Short abstract
Background
Motor recovery following a multiple sclerosis (MS) relapse depends on mechanisms of tissue repair but also on the capacity of the central nervous system for compensating of permanent damage.
Objectives
We aimed to investigate changes in corticospinal plasticity and interhemispheric connections after a relapse of MS using transcranial magnetic stimulation (TMS).
Methods
Twenty healthy and 13 relapsing–remitting MS subjects with a first motor relapse were included. TMS mapping and ipsilateral silent period (iSP) were performed after relapse and at 6-month follow-up.
Results
Strength and dexterity of the paretic hand were impaired at baseline and improved over time. After relapse, mapamplitude and mapdensity were decreased for the ipsilesional-corticospinal tract (IL-CST) while expanded for the contralesional-CST (CL-CST). At follow-up, map parameters normalized for the CL-CST independently from recovery while the increase of outputs from the IL-CST was associated with straight and dexterity improvement. iSP measurements were impaired in MS irrespective of the phase of the disease. Prolonged iSPduration at baseline was associated with less dexterity recovery.
Conclusions
After a motor relapse, TMS mapping shows acute changes in corticospinal excitability and rearrangements of motor outputs. iSP is less influenced by the phase of disease but may better predict recovery, possibly reflecting the integrity of interhemispheric motor networks.
Keywords: TMS, mapping, iSP, multiple sclerosis, relapse
Introduction
Tissue repair after a multiple sclerosis (MS) relapse depends on several factors, such as resolution of inflammation and oedema, and restoration of conduction to remyelinated axons or to persistently demyelinated axons.1 Recovery of symptoms is, however, highly variable, and seems to be only partially related with structural central nervous system (CNS) damage.2 Growing evidence highlights the role of cortical reorganization and neural plasticity in recovery for compensating axonal loss and structural damage.1,3 Functional magnetic resonance imaging studies (fMRI) in MS indicate that functional plasticity is a dynamic phenomenon.4 An increased recruitment of motor areas located in the contralesional hemisphere during movement of the impaired upper limb has been reported in subjects with an acute motor relapse. After 1 year, patients with good clinical recovery had relateralization of motor networks to the previously affected hemisphere, whereas those patients with poor clinical recovery continued to show recruitment of motor areas in the contralesional hemisphere.3 Transcranial magnetic stimulation (TMS) has been used in MS to principally investigate corticomotor conduction time. TMS offers also the possibility to study acute and rapid plastic rearrangements of cortical motor outputs and interhemispheric connections in physiological or pathological conditions.5–7 Thickbroom and colleagues evaluated the topographic organization of the primary motor cortex in relapsing–remitting multiple sclerosis (RRMS). The authors found that delayed conduction was associated with more laterally placed maps that was interpreted as a process of neural plasticity associated with axonal damage in MS.8 Moreover, abnormalities of interhemispheric conduction have been detected in RRMS using the ipsilateral silent period (iSP) technique.9 In both cases, subjects were investigated in the remitting phase of the disease. The aim of the present study was to evaluate early changes of the map of outputs from the two motor cortices and interhemispheric connections in RRMS after acute relapse conditioning motor impairment of one upper limb. We also tested whether these changes correlated with indices of motor function and with motor recovery.
Materials and methods
Population
Thirteen patients (F/M: 9/4, aged 33.3 ± 7.1) with motor impairment of one upper limb (left/right = 6/7) due to an acute relapse of clinically definite MS according to McDonald criteria10 were included. All subjects had symptoms for no more than 10 days and showed a new active lesion at brain or cervical MRI consistent with the acute motor deficit. Nine subjects were under corticosteroid treatment at baseline evaluation (T1). Follow-up (T2) was performed on average at 6 months (range 4–8); during this period subjects were free from new relapses. One MS subject did not perform TMS assessment at T2 because of pregnancy (Table 1). Twenty healthy volunteers matched according to gender and age (F/M:13/7, aged 30.3 ± 8.8) were also studied. Subjects gave their written informed consent before participating in the study, which was approved by our local Ethics Committee.
Table 1.
Demographic data and clinical features.
| Age | Gender | Disease duration | Relapse | MRI lesion load | Symptomatic lesion site | Time from relapse | Corticosteroid intake | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | (years) | F/M | MS | (months) | N° | N° | EDSS | (days) | (days) | |
| 1 | 31 | F | RR | 79 | 1 | 13 | 1.5 | Cervical | 7 | 3 |
| 2 | 23 | M | RR | 30 | 3 | 16 | 1.5 | Cervical | 6 | 3 |
| 3 | 49 | M | RR | 89 | 1 | 10 | 1.0 | Cervical | 5 | 1 |
| 4 | 35 | F | RR | 144 | 4 | 30 | 3.0 | ST | 5 | 0 |
| 5 | 30 | F | RR | 14 | 3 | 24 | 2.0 | Cervical | 5 | 0 |
| 6 | 30 | F | RR | 106 | 3 | 20 | 1.5 | Cervical | 3 | 0 |
| 7 | 29 | F | RR | 12 | 1 | 9 | 0.0 | Cervical | 9 | 5 |
| 8 | 33 | F | RR | 227 | 2 | 27 | 3.0 | Cervical | 10 | 5 |
| 9 | 35 | M | RR | 144 | 6 | 11 | 2.0 | Cervical | 10 | 4 |
| 10 | 42 | F | RR | 98 | 4 | 30 | 2.5 | ST | 7 | 5 |
| 11 | 37 | M | RR | 7 | 1 | 12 | 1.0 | Cervical | 10 | 3 |
| 12 | 23 | F | RR | 4 | 1 | 7 | 0.0 | ST | 10 | 5 |
| 13 | 37 | F | RR | 288 | 4 | 15 | 3.0 | ST | 7 | 2 |
| m/sd | 33.3±7.1 | 9/4 | 95.5±88.3 | 2.6±1.6 | 17.23±8.11 | 1.6±1 | 7.2±2.3 |
F: female; M: male; ST: supratentorial
Clinical assessment
Patients received a complete neurological examination including Medical Research Council (MRC) grading (where 0 indicates no movement and 5 normal strength) of abductor pollicis brevis (APB) and abductor digiti minimi (ADM). Arm dexterity was assessed with the nine-hole peg test (NHPT) which is a reliable measure commonly used in neurological diseases, such as MS and stroke.11,12 Furthermore, NHPT has been already used as measure of motor dexterity in previous studies for evaluating longitudinal changes after acute motor relapse of one side in MS.3,13 We performed NHPT for both sides in MS subjects and controls.
Transcranial magnetic stimulation
TMS was delivered with a Magstim 200 simulator (Magstim Company, Ltd., Whitland, Dyfed, UK) connected to a figure-of-eight coil (70 mm of external diameter). The coil was positioned over the best scalp location for optimal motor evoked potentials (MEPs) to the contralateral hand muscle. Electromyogram (EMG) was recorded from APB and ADM muscles using Ag/AgCl surface electrodes in a belly-tendon montage. For measurement of iSPs, 15 stimuli were applied at an intensity of 90% of the maximal stimulator output to ensure maximal activation of transcallosal neurons while subjects were performing a voluntary maximal contraction of the ipsilateral APB muscle.6,7,14,15 This intensity was selected because it produces stable plateau values for iSPonset latency and iSPduration.16,17
For the cortical map registration, we used a grid centred on the vertex. Intersection points of the grid lines were spaced 1 cm apart and served as visual references for coil positioning. APB and ADM muscles were simultaneously and bilaterally recorded. The cortical hotspot was defined as the grid node eliciting optimal MEPs on at least one of the contralateral APB or ADM muscles. For each side, resting motor threshold (RMT) was measured as the minimal intensity evoking MEPs in at least one of the two muscles with amplitude of 50 μV or higher in 5 out of 10 stimuli. TMS mapping was performed at intensity of 115% of RMT, by stimulating every 4 s at adjacent grid nodes starting from the hotspot until no MEPs were evoked. Four sequential MEPs were obtained for each node.6,7 The level of background EMG activity was constantly monitored during the experiment, controlling for muscle relaxation during both RMT detection and TMS mapping. Data were acquired using the SynAmp/SCAN 4.3 system (Compumedics Germany GmbH, Singen, Germany).
Offline data analyses
Considering TMS mapping, the peak-to-peak amplitudes of four MEPs obtained from the stimulation of each scalp position were measured and averaged. Then we calculated:
Maparea (cm2), as the number of responsive sites at which a MEP of amplitude over 50 μV was evoked.
Mapamplitude (µV), as ∑ iMEPi e where MEPi is the amplitude at i-th grid point.
Mapdensity, as mapamplitude/maparea (µV/cm2).
where MEPX/MEPY are the amplitudes of the MEP value at X/Y-th grid position.
Data from both muscles were averaged and, for controls, measurements obtained from the left and right hemispheres were averaged.
Ipsilateral MEPs to stimulation of the unlesioned corticospinal tract (CST) were also checked.
The iSP was quantified in the average of the 15-single rectified-EMG traces. The iSPonset was defined as the time point, after TMS pulse, with EMG activity constantly smaller than the averaged baseline EMG contraction (pre-stimulus between –50 and –20 ms). The iSPoffset was defined as the first time point after iSPonset in which the level of EMG activity regained the baseline value. The iSPduration was the difference between iSPoffset and iSPonset. The iSParea (in mV*s) was calculated as [iSPamplitude, defined as mean EMG level between iSPonset and iSPoffset * ISPduration].15 A good reliability has been demonstrated for iSPduration.18 Furthermore, no effects of varying the magnitude of the contraction have been found on the time course of the iSP or on the depth of inhibition but only when expressed as a percentage of the baseline EMG level.19 To reduce inter-subject and inter-session variability related to the degree of pre-stimulus contraction, iSParea was normalized for the rectified baseline EMG activity between –50 ms and –20 ms pre-stimulus as follows:
Statistical analysis
Data were analysed with IBM SPSS Statistics 22.0.
A mixed factorial ANOVA designed for repeated measurements was used for clinical and neurophysiological variables using ‘time’ (T1 = baseline and T2 = follow-up) and ‘side’ (affected and unaffected) as a within-subjects factors and ‘group’ (MS and controls) as a between-subjects factor. According to sample distribution to the Kolmogorov–Smirnov test, post-hoc analyses were performed using parametric tests for neurophysiological parameters and non-parametric tests for clinical measurements. Spearman’s correlation coefficient was used to test the relationship between clinical and neurophysiological variables. Significance level was set at p ≤ 0.05.
Results
Clinical evaluation
The ANOVA analysis performed for the NHPT showed a significant effect of ‘group’ factor (F1,31 = 33.7, p < 0.0001), MS subjects being slower than controls independently from the side and the time of evaluation. A significant interaction among ‘group’, ‘side’ and ‘time’ factors (F1,31 = 46.3, p < 0.0001) was also obtained. MS subjects performed the NHPT with the affected side slower than with the unaffected at both T1 (z12 = 3.06, p = 0.002) and T2 (z12 = 3, p = 0.003), although significantly recovered over time (z12 = 3, p = 0.002). MRC score showed the same trend (ANOVA ‘side’ and ‘time’ effect: F1,12 = 27.9, p < 0.0001), hand muscles were weaker for the affected than the unaffected side at T1 (z12 = –3.2, p = 0.001) and T2 (z12 = –2.6, p = 0.007), but significantly recovered over time (z12 = –3, p = 0.003).
TMS mapping
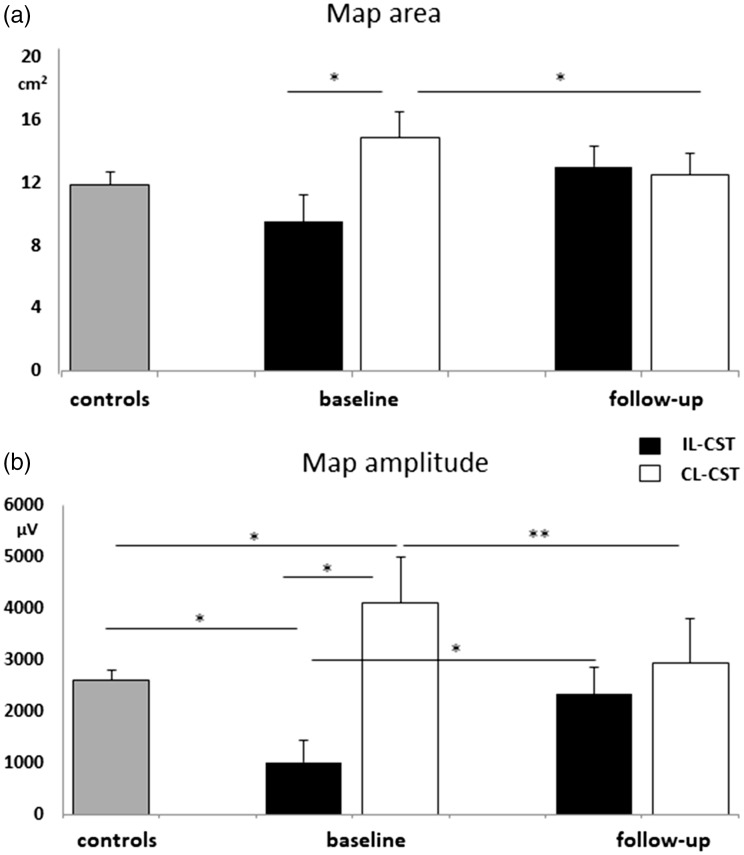
At baseline, three subjects showed no MEPs to stimulation of the ipsilesional-CST (IL-CST) (subjects 4, 5, 12), and subject 7 showed MEPs of amplitude lower than 50 µV at maximal stimulator output so that TMS mapping was not performed. For all these subjects MEPs recovered at follow-up. RMT was significantly higher for MS than for control (‘group’ effect: F1,26 = 4.9, p = 0.34), independently from the side and time of evaluation. For map parameters a significant interaction among ‘group’, ‘side’ and ‘time’ factors was obtained (maparea: F1,26 = 17.1, p < 0.0001; mapamplitude: F1,26 = 30.4, p < 0.0001 and mapdensity: F1,26 = 11.4, p = 0.002). Maparea to the IL-CST resulted in significantly lower in comparison with the contralesional-CST (CL-CST) (t8: –3.3, p = 0.01) and mapamplitude in comparison with the CL-CST and control (t8: –2.8, p = 0.02 and t27: –2.7, p = 0.01, respectively). On the contrary, mapamplitude and mapdensity to the CL-CST were significantly increased compared with controls (t31: 2.2, p = 0.031 and t31: 2.1, p = 0.038, respectively) and a trend was observed for maparea (t31: 1.9, p = 0.06). Over time, mapamplitude significantly increased for the IL-CST (t8: –2.8; p = 0.022) while all the map parameters decreased for the CL-CST (maparea: t11: 2.7, p = 0.019 mapamplitude t11: 3.4; p = 0.005 and mapdensity t11: 3, p = 0.010) (Figure 1 and 2).
Figure 1.
Cortical map parameters in MS patients and controls. (a) greater maparea to the contralesional-corticospinal tract (CL-CST) in comparison with the ipsilesional-corticospinal tract (IL-CST) at baseline that significantly decrease at follow-up. (b) reduced mapamplitude to the IL-CST in comparison with the CL-CST and controls and increased mapamplitude to the CL-CST in comparison with controls at baseline. Significant mapamplitude increase for the IL-CST and decrease for the CL-CST at follow-up. *p < 0.05; **p < 0.005
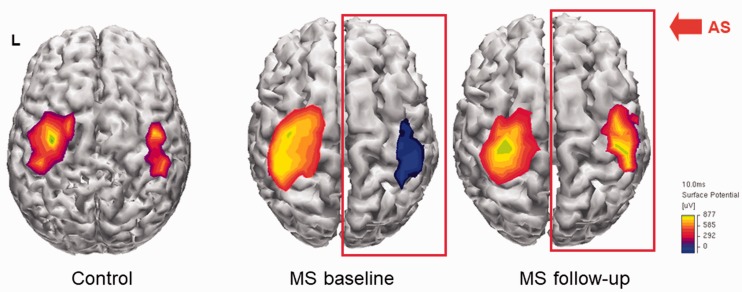
Figure 2.
Example of cortical motor mapping from a single patient. MEPs were obtained from the APB muscle of both sides by TMS of the contralateral motor cortex. MEPs amplitudes higher than 50 µV were interpolated and projected on an average brain cortical surface reconstruction using Curry software V4.6.
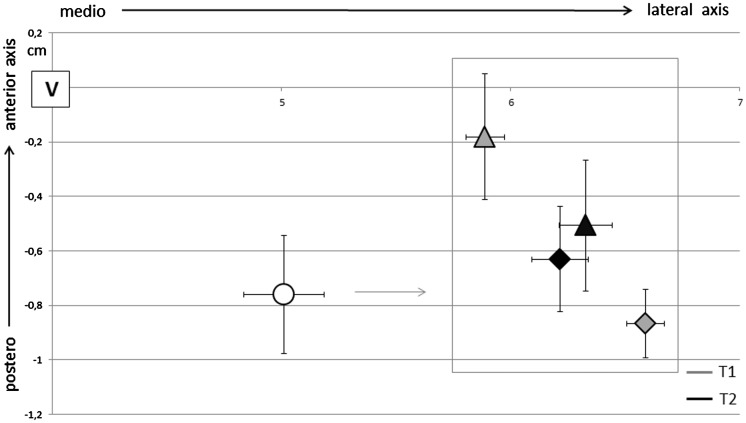
The analysis of CoGs position along the medio-lateral axis showed a significant effect of ‘group’ factor (F1,26 = 16.8, p < 0.0001), CoGs in MS being laterally displaced compared with controls independently from the side and time of evaluation. An interaction among ‘group’, ‘side’ and ‘time’ factors (F1,26 = 54.1, p < 0.0001) was also obtained. At baseline CoG of the unaffected side was more medial than the affected (t8: 6.6, p < 0.0001) and at follow-up it lateralized (t11: –3.1, p = 0.01). Considering the antero-posterior axis, a significant interaction among ‘group’, ‘side’ and ‘time’ factors (F1,26 = 40.2, p < 0.0001) was obtained. CoG of the unaffected side was anteriorly positioned with respect to the ipsilesional side at T1 (t8: –2.5, p = 0.03) and over time it shifted posteriorly (t11: 2.5, p = 0.05). At baseline, the greater the map volume of the CL-CST the greater anteriorly map shift (r = 0.65, p = 0.015). There were no significant differences in CoG position between MS subjects and controls in the anterior–posterior axis (Figure 3). In our patients, iMEPs to stimulation of the CL-CST were never elicited.
Figure 3.
CoGs position in respect with vertex for controls (circular marker) and MS subjects (triangle for the unaffected side and rhombus for the affected side) at baseline (T1) and follow-up (T2). CoGs in MS subjects were laterally displaced in comparison with controls (p < 0.0001). At baseline, CoG of the unaffected side was more medially (p < 0.0001) and anteriorly (p = 0.03) positioned than in the affected side in MS. At follow-up CoGs positions of the two hemispheres were more symmetric.
iSP
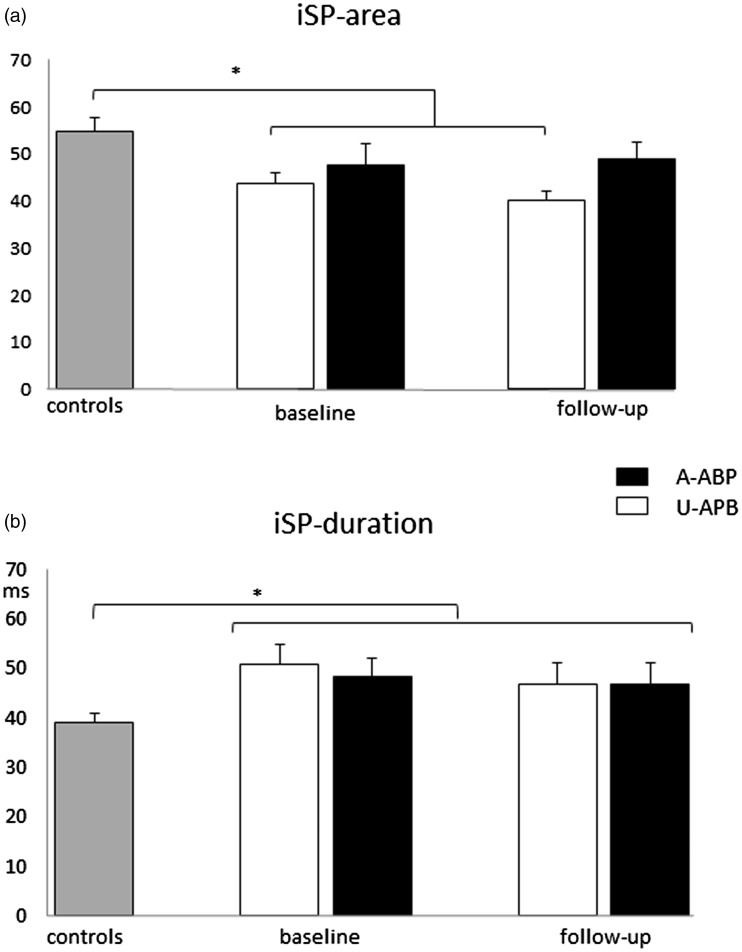
From the ANOVA analysis we obtained a ‘group’ effect (F1,29 = 7, p = 0.01), and a significant interaction between ‘group’ and ‘side’ factors (F1,29 = 7.7, p = 0.01), and iSParea on the unaffected APB was significantly lower in comparison with controls (t28: –3; p = 0.006). Only a significant effect of ‘group’ factor was obtained for iSPonset and iSPduration; MS showed delayed iSPonset and longer iSPduration than controls independently of the side and time of evaluation (F1,29 = 10.1; p = 0.003 and F1,29 = 9,2; p = 0.005, respectively) (Figure 4).
Figure 4.
Ipsilateral silent period parameters (area-a and duration-b) in controls and MS on the affected (A-APB) and unaffected APB (U-APB). *p < 0.05.
Correlation of clinical measurements and neurophysiological parameter
Expanded Disability Status Scale (EDSS) in the relapsing as well as in the remitting phase directly correlated with disease duration (r = 0.77, p = 0.002 and r = 0.87, p < 0.001, respectively), the number of relapses (r = 0.78, p = 0.001 and r = 0.75, p = 0.003, respectively), and the MRI lesion load (r = 0.69, p = 0.009 and r = 0.77, p = 0.002, respectively). No correlations were found with neurophysiological parameters.
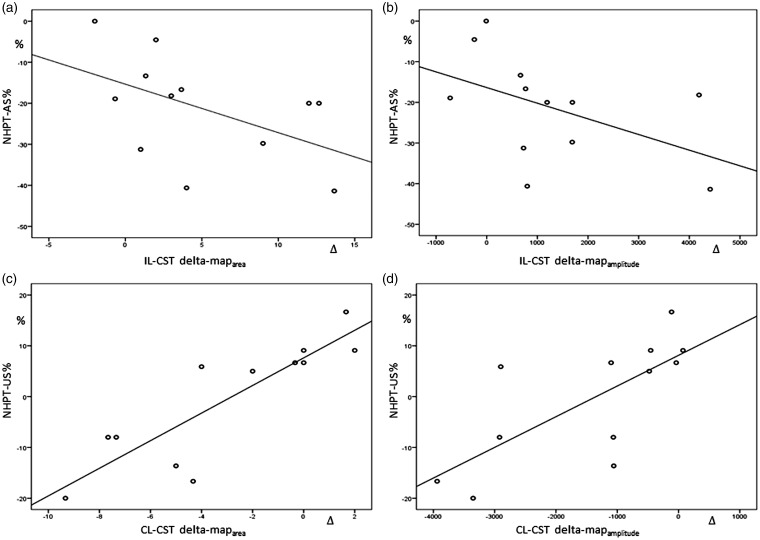
Map parameters were not influenced by lesion site (cervical or supratentorial), disease duration, corticosteroids intake or number of relapses. At baseline, map parameters to the CL-CST did not correlated with motor performance of both sides, while maparea and mapamplitude to the IL-CST correlated with MRC score (r = 0.55, p = 0.001 and r = 0.56, p = 0.001) and NHPT of the affected side (r = −0.46, p = 0.007 and r = −0.53, p = 0.001). Baseline values of map parameters to the IL-CST and CL-CST did not correlated with motor recovery of the paretic arm. The over-time increase in mapdensity to the IL-CST correlated with MRC improvement at follow-up (r = 0.62, p = 0.02); NHPT improvement weakly correlated with the over-time increase of maparea and mapamplitude (r = −0.6, p = 0.037 and r = −0.5, p = 0.044, respectively). Interestingly, reduction of maparea and mapamplitude to the CL-CST was associated with an amelioration in performing NHPT with the unlesioned side (r = 0.9, p < 0.001 and r = 0.8, p = 0.003) (Figure 5).
Figure 5.
NHPT improvement for the affected side (AS) correlated with the over-time increase of maparea (a) and mapamplitude (b) to the IL-CST (r = −0.6, p = 0.037 and r = −0.5, p = 0.044, respectively). Amelioration in performing NHPT with the unlesioned side (US) correlated with reduction of maparea (c) and mapamplitude (d) to the CL-CST (r = 0.9, p < 0.001 and r = 0.8, p = 0.003, respectively).
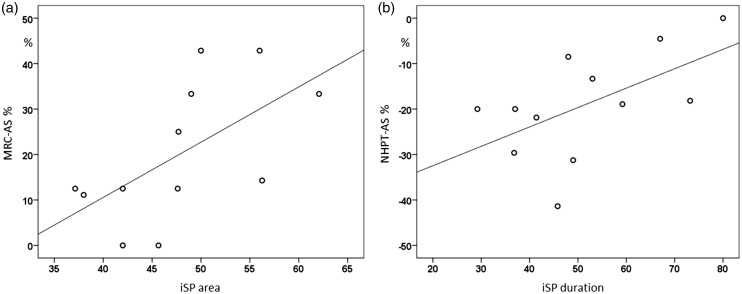
ISParea at baseline negatively correlated with the number of relapses (r = –0.6, p = 0.038). Greater iSParea on the affected hand at baseline directly correlated with muscular straight improvement (r = 0.7, p = 0.015) and iSPduration with NHPT improvement at follow-up (r = 0.6, p = 0.035) (Figure 6). No significant correlations were obtained between cortical map parameters and iSP measurements.
Figure 6.
(a) iSParea on the affected APB at baseline directly correlated with muscular straight improvement of the affected side (AS) (r = 0.7, p = 0.015) and (b) iSPduration with NHPT improvement of the AS at follow-up (r = 0.6, p = 0.035).
Discussion
TMS mapping provides complementary information about the integrity of the CST and changes of corticospinal excitability after brain lesions. Our data indicate that acute relapse of MS involving the CST of one side determinates bi-hemispheric changes of corticospinal excitability. In particular, we observed an enlargement of the cortical motor representation of hand muscles of the unaffected side which was associated with a displacement of CoGs towards more frontal regions. This anterior shift could reflect some degree of cortical plasticity and perhaps an activation of premotor areas.20 This latter phenomenon has been described in fMRI studies on remission–relapsing or secondary progressive MS;3,21 however, the role played by the different brain areas in terms of compensation or restoration of function is controversial. TMS mapping provides an indication of cortical regions directly projecting to the target muscle.8 In this study, iMEPs to stimulation of the CL-CST have not been observed. Furthermore, we found that early hyperexcitability of the unlesioned CST is irrespective of the severity of motor deficit or lesion site and it decreases over time (around 6 months), becoming closer to that of controls independently from motor recovery. These data suggest that, in the relapsing phase of the disease, changes of corticospinal excitability of the unlesioned side do not reflect a true functional reorganization. Motor cortex hyperexcitability could be the expression of a disinhibition, derived from changes in either transcallosal or intracortical circuits. Transcallosal connections have been investigated in this study using iSP, which is a measure of the interhemispheric control of voluntary cortical motor output.22 We found a reduction of iSP parameters reflecting a declined efficiency of interhemispheric inhibition. However, the hyperexcitability of the unlesioned hemisphere does not directly correlate with interhemispheric inhibitions, suggesting that this could not be the only mechanism involved. Although intracortical circuits have not been tested in this study, a diffused lack of normal gamma-aminobutyric acid (GABA) receptors-mediated intracortical inhibitory activity after acute relapse has been demonstrated in a previous study.23 As MEPs mainly originate from the stimulation of excitatory axons impinging on the CST,24 our finding of increased map of output from the CL-CST supports the idea that the relapsing phase of MS is paralleled by an imbalance between glutamate and GABA transmission, favouring excitation processes. It is, therefore, likely that acute hyperexcitability of the contralesional side could be the effect of a diffuse and unspecific inflammatory status. In this regard, rapid functional changes in the excitability of cortical motor circuits have been obtained immediately after high-dose steroids intake.25 We did not observe similar effects on map parameters probably because steroid intake was not standardized and follow-up was performed several months later. The over-time hyperexcitability decrement was associated with an improvement in performing NHPT with the ipsi-lesional hand, suggesting that acute disinhibition of the unlesioned hemisphere could transitorily affect bi-hemispherical cortical networks involved in the more complex unilateral motor tasks such as NHPT.5,26
Considering the IL-CST, TMS mapping parameters at baseline were strongly affected by the acute damage of the CST by lesion; for three subjects a severe conduction block was observed and for the remaining subjects the map of outputs was reduced. We observed a kind of mismatch between two map parameters, mapamplitude was much more severely decreased than maparea. This indicates that the cortical excitable area of hand muscles (as maparea) was almost preserved, being the cortical region not involved in acute relapse. Reduction of map amplitudes could be, instead, the effect of impaired temporal or spatial summation of impulses arriving at the spinal motoneuron pool due to slowed axonal conduction or conduction block.1,27,28 Reduction of the map of output from the affected side strongly correlated with the degree of motor impairment in the relapsing phase, but was not strictly predictive of poor recovery. This latter finding leads to several considerations. After acute relapse, the alteration of axonal conduction to TMS could be related to transitory oedema rather than to demyelination, which cannot be determined with certainty.27 Second, it is likely that impairment of baseline MEPs is representative of the degree of corticospinal damage produced by an acute lesion but is not able to predict the efficiency of the CNS in restoring the conduction to remyelinated axons or to persistently demyelinated axons. Third, behavioural recovery could be related to tissue repair as well as to mechanisms of brain plasticity occurring when tissue repair is incomplete.29 In a previous MRI study, no correlations were found between the pseudotumoral lesion volume involving the CST and motor performance.3 Recovery of symptoms scarcely correlated with structural CNS damage, as measured using MRI.2 The evaluation of synaptic plasticity seems, instead, to predict long-term recovery (up to 12 weeks). For instance, normal synaptic plasticity, measured during a relapse of MS with the paired associative stimulation protocol, has been associated with complete recovery, and impaired plasticity with incomplete or absent recovery.29 From our data we found that MEPs recovered over time for all subjects, and map parameters significantly increased for most of them. The increase of mapdensity over the affected side correlated with the improvement of hand strength. The enlargement of the map of outputs from the affected hemisphere could be related to the restoration of conduction to corticospinal axons. Otherwise, TMS may activate a larger number of cortical interneurons that connect to the same unlesioned corticospinal neurons,30 indicating a mechanism of cortical plasticity. The amelioration in hand dexterity weakly correlated with the increase of output from the IL-CST. As previously reported, the NHPT task involves bi-hemispheric networks.26 Studying transcallosal connections using TMS offers the possibility to detect clinically disabling as well as subclinical alteration of interhemispheric functional connectivity in MS.31 Interestingly, from the iSP analysis, we found that prolonged inhibition of the voluntary movement of the paretic muscle to stimulation of the ipsilateral motor cortex, at baseline, negatively correlated with hand dexterity improvement. A prolongation of iSPduration could be explained by involvement of either the transcallosal connections between the two motor cortices or the CST that originates from the non-stimulated motor cortex. As described by Jung and colleagues, iSPduration is the most sensitive among the iSP measures in MS.9 For instance, partial demyelination of callosal fibres or CST fibres projecting to the target muscle could result in a normal iSPonset from interruption of voluntary activity in normally conducting fibres, but dispersed iSP transmission and delayed resumption of voluntary activity along demyelinated fibres.9 Considering this, iSPduration could be influenced by acute and chronic neuronal damage involving interhemispheric connection through corpus callosum and corticospinal projections as well. In this respect, it may be representative of the integrity of hemispheric functional motor networks and therefore a good predictor of recovery.
Conclusions
We obtained bi-hemispheric chances of corticospinal excitability after acute motor relapse of one upper limb consisting of a reduction of outputs from the ipsilesional side and in a transitory hyperexcitability of the contralesional side. The increase of the map of outputs from the IL-CST was associated with hand straight and weakly with dexterity improvement. iSP measurements were less influenced by the time after relapse, but were able to predict motor hand recovery, possibly reflecting the integrity of interhemispheric functional motor networks.
Contributor Information
Raffaella Chieffo, Department of Neurorehabilitation and Department of Clinical Neurophysiology, Hospital San Raffaele, Milan, Italy; Experimental Neurophysiology Unit, Institute of Experimental Neurology (INSPE), San Raffaele Scientific Institute, Milan, Italy.
Laura Straffi, University Vita-Salute San Raffaele, Milan, Italy.
Alberto Inuggi, Experimental Neurophysiology Unit, Institute of Experimental Neurology (INSPE), San Raffaele Scientific Institute, Milan, Italy.
Elisabetta Coppi, University Vita-Salute San Raffaele, Milan, Italy.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the FISM (Fondazione Italiana Sclerosi Multipla). Grant number: FISM07/R/8.
References
- 1.Smith KJ, McDonald WI. The pathophysiology of multiple sclerosis: The mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci 1999; 354(1390): 1649–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippi M, Grossman RI. MRI techniques to monitor MS evolution: The present and the future. Neurology 2002; 58(8): 1147–1153. [DOI] [PubMed] [Google Scholar]
- 3.Mezzapesa DM, Rocca MA, Rodegher M, et al. Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp 2008; 29(5): 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi M, Preziosa P, Rocca MA. Brain mapping in multiple sclerosis: Lessons learned about the human brain. Neuroimage 2017; 190: 32--2. [DOI] [PubMed] [Google Scholar]
- 5.Chieffo R, Inuggi A, Straffi L, et al. Mapping early changes of cortical motor output after subcortical stroke: A transcranial magnetic stimulation study. Brain Stimul 2013; 6(3): 322–329. [DOI] [PubMed] [Google Scholar]
- 6.Chieffo R, Straffi L, Inuggi A, et al. Motor cortical plasticity to training started in childhood: The example of piano players. PLoS ONE 2016; 11(6): e0157952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppi E, Houdayer E, Chieffo R, et al. Age-related changes in motor cortical representation and interhemispheric interactions: A transcranial magnetic stimulation study. Front Aging Neurosci 2014; 6: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thickbroom GW, Byrnes ML, Archer SA, et al. Corticomotor organisation and motor function in multiple sclerosis. J Neurol 2005; 252(7): 765–771. [DOI] [PubMed] [Google Scholar]
- 9.Jung P, Beyerle A, Humpich M, et al. Ipsilateral silent period: A marker of callosal conduction abnormality in early relapsing-remitting multiple sclerosis? J Neurol Sci 2006; 250(1–2): 133–139. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–127. [DOI] [PubMed] [Google Scholar]
- 11.Kragt JJ, van der Linden F a. H, Nielsen JM, et al. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Mult Scler 2006; 12(5): 594–598. [DOI] [PubMed] [Google Scholar]
- 12.Chen H-M, Chen CC, Hsueh I-P, et al. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair 2009; 23(5): 435–440. [DOI] [PubMed] [Google Scholar]
- 13.Wirsching I, Buttmann M, Odorfer T, et al. Altered motor plasticity in an acute relapse of multiple sclerosis. Eur J Neurosci 2018; 47(3): 251–257. [DOI] [PubMed] [Google Scholar]
- 14.Spagnolo F, Coppi E, Chieffo R, et al. Interhemispheric balance in Parkinson’s disease: A transcranial magnetic stimulation study. Brain Stimul 2013; 6(6): 892–897. [DOI] [PubMed] [Google Scholar]
- 15.Trompetto C, Bove M, Marinelli L, et al. Suppression of the transcallosal motor output: A transcranial magnetic stimulation study in healthy subjects. Exp Brain Res 2004; 158(2): 133–140. [DOI] [PubMed] [Google Scholar]
- 16.Meyer BU, Röricht S, Gräfin von Einsiedel H, et al. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 1995; 118 (Pt 2): 429–440. [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 2003; 89(3): 1256–1264. [DOI] [PubMed] [Google Scholar]
- 18.Fleming MK, Newham DJ. Reliability of transcallosal inhibition in healthy adults. Front Hum Neurosci 2017. [accesssed April 2019]; 10. Available at: https://www.frontiersin.org/articles/10.3389/fnhum.2016.00681/full [DOI] [PMC free article] [PubMed]
- 19.Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol 1992; 453: 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delvaux V, Alagona G, Gérard P, et al. Post-stroke reorganization of hand motor area: A 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol 2003; 114(7): 1217–1225. [DOI] [PubMed] [Google Scholar]
- 21.Rocca MA, Colombo B, Falini A, et al. Cortical adaptation in patients with MS: A cross-sectional functional MRI study of disease phenotypes. Lancet Neurol 2005; 4(10): 618–626. [DOI] [PubMed] [Google Scholar]
- 22.Giovannelli F, Borgheresi A, Balestrieri F, et al. Modulation of interhemispheric inhibition by volitional motor activity: An ipsilateral silent period study. J Physiol (Lond) 2009; 587(Pt 22): 5393–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caramia MD, Palmieri MG, Desiato MT, et al. Brain excitability changes in the relapsing and remitting phases of multiple sclerosis: A study with transcranial magnetic stimulation. Clin Neurophysiol 2004; 115(4): 956–965. [DOI] [PubMed] [Google Scholar]
- 24.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994; 91(2): 79–92. [DOI] [PubMed] [Google Scholar]
- 25.Ayache SS, Créange A, Farhat WH, et al. Relapses in multiple sclerosis: Effects of high-dose steroids on cortical excitability. Eur J Neurol 2014; 21(4): 630–2. [DOI] [PubMed] [Google Scholar]
- 26.Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: Representation of movement difficulty or memory load? Clin Neurophysiol 2003; 114(4): 605–613. [DOI] [PubMed] [Google Scholar]
- 27.Kukowski B. Duration, configuration and amplitude of the motor response evoked by magnetic brain stimulation in patients with multiple sclerosis. Electromyogr Clin Neurophysiol 1993; 33(5): 295–297. [PubMed] [Google Scholar]
- 28.Hardmeier M, Leocani L, Fuhr P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. Mult Scler 2017; 23(10): 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori F, Kusayanagi H, Nicoletti CG, et al. Cortical plasticity predicts recovery from relapse in multiple sclerosis. Mult Scler 2014; 20(4): 451–457. [DOI] [PubMed] [Google Scholar]
- 30.Topka H, Cohen LG, Cole RA, et al. Reorganization of corticospinal pathways following spinal cord injury. Neurology 1991; 41(8): 1276–1283. [DOI] [PubMed] [Google Scholar]
- 31.Codecà C, Mori F, Kusayanagi H, et al. Differential patterns of interhemispheric functional disconnection in mild and advanced multiple sclerosis. Mult Scler 2010; 16(11): 1308–1316. [DOI] [PubMed] [Google Scholar]