Abstract
Many studies have shown that honey with high phenolic contents prevents cancer formation. Furthermore, recent studies have demonstrated that honey can be used for the treatment of cancer as well as cancer prevention. Antineoplastic effects of honey are often associated with their antioxidant phenolic contents. However, very few studies have dealt with the association of phenolic contents of honeys in terms of antiproliferative effects. The aim of this study was, therefore, to elucidate the cytotoxic, genotoxic, apoptotic, and reactive oxygen species (ROS) generating effects of honey samples on the basis of their phenolic and flavonoid contents. Fourteen different honey varieties were collected from various parts of Turkey, and their characteristics regarding total phenols, flavonoids, and antioxidant contents were determined to test their effects on gastric cancer cells (AGS). For convenience, 2 honey varieties were selected, namely, Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) having the highest phenolic and antioxidant content and Canakkale multifloral honey (MFH-C) with the lowest phenolic and antioxidant content. Levels of 11 different phenolic compounds in QPHH-IM and MFH-C samples were determined by LC-MS/MS. AGS cells were incubated with different concentrations of QPHH-IM and MFH-C for 24 hours, then the cell viability, DNA damage, apoptosis, and generation of ROS were determined. We found that QPHH-IM had more cytotoxic, genotoxic, and apoptotic effects than that of MFH-C. We think that these effects are probably related to pro-oxidant activities due to the high phenolic contents present. Therefore, further research on high-phenolic honey may contribute to the future development of cancer therapeutics.
Keywords: Quercus pyrenaica, honey, gastric carcinoma, apoptosis, DNA damage, reactive oxygen species, apitherapy
Introduction
Gastric cancer (GC) is one of the most common cancer types and an important health problem as the second leading cause of cancer death worldwide.1 Adenocarcinoma is the most common type in approximately 90% of diagnosed GC cases. Given the current limitations in chemotherapy, radiotherapy, and surgical treatment, there is an increasing interest in complementary/alternative medicine approaches for gastric and other types of cancer.2 The most important concern with anticancer drugs is their toxicity as side effects after treatments. However, natural compounds have been considered to be less toxic.
Honey is a natural product of honey bees, Apis mellifera. Honeydew honey (HH) is a type of honey obtained from the excretions of plant-sucking insects found on living parts of the plant or from their secretions from the living parts of plants.3 Polyphenolic compounds and phenolic acids found in honey vary according to geographical and climatic conditions. Some of these compounds have been reported as a specific marker for the botanical origin of honey.4 Due to its geographic location and ideal climatic conditions, Turkey is one of the most important producers of honey in the world.
Recently, honey has been tested and approved for its functional and biological properties such as antioxidant, anti-inflammatory, antibacterial, antiviral, anti-ulcer activities as well as antilipidemic and anticancer properties.5 In particular, the antioxidant properties of honey were shown to contribute to the prevention of various acute and chronic disorders such as diabetes, inflammatory disorders, and cancer.6 Phenolic acids and flavonoids are responsible for the antioxidant activity of honey.6 Flavonoids are well known to have antineoplastic effects due to their ability to scavenge free radicals.7 However, in recent years, researchers have focused on antiproliferative, genotoxic, and apoptotic effects as well as antioxidant and antineoplastic properties of honey. Antiproliferative effects have been demonstrated in a variety of cancer cell lines and tissues such as breast,8 colorectal, prostate, endometrial, and oral cancer.9 Furthermore, polyphenolic compounds in honey have also been considered to be one of the main factors responsible for the antiproliferative activity. However, the mechanisms of these opposite effects and their relation to the type and polyphenolic contents of honeys have not been elucidated in detail.
The aim of this study was to investigate the cytotoxic, genotoxic, apoptotic, and reactive oxygen species (ROS) generating effects of 2 different honey samples that were selected on the basis of their phenolic and flavonoid contents on GC cells.
Materials and Methods
Honey Samples
Fourteen different honey samples derived from chestnut, pine, cedar, oak, and multifloral were obtained from honey manufacturers from different regions of Turkey in 2018. The honey samples were stored at 4°C in the dark until analyses and dissolved with distilled water just before use for the biochemical and molecular analysis.
Chemicals
Human AGS cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Fetal bovine serum, penicillin-streptomycin (10 000 U/mL), 0.25% trypsin-EDTA, and phenol red were supplied by Life Technologies (Carlsbad, CA). Ham’s F-12K (Kaighn’s) medium was obtained from Gibco/Invitrogen Corporation (Carlsbad, CA). Bax, Bcl-2, caspase-3, and P-53 primer antibodies were provided by Santa Cruz Biotechnologies (Santa Cruz, CA), and ATP-Glo cell viability assay kit was provided by Promega (Madison, WI). Other chemicals such as 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH2-DA), ethidium bromide (EB), acridine orange (AO), ninhydrin, acetic acid, aluminum chloride (AlCl3), cadmium chloride hemi (pentahydrate), (+)-catechin, methanol sodium hydroxide (NaOH), gallic acid, L-leucine, 2,4,6-tripyridyl-S-triazine (TPTZ), sodium nitrite (NaNO2), potassium persulfate (K2SO4), ferric chloride (FeCl3), sodium chloride (NaCl), sodium carbonate (Na2CO3), ammonium ferrous sulfate, phosphoric acid (H3PO4), Coomassie Brilliant Blue, and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Chloroform, acetone, and methanol were purchased from Merck (Darmstadt, Germany). Standards in liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis were caffeic acid (98%, Sigma-Aldrich), p-coumaric acid (98% Sigma Aldrich), kaempferol (99%, Sigma-Aldrich), penduletin (95%, Supelco), apigenin (95%, Sigma-Aldrich), acacetin (95%, Sigma-Aldrich), luteolin (95%, Sigma-Aldrich), diosmetin (95%, Sigma-Aldrich), nepetin (98%, Supelco), taxifolin (85% Sigma-Aldrich), and eupatilin (98%, Sigma-Aldrich).
Determination of Total Polyphenol, Flavonoid, Antioxidant, Glucose, and Fructose Contents of Honey Samples
The Folin-Ciocalteu method10 was used to determine total phenolic content of honey samples. One gram of honey sample was dissolved in 10 mL of distilled water and filtered through filter paper. Fifty microliters of filtered honey sample and 250 µL of 0.2 N Folin-Ciocalteu reagent was mixed with vortex and kept for 5 minutes at room temperature. Then, it was mixed with 200 µL of 0.7 mol L−1 Na2CO3. After incubation at room temperature for 2 hours, the absorbance of the reaction mixture was measured at 760 nm against a blank using a Varioskan Flash Multimode Reader (Thermo Scientific, Waltham, MA). Gallic acid (0-300 mg L−1) was used as standard to produce the calibration curve. The mean of 3 readings was used, and the total phenolic content was expressed in mg of gallic acid equivalents per100 g honey.
The total flavonoid content of the honey samples was determined according to colorimetric assay method developed by Zhishen et al.11 Fifty microliters of filtered honey samples was mixed with 250 µL of distilled water and 15 µL of a 5% NaNO2 solution. After 6 minutes, 30 µL of 10% AlCl3 solution was added, then 100 µL 1 mol L−1 NaOH was added, and the solution was incubated for a further 5 minutes at room temperature. The reaction mixture was mixed well, and the intensity of the red flavonoid-aluminum complex was measured at 510 nm using a Varioskan Flash Multimode Reader (Thermo Scientific). A standard curve of catechin was drawn within a concentration range of 5 to 50 mg/L. Total flavonoid content was expressed as mg of (+)-catechin equivalents per 100 g of honey.
The total antioxidant capacity was determined according to the photometric method developed by Erel.12 Briefly, 1 g of honey sample was dissolved by stirring in 1 mL of distilled water and then 5 µL of sample was added to 500 µL of ABTS+ reagent. The mixture was incubated at room temperature for 90 seconds, and the color inhibition of the ABTS+ radical was measured at 734 nm using a Varioskan Flash Multimode Reader (Thermo Scientific). Results were expressed in mmol trolox equivalents per 100 g of honey.
The amount of glucose present in honey samples was determined using the commercial kit working with the glucose oxidase method (Abbott Diagnostics, Lake Forest, IL). For measurement, 5 µL of sample or standard was mixed with 500 µL of reagent and incubated for 10 minutes at 37°C. The absorbance of the samples or standards was read against a blank within 60 minutes at 520 nm using a Multimode Reader (Varioskan Flash, Thermo Scientific).
Fructose levels in honey samples were measured by photometric method.13 For the samples solution, 0.2 mL of resorcinol reagent was added to the honey sample and mixed well. Then, 0.2 mL of dilute HCl was added to it. For the standard solutions, 0.4, 0.6, 0.8, and 1.0 mg mL−1 fructose were prepared in 0.2 mL resorcinol and 0.2 mL of diluted HCl. The blank consisted of 0.2 mL resorcinol and 0.2 mL of diluted HCl. The standard, blank, and the sample tubes were incubated in a water bath at 80°C for about 10 minutes, and then the tubes were removed from the water bath and cooled down with tap water for 5 minutes. It was then read against the blank at 520 nm in 30 minutes using the Varioskan Flash Multimode Reader (Thermo Scientific).
Measurement of Phenolic Contents of Honeys by High-Performance LC-MS/MS
Ten grams of honey samples were extracted with 3 × 40 mL n-BuOH-water-CHCl3. After the separation of phases, the organic phase was collected and evaporated until dryness. The residue was weighed to 10 mL in a volumetric flask and dissolved in 5 mL of MeOH in ultrasonic bath. Then, 100 µL of curcumin solution (from 100 ppm stock solution) was added as an internal standard and diluted to the volume with mobile phase, mixed and warmed to get a clear solution. The solution was filtered through a 0.45-µm Millipore Millex-HV filter, and the final solution (1 mL) was transferred into a capped auto sampler vial, from which 10 µL of sample was injected to LC for each run. The samples in the auto sampler were kept at 15°C during the experiment.14,15
LC-MS/MS experiments were performed on a Zivak Multitasker and Zivak Tandem Gold Triple quadrupole (Istanbul, Turkey) mass spectrometer equipped with a Fortis C18 column (150 × 3.0 mm id, 5 µm particle size). The mobile phase was composed of water (A, 0.1% formic acid) in water (B, 0.1% formic acid in methanol), the gradient program of which was 0 to 1.00 minute 70% A and 30% B, 1.01 to 20.00 minutes 100% B, and finally 20.01 to 25.00 minutes 70% A and 30% B. The flow rate of the mobile phase was 0.30 mL/min, and the column temperature was set to 30°C. The injection volume was 10 µL.15,16
The best mobile phase solution was determined to be a gradient of acidified methanol and water system. Such a mobile phase was found to be satisfactory for the ionization abundance and separation of the compounds. The best ionization of small and relatively polar antioxidants was obtained by electrospray ionization (ESI) source.16,17 The optimum ESI parameters were determined as 2.40 mTorr CID gas pressure, 5000.00 V ESI needle voltage, 600.00 V ESI shield voltage, 300.00°C drying gas temperature, 50.00°C API housing temperature, 55 psi nebulizer gas pressure, and 40.00 psi drying gas pressure.
During the validation experiments, curcumin was used as an internal standard. The validation parameters consisted of linearity, repeatability, recovery, limit of detection, and limit of quantification experiments. The linearity for each compound for the reported method was determined by analyzing standard solutions (discussed later). A detailed methodology of uncertainty evaluations are available in the literature.15,16
Cell Culture Studies
Quercus pyrenaica Honeydew Honey from Ida Mountains (QPHH-IM) and multifloral honey from Canakkale (MFH-C) possessing the highest and lowest phenolic, flavonoid, and antioxidant contents, respectively, were selected from 14 honey types, and cytotoxic, genotoxic, apoptotic, and ROS generating effects were tested on AGS cells via in vitro cell culture studies.
Human AGS cells are commonly used as a GC model for human stomach research. These cells were cultured in Ham’s F-12 (Kaighn’s) medium. In our study, the medium was supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin). The cells were incubated at 37°C in a humidified atmosphere of 5% CO2. When the cells became almost confluent in 75 cm2 plastic flasks, they were harvested weekly. For the experiments, the AGS cells were plated in a 96-well plate at a density of 15 × 103 cells mL−1 and a 6-well plate at a density of 18 × 104 cells mL−1.
Cell Viability Assay
Cell Titer-Glo Luminescent Cell Viability Test Kit (Promega) was used to measure cell viability level. This method determines the degree of cell viability in proportion to the amount of ATP. For analysis, AGS cancer cells (1.5 × 103 cells well−1) were plated on 96-well plates. After 24 hours, the cells were incubated with different concentrations (range = 0.25% to 5% w/v) of QPHH-IM and MFH-C. After incubation, the luciferin derivative and cell lysis solution were added as substrates. The luciferin derivative converts a light signal proportional to the current amount of ATP. Luminescence was measured using a Varioskan Flash Multimode Reader (Thermo Scientific) and normalized to control.
Intracellular Reactive Oxygen Species Measurement
The intracellular ROS production levels were measured by fluorometric method using a probe, 2′,7′-dichlorofluorescein diacetate (H2DCF-DA, Sigma, MO). Cells (1.5 × 105 cells/well) were seeded in each well of 96 wells. After 24 hours, they were treated with QPHH-IM and MFH-C at different concentrations (0.25% to 5%) and incubated for another 24 hours. The cells were washed with phosphate-buffered saline (PBS) and incubated with 5 µM H2DCF-DA for 30 minutes at 37°C in the dark. The cells were then washed, resuspended in PBS, and measured for the ROS contents using a fluorimeter (Varioskan Flash Multimode Reader, Thermo Scientific) and normalized to control.
Genotoxicity Assay
Alkaline single cell gel electrophoresis assay (Comet Assay) was carried out with a slight modification of the method of Singh et al18 to assess the genotoxic effects of honey on AGS cells. AGS cells were plated on 6-well cell culture plates (approximately 2 × 105 cells per well) containing cell culture medium and incubated at 37°C in 5% CO2 for 24 hours. Then, the honey samples below IC50 (50% inhibitory) concentrations were added and incubated for another 24 hours. Cells were rinsed with PBS after incubation, collected using trypsin/EDTA for 4 minutes at 4°C, and centrifuged at 400g for 5 minutes at 4°C. The cells were rinsed with PBS after incubation, collected using trypsin/EDTA, and centrifuged at 400g for 5 minutes at 4°C. The supernatant was drained, and the cell density was adjusted to 2 × 105 cells/mL using cold PBS. Ninety microliters of 0.6% low melting point agarose and 10 µL cell suspension were mixed and placed on 1% normal melting point agarose precoated slides. They were allowed to solidify on a cold tray for a few minutes, and the slides were then placed in lysis buffer, pH 10 (1% Triton X-100, 2.5 M NaCl, 10 mmol L−1 Tris, 0.1 mol L−1 EDTA, Sigma-Aldrich) for 1 hour on ice in dark conditions. The slides were then incubated in alkaline solution (0.3 M NaOH, 1 mM EDTA, Sigma-Aldrich) for 40 minutes at dark conditions in the presence of cooling blocks to unwind the DNA. Electrophoresis was performed at 0.72 V/cm (26 V, 300 mA) for 25 minutes at 4°C. The slides were neutralized in Tris buffer (0.4 M Tris, pH = 7.5) for 5 minutes and then dehydrated with ethanol before staining. The slides were then stained with EB (2 µg/mL in distilled H2O, 70 µL/slide), coated with a coverslip, and scored with a fluorescence microscope (Leica DM 1000, Solms, Germany) using the Comet assay IV software (Perceptive Instruments, Suffolk, UK).
Measurements of Apoptosis Indicators
Acridine orange/EB are DNA-specific dyes. AO/EB double staining was developed by McGahon et al.19 The cells undergoing apoptosis are differentiated from the viable cells by the morphological changes of apoptotic nuclei. AO and EB are DNA-intercalating dyes. AO is taken up by both living and dead cells and stains double-stranded and single-stranded nucleic acids.20 AO spreads green fluorescence on stimulation at 480 to 490 nm from live cells while being diffused into dsDNA. Briefly, 2 × 105 cells/well were seeded in 6-well plates and incubated for 24 hours. Then, the honey samples below IC50 concentrations were added and incubated for another 24 hours. Following honey treatment, the cells were collected and washed with PBS followed by staining with 1:1 mixture of AO/EB (100 µg/mL). Triplicate samples of 100 cells each were counted and scored for the incidence of apoptotic chromatin condensation using a fluorescent microscope (Leica DM 1000, Solms, Germany).
Immunoblotting Analysis
AGS cancer cells were seeded on 6-well plates at 1.5 × 105 cells per well and incubated for 24 hours. They were then treated with honeys according to their IC50 values. After 24 hours of incubation, the cells were harvested and prepared in NP-40 cell lysis buffer (2 mM Tris-HCl pH 7.5, 150 mM NaCl, 10% glycerol, and 0.2% NP-40 plus a protease inhibitor cocktail) for 30 minutes on ice and centrifuged at 14 000 × g (Beckman Coulter, Krefeld, Germany) for 10 minutes at 4°C. The final supernatant was then used as the cytosolic fraction. The protein concentration of the supernatant was determined using the Bradford protein assay method.21 Proteins from cellular supernatants were separated on 8% to 10% polyacrylamide gel and transferred to a nitrocellulose membrane using the Trans-blot SD semipermeable electrophoretic transfer cell (Bio-Rad, Hercules, CA). Tris-HCl buffered saline with Tween 20 (TBST) with 5% nonfat milk were used for blocking the membrane. The primary antibodies, P-53, caspase-3, Bax, Bcl2, and Nf-κB (1/500 dilution), were used after a night incubation (4°C). All samples were also blotted for β-actin to normalize protein amounts. TBST was used for washing the membrane and incubated with horseradish peroxidase–conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) for another hour. Immunolabelled proteins were visualized with Pierce ECL Western staining substrate (Thermo Scientific) and captured with an imaging system (Vilber Lourmat Sté, Collégien, France).
Statistical Data Treatment
The experiments were repeated 3 times, and the results were expressed as mean value ± standard deviation (mean ± SD). Statistical evaluation was performed using analysis of variance (1-way ANOVA). Differences with a probability value of P < .05 were considered statistically significant. IC50 values of honeys over the cell lines were calculated by nonlinear regression analysis. The statistical analysis was performed by using Statistical Package for Social Sciences (SPSS) version 21.
Results
Total Phenol, Flavonoids, Antioxidant, and Carbohydrate Contents
Total phenol and flavonoid contents of 14 different honey samples were compared in terms of phenol, flavonoid, and antioxidant content as well as glucose and fructose. From the different honey types, QPHH-IM showed the highest activity while MFH-C showed the lowest activity (Table 1). Hence, these 2 honey types were selected for further analysis.
Table 1.
Total Phenol and Flavonoid Contents and the Antioxidant Capacity for 14 Different Honey Samples. Ida Mountains Quercus pyrenaica Honeydew Honey (QPHH-IM), Chestnut Honeydew Honey (CNHH), Pine Honeydew Honey (PHH), Multifloral Honey (MFH).
| Honeys | Total Phenolic Content (mg GAE/100 g) | Total Flavonoid Content (mg QE/100 g) | Total Antioxidant Capacity (Inhibition of ABTS %) |
|---|---|---|---|
| CNHH (Düzce) | 79.96 ± 6.02 | 45.39 ± 4.80 | 85.96 ± 0.35 |
| CNHH (Bursa) | 89.52 ± 5.51 | 48.38 ± 8.00 | 86.75 ± 0.36 |
| CNHH (Rize) | 84.50 ± 4.01 | 46.52 ± 3.12 | 85.81 ± 0.22 |
| CNHH (Balıkesir) | 75.36 ± 5.10 | 48.43 ± 5.95 | 85.73 ± 0.19 |
| CNHH (Kastamonu) | 98.83 ± 10.15 | 53.59 ± 3.02 | 86.07 ± 0.15 |
| CNHH (Kocaeli) | 76.36 ± 6.15 | 46.31 ± 4.85 | 85.66 ± 0.29 |
| QPHH-IM | 115.41 ± 9.95* | 77.36 ± 7.25* | 89.36 ± 0.16* |
| MFH (Havran) | 90.36 ± 4.15 | 47.37 ± 4.95 | 85.98 ± 0.05 |
| PHH (Muğla) | 77.63 ± 7.51 | 45.16 ± 5.01 | 85.71 ± 0.18 |
| PHH (IM) | 78.40 ± 6.95 | 49.84 ± 4.65 | 86.07 ± 0.24 |
| MFH (Çanakkale) | 67.66 ± 2.87 | 42.69 ± 2.71 | 84.09 ± 0.20 |
| MFH (Balıkesir) | 74.36 ± 3.95 | 54.25 ± 4.15 | 88.23 ± 0.17 |
| MFH (Bayburt) | 88.36 ± 3.95 | 52.84 ± 2.61 | 88.68 ± 0.06 |
| MFH (Black Sea) | 75.36 ± 5.00 | 48.13 ± 3.95 | 88.39 ± 0.05 |
The significant difference between total phenol, flavonoid and antioxidant contents of QPHH-IM and MFH. Honey samples with highest and lowest phenolic, flavonois and antioxidant contents demonstrated with boldface.
Sugar is known to affect cell proliferation. Therefore, glucose and fructose contents of all honeys were also measured to exclude the possible effects of sugar on the cells. The results of the glucose and fructose contents and fructose/glucose ratios of the honey samples are presented in Table 2.
Table 2.
Glucose, Fructose, Fructose + Glucose Levels in 100 g Honey Samples and Fructose/Glucose Ratio of the Samples.
| Honeys | Glucose (g %) | Fructose (g %) | Glucose + Fructose (g %) | Fructose/Glucose Ratio |
|---|---|---|---|---|
| CNHH (Düzce) | 23.76 ± 1.5 | 35.9 ± 0.8 | 59.70 ± 2.1 | 1.5 ± 0.1 |
| CNHH (Bursa) | 23.83 ± 1.3 | 41.8 ± 0.7 | 65.67 ± 2.0 | 1.8 ± 0.2 |
| CNHH (Rize) | 22.45 ± 0.9 | 23.5 ± 0.6 | 45.96 ± 1.8 | 1.0 ± 0.1 |
| CNHH (Balıkesir) | 26.49 ± 1.2 | 15.4 ± 0.5 | 41.85 ± 1.7 | 0.6 ± 0.1 |
| CNHH (Kastamonu) | 14.66 ± 0.8 | 22.3 ± 0.4 | 36.95 ± 1.3 | 1.5 ± 0.3 |
| CNHH (Kocaeli) | 32.46 ± 1.4 | 26.8 ± 0.5 | 59.25 ± 1.8 | 0.8 ± 0. 2 |
| QPHH-IM | 33.98 ± 1.5 | 34.2 ± 0.7 | 68.16 ± 2.1 | 1.0 ± 0.3 |
| MFH (Havran) | 28 ± 1.2 | 65.5 ± 1.1 | 93.47 ± 2.2 | 2.3 ± 0.2 |
| PHH (Muğla) | 21.71 ± 1.3 NS | 28.1 ± 0.9 NS | 49.77 ± 1.9 NS | 1.3 ± 0.1 NS |
| PHH (IM) | 44.75 ± 1.8 | 21.5 ± 0.7 | 66.27 ± 3.1 | 0.5 ± 0.2 |
| MFH(Çanakkale) | 32.9 ± 1.5 | 33.6 ± 0.8 | 66.50 ± 2.5 | 1.0 ± 0.3 |
| MFH (Balıkesir) | 25.72 ± 1.4 | 36.5 ± 1.1 | 62.24 ± 2.8 | 1.4 ± 0.2 |
| MFH (Bayburt) | 19.81 ± 1.3 | 28.6 ± 1.2 | 48.41 ± 2.1 | 1.4 ± 0.1 |
| MFH (Black Sea) | 33.98 ± 1.2 | 33.1 ± 1.3 | 67.08 ± 2.0 | 1.0 ± 0.2 |
Abbreviations: CNHH, chestnut honeydew honey; QPHH-IM, Ida Mountains Quercus pyrenaica honeydew honey; MFH, multifloral honey; PHH, pine honeydew honey. Selected two honey samples according to the total phenol and flavonoid content showed with boldface.
As shown in Table 2, there was no significant difference in terms of glucose and fructose levels between QPHH-IM and MFH-C; these honey types exhibited the highest and lowest phenolic contents (Table 1).
Eleven different phenolic compound levels of QPHH-IM and MFH-C samples were determined by LC-MS/MS method, and related data are shown in Table 3. Detailed information on method validation and uncertainty evaluation, LC-MS/MS parameters, and representative chromatograms for QPHH-IM and MFH-C samples are given in supplementary material (Supplement 1, available online).
Table 3.
Validation and Uncertainty Parameters of for LC-MS/MS Method.
| Compound | Linear Regression | R 2 | Recovery | LOD/LOQ (mg/kg) | U95 (%) | QPHH-IM (mg/kg) | MFH-C (mg/kg) |
|---|---|---|---|---|---|---|---|
| Salicylic acid | y = +0.2121x + 0.04 | 0.99 | 94.3 | 0.7/3.5 | 18.2 | 60.4 | 4.4 |
| Caffeic acid | y = +0.2543x + 0.01 | 0.96 | 92.8 | 1/5.0 | 20.6 | 6.0 | <LOQ |
| Kaempferol | y = +0.0095x − 0.00 | 0.96 | 93.3 | 0.3/1.5 | 12.1 | 15.2 | <LOQ |
| Penduletin | y = +0.1385x − 0.00 | 0.99 | 100.1 | 0.6/3.1 | 7.8 | 1.2 | <LOQ |
| Apigenin | y = +0.1329x + 0.05 | 0.98 | 99.7 | 1.1/6.0 | 10.8 | 5.9 | <LOQ |
| Acacetin | y = +0.6369x + 0.07 | 0.98 | 95.3 | 1.2/6.0 | 5.7 | 7.0 | 2.5 |
| Luteolin | y = +0.2217x + 0.03 | 0.98 | 99.8 | 0.7/3.5 | 4.2 | 3.7 | <LOQ |
| Diosmetin | y = +1.1820x + 0.32 | 0.98 | 100.2 | 0.6/3.0 | 3.8 | 0.7 | <LOQ |
| Taxifolin | y = +0.0735x + 0.000 | 0.97 | 91.8 | 3.1/15.0 | 10.1 | 4.2 | <LOQ |
| Eupatilin | y = +0.5231x + 0.07 | 0.98 | 96.2 | 0.9/4.0 | 15.7 | <LOQ | <LOQ |
| Nepetin | y = +0.3282x − 0.06 | 0.98 | 100.1 | 2.2/11.0 | 10.6 | 0.6 | <LOQ |
Abbreviations: LC-MS/MS, liquid chromatography–tandem mass spectrometry; LOD, limit of detection; LOQ, limit of quantification; QPHH-IM, Ida Mountains Quercus pyrenaica honeydew honey; MFH-C, Canakkale multifloral honey.
As seen from Table 3, of the 11 phenolic compounds, only 2 phenolic compounds were found in light-colored MFH-C above the detection limit, while in dark-looking QPHH-IM only eupatilin levels were below the detection limit. Hence, these 2 honey types were selected for further analysis. Salicylic acid level was 14-fold higher, and acacetin level was 3-fold higher in QPHH-IM than in MFH-C samples.
Cell Viability Assessment
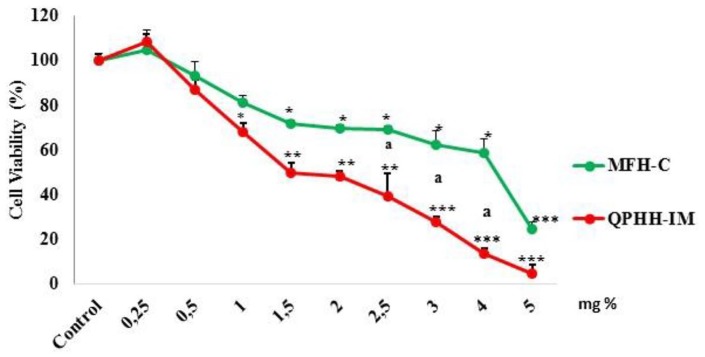
The cell viability test was performed with AGS cells for 24 hours to evaluate the effect of QPHH-IM and MFH-C on cell growth. After incubation, the cytotoxic effect of honey was measured by ATP cell viability test. When cell viability of the control cells was accepted as 100%, the cell viability increased to 108% at a concentration of 0.25% relative to the control 24 hours after addition of the honeys. At doses above this concentration, cytotoxic activity increased in a concentration-dependent manner (P < .001). Higher doses of QPHH-IM resulted in greater cellular death than in MFH-C in AGS cells (P < .05). The IC50 concentrations were calculated as 17 and 45 mg/mL (1.7% and 2.5% w/v) for the QPHH-IM and for the MFH-C in AGS cells, respectively (Figure 1). These data indicate that both QPHH-IM and MFH-C have proliferative effects at lower concentrations; on the other hand, QPHH-IM with high phenolic content was more cytotoxic than MFH-C with low phenolic content at their higher concentration.
Figure 1.
Cells were treated with 0, 0.25, 0.5, 1, 1.5, 2, 2.5 3, 4, and 5% mg Canakkale multifloral honey (MFH-C) and Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) and incubated for 24 hours. The ATP cell viability test was used to assess the cell viability. The percentage of cell viability was calculated by normalizing with a control panel. Significant differences compared with the control are indicated by *P < .05 and **P < .01.
Reactive Oxygen Species Generation Assessment
We measured intracellular ROS formation by fluorometric method using the H2DCF-DA probe. Low dose of honey samples (0.25%) decreased the intracellular ROS production in cancer cells (P < .05). However, ROS production significantly increased at higher doses of the samples (1.5% for QPHH-IM and 5% for MFH-C [Figure 2]).
Figure 2.
Effects of Canakkale multifloral honey (MFH-C) and Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) on the morphological changes in AGS cells. Cells were incubated with various concentrations of honeys for 24 hours and stained with AO/EB to observe the morphology. Significant differences compared with the control are indicated by *P < .05 and **P < .01.
There were close negative relationships between cell viability and ROS generating activity in both honeys (r = −0.839, P < .001, for QPHH-IM and r = −0.853, P < .001, for MFH-C in AGS cells).
Genotoxic Assessment
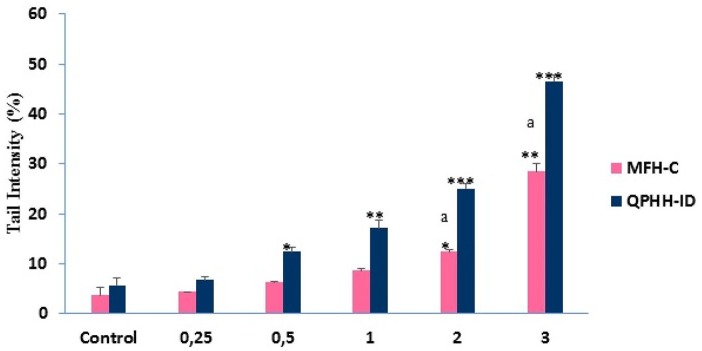
For DNA damage analysis, AGS cells were treated with different doses of honey samples for 24 hours and the DNA damage was measured via the Comet Assay method. Damaged DNA appears in a bright head and comet, while undamaged DNA appears to be only round. After incubation, the % tail intensity significantly increased with the increasing doses of honey samples and, when compared with MFH-C, DNA damage levels were significantly higher in QPHH-IM at higher concentrations (Figure 3).
Figure 3.
AGS cells were treated with different concentrations of Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) and Canakkale multifloral honey (MFH-C) for 24 hours, and there were significant changes in the tail % of DNA according to the control with the increasing concentrations. Significant differences are indicated by *P < .05, **P < .01, and ***P < .001. Significant differences between QPHH-IM and MFH-C are indicated by “a.”
These findings indicate that DNA damage level in cancer cells is related to the honey sample concentrations and their phenolic contents.
Apoptosis Assessment
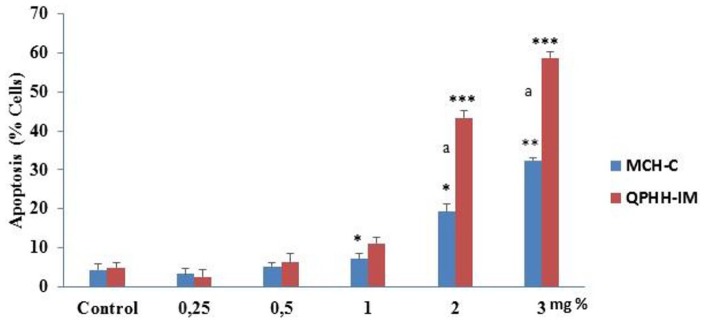
Apoptosis is important in determining tumor formation and resistance to treatment. In our study, we performed AO/EB double staining and Western blot methods in order to evaluate apoptotic effects of different concentrations of honeys in cancer cells. AGS cells were incubated with both honey for 24 hours to demonstrate the morphological characteristics of apoptosis on cells. Cells were then stained with AO/EB double staining and examined under fluorescence microscopy (Figure 4). As shown in Figure 4, after 24 hours of incubation, as the administered dose of both honeys increased, the green-looking viable cell ratios decreased and the yellow-orange–looking apoptotic cell ratios increased. High doses of QPHH-IM caused more apoptosis in cancer cells than MFH-C (Figure 4).
Figure 4.
Apoptotic activity of honeys on AGS cell lines. Cells were treated with different concentrations of honeys (0.25% to 5%) for 24 hours. Acridine orange/ethidium bromide (AO/EB) method was used and apoptotic and live cells were analyzed by fluorescence microscopy. Data presented were mean ± SD (n = 3). According to the control, significant differences are indicated by *P < .05, **P < .01, and ***P < .001. Significant differences between Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) and Canakkale multifloral honey (MFH-C) are indicated by “a.”
Western Blotting Results
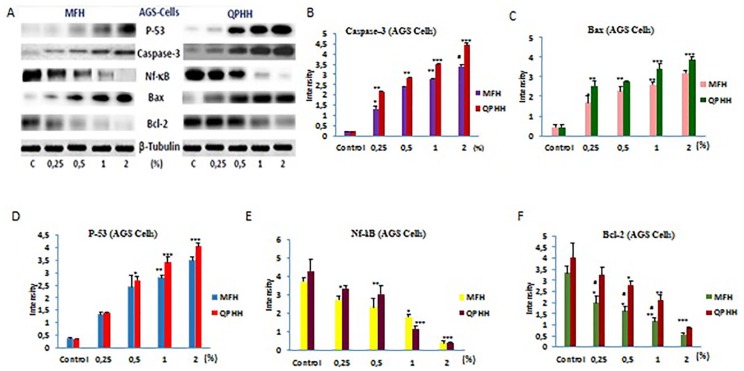
In order to investigate the relationship between the cytotoxic effects of honeys and apoptosis signaling pathways, the expression levels of P-53, caspase-3, Bax, Nf-κB, and Bcl-2 proteins were analyzed by the Western blotting method. For this purpose, AGS cells were treated with honey at different concentrations for 24 hours. Cell extracts were used for Western blotting. The β-tubulin was used as control. The results showed that both MFH and QPH increased expression levels of P-53, caspase-3, and Bax protein in AGS cancer cells, while decreasing expression levels of Nf-κB and Bcl-2 (Figure 5).
Figure 5.
Proapoptotic and antiapoptotic signal pathways AGS cells were treated with Ida Mountains Quercus pyrenaica honeydew honey (QPHH-IM) and Canakkale multifloral honey (MFH-C) for 24 hours. A, indicates western blotting images; B, graphic indicates caspase-3; C, graphic indicates Bax; D, graphic indicates P-53; E, graphic indicates Nf-κB; F, graphic indicates Bcl-2. Cell lysate were used to demonstrate apoptotic and antiapoptotic signaling pathways protein expression. Significant differences according to the control are indicated by *P < .05, **P < .01, and ***P < .001.
However, expression levels were significantly higher in QPHH-IM than in MFH-C at increasing honey sample concentrations.
Discussion
The biological properties of honeys have been studied extensively. While most of the previous studies related to honey’s antioxidant and free radical scavenging properties, studies on prooxidant properties have recently increased.22 In general, these adverse effects have been associated with phenolic contents of honey. However, the mechanism(s) of these opposite effects have not yet been fully understood. In addition, there is no study investigating the relationship between the therapeutic effects of honey on gastric adenocarcinoma cells and their phenolic contents. Some studies consider that dark honeys, including the HH, tend to have higher amounts of phenolic compounds,23,24 but studies on this issue are doubtful. Therefore, we selected these 2 types of honey (QPHH-IM and MFH-C) based on total phenol, flavonoid, and antioxidant contents from 14 different honeys. In addition, we also measured 11 different phenolic compounds in both QPHH-IM and MFH-C samples. These results demonstrated that total and separately measured phenolic compound levels supported each other and that QPHH-IM has a very rich content relative to MFH-C in terms of phenolic compounds.
Cancer cells have a different metabolism than normal cells, and glucose is the primary source of energy for the growth and proliferation of these cells.25 Diets that are high in sugar may potentially cause a metabolic switch from oxidative phosphorylation to glycolysis in tumor cells, which confers the ability to grow in hypoxic environments, fuels tumor growth and invasion, and prevents apoptosis.26 Carbohydrates are the main constituents, comprising about 95% of the honey dry weight, and the main sugars are the monosaccharides fructose and glucose.27 To rule out the possible effects of sugar on the cells, we measured the glucose and fructose content of honeys before cell culture analysis. There was no significant difference between QPHH-IM and MFH-C in terms of glucose and fructose content. Therefore, we think that biological effects of both honeys on AGS cells may be caused by other substances such as non-sugar phenolic compounds.
We investigated cytotoxic, genotoxic, and apoptotic activities and ROS production capacity in human gastric adenocarcinoma cells in order to understand the relationship between phenolic contents and antiproliferative effects. We have demonstrated that QPHH-IM inhibit cell proliferation significantly at concentrations as low as 0.4% (w/v) in AGS cells and antiproliferative activity increased in a dose-dependent manner. We found 50% inhibition after 24 hours incubation of AGS cells with the 1.7% final concentration for QPHH-IM and 2.5% for MFH-C using ATP-Glo cell viability assay kit. The well-known honeys that have antiproliferative activity on different cancer cells are manuka honey,28 tualang honey,29 and gelam honey.30 Cytotoxic doses of these honeys were quite different and vary according to the cell types. Fernandez-Cabezudo et al31 found inhibition of cell proliferation at final concentrations of 0.6% manuka honey. They found 40% inhibition after 24 hours incubation of MCF-7 cells with 5% final concentration of honey. Tualang honey was also shown to exhibit antiproliferative effects on oral squamous and osteosarcoma cell lines and IC50 concentration of 4% (oral squamous cell line) and 3.5% (osteosarcomas cell line).32 These results demonstrated that QPHH-IM can be a more potent cytotoxic to cancer cells than manuka and tualang honeys.
To better understand the mechanisms of antiproliferative effects of these honeys, we analyzed genotoxic, apoptotic, and ROS production activities on gastric adenocarcinoma cells. We have shown that QPHH-IM decreased ROS generation at the concentration of 0.25% (w/v) in AGS cells, and above this concentration, ROS levels begun to increase in a dose-dependent manner. ROS generating activity was higher in QPHH-IM–exposed cells than in MFH-C. In addition, there was a close negative relationship between cell viability and ROS generating activity (r = −0.839, P < .001, for QPHH-IM and r = −0.853, P < .001, for MFH-C on AGS cells). Generally, it has been known that honeys have phenolic compounds that are the main source of antioxidative and free radical scavenging effects.33 On the other hand, honey increases ROS production and shows cell death activity in cancer cells with indications that phenolic compounds are responsible for the increases of ROS production by the prooxidant activity of honey.34 In fact, there is supportive evidence that ROS may have a proliferative or cytotoxic effect on cancer cells. It has been shown that low levels of ROS increase cell proliferation.35 On the other hand, high levels of ROS increase DNA damage, apoptosis, and cell death.36 Normally, phenolic compounds are antioxidants and may inhibit oxidative damage as a consequence of their ability to inhibit ROS. Under certain conditions, however, such as low pH, high phenolic concentrations, and the presence of redox-active transition metals (Fe and Cu), phenolic compounds exhibit prooxidant activity. In particular, the Cu+2 concentration in cancer cells is higher than normal cells, making them more susceptible to the prooxidant activity of phenolic compounds. In the presence of Cu2+, the prooxidant activity of phenolic compounds is supposed to progress via generating OH− radical in a Fenton-type reaction, which eventually leads to DNA damage and apoptosis in cancer cells.37
DNA damage has been investigated in order to understand the mechanisms by which high doses of honeys in cancer cells cause cytotoxicity. We used the comet assay technique to measure the genotoxic effects of both honeys on AGS cells. This is one of the most important methods for the evaluation of DNA damage of different active substances in different cells.38 In this study, we found that QPHH-IM levels above 0.5% caused DNA damage and the same QPHH-IM doses resulted in higher DNA damage than MFH-C in AGS cells. As far as we know, there is no prior experimental evidence that high doses of honeys in GC cells cause DNA damage. The vast majority of the studies have been concerned with the protective effects of honey on DNA damage.39 However, it has been shown that gelam and tualang honeys induced DNA damage in different cancer cells in a dose-dependent manner.40 Our results are consistent with the results of these studies.
Morphological, biochemical, and molecular changes related to apoptosis in cells can be measured by different methods. In the study, apoptosis was analyzed by AO/EB double staining. Apoptotic, necrotic, and living cells can be distinguished by this method. The results of the present work revealed that while high doses of honeys increased apoptosis in AGS cancer cells, QPHH-IM with high phenolic content induced apoptosis more than that of MFH-C with low phenolic content. It has been reported that the anticancer drugs can kill the cancer cells by stimulating the apoptotic pathways.41 Phenolic compounds can affect the cellular redox status because of their prooxidant properties. This can lead to cell death as a result of DNA damage and apoptotic activity.41 Our results are consistent with the results of other recent studies.42 The vast majority of chemotherapists used in cancer treatment show their effects by inducing apoptosis.43 Although the mechanism has not yet been fully understood, studies have shown that honey has antiproliferative effects by inducing apoptosis in cancer cells as well as by multiple cell signaling pathways.44 A recent study to understand honey’s molecular mechanism of colon cancer cell growth inhibition has shown that honey-induced apoptosis upregulates P-53 and is accompanied by modulating the expression of proapoptotic and antiapoptotic proteins.45 Our results are similar to previous studies. We also found that both honey species cause a decrease in Bcl-2 signal expression and an increase in apoptotic P-53, Bax, and caspase-3 signal expression levels. These effects of QPHH-IM were more pronounced than MFH-C, especially at high doses.
NF-κB has an important role in the regulation of intracellular signal transduction and protein expression of various genes in the cell nucleus.46 Exceptional NF-κB activation is associated with the stimulation of proliferation and protection against apoptosis in malignant cells.47 Recent studies have focused on the inhibitory effect of honey on inflammatory-mediated NF-κB activation.48,49 We also showed that NF-κB expression levels decreased with the increasing concentrations of honey, and the degree of inhibition with QPHH-IM was significantly higher than MFH-C, especially at high doses. These results show that honey has not only inhibitory effect on inflammation but also antiproliferative effect on cancer cells.
Conclusion
Data showed that low concentrations of honey samples had proliferative effects due to their antioxidant activity, whereas high concentrations had cytotoxic, genotoxic, and apoptotic effects due to their prooxidant activities in cancer cells. All these effects were higher with QPHH-IM application possessing the high phenolic content when compared with that of MFH-C, which possesses low phenolic content on AGS cancer cells. These preliminary results suggest that high-phenolic honey may contribute to the future development of cancer therapeutics.
Supplemental Material
Supplemental material, Supplement_1_EDITS for Quercus pyrenaica Honeydew Honey With High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells by Abdurrahim Kocyigit, Gokhan Aydogdu, Ezgi Balkan, Vildan Betül Yenigun, Eray Metin Guler, Huri Bulut, Fatmanur Koktasoglu, Ahmet Ceyhan Gören and Ali Timucin Atayoglu in Integrative Cancer Therapies
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abdurrahim Kocyigit  https://orcid.org/0000-0003-2335-412X
https://orcid.org/0000-0003-2335-412X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7:52517-52529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pita-Calvo C, Vázquez M. Differences between honeydew and blossom honeys: a review. Trends Food Sci Technol. 2017;59:79-87. [Google Scholar]
- 4. Tomás-Barberán FA, Martos I, Ferreres F, Radovic BS, Anklam E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J Sci Food Agric. 2001;81:485-496. [Google Scholar]
- 5. Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol. 2008;46:3774-3779. [DOI] [PubMed] [Google Scholar]
- 6. Hassan MI, Mabrouk GM, Shehata HH, Aboelhussein MM. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr Cancer Ther. 2012;11:354-363. [DOI] [PubMed] [Google Scholar]
- 7. Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:1-31. [DOI] [PubMed] [Google Scholar]
- 8. Fauzi AN, Norazmi MN, Yaacob NS. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem Toxicol. 2011;49:871-878. [DOI] [PubMed] [Google Scholar]
- 9. Tsiapara AV, Jaakkola M, Chinou I, et al. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: profile analysis of extracts. Food Chem. 2009;116:702-708. [Google Scholar]
- 10. Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152-178. [Google Scholar]
- 11. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555-559. [Google Scholar]
- 12. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277-285. [DOI] [PubMed] [Google Scholar]
- 13. AOAC International. Sugars and Sugar Products. Vol 2 Gaithersburg, MD: AOAC International; 2000:22-33. [Google Scholar]
- 14. Baki S, Tufan AN, Altun M, Özgökçe F, Güçlü K, Özyürek M. Microwave-assisted extraction of polyphenolics from some selected medicinal herbs grown in Turkey. Rec Nat Prod. 2017;12:29-39. [Google Scholar]
- 15. Yılmaz H, Çarıkçı S, Kılıç T, Dirmenci T, Arabacı T, Gören AC. Screening of chemical composition, antioxidant and anticholinesterase activity of section Brevifilamentum of Origanum (L) species. Rec Nat Prod. 2017;11:439-455. [Google Scholar]
- 16. Han H, Yılmaz H, Gülçin İ. Antioxidant activity of flaxseed (Linum usitatissimum L) shell and analysis of its polyphenol contents by LC-MS/MS. Rec Nat Prod. 2018;12:397-402. [Google Scholar]
- 17. Çarıkçı S, Kılıç T, Özer Z, Dirmenci T, Arabacı T, Gören AC. Quantitative determination of some phenolics in Origanum laevigatum Boiss extracts via validated LC-MS/MS method and antioxidant activity. J Chem Metrol. 2018;12:121-127. [Google Scholar]
- 18. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184-191. [DOI] [PubMed] [Google Scholar]
- 19. McGahon AJ, Martin SJ, Bissonnette RP, et al. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 1995;46:153-185. [DOI] [PubMed] [Google Scholar]
- 20. Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [DOI] [PubMed] [Google Scholar]
- 22. Scepankova H, Saraiva JA, Estevinho LM. Honey health benefits and uses in medicine. In: Alvarez-Suarez JM. ed. Bee Products—Chemical and Biological Properties. Cham, Switzerland: Springer; 2017:83-96. [Google Scholar]
- 23. Al ML, Daniel D, Moise A, Bobis O, Laslo L, Bogdanov S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112:863-867. [Google Scholar]
- 24. Bertoncelj J, Doberšek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822-828. [Google Scholar]
- 25. Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Mjiyad N, Caro-Maldonado A, Ramírez-Peinado S, Muñoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253-264. [DOI] [PubMed] [Google Scholar]
- 27. Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27:677-689. [DOI] [PubMed] [Google Scholar]
- 28. Afrin S, Giampieri F, Gasparrini M, et al. The inhibitory effect of manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: the suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018;9:2145-2157. [DOI] [PubMed] [Google Scholar]
- 29. Syazana MSN, Halim AS, Gan SH, Shamsuddin S. Antiproliferative effect of methanolic extraction of tualang honey on human keloid fibroblasts. BMC Complement Altern Med. 2011;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hakim L, Alias E, Makpol S, Ngah WZW, Morad NA, Yusof Y. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pac J Cancer Prev. 2014;15:4651-4657. [DOI] [PubMed] [Google Scholar]
- 31. Fernandez-Cabezudo MJ, El-Kharrag R, Torab F, et al. Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS One. 2013;8:e55993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghashm AA, Othman NH, Khattak MN, Ismail NM, Saini R. Antiproliferative effect of tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complment Altern Med. 2010;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513-518. [Google Scholar]
- 34. Afrin S, Forbes-Hernandez TY, Gasparrini M, et al. Strawberry-tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: a comparison with manuka honey. Int J Mol Sci. 2017;18:E613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173-186. [DOI] [PubMed] [Google Scholar]
- 36. Kocyigit A, Guler EM. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell Mol Biol. 2017;63:97-105. [DOI] [PubMed] [Google Scholar]
- 37. Azmi AS, Bhat SH, Hadi S. Resveratrol-Cu (II) induced DNA breakage in human peripheral lymphocytes: implications for anticancer properties. FEBS Lett. 2005;579:3131-3135. [DOI] [PubMed] [Google Scholar]
- 38. Liao W, McNutt MA, Zhu WG. The comet assay: a sensitive method for detecting DNA damage in individual cells. Methods. 2009;48:46-53. [DOI] [PubMed] [Google Scholar]
- 39. Cheng N, Wang Y, Cao W. The protective effect of whole honey and phenolic extract on oxidative DNA damage in mice lymphocytes using comet assay. Plant Foods Hum Nutr. 2017;72:388-395. [DOI] [PubMed] [Google Scholar]
- 40. Wen CTP, Hussein SZ, Abdullah S, Karim NA, Makpol S, Yusof YAM. Gelam and nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac J Cancer Prev. 2012;13:1605-1610. [DOI] [PubMed] [Google Scholar]
- 41. Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233-239. [DOI] [PubMed] [Google Scholar]
- 42. Jubri Z, Narayanan NNN, Karim NA, Ngah WZW. Antiproliferative activity and apoptosis induction by gelam honey on liver cancer cell line. Int J Appl Sci Technol. 2012;2:135-141. [Google Scholar]
- 43. Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337-343. [DOI] [PubMed] [Google Scholar]
- 44. Kumar Jaganathan S, Balaji A, Vellayappan MV, et al. A review on antiproliferative and apoptotic activities of natural honey. Anticancer Agents Med Chem. 2015;15:48-56. [DOI] [PubMed] [Google Scholar]
- 45. Afrin S, Giampieri F, Forbes-Hernández TY, et al. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic Biol Med. 2018;126:41-54. [DOI] [PubMed] [Google Scholar]
- 46. Chen L, Teng H, Jia Z, et al. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: the most recent evidence. Crit Rev Food Sci Nutr. 2018;58:2908-2924. [DOI] [PubMed] [Google Scholar]
- 47. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218-233. [DOI] [PubMed] [Google Scholar]
- 48. Hamad R, Jayakumar C, Ranganathan P, et al. Honey feeding protects kidney against cisplatin nephrotoxicity through suppression of inflammation. Clin Exp Pharmacol Physiol. 2015;42:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim HN, Park SB, Kim JD, Jeong HJ, Jeong JB. Acacia honey exerts anti-inflammatory activity through inhibition of NF-kB and MAPK/ATF2 signaling pathway in LPS-Stimulated RAW264. 7 cells. Paper presented at: International Symposium on Natural Products Industry and 2018 Autumn Conference of Korean Society of Plant Resources; September 17-19, 2018; Seoul, Korea. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_1_EDITS for Quercus pyrenaica Honeydew Honey With High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells by Abdurrahim Kocyigit, Gokhan Aydogdu, Ezgi Balkan, Vildan Betül Yenigun, Eray Metin Guler, Huri Bulut, Fatmanur Koktasoglu, Ahmet Ceyhan Gören and Ali Timucin Atayoglu in Integrative Cancer Therapies