Abstract
BACKGROUND
Blood pressure (BP) and cholesterol are major modifiable risk factors for cardiovascular disease (CVD), but effects of exposures during young adulthood on later life CVD risk have not been well quantified.
OBJECTIVE
The authors sought to evaluate the independent associations between young adult exposures to risk factors and later life CVD risk, accounting for later life exposures.
METHODS
The authors pooled data from 6 U.S. cohorts with observations spanning the life course from young adulthood to later life, and imputed risk factor trajectories for low-density lipoprotein (LDL) and high-density lipoprotein cholesterols, systolic and diastolic BP starting from age 18 years for every participant. Time-weighted average exposures to each risk factor during young (age 18 to 39 years) and later adulthood (age ≥40 years) were calculated and linked to subsequent risks of coronary heart disease (CHD), heart failure (HF), or stroke.
RESULTS
A total of 36,030 participants were included. During a median follow-up of 17 years, there were 4,570 CHD, 5,119 HF, and 2,862 stroke events. When young and later adult risk factors were considered jointly in the model, young adult LDL ≥100 mg/dl (compared with <100 mg/dl) was associated with a 64% increased risk for CHD, independent of later adult exposures. Similarly, young adult SBP ≥130 mm Hg (compared with <120 mm Hg) was associated with a 37% increased risk for HF, and young adult DBP ≥80 mm Hg (compared with <80 mm Hg) was associated with a 21% increased risk.
CONCLUSIONS
Cumulative young adult exposures to elevated systolic BP, diastolic BP and LDL were associated with increased CVD risks in later life, independent of later adult exposures.
Keywords: blood pressure, cholesterol, coronary heart disease, heart failure, stroke, young adulthood
Blood pressure (BP) and cholesterol are major modifiable cardiovascular disease (CVD) risk factors and key components of risk prediction algorithms (1–4). Compared with BP or cholesterol measured at the time of risk assessment (usually in middle or older age), average levels over many years may more accurately capture an individual’s long-term risk factor exposure history and may offer additional prognostic information (5–10). Young adult exposures to elevated BP and cholesterol are associated with subclinical atherosclerosis in middle age as well as risk for CVD events that occur decades later (8–15). However, it is unclear whether exposures to risk factors during young adulthood contribute independently to future CVD risk above and beyond later life exposures (14). Because most cohort studies are restricted in their baseline age range, and few cohorts followed participants from young adulthood to later life, it has been challenging to elucidate independent contributions of risk factor exposures during early versus later adulthood because few cohorts measured both young adult exposures and substantial numbers of CVD events, which primarily occur much later in life.
By pooling and harmonizing data from 6 prospective cohort studies with repeated risk factor measurements, our study sought to model complete risk factor trajectories starting at 18 years of age until the end of follow-up for all study participants, and used those trajectories to estimate the independent associations of risk factor exposures during young adulthood (18 to 39 years of age) and later adulthood (≥40 years of age) with subsequent risks of coronary heart disease (CHD), heart failure (HF), and stroke.
METHODS
STUDY DESIGN AND COHORTS.
The present analysis was based on data from 6 large, community-based, prospective cohort studies: 1) the ARIC (Atherosclerosis Risk In Communities) study (16); 2) the CARDIA (Cardiovascular Risk Development in Young Adults) study (17); 3) the CHS study (Cardiovascular Health Study) (18); 4) the FHS-O cohort (Framingham Heart Study Offspring Cohort) (19); 5) the Health ABC (Health, Aging and Body Composition) study (20); and 6) the MESA study (Multi-Ethnic Study of Atherosclerosis) (21). Details of the design of each study are reported in the Online Methods in the Online Appendix. All data were centralized at Columbia University for pooling, harmonization, and analysis, as part of the NHLBI (National Heart, Lung, and Blood Institute) Pooled Cohorts study (22). The present analysis was restricted to participants ≥18 years of age without known CVD at baseline and with at least 1 nonmissing value for each CVD risk factor (Online Figure 1). The final sample size comprised 36,030 individuals.
CLINICAL DATA COLLECTION AND FOLLOW-UP FOR CVD EVENTS.
Details of clinical data collection and events follow-up are reported in the Online Methods. The primary CVD risk factors of interest in the current analysis were systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. The primary outcomes of interest for our analysis were incident CHD (defined as myocardial infarction or CHD death), HF, and stroke (ischemic or hemorrhagic). Events were ascertained and adjudicated using each cohort’s specific protocol, and the details are provided in Online Table 1.
IMPUTATION OF CVD RISK FACTORS ACROSS THE LIFE COURSE.
Most studies are restricted in age range and therefore did not directly measure CVD risk factors during both early and later life (for example, participants in the ARIC study were enrolled after 45 years of age, and therefore, their CVD risk factor levels before age 45 years were not observed). We previously developed a method to impute risk factors across the life course (14,23). Details of the method have been described elsewhere (23). Briefly, we pooled data from multiple cohorts (which together span the adult life course), and leveraged the risk factor patterns observed in the younger cohorts to impute unobserved young adult exposures in the older cohorts, and vice versa. We used linear mixed models to estimate latent trajectories underlying the observed values for each participant, and imputed risk factor levels annually from age 18 years through the end of follow-up for each participant. Examples of imputed LDL trajectories for 18 randomly selected participants (3 participants per study) are illustrated in Online Figure 2. Results of the validation of the imputation method are shown in Online Figure 3.
STATISTICAL ANALYSES.
Using the imputed trajectories, we calculated period-specific time-weighted averages (TWAs) of SBP, DBP, LDL, and HDL levels as summary measures of young (18 to 39 years of age) and later adult (≥40 years of age) exposures to CVD risk factors (Online Figure 2).
We used Cox proportional hazards models for each CVD outcome to calculate hazard ratios (HRs) for young adult and later life risk factor exposures. We used age as the time scale, with the origin for time to event set at the first in-person visit or age 40 years, whichever occurred later. Each Cox model was stratified by study cohort and adjusted for race/ethnicity, sex, birth year, body mass index, smoking status, cigarettes smoked per day, diabetes, years with diabetes, use of lipid-lowering and antihypertensive medications, and the early and later adult TWAs of other primary CVD risk factors of interest. For example, the model for LDL cholesterol was simultaneously adjusted for early and later adult exposures to SBP, DBP, and HDL, as well as for other time-varying covariates mentioned earlier in the text. The proportional hazards assumption was checked by plotting the log(−log(survival)) versus log(survival time) and by using Schoenfeld residuals. Tests for linear trend in association with the outcome across the categories of each risk factor were conducted by including a variable with the median level of each category in the models.
To account for estimation error in imputed risk factors trajectories and TWAs, we used multiple imputation techniques based on parametric bootstrap to obtain 30 imputed datasets. Survival analyses of the associations between risk factors and CVD outcomes were performed on each imputed dataset, and a summary HR and corresponding 95% confidence interval (CI) were calculated across all 30 imputations using established methods (the summary HR and 95% CI can be interpreted the same way as those generated from a standard Cox model) (24).
To examine the robustness and consistency of our findings, we performed several sensitivity analyses. These included examining the associations between non-HDL cholesterol and CVD outcomes instead of LDL; further adjusting for the most recent directly observed value carried forward; stratifying by sex and race (whites and blacks); excluding individuals who ever used antihypertensive or lipid-lowering medications; repeating analyses by cohort, and leaving out 1 cohort at a time to confirm that our findings were not driven by any single study. All analyses were performed using STATA version 14 (StataCorp LP, College Station, Texas).
RESULTS
The average observed age of study participants at their first in-person examination was 52.7 years, ranging from 24.9 years in the CARDIA study to 73.5 years in the Health ABC study (Table 1). Men composed 44.5% of all participants, and 68.5% self-identified as white. The majority of participants (95%) contributed >1 direct measurement over time (mean 5.1 per person) (Table 2). Time-weighted average measurements of SBP, DBP, LDL, and HDL from young adulthood were strongly correlated with their later life averages (Online Table 2). Although relatively few participants had BP ≥130/80 mm Hg or LDL ≥160 mg/dl during young adulthood, many of them were exposed to nonoptimal levels of risk factors, particularly LDL ≥100 but <160 mg/dl (Online Table 3).
TABLE 1.
Characteristics of Study Participants at the First In-Person Examination
| Total (N = 36,030) | ARIC (n = 13,325) | CARDIA (n = 4,669) | CHS (n = 4,301) | FHS-O (n = 4,905) | Health ABC (n = 2,135) | MESA (n = 6,695) | |
|---|---|---|---|---|---|---|---|
| Year of enrollment | 1987-1989 | 1985-1986 | 1989-1990 | 1971-1975 | 1997-1998 | 2000-2002 | |
| Age range at study enrollment, yrs | 45-64 | 18-30 | ≥65 | 5-70 | 70-79 | 45-84 | |
| Age, yrs | 52.7 ± 16.6 | 54.0 ± 5.7 | 24.9 ± 3.6 | 72.4 ± 5.4 | 36.5 ± 9.9 | 73.5 ± 2.8 | 62.2 ± 10.2 |
| Race | |||||||
| White | 24,681 (68.5) | 9,977 (74.9) | 2,325 (49.8) | 3,644 (84.7) | 4,905 (100.0) | 1,258 (58.9) | 2,572 (38.4) |
| Black | 9,015 (25.0) | 3,306 (24.8) | 2,344 (50.2) | 630 (14.6) | 0 (0.0) | 877 (41.1) | 1,858 (27.8) |
| Hispanic | 1,469 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1,469 (21.9) |
| Other | 865 (2.4) | 42 (0.3) | 0 (0.0) | 27 (0.6) | 0 (0.0) | 0 (0.0) | 796 (11.9) |
| Sex | |||||||
| Female | 19,995 (55.5) | 7,484 (56.2) | 2,592 (55.5) | 2,628 (61.1) | 2,557 (52.1) | 1,193 (55.9) | 3,541 (52.9) |
| Male | 16,035 (44.5) | 5,841 (43.8) | 2,077 (44.5) | 1,673 (38.9) | 2,348 (47.9) | 942 (44.1) | 3,154 (47.1) |
| Smoking | |||||||
| Never | 16,701 (46.4) | 5,765 (43.3) | 2,662 (57.0) | 2,090 (48.6) | 1,757 (35.8) | 1,009 (47.3) | 3,418 (51.1) |
| Former | 10,769 (29.9) | 4,190 (31.4) | 620 (13.3) | 1,685 (39.2) | 945 (19.3) | 910 (42.6) | 2,419 (36.1) |
| Current | 8,526 (23.7) | 3,365 (25.3) | 1,362 (29.2) | 523 (12.2) | 2,203 (44.9) | 216 (10.1) | 857 (12.8) |
| Cigarettes per day, current smokers | 18.7 ± 12.1 | 20.9 ± 12.4 | 13.1 ± 9.2 | 16.6 ± 9.5 | 21.6 ± 12.4 | 14.3 ± 10.0 | 13.6 ± 10.5 |
| Body mass index, kg/m2 | 26.8 ± 5.2 | 27.5 ± 5.3 | 24.5 ± 5.0 | 26.6 ± 4.6 | 25.2 ± 4.3 | 27.4 ± 4.9 | 28.3 ± 5.5 |
| Systolic blood pressure, mm Hg | 123.4 ± 20.0 | 120.8 ± 18.6 | 110.3 ± 10.9 | 136.4 ± 21.4 | 121.6 ± 16.2 | 135.7 ± 20.7 | 126.6 ± 21.5 |
| Diastolic blood pressure, mm Hg | 72.9 ± 11.2 | 73.6 ± 11.2 | 68.6 ± 9.5 | 71.3 ± 11.5 | 78.5 ± 10.8 | 71.6 ± 11.7 | 71.9 ± 10.3 |
| LDL cholesterol, mg/dl | 126.8 ± 37.1 | 137.2 ± 39.1 | 109.5 ± 31.2 | 130.0 ± 35.5 | 127.8 ± 36.7 | 123.0 ± 34.4 | 117.2 ± 31.4 |
| HDL cholesterol, mg/dl | 52.6 ± 15.9 | 52.1 ± 17.0 | 53.2 ± 13.0 | 55.6 ± 15.7 | 51.9 ± 16.2 | 55.4 ± 17.1 | 51.0 ± 14.8 |
| Diabetes | 3,070 (8.5) | 1,251 (9.4) | 27 (0.6) | 559 (13.0) | 80 (1.6) | 307 (14.4) | 846 (12.6) |
| Hypertension medication use | 8,798 (24.4) | 3,443 (25.8) | 35 (0.7) | 1,679 (39.0) | 149 (3.0) | 989 (46.3) | 2,503 (37.4) |
| Lipid medication use | 1,856 (5.2) | 329 (2.5) | 0 (0.0) | 194 (4.5) | 21 (0.4) | 221 (10.4) | 1,091 (16.3) |
Values are mean ± SD or n (%), unless otherwise indicated.
ARIC = Atherosclerosis Risk in Communities; CARDIA = Cardiovascular Risk Development in Young Adults; CHS = Cardiovascular Health Study; FHS-O = Framingham Heart Study Offspring Cohort; HDL = high-density lipoprotein cholesterol; Health ABC = Health, Aging and Body Composition; LDL = low-density lipoprotein cholesterol; MESA = Multi-Ethnic Study of Atherosclerosis.
TABLE 2.
Study Observation Period and Number of Events
| Total (N = 36,030) | ARIC (n = 13,325) | CARDIA (n = 4,669) | CHS (n = 4,301) | FHS-O (n = 4,905) | Health ABC (n = 2,135) | MESA (n = 6,695) | |
|---|---|---|---|---|---|---|---|
| Median follow-up, yrs | 17 | 26 | 16 | 14 | 31 | 14 | 14 |
| Number of in-person exams | 5.1 ± 2.4 | 4.0 ± 1.1 | 7.5 ± 1.9 | 3.0 ± 0.9 | 6.6 ± 2.6 | 9.4 ± 2.6 | 4.3 ± 1.1 |
| Number of in-person exams before age 40 yrs | 0.8 ± 1.7 | 0 | 4.8 ± 1.2 | 0 | 1.2 ± 1.3 | 0 | 0 |
| Number of events | |||||||
| CHD | 4,570 | 1,783 | 84 | 1,272 | 631 | 400 | 400 |
| Heart failure | 5,119 | 2,509 | 64 | 1,444 | 453 | 347 | 302 |
| Stroke | 2,862 | 1,073 | 60 | 865 | 354 | 248 | 262 |
Values are n or mean ± SD, unless otherwise indicated.
CHD = coronary heart disease; other abbreviations as in Table 1.
During a median follow-up of 17 years, there were a total of 4,570 incident CHD, 5,119 HF, and 2,862 stroke events (Table 2). When young adult and later life risk factor exposures were considered jointly in the same model, exposures to elevated DBP and LDL during young adulthood were associated with an increased risk of CHD, independent of later life exposures (Central Illustration, Figure 1). Specifically, compared with DBP <70 mm Hg, multivariable-adjusted HRs for CHD were 1.08 (95% CI: 0.94 to 1.24) for DBP 70 to 79 mm Hg, 1.21 (95% CI: 1.02 to 1.43) for DBP 80 to 89 mm Hg, and 1.21 (95% CI: 0.62 to 2.38) for DBP ≥90 mm Hg (p value for trend = 0.04). Compared with LDL <100 mg/dl, adjusted HRs were 1.62 (95% CI: 1.25 to 2.10) for LDL 100 to 129 mg/dl, 1.89 (95% CI: 1.43 to 2.50) for LDL 130 to 159 mg/dl, and 2.03 (95% CI: 1.47 to 2.82) for LDL ≥160 mg/dl (p value for trend <0.001). When the top 3 LDL categories were combined, young adult exposure to LDL ≥100 mg/dl was associated with an adjusted HR of 1.64 (95% CI: 1.27 to 2.11) for CHD, compared with LDL <100 mg/dl.
CENTRAL ILLUSTRATION. Associations of Blood Pressure and Cholesterol Levels During Young Adulthood With Cardiovascular Events Later in Life.
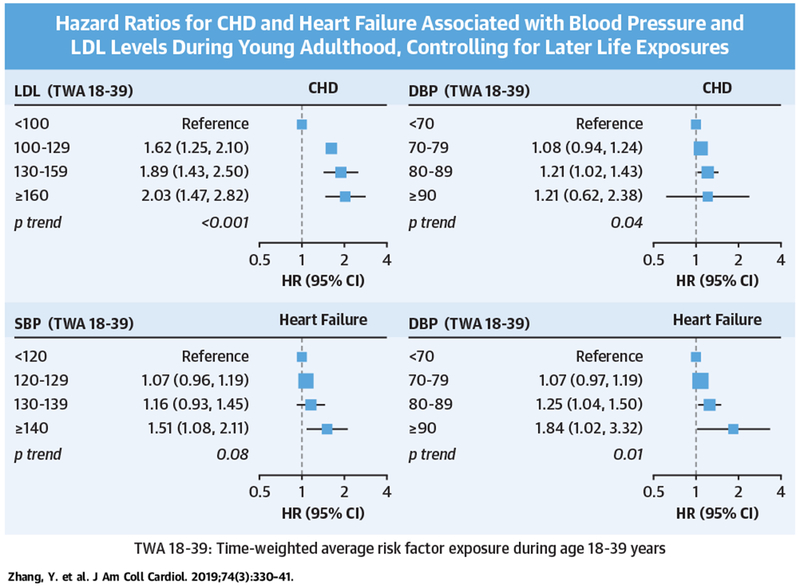
Study of 36,030 U.S. adults found that exposures to elevated SBP, DBP, and LDL during young adulthood (18 to 39 years of age) were associated with increased CHD and heart failure risks in later life, independent of later adult exposures. BP = blood pressure; CHD = coronary heart disease; CI = confidence interval; DBP = diastolic blood pressure; HR = hazard ratio; LDL = low-density lipoprotein; SBP = systolic blood pressure; TWA = time-weighted average.
FIGURE 1. Associations Between Young Adult and Later Adult Risk Factor Exposures and Incident CHD.
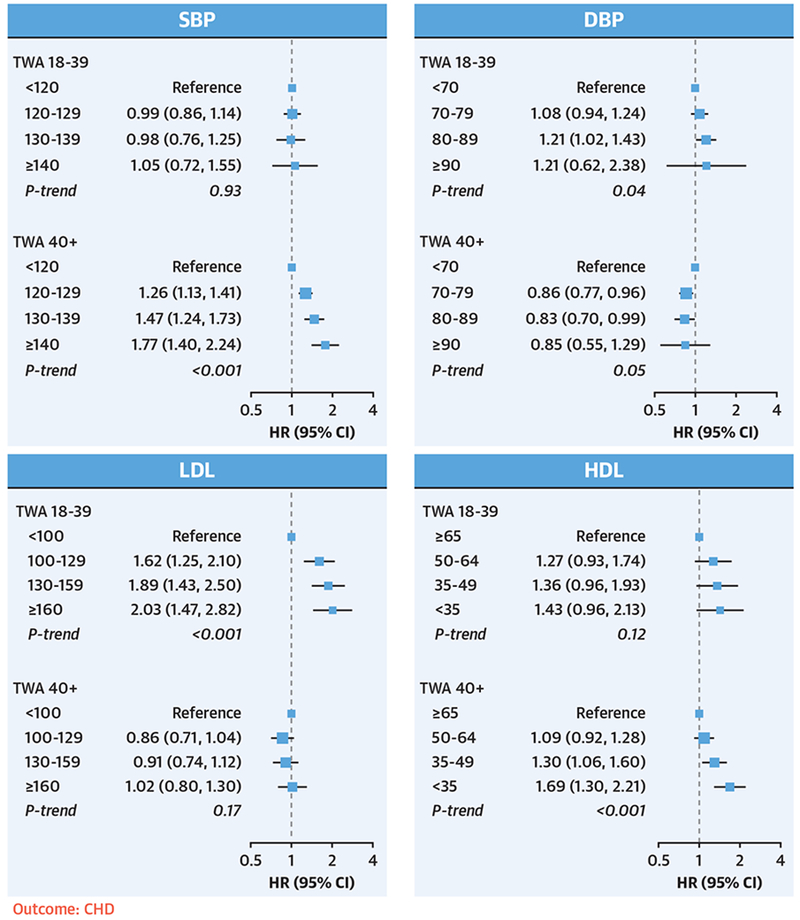
TWA exposures to SBP, DBP, LDL, and HDL from young adulthood (18 to 39 years of age) and later adulthood (≥40 years of age) were included simultaneously in the same model. Models were stratified by study cohort and adjusted for race/ethnicity, sex, birth year, BMI, smoking status, cigarettes smoked per day, diabetes, years with diabetes, use of lipid-lowering and antihypertensive medications, and the early and later adult TWAs of other risk factors. Exposures to elevated DBP and LDL during young adulthood were associated with an increased risk of CHD, independent of later life exposures. BMI = body mass index; CHD = coronary heart disease; CI = confidence interval; DBP = diastolic blood pressure; HDL = high-density lipoprotein; HR = hazard ratio; LDL = low-density lipoprotein; SBP = systolic blood pressure; TWA = time-weighted average.
Nonoptimal SBP and DBP in young adulthood were independently associated with subsequent risk of HF (Figure 2). Compared with SBP <120 mm Hg, young adult SBP ≥130 mm Hg was associated with an adjusted HR of 1.37 (95% CI: 1.17 to 1.61) for HF. Compared with DBP <80 mm Hg, young adult DBP ≥80 mm Hg was associated with an HR of 1.21 (95% CI: 1.04 to 1.41) for HF.
FIGURE 2. Associations Between Young Adult and Later Adult Risk Factor Exposures and Incident HF.
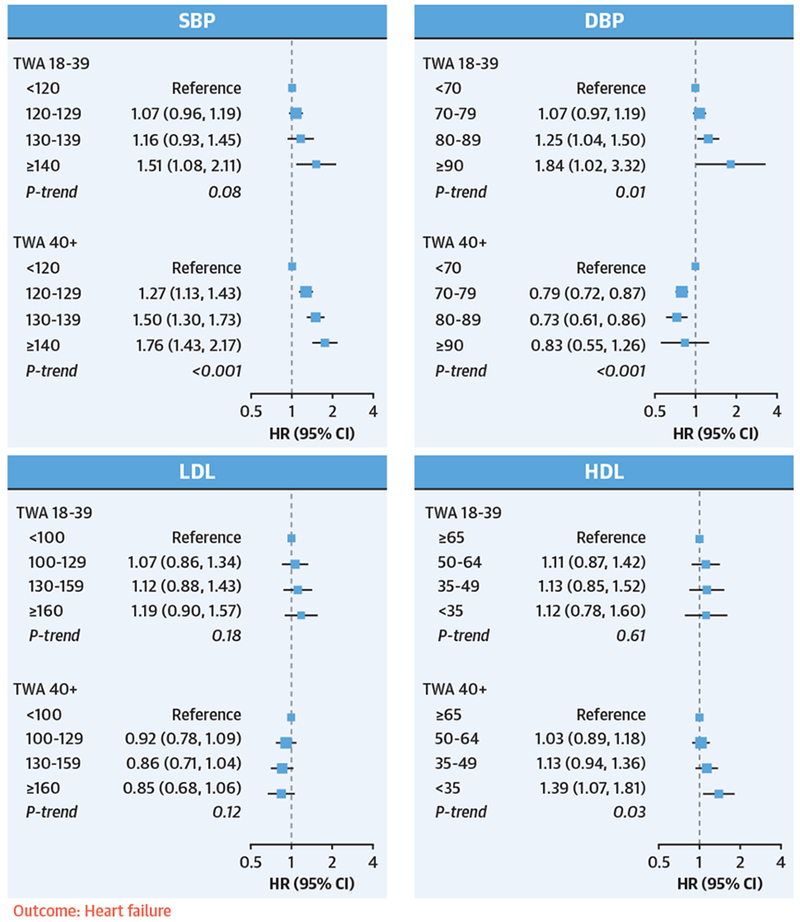
TWA exposures to SBP, DBP, LDL, and HDL from young adulthood (18 to 39 years of age) and later adulthood (≥40 years of age) were included simultaneously in the same model. Model adjustments were the same as in Figure 1. Exposures to elevated SBP and DBP during young adulthood were associated with an increased risk of heart failure, independent of later life exposures. HF = heart failure; other abbreviations as in Figure 1.
For stroke events, none of the young adult exposures were independently associated with incident stroke, whereas later adult exposures to high SBP or DBP were strong predictors of stroke risk (Figure 3).
FIGURE 3. Associations Between Young Adult and Later Adult Risk Factor Exposures and Incident Stroke.
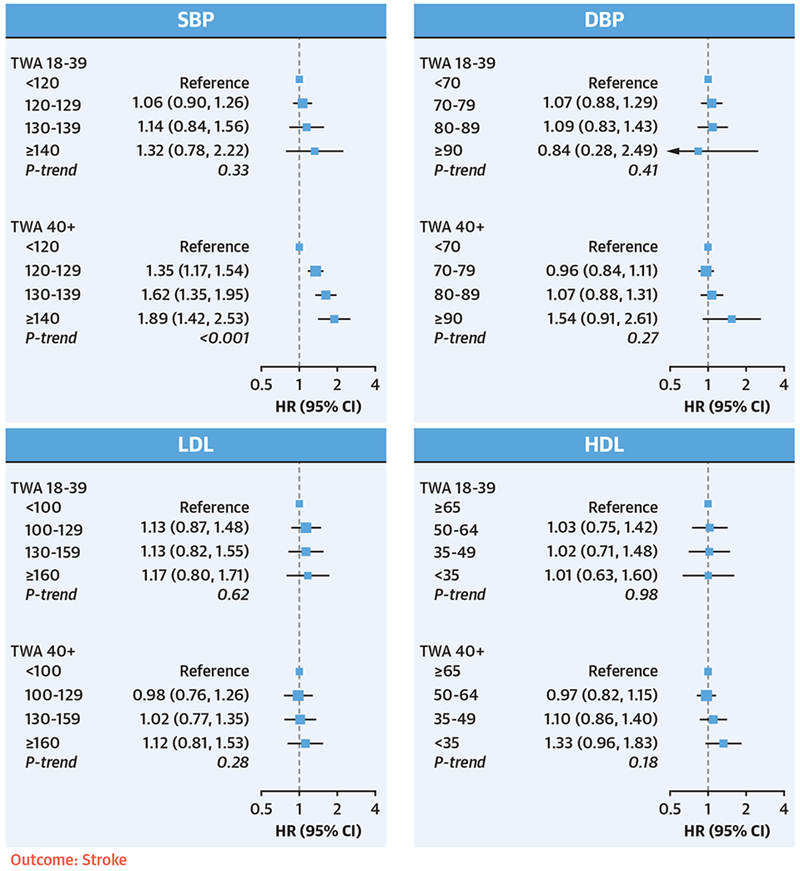
TWA exposures to SBP, DBP, LDL, and HDL from young adulthood (18 to 39 years of age) and later adulthood (≥40 years of age) were included simultaneously in the same model. Model adjustments were the same as in Figure 1. None of the young adult risk factors were independently associated with incident stroke, whereas later adult exposures to high SBP and DBP were strongly associated with stroke risk. Abbreviations as in Figure 1.
We found similar patterns of association in sensitivity analyses. The associations between non-HDL cholesterol and incident CVD events were largely consistent with what we saw with LDL (Online Figure 4), When further adjusting for the most recent directly observed exposures in the models, the associations between young adult exposure and CVD outcomes remained unchanged, whereas the strength of associations between later adult exposures and CVD outcomes were slightly attenuated (Online Figures 5 to 7). The patterns of the associations were similar among men and women (Online Figures 8 to 10), and among whites and blacks (Online Figures 11 to 13; all p-interactions by sex or by race were >0.10). Among the 9,955 participants who never used antihypertensive or lipid-lowering medications, young adult DBP and LDL remained significantly associated with CHD and HF events with even stronger associations compared with the main analysis (Online Figures 14 to 16). The analyses leaving out 1 study at a time (Online Figures 17 to 20) or by each study (Online Figures 21 to 24) also found consistent results.
DISCUSSION
In this analysis of pooled data from 6 large prospective U.S. cohort studies, we found that young adult exposures to elevated DBP and LDL were associated with incident CHD, and young adult exposure to elevated SBP and DBP were associated with incident HF, independent of later adult exposures (Central Illustration). These findings suggest that exposures to elevated SBP, DBP, and LDL during young adulthood contribute independently to later life CHD and HF risks.
Elevated BP is a well-established CVD risk factor and consistently proved to be a key young adult and later adult CVD risk factor in our analyses (1–4). Past studies have shown that early life exposure to high BP are associated with signs of subclinical atherosclerosis at middle age (8,9). In the CARDIA study, both cumulative BP exposure and long-term BP trajectories during young adulthood were associated with coronary atherosclerosis 2 decades later, independent of mid-life BP levels (8,9). Similarly, in 18,881 men from the Harvard Alumni Health Study, high BP in young adulthood was associated with allcause, CVD, and CHD mortality, after adjusting for middle-age hypertension (11). Although these studies controlled for current BP in their analyses, they did not tease apart the relative importance of early versus later life exposures in predicting future CVD risk (14). Our analysis modeled average young adult and later adult BP exposures jointly, and found that both early and later adult BP were associated with subsequent risks of CHD and HF, with DBP being the dominant predictor in early adulthood and SBP in later adulthood. This is consistent with findings from the Framingham Heart Study, which showed that DBP was a stronger predictor of CHD risk than SBP in participants <50 years of age, and there was a gradual shift from DBP to SBP as predictors of CHD with increasing age (25). It may be that diminished peripheral amplification of SBP due to pulse wave reflection in young adults confounds detection of those with moderately raised central SBP in this age group (25). As a consequence, peripheral DBP may be superior to SBP in predicting CVD risks in young adults (25). With age- and atherosclerosis-dependent increases in large artery stiffness, the difference between central and peripheral SBP narrows, thereby improving the predictive utility of peripheral SBP while diminishing that of DBP (25). The J-shaped association between later adult DBP and CHD risk observed in our analysis is consistent with previous studies and may represent an epiphenomenon of increased arterial stiffness in older adults leading to a higher peripheral SBP, a lower DBP, and a wider pulse pressure (25–27).
Elevated levels of LDL cholesterol in young adulthood were strongly and independently associated with later life CHD in our analysis. Early reports from large prospective cohort studies demonstrated that LDL measured once during young adulthood was associated with CHD events decades later; however, these analyses did not account for later life LDL (12,15). The CARDIA study reported that nonoptimal levels of LDL cholesterol during young adulthood were associated with signs of subclinical coronary atherosclerosis in middle age independent of mid-life LDL levels (10). The Framingham Offspring Study showed that cumulative exposure to hyperlipidemia during ages 35 to 55 years was associated with an increased risk of later life CHD in a dose-dependent fashion, after controlling for non-HDL cholesterol at 55 years of age (13). Our study extends these previous reports by further delineating and quantifying the independent contributions of average young adult versus later adult LDL exposure to future CHD, HF, and stroke risk.
The stronger association between young adult LDL and subsequent CHD risk, and weaker association for later adult LDL is likely explained by the relatively high heritability of LDL, particularly during young adulthood (heritability >70%) (28,29), compared with other CVD risk factors such as BP and HDL (30). Thus, exposure to high LDL levels during young adulthood is more likely driven by genetic determinants, and genetically determined LDL level is an important cause of atherosclerosis early in life; whereas later in life, atherosclerosis is likely driven by the same behavioral and environmental factors that affect the other risk factors included in our models (i.e., BP, diabetes, and smoking) (30–32). Mendelian randomization studies demonstrated that early life LDL is a strong causal determinant of CHD risk that cannot be captured by later life LDL alone (33–35). Prolonged low LDL levels beginning early in life due to genetic variation is associated with a substantially greater reduction in CHD risk compared with pharmacological LDL-lowering later in life (33–35). Because participants were more likely to be started on lipid-lowering medication as they grew older, the higher prevalence of lipid medication use during later adulthood may have also contributed to the weaker association between later life LDL and CHD. However, when restricting our analysis to participants who never used lipid-lowering medications, we observed a similar pattern of associations and in fact an even stronger association between early adult LDL and CHD.
Our results add to accumulating evidence that young adulthood is a critical period when exposure to suboptimal BP or cholesterol is particularly harmful, and maintaining optimal levels of BP and LDL throughout young adulthood could yield substantial lifetime CVD prevention benefits (14). However, young adults are difficult to reach by way of traditional, clinic-based preventive programs: they are transitioning between pediatric and adult-centered models of care (36); they often lack health insurance or experience frequent gaps in insurance coverage (37); and their use of ambulatory medical care and adherence to preventive health guidelines are the lowest of any age group (36,38). Data from National Health and Nutrition Examination Surveys showed that young adults lagged behind in awareness, treatment, and control of high BP and LDL compared with middle-aged and older adults (39,40), and were especially unlikely to be aware of borderline levels of BP and cholesterol, which were associated with future risk of CHD and HF in our study (41). Even when informed about a cholesterol screening result that indicates they are at high risk for future CVD, young adults may discount the importance of such results for their current health, believing they have time to change their health behaviors and mitigate their risk (42). Implementing preventive programs targeting individual young adults will require novel prevention program models that are community- and/or web-based, patient-centered, mobile, and account for cognitive bias in future CVD risk perception. Population-wide policies to promote healthful foods, modify the built environment to promote physical activity, and lower structural barriers to accessing healthy lifestyle choices can augment individual-level screening and treatment.
Current U.S. guidelines for treatment of high cholesterol and high BP advise using atherosclerotic CVD (ASCVD) risk to guide treatment decisions (43,44). The 2017 American College of Cardiology/American Heart Association guideline for high BP recommends pharmacological treatment for stage one hypertension (SBP 130 to 139 mm Hg or DBP 80 to 89 mm Hg) for younger adults without chronic kidney disease or diabetes only if 10-year ASCVD risk is ≥10% (43). The 2018 American College of Cardiology/American Heart Association cholesterol guideline emphasizes the importance of assessment of 30-year or lifetime ASCVD risk in young adults, but statin treatment of high cholesterol is recommended for young adults with LDL <190 mg/dl only if with long-standing diabetes or a concomitant higher-risk condition (44). The majority of the young adults with nonoptimal risk factors have low 10-year ASCVD risk and are not likely to receive either pharmacological interventions or advice to pursue lifestyle measures (i.e., weight loss, heart-healthy diet), if they are screened at all (45–47). Pharmacological BP and LDL lowering may benefit selected young adults at high risk for premature ASCVD (36,47,48). The currently ongoing ECAD (Eliminate Coronary Artery Disease) trial is designed to address the question of whether incident ASCVD events can be more effectively prevented by early initiation of statin-based LDL lowering in young and middle-aged adults who are not yet candidates for guideline-based pharmacological LDL lowering due to low 10-year ASCVD risk (47).
STUDY STRENGTHS AND LIMITATIONS.
Our study has several strengths. By pooling and harmonizing data from multiple prospective cohort studies with repeated observations that span the adult life course, we were able to model long-term risk factor trajectories from age 18 years for each individual, and tease apart the independent contributions of exposures during early versus later life to future CVD risk. High-quality risk factor and outcome assessments, large sample size, and long follow-up duration also allowed us to more reliably estimate the associations between CVD risk factors with less common outcomes such as stroke, controlling for confounding from a comprehensive set of variables.
A few limitations of this study need to be considered. Our study relied on imputed risk factor levels before 40 years of age, because the majority of the cardiovascular cohort studies are restricted in age range and did not measure risk factors during both early and later life. Future studies are needed to validate our findings in cohorts with longer follow-up spanning from young adulthood to later life. The risk factor trajectories and TWAs are subject to imputation error; however, imputation error in our study is likely nondifferential, and trajectory estimates for individuals with relatively fewer observed measurements are subject to extra “shrinkage” toward the sample means (14). Therefore, our estimates of the association between risk factors and CVD outcomes are likely conservative and biased towards the null. Our study reported nominal p values without adjustment for multiple testing. However, we performed various sensitivity analyses and found largely consistent results across the board, supporting the robustness of the main findings.
CONCLUSIONS
This pooled U.S. cohorts study of over 36,000 participants found that young adult exposures to raised DBP and LDL levels were associated with later life CHD risk, and young adult SBP and DBP were associated with later life HF risk, independent of later adult exposures. These findings suggest that investment now in programs to control modifiable risk factors during young adulthood has the potential to reduce the future burden of CVD.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Young adults with elevated Levels of blood pressure and Low-density Lipoprotein cholesterol are at greater risk of cardiovascular disease Later in adulthood, independent of subsequent exposure to these risk factors.
TRANSLATIONAL OUTLOOK:
Cardiovascular disease prevention programs should be coupled with policies that promote risk awareness and reduce barriers to accessing healthy Lifestyle choices among young adults.
ACKNOWLEDGMENTS
The authors thank the investigators, staff, and participants of all 6 cohorts for their valuable contributions. In addition, the authors would like to thank the Cross-Cohort Collaboration Consortium (CCC) for their promotion and support of this multicohort effort.
This work was supported by National Institutes of Health (NIH) grant R01 HL130500 (to Dr. Moran). The ARIC has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), NIH, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. The CARDIA study is conducted and supported by contracts from the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). The CARDIA study is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This paper has been reviewed by CARDIA for scientific content. The CHS study was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The FHS-O study is conducted and supported by the NHLBI in collaboration with Boston University (Contract No. N01-HC-25195 and HSN268201500001I). The Health ABC study was supported by NIA contracts #N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459. The MESA study was supported by NHLBI contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169, and by National Center for Advancing Translational Sciences grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Yaffe is supported by NIA grant 1RF1AG054443; and has served on a Data Safety Monitoring Board for Eli Lily. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ASCVD
atherosclerotic cardiovascular disease
- BP
blood pressure
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- HF
heart failure
- HR
hazard ratio
- LDL
low-density lipoprotein
- SBP
systolic blood pressure
- TWA
time-weighted average
Footnotes
APPENDIX For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63 Pt B:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611–9. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 2008; 118:2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri S, Wolf PA, Beiser A, et al. Elevated midlife blood pressure increases stroke risk in elderly persons: the Framingham Study. Arch Intern Med 2001;161:2343–50. [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension 2007;50:869–76. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation 2002;105:48–53. [DOI] [PubMed] [Google Scholar]
- 8.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014; 311:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pletcher MJ, Bibbins-Domingo K, Lewis CE, et al. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med 2008;149:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pletcher MJ, Bibbins-Domingo K, Liu K, et al. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med 2010;153: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol 2011;58:2396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klag MJ, Ford DE, Mead LA, et al. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med 1993;328:313–8. [DOI] [PubMed] [Google Scholar]
- 13.Navar-Boggan AM, Peterson ED, D’Agostino RB Sr., Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015;131:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: the Framingham Offspring study. PLoS One 2016;11:e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000;284:311–8. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring study. Am J Epidemiol 1979;110:281–90. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–65. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 22.Oelsner EC, Balte PP, Cassano PA, et al. Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI pooled cohorts study. Am J Epidemiol 2018;187:2265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol 2018. December 7 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 25.Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9. [DOI] [PubMed] [Google Scholar]
- 26.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144: 884–93. [DOI] [PubMed] [Google Scholar]
- 27.Protogerou AD, Safar ME, Iaria P, et al. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension 2007; 50:172–80. [DOI] [PubMed] [Google Scholar]
- 28.Pietilainen KH, Soderlund S, Rissanen A, et al. HDL subspecies in young adult twins: heritability and impact of overweight. Obesity (Silver Spring) 2009;17:1208–14. [DOI] [PubMed] [Google Scholar]
- 29.Souren NY, Paulussen AD, Loos RJ, et al. Anthropometry, carbohydrate and lipid metabolism in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia 2007;50: 2107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elder SJ, Lichtenstein AH, Pittas AG, et al. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res 2009;50: 1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension 2002;40:7–12. [DOI] [PubMed] [Google Scholar]
- 32.Bodurtha JN, Chen CW, Mosteller M, Nance WE, Schieken RM, Segrest J. Genetic and environmental contributions to cholesterol and its subfractions in 11-year-old twins. The Medical College of Virginia Twin Study. Arterioscler Thromb 1991;11:844–50. [DOI] [PubMed] [Google Scholar]
- 33.Ference BA, Mahajan N. Reply: to PMID 23083789. J Am Coll Cardiol 2013;61:1931–2. [DOI] [PubMed] [Google Scholar]
- 34.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol 2010;55: 2833–42. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JC, Boerwinkle E, Mosley TH Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72. [DOI] [PubMed] [Google Scholar]
- 36.Gooding H, Johnson HM. The unchartered frontier: preventive cardiology between the ages of 15 and 35 years. Curr Cardiovasc Risk Rep 2016; 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMorrow S, Kenney GM, Long SK, Anderson N. Uninsurance among young adults continues to decline, particularly in Medicaid expansion states. Health Aff (Millwood) 2015;34: 616–20. [DOI] [PubMed] [Google Scholar]
- 38.Fortuna RJ, Robbins BW, Halterman JS. Ambulatory care among young adults in the United States. Ann Intern Med 2009;151:379–85. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension 2017;70:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyre AD, Muntner P, Menke A, Raggi P, He J. Trends in ATP-III-defined high blood cholesterol prevalence, awareness, treatment and control among U.S. adults. Ann Epidemiol 2007;17:548–55. [DOI] [PubMed] [Google Scholar]
- 41.Bucholz EM, Gooding HC, de Ferranti SD. Awareness of cardiovascular risk factors in u.s. young adults aged 18-39 years. Am J Prev Med 2018;54:e67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gooding HC, Sheldrick RC, Leslie LK, Shah S, de Ferranti SD, Mackie TI. Adolescent perceptions of cholesterol screening results: “young invincibles” or developing adults? J Adolesc Health 2016;59:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:2199–269. [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol 2019;74:e285–350. [Google Scholar]
- 45.Patel KK, Taksler GB, Hu B, Rothberg MB. Prevalence of elevated cardiovascular risks in young adults: a cross-sectional analysis of national health and nutrition examination surveys. Ann Intern Med 2017;166:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM, Cook NR. Cholesterol evaluation in young adults: absence of clinical trial evidence is not a reason to delay screening. Ann Intern Med 2017;166:901–2. [DOI] [PubMed] [Google Scholar]
- 47.Domanski MJ, Fuster V, Diaz-Mitoma F, et al. Next steps in primary prevention of coronary heart disease: rationale for and design of the ECAD trial. J Am Coll Cardiol 2015;66: 1828–36. [DOI] [PubMed] [Google Scholar]
- 48.Berry JD, Liu K, Folsom AR, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the Coronary Artery Risk Development in Young Adults study and Multi-Ethnic Study of Atherosclerosis. Circulation 2009;119: 382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


