Abstract
Objectives
To explore the trends and the predictors of incident malignant cancer among patients with inflammatory bowel disease [IBD].
Methods
We identified a cohort of all patients with incident IBD in Quebec, Canada, from 1998 to 2015, using provincial administrative health-care databases [RAMQ and Med-Echo]. Annual incidence rates [IRs] of cancer were calculated using Poisson regression and were compared with those of the Quebec population using standardized incidence ratios [SIRs ]. Temporal trends in these rates were evaluated by fitting generalized linear models. Conditional logistic regression was used to estimate odds ratios [ORs] for predictors associated with cancer development.
Results
The cohort included 35 985 patients with IBD, of which 2275 developed cancers over a mean follow-up of 8 years (IR 785.6 per 100 000 persons per year; 95% confidence interval [CI] 754.0–818.5). The rate of colorectal cancer decreased significantly from 1998 to 2015 [p < 0.05 for linear trend], but the incidence remained higher than expected, compared with the Quebec population [SIR 1.39; 95% CI 1.19–1.60]. Rates of extraintestinal cancers increased non-significantly over time [p = 0.11 for linear trend]. In the IBD cohort, chronic kidney disease [OR 1.29; 95% CI 1.17–1.43], respiratory diseases [OR 1.07; 95% CI 1.02–1.12], and diabetes mellitus [OR 1.06; 95% CI 1.01–1.11] were associated with an increase in the incidence of cancer.
Conclusions
The decreasing rates of colorectal cancer suggest improved management and care in IBD. Further studies are needed to explore the impact of comorbid conditions on the risk of cancer in IBD.
Keywords: Inflammatory bowel diseases, cancer, incidence
1. Introduction
Inflammatory bowel disease [IBD], including Crohn’s disease [CD] and ulcerative colitis [UC], is most prevalent in western societies such as Europe and North America.1 Canada, in particular, has one of the highest rates of IBD, worldwide.2 The etiology of these diseases remains largely unclear, and it is suspected that IBD develops through the interaction of numerous factors, including genetics, diet, and lifestyle habits such as smoking and physical activity.3 The English: UK language contains missing user dictionary files. Please verify the user dictionary paths in the Dictionary Preferences panel. Notwithstanding economic costs related to hospitalizations, surgeries, medications, and other treatment strategies, the risk of mortality has been reported to be up to 54% higher among IBD patients, compared with the general population.4 A significant portion of the health burden in IBD stems from its association with an increased risk of cancer.5 Indeed, chronic inflammation is a known risk factor for the development of cancer, and IBD treatments, such as immunosuppressant medications, may also independently increase the risk of cancer in IBD.6–11 These findings may differ depending on the specific cancer and/or immunosuppressant.
Aside from our previous work addressing the risk of lymphoma and skin cancer associated with IBD medications,12 the direct association between IBD and cancer has yet to be explored in the province of Quebec. The objective of our study was to explore the trends in the incidence of malignant cancer among Quebec patients with IBD, and to compare these rates of events with those of the general population. We also sought to identify the clinical factors associated with cancer development in IBD.
2. Methods
2.1. Data source
This study was conducted using administrative health-care data from the province of Quebec, which provides universal health-care coverage for nearly all Quebec residents [over 8 million individuals]. The Régie de l’Assurance Maladie du Québec [RAMQ] database contains information on patient demographics and records of physician billings for medical services and procedures, and the Med-Echo database contains hospital discharge records. These data are coded using International Classification of Diseases [ICD] 9 and 10 codes. Drug prescriptions that are issued under the provincial drug insurance plan are also captured in the RAMQ database, and are identified by the corresponding Drug Identification Number [DIN].
2.2. Ethical approval
This study was approved by the Research Ethics Committee of the Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec.
2.3. Study population
Using the RAMQ and Med-Echo databases, we retrieved data from between 01 January 1996 and 31 December 2015, for all patients ever diagnosed or hospitalized with IBD within this time period. From this population, we identified patients aged 18 or older and meeting the IBD definition criteria between 01 January 1998 and 31 December 2015. Data from the years 1996 and 1997 were used to distinguish incident and prevalent disease. The IBD definition was based on a previously validated algorithm.13 Briefly, patients were defined as having IBD if: [i] they were discharged from the hospital with a principal diagnosis of CD or UC; or [ii] they had records of at least four physician billings within a 2-year interval, each with a diagnosis of CD or UC. Diagnoses were made using the appropriate ICD codes for UC [ICD-9 556; ICD-10 K51] and CD [ICD-9 555; ICD-10 K50], through which we further subdivided the group into patients with CD, patients with UC, or unclassified IBD patients. The date of IBD diagnosis was considered the date of first hospital discharge or the date of the fourth physician billing, whichever came first. We limited the study cohort to patients with incident IBD, defined as having no IBD billings or hospitalizations in the 2 years prior to their disease-defining interval, as well as to patients with at least 1 day of follow-up [i.e. to account for death recorded prior to or on the same day as IBD diagnosis]. Patients were followed starting from the date of IBD diagnosis until the date of outcome occurrence, death from any cause, or end of the study period [31 December 2015], whichever occurred first.
2.4. Outcome definition
The outcome of interest was a first hospital discharge with a primary diagnosis of incident malignant cancer, which was identified using the appropriate ICD codes. Hospitalization data was used in order to achieve a greater sensitivity over the use of physician billing data. Events were defined as incident if the patient had no previous history of the same cancer type. Extraintestinal and intestinal cancers were also evaluated separately, as secondary outcomes.
2.5. Covariates
In order to describe the baseline profile of our IBD cohort, we identified patient demographic characteristics, risk factors, and comorbidities from within the year prior to cohort entry. Demographic characteristics included age, sex, and area of residence [rural or urban].14 Risk factors and comorbidities included rheumatoid arthritis, inflammatory skin conditions, other autoimmune/inflammatory disorders [systemic lupus erythematosus, ankylosing spondylitis, carditis, multiple sclerosis], mental illnesses [anxiety, depression], respiratory diseases [asthma, bronchitis, chronic obstructive pulmonary disease], vascular diseases [peripheral vascular disease, venous thromboembolism], liver disease, cancer, chronic kidney disease [CKD], diabetes mellitus, dyslipidemia, hypertension, and obesity. We also identified the number of previous hospitalizations, as a measure of overall health and disease severity. All covariates were defined using all available data in patients’ medical records, with the exception of cancer, which was defined using records of primary hospital discharges only.
2.6. Statistical analyses
2.6.1. Trends in cancer incidence
We used Poisson regression to calculate the yearly incidence rates [IRs] of cancer within our cohort, both as a function of calendar time, and as a function of time since IBD. Changes in these rates over time were assessed by fitting generalized linear models, and were presented graphically as 3-year centred moving averages from 1999 to 2014. We also used indirect standardization to calculate age- and sex-standardized incidence ratios [SIRs] comparing the number of observed cancer events within our study cohort, with the number of expected events using the Quebec population as reference. Age- and sex-specific rates of cancer in Quebec were obtained from Statistics Canada,15 and expected events were calculated by applying these rates to our study population. Data on the incidence rate of cancer in Quebec were not available from 2011 onwards, and this analysis was therefore restricted to cancer events occurring between 1998 and 2010.
2.6.2. Factors associated with cancer incidence
We identified patient characteristics associated with incident cancer development in IBD, by comparing cancer cases with matched controls. Within our cohort of incident IBD patients, for all those experiencing a cancer event during follow-up, we identified up to 10 controls who did not have cancer as at the corresponding event calendar date. Cases and controls were matched on age [within one year], sex, and calendar date of IBD diagnosis [within 1 year]. In subgroup analyses, cases and controls were also matched according to IBD phenotype. The index date was the date of cancer diagnosis for cases, and the corresponding calendar date for matched controls. Conditional stepwise logistic regression was used to identify the covariates associated with cancer development, measured in the year prior to the index date. In addition to all aforementioned covariates, prescriptions for immunosuppressant medications and biologics [tumor necrosis factor [TNF] inhibitors, corticosteroids, 5-aminosalicylic acid, thiopurines, methotrexate, others] were also included in the model. A significance level of 0.25 was required for covariates to enter the model, and a significance level of 0.15 was required for covariates to be retained. In sensitivity analyses, covariates were measured in the 1-year period ending 6 months and 12 months prior to the index date, in order to explore the potential for reverse causality related to increased surveillance among patients presenting early signs or symptoms of cancer.
All analyses were conducted using SAS version 9.4 [SAS Institute Inc., Cary, North Carolina].
3. Results
3.1. Cohort description
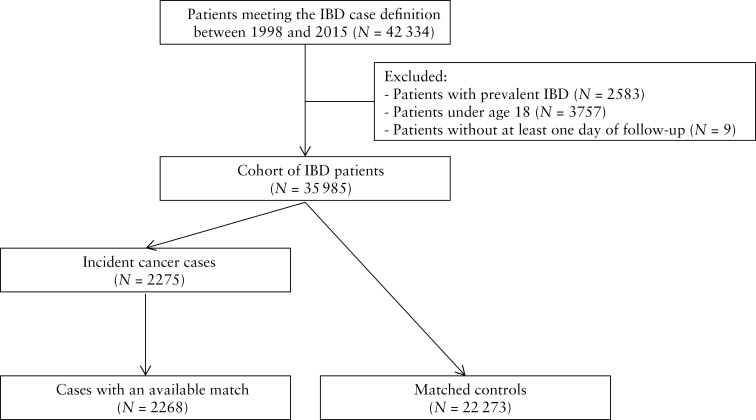
We identified 42 334 patients meeting the IBD definition between 1998 and 2015 [Figure 1]. After excluding patients under 18 years of age, those with prevalent IBD, and those with less than 1 day of follow-up, our final cohort comprised 35 985 adult patients with a mean age of 45 years. The mean follow-up time was 8 years, for a total of 289 593 person-years of follow-up. Altogether, we identified 20 644 [57.4%] patients with CD, 14 000 [38.9%] patients with UC, and 1341 [3.7%] patients who could not be classified as either CD or UC [Table 1]. Compared with patients with UC, those with CD tended to be younger at the time of IBD and were predominantly female. They also had a lower prevalence of vascular disease and hypertension at baseline.
Figure 1.
Cohort definition and matching flowchart
Table 1.
Baseline characteristics of IBD patients in Quebec.
| IBD overall | Crohn’s disease | Ulcerative colitis | |
|---|---|---|---|
| [n = 35 985] | [n = 20 644] | [n = 14 000] | |
| Demographic characteristics | |||
| Age [years] | |||
| 18–30 | 9380 [26.1] | 6270 [30.4] | 2737 [19.6] |
| 31–40 | 6578 [18.3] | 3778 [18.3] | 2568 [18.3] |
| 41–50 | 6683 [18.6] | 3730 [18.1] | 2697 [19.3] |
| 51–60 | 5678 [15.8] | 3030 [14.7] | 2433 [17.4] |
| 61–70 | 3937 [10.9] | 2036 [9.9] | 1770 [12.6] |
| 71–80 | 2552 [7.1] | 1246 [6.0] | 1210 [8.6] |
| ≥81 | 1177 [3.3] | 554 [2.7] | 585 [4.2] |
| Sex | |||
| Men | 16 563 [46.0] | 8900 [43.1] | 6997 [50.0] |
| Women | 19 422 [54.0] | 11 744 [56.9] | 7003 [50.0] |
| Year of IBD diagnosis | |||
| 1998–2000 | 6043 [16.8] | 3212 [15.6] | 2608 [18.6] |
| 2001–2003 | 6247 [17.4] | 3538 [17.1] | 2452 [17.5] |
| 2004–2006 | 5681 [15.8] | 3422 [16.6] | 2063 [14.7] |
| 2007–2009 | 5708 [15.9] | 3268 [15.8] | 2211 [15.8] |
| 2010–2012 | 5936 [16.5] | 3430 [16.6] | 2277 [16.3] |
| 2013–2015 | 6370 [17.7] | 3774 [18.3] | 2389 [17.1] |
| Area of residence | |||
| Urban | 28 800 [80.0] | 16 522 [80.0] | 11 197 [80.0] |
| Rural | 6661 [18.5] | 3822 [18.5] | 2596 [18.5] |
| Unknown | 524 [1.5] | 300 [1.5] | 207 [1.5] |
| Clinical characteristics | |||
| Other inflammatory/autoimmune diseases | |||
| Rheumatoid arthritis | 553 [1.5] | 350 [1.7] | 184 [1.3] |
| Inflammatory skin diseases | 3257 [9.1] | 1849 [9.0] | 1259 [9.0] |
| Other | 847 [2.4] | 586 [2.8] | 232 [1.7] |
| Chronic kidney disease | 552 [1.5] | 272 [1.3] | 260 [1.9] |
| Diabetes mellitus | 2577 [7.2] | 1310 [6.3] | 1168 [8.3] |
| Dyslipidemia | 2547 [7.1] | 1205 [5.8] | 1243 [8.9] |
| Hypertension | 5339 [14.8] | 2695 [13.1] | 2440 [17.4] |
| Obesity | 837 [2.3] | 466 [2.3] | 341 [2.4] |
| Mental illnesses | 4593 [12.8] | 2721 [13.2] | 1693 [12.1] |
| Respiratory diseases | 4421 [12.3] | 2364 [11.5] | 1893 [13.5] |
| Vascular diseases | 5406 [15.0] | 2445 [11.8] | 2721 [19.4] |
| Liver diseases | 1043 [2.9] | 565[2.7] | 433 [3.1] |
| Cancer | |||
| Intestinal | 90 [0.3] | 58 [0.3] | 30 [0.2] |
| Extraintestinal | 225 [0.6] | 117 [0.6] | 99 [0.7] |
| Number of hospitalizations | |||
| 0 | 28 346 [78.8] | 16 020 [77.6] | 11 304 [80.7] |
| 1 | 5258 [14.6] | 3209 [15.5] | 1822 [13.0] |
| 2 | 1473 [4.1] | 881 [4.3] | 530 [3.8] |
| ≥3 | 912 [2.5] | 537 [2.6] | 345 [2.5] |
All values are presented as n [%], unless otherwise specified.
3.2. Trends in cancer incidence
A total of 2275 incident malignant cancer events occurred during follow-up (IR 785.6 per 100 000 person-years; 95% confidence interval [CI] 754.0–818.5), over 80% of which were extraintestinal. Cancers of the respiratory system [lungs, bronchi, trachea] were the most frequent [13.6%, IR 107.0 per 100 000 person-years], followed by breast cancer [12.7%, IR 99.8 per 100 000 person-years], colorectal cancer [12.6%, IR 99.1 per 100 000 person-years], and non-melanoma skin cancer [NMSC] [10.2%, IR 80.5 per 100 000 person-years], altogether comprising nearly 50% of all events. Cases of melanoma comprised 2% of cancer events [IR 18.3 per 100 000 person-years].
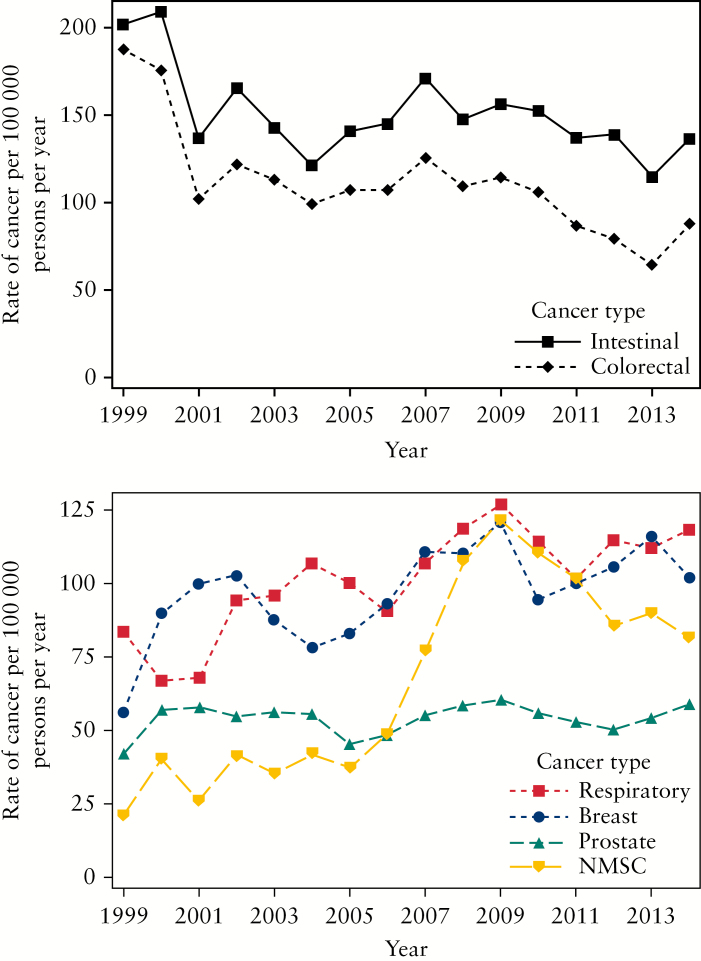
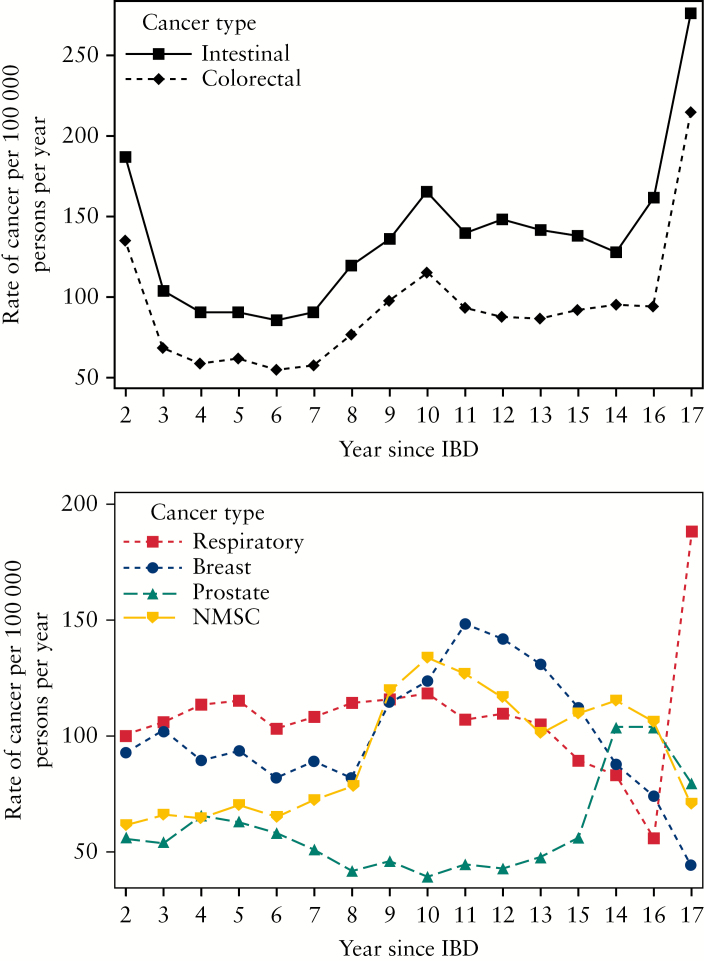
Overall, the incidence of cancer in IBD was relatively stable over the study period, from 1998 to 2015. There was a decreasing trend for intestinal cancers, mainly due to a significant decline in the rate of colorectal cancer [p < 0.05 for linear trend] [Figure 2]. In contrast, there was an increasing trend for extraintestinal cancers, including breast cancer and respiratory cancers, with a significant increase in the rate of NMSC over time [p < 0.001 for linear trend]. When looking at occurrence of cancer as a function of time since IBD diagnosis, the rate of colorectal cancer was elevated in the earlier and in the later years after diagnosis, and also increased notably from 7 to 10 years after diagnosis [Figure 3]. Similarly, the rates of NMSC and breast cancer increased 8 to 11 years after IBD diagnosis, and decreased thereafter. Overall, these findings were unchanged when stratified by IBD phenotype, although the low number of events, particularly among patients with UC, precludes a valid interpretation of some of these results [Supplementary Figures 1–4].
Figure 2.
Crude rates of intestinal [above] and extraintestinal [below] cancers as a function of calendar time, presented as 3-year centred moving averages from 1999 to 2014.
NMSC, non-melanoma skin cancer.
Figure 3.
Crude rates of intestinal [above] and extraintestinal [below] cancers as a function of time since IBD, presented as three-year moving averages.
NMSC, non-melanoma skin cancer.
From 1998 to 2010, the overall incidence of cancer was significantly higher than expected in our cohort of IBD patients, based on the rates of cancer in the general Quebec population [Table 2]. This elevated risk appeared to be driven by a higher incidence of NMSC [SIR 28.81; 95% CI 22.06–35.56] and colorectal cancer [SIR 1.73; 95% CI 1.40–2.06] among patients with CD, which were modestly offset by a lower incidence of respiratory cancers among patients with UC [SIR 0.75; 95% CI 0.57–0.94].
Table 2.
Standardized incidence ratios [SIRs] and 95% confidence intervals, evaluating the risk of cancer events in IBD compared with in the general Quebec population
| IBD overall | Crohn’s disease | Ulcerative colitis | |
|---|---|---|---|
| All cancers | 1.20 [1.13–1.26] | 1.39 [1.28–1.49] | 1.00 [0.91–1.09] |
| Extraintestinal cancers | 1.16 [1.08–1.23] | 1.33 [1.22–1.44] | 0.99 [0.89–1.09] |
| Breast cancer | 1.13 [0.96–1.31] | 1.04 [0.82–1.27] | 1.21 [0.93–1.50] |
| Prostate cancer | 0.92 [0.73–1.12] | 0.92 [0.62–1.22] | 1.00 [0.72–1.27] |
| Respiratory cancers | 0.95 [0.80–1.09] | 1.16 [0.93–1.39] | 0.75 [0.57–0.94] |
| Non-melanoma skin cancer | 22.62 [18.27–26.96] | 28.81 [22.06–35.56] | 15.51 [10.05–20.97] |
| Intestinal cancers | 1.36 [1.19–1.53] | 1.66 [1.39–1.94] | 1.01 [0.79–1.22] |
| Colorectal cancer | 1.39 [1.19–1.60] | 1.73 [1.40–2.06] | 1.02 [0.77–1.27] |
3.3. Factors associated with cancer incidence
All but seven cases were matched to up to 10 controls on age, sex, date of IBD diagnosis, and calendar time [Table 3]. Overall, cases of cancer were more likely to have CKD (odds ratio [OR] 1.29; 95% CI 1.17–1.43), respiratory diseases [OR 1.07; 95% CI 1.02–1.12], and diabetes mellitus [OR 1.06; 95% CI 1.01–1.11], and they were also hospitalized more frequently [OR 1.05; 95% CI 1.03–1.07]. Similar findings were obtained when matching and stratifying on IBD phenotype, as well as in sensitivity analyses lagging the measurement of covariates by 6 months and by 12 months prior to the index date [Supplementary Tables 1–2].
Table 3.
Characteristics of matched cancer cases and controls with IBD
| Cases | Controls | Multivariate | |
|---|---|---|---|
| [n = 2268] | [n = 22,273] | OR [95% CI]* | |
| Demographic characteristics | |||
| Age [years] | -- | ||
| 18–30 | 139 [6.1] | 1387 [6.2] | |
| 31–40 | 219 [9.7] | 2185 [9.8] | |
| 41–50 | 410 [18.1] | 4095 [18.4] | |
| 51–60 | 554 [24.4] | 5571 [25.0] | |
| 61–70 | 518 [22.8] | 5171 [23.2] | |
| 71–80 | 347 [15.3] | 3313 [14.9] | |
| ≥81 | 81 [3.6] | 551 [2.5] | |
| Sex | -- | ||
| Men | 1089 [48.0] | 10,604 [47.6] | |
| Women | 1179 [52.0] | 11,669 [52.4] | |
| Year of IBD diagnosis | -- | ||
| 1998–2000 | 637 [28.1] | 6347 [28.5] | |
| 2001–2003 | 590 [26.0] | 5724 [25.7] | |
| 2004–2006 | 428 [18.9] | 4234 [19.0] | |
| 2007–2009 | 312 [13.8] | 3079 [13.8] | |
| 2010–2012 | 209 [9.2] | 2075 [9.3] | |
| 2013–2015 | 92 [4.1] | 814 [3.7] | |
| Area of residence | NS | ||
| Urban | 1828 [80.6] | 17 907 [80.4] | |
| Rural | 440 [19.4] | 4366 [19.6] | |
| Unknown | 0 [0.0] | 0 [0.0] | |
| Clinical characteristics | |||
| IBD phenotype | |||
| Crohn’s disease | 1307 [57.6] | 11,590 [52.0] | 1.03 [0.95–1.11] |
| Ulcerative colitis | 887 [39.1] | 9831 [44.1] | 0.99 [0.92–1.07] |
| Unclassified | 74 [3.3] | 852 [3.8] | 1.00 [ref.] |
| Other inflammatory/autoimmune diseases | |||
| Rheumatoid arthritis | 40 [1.8] | 456 [2.0] | NS |
| Inflammatory skin diseases | 204 [9.0] | 1669 [7.5] | NS |
| Other | 63 [2.8] | 440 [2.0] | NS |
| Chronic kidney disease | 83 [3.7] | 496 [2.2] | 1.29 [1.17–1.43] |
| Diabetes mellitus | 306 [13.5] | 2443 [11.0] | 1.06 [1.01–1.11] |
| Dyslipidemia | 172 [7.6] | 1434 [6.4] | NS |
| Hypertension | 524 [23.1] | 4316 [19.4] | 0.97 [0.93–1.00] |
| Obesity | 41 [1.8] | 389 [1.7] | NS |
| Mental illnesses | 265 [11.7] | 2229 [10.0] | NS |
| Respiratory diseases | 375 [16.5] | 2703 [12.1] | 1.07 [1.02–1.12] |
| Vascular diseases | 298 [13.1] | 1848 [8.3] | 1.05 [0.99–1.11] |
| Liver diseases | 113 [5.0] | 440 [2.0] | NS |
| Immunosuppressant/biologics use | 1039 [45.8] | 9141 [41.0] | NS |
| 5-aminosalicylic acid | 673 [29.7] | 6338 [28.5] | |
| Corticosteroids | 514 [22.7] | 4023 [18.1] | |
| Thiopurines | 297 [13.1] | 2155 [9.7] | |
| Anti-TNF | 80 [3.5] | 690 [3.1] | |
| Methotrexate | 41 [1.8] | 476 [2.1] | |
| Other** | 0 [0.0] | 4 [0.0] | |
| Number of hospitalizations | 1.05 [1.03–1.07] | ||
| 0 | 1450 [63.9] | 16,825 [75.5] | |
| 1 | 477 [21.0] | 3394 [15.2] | |
| 2 | 200 [8.8] | 1294 [5.8] | |
| ≥3 | 141 [6.2] | 760 [3.4] |
OR, odds ratio; anti-TNF, tumor necrosis factor inhibitors; NS, not selected.
*Results of the conditional logistic stepwise regression model, including all presented variables, with age, sex, year of IBD diagnosis, and calendar time as matching factors.
**Use of other immunosuppressant therapies and/or biologics, including anti-integrins [vedolizumab and natalizumab], ustekinumab, and other therapies [rituximab, cellcept].
4. Discussion
Over the course of the 18-year study period, rates of cancer in IBD were relatively stable; extraintestinal cancers increased non-significantly, and intestinal cancers decreased non-significantly. For colorectal cancer, breast cancer, and NMSC, the risk of events increased 7 to 8 years after IBD diagnosis. Moreover, our findings suggest a higher incidence of cancer in IBD compared with in the general population, mainly owing to an elevated risk of NMSC and colorectal cancer in patients with CD. Finally, compared with matched controls, cases of cancer in IBD were more likely to have CKD, respiratory diseases, or diabetes mellitus, and they were also hospitalized more frequently.
The incidence of IBD-related cancers reported in this study was comparable with that in previous reports. According to a recent review, meta-analyses had estimated the incidence of colorectal cancer at 300 and 50 cases per 100 000 person-years overall, for patients with UC and CD, respectively.16 However, with an average follow-up of 8 years, our IR of 99.8 is in line with other estimates at 100 cases per 100 000 person-years in the first decade of disease.17 Similarly, the rates of NMSC and melanoma have been reported at 733 and 27.5 per 100 000 person-years, respectively, both higher than the rates observed in our cohort.18,19 The difference may again be attributable to the relatively short duration of follow-up in our study, in addition to the low reported use of thiopurines and anti-TNFs in our cohort, which are known to increase the risk of both types of skin cancers. The expected lower rates of sun exposure in Canada compared with that of other countries may have also been a contributing factor.
The positive association between IBD and colorectal cancer has been extensively demonstrated,20 and is reaffirmed in this study. Nevertheless, we have observed a significant decrease in the rates of colorectal cancer in IBD over time, comparable with previous findings demonstrating a decline in mortality related to intestinal cancers and diseases in Quebec, from 1998 to 2008.21 Taken together, these findings suggest significant improvements over the last two decades in the screening, treatment, and management of colorectal cancer in IBD, as well as of IBD itself. Contrary to most available evidence,22,23 we did not find an elevated risk of colorectal cancer among patients with UC, specifically, compared with the general population. Owing to the lower number of events among these patients, we may not have had sufficient power to detect such differences.17 It is also possible that our findings may reflect a true lack of association between colorectal cancer and UC, as suggested by others, as a result of improved surveillance and management practices.24,25
Compared with the general population, we also observed elevated rates of NMSC in CD and UC, which are supported by findings from previous studies.26,27 Our calculated SIR may be overestimated, as NMSC may not be routinely reported in cancer registries.28 At the same time, NMSC does not frequently result in hospitalization, which may have led to an underestimation of the true number of NMSC events in our IBD cohort. The increasing rate of events from 1998 to 2009 may reflect increasing use of thiopurines and other immunosuppressant therapies, which have been shown to play a significant role in the development of NMSC.29 While the rate of NMSC was observed to decrease from 2009 onwards, it is not clear whether this trend reflects intentional efforts to mitigate this risk in IBD, and future temporal studies are needed to establish the nature of this change. Finally, our results demonstrate a lower rate of respiratory cancers among patients with UC compared with the general population, with similar findings having been reported previously.30,31 This negative association may be attributable to differences in smoking behaviour.32 Indeed, in a 2015 study from Switzerland, the rate of active smoking was up to 60% lower among patients with UC [15.3%], compared both with patients with CD [39.6%] and the general population [27%].33
In our study cohort, colorectal cancer was observed to increase from 7 to 10 years after IBD diagnosis. These findings are in accordance with evidence-based guidelines pertaining to colorectal screening in IBD, which have suggested that regular surveillance should begin up to 8 to 10 years following the symptoms or diagnosis of IBD.34,35 We suspect that the elevated rate of events observed in the first several years of IBD diagnosis may reflect a bias due to prevalent cases of mild IBD, who did not meet our case definition criteria until symptoms of cancer prompted further medical care. While the substantial increase in the later years after IBD diagnosis is also questionable, this may reflect a true trend in the incidence of colorectal cancer in IBD. Indeed, a previous study has suggested an exponential increase in the rate of events with progressive duration of disease.36 Unlike colorectal cancer, screening guidelines for other cancers such as NMSC or breast cancer in IBD patients have yet to be proposed. Based on our present findings, screening activities may be warranted in the 7 to 10 years after IBD diagnosis, especially in the case of NMSC, because the incidence of these events was significantly higher than expected in our IBD cohort, compared with in the Quebec population. Nevertheless, additional research is needed to establish the benefits and harms associated with such screening strategies.
It has been suggested that CKD may be both a risk factor and an outcome of incident cancer in the general population,37 and it is thus unsurprising to have identified CKD as a predictor of cancer in our IBD cohort. However, to our knowledge, no previous studies have directly assessed the association between CKD and cancer in IBD, specifically. The situation is similar with respect to respiratory diseases and diabetes mellitus. In the latter case, the biological mechanism for the interplay between IBD, diabetes mellitus, and colorectal cancer has been explored.38 However, on a population level, while the positive association between these diseases and cancers has been well studied overall,39–41 there appears to be little epidemiological evidence in patients with IBD. Thus, further population-based research is needed to ascertain the relationship between these comorbid conditions and the risk of cancer development within this vulnerable subgroup.
Our study presents a number of strengths, being one of few to describe the clinical profile of patients with IBD, as well as the first to explore the trends in the incidence of cancer associated with IBD in Quebec. Moreover, our analyses are based on data from all patients registered with the RAMQ, which comprises all Quebec residents. Our findings may therefore be directly used to inform health-care policy and decision-making at the population level. In terms of limitations, our analyses were not able to account for certain risk factors and comorbidities that may influence patients’ risk of cancer, such as smoking, family history of IBD and/or cancer, IBD severity, or CD/UC phenotype, due to the administrative nature of the RAMQ database. Also, Quebec’s public drug insurance plan covers ~40% of the Quebec population. Therefore, use of immunosuppressant medications or biologics may have been underestimated in our study population, precluding a precise estimate as to the effect of these medications on cancer development in IBD. Finally, considering the significance of IBD duration as a risk factor for cancer, the relatively short follow-up time in our study may not have been sufficient to observe the development of cancer among the patients in our cohort.
5. Conclusion
In addition to demonstrating an elevated risk of cancer in IBD, as shown elsewhere, the results of our study highlight the clinical profile of IBD patients in Quebec, as well as the characteristics that may be associated with cancer development in IBD. Moreover, we observed significant temporal trends in the rates of cancers over time. We expect that these findings will inform the management of IBD and associated cancer outcomes, although further studies with longer follow-up are needed to affirm these results, as well as to explore the impact of comorbid conditions on the risk of cancer in IBD.
Funding
This research was funded by an operating grant [#396849] from the Canadian Institutes of Health Research [CIHR].
Conflict of Interest
None declared.
Author Contributions
SL was involved in the concept and design of the study, analysis, interpretation of data, and was responsible for drafting the manuscript; MV, AB and PLL were involved in interpretation of data and critically revising the manuscript; LA and SS were involved in the statistical analysis, interpretation of data, revising and editing the manuscript; PB was involved in the concept and design of the study, interpretation of data, and drafting and revising the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Acknowledgments
PLL was supported by the CAS Research Fund of the Department of Medicine, McGill University Health Centre.
References
- 1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- 2. Rocchi A, Benchimol EI, Bernstein CN, et al. . Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol 2012;26:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 4. Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology 2003;125:1583–90. [DOI] [PubMed] [Google Scholar]
- 5. Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322–37. [DOI] [PubMed] [Google Scholar]
- 6. O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis 2010;16:1411–20. [DOI] [PubMed] [Google Scholar]
- 7. Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonovas S, Fiorino G, Allocca M, et al. . Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–97.e10. [DOI] [PubMed] [Google Scholar]
- 9. Nyboe Andersen N, Pasternak B, Basit S, et al. . Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA 2014;311:2406–13. [DOI] [PubMed] [Google Scholar]
- 10. Biancone L, Orlando A, Kohn A, et al. . Infliximab and newly diagnosed neoplasia in Crohn’s disease: a multicentre matched pair study. Gut 2006;55:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441–52. [DOI] [PubMed] [Google Scholar]
- 12. Kopylov U, Vutcovici M, Kezouh A, Seidman E, Bitton A, Afif W. Risk of lymphoma, colorectal and skin cancer in patients with IBD treated with immunomodulators and biologics: A Quebec claims database study. Inflamm Bowel Dis 2015;21:1847–53. [DOI] [PubMed] [Google Scholar]
- 13. Rezaie A, Quan H, Fedorak RN, Panaccione R, Hilsden RJ. Development and validation of an administrative case definition for inflammatory bowel diseases. Can J Gastroenterol 2012;26:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puderer H, Mechanda K. How Postal Codes Map to Geographic Areas 2007. http://www.statcan.gc.ca/pub/92f0138m/92f0138m2007001-eng.htm Accessed July 14, 2018.
- 15. Statistics Canada. Table 13-10-0111-01: Number and rates of new cases of primary cancer, by cancer type, age group and sex, Canada, provinces and territories, annual, 1992 to 2013. CANSIM [database] https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011101. Accessed July 14, 2018. [Google Scholar]
- 16. Sebastian S, Yuksel ES, Gasche C, et al. . Colorectal cancer in inflammatory bowel disease: Results of the 3rd ECCO pathogenesis scientific workshop [I]. J Crohns Colitis 2014;8:5–18. [DOI] [PubMed] [Google Scholar]
- 17. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long MD, Kappelman MD, Pipkin CA. Nonmelanoma skin cancer in inflammatory bowel disease: a review. Inflamm Bowel Dis 2011;17:1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh S, Nagpal SJ, Murad MH, et al. . Inflammatory bowel disease is associated with an increased risk of melanoma: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:210–8. [DOI] [PubMed] [Google Scholar]
- 20. Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 2009;29:2727–37. [PubMed] [Google Scholar]
- 21. Bitton A, Vutcovici M, Sewitch M, Suissa S, Brassard P. Mortality trends in Crohn’s disease and ulcerative colitis: A population-based study in Québec, Canada. Inflamm Bowel Dis 2016;22:416–23. [DOI] [PubMed] [Google Scholar]
- 22. Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990;323:1228–33. [DOI] [PubMed] [Google Scholar]
- 23. Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in ulcerative colitis patients. Int J Cancer 2008;123:1417–21. [DOI] [PubMed] [Google Scholar]
- 24. Andersen NN, Jess T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J Gastroenterol 2013;19:7561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: A population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol 2004;2:1088–95. [DOI] [PubMed] [Google Scholar]
- 26. Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2010;8:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh H, Nugent Z, Demers AA, Bernstein CN. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology 2011;141:1612–20. [DOI] [PubMed] [Google Scholar]
- 28. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166:1069–80. [DOI] [PubMed] [Google Scholar]
- 29. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143:390–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karlén P, Löfberg R, Broström O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: A population-based cohort study. Am J Gastroenterol 1999;94:1047–52. [DOI] [PubMed] [Google Scholar]
- 31. Masala G, Bagnoli S, Ceroti M, et al. . Divergent patterns of total and cancer mortality in ulcerative colitis and Crohn’s disease patients: the Florence IBD study 1978–2001. Gut 2004;53:1309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D’Andrea N, Vigliarolo R, Sanguinetti CM. Respiratory involvement in inflammatory bowel diseases. Multidiscip Respir Med 2010;5:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Misselwitz B, Manser CN, Rogler G, et al. . High rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation—data from the Swiss IBD cohort study. J Crohns Colitis 2015;9:819–29. [DOI] [PubMed] [Google Scholar]
- 34. Eaden JA, Mayberry JF. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 2002;51:v10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:314–21. [DOI] [PubMed] [Google Scholar]
- 36. Lashner BA, Hanauer SB, Silverstein MD. Optimal timing of colonoscopy to screen for cancer in ulcerative colitis. Ann Intern Med 1988;108:274–8. [DOI] [PubMed] [Google Scholar]
- 37. Stengel B. Chronic kidney disease and cancer: A troubling connection. J Nephrol 2010;23:253–62. [PMC free article] [PubMed] [Google Scholar]
- 38. Jurjus A, Eid A, Al Kattar S, et al. . Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: the links. BBA Clin 2016;5:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giovannucci E, Harlan DM, Archer MC, et al. . Diabetes and cancer: A consensus report. Diabetes Care 2010;33:1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denholm R, Schüz J, Straif K, et al. . Is previous respiratory disease a risk factor for lung cancer? Am J Respir Crit Care Med 2014;190:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.