 were the main sources of heterogeneity according to the sensitivity analysis. After omitting these two studies, the heterogeneity decreased from 83.8% to 0.0%. The omission of the two studies did not affect the statistical conclusion.
Figure 17.
Tumor necrosis factor alpha.
CI, confidence interval; SMD, standard mean; TNF-α, tumor necrosis factor alpha.
Figure 18.
Leptin.
CI, confidence interval; SMD, standard mean.
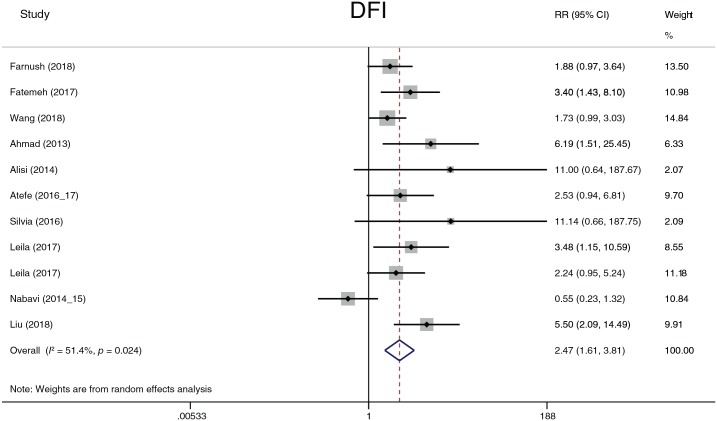
The effect of probiotics on DFI
Finally, we collected the number of patients whose liver fat infiltration was restored at the treatment endpoint and summarized the data by relative risk (Figure 19). According to the overall effects (2.47, 1.61–3.81, p < 0.001), there existed a significant association between probiotic treatment and a decrease in fat infiltration, and probiotic treatment improved liver fat infiltration. Heterogeneity (I2 = 51.4%, p = 0.024) was detected among the studies. No study showed significant heterogeneity with any other study according to the sensitivity analysis. A subgroup analysis was performed according to race and treatment duration. When evaluated by continent, the heterogeneity of the Europe group decreased (I2 = 0.0%, p = 0.995) and that of the Asia group (I2 = 55.2%, p = 0.022) did not change. When evaluted by treatment duration, heterogeneity decreased in the 12-week group (I2 = 0.0%, p = 0.606), and the p value of the chi-squared test increased in the 24-week group (I2 = 57.3%, p = 0.126).
Figure 19.
Degree of liver fat infiltration.
CI, confidence interval; DFI, degree of liver fat infiltration; RR, relative risk.
Publication bias assessment
For each parameter, only when the included groups exceeded 10 was the publication bias assessed by Egger’s test. The publication bias of weight (p = 0.670), BMI (p = 0.050), WC (p = 0.077), ALT (p = 0.415), AST (p = 0.761), Glu (p = 0.445), TC (p = 0.288), LDL-C (p = 0.732), HDL-C (p = 0.1916), TG (p = 0.801), and DFI (p = 0.083) was assessed. No significant publication bias was detected.
Discussion
This systematic review with meta-analysis focused on nearly all known related data sorted by six aspects: anthropometric parameters, liver function, lipid profiles, glucose profiles, cytokines, and DFI. In regard to the anthropometric parameters, probiotics decreased weight (p = 0.035) and BMI (p = 0.030). Being overweight and having a high BMI are associated with NAFLD;64–67 however, the details on this association are unclear. It is difficult to determine whether NAFLD is only accompanied by overweight or whether being overweight plays a role in NAFLD pathology. Sarr and colleagues8 reported that a low birth weight (LBW) is independently associated with hepatic steatosis in guinea pigs by affecting gene expression and the metabolomic profile in early adulthood. Bugianesi and colleagues68 reported that an LBW increased the risk of hepatic steatosis in childhood. Moreover, being overweight in childhood will increase the risk of NAFLD in adulthood.69 Weight seems to play different roles in newborns, fetuses, and children or adults, but the mechanism requires further research. With regards liver function, probiotics improved ALT, AST, ALP, and γ-GT. AST, ALT and γ-GT are signs of hepatic cell injury; elevated γ-GT and ALP levels are related to biliary obstruction (e.g. hepatic cirrhosis induced by NAFLD). ALT indicates the worst metabolic profile and persistent NAFLD according to recent research.70 Elevated liver enzymes (ALT, AST, and γ-GT) could also be related to liver disease mortality.71 Therefore, probiotics are beneficial for hepatic cell protection. In regard to the lipid profile, probiotics deceased TC, LDL-C, and TG, all of which are the main risk factors of NAFLD. Lipids were detected in all liver tissues of NAFLD patients. Cholesterol and TG can accumulate in hepatic cells and liver tissue and induce deleterious hypoxic and nitric oxide signal-transduction pathways.72,73 LDL-C metabolism is associated with steatotic liver tissue, and lipid heterogeneity exists between steatotic and nonsteatotic liver tissue (i.e. the lipid composition or metabolism may be different) which may help us understand the mechanisms of lipid accumulation in NAFLD.74 Considering the association of hyperlipidemia with NAFLD, statin treatment targeting dyslipidemia seems promising,75 but its safety is debatable.76,77 Probiotic treatment could be a promising supplemental therapeutic method targeting dyslipidemia. In regard to plasma glucose, probiotics decreased Glu and insulin levels. Diabetes and NAFLD are like ‘twins’. NAFLD patients are usually insulin resistant, similar to diabetic patients, and diabetic patients usually have fatty liver.78,79 In a high-fat-diet mouse model of obesity, diabetes was shown to worsen NAFLD.80 The inflammation associated with diabetes also accelerates NASH. Improving IR and hyperglycemia will be beneficial for NAFLD patients. In regard to serum inflammation, although the summarized data indicate that probiotics decreased TNF-α and leptin levels, the results and conclusion of this section must be reviewed carefully, given the deficient data and considerable heterogeneity. TNF-α is an inflammatory factor activated by nuclear factor-kappa B, promotes NASH, and accelerates IR.81,82 Leptin is a debatable factor in NAFLD and obese patients, and can regulate energy homeostasis.83,84 Animal trials, human trials, and reviews seem to support the application of leptin or its efficacy in obesity or NAFLD considering its beneficial effect on IR and liver fat accumulation.85–90 However, Imajo and colleagues91 reported that obesity-induced leptin plays a crucial role in NASH progression via enhanced responsivity to endotoxin, and Polyzos and colleagues92 concluded that a high circulating leptin level was associated with severity of NAFLD. Above all, the effect of leptin on NAFLD still needs further investigation. Finally, we found that NAFLD patients receiving probiotic treatment were more likely to exhibit reduced DFI. Although liver fat accumulation does not cause clinical symptoms in most instances, liver fat is reported to disrupt glycerol metabolism93 and is associated with impaired cardiac and autonomic function, inflammation and oxidative stress.94,95 We believe that DFI should also be a therapeutic goal.
Although probiotics seem to improve NAFLD in many aspects, the specific mechanism is unclear. Microbial products (e.g. the short-chain fatty acid acetate, butyrate and propionate), microbial enzymes (e.g. bile salt hydrolase) and the dysbiosis-induced dysregulation of gut endothelial barrier function seem promising in explaining the mechanism involved.96–98 Considering the complex and multiple effects of probiotics on NAFLD, we believe it is unlikely that any single bacteria can generate sufficient treatment efficiency. However, we need more animal or human trials to investigate the effect of a single bacteria on NAFLD to understand the effect of every bacterium and then pack the most efficient probiotics for clinical application. Regretfully, most studies have investigated only the effect of multistrain probiotics because this kind of study is more likely to achieve positive results. In addition, the bacteria investigated differed among the studies. Although our study indicates that probiotics are a promising treatment for NAFLD, the subgroup analysis did not explain all sources of heterogeneity. Moreover, the degree of improvement in each parameter was moderate, and the included subjects were mainly from Asia. The conclusion of the current meta-analysis should be cautiously reviewed when considering race.
Conclusion
Probiotics can reduce weight and BMI, improve liver function, decrease plasma lipid and glucose levels, alleviate inflammation and restore liver fat infiltration. Probiotic treatment or supplementation is a promising therapeutic method for NAFLD.
Acknowledgments
YT, QBY and HDH conceived and designed the study. HDH is the guarantor of the article. YT, JH and WYZ designed the search strategy, searched the database, screened the articles, assessed the risk of bias, extracted and synthesized the data. SQ designed the data extraction table and edited the figures in this study. YXY and HR edited partial clinical theses. All authors have approved the final manuscript and declare that they have no conflicts of interest.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Natural Science Foundation of China (grant no. 81171560), the ‘Par-Eu Scholars Program’ of Chongqing City and the National Science and Technology Major Project of China (grant no. 2012ZX10002007001).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Huaidong Hu  https://orcid.org/0000-0003-2332-2304
https://orcid.org/0000-0003-2332-2304
Contributor Information
Yao Tang, Department of Clinical Nutrition, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China; Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Juan Huang, Department of Clinical Nutrition, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China; Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Wen yue Zhang, Department of Clinical Nutrition, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China; Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Si Qin, Center for Endocrine Diseases, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Yi xuan Yang, Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Hong Ren, Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Qin-bing Yang, Department of Clinical Nutrition, Tsinghua University, Beijing, China.
Huaidong Hu, Department of Clinical Nutrition, The Second Affiliated Hospital of Chongqing Medical University, No.76, Linjiang Road, Chongqing 400010, China.
References
- 1. Mofidi F, Poustchi H, Yari Z, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr 2017; 117: 662–668. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012; 91: 319–327. [DOI] [PubMed] [Google Scholar]
- 4. Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012; 96: 727–734. [DOI] [PubMed] [Google Scholar]
- 5. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010; 5: 145–171. [DOI] [PubMed] [Google Scholar]
- 6. Fan JG, Cao HX. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2013; 28(Suppl. 4): 81–87. [DOI] [PubMed] [Google Scholar]
- 7. Savari F, Mard SA, Badavi M, et al. A new method to induce nonalcoholic steatohepatitis (NASH) in mice. BMC gastroenterology 2019; 19: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarr O, Mathers KE, Zhao L, et al. Western diet consumption through early life induces microvesicular hepatic steatosis in association with an altered metabolome in low birth weight Guinea pigs. J Nutr Biochem 2019; 67: 219–233. [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Jaramillo M, Spooner MH, Lohr CV, et al. Lipidomic and transcriptomic analysis of western diet-induced nonalcoholic steatohepatitis (NASH) in female Ldlr-/- mice. PLoS One 2019; 14: e0214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gabbia D, Roverso M, Guido M, et al. Western diet-induced metabolic alterations affect circulating markers of liver function before the development of steatosis. Nutrients 2019; 11 pii: E1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 12. Li Z, Xue J, Chen P, et al. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol 2014; 29: 42–51. [DOI] [PubMed] [Google Scholar]
- 13. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148: 547–555. [DOI] [PubMed] [Google Scholar]
- 14. Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998; 114: 842–845. [DOI] [PubMed] [Google Scholar]
- 15. Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci 2014; 15: 8591–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 17. Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther 2012; 36: 815–823. [DOI] [PubMed] [Google Scholar]
- 18. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017; 67: 829–846. [DOI] [PubMed] [Google Scholar]
- 19. Doumas M, Imprialos K, Dimakopoulou A, et al. The role of statins in the management of nonalcoholic fatty liver disease. Curr Pharm Des 2018; 24: 4587–4592. [DOI] [PubMed] [Google Scholar]
- 20. Xiang Z, Chen YP, Ma KF, et al. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: a systematic review. BMC Gastroenterol 2013; 13: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khoo J, Hsiang JC, Taneja R, et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int Epub ahead of print 2019. DOI: 10.1111/liv.14065. [DOI] [PubMed] [Google Scholar]
- 22. McPherson S, Wilkinson N, Tiniakos D, et al. A randomised controlled trial of losartan as an anti-fibrotic agent in non-alcoholic steatohepatitis. PLoS One 2017; 12: e0175717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato K, Gosho M, Yamamoto T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition 2015; 31: 923–930. [DOI] [PubMed] [Google Scholar]
- 24. Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2017; 15: 1940–1949e1948. [DOI] [PubMed] [Google Scholar]
- 25. Jump DB, Lytle KA, Depner CM, et al. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol Ther 2018; 181: 108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sridharan K, Sivaramakrishnan G, Sequeira RP, et al. Pharmacological interventions for non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Postgrad Med J 2018; 94: 556–565. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKibbon KA, Wilczynski NL, Haynes RB. Retrieving randomized controlled trials from medline: a comparison of 38 published search filters. Health Info Libr J 2009; 26: 187–202. [DOI] [PubMed] [Google Scholar]
- 30. Haynes RB, McKibbon KA, Wilczynski NL, et al. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 2005; 330: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wieland LS, Robinson KA, Dickersin K. Understanding why evidence from randomised clinical trials may not be retrieved from Medline: comparison of indexed and non-indexed records. BMJ 2012; 344: d7501. [DOI] [PubMed] [Google Scholar]
- 32. Royle PL, Waugh NR. Making literature searches easier: a rapid and sensitive search filter for retrieving randomized controlled trials from PubMed. Diabet Med 2007; 24: 308–311. [DOI] [PubMed] [Google Scholar]
- 33. Royle P, Waugh N. A simplified search strategy for identifying randomised controlled trials for systematic reviews of health care interventions: a comparison with more exhaustive strategies. BMC Med Res Methodol 2005; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rafraf M, Nabavi S, Somi MH, et al. The effect of probiotic and conventional yogurt consumptions on anthropometric parameters in individuals with non alcoholic fatty liver disease. J Babol Univ Med Sci 2014; 16: 55–62. [Google Scholar]
- 35. Nabavi S, Rafraf M, Somi MH, et al. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 2014; 97: 7386–7393. [DOI] [PubMed] [Google Scholar]
- 36. Nabavi S, Rafraf M, Somi MH, et al. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD). J Funct Foods 2015; 18: 684–691. [Google Scholar]
- 37. Eslamparast T, Poustchi H, Zamani F, et al. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 2014; 99: 535–542. [DOI] [PubMed] [Google Scholar]
- 38. Kobyliak N, Abenavoli L, Falalyeyeva T, et al. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: a randomized clinical study. Minerva Med 2018; 109: 418–428. [DOI] [PubMed] [Google Scholar]
- 39. Kobyliak N, Abenavoli L, Mykhalchyshyn G, et al. A Multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestin Liver Dis 2018; 27: 41–49. [DOI] [PubMed] [Google Scholar]
- 40. Wong VW, Won GL, Chim AM, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol 2013; 12: 256–262. [PubMed] [Google Scholar]
- 41. Cakir M, Aksel Isbilen A, Eyupoglu I, et al. Effects of long-term synbiotic supplementation in addition to lifestyle changes in children with obesity-related non-alcoholic fatty liver disease. Turk J Gastroenterol 2017; 28: 377–383. [DOI] [PubMed] [Google Scholar]
- 42. Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes (Lond) 2018; 42: 147–155. [DOI] [PubMed] [Google Scholar]
- 43. Miccheli A, Capuani G, Marini F, et al. Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes (Lond) 2015; 39: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 44. Asgharian A, Askari G, Esmailzade A, et al. The effect of symbiotic supplementation on liver enzymes, c-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: a clinical trial. Int J Prev Med 2016; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asgharian A, Mohammadi V, Gholi Z, et al. The effect of synbiotic supplementation on body composition and lipid profile in patients with NAFLD: a randomized, double blind, placebo-controlled clinical trial study. Iran Red Crescent Med J 2017; 19(4): e42902. [Google Scholar]
- 46. Ekhlasi G, Kolahdouz Mohammadi R, Agah S, et al. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci 2016; 21: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ekhlasi G, Zarrati M, Agah S, et al. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J 2017; 16: 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Javadi L, Ghavami M, Khoshbaten M, et al. The effect of probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: a double blind randomized clinical trial. Iran Red Crescent Med J 2017; 19. [Google Scholar]
- 49. Javadi L, Ghavami M, Khoshbaten M, et al. The potential role of probiotics or/and prebiotic on serum lipid profile and insulin resistance in alcoholic fatty liver disease: a double blind randomized clinical trial. CJMB 2017; 4(3): 131–138. [Google Scholar]
- 50. Shavakhi A, Minakari M, Firouzian H, et al. Effect of a probiotic and metformin on liver aminotransferases in non-alcoholic steatohepatitis: a double blind randomized clinical trial. Int J Prev Med 2013; 4: 531–537. [PMC free article] [PubMed] [Google Scholar]
- 51. Ahn SB, Jun DW, Kang BK, et al. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep 2019; 9: 5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014; 39: 1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bakhshimoghaddam F, Shateri K, Sina M, et al. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr 2018; 148: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 54. Behrouz V, Jazayeri S, Aryaeian N, et al. Effects of probiotic and prebiotic supplementation on leptin, adiponectin, and glycemic parameters in non-alcoholic fatty liver disease: a randomized clinical trial. Middle East J Dig Dis 2017; 9: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Famouri F, Shariat Z, Hashemipour M, et al. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr 2017; 64: 413–417. [DOI] [PubMed] [Google Scholar]
- 56. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients 2016; 8 pii: E397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 2012; 57: 545–553. [DOI] [PubMed] [Google Scholar]
- 58. Manzhalii E, Virchenko O, Falalyeyeva T, et al. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: a pilot trial. J Dig Dis 2017; 18: 698–703. [DOI] [PubMed] [Google Scholar]
- 59. Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011; 15: 1090–1095. [PubMed] [Google Scholar]
- 60. Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol 2018; 24: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sepideh A, Karim P, Hossein A, et al. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr 2016; 35: 500–505. [DOI] [PubMed] [Google Scholar]
- 62. Yanghe L, Dengyi L, Bei Zheng, et al. Efficacy of bacillus subtilis duplex and polyene phosphatidylcholine combination in treatments of patients with nonalcoholic fatty liver diseases. Journal of Practical Hepatology 2018; 21: 4. [Google Scholar]
- 63. Wang W, Shi LP, Shi L, et al. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi 2018; 57: 101–106. [DOI] [PubMed] [Google Scholar]
- 64. Pang Y, Kartsonaki C, Turnbull I, et al. Adiposity in relation to risks of fatty liver, cirrhosis and liver cancer: a prospective study of 0.5 million Chinese adults. Sci Rep 2019; 9: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep 2018; 8: 15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu J, Xu H, He X, et al. Six-year changes in the prevalence of obesity and obesity-related diseases in Northeastern China from 2007 to 2013. Sci Rep 2017; 7: 41518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bischoff SC, Boirie Y, Cederholm T, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr 2017; 36: 917–938. [DOI] [PubMed] [Google Scholar]
- 68. Bugianesi E, Bizzarri C, Rosso C, et al. Low Birthweight increases the likelihood of severe steatosis in pediatric non-alcoholic fatty liver disease. Am J Gastroenterol 2017; 112: 1277–1286. [DOI] [PubMed] [Google Scholar]
- 69. Yan Y, Hou D, Zhao X, et al. Childhood adiposity and nonalcoholic fatty liver disease in adulthood. Pediatrics 2017; 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sung KC, Lee MY, Lee JY, et al. Utility of ALT concentration in men and women with nonalcoholic fatty liver disease: cohort study. J Clin Med 2019; 8 pii: E445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology 2016; 63: 1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tirosh O. Hypoxic Signaling and cholesterol lipotoxicity in fatty liver disease progression. Oxid Med Cell Longev 2018; 2018: 2548154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 2017; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ščupáková K, Soons Z, Ertaylan G, et al. Spatial systems lipidomics reveals nonalcoholic fatty liver disease heterogeneity. Anal Chem 2018; 90: 5130–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tziomalos K, Athyros VG, Paschos P, et al. Nonalcoholic fatty liver disease and statins. Metabolism 2015; 64: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 76. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek atorvastatin and coronary heart disease evaluation (GREACE) study: a post-hoc analysis. Lancet 2010; 376: 1916–1922. [DOI] [PubMed] [Google Scholar]
- 77. Calderon RM, Cubeddu LX, Goldberg RB, et al. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc 2010; 85: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017; 14: 32–42. [DOI] [PubMed] [Google Scholar]
- 79. Khan RS, Bril F, Cusi K, et al. Modulation of insulin resistance in NAFLD. Hepatology. Epub ahead of print 2018. DOI: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 80. Lo L, McLennan SV, Williams PF, et al. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J Hepatol 2011; 55: 435–444. [DOI] [PubMed] [Google Scholar]
- 81. Chen Z, Yu R, Xiong Y, et al. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis 2017; 16: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab 2014; 99: 39–48. [DOI] [PubMed] [Google Scholar]
- 83. Munzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism 2015; 64: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism 2015; 64: 60–78. [DOI] [PubMed] [Google Scholar]
- 85. Rodríguez A, Moreno NR, Balaguer I, et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci Rep 2015; 5: 12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007; 92: 532–541. [DOI] [PubMed] [Google Scholar]
- 87. Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract 2010; 16: 324–333. [DOI] [PubMed] [Google Scholar]
- 88. Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev 2013; 34: 377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamamoto-Kataoka S, Ebihara K, Aizawa-Abe M, et al. Leptin improves fatty liver independently of insulin sensitization and appetite suppression in hepatocyte-specific Pten-deficient mice with insulin hypersensitivity. Horm Metab Res 2015; 47: 168–175. [DOI] [PubMed] [Google Scholar]
- 90. Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism 2015; 64: 146–156. [DOI] [PubMed] [Google Scholar]
- 91. Imajo K, Fujita K, Yoneda M, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 2012; 16: 44–54. [DOI] [PubMed] [Google Scholar]
- 92. Polyzos SA, Aronis KN, Kountouras J, et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 2016; 59: 30–43. [DOI] [PubMed] [Google Scholar]
- 93. Jin ES, Browning JD, Murphy RE, et al. Fatty liver disrupts glycerol metabolism in gluconeogenic and lipogenic pathways in humans. J Lipid Res 2018; 59: 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Houghton D, Zalewski P, Hallsworth K, et al. The degree of hepatic steatosis associates with impaired cardiac and autonomic function. J Hepatol. Epub ahead of print 13 February 2019. DOI: 10.1016/j.jhep.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 95. Fricker ZP, Pedley A, Massaro JM, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham Heart Study. Clin Gastroenterol Hepatol 2019; 17: 1157–1164e1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Canfora EE, Meex RCR, Venema K, et al. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 2019; 15: 261–273. [DOI] [PubMed] [Google Scholar]
- 97. Safari Z, Gerard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci 2019; 76: 1541–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gonzalez FJ, Jiang C, Patterson AD. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 2016; 151: 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]