Abstract
Background:
Demographic disparities have been described for survival after thyroid cancer surgery using national registries and databases. At the institution level, we hypothesized that assessing survival after thyroid cancer surgery in a long-term cohort with diverse gender and racial groups would reveal disparities in survival.
Methods:
We examined medical records of patients with papillary or follicular thyroid cancer undergoing thyroidectomy, lobectomy, and other surgical procedures from 1971 to 2016 at a tertiary referral center. We obtained information on demographics, cancer stage, procedure, and radioactive iodine (RAI). We measured survival using Kaplan-Meier estimates and Cox proportional hazards models.
Results:
A total of 1440 (91%) patients with papillary cancer and 144 (9%) patients with follicular thyroid cancer underwent total thyroidectomy (1297, 82%), lobectomy (261, 16.5%), and other surgical procedures (26, 1.5%). Most patients (1131, 71%) were woman, and 909 (57%) were older than 45 years. Race/ethnicity included 805 (51%) white, 161 (10%) African Americans, and 618 (39%) other race/ethnicities. Both 10- and 20-year survival rates in nonwhite males were worse compared with nonwhite females (P < .0001). After controlling for age, cancer type, stage, surgical procedure, RAI, and year of surgery, nonwhite males had a higher mortality risk compared with nonwhite females (P = .0376, confidence interval (CI): 1.03-2.43), white males (P < .0001, CI: 1.88-6.54), and white females (P < .0001, CI: 3.31-9.90).
Conclusions:
Our diverse cohort demonstrates significant gender and racial disparities in survival after thyroid cancer surgery. To improve health outcomes and reduce health disparities among nonwhite males, interventions and long-term care management should target potentially modifiable causes of worse outcomes in this group.
Keywords: Thyroid cancer, gender, race and disparity
Introduction
Thyroid cancer is the most rapidly increasing cancer in the United States for both men and women with age-adjusted incidence that increased from 11.03 per 100 000 in 2006 to 14.3 per 100 000 in 2009.1 These changes have been attributed to the increase in detection of smaller thyroid cancers especially papillary thyroid carcinoma using high-resolution neck images.2-4 Despite this increase in the incidence rate, the mortality rate of thyroid cancer remains unchanged at 0.5 per 100 000 population.5
Generally, racial disparities in disease incidence and prevalence, mortality, burden, and health care outcomes have been well documented in a multitude of acute and long-term diseases including sepsis,6 heart failure,7 and cancer.8,9 Racial and ethnic disparities in health care exist and have been shown to be associated with worse outcomes in many cases as reported by the comprehensive Institute of Medicine (IOM).10 Although the growing prevalence and incidence rates of thyroid cancer are similar among races, except among Native Americans, there is evidence to suggest a significant survival difference among races at 5 years.11,12 Some studies point to the fact that racial disparities exist in the types of treatment and outcomes in thyroid cancers as the reason for this difference,13 whereas others point to the role of socioeconomic status14 and variations in clinical and pathological characteristics of thyroid cancers.15
However, many of these studies12-15 have used publicly available national databases and registry such as Surveillance, Epidemiology, and End Results (SEER) and California Cancer Registry. These disparities in survival among patients with thyroid cancer may also exist at the institution level. Therefore, using existing data at a large tertiary referral center, we hypothesized that disparities in survival after thyroid cancer surgery exist in a long-term follow-up cohort of patients with diverse gender and racial groups.
Patients and Methods
Data source and patients
This study used the Tumor Registry Database at The University of Alabama at Birmingham (UAB). Data were inquired for all patients with differentiated thyroid cancer, papillary thyroid cancer (PTC), and follicular thyroid cancer (FTC), from 1975 to 2016 who underwent any type of thyroid surgery at the UAB and/or were referred for further management after their thyroid surgery. The UAB Institutional Review Board for Human Use committee approved this retrospective study. Those patients with histological type other than PTC or FTC or with metastatic disease on presentation were excluded from the analysis. This database contains information about patients’ demographics including age, sex, race, tumor clinical and pathological stages, and mortality.
Outcomes measurement
We divided the cohort into 4 groups: white males, white females, nonwhite males, and nonwhite females. The overall 10- and 20-year survival rates were evaluated among these groups. Patients’ survival rate was obtained from the Tumor Registry Database and calculated from the date of diagnosis. Based on the result of the nonadjusted model, mortality risk of the group with the worst overall 10- and 20-year survival rate, nonwhite males, was compared with the rest of the cohort, white males, white females (reference group), and nonwhite females. Factors included in the adjusted model are age, cancer type, stage (AJCC 7th Ed Cancer Staging Manual, 2010), surgical procedure, radioactive iodine (RAI), and year of surgery.
Statistical analysis
Continuous variables are summarized as mean ± SD and t tests were used to evaluate association for outcome. Chi-square was used for categorical variables. Age was divided to 2 groups, younger than 45 (⩽45) and older than 45 (>45) to be in line with clinical staging criteria for thyroid cancer before the new American Thyroid Association (ATA) 2015 guidelines. Cox proportional hazard models were used for multivariable analyses. We did not conduct any univariate analysis and variables included in the multivariable analysis were those that have been shown to affect survival among patients with differentiated thyroid cancer. We used the Kaplan-Meier method and log rank tests to compare unadjusted overall survival between different racial/gender groups and parametric hazard analysis to identify risk factors for mortality. Of note, because race is a fixed parameter, and the testing of time-varying parameters’ interactions was insignificant, we did not test for time interaction in our method. P value with significance level set at P < .05, adjusted hazard ratio (HR), and 95% confidence interval (CI) are reported. Data management and analysis were performed using SAS software, Version 9.4. (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and clinical characteristics
Over the past 45 years, a total of 1584 patients with well differentiated thyroid cancer were identified. Of those, there were 1440 (91%) patients with papillary cancer and 144 (9%) patients with follicular thyroid cancer. A total of 1131 (71%) patients were woman and 909 (57%) were older than 45 years with no significant difference in age/sex among groups. Race/ethnicity included 805 (51%) white, 161 (10%) African Americans, and 618 (39%) other groups. Thyroid surgery was divided between total thyroidectomy (n = 1297 patients [82%]), lobectomy (n = 261 patients [16.5%]), and other surgical procedures (n = 26 [1.5%]). A total of 767 (48%) received RAI. The patients’ characteristics are displayed in Table 1. A total of 313 patients (20%) had a stage III or greater disease, and lymph nodes were positive in 340 patients (22%) (Table 1). Table 2 demonstrates the difference in clinical presentation and management of thyroid cancer per race-gender group (white females, white males, nonwhite females, and nonwhite males). Nonwhite males were older than 55 years at diagnosis, presented with more positive lymph node metastasis and advanced clinical stage compared with white females, and less likely to receive RAI or undergo total thyroidectomy (Table 2).
Table 1.
Patients’ demographics and characteristics.
| Total N (n = 1584) | % | |
|---|---|---|
| Age | ||
| ⩽45 | 909 | 63.5 |
| Gender | ||
| Female | 1131 | 71 |
| Race | ||
| White | 805 | 51 |
| Black | 161 | 10 |
| Other | 618 | 39 |
| Histology | ||
| Papillary | 1440 | 91 |
| Follicular | 144 | 9 |
| Lymph node metastasis | ||
| Positive | 340 | 22 |
| TNM stage | ||
| I | 795 | 50 |
| II | 210 | 13 |
| III | 222 | 14 |
| IV | 91 | 6 |
| RAI | ||
| Yes | 767 | 48 |
| Treatment strategy | ||
| Total thyroidectomy | 1297 | 82 |
| Lobectomy | 261 | 16.5 |
| Other procedure | 26 | 1.5 |
Abbreviations: RAI, radioactive iodine; TNM, tumor, node, metastasis.
Table 2.
Demographics and clinical characteristics of the cohort per race-gender group.
| Total N (n = 1584) | White female (n = 554) | White male (n = 251) | Nonwhite female (n = 577) | Nonwhite male (n = 202) | P value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ⩾55 | 572 (36%) | 177 (32%) | 123 (49%) | 178 (31%) | 94 (47%) | <.0001 |
| Histology | ||||||
| Papillary | 1440 (91%) | 514 (93%) | 238 (95%) | 505 (88%) | 183 (91%) | .0019 |
| Follicular | 144 (9%) | 40 (7%) | 13 (5%) | 72 (12%) | 19 (9%) | |
| Lymph node metastasis | ||||||
| Positive | 340 (27%) | 127 (27%) | 82 (38%) | 86 (20%) | 45 (30%) | <.0001 |
| TNM stage | ||||||
| I | 795 (50%) | 324 (59%) | 121 (48%) | 271 (47%) | 79 (39%) | <.0001 |
| II | 210 (13%) | 71 (13%) | 31 (12%) | 85 (15%) | 23 (11%) | |
| III | 222 (14%) | 72 (13%) | 45 (18%) | 71 (12%) | 34 (17%) | |
| IV | 91 (6%) | 29 (5%) | 27 (11%) | 21 (4%) | 14 (7%) | |
| Unknown | 266 (17%) | 58 (10%) | 27 (11%) | 129 (22%) | 52 (26%) | |
| RAI | ||||||
| Yes | 767 (48%) | 268 (48%) | 138 (55%) | 275 (48%) | 86 (43%) | .0651 |
| Treatment strategy | ||||||
| Total thyroidectomy | 836 (53%) | 305 (55%) | 133 (53%) | 294 (51%) | 104 (51%) | <.0001 |
| Lobectomy | 232 (15%) | 60 (11%) | 19 (8%) | 112 (19%) | 41 (20%) | |
Abbreviations: RAI, radioactive iodine; TNM, tumor, node, metastasis.
Outcomes analyses
When analyzed by race and gender, the 10- and 20-year survival in nonwhite males of 81.8% and 63.5%, respectively, was significantly lower than that of nonwhite females (90.5% and 66.0%), white males (92.4% and 82.3%), and white females (95.8% and 90.8%; P < .0001; Figure 1). The median survival for nonwhite males, nonwhite females, white males, and white females is 6.7, 6.8, 4.6, and 5.2 years, respectively. The median follow-up for the total cohort was 5.9 years. We used univariate analysis to identify variables that have an effect on survival. After controlling for age, cancer type, stage, surgical procedure, RAI, and year of surgery, nonwhite males had a higher mortality risk with HR of 1.58 compared with nonwhite females (P = .0376, CI: 1.03-2.43), HR of 3.50 compared with white males (P < .0001, CI: 1.88-6.54), and HR of 4.78 compared with white females (P < .0001, CI: 3.31-9.9) (Table 3). In a univariate cox regression model, only age ⩾55 was associated with statistically significant increased HR of worse outcome (HR: 2.84, 95% CI: 1.13-7.12, P = .03; Table 4).
Figure 1.
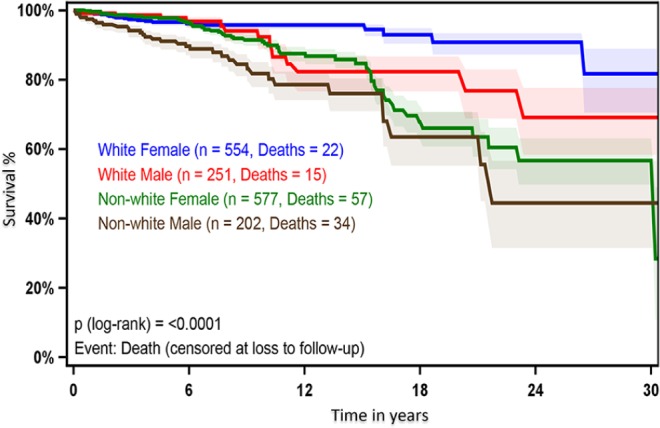
Kaplan-Meier curve of overall survival by race and gender. Survival rate (y-axis) over the study period (x-axis). Blue line represents white females, red line represents white males, green line represents nonwhite females, and brown line represents nonwhite males. Significance levels were set at P < .05.
Table 3.
Adjusted mortality risk after thyroid surgery among patients in the cohort by age and sex.
| Mortality risk | HR | 95% CI | P value |
|---|---|---|---|
| Nonwhite male vs white female | 4.78 | (3.31-9.90) | <.0001 |
| Nonwhite male vs white male | 3.50 | (1.88-6.54) | <.0001 |
| Nonwhite male vs nonwhite female | 1.58 | (1.03-2.43) | .0376 |
| Nonwhite male | Reference |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 4.
Predictors of worse outcome and survival among nonwhite males.
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Age | |||
| ⩾55 | 2.84 | 1.13-7.12 | .03 |
| Histology | |||
| Papillary vs follicular | 0.90 | 0.18-4.56 | .90 |
| Lymph node metastasis | |||
| Positive | 1.81 | 0.53-6.14 | .34 |
| TNM stage | |||
| II vs I | 1.00 | 0.25-4.02 | .99 |
| III vs I | 1.11 | 0.31-0.99 | .87 |
| IV vs I | 2.20 | 0.52-9.27 | .28 |
| RAI | |||
| Yes | 0.42 | 0.17-1.07 | .07 |
| Treatment strategy | |||
| Lobectomy vs total thyroidectomy | 0.70 | 0.20-2.50 | .57 |
Note: Bold values in the table are statistically significant.
Abbreviations: CI, confidence interval; RAI, radioactive iodine; TNM, tumor, node, metastasis.
Discussion
Using a large tumor registry database from 1975 to 2016 at a busy tertiary referral center that provides care for diverse gender and racial groups, this study demonstrates a gender and racial disparity in survival after surgery among patients with papillary and follicular thyroid cancer. Our results show that nonwhite males have worse 10- and 20-year survival rates when compared with white males, white females, and nonwhite female with a higher mortality risk compared with these groups.
Although not investigated in our study, several factors such as genetic and molecular differences between races and genders, disease presentation and socioeconomic factors likely play an important role in the observed disparities in survival among minorities with thyroid cancer.12 A meta-analysis showed a difference in BRAF status by race/ethnicity and gender. As BRAF mutations are associated with aggressive tumor and disease behaviors, and therefore worse outcome, however, the contribution of this mutation to outcomes in our study cannot be measured.16
Advanced presentation and tumors with aggressive features such as extrathyroidal extension, with corresponding worse prognosis, have been found more prevalent among patients with ethnic minorities.17 In one study of 25 945 patients with differentiated thyroid cancer that used the California Cancer Registry, minority groups were found to have a higher percentage of metastatic and regional disease at presentation, as compared with white patients.14 These findings are in alignment with our results in which we showed that nonwhite males are more likely to present with more positive lymph node metastasis, advance clinical stage and less likely to receive RAI or undergo total thyroidectomy compared with white females. Consistent with our findings, this study found lower overall and adjusted survival rates among black patients. Similarly, Moo-Young et al15 found that African Americans aged <45 years are less likely to have lymph node involvement but had the largest tumor size in presentation. Furthermore, in this study, advanced stage IV disease was found to be more prevalent among Asian Americans.
Socioeconomic status has also been shown to play a vital role in disparities in presentation and outcomes among patients with thyroid cancer. In one study, only 2% of patients had insurance at the public hospital where 96% of patients were ethnic minorities, compared with 85% with insurance at the university hospital.18 Furthermore, investigating the sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults, Keegan et al have found that African American or Hispanic race/ethnicity (vs non-Hispanic whites) had worse thyroid cancer-specific survival. This study showed worse thyroid cancer-specific survival among adolescent and young adult men from low socioeconomic status neighborhoods and nonmetropolitan areas; however, these findings did not hold true among women.
In addition, in the SEER database, African American patients had worse overall and adjusted survival vs nonblack patients among 17 668 patients diagnosed with papillary thyroid microcarcinoma between 1988 and 2009,19 which is consistent with our findings.
This study has several limitations. First, we were not able to adjust for some clinical and tumor pathological features that may affect patients’ survival, such as extrathyroidal tumor extension, capsular invasion, and angioinvasion. Second, the Tumor Registry Database is missing information on patients’ socioeconomic status, such as annual income, level of education, insurance type, and marital status, whcich have a direct effect on disparities among different races. Third, we could not obtain disease-specific survival to accurately estimate thyroid cancer-related survival. Still, our overall survival rate was consistent with previous publications.
Conclusions
Our diverse cohort demonstrates significant gender and racial disparities in survival after thyroid cancer surgery. To improve health outcomes and reduce disparities among nonwhite males, interventions and long-term care management should target potentially modifiable causes of worse outcomes in this group, and future research should be directed to further understand why disparities exist among minorities.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AA: wrote paper, analyzed data.
SKC: data collection.
RX: data analysis, reviewed paper.
BML: reviewed paper.
CJB: reiviewed paper.
JKK: reviewed paper.
HC: supervised study, reviewed data and paper.
ORCID iDs: Ammar Asban  https://orcid.org/0000-0003-3031-4521
https://orcid.org/0000-0003-3031-4521
References
- 1. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317-322. [DOI] [PubMed] [Google Scholar]
- 2. Leenhardt L, Bernier MO, Boin-Pineau MH, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150:133-139. [DOI] [PubMed] [Google Scholar]
- 3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254:653-660. [DOI] [PubMed] [Google Scholar]
- 5. Ries LAG, Melbert D, Krapcho M, Bethesda MD. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; https://seer.cancer.gov/archive/csr/1975_2005/. [Google Scholar]
- 6. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [DOI] [PubMed] [Google Scholar]
- 7. Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. 2016;4:885-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102:953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulhern KC, Wahl TS, Goss LE, et al. Reduced disparities and improved surgical outcomes for Asian Americans with colorectal cancer. J Surg Res. 2017;218: 23-28. [DOI] [PubMed] [Google Scholar]
- 10. Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences. In: Wizemann TM, Pardue ML, eds. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: National Academies Press; 2001:10-13. [PubMed] [Google Scholar]
- 11. Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579-E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollenbeak CS, Wang L, Schneider P, Goldenberg D. Outcomes of thyroid cancer in African Americans. Ethn Dis. 2011;21:210-215. [PubMed] [Google Scholar]
- 13. Roche AM, Fedewa SA, Chen AY. Association of socioeconomic status and race/ethnicity with treatment and survival in patients with medullary thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2016;142:763-771. [DOI] [PubMed] [Google Scholar]
- 14. Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moo-Young TA, Panergo J, Wang CE, et al. Variations in clinicopathologic characteristics of thyroid cancer among racial ethnic groups: analysis of a large public city hospital and the SEER database. Am J Surg. 2013;206:632-640. [DOI] [PubMed] [Google Scholar]
- 16. Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38-46. [DOI] [PubMed] [Google Scholar]
- 17. Lim II, Hochman T, Blumberg SN, Patel KN, Heller KS, Ogilvie JB. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keegan TH, Grogan RH, Parsons HM, et al. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25:635-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Megwalu UC, Saini AT. Racial disparities in papillary thyroid microcarcinoma survival. J Laryngol Otol. 2017;131:83-87. [DOI] [PubMed] [Google Scholar]


