Abstract
Background and Aims
In vitro studies using immortalised cancer cell lines showed that butyrate has an overall positive effect on epithelial barrier integrity, but the physiological relevance of cancer cell lines is limited. We developed epithelial monolayers from human tissue samples of patients with ulcerative colitis [UC] to assess the effect of butyrate on epithelial barrier function.
Methods
A protocol to establish monolayers from primary epithelial cells of UC patients [n = 10] and non-UC controls [n = 10] was optimised. The monolayers were treated with 8 mM sodium butyrate ± tumour necrosis factor alpha [TNFα] and type II interferon [IFNγ] for 48 h. Changes in transepithelial electrical resistance were monitored. Barrier gene expression levels were measured. Inflammatory proteins in the supernatant of the cells were quantified with OLINK.
Results
We demonstrated that primary monolayer cultures can be grown within 1 week of culture with robust resistance values and polarised tight junction expression. Butyrate treatment of the cultures increased resistance but was detrimental in combination with TNFα and IFNγ. The combined treatment further induced even higher IL8 mRNA and inflammatory protein secretion than for the inflammatory mediators alone. The observed effects were similar in cultures from patients and non-UC controls, suggesting that there were no patient-specific responses responsible for these findings.
Conclusions
We found that butyrate does not protect against inflammation-induced barrier dysfunction and even worsens its effects in primary epithelial monolayers of UC patients and controls. The basic mechanisms of butyrate should therefore be reconsidered in future studies, in particular in patients with active inflammation and pre-existing barrier defects as is known for UC.
Keywords: Butyrate, ulcerative colitis, primary epithelial monolayers
1. Introduction
Ever since the first observations of increased intestinal permeability in patients with inflammatory bowel disease [IBD], dysfunction of the intestinal barrier has been recognised to play a major role in the multifactorial pathogenesis of IBD.1,2 Besides the mucus layer, the intestinal epithelial cells represent a key feature of the intestinal barrier where they provide a physical and immunological defence mechanism to protect the host against invading pathogenic microorganisms and potentially harmful molecules. Regulation of the intestinal barrier is highly complex and involves several internal and exogenous factors including the gut microbiota and its metabolites.3,4
In IBD patients, alterations in the gut microbiota, also termed dysbiosis, have been defined as decreased microbial diversity compared with healthy individuals and changes in abundance of specific bacterial taxa.5 Among these, for example, a decrease of short-chain fatty acid-producing bacteria belonging to the phylum Firmicutes has been consistently found in stools of IBD patients.5–7 Short-chain fatty acids are the primary end products of fermentation of non-digestible carbohydrates in the large intestine, and provide a direct mechanistic link between intestinal dysbiosis, barrier dysfunction, and IBD pathogenesis.8 Whereas acetate has the highest concentrations in the gut, butyrate is the most well-known short-chain fatty acid for its pleiotropic effects.
Butyrate is the major energy source for colonocytes, and was shown to be involved in anti-inflammatory processes, oxidative stress pathways, regulation of cell proliferation and differentiation, colonic defence, satiety, immune regulation, and intestinal barrier function.9 Given these reported positive effects and the proven lack of butyrate-producing bacteria in IBD patients, supplementation of butyrate has been repeatedly proposed in the management of IBD colitis. However, the clinical effects of butyrate treatment, in particular using enemas, have been inconsistent across studies ranging from beneficial effects on inflammatory parameters to very mild or no improvement.10–15 Data from murine colitis models have also been contradictory and even showed influence on tumour formation in mice, stressing the need for additional studies on the basic mechanisms of butyrate and other short-chain fatty acids.16–21
With regard to the effect of butyrate on intestinal barrier function, most researchers are using intestinal cell lines including Caco-2 and HT-29 monolayers as model systems. In these models, butyrate enhances epithelial barrier function and tight junction protein expression, although the observed changes are often dependent on the concentration of butyrate and the type of cells.9,22 Caco-2 cells have the advantage that they are easy to culture and differentiate spontaneously to enterocytes, but they are derived from a human colon carcinoma and thus do not fully resemble the expression patterns and behaviour of normal intestinal epithelial cells.23
Recently, there is a growing interest in the use of primary intestinal epithelial cells as an alternative in vitro model for studies of human intestinal epithelia.24 Although these models have been challenging due to limitations in cell viability and cell number, recent advances with organoid cultures derived from human biopsies have overcome these hurdles.25 Using the proliferative power of epithelial spheroids to expand primary epithelial cells of patients on Transwell filters, the group of VanDussen et al. previously showed that adherence of specific pathogens to primary cells was enhanced compared with immortalised cells, again confirming the importance of the origin of the cells.26
Considering the lack of results in human primary tissue for butyrate and the inconsistent clinical data in IBD, we studied the effects of butyrate treatment on primary intestinal epithelial monolayers of patients, to untangle the mechanisms of the metabolite on epithelial barrier function. We first optimised a protocol to obtain epithelial cell cultures from endoscopically derived biopsies of patients with ulcerative colitis [UC] in Transwell inserts. Using this model, we then analysed the effect of butyrate in combination with the inflammatory mediators tumour necrosis factor alpha [TNFα] and interferon type II [IFNγ] on barrier function including changes in: epithelial resistance; gene expression levels of selected genes related to the intestinal barrier; and inflammatory protein secretion in the supernatant of the cell monolayers.
2. Materials and Methods
2.1. Human biopsy collection and ethical statement
Mucosal biopsies from macroscopically non-inflamed colon were obtained during routine endoscopy from 10 UC patients and 10 non-UC controls at the University Hospitals Leuven. Previous inflammation at the site of biopsy extraction for UC patients was not excluded, nor active inflammation at other parts of the large intestine. Non-UC controls were individuals undergoing colonoscopy for surveillance of colorectal polyps or abdominal discomfort, but without abnormalities found. The biopsies were collected in ice-cold basal medium [BM] (DMEM:F12 1:1 Mixture [Lonza, Basel, Switzerland] supplemented with 1x GlutaMAX [Gibco, Thermo Fisher Scientific, Waltham, MA, USA], 10 mM HEPES [Gibco], and 100 U/ml + 100 μg/ml penicillin/streptomycin [Gibco]), and processed within 2 h for crypt isolation.
All individuals gave written informed consent before sample collection [S53684/B322201213950 approved by the Ethics Committee of the University Hospitals Leuven]. Baseline characteristics of the individuals are given in Table 1.
Table 1.
Baseline characteristics.
| UC [n = 10] | Non-UC controls [n = 10] | |
|---|---|---|
| Male/female [%] | 5/5 [50/50] | 4/6 [40/60] |
| Median [IQR] age at inclusion [years] | 47.3 [33.9–57.6] | 58.6 [49.2–70.7] |
| Median [IQR] age at diagnosis [years] | 32 [28–38] | NA |
| Median [IQR] disease duration [years] | 10.5 [4.3–15.3] | NA |
| Disease activity at inclusiona | ||
| Inactive [0–1] | 5 | NA |
| Active [2–3] | 5 | NA |
| Medication use [%] | ||
| 5-aminosalicylates | 6 [60] | NA |
| Corticosteroids | 1 [10] | NA |
| Immunomodulators | 1 [10] | NA |
| Biologics | 5 [50] | NA |
| Previous UC-related surgery [%] | 0 [0] | NA |
UC, ulcerative colitis; IQR, interquartile range; NA, not applicable.
aDisease activity was based on the overall Mayo endoscopic subscore.
2.2. Intestinal crypt isolation and organoid culture
Intestinal crypts were isolated from 4–6 colon biopsies per individual as described before.27 At the end of the procedure, the crypts were re-suspended in Matrigel [Growth Factor Reduced, phenol-red-free, Corning, NY, USA] diluted with basal medium [50/50%].28 To allow the formation of 3D organoid structures, three droplets [13.3 μl] of each cell suspension were plated in every well of a 24-well tissue culture plate [8–12 wells/4–6 biopsies dependent on biopsy size and isolation efficiency]. After polymerisation of the Matrigel mixture in a humidified incubator at 37°C and 5% CO2 for at least 30 min, 500 μl human expansion medium [HM] [see Supplementary Table 1, available as Supplementary data at ECCO-JCC online for a list with the components] was added to the wells to let the organoids grow over time. The medium was replaced every 48 h, and the organoids were split after 7–10 days [usually 1:3 split] depending on their growth rate. Of every new culture, at least four wells with a low passage number [P2-P10] were pelleted, re-suspended in 1 ml Recovery Cell Culture Freezing Medium [Gibco] and stored in liquid nitrogen to obtain an organoid stock. Different sample aliquots were stored from each culture, allowing for future or additional experiments within the same individual. For each monolayer experiment, one sample aliquot with colonic organoids was slowly thawed, re-suspended in Matrigel mixture, and expanded until sufficient wells were obtained.
2.3. Primary epithelial cell monolayer culture
The protocol to form primary epithelial cell monolayers on membrane inserts from organoids was based on the protocol from VanDussen et al. with slight modifications.26 In brief, 6.5 mm Transwell inserts [CLS3470, 0.4-μm pore PET membrane, Corning Costar] were coated with 0.1 mg/ml collagen type I [rat tail, Corning] diluted in 0.02 M acetic acid for 24 h at 37°C. The day after, the Transwells were rinsed three times with phosphate buffered saline [PBS] and pre-incubated with 50% HM [diluted with BM] and 10 μM Rho-associated kinase [ROCK] inhibitor [Y-27632, Selleckchem, Munich, Germany] for at least 2 h. On the same day [usually 3 days after splitting], approximately three wells with organoids of a 24-well plate per Transwell were harvested and dissociated mechanically by pipetting up and down 10 times using a 200-μl tip on top of a 1000-μl tip and pipette. All organoids were harvested between passage numbers 2 and 10. The organoid fractions were subsequently washed in 0.5-mM EDTA/PBS solution and centrifuged for 5 min at 350 g. The fractions were then treated with 0.25% trypsin/EDTA [Gibco] for 5 min at 37°C in a water bath. The fractions were further dissociated mechanically with a pipette until a homogeneous solution without visible aggregates was obtained. We also checked the fractions regularly under a standard light microscope to evaluate the size of the aggregates. With cell aggregates of 2–10 cells, trypsin was inactivated using an excess of BM supplemented with 10% fetal bovine serum. The cells were spun down in the solution at 350 g for 5 min. The pellet was again re-suspended in BM and the number of aggregates was manually counted. After counting, the cells were centrifuged and finally dissolved in the correct volume of 50% HM + ROCK inhibitor to seed approximately 1 x 106 cells in 100 μl in each Transwell insert [apical compartment]. The lower compartment of the wells was filled with 600 μl of 50% HM + ROCK inhibitor. To let the aggregates attach and grow, the cells were kept at 37°C in a humidified incubator with 5% CO2. After 24 h, dead and unattached cells were washed away by carefully pipetting up and down without touching the membrane. The medium in the apical [200 μl] and basolateral compartments [600 μl] was refreshed with 50% HM without ROCK inhibitor, and this was repeated every other day until confluent and polarised monolayers were formed. Imaging of the living cell monolayers was performed with a Zeiss Axiovert 40 CFL microscope [Carl Zeiss Inc., Oberkochen, Germany] and AxioCam Mrc5 camera [AxioVision Rel 4.8 software].
2.4. Haematoxylin and eosin staining and immunofluorescence
Stainings were performed either on transverse sections or on whole mounts of the monolayers. Primary antibodies for immunofluorescence included mouse anti-ZO1 antibody [1/50; 33–9100, Thermo Scientific], rabbit anti-claudin-3 antibody [1/200; ab52231, Abcam, Cambridge, UK], rabbit anti-mucin 2 [1/150; SC-15334, Santa Cruz Biotechnology, Dallas, TX, USA], and mouse anti-human Ki67 [1/150; MONX10283, Monosan, Sanbio, Uden, The Netherlands]. Additional details are provided in Supplementary Methods, available as Supplementary data at ECCO-JCC online.
2.5. Sodium butyrate and cytokine treatment
Primary intestinal epithelial cultures were treated with a physiological concentration of 8 mM sodium butyrate [Sigma-Aldrich, St Louis, MO, USA] dissolved in 200 µl 50% HM medium for 48 h. To further evaluate its effect in the presence of inflammatory stimuli, this condition was repeated in combination with 25 ng/ml recombinant human TNFα [Invivogen, Toulouse, France] and 25 ng/ml IFNγ [Invitrogen] in 600 µl 50% HM medium. The concentrations of the inflammatory stimuli were based on literature29–34 and a pilot study in our laboratory with 50 ng/ml of the components [Supplementary Figures 1 and 2, available as Supplementary data at ECCO-JCC online]. Cells grown in 50% HM were used as negative control condition. All conditions were tested in duplicate and the treatments were initiated when the cell monolayers showed polarisation based on resistance and visual evaluation [usually 5–7 days after seeding in the Transwells]. Sodium butyrate was added to the medium of the upper [apical or luminal side] compartment of the Transwells, and TNFα and IFNγ were administered to the medium of the basolateral compartment of the 24-well culture plates. Cells were treated for 48 h, after which they were used for RNA extraction. The apical and basolateral media were harvested and stored at -80°C for protein measurements.
2.6. Transepithelial electrical resistance measurements
Transepithelial electrical resistance [TEER] measurements were performed using an EVOM2 epithelial Volt/Ohm meter and STX2 chopstick electrode set [World Precision Instruments, Sarasota, FL, USA]. All measurements were performed in triplicate and corrected with the resistance value of an empty Transwell insert. Final values were calculated as the average resistance multiplied by the area [0.33 cm2] of the Transwell membrane and expressed as Ω.cm2.
2.7. Gene expression analysis by quantitative reverse transcription
Cells were immersed in RNA lysis buffer [Qiagen, Hilden, Germany] with 2-mercaptoethanol [Sigma-Aldrich], and lysates were kept at -80°C until RNA extraction. Total RNA isolation was performed using the RNeasy Mini Kit [Qiagen]. Complementary DNA was synthesised from 0.22 μg total RNA using the RevertAid TM H Minus First strand cDNA Synthesis kit [Fermentas, Thermo Fisher Scientific] according to the manufacturer’s protocol. The expression of some of the major genes involved in intestinal barrier function was studied: claudin 1 [CLDN1], claudin 2 [CLDN2], claudin 8 [CLDN8], occludin [OCLN], zonula occludens 1 [ZO1], and mucin 2 [MUC2]. Hypoxia-inducible factor 1A [HIF1A] was also measured as upstream mediator of tight junction function. Interleukin-8 [IL8] was added as marker for inflammation, and vilin-1 [VIL1] and antigen Ki-67 [MKI67] were quantified as gut epithelial cell and proliferation marker, respectively. Primers and dual-labelled probes [Sigma, Supplementary Table 2, available as Supplementary data at ECCO-JCC online] were custom designed using OligoAnalyzer 3.1 software [Integrated DNA technologies]. The SensiFast Probe No-ROX Kit [GC Biotech, Alphen aan den Rijn, The Netherlands] was applied according to the manufacturer’s instructions. All reactions were performed in duplicate on a 7500 Fast Real-Time PCR machine [Applied Biosystems, Ghent, Belgium]. Results are presented as relative mRNA levels to the endogenous reference gene beta-actin [ACTB], and were calculated based on the Pfaffl method.35
2.8. Relative quantification of inflammation-related proteins in culture supernatant
The apical and basolateral media samples were analysed for inflammation-related proteins using the Proseek Multiplex Inflammation panel from OLINK Proteomics [Uppsala, Sweden]. All samples passed the quality control checks from OLINK. For statistical comparison, proteins with missing data frequencies above 25% for the apical and/or basolateral media samples were excluded, leaving 40 proteins for analysis. Unsupervised clustering of the overall protein compositions of the media samples was performed using principal component analysis. Protein levels are presented as normalised protein expression [NPX] values following an inter-plate control normalisation procedure. Fold changes between the treatment groups were calculated as 2ΔNPX. Proteins with >2-fold change difference and adjusted p-values <0.05 were considered differentially expressed.
2.9. Statistical analysis
Statistical analysis was performed using Graphpad Prism 7 software [La Jolla, CA, USA] and R 3.4.1 [R Foundation, Vienna, Austria]. Continuous data were not normally distributed and were therefore presented as median with interquartile [IQR] ranges. Comparisons between treatment groups and time-dependent analyses of TEER and gene expression levels were performed using paired Friedman tests and post-hoc Dunn’s tests adjusted for the number of comparisons in each analysis. Mann-Whitney U tests were used to compare the data between UC patients and non-UC controls. Comparisons of the protein markers in the treatment groups were conducted using paired Wilcoxon tests with correction for multiple testing according to the Benjamini-Hochberg false discovery rate procedure. An adjusted significance level of 0.05 [adj.p] was used in all analyses unless otherwise stated.
3. Results
3.1. Establishment of tight monolayer cultures from primary intestinal epithelial cells in Transwells is feasible within 1 week
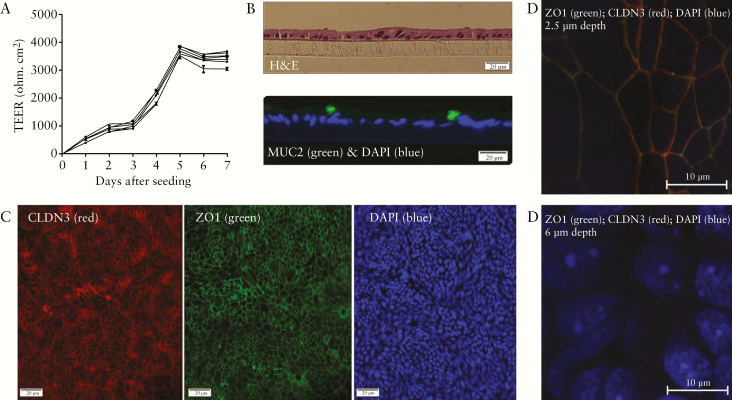
We first set out to obtain confluent monolayer cultures from primary intestinal epithelial cells of human colon organoids from UC patients and non-UC controls in Transwells. To evaluate the efficiency of our procedure, we measured TEER every 24 h after cell seeding, and performed haematoxylin and eosin [H&E] and immunostaining. First, we observed that the TEER values rapidly increased a few days after seeding in all cultures [Figure 1A]. Although the absolute TEER values differed substantially between cultures from different individuals, all cultures reached high and stable values within 5–7 days after cell seeding [at least 1500 Ω.cm2]. There was no difference between baseline TEER values and TEER increases in time from cultures of UC patients versus non-UC controls. To confirm that we obtained a single layer of intestinal epithelial cells at this stage, we performed H&E staining on paraffin-embedded sections of the monolayers which showed that we attained adjacent cells without visible multicellular structures [Figure 1B]. H&E staining of whole mounts of the cultures confirmed confluency of the cultures across the Transwell membranes, as suggested earlier by the stable TEER values [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online]. With immunostaining, we demonstrated that different cell types of the intestinal epithelium were present, including MUC2-expressing goblet cells [Figure 1B] and proliferating cells expressing Ki67 [Supplementary Figure 4, available as Supplementary data at ECCO-JCC online]. Furthermore, the cell cultures formed tight junction proteins, including ZO1 and CLDN3 as demonstrated by immunofluorescent staining of whole mounts of the monolayers [Figure 1C]. The apical-basolateral polarisation of the cells was confirmed using Z-stack images. At a depth of 2.5 µm, ZO1 and CLDN3 showed a clear fluorescent signal with co-localisation [as indicated by the overlap of the fluorescent signals], whereas this signal disappeared when moving towards the basolateral side where the DAPI-stained nuclei became clearly visible at a depth of 6 µm [Figure 1D].
Figure 1.
Characterisation of the primary monolayer cultures. [A] Graphical representation of the absolute TEER values of eight Transwell replicates of a representative cell culture from a UC patient showing that a rapid increase in TEER was followed by a stable phase after 5–7 days, indicative of epithelial polarisation. Mean values of triplicate measurements with 95% confidence intervals are shown for each culture well. [B] Cross-sectional views of paraffin-embedded Transwell cultures confirmed that we obtained cell monolayers [upper panel] including MUC2-expressing cells [lower panel]. [C] The cell cultures covered the whole area of the Transwell filters and formed tight junctions including CLDN3 and ZO1. [D] Z-stack images demonstrated apical co-expression of the tight junction proteins ZO1 and CLDN3, whereas nuclear staining was only observed at a lower depth, indicative of correct polarisation of the monolayers. All stainings were performed on representative confluent cultures from UC patients and/or non-UC controls [no difference dependent on disease status]. TEER, transepithelial electrical resistance; UC, ulcerative colitis; MUC2, mucin 2; CLDN3, claudin 3; ZO1, zonula occludens 1; DAPI, 4’,6-diamidino-2-phenylindole.
3.2. Butyrate is detrimental for intestinal barrier integrity and cell appearance in the presence of inflammatory mediators
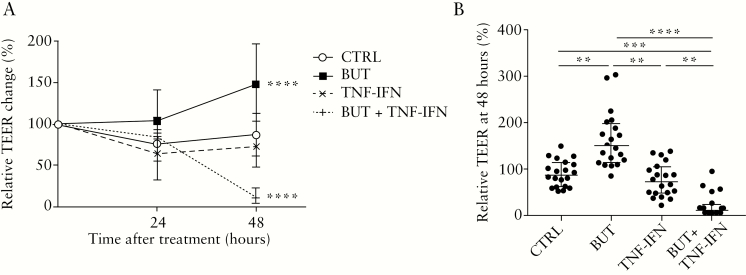
When adding 8mM butyrate to the primary monolayer cultures, we found an overall beneficial effect on TEER of the cells, with a significant increase over a time period of 48 h compared with the negative control condition (two-way analysis of variance [ANOVA], adj.p <0.0001; Figure 2A). Although medium complemented with TNFα and IFNγ alone had a very limited negative effect on TEER over time [two-way ANOVA, adj.p = 0.14; Figure 2A], co-incubation of the monolayers with these inflammatory mediators and butyrate led to a large, significant drop in TEER [two-way ANOVA, adj.p <0.0001; Figure 2A]. The changes in TEER were not dependent on the disease status of the cultures [UC versus non-UC], since the relative TEER changes did not differ at any time point or treatment condition between monolayers from UC patients and non-UC controls [Mann-Whitney U test, adj.p >0.05; Supplementary Figure 5, available as Supplementary data at ECCO-JCC online]. Pairwise comparisons at 48 h between the treatment groups confirmed that medium complementation with butyrate without inflammatory stimuli was superior to all other treatments in terms of relative TEER change, with a median increase of 62.2% versus the control condition (148.5 [113.8–197.5] % versus 86.3 [61.1–113.1] %; adj.p <0.01; Figure 2B). On the other hand, butyrate was detrimental in the presence of inflammation compared with all other treatments, with a median decrease in TEER of 75.7% versus the control condition (10.6 [4.1–22.7] % versus 86.3 [61.1–113.1] %; adj.p <0.001; Figure 2B). Induction by TNFα and IFNγ alone decreased median TEER values with 14.1%, but this was not significantly different from the control condition (10.6 [72.2 [47.7–104.3] versus 86.3 [61.1–113.1] %; adj.p >0.05; Figure 2B).
Figure 2.
Effect of butyrate ± TNFα and IFNγ on TEER in monolayer cultures of UC patients [n = 10] and non-UC controls [n = 10] during the whole treatment period [A] and at the end of treatment [B].[A] Complementation with butyrate ± TNFα and IFNγ induced significant changes in TEER over time compared with the untreated control condition [two-way ANOVA, p <0.0001]. [B] At 48 h, treatment had a significant effect on TEER [Friedman test, p <0.0001] with higher values for the cells treated with butyrate compared with all others, but butyrate had the opposite effect in the presence of TNFα and IFNγ as inflammatory mediators compared with all other treatment conditions [Dunn tests, adj.p <0.05]. TEER is given as percentage change to the initial values of the cultures at the start of treatment [0 h]. Data from UC patients and non-UC controls were merged for analysis. Data are shown as medians with interquartile ranges. Each treatment condition was tested in duplicate. Significant comparisons of the post-hoc tests are indicated. TEER, transepithelial electrical resistance; UC, ulcerative colitis; TNF, tumour necrosis factor; IFN, interferon; ANOVA, analysis of variance; CTRL, negative control [medium]; BUT, 8 mM butyrate; TNF-IFN, 25ng/ml TNFα and 25ng/ml IFNγ; BUT + TNF-IFN, 8 mM butyrate + 25ng/ml TNFα and 25ng/ml IFNγ. **adj.p <0.01; ***adj.p <0.001; ****adj.p <0.0001.
Brightfield microscopic images of the cultures at the end of the treatments were in agreement with the TEER findings and showed confluent monolayers for the untreated control condition and butyrate treatment condition, whereas the morphology of the cells was slightly altered in the presence of TNFα and IFNγ, and even more so in the combined treatment condition [Supplementary Figure 6, available as Supplementary data at ECCO-JCC online].
3.3. Butyrate treatment has divergent though disease-independent effects on epithelial barrier gene expression
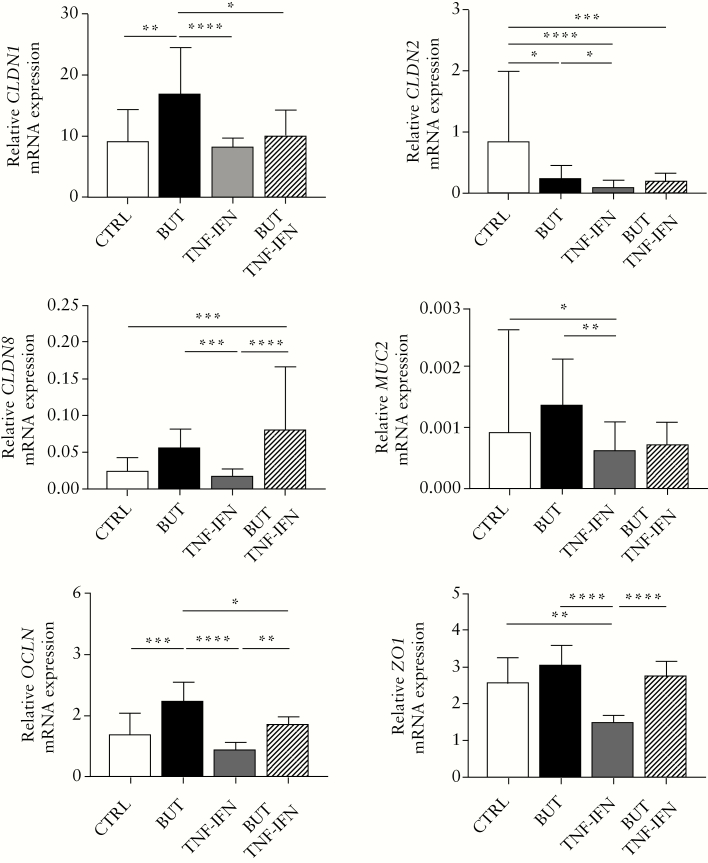
Given the observed TEER and microscopic changes, we also evaluated the mRNA expression levels of a selection of intestinal barrier genes using quantitative real-time polymerase chain reaction [qRT-PCR] in the treated cultures. Overall, treatment had a significant effect on the expression of all studied genes related to intestinal barrier function [Friedman test, p <0.0001, Figure 3]. More specifically, butyrate treatment induced higher median expression levels of almost all barrier genes, though significantly only for CLDN1 and OCLN and except for CLDN2, which was significantly downregulated as compared with the control condition [post-hoc Dunn test, adj.p <0.05]. Treatment with TNFα and IFNγ resulted in generally lower barrier gene expression values compared with the negative control cultures, with a significant effect for CLDN2, MUC2 and ZO1 [post-hoc Dunn test, adj.p <0.05]. The combination of TNFα and IFNγ with butyrate versus the control condition also showed significantly lower mRNA expression for CLDN2, whereas CLDN8 expression was significantly higher [post-hoc Dunn test, adj.p <0.05]. The mRNA gene expression changes for the additionally tested genes HIF1A, IL8, MKI67, and VIL1 are given in Supplementary Figure 7 available as Supplementary data at ECCO-JCC online. To note, the inflammatory marker IL8 was significantly increased upon incubation with butyrate alone, and even more in combination with TNFα and IFNγ when compared with the non-treated control samples [post-hoc Dunn test, adj.p <0.05].
Figure 3.
Bar plots showing the relative mRNA expression levels of six intestinal barrier genes as evaluated with qRT-PCR in monolayer cultures from UC patients [n = 10] and non-UC controls [n = 10] treated with medium [CTRL, white bars], 8 mM sodium butyrate [BUT, black bars], 25 ng/ml TNFα and IFNγ [TNF-IFN, grey bars], or 8 mM sodium butyrate + 25 ng/ml TNFα and IFNγ [BUT + TNF-IFN, striped line bars]. Treatment had a significant effect on all barrier gene levels [Friedman test], with the effect depending on the gene and treatment condition [post-hoc Dunn’s tests with adjustment for multiple comparisons]. The expression levels were normalised to beta-actin [ACTB], and results are shown as medians with interquartile ranges. Significant differences for the post-hoc tests are indicated. UC, ulcerative colitis; TNF, tumour necrosis factor; IFN, interferon; qRT_PCR, quantitative real-time polymerase chain reaction; CTRL, negative control; BUT, 8 mM butyrate. *adj.p <0.05; **adj.p <0.01; ***adj.p <0.001; ****adj.p <0.0001.
The expression values of all genes for the control condition did not differ between patients and non-UC controls [Mann-Whitney U test, adj.p >0.05], showing that there were no baseline differences for the selected genes between both groups. Second, none of the genes showed differential responses to the treatments based on disease status of the cultures [Mann-Whitney U test, adj.p >0.05]. Only for CLDN1, we found slightly higher mRNA levels upon butyrate treatment in UC patients compared with the non-UC controls, but this did not remain significant when corrected for multiple testing [Mann-Whitney U test, unadj.p = 0.01; Supplementary Figure 8, available as Supplementary data at ECCO-JCC online].
3.4. Butyrate induces high inflammatory protein expression upon induction with TNFα and IFNγ
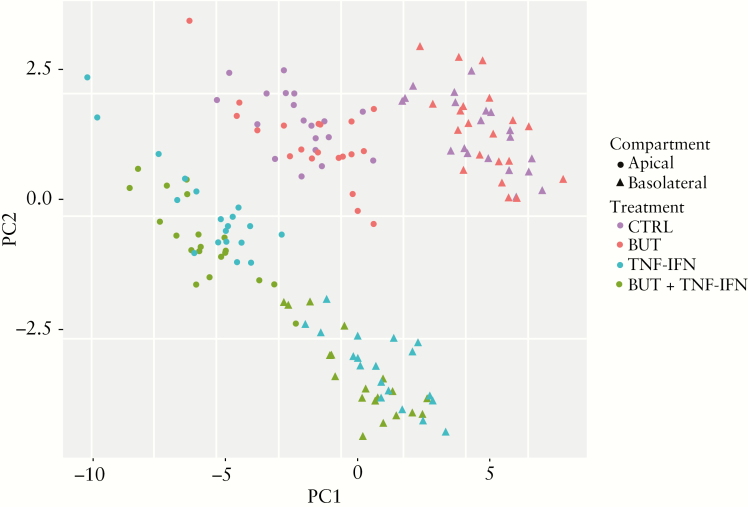
To evaluate the expression of secreted proteins upon cell treatment, we measured a panel of 40 inflammation-related proteins in the upper and lower compartment media of the treated monolayers. Unsupervised clustering showed that the negative control condition and butyrate-treated samples largely overlapped and were distinct from the samples treated with the inflammatory mediators TNFα and IFNγ and its combination with butyrate (principal component analysis [PCA]; Figure 4). The PCA plot furthermore showed a second separation of the samples dependent on the medium compartment [apical versus basolateral]. Statistical analysis of the clusters confirmed that treatment and medium compartment had a significant influence on the sample protein compositions [Adonis test adj.p = 0.002 for both]. Pairwise comparisons of the treatment conditions showed that the samples treated with TNFα and IFNγ differed from the negative control samples and the combination treatment with butyrate [adj.p = 0.002 and 0.004, respectively]. The latter samples also were different from the negative control samples [adj.p = 0.002], whereas butyrate treatment alone did not, reflected by the large overlap [adj.p = 0.10]. Likewise, there was no difference between media samples of cultures from UC patients and non-UC controls [Adonis test diagnosis adj.p = 0.32].
Figure 4.
Principal component analysis [n = 160] of inflammation-related proteins in the apical [circles] and basolateral [triangles] media of primary monolayer cultures from UC patients [n = 10] and non-UC controls [n = 10] treated with medium [negative control; CTRL], 8 mM sodium butyrate [BUT], 25 ng/ml TNFα and IFNγ [TNF-IFN], or the combination of 8 mM sodium butyrate and 25 ng/ml TNFα and IFNγ [BUT + TNF-IFN]. The apical [n = 80, 20 individuals/four treatment conditions] and basolateral [n = 80, 20 individuals/four treatment conditions] media samples were collected after 48 h and evaluated with the OLINK Inflammation panel which quantified 40 inflammation-related proteins. Unsupervised clustering shows distinct protein compositions according to treatment condition and medium compartment [apical versus basolateral]. UC, ulcerative colitis; TNF, tumour necrosis factor; IFN, interferon.
When subdividing the apical and basolateral media samples to investigate which proteins were differentially expressed upon cell treatment, we found that butyrate [BUT] induced dysregulated expression of six and two proteins compared with the negative control treatment condition in the apical and basolateral medium, respectively (>2-fold change, paired Wilcoxon adj.p <0.05; BUT versus CTRL [see Table 2]). Most proteins were significantly downregulated [italics] upon butyrate incubation, except for DNER and TNFRS9 which were higher in the apical media samples compared with the non-treated samples [indicated with an asterisk]. The number of differentially expressed proteins further increased to 11 [apical] and 14 [basolateral] when comparing the TNFα/IFNγ-treated samples versus the non-treated control samples (>2-fold change, paired Wilcoxon adj.p <0.05; TNF-IFN versus CTRL [Table 2]). All proteins were significantly upregulated for these comparisons. For the combination treatment of butyrate and TNFα and IFNγ, we identified a total of 18 and 13 significantly altered proteins in the apical and basolateral media, respectively, as compared with the negative control media samples of these cultures (>2-fold change, paired Wilcoxon adj.p <0.05; BUT + TNF-IFN vs CTRL [Table 2]). The majority of proteins were common with those seen for the treatment condition with TNFα and IFNγ alone, although generally higher increases in protein concentrations were observed for the combination with butyrate versus the control condition. A Venn diagram showing the distribution of the differentially expressed proteins in the different treatment groups is given in Supplementary Figure 9, available as Supplementary data at ECCO-JCC online.
Table 2.
Differentially expressed proteins in apical [n = 80, 20 individuals/four treatment conditions] and basolateral media samples [n = 80, 20 individuals/four treatment conditions] from treated monolayer cultures of UC patients [n = 10] and non-UC controls [n = 10] versus the negative control condition of these cultures.
| Apical | Basolateral | ||||||
|---|---|---|---|---|---|---|---|
| Protein symbol | UniProt ID | BUT vs CTRL | TNF-IFN vs CTRL | BUT + TNF-IFN vs CTRL | BUT vs CTRL | TNF-IFN vs CTRL | BUT + TNF-IFN vs CTRL |
| 4E-BP1 | Q13541 | 2.64* | |||||
| ADA | P00813 | 2.62* | |||||
| CASP8 | Q14790 | 4.66* | |||||
| CCL20 | P78556 | 2.07* | 36.41* | 53.45* | |||
| CD40 | P25942 | 6.55* | 8.02* | 2.70* | |||
| CDCP1 | Q9H5V8 | 2.10* | 3.29* | ||||
| CSF1 | P09603 | 3.67* | 2.69* | ||||
| CXCL1 | P09341 | 0.43 | 15.88* | 15.14* | |||
| CXCL10 | P02778 | 0.33 | 26.47* | 36.20* | 22.01* | 25.44* | |
| CXCL11 | O14625 | 10.57* | 18.87* | 107.45* | 199.65* | ||
| CXCL5 | P42830 | 31.76* | 48.12* | ||||
| CXCL6 | P80162 | 2.67* | 8.77* | ||||
| CXCL9 | Q07325 | 48.00* | 53.70* | 306.95* | 364.06* | ||
| DNER | Q8NFT8 | 2.37* | |||||
| Flt3L | P49771 | 2.16* | |||||
| IL18R1 | Q13478 | 6.10* | 2.57* | 2.41* | |||
| IL7 | P13232 | 2.10* | |||||
| IL8 | P10145 | 10.45* | 15.92* | ||||
| LIF | P15018 | 0.11 | 3.86* | 0.30 | 0.38 | 4.17* | |
| MCP1 | P13500 | 0.39 | 0.49 | 3.80* | |||
| SCF | P21583 | 2.34* | |||||
| STAMPB | O95630 | 3.37* | |||||
| TGFα | P01135 | 2.58* | 6.26* | ||||
| TNFRSF9 | Q07011 | 2.30* | 9.97* | 9.36* | 23.75* | 19.58* | |
| TRAIL | P50591 | 14.96* | 4.36* | 9.46* | 2.79* | ||
| uPA | P00749 | 0.28 | 0.34 | ||||
Up- [asterisk] and downregulated [italics] proteins are indicated [>2-fold change and adjusted p <0.05].
UC, ulcerative colitis; vs CTRL, versus negative control [medium]; TNF, tumour necrosis factor; IFN, interferon; BUT, 8 mM butyrate; TNF-IFN, 25ng/ml TNFα and 25ng/ml IFNγ; BUT + TNF-IFN, 8m M butyrate + 25ng/ml TNFα and 25ng/ml IFNγ.
4. Discussion
In this study, we could efficiently obtain confluent and polarised monolayer cultures in Transwell inserts from primary epithelial cells of UC patients and non-UC controls. Using this model to better mimic the physiological situation than earlier studies with immortalised cancer cell lines, we first confirmed that butyrate has a beneficial effect on barrier resistance, but we also observed a surprising, negative effect of butyrate on TEER and visual appearance of the epithelial cell monolayers in the presence of the inflammatory mediators TNFα and IFNγ. The combined treatment of butyrate with TNFα and IFNγ also induced a strong upregulation of IL8 mRNA expression and many inflammatory proteins. These treatment responses were, however, not dependent on disease-related signatures of the epithelial cells, because cultures from UC patients did not differ at any point from those of non-UC controls, similarly not at baseline. Together our results underscore the need of revisiting the basic mechanisms of butyrate with improved human model systems.
The use of primary intestinal epithelial monolayer cultures in permeable inserts has been of interest to a growing number of researchers.24,26,36–38 The protocol enables investigation of mechanisms and responses in a patient-specific manner with easy access to the apical and basolateral side of epithelial cells, which is a great advantage as compared with the use of organoid cultures where the lumen is enclosed. Similar to the results of VanDussen et al. whose protocol and expertise were used as guidance,26 we obtained polarised monolayers consisting of a mix of differentiated intestinal epithelial cells, including colonocytes and MUC2-producing cells, and proliferative Ki67-positive cells. H&E staining confirmed confluency of the monolayers across the Transwell inserts, which was fundamental for the set up of this study. As evidenced by the high TEER values, the monolayers also showed clear tight junction protein expression including CLDN3 and ZO1.
Butyrate has generally been seen as a positive regulator of intestinal barrier function.39–42 Peng et al., however, showed that the effect of butyrate can also be paradoxical dependent on the applied dose: whereas low concentrations [2 mM] of butyrate promoted barrier function in Caco-2 cells, high concentrations [8 mM] induced intestinal cell apoptosis and reduced TEER.43 Here, epithelial monolayers were exposed to 8 mM butyrate which was considered a physiologically relevant concentration for primary epithelial colon cells based on literature, and indeed showed an evident, increased TEER without induction of apoptosis.
Researchers have shown that primary colon cells can tolerate higher concentrations of butyrate compared with cancer cells, due to differences in cell origin and behaviour, again confirming the importance of a primary human tissue model.44 An intermediate dose of 4 mM also demonstrated higher TEER values in our cultures, although the percentage increase was much lower at the end of the treatment period [Supplementary Figure 10, available as Supplementary data at ECCO-JCC online]. Since intestinal barrier function should not be assessed with TEER measurements alone, which mostly reflect permeability to ions,45 we also investigated changes in expression of tight junction proteins. The TEER-associated changes in mRNA expression of some well-known epithelial barrier genes showed increased values for all but one barrier gene in the butyrate-treated cultures as compared with the negative controls, with the strongest upregulations seen for CLDN1 and OCLN. CLDN2 was the only gene that was significantly downregulated.
As opposed to CLDN1 and OCLN, but also CLDN8, MUC2, and ZO1 that are encoding barrier-enhancing proteins, CLDN2 is a pore-forming tight junction protein and thus barrier-deteriorating.46 These results are in line with previous studies, although for CLDN1, increased mRNA expression levels in IBD patients with active clinical disease have also been observed.47,48 This would imply that CLDN1 levels are associated with barrier dysfunction in active IBD, but this is in contrast with the observed increased TEER values in the cultures. A potential explanation could be that the function of CLDN1 is dual and dependent on the disease environment or other tight junction proteins. Although part of the barrier-forming tight junction complex in steady-state conditions, its role might be altered during the disease process. For the upstream regulator of the tight junctions, HIF1A, increased mRNA levels were found in the butyrate-treated cultures as compared with the untreated controls, again suggesting a protective effect of the metabolite for epithelial barrier function as previously also seen in Caco-2 cells and mice models.49,50 This positive effect is further confirmed by increased expression of the epithelial cell marker VIL1 and a known effect on epithelial cell differentiation as reflected by the lower MKI67 values.51 At the same time, however, the mRNA expression of IL8 was significantly increased in butyrate-treated cultures as compared with untreated control cultures, independent of co-stimulation with the inflammatory mediators TNFα and IFNγ. Albeit this has been described before, the mechanism behind this effect remains to be elucidated.41
Since inflammation is a central process of IBD, we included TNFα and IFNγ as pro-inflammatory mediators in our system. The TEER values with these mediators alone decreased by 14.1% as compared with the negative control cultures. This effect was modest, as also reflected by the unchanged IL8 mRNA expression values in these cultures at 48 h. We did, however, observe a visual effect on the appearance of the cell monolayers, suggesting that we nonetheless induced a pro-inflammatory environment which was also implied by the higher levels of the inflammatory proteins. Previous studies have shown that combined TNFα and IFNγ treatment indeed provokes an inflammatory milieu in a synergistic manner in monolayer cultures, and can be associated with increased paracellular permeability and displacement or downregulation of tight junction proteins.52 Compared with most other studies that are using concentrations ranging from 50 ng/ml to 100 ng/ml, the TNFα and IFNγ concentrations in our study were rather low since we wanted to limit profound epithelial damage in this condition due to cell viability decreases.29,53 When using double concentrations [50 ng/ml] of the cytokines, the gene expression alterations showed similar trends with higher relative changes, but also more extreme TEER decreases [Supplementary Figures 1 and 2]. Because the epithelial cells were derived from non-inflamed areas in the UC patients, we did not expect to see high baseline inflammatory marker expression levels. Indeed, the non-treated cells showed low IL8 expression and had robust and high TEER values. Furthermore, the mRNA levels of IL8 were not different when compared with those from non-UC controls. To create an inflammatory milieu in the cell monolayers, addition of the stimuli was thus needed in both cultures from UC patients and non-UC controls. Additionally, data from our group have previously shown that the inflammatory status of mucosal biopsies is not propagated to organoid cultures, and an external stimulus is needed for continued expression of this status.54 Conversely, it is possible that UC patients have imprinted alterations in other genes of the colonic epithelium, driving pathology and altering their responses to stimuli.55 Yet, we did not find evidence for this for the epithelial barrier genes that we selected.
Our most surprising results were observed for the combination treatment of butyrate with the pro-inflammatory cytokines TNFα and IFNγ, the condition which we believe is most relevant in the context of IBD. In a Caco-2 cell model, Eeckhaut et al. demonstrated that supernatant of Butyricicoccus pullicaecorum—a major butyrate producer—prevented the loss of TEER and increase in IL8 secretion induced by TNFα and IFNγ.56 We however found here that butyrate worsens TEER and cell appearance in the presence of these inflammatory mediators, and IL8 mRNA gene expression was highly upregulated as compared with the negative control media samples. The effects on barrier gene mRNA levels were less pronounced, with most having levels comparable to the control medium condition. The discrepancies between the detrimental effects in our study with previously published data are probably associated with the fact that earlier studies always used cancer cell lines as in vitro models, which do not reflect the normal physiology of primary human colon epithelial cells as previously discussed, and thus do not give us the whole picture of the epithelial functions of butyrate, in particular during inflammation.57 This is further evidenced by a study of Chang et al. who showed that butyrate could not ameliorate colitis in diamino-diphenyl-sulphone [DDS]-treated mice.17 A potential mechanism behind these observations and the data we present was recently also described by the group of Kaiko et al., who identified that colonic crypts protect stem cells in vivo from high butyrate concentrations.58 The overlying layer with differentiated colonocytes would serve as a metabolic barrier and prevent the metabolite reaching the progenitor cells in the crypts where it inhibits stem cell proliferation. They suggest that exposure of proliferating cells to butyrate in cases of mucosal injury can delay wound repair—a mechanism which could also be responsible for the detrimental effects seen in the cultures from this study which are concomitantly exposed to the inflammatory mediators TNFα and IFNγ.
Quantification of a panel of inflammation-related proteins in the apical and basolateral media samples of the cell cultures confirmed that butyrate does not counteract the induction of a pro-inflammatory milieu by TNFα and IFNγ, but induced even higher levels of many inflammatory proteins as compared with the condition with TNFα and IFNγ alone. There was also a significant upregulation of the apoptosis-related protein CASP8 for the combination treatment condition which might explain some of the effects seen on cell morphology.
Finally, given that more than 200 genetic loci have been associated with IBD, and part of these loci have been attributed to epithelial barrier genes and intestinal integrity pathways, UC patients could have an increased genetic predisposition to functional barrier defects.59,60 In a previous study from our group, we observed measurable defects in epithelial ER stress handling in patients with many IBD-associated endoplasmic reticulum [ER] stress variants versus those carrying less variants.27 Likewise, we here evaluated whether UC patients had different TEER and barrier gene levels compared with non-UC controls who are expected to carry substantially less disease-associated alleles. Although we found large inter-individual differences in TEER and barrier gene levels, there was no general difference for the median levels between UC patients and non-UC controls. Neither baseline differences nor altered response rates to the different treatment conditions were observed, indicating that patients do not need higher/other butyrate concentrations or are more sensitive than healthy controls. We however did not specifically evaluate the genetic variants in the included patients, which would allow stratification of the patients in genetic risk groups and could possibly show a better distinction.
In conclusion, we developed a valuable ex vivo approach to assess the effect of different molecules/therapies on barrier function of primary epithelial cells from IBD patients. Using this model, we demonstrated that butyrate supplementation could not counteract the negative effects of TNFα and IFNγ on epithelial barrier function, but even worsened the negative effects on TEER, and strongly upregulated inflammatory mRNA and protein expression. We suggest that the addition of the inflammatory mediators induced barrier dysfunction and mucosal damage mechanisms in the cultures, which make the cells oversensitive to butyrate with subsequent inhibition of cell proliferation and repair, as recently shown in intestinal crypts. Together our observations confirm that additional studies are needed to provide a more nuanced understanding of the effects of butyrate on epithelial barrier function.
Funding
This work was supported by a European Research Council [ERC] Advanced Grant [ERC-2015-AdG, 694679, CrUCCial].
Conflict of Interest
SV reports research support from Merck, Abbvie, Pfizer, Takeda, and Janssen; consultancy fees from Abbvie, MSD, Prometheus Laboratories, Tillotts Pharma, EliLilly, Pfizer, Takeda, Janssen, Celgene, Ferring Pharmaceuticals, Galagapos, Gilead, Hospira, MundiPharma, Second Genome, Biogen; and lecture fees from Abbvie, Pfizer, Takeda, Janssen, Ferring Pharmaceuticals, Galagapos, and Hospira. MF reports grant support from Janssen and Takeda; consulting fees from Abbvie, Boehringer-Ingelheim, Ferring, Janssen, MSD; and speaker fees from Abbvie, Boehringer-Ingelheim, Chiesi, Falk, Ferring, Janssen, Mitsubishi Tanabe, MSD, Takeda, Tillotts, Zeria. The remaining authors have no conflicts of interest regarding this manuscript.
Authorx Contributions
MV: study design, data collection, data analysis, writing first draft of the paper. TL: data collection and critical revision of the manuscript. WV: study design, data collection and critical revision of the manuscript. KA: data collection and critical revision of the manuscript. ASR: data collection and critical revision of the manuscript. RF: study design and critical revision of the manuscript. IC: study design and critical revision of the manuscript. MF: study design, patient recruitment, and critical revision of the manuscript. SV: study design, patient recruitment, data analysis, and critical revision of the manuscript. All authors approved the final version of the manuscript for publication.
Supplementary Material
Acknowledgments
The authors would like to thank Paulien Peetermans, Kaline Arnauts, and Anabela Santo Ramalho for the supply of the human expansion medium and technical help with organoid culturing. We would also like to thank An-Sofie Desmet and Valérie Van Steenbergen from the Lab of Enteric NeuroScience [LENS] of Prof. Pieter Vanden Berghe for their help with the immunostaining and microscopy.
References
- 1. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 2. Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2017;4:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 2017;11:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 5. Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017;14:573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014;63:1275–83. [DOI] [PubMed] [Google Scholar]
- 7. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27:104–19. [DOI] [PubMed] [Google Scholar]
- 10. Breuer RI, Buto SK, Christ ML, et al. Rectal irrigation with short-chain fatty acids for distal ulcerative colitis. Preliminary report. Dig Dis Sci 1991;36:185–7. [DOI] [PubMed] [Google Scholar]
- 11. Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992;103:51–6. [DOI] [PubMed] [Google Scholar]
- 12. Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther 1996;10:729–36. [DOI] [PubMed] [Google Scholar]
- 13. Vernia P, Marcheggiano A, Caprilli R, et al. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther 1995;9:309–13. [DOI] [PubMed] [Google Scholar]
- 14. Vernia P, Annese V, Bresci G, et al. ; Gruppo Italiano per lo Studio del Colon and del Retto Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest 2003;33:244–8. [DOI] [PubMed] [Google Scholar]
- 15. Breuer RI, Soergel KH, Lashner BA, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut 1997;40:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belcheva A, Irrazabal T, Robertson SJ, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014;158:288–99. [DOI] [PubMed] [Google Scholar]
- 17. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 2014;111:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res 2009;58:111–9. [DOI] [PubMed] [Google Scholar]
- 19. Kespohl M, Vachharajani N, Luu M, et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front Immunol 2017;8:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vieira EL, Leonel AJ, Sad AP, et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 2012;23:430–6. [DOI] [PubMed] [Google Scholar]
- 21. Chen G, Ran X, Li B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 2018;30:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plöger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012;1258:52–9. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay E, Auclair J, Delvin E, et al. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J Cell Biochem 2006;99:1175–86. [DOI] [PubMed] [Google Scholar]
- 24. Kauffman AL, Gyurdieva AV, Mabus JR, Ferguson C, Yan Z, Hornby PJ. Alternative functional in vitro models of human intestinal epithelia. Front Pharmacol 2013;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noben M, Vanhove W, Arnauts K, et al. Human intestinal epithelium in a dish: Current models for research into gastrointestinal pathophysiology. United European Gastroenterol J 2017;5:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. VanDussen KL, Marinshaw JM, Shaikh N, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015;64:911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vanhove W, Nys K, Arijs I, et al. Biopsy-derived intestinal epithelial cell cultures for pathway-based stratification of patients with inflammatory bowel disease. J Crohns Colitis 2018;12:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noben M. Intestinal organoids in inflammatory bowel diseases. Intestinale Organoids in Inflammatoire Darmziekten PhD thesis. 2017.
- 29. Boesmans L, Ramakers M, Arijs I, et al. Inflammation-induced downregulation of butyrate uptake and oxidation is not caused by a reduced gene expression. J Cell Physiol 2015;230:418–26. [DOI] [PubMed] [Google Scholar]
- 30. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005;166:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS One 2013;8:e61944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Phuong Nguyen T, Fenyvesi F, Remenyik J, et al. Protective effect of pure sour cherry anthocyanin extract on cytokine-induced inflammatory caco-2 monolayers. Nutrients 2018,. Jul 3. doi: 10.3390/nu10070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warhurst AC, Hopkins SJ, Warhurst G. Interferon gamma induces differential upregulation of alpha and beta chemokine secretion in colonic epithelial cell lines. Gut 1998;42:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van De Walle J, Hendrickx A, Romier B, Larondelle Y, Schneider YJ. Inflammatory parameters in caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol In Vitro 2010;24:1441–9. [DOI] [PubMed] [Google Scholar]
- 35. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol 2014;7:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kozuka K, He Y, Koo-McCoy S, et al. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Reports 2017;9:1976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, DiSalvo M, Gunasekara DB, et al. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol 2017;4:165–182.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mariadason JM, Barkla DH, Gibson PR. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol 1997;272:G705–12. [DOI] [PubMed] [Google Scholar]
- 40. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139:1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 2017;12:e0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng L, Kelly CJ, Battista KD, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J Immunol 2017;199:2976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res 2007;61:37–41. [DOI] [PubMed] [Google Scholar]
- 44. Sauer J, Richter KK, Pool-Zobel BL. Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J Nutr Biochem 2007;18:736–45. [DOI] [PubMed] [Google Scholar]
- 45. Farré R, Vicario M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol 2017;239:193–217. [DOI] [PubMed] [Google Scholar]
- 46. Landy J, Ronde E, English N, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 2016;22:3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vancamelbeke M, Vanuytsel T, Farré R, et al. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Devriese S, Eeckhaut V, Geirnaert A, et al. Reduced mucosa-associated butyricicoccus activity in patients with ulcerative colitis correlates with aberrant Claudin-1 expression. J Crohns Colitis 2017;11:229–36. [DOI] [PubMed] [Google Scholar]
- 49. Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015;17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mariadason JM, Velcich A, Wilson AJ, Augenlicht LH, Gibson PR. Resistance to butyrate-induced cell differentiation and apoptosis during spontaneous Caco-2 cell differentiation. Gastroenterology 2001;120:889–99. [DOI] [PubMed] [Google Scholar]
- 52. Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 2009;1788:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pedersen G, Saermark T, Horn T, Giese B, Bendtzen K, Brynskov J. Cytokine-induced impairment of short-chain fatty acid oxidation and viability in human colonic epithelial cells. Cytokine 2000;12:1400–4. [DOI] [PubMed] [Google Scholar]
- 54. Noben M, Verstockt B, de Bruyn M, et al. Epithelial organoid cultures from patients with ulcerative colitis and Crohn’s disease: a truly long-term model to study the molecular basis for inflammatory bowel disease? Gut 2017;66:2193–5. [DOI] [PubMed] [Google Scholar]
- 55. Dotti I, Mora-Buch R, Ferrer-Picón E, et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut 2017;66:2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eeckhaut V, Machiels K, Perrier C, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013;62:1745–52. [DOI] [PubMed] [Google Scholar]
- 57. Cushing K, Alvarado DM, Ciorba MA. Butyrate and mucosal inflammation: new scientific evidence supports clinical observation. Clin Transl Gastroenterol 2015;6:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaiko GE, Ryu SH, Koues OI, et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016;167:1137. [DOI] [PubMed] [Google Scholar]
- 59. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium [IIBDGC] Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.