Key Points
Question
How does the survival of patients undergoing excision of stage I melanoma (defined by the American Joint Committee on Cancer Cancer Staging Manual, 8th edition) with Mohs micrographic surgery compare with that of patients receiving traditional wide margin excision?
Findings
In this cohort analysis of the National Cancer Database, treatment of early-stage invasive melanoma with Mohs micrographic surgery was associated with moderately improved overall survival compared with traditional wide margin excision.
Meaning
These findings suggest that Mohs micrographic surgery may provide an alternative to wide margin excision for appropriately selected cases of early-stage invasive melanoma without compromising patient survival.
This cohort study compares overall survival of patients undergoing Mohs micrographic surgery vs traditional wide margin excision for early-stage invasive melanoma in the National Cancer Database.
Abstract
Importance
Melanoma is among the most common malignant neoplasms in the United States, with 91 270 cases estimated to be diagnosed in 2018. Since 2012, Mohs micrographic surgery (MMS) has gained popularity in the treatment of melanoma in situ. Although current guidelines for invasive melanoma without nodal metastases recommend surgery with wide margin excision (WME), use of MMS for this disease has increased as well, particularly in early stages. How the survival outcomes after each procedure compare with one another for early-stage invasive melanoma is unknown to date.
Objective
To evaluate overall survival of patients with stage I melanoma defined by the American Joint Committee on Cancer Cancer Staging Manual, 8th edition (AJCC-8) after MMS vs traditional WME.
Design, Setting, and Analysis
This retrospective cohort study includes all patients with AJCC-8 stage I melanoma who underwent MMS or WME in the National Cancer Database with a diagnosis from January 1, 2004, through December 31, 2014. The National Cancer Database includes all reportable cases from Commission on Cancer–accredited facilities and represents approximately 50% of all newly diagnosed melanoma cases in the United States. Data were analyzed from November 13, 2018, through June 9, 2019.
Exposures
MMS vs WME.
Main Outcomes and Measures
Overall survival evaluated using multivariable Cox proportional hazards regression analysis.
Results
A total of 70 319 eligible patients (52.3% male and 47.7% female; median [SD] age, 57.0 [16.2] years) were identified, including 67 085 treated with WME and 3234 treated with MMS. Multivariable Cox proportional hazards regression survival analysis controlling for clinical and tumor factors revealed that treatment with MMS was associated with a modest improvement in overall survival relative to WME (hazard ratio [HR], 0.86; 95% CI, 0.76-0.97). Propensity score–matched analysis of cohorts of patients treated with MMS vs WME also found modestly improved survival for those treated with MMS (HR, 0.82; 95% CI, 0.68-0.98). Academic facilities were more likely to use MMS than nonacademic facilities (odds ratio, 2.03; 95% CI, 1.88-2.18).
Conclusions and Relevance
These findings suggest that Mohs surgery may provide an alternative approach to traditional WME for appropriately selected cases of AJCC-8 stage I melanoma without compromising patient survival.
Introduction
Melanoma is among the most common malignant neoplasms in the United States, with 91 270 cases estimated to be diagnosed in 2018.1 For cases of invasive melanoma without nodal or extralymphatic metastasis, current national guidelines recommend treatment with wide margin excision (WME) and consideration of sentinel lymph node biopsy, depending on patient preference and comorbidities.2 Although not currently espoused by guidelines for this indication, use of Mohs micrographic surgery (MMS) has increased as an alternative to WME.3 Some controversy remains as to the potential effects of the 2 surgical techniques on patient survival, with proponents of MMS pointing to its complete margin evaluation and critics of MMS concerned about residual disease in tumors without contiguous growth patterns.4,5
Most of the work studying the efficacy of MMS for the treatment of melanoma has focused on its use for melanoma in situ, particularly the lentigo maligna subtype that, owing to subclinical tumor extensions, frequently recurs after traditional excision.6,7 For these tumors, treatment with traditional MMS or staged surgical excisions with permanent sections has demonstrated local control rates higher than those obtained with WME.8,9,10 Based on the evidence available at the time, the American Academy of Dermatology/American College of Mohs Surgery/American Society for Dermatologic Surgery Association/American Society for Mohs Surgery Ad Hoc Task Force on Appropriate Use Criteria for Mohs surgery11 concluded that MMS was appropriate for all melanoma in situ lesions, with the exception of lesions on the trunk or extremities, which were classified as uncertain by the task force. The guidelines do not offer specific recommendations on the use of MMS for invasive melanoma, however, and this is an area of active investigation. Retrospective studies from individual institutions of early-stage melanoma treated with MMS have shown rates of recurrence and survival similar to those of melanoma treated with WME.12,13,14 National registry studies of invasive melanoma have also not shown any significant difference in survival between MMS and WME, but these studies have not focused on stage I invasive melanoma.15,16
We sought herein to investigate the association of the type of surgical excision—WME or MMS—with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual, 8th edition (AJCC-8) stage I invasive melanoma. We limited our analysis to stage I tumors because we hypothesized that these patients are least likely to have undetected disseminated disease at presentation and therefore most likely to benefit from primary tumor excision with the complete microscopic margin control provided by MMS. We had a secondary aim of investigating the factors that influence the type of surgical excision (MMS vs WME) performed for individual cases. To accomplish this, we studied a national cohort of 70 319 patients from the Commission on Cancer’s National Cancer Database (NCDB), with diagnosis from January 1, 2004, through December 31, 2014.
Methods
Data Source
Data originated primarily from the NCDB, with diagnosis from January 1, 2004, through December 31, 2014.17 This study was determined to be exempt from institutional review by the Yale Human Investigation Committee, which waived the need for informed consent for the use of publicly available data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
We identified adults with melanoma (n = 525 271) with a primary site in the skin using the International Classification of Diseases for Oncology, 3rd Edition, histology codes 8720 to 8780 and primary site codes C44.0 to C44.9. We excluded patients if they had previous or other cancer diagnoses (n = 147 024); did not have their primary tumor excised (n = 19 714); received excisions other than WME or MMS (n = 130 966); had tumors defined as in situ (n = 44 643); had nodal (n = 6204) or extralymphatic (n = 1286) distant metastases at the time of presentation; had AJCC-8 pathologic stage II or greater disease (n = 40 742); received any adjuvant therapy, including chemotherapy (n = 672), immunotherapy (n = 2096), or radiotherapy (n = 497); had missing or incomplete follow-up information (n = 10 716); or were missing data on surgical excision (n = 50), adjuvant therapies (n = 3985), clinical N (n = 38 103) or M (n = 1641) staging, Breslow thickness (n = 3511), or tumor ulceration status (n = 3102). All inclusion and exclusion criteria were determined a priori.
Patients were defined as having received WME if they received a primary excision or reexcision after diagnostic biopsy with margins greater than 1 cm. Patients were included in the MMS group if they received MMS as the primary treatment or as definitive surgery after diagnostic biopsy, regardless of the size of margins.
Statistical Analysis
Data were analyzed from November 13, 2018, through June 9, 2019. We performed Kaplan-Meier analysis stratified by type of excision. Our primary outcome was overall survival. Sensitivity analysis was also performed for cases excluded owing to missing data. We also conducted multivariable survival analyses using Cox proportional hazards regression models. Hazard ratios (HRs) calculated from these models refer to the relative risk of death. All patient, tumor, and treatment variables outlined in Table 1 were tested for appropriateness for inclusion as covariates in these models using Akaike information criterion minimization.18 Akaike information criterion minimization ensures parsimony of the multivariable model. We additionally performed a propensity score–matched analysis of patients who received WME and MMS. This method controls for differences in outcomes due to systemic differences between treatment groups.19,20,21,22,23,24,25,26 Scores were calculated using a logistic regression with the factors outlined in Table 1 except for excision type, with groups generated using a 1-to-1 nearest-neighbor match without replacement. We also performed a multivariable logistic analysis of factors associated with the type of excision received. This model was also optimized using Akaike information criterion minimization. Although cases treated with narrow margin excision (NME) were excluded from the primary analysis owing to WME being the standard of care for comparison with MMS, a sensitivity analysis was performed including cases treated with NME. Statistical significance was determined at 2-tailed P < .05. Data analysis was performed using Stata, version 13 (StataCorp LP).
Table 1. Characteristics of the Analytic Sample.
| Variable | Treatment Groupa | ||||
|---|---|---|---|---|---|
| All (N = 70 319) | Before Propensity Score Matching | After Propensity Score Matching | |||
| WME (n = 67 085) | MMS (n = 3234) | WME (n = 2589) | MMS (n = 2589) | ||
| Age, median (SD) | 57.0 (16.2) | 57.0 (16.2) | 61.0 (16.3) | 60.0 (15.8) | 60.0 (15.8) |
| Sex | |||||
| Male | 36 810 (52.3) | 35 036 (52.2) | 1774 (54.9) | 1431 (55.3) | 1431 (55.3) |
| Female | 33 509 (47.7) | 32 049 (47.8) | 1460 (45.1) | 1158 (44.7) | 1158 (44.7) |
| Race/ethnicity | |||||
| White | 68 104 (96.9) | 64 983 (96.9) | 3121 (96.5) | 2493 (96.3) | 2504 (96.7) |
| Black | 211 (0.3) | 197 (0.3) | 14 (0.4) | 7 (0.3) | 8 (0.3) |
| Hispanic | 662 (0.9) | 639 (1.0) | 23 (0.7) | 25 (1.0) | 16 (0.6) |
| Asian or Pacific Islander | 133 (0.2) | 129 (0.2) | 4 (0.1) | 4 (0.2) | 4 (0.2) |
| Other or unknown | 1209 (1.7) | 1137 (1.7) | 72 (2.2) | 60 (2.3) | 57 (2.2) |
| Charlson-Deyo comorbidity scoreb | |||||
| 0 | 62 861 (89.4) | 59 891 (89.3) | 2970 (91.8) | 2477 (95.7) | 2480 (95.8) |
| 1 | 6370 (9.1) | 6147 (9.2) | 223 (6.9) | 110 (4.2) | 108 (4.2) |
| 2 | 832 (1.2) | 803 (1.2) | 29 (0.9) | 2 (0.1) | 1 (0.03) |
| ≥3 | 256 (0.4) | 244 (0.4) | 12 (0.4) | 0 | 0 |
| Insurance | |||||
| Private | 44 900 (63.9) | 43 044 (64.2) | 1856 (57.4) | 1613 (62.3) | 1612 (62.3) |
| Government | 22 721 (32.3) | 21 470 (32.0) | 1251 (38.7) | 942 (36.4) | 943 (36.4) |
| None | 1545 (2.2) | 1472 (2.2) | 73 (2.3) | 23 (0.9) | 22 (0.8) |
| Unknown | 1153 (1.6) | 1099 (1.6) | 54 (1.7) | 11 (0.4) | 12 (0.5) |
| Facility type | |||||
| Nonacademic | 43 343 (61.6) | 41 951 (62.5) | 1392 (43.0) | 1158 (44.7) | 1157 (44.7) |
| Academic | 26 976 (38.4) | 25 134 (37.5) | 1842 (57.0) | 1431 (55.3) | 1432 (55.3) |
| Anatomical site | |||||
| Trunk | 23 770 (33.8) | 22 991 (34.3) | 779 (24.1) | 703 (27.2) | 702 (27.1) |
| Upper limb or shoulder | 18 387 (26.1) | 17 711 (26.4) | 676 (20.9) | 572 (22.1) | 575 (22.2) |
| Lower limb or hip | 14 426 (20.5) | 13 962 (20.8) | 464 (14.3) | 378 (14.6) | 377 (14.6) |
| Face | 8460 (12.0) | 7443 (11.1) | 1017 (31.4) | 757 (29.2) | 757 (29.2) |
| Scalp or neck | 4917 (7.0) | 4640 (6.9) | 277 (8.6) | 174 (6.7) | 174 (6.7) |
| Overlapping or NOS | 359 (0.5) | 338 (0.5) | 21 (0.6) | 5 (0.2) | 4 (0.2) |
| Histologic subtype | |||||
| Superficial spreading | 28 590 (40.7) | 27 502 (41.0) | 1088 (33.6) | 928 (35.8) | 926 (35.8) |
| Lentigo maligna | 3774 (5.4) | 4252 (6.3) | 552 (17.1) | 350 (13.5) | 351 (13.6) |
| Nodular | 2720 (3.9) | 2658 (4.0) | 62 (1.9) | 15 (0.6) | 17 (0.7) |
| Malignant acral lentiginous | 610 (0.9) | 589 (0.9) | 21 (0.6) | 3 (0.1) | 4 (0.2) |
| Malignant desmoplastic | 450 (0.6) | 431 (0.6) | 19 (0.6) | 3 (0.1) | 3 (0.1) |
| Spindle cell | 430 (0.6) | 414 (0.6) | 16 (0.5) | 2 (0.1) | 2 (0.1) |
| Other or NOS | 33 745 (48.0) | 32 239 (48.1) | 1506 (46.6) | 1288 (49.7) | 1288 (49.8) |
| Ulceration | |||||
| Not present | 67 291 (95.7) | 64 159 (95.6) | 3132 (96.8) | 2563 (99.0) | 2559 (98.8) |
| Present | 3028 (4.3) | 2926 (4.4) | 102 (3.2) | 126 (4.9) | 30 (1.2) |
| Breslow thickness, mm | |||||
| <0.80 | 45 167 (64.2) | 42 569 (63.5) | 2598 (80.3) | 2154 (83.2) | 2156 (83.3) |
| 0.80-1.00 | 8916 (12.7) | 8633 (12.9) | 283 (8.8) | 178 (6.9) | 176 (6.8) |
| 1.01-2.00 | 16 236 (23.1) | 15 883 (23.7) | 353 (10.9) | 257 (9.9) | 257 (9.9) |
| Type of excision | |||||
| WME | 67 085 (95.4) | 67 085 (100.0) | NA | 2589 (100.0) | NA |
| MMS | 3234 (4.6) | NA | 3234 (100.0) | NA | 2589 (100.0) |
Abbreviations: MMS, Mohs micrographic surgery; NA, not applicable; NOS, not otherwise specified; WME, wide margin excision.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100.
Lower score indicates a lower comorbidity burden; higher score, a higher comorbidity burden.
Results
The demographic and clinical characteristics of the study sample are presented in Table 1 (52.3% male and 47.7% female; median [SD] age, 57.0 [16.2] years). Most of the patients were white (96.9%) with a Charlson-Deyo comorbidity score of 0 (89.4%). Most patients were treated at nonacademic centers (61.6%) and held private insurance (63.9%). The most common anatomical subsite was the trunk (33.8%), followed by the upper limb or shoulder (26.1%), the lower limb or hip (20.5%), and the face (12.0%). Among the most common histologic tumor subtypes were superficial spreading melanoma (40.7%), lentigo maligna melanoma (5.4%), and nodular melanoma (3.9%), although the most common subtype was other or not otherwise specified (48.0%). Most tumors were smaller than 0.8 mm, with a median (SD) Breslow thickness of 0.58 (0.48) mm. Wide margin excision was used in a much larger proportion of cases (95.4%) than MMS (4.6%). Mean (SD) follow-up was 4.81 (2.86) years, with a maximum follow-up of 13.09 years. Sensitivity analysis of patients with known survival data who were excluded owing to missing data revealed that cases without missing data demonstrated slightly improved survival. Overall survival at 3 years was 95.2% (SE, 0.1%); at 5 years, 90.9% (SE, 0.1%); and at 10 years, 80.0% (SE, 0.3%) for the analytic cohort. Among cases excluded owing to missing data, 3-year overall survival was 93.8% (SE, 0.1%); 5-year overall survival, 89.4% (SE, 0.2%); and 10-year survival, 79.2% (SE, 0.2%).
On Kaplan-Meier univariable analysis, patients treated with MMS demonstrated similar overall survival relative to those treated with WME (HR, 1.01; 95% CI, 0.90-1.14). Among patients treated with WME, 3-year overall survival was 95.2% (SE, 0.1%); 5-year overall survival, 90.9% (SE, 0.1%); and 10-year overall survival, 80.0% (SE, 0.3%). Among patients treated with MMS, 3-year overall survival was 95.4% (SE, 0.4%); 5-year overall survival, 90.5% (SE, 0.7%); and 10-year overall survival, 79.2% (SE, 1.6%).
After controlling for covariates via multivariable and propensity score–matched analyses, patients treated with MMS were found to have improved overall survival compared with those treated with WME. Multivariable Cox proportional hazards regression modeling (Table 2) demonstrated improved overall survival for those treated with MMS as opposed to WME (HR, 0.86; 95% CI, 0.76-0.97). Subgroup analyses of patients with stages IA (HR, 0.88; 95% CI, 0.77-1.01) and IB (HR, 0.78; 95% CI, 0.56-1.07) disease revealed nonsignificant improved overall survival for patients receiving MMS. Analysis including only patients with primary lesions located on the head and neck similarly demonstrated improved overall survival for patients treated with MMS as opposed to WME (HR, 0.81; 95% CI, 0.69-0.96). Treatment with MMS was associated with similar overall survival to treatment with WME (HR, 0.92; 95% CI, 0.77-1.11) for patients with primary lesions outside the head and neck.
Table 2. Multivariable Analysis of Factors Associated With Overall Survivala.
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Age | 1.08 (1.07-1.08) | <.001 |
| Sex | ||
| Male | 1.40 (1.33-1.48) | <.001 |
| Female | 1 [Reference] | NA |
| Charlson-Deyo comorbidity scoreb | ||
| 0 | 1 [Reference] | NA |
| 1 | 1.49 (1.40-1.59) | <.001 |
| 2 | 2.48 (2.18-2.82) | <.001 |
| ≥3 | 3.55 (2.88-4.37) | <.001 |
| Insurance | ||
| Private | 1 [Reference] | NA |
| Government | 1.28 (1.19-1.37) | <.001 |
| None | 2.02 (1.66-2.46) | <.001 |
| Unknown | 1.15 (0.95-1.39) | .15 |
| Facility type | ||
| Nonacademic | 1 [Reference] | NA |
| Academic | 0.93 (0.88-0.97) | .003 |
| Anatomical site | ||
| Trunk | 1 [Reference] | NA |
| Upper limb or shoulder | 0.87 (0.81-0.93) | <.001 |
| Lower limb or hip | 0.79 (0.72-0.86) | <.001 |
| Face | 1.20 (1.12-1.29) | <.001 |
| Scalp or neck | 1.31 (1.20-1.42) | <.001 |
| Overlapping or NOS | 1.69 (1.31-2.19) | <.001 |
| Histologic subtype | ||
| Superficial spreading | 1 [Reference] | NA |
| Lentigo maligna | 1.00 (0.91-1.10) | >.99 |
| Nodular | 1.63 (1.47-1.81) | <.001 |
| Malignant acral lentiginous | 1.54 (1.24-1.92) | <.001 |
| Malignant desmoplastic | 1.01 (0.80-1.27) | .95 |
| Spindle cell | 1.13 (0.90-1.42) | .30 |
| Other or NOS | 1.07 (1.01-1.13) | .02 |
| Ulceration | ||
| Not present | 1 [Reference] | NA |
| Present | 2.24 (2.06-2.43) | <.001 |
| Breslow thickness, mm | ||
| <0.80 | 1 [Reference] | NA |
| 0.80-1.00 | 1.09 (1.01-1.17) | .04 |
| 1.01-2.00 | 1.46 (1.38-1.55) | <.001 |
| Type of excision | ||
| WME | 1 [Reference] | NA |
| MMS | 0.86 (0.76-0.97) | .02 |
Abbreviations: HR, hazard ratio; MMS, Mohs micrographic surgery; NA, not applicable; NOS, not otherwise specified; WME, wide margin excision.
Patient race/ethnicity was excluded from the multivariable model after Akaike information criterion minimization.
Lower score indicates a lower comorbidity burden; higher score, a higher comorbidity burden.
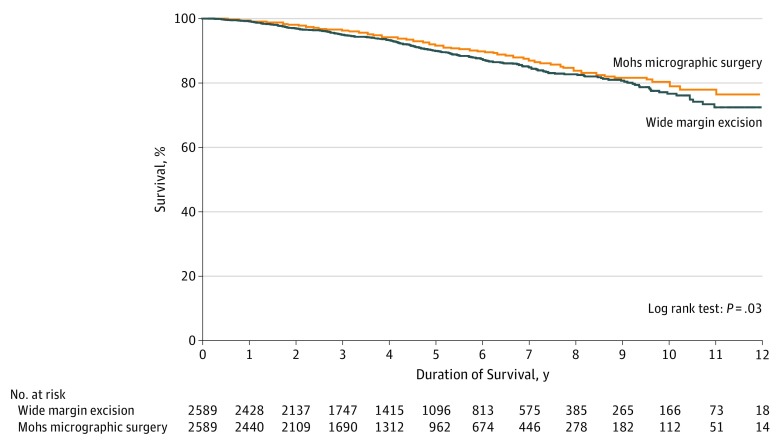
The demographic and clinical characteristics of propensity score–matched cohorts of patients treated with WME and MMS are presented in Table 1. Analysis of these cohorts also revealed improved overall survival for patients treated with MMS compared with those treated with WME (HR, 0.82; 95% CI, 0.68-0.98). Survival curves of these matched cohorts are presented in the Figure. Within these cohorts, 3-year overall survival was 95.0% (SE, 0.5%), 5-year overall survival was 90.0% (SE, 0.7%), and 10-year overall survival was 76.7% (SE, 1.7%) among patients treated with WME. Among those treated with MMS, 3-year overall survival was 96.3% (SE, 0.4%), 5-year overall survival was 91.8% (SE, 0.7%), and 10-year overall survival was 80.4% (SE, 1.8%). Sensitivity analysis using a multivariable Cox proportional hazards regression model that included cases treated with NME revealed no survival difference between MMS and NME. Exclusion of cases found to have positive pathologic lymph nodes after presentation did not change the results.
Figure. Overall Survival for Propensity Score–Matched Cohorts of Patients Treated Using Mohs Micrographic Surgery vs Wide Margin Excision.
We additionally performed a subgroup analysis of low-risk patients. Multivariable survival analysis of cases with a Breslow thickness of less than 0.80 mm with or without tumor ulceration using a Cox proportional hazards regression model demonstrated nonsignificant improved overall survival with MMS as opposed to WME (HR, 0.87; 95% CI. 0.75-1.00). Multivariable survival analysis of cases without tumor ulceration revealed improved overall survival for patients receiving MMS compared with those who received WME (HR, 0.85; 95% CI, 0.75-0.97).
Several factors were found to be associated with the type of excision used (Table 3). Mohs micrographic surgery was more likely to be used for the eldest group of patients (≥75 years) compared with the youngest (<55 years) (odds ratio [OR], 1.16; 95% CI, 1.04-1.30). Male patients were less likely to receive MMS than female patients (OR, 0.91; 95% CI, 0.85-0.99). Patient insurance status was not found to be associated with likelihood of MMS treatment and was excluded from the model after Akaike information criterion minimization, whereas black patients were found to be more likely to receive MMS than white patients (OR, 1.87; 95% CI, 1.07-3.28). Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS (OR, 2.03; 95% CI, 1.88-2.18). Results also indicate that MMS is less commonly used for more aggressive subtypes of melanoma, with lower rates of use for nodular melanoma compared with superficial spreading melanoma (OR, 0.75; 95% CI, 0.57-0.97), as well as for tumors with Breslow thickness of 0.80 to 1.00 mm (OR, 0.58; 95% CI, 0.51-0.66) or 1.01 to 2.00 mm (OR, 0.37; 95% CI, 0.33-0.42) compared with tumors with Breslow thickness of less than 0.80 mm. Conversely, lentigo maligna melanomas were nearly twice as likely to be excised by MMS than superficial spreading melanomas (OR, 1.95; 95% CI, 1.73-2.21). Variation was noted in the use of MMS by the anatomical subsite of disease. Facial tumors were the most likely to be excised with MMS, more than 3 times as likely as truncal tumors (OR, 3.17; 95% CI, 2.86-3.52). Scalp and neck tumors were also more likely to be removed by MMS than truncal tumors (OR, 1.60; 95% CI, 1.38-1.85).
Table 3. Multivariable Analysis of Factors Associated With Odds of Receiving MMSa.
| Variable | OR (95% CI)b | P Value |
|---|---|---|
| Age, y | ||
| ≤54 | 1 [Reference] | NA |
| 55-64 | 1.07 (0.97-1.17) | .20 |
| 65-74 | 1.08 (0.98-1.20) | .13 |
| ≥75 | 1.16 (1.04-1.30) | .006 |
| Sex | ||
| Male | 0.91 (0.86-0.99) | .02 |
| Female | 1 [Reference] | NA |
| Race | ||
| White | 1 [Reference] | NA |
| Black | 1.87 (1.07-3.28) | .03 |
| Hispanic | 0.76 (0.50-1.17) | .21 |
| Asian or Pacific Islander | 0.76 (0.28-2.07) | .59 |
| Other or unknown | 1.19 (0.93-1.53) | .16 |
| Charlson-Deyo comorbidity scorec | ||
| 0 | 1 [Reference] | NA |
| 1 | 0.72 (0.63-0.83) | <.001 |
| 2 | 0.67 (0.46-0.98) | .04 |
| ≥3 | 0.93 (0.51-1.69) | .81 |
| Facility type | ||
| Nonacademic | 1 [Reference] | NA |
| Academic | 2.03 (1.88-2.18) | <.001 |
| Anatomical site | ||
| Trunk | 1 [Reference] | NA |
| Upper limb or shoulder | 1.09 (0.98-1.21) | .11 |
| Lower limb or hip | 0.97 (0.86-1.09) | .59 |
| Face | 3.17 (2.86-3.52) | <.001 |
| Scalp or neck | 1.60 (1.38-1.85) | <.001 |
| Overlapping or NOS | 1.62 (1.03-2.54) | .04 |
| Histologic subtype | ||
| Superficial spreading | 1 [Reference] | NA |
| Lentigo maligna | 1.95 (1.73-2.21) | <.001 |
| Nodular | 0.75 (0.57-0.97) | .03 |
| Malignant acral lentiginous | 0.99 (0.63-1.55) | .96 |
| Malignant desmoplastic | 0.97 (0.61-1.56) | .91 |
| Spindle cell | 0.93 (0.56-1.55) | .78 |
| Other or NOS | 1.08 (1.00-1.17) | .07 |
| Ulceration | ||
| Not present | 1 [Reference] | NA |
| Present | 0.66 (0.54-0.81) | <.001 |
| Breslow thickness, mm | ||
| <0.80 | 1 [Reference] | NA |
| 0.80-1.00 | 0.58 (0.51-0.66) | <.001 |
| 1.01-2.00 | 0.37 (0.33-0.42) | <.001 |
Abbreviations: MMS, Mohs micrographic surgery; NA, not applicable; NOS, not otherwise specified; OR, odds ratio.
Patient insurance status was excluded from the multivariable model after Akaike information criterion minimization.
Indicates in reference to wide margin excision.
Lower score indicates a lower comorbidity burden; higher score, a higher comorbidity burden.
Discussion
Although this study is the first, to our knowledge, to report improved survival for early-stage (AJCC-8 stage I) invasive melanoma treated with MMS rather than WME, our findings are similar to those of several studies14,15,16 that have suggested that MMS is noninferior to WME for localized invasive melanoma. A recent study of all subtypes of melanoma (melanoma in situ, localized invasive melanoma, invasive melanoma with regional spread, and invasive melanoma with distant metastasis)15 found that MMS was noninferior to WME and NME.
Historically, one of the most significant barriers to the use of MMS for melanoma excision was the difficulty in interpreting melanoma with frozen sections. However, the rise of immunostains, in particular melanoma antigen recognized by T cells (MART-1), has made the histopathological interpretation of melanoma more feasible.4,27,28,29,30 Recently, several single-institution studies12,13,14 have reported excellent outcomes with invasive melanoma treated with MMS and MART-1 immunostaining, with local recurrence rates ranging from 0 to 1.43%.
We identified several factors associated with the use of MMS for invasive melanoma as opposed to traditional excision. Although previous reports studying the use of MMS in nonmelanoma skin cancer have suggested that patient insurance is associated with the rate of use of MMS, patient insurance was found not to be significantly associated with the use of MMS in our cohort of patients with AJCC-8 stage I invasive melanoma.31 In addition, although previous work32 has demonstrated less use of MMS for nonmelanoma skin cancer in black patients, such patients in our cohort were more likely to receive MMS than white patients. These findings may be owing to the controversy surrounding the use of MMS for invasive melanoma and will need to be monitored should MMS gain more widespread acceptance as a potential treatment. Why academic centers were found to be almost twice as likely to use MMS than were nonacademic centers is not clear.
Limitations
There are several limitations to this study. First, we did not have access to data on the type of stains that were used in margin evaluation for the WME or MMS cohorts. The frequency of immunostain use with MMS is unknown in this cohort, but those cases that did not use immunostains likely would have biased our results toward detecting no difference in survival or poorer survival for cases treated with MMS. Further, although the NCDB captures approximately 70% of new cancer cases in the United States, recent work suggests that the coverage for incident melanoma is closer to 50%, likely owing to more outpatient treatment of melanoma compared with other malignant neoplasms.33 The NCDB is designed as a large convenience sample, capturing cases only at Commission on Cancer–accredited facilities, rather than a population-based sample such as the Surveillance, Epidemiology, and End Results registry. Although this limitation is less of a concern for analyses of treatment outcomes than incidence studies, this distinction may make our results less applicable to melanoma treatment outside of Commission on Cancer–accredited institutions. In addition, owing to the nature of the NCDB, we did not have access to data on recurrence or reexcision. Particularly because of the potential benefit of MMS to provide lower recurrence rates with less tissue removal, this aspect of these 2 procedures should be assessed in future studies. Furthermore, although we controlled for key tumor risk features such as histologic subtype, ulceration, and Breslow thickness, patients who received MMS may have had divergent rates of additional high-risk features that were not captured in our analysis, such as increased mitotic rate or absent tumor-infiltrating lymphocytes. Thus, controlling for these variables might reveal that MMS is associated with better or worse survival than suggested by our analysis.
In addition, although 1 cm is the recommended excision margin for stage IA melanoma, these patients were excluded from our primary analysis owing to NCDB skin surgery coding that included all patients with margins of 1 cm or less in the same group. For stage IB disease, the recommended excision margin is 1 to 2 cm. This recommended excision margin is included in our analysis of WME (>1 cm), but we were unable to determine the exact margins used that were greater than 1 cm for these cases. Finally, results generated from such retrospective registry studies are hypothesis generating in nature and must be further studied via clinical trials because of previous observational studies being inconsistently reproduced with randomized clinical trials.34
Conclusions
In this analysis of a large sample of early-stage invasive melanomas, we found that treatment with MMS was associated with a modest survival advantage compared with treatment with traditional WME. These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma.
References
- 1.American Cancer Society Cancer Facts & Figures 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Published January 2018. Accessed December 2018.
- 2.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN guidelines): melanoma (version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Published March 12, 2019. Accessed July 9, 2018.
- 3.Viola KV, Rezzadeh KS, Gonsalves L, et al. . National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72(6):1060-1065. doi: 10.1016/j.jaad.2015.02.1122 [DOI] [PubMed] [Google Scholar]
- 4.Hui AM, Jacobson M, Markowitz O, Brooks NA, Siegel DM. Mohs micrographic surgery for the treatment of melanoma. Dermatol Clin. 2012;30(3):503-515. doi: 10.1016/j.det.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu D, Fathi R, Srivastava D, Nijhawan RI. Current perspectives on Mohs micrographic surgery for melanoma. Clin Cosmet Investig Dermatol. 2018;11:309-320. doi: 10.2147/CCID.S137513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 1995;33(6):923-936. doi: 10.1016/0190-9622(95)90282-1 [DOI] [PubMed] [Google Scholar]
- 7.Tsao H, Pehamberger H, Sober A. Precursor lesions and markers of increased risk for melanoma In: Balch CM, Houghton AN, Sober AJ, Seng-jaw S, eds. Cutaneous Melanoma. St Louis, MO: Quality Medical Publishing; 1998: 65-79. [Google Scholar]
- 8.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438-444. doi: 10.1016/j.jaad.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 9.Hilari H, Llorca D, Traves V, et al. . Conventional surgery compared with slow Mohs micrographic surgery in the treatment of lentigo maligna: a retrospective study of 62 cases. Actas Dermosifiliogr. 2012;103(7):614-623. doi: 10.1016/j.ad.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 10.de Vries K, Greveling K, Prens LM, et al. . Recurrence rate of lentigo maligna after micrographically controlled staged surgical excision. Br J Dermatol. 2016;174(3):588-593. doi: 10.1111/bjd.14325 [DOI] [PubMed] [Google Scholar]
- 11.Connolly SM, Baker DR, Coldiron BM, et al. ; Ad Hoc Task Force; Ratings Panel . AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550. doi: 10.1016/j.jaad.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Valentín-Nogueras SM, Brodland DG, Zitelli JA, González-Sepúlveda L, Nazario CM. Mohs micrographic surgery using MART-1 immunostain in the treatment of invasive melanoma and melanoma in situ. Dermatol Surg. 2016;42(6):733-744. doi: 10.1097/DSS.0000000000000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etzkorn JR, Sobanko JF, Elenitsas R, et al. . Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72(5):840-850. doi: 10.1016/j.jaad.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 14.Degesys CA, Powell HB, Hsia LB, Merritt BG. Outcomes for invasive melanomas treated with Mohs micrographic surgery: a retrospective cohort study. Dermatol Surg. 2019;45(2):223-228. doi: 10.1097/DSS.0000000000001658 [DOI] [PubMed] [Google Scholar]
- 15.Trofymenko O, Bordeaux JS, Zeitouni NC. Melanoma of the face and Mohs micrographic surgery: nationwide mortality data analysis. Dermatol Surg. 2018;44(4):481-492. doi: 10.1097/DSS.0000000000001429 [DOI] [PubMed] [Google Scholar]
- 16.Elias ML, Lambert WC. Surgical management of localized melanoma: a National Cancer Database retrospective review [published online March 25, 2019]. Br J Dermatol. doi: 10.1111/bjd.17901 [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683-690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716-723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 19.Normand ST, Landrum MB, Guadagnoli E, et al. . Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. doi: 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 20.Cheraghlou S, Kuo P, Mehra S, et al. . Adjuvant therapy in major salivary gland cancers: analysis of 8580 patients in the National Cancer Database. Head Neck. 2018;40(7):1343-1355. doi: 10.1002/hed.24984 [DOI] [PubMed] [Google Scholar]
- 21.Tsutani Y, Miyata Y, Nakayama H, et al. . Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score–matched analysis in a multicenter study. J Thorac Cardiovasc Surg. 2013;146(2):358-364. doi: 10.1016/j.jtcvs.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Verstegen NE, Oosterhuis JW, Palma DA, et al. . Stage I-II non–small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24(6):1543-1548. doi: 10.1093/annonc/mdt026 [DOI] [PubMed] [Google Scholar]
- 23.Dehejia RH, Wahba S. Propensity score–matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151-161. doi: 10.1162/003465302317331982 [DOI] [Google Scholar]
- 24.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 25.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 26.Cheraghlou S, Agogo GO, Girardi M. Treatment of primary nonmetastatic melanoma at high-volume academic facilities is associated with improved long-term patient survival. J Am Acad Dermatol. 2019;80(4):979-989. doi: 10.1016/j.jaad.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 27.Zalla MJ, Lim KK, Dicaudo DJ, Gagnot MM. Mohs micrographic excision of melanoma using immunostains. Dermatol Surg. 2000;26(8):771-784. doi: 10.1046/j.1524-4725.2000.00081.x [DOI] [PubMed] [Google Scholar]
- 28.Miller CJ, Sobanko JF, Zhu X, Nunnciato T, Urban CR. Special stains in Mohs surgery. Dermatol Clin. 2011;29(2):273-286, ix. doi: 10.1016/j.det.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 29.Blessing K, Sanders DS, Grant JJ. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998;32(2):139-146. doi: 10.1046/j.1365-2559.1998.00312.x [DOI] [PubMed] [Google Scholar]
- 30.Albertini JG, Elston DM, Libow LF, Smith SB, Farley MF. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28(8):656-665. [DOI] [PubMed] [Google Scholar]
- 31.Gaston DA, Naugle C, Clark DP. Mohs micrographic surgery referral patterns: the University of Missouri experience. Dermatol Surg. 1999;25(11):862-866. doi: 10.1046/j.1524-4725.1999.99037.x [DOI] [PubMed] [Google Scholar]
- 32.Viola KV, Jhaveri MB, Soulos PR, et al. . Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148(4):473-477. doi: 10.1001/archdermatol.2011.2456 [DOI] [PubMed] [Google Scholar]
- 33.Mallin K, Browner A, Palis B, et al. . Incident cases captured in the national cancer database compared with those in US population based central cancer registries in 2012-2014. Ann Surg Oncol. 2019;26(6):1604-1612. doi: 10.1245/s10434-019-07213-1 [DOI] [PubMed] [Google Scholar]
- 34.Soni PD, Hartman HE, Dess RT, et al. . Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol. 2019;37(14):1209-1216. doi: 10.1200/JCO.18.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]