Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a class of incretin mimetics with multisystem cardiometabolic effects. Six GLP-1RAs are approved for improving glycemic control by the U.S. Food and Drug Administration (FDA). Four therapies (albiglutide, dulaglutide, liraglutide, and semaglutide) have been demonstrated to reduce cardiovascular events in high-risk patients with type 2 diabetes mellitus (T2DM) in cardiovascular outcomes trials (, , , and ). Subsequently, the FDA expanded the labeling of liraglutide to lower major adverse cardiovascular events in T2DM and established cardiovascular disease (CVD). While GLP-1RAs have been in clinical use for more than a decade, utilization by cardiologists is unknown. We aimed to describe specialty-specific prescription patterns of GLP-1RAs at a tertiary care hospital system.
We identified all first-time prescriptions of GLP-1RAs on formulary (albiglutide, dulaglutide, exenatide [immediate-release/extended-release], and liraglutide) across the multicenter Partners HealthCare system from April 2005 (FDA approval of first GLP-1RA) to August 2018. Two recently approved GLP-1RAs (semaglutide and lixisenatide) are not yet on formulary, and albiglutide has been withdrawn from the market. Statistical analyses were performed with STATA 14.1 (StataCorp, College Station, Texas). The Partners HealthCare Institutional Review Board approved the study.
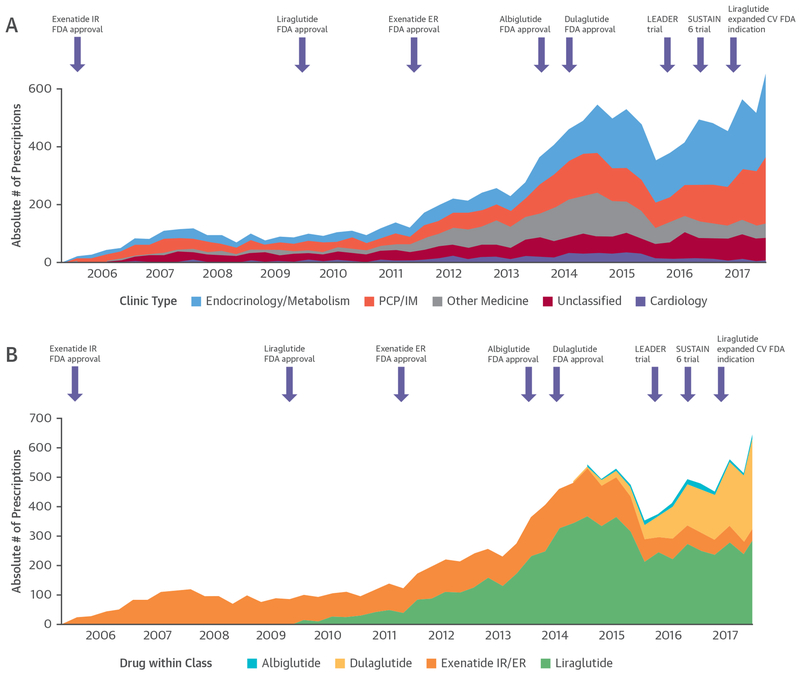
Overall, 7,609 patients were prescribed GLP-1RAs (median age 61 years [25th to 75th percentiles: 53 to 69 years], 54% women, 76% white, 34% CVD). Most patients (64%) prescribed GLP-1RA by cardiologists had CVD, whereas rates were lower for other specialties (23% to 40%). The median number of background glucose-lowering therapies was 2 (25th to 75th percentiles: 1 to 2). Background therapy with insulin (54%) or metformin (64%) were common, whereas few patients were on sodium-glucose cotransporter 2 inhibitors (4%) or dipeptidyl peptidase-4 inhibitors (4%). From 2005 to 2015, quarterly GLP-1RA prescriptions rose steadily, followed by a decrease in the first half of 2016, and a subsequent increase from the second half of 2016 onward (Figure 1).
FIGURE 1. New Quarterly Glucagon-Like Peptide-1 Receptor Agonist Prescriptions By Prescriber Type and Specific Drug Within Class.
Prescriber type (A) and specific drug within class (B). Purple arrows indicate key U.S. Food and Drug Administration (FDA) regulatory approvals and landmark cardiovascular outcomes trials. CV = cardiovascular; ER = extended release; IM = internal medicine; IR = immediate-release; LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; PCP = primary care physician; SUSTAIN-6 = Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes.
The specialty-specific prescriber rates were: endocrinology (33%), primary care (29%), other specialties (16%), cardiology (4.5%), and unclassified (17.5%) (Figure 1A). In the year after expansion of the FDA labeling for liraglutide, endocrinologists and primary care physicians prescribed 43% and 33%, respectively, while cardiologists accounted for 2% of prescriptions.
From 2005 to 2018, liraglutide was most commonly prescribed (50%), followed by exenatide (34%), dulaglutide (16%), and albiglutide (<1%) (Figure 1B). Liraglutide prescription rates rose steadily from initial FDA approval in 2010 to 2015. Subsequently, relative prescription of both liraglutide and exenatide decreased, while prescription of dulaglutide increased. By the second quarter of 2018, dulaglutide was most common (51%).
During a 13-year time span in a multicenter health system, cardiologists accounted for <5% of new prescriptions of GLP-1RAs, while endocrinologists and primary care physicians initiated >60%. In the year after regulatory broadening of the indication for liraglutide, cardiologists continued to prescribe GLP-1RAs infrequently, and use of liraglutide was relatively unchanged.
It is encouraging that use of GLP-1RAs has been increasing overall in recent years. The initial decline in prescription of GLP-1RAs in early 2016 may have been related to competing sodium-glucose cotransporter 2 inhibitor prescription, another class with established beneficial cardiovascular effects (1). However, despite these favorable trends, cardiologists infrequently prescribed either class of therapies in our health system (1). Lack of practical knowledge, concerns regarding potential adverse effects, patient fear of injections, cost/coverage issues, uncertainties about overstepping interdisciplinary boundaries, and added time to clinical care may all contribute to sluggish uptake in the cardiology community. Advances in patient-friendly injection designs (which may have contributed to recent expanded use of dulaglutide) and the prospect of an oral GLP-1RA (as the development program of oral semaglutide is nearing completion) may alleviate select barriers to uptake. However, global efforts to improve cardiologist engagement with comprehensive T2DM care are needed.
Our descriptive study is subject to limitations. We were unable to determine drug indications (obesity vs. glucose-lowering). CVD status was determined by administrative coding and may be subject to misclassification. Initial prescription outside the health system may have been missed. Despite the tertiary-care practice setting, Partners HealthCare does encompass >10 health care entities.
In current clinical practice, <5% of GLP-1RA prescriptions are initiated by cardiologists. Increased awareness and development of streamlined multidisciplinary care approaches (2) and implementation pathways (3) may improve uptake. As high-risk patients with T2DM are commonly encountered in cardiology practices, we believe cardiologists are uniquely positioned to participate in integration of this evidence-based but underutilized class of cardiometabolic therapies to advance comprehensive cardiovascular care.
Acknowledgments
Please note: Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Institutes of Health/National Center for Advancing Translational Sciences Award UL 1TR002541); and has served on advisory boards for AstraZeneca, Bayer AG, and Baxter Healthcare. Drs. Patel and Qamar are supported by NHLBI T32 postdoctoral training grants (T32HL069771 and T32HL007604). Dr. Qamar is supported by the American Heart Association Strategically Focused Research Network in Vascular Disease grant (18SFRN3390085). Dr. Januzzi is supported in part by the Hutter Family Professorship; has received grant support from Abbott, Cleveland Heart Labs, Singulex, and Prevencio; has received consulting income from Roche Diagnostics, Critical Diagnostics, and Novartis; and has participated in clinical endpoint committees/data or safety monitoring boards for Novartis, Amgen, GE, Janssen, Pfizer, and Boehringer Ingelheim. Dr. Scirica has received research grants via Brigham and Women’s Hospital from AstraZeneca, Eisai, Novartis, and Merck; has received consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr. Reddy’s Laboratories, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Merck, Novo Nordisk, Sanofi, and St. Jude’s Medical; and has equity in Health [at] Scale. Dr. Butler has received research support from the National Institutes of Health and European Union; and has served as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, StealthPeptide, SC Pharma, Vifor Pharma, and ZS Pharma. Dr. Cannon has received research grants from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, and Merck; and has received consulting fees from Alnylam, Amarin, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Janssen, Kowa, Merck, Pfizer, Regeneron, and Sanofi. Dr. Bhatt has served on the Advisory Board of Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; has served on the Board of Directors of Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; has served as Chair of the American Heart Association Quality Oversight Committee, NCDR-ACTION Registry Steering Committee, and VA CART Research and Publications Committee; has served on the Data Monitoring Committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and the Population Health Research Institute; has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (COMPASS clinical trial steering committee funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); has served as Deputy Editor of Clinical Cardiology; has received research funding from Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has served as site co-investigator for Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; is a Trustee of the American College of Cardiology; and has performed unfunded research for FlowCo, Merck, Novo Nordisk, PLx Pharma, and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Prakash Deedwania, MD, served as Guest Editor for this paper.
REFERENCES
- 1.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–2. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the ADA and the EASD. Diabetologia 2018;61:2461–98. [DOI] [PubMed] [Google Scholar]
- 3.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the ACC Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–23. [DOI] [PMC free article] [PubMed] [Google Scholar]