Abstract
Cylindrospermopsin (CYN) is a toxin associated with numerous species of freshwater cyanobacteria throughout the world. It is postulated to have caused an episode of serious illnesses in Australia through treated drinking water, as well as lethal effects in livestock exposed to water from farm ponds. Toxicity included effects indicative of both hepatic and renal dysfunction. In humans, symptoms progressed from initial hepatomegaly, vomiting, and malaise to acidosis and hypokalemia, bloody diarrhea, and hyperemia in mucous membranes. Laboratory animal studies predominantly involved the intraperitoneal (i.p.) route of administration and confirmed this pattern of toxicity with changes in liver enzyme activities and histopathology consistent with hepatic injury and adverse renal effects. The aim of this study was designed to assess subchronic oral exposure (90 d) of purified CYN from 75 to 300 μg/kg/d in mouse. At the end of the dosing period, examinations of animals noted (1) elevated organ to body weight ratios of liver and kidney at all dose levels, (2) treatment-related increases in serum alanine aminotransferase (ALT) activity, (3) decreased blood urea nitrogen (BUN) and cholesterol concentrations in males, and (4) elevated monocyte counts in both genders. Histopathological alterations included hepatocellular hypertrophy and cord disruption in the liver, as well as renal cellular hypertrophy, tubule dilation, and cortical tubule lesions that were more prominent in males. A series of genes were differentially expressed including Bax (apoptosis), Rpl6 (tissue regeneration), Fabp4 (fatty acid metabolism), and Proc (blood coagulation). Males were more sensitive to many renal end points suggestive of toxicity. At the end of exposure, toxicity was noted at all dose levels, and the 75 μg/kg group exhibited significant effects in liver and kidney/body weight ratios, reduced BUN, increased serum monocytes, and multiple signs of histopathology indicating that a no-observed-adverse-effect level could not be determined for any dose level.
Introduction
Cyanobacteria are photosynthetic organisms that are found on all continents and virtually all ecosystems, but are primarily inhabitants of both freshwater and saltwater. There are many species that produce chemicals that are toxic to mammals (Zurawell et al. 2005). One of the most widespread freshwater toxins is the alkaloid, cylindrospermopsin (CYN), a tricyclic guanidine joined to uracil by a carbon bridge (Ohtani, Moore, and Runnegar 2002), which has been associated with severe toxicity in humans and livestock. The discovery of CYN followed an episode of poisoning that took place in the Palm Island community of Australia during 1979 (Byth 1980). The drinking water in the town was obtained from a reservoir and it was observed that people using well water did not become ill (Hawkins et al. 1985). The reservoir was thought to have had an algal bloom for 2 months since the water had taste and odor issues during that time, and was treated with the algaecide, copper sulfate (CuSO4). One week after the application of the algaecide, individuals began to get sick and 138 eventually required hospital treatment. The initial symptoms included constipation, vomiting, anorexia, headache, hepatomegaly, glucosuria, proteinuria, and ketonuria. In 1 to 3 d following the initial signs, the illness progressed and severe electrolyte imbalance was noted including hypokalemia and resultant acidotic shock and hypovolemia; diarrhea, often containing blood that persisted up to three weeks; accompanied by hyperemia or bleeding mucous membranes. Subsequently, CYN was associated with the death of cattle after ingestion of water with an ongoing cyanobacterial bloom. The animals exhibited symptoms that included hepatomegaly, hepatic degeneration and necrosis, and extensive intestinal hemorrhages (Saker, Thomas, and Norton 1999; Thomas et al. 1998).
In mouse, the possibility of a toxicity being initiated by toxins produced by cyanobacteria in the reservoir was tested using a variety of cultures of cyanobacterial species present in the reservoir. Freeze-dried cultures of Cylindrospermopsis raciborskii administered intraperitoneally in mice produced hepatic necrosis and adverse effects in a variety of other organs (Hawkins et al. 1985). CYN was isolated (Ohtani, Moore, and Runnegar 2002), the alkaloid considered to be the primary toxin produced by C. raciborskii, and in subsequent years, evidence indicated that both lyophilate extracts of CYN-containing cyanobacteria and purified CYN induced a spectrum of adverse effects in mice that were similar to those seen in the affected human population. Hepatic toxicity was characterized by changes in levels of serum enzymes that are indicative of hepatic injury as well as histopathology, demonstrating varying degrees of centrilobular injury (Chernoff et al. 2010, 2014; Harada et al. 1994; Hawkins et al. 1985; Zurawell et al. 2005). Renal effects that were observed included interstitial nephritis and/or tubular epithelial necrosis (Falconer et al. 1999; Hawkins et al. 1985). Other adverse effects included CYN induced bleeding in the gastrointestinal tract (GIT) (Chernoff et al. 2010; Hawkins et al. 1985; Seawright et al. 1999), lungs (Bernard et al. 2003; Hawkins et al. 1985), heart (Hawkins et al. 1985; Seawright et al. 1999), and periorbital sinus and tail tips (Chernoff et al. 2010; Rogers et al. 2007; Shaw et al. 2000).
The widespread occurrence of CYN in bodies of freshwater and its ability to bioaccumulate in invertebrate and vertebrate species (Kinnear, Duivenvoorden, and Fabbro 2009; White et al. 2006, 2007) raised concerns regarding its potential to contaminate drinking water supplies (De La Cruz et al. 2013; Poniedziałek, Rzymski, and Kokociński 2012). CYN is now known to be produced by cyanobacterial species globally including C. raciborskii in Australia, New Zealand, Thailand, and the USA (Burns 2008; Li et al. 2001b; Ohtani, Moore, and Runnegar 2002; Sterling and Quilliam 2001), Anabaena bergii and Lyngbya wollei in Australia (Fergusson and Saint 2003; Seifert et al. 2007), Umezakia natans in Japan (Harada et al. 1994), Chrysosporum (Aphanizomenon) ovalisporum in Israel (Banker et al. 1997), Raphidiopsis curvata in China (Li et al. 2001a), Chrysosporum (Aphanizomenon) flosaquae in Germany (Preussel et al. 2006), Anabaena lapponica in Finland (Spoof et al. 2006), Oscillatoria sp. in France and Portugal (Bellem, Nunes, and Morais 2013; Mazmouz et al. 2010), and Hormoscilla pringsheimii in Europe (Bohunická et al. 2015). In the USA, CYN occurs widely in numerous lakes (Distressed Watershed Designation Analysis, 2011; Loftin et al. 2016). In 2015, the United States Environmental Protection Agency developed Drinking Water Health Advisories for CYN as informal technical guidance for protection of public health for contaminants that affect drinking water quality, but are not regulated under the Safe Drinking Water Act. The 10-d value for CYN is 0.7 μg/L for bottle-fed infants and young children of pre-school age, and 3 μg/L for school-age children through adults (USEPA Drinking Water Health Advisory, 2015).
The known toxicity of CYN coupled with its wide distribution points to the need for in vivo mammalian studies that assess the potential toxicity of this microcystin in the environment. The majority of studies examining the toxicity of CYN used extracts of C. raciborskii cultures and/or the i.p. route of administration, neither of which is completely satisfactory to reach definitive quantitative risk assessments as extracts contain a variety of compounds whose identities and toxicities are unknown. Further, the i.p. route is inappropriate for extrapolation to oral intake in the environment. Toxicity induced by extracts is difficult to correlate with CYN levels as extract toxicity is greater than that which may be attributed to the CYN that is present (Falconer et al. 1999; Hawkins et al. 1997). Other investigators demonstrated that strains of C. raciborskii that did not produce CYN also initiate similar types of adverse effects. Hepatocellular toxicity including necrosis occurred in mice following i.p. injections of lyophilized extracts of C. raciborskii from France at 20 mg/kg (Bernard et al. 2003), at 800 mg/kg from a German strain (Fastner et al. 2003), and at >1300 mg/kg from a strain found in Portugal (Saker et al. 2003), none of which produced detectable CYN. Humpage and Falconer (2003), using male mice exposed to purified CYN obtained from C. raciborskii at 0, 30, 60, 120, or 240 μg/kg/d, by gavage for 11 weeks found effects that included minor histopathological changes in the liver, significant reductions in protein/creatinine concentrations in urine at doses of ≥120 μg/kg, increased kidney weights at ≥60 μg/kg/d, and damage to the proximal tubules at 240 μg/kg/d.
Previously, Chernoff et al. (2010, 2014) demonstrated that i.p. injections of CYN in adult mice elevated serum alterations indicative of hepatic injury, histological changes in the liver, and alterations in gene expression patterns associated with inflammation and hemorrhage. CYN is a developmental toxin, producing prenatal and postnatal toxicity in fetuses and pups when administered to pregnant mice during the second half of gestation (Rogers et al. 2007). Fetal toxicity is characterized by in utero lethality, premature birth on GD17, and associated reduced pup birth weight; bleeding in GIT and tail; and decreased postnatal survival and long-term growth retardation. Fetal and postnatal bleeding in the GIT and tail tip, and lethality are similar to effects noted in adults, but severity of manifestations after in utero exposure is greater and fetal/postnatal effects often occurred in asymptomatic females (Chernoff et al. 2014; Rogers et al. 2007).
The purpose of this study was to (1) expose mice of both genders to CYN administered by gavage daily for a 90-d period and (2) identify and assess CYN-induced adverse health consequences. The protocol used was a modified Repeated Dose 90-Day Oral Toxicity Study as outlined in OECD Test No. 408 (OECD 1998).
Materials and methods
Animals
Eighty CD-1 (Swiss-Webster) mice, equal numbers of males and females, were obtained from Charles River Laboratories (Raleigh,NC, USA). The animals arrived at the National Health and Environmental Effects Research Laboratory (NHEERL) animal facility postweaning and were allowed to acclimate for 5 d, and dosing was begun at 30 d of age. Animals were housed singly in polycarbonate cages on heat-treated pine shaving bedding in animal rooms with a controlled temperature range (22–26°C) and a 12:12-h light–dark cycle. Animals were fed commercial rodent chow (Purina Prolab) and water ad libitum. Treatment was begun with two identical groups, 1 week apart. All studies were conducted after approval by the Institutional Animal Care and Use Committee, NHEERL, using recommendations of the 2011 NRC “Guide for the Care and Use of Laboratory Animals,” the Animal Welfare Act, and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
Compound
CYN used in these investigations was Lot #13 supplied by GreenWater Laboratories (Palatka, FL, USA). The purified toxin (C15H21N5O7S; CAS number 143545–90-8, molecular weight 415.42) was characterized by liquid chromatography/mass spectrometry (LC-MS) and LC using photodiode array detection. The toxin was shown to be free from organic impurities and present as the sodium salt. The estimated purity was >95%. Quantitation was based on absorbance at 262 nm with a molar extinction coefficient of 9800 L/mol and also compared quantitatively to HPLC-PDA/UV data achieved from a certified calibration solution acquired from the National Research Council of Canada (CRM-CYN).
Experimental design
Animals were dosed with CYN by successive daily oral doses administered via feeding needles. Four dose levels 0, 75, 150, and 300 μg/kg/d were employed. The number of animals ranged from 18 to 20 per group, divided equally between males and females. The CYN-treated animals received the toxin in 0.2 ml of filtered water/dose and controls received the vehicle (0.2 ml/d) alone. End points measured during the dosing period included body weight and clinical signs of toxicity obtained at least three times each week. Dose solutions were formulated biweekly and adjusted to reflect weight changes in the treated groups. After the 90-d dosing was completed, all animals were anesthetized by CO2 inhalation, weighed and euthanized by exsanguination. Necropsies were performed on all animals, and data obtained are detailed below.
Blood collection
Animals were anesthetized with CO2, and blood for hematology and clinical chemistry was obtained transdermally from the heart with a 25 g, 5/8 in. needle attached to a 1 ml syringe. Whole blood for hematology was transferred to a 0.5ml tube containing EDTA as the anticoagulant. Whole blood for clinical chemistry was transferred to a 0.5 ml serum separator tube, allowed to clot for approximately 1 h, and centrifuged at 1300 × g for 10min to separate the serum. Serum was transferred to an auto-analyzer cup and stored at –80ºC.
Clinical chemistry
All serum clinical chemistry analyses were carried out using the Beckman-Coulter Olympus AU400E analyzer (Irving, TX, USA). Hepatic injury was assessed by determining the serum activities of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and serum concentrations of total bile acids (TBAs). Markers of renal injury included serum concentrations of blood urea nitrogen (BUN) and creatinine. Serum glucose, cholesterol, triglycerides, total protein, and albumin were also measured as markers of general toxicity. All assays except TBA were performed using reagents obtained from the instrument manufacturer; reagents for TBA analyses were obtained from Sekisui Diagnostics (Lexington, MA, USA).
Hematology
Whole blood samples collected for hematology were analyzed for a complete blood count using an automated analyzer (Procyte DX, IDEXX Laboratories, Inc., Westbrook, ME, USA). The variables of complete blood count included the hematocrit (Hct), erythrocyte count (RBC), hemoglobin concentration (Hgb), mean corpuscular volume, mean corpuscular hemoglobin concentration, platelet count, total and differential white blood cell counts, and reticulocyte count.
Histopathological evaluation
At necropsy, tissue samples from the liver, kidneys, thymus, heart, spleen, intestine, pancreas, testes, and any other organs that showed gross changes were examined and fixed in >10× volume 10% neutral buffered formalin (NBF). Liver, both kidneys, and testes were weighed and examined for visible lesions. Liver tissue samples, approximately 5 mm thick, were obtained from both the median and left lobes. Both kidneys were collected from the same animals: the left kidney cut longitudinally and the right cross sectionally. Tissues were placed in NBF for at least 24 h and then transferred to 70% ethanol. Sections of the selected tissues from each animal were evaluated. Tissues were routinely processed, embedded in paraffin, sectioned at approximately 5 μm, stained with hematoxylin and eosin (H&E), and examined microscopically. All slides were read by one pathologist (E.M. Whitley). The scoring system consisted of “blind” (no knowledge of treatment group) evaluation of the tissues and assigning a semi-quantitative score scheme with 0 = no lesion to 4 = severe lesion with respect to changes in organ structure, cell types, vasculature, and presence of inflammation (see the supplementary table). After an initial review, selected tissues were reevaluated and harmonized with initial scores.
Gene expression analysis
Gene expression analysis was conducted on a series of genes whose proteins are involved in various processes that may be affected by CYN exposure, including metabolism, apoptosis, liver regeneration, and coagulation. Liver samples were harvested and stored in RNA later (Life Technologies AM7021) until extraction with TRI reagent (Sigma T9424) according to the manufacturer’s protocol. RNA pellets were resuspended in nuclease-free H2O, DNased (RQ1 DNase, Promega M6101) and quantitated with Quant-iT RiboGreen RNA assay kit (Life Technologies R11490). RNA was reverse transcribed (Life Technologies High Capacity cDNA Reverse Transcription Kit 4374966), and 25 ng equivalent cDNA was amplified in a 12 μl volume using TaqMan Gene Expression Assays (Life Technologies, Foster City, CA) and TaqMan Gene Expression PCR Master Mix (Life Technologies 4369510). Amplification was performed on an ABI model 7900HT sequence detection system. All samples were run in duplicate. Data were analyzed by the 2–ΔΔCt method. The following TaqMan assays (Life Technologies) were used: Bcl2-associated X protein (Bax), Mm00432051_m1; fatty acid-binding protein 4(Fabp4), Mm00445878_m1; c-Jun, Mm00495062_s1; Kallikrein b1 (Klkb1), Mm00434658_m1; nuclear protein 1 (Nupr1), Mm00498104_m1; protein C (Proc), Mm00435966_m1; 60S ribosomal protein L6 (Rpl6), Mm01198491_g1; thrombospondin 1 (Thbs1), Mm00449032_g1; thrombopoietin (Thpo), Mm00437040_m1; tumor protein 53 (Trp53), Mm01731290_g1; and beta-2-microglobulin (B2M), Mm00437762_m1. B2M was used as the reference gene based on NormFinder analysis of control and high-dose groups.
Statistical evaluation
Single time point variables were analyzed separately for each gender, and all analyses were conducted utilizing SAS/Stat® v.13.1 software (2013). The SAS® procedures Freq, NPar1Way, and GLM were used. A p value of <.05 was considered statistically significant. No adjustments for multiple comparisons were employed, as the purpose of the investigation was to examine the pattern of response across the end points, rather than statistical significance of any single variable. All variables were tested for any difference among dose groups collectively, and each dose group was also tested for any difference from control. Variables were also tested for a trend in response across increasing dose groups.
Differences among treatment groups for categorical variables were tested using chi-square, Wilcoxon, and Kruskal–Wallis tests. Evidence of a trend across dose was tested using the Mantel–Haenszel trend chi-square. Differences in scores among dose groups for pathology end points were analyzed using Cochran–Mantel–Haenszel (CMH) chi-square statistics; the null hypothesis of no difference in mean scores among the dose groups was tested as well as the test for correlation (or trend) with increasing dose. Pathology summary variables (liver sum and kidney sum) were analyzed both with the CMH statistics as above and with two-way Analysis of Variance as detailed for continuous variables. In testing for gender-related differences in increasing cortical lesions across doses, a regression model was used to test differences in slopes.
Continuous variables were first assessed, on both the linear and log scales, for normality and homogeneity of variance across the dose groups, using Levene’s test and the Shapiro–Wilk test. The scale that best satisfied these assumptions was utilized in ANOVA and regression analyses. Continuous variables that contained several outliers or were exceedingly non-normal were also analyzed with the Wilcoxon and Kruskal–Wallis tests. Each continuous variable was first analyzed with a two-way factorial ANOVA, looking for effects of block, dose, and/or any interaction between block and dose. If an interaction between block and dose was not present, the interaction was removed from the model, and a two-way main effect analysis was employed. If there was an interaction, a one-way ANOVA was performed separately for each block. A regression analysis was used to test for a trend across dose. As in the ANOVA models, block and interaction between block and dose were included with dose as predictors in the first model. If the interaction was not significant, it was removed from the model. If there was significance, separate simple regressions for each block were run for that variable.
Results
Clinical findings
A significant decreasing trend in body weight across dose was observed in females at necropsy, and the 300 μg/kg group was significantly lower than controls (Table 1). No significant weight differences were detected in males. The appearance of treated animals did not differ from controls and no overt signs of toxicity were found during the course of the study. Gross pathology of liver observed during necropsy included dark congested areas and a reticulated pattern in all male treated groups and in the 150 and 300 μg/kg females.
Table 1.
Selected Organ Weight and Organ Weight to Body Weight Ratios in Mice in the 90-d Gavage Study of CYNa
| Dose (μg/kg) | 0 | 75 | 150 | 300 |
|---|---|---|---|---|
| Male | ||||
| n | 10 | 10 | 9 | 10 |
| Necropsy weight | 40.6 ± 1.4 | 40.4 ± 0.9 | 38.1 ± 0.6 | 39.3 ± 0.7 |
| Liver | ||||
| Absolute | 1.96 ± 0.07 | 2.37 ± 0.08** | 2.36 ± 0.05** | 2.70 ± 0.06** |
| Relative | 0.048 ± 0.002 | 0.059 ± 0.001** | 0.062 ± 0.001** | 0.069 ± 0.001** |
| Kidney | ||||
| Absolute | 0.62 ± 0.03 | 0.88 ± 0.03** | 0.89 ± 0.04** | 0.84 ± 0.05** |
| Relative | 0.015 ± 0.001 | 0.022 ± 0.001** | 0.023 ± 0.001** | 0.022 ± 0.001** |
| Testes | ||||
| Absolute | 0.248 ± 0.009 | 0.246 ± 0.011 | 0.252 ± 0.010 | 0.277 ± 0.013* |
| Relative | 0.0061 ± 0.0002 | 0.0061 ± 0.0002 | 0.0067 ± 0.0002 | 0.0071 ± 0.0004** |
| Female | ||||
| n | 9 | 10 | 10 | 10 |
| Necropsy weight | 33.5 ± 1.7 | 31.8 ± 1.1 | 30.5 ± 0.6 | 29.8 ± 0.7* |
| Liver | ||||
| Absolute | 1.64 ± 0.11 | 1.66 ± 0.07 | 1.88 ± 0.09* | 1.87 ± 0.05* |
| Relative | 0.049 ± 0.001 | 0.052 ± 0.001 | 0.062 ± 0.003** | 0.063 ± 0.001** |
| Kidney | ||||
| Absolute | 0.38 ± 0.015 | 0.39 ± 0.01 | 0.40 ± 0.02 | 0.39 ± 0.01 |
| Relative | 0.012 ± 0.000 | 0.012 ± 0.000 | 0.013 ± 0.001* | 0.013 ± 0.000** |
Necropsy body weight and organ weights are given in grams (g); relative organ weights (organ-weight-to-body-weight ratios) are given as g organ weight/g body weight (mean ± standard error).
Significant differences (P ≤0.05) from control using Wilcoxon and Kruskal-Wallis tests.
Significant differences (P ≤0.01) from control using Wilcoxon and Kruskal-Wallis tests.
Organ and organ/body weight ratios
Data for organ weight-related effects are summarized in Table 1. Both absolute and relative liver weights were significantly increased at all dose levels in males and at 150 μg/kg and 300 μg/kg toxin concentrations in females. Absolute and relative kidney weights were also elevated in males at all cyanobacterial toxin levels. In females, there were no marked differences in absolute kidney weights between groups, but the relative kidney weight was significantly increased at two highest toxin concentrations. A treatment-dependent rise in the absolute and relative weights of testes occurred at 300 μg/kg.
Clinical chemistries
The serum clinical chemistry results are summarized in Table 2. Serum activities of liver enzymes, suggestive of hepatic damage or altered function, were significantly elevated in high-dose male and female groups. In females, ALP activity exhibited a significant increase in the high-dose group compared to controls. Along with serum TBA concentrations, serum ALP activity is typically used as an indicator of cholestasis. However, in this study, TBA levels were not markedly affected. Although ALP activity rise was significant (38%), it is not clear whether the ALP increase was reflective of a cholestatic event. In males, ALT activity demonstrated a significant elevation (64%) in the 300 μg/kg group. Males exhibited a significant dose-related reduction in serum cholesterol concentration at both 150 μg/kg and 300 μg/kg compared to controls. A significant decrease in triglyceride levels occurred in males receiving 300 μg/kg. Male BUN levels in all dose groups were significantly lowered, and there was a significant dose-related trend. The only significant effect BUN in females was a significant fall in the 150 μg/kg dose group.
Table 2.
Clinical chemistry data for mice in the 90-day gavage study of CYNa
| Dose (μg/kg) | 0 | 75 | 150 | 300 |
|---|---|---|---|---|
| Male | ||||
| n | 10 | 10 | 9 | 10 |
| ALP (IU/L) | 38.3 ± 3.3 | 31.6 ± 0.7 | 35.4 ± 2.7 | 46.1 ± 5.0 |
| ALT (IU/L) | 68.6 ± 7.3 | 56.5 ± 5.4 | 63.2 ± 11.9 | 112.7 ± 14.0* |
| TBA (μmol/L) | 0.96 ± 0.21 | 1.05 ± 0.20 | 0.98 ± 0.13 | 1.26 ± 0.29 |
| BUN (mg/dL) | 23.6 ± 2.3 | 17.8 ± 0.8* | 16.8 ± 1.7** | 16.2 ± 0.9** |
| Creatinine (mg/dL) | 0.16 ± 0.02 | 0.10 ± 0.00 | 0.16 ± 0.02 | 0.15 ± 0.02 |
| Glucose (mg/dL) | 174.4 ± 8.1 | 184.4 ± 8.3 | 167.2 ± 7.1 | 170.4 ± 8.4 |
| Albumin (g/dL) | 2.8 ± 0.08 | 3.1 ± 0.04* | 2.9 ± 0.08 | 2.9 ± 0.06 |
| Total protein (g/dL) | 5.5 ± 0.2 | 5.5 ± 0.1 | 5.4 ± 0.2 | 5.2 ± 0.1 |
| Cholesterol (mg/dL) | 148.7 ± 12.4 | 130.3 ± 6.4 | 114.3 ± 5.1** | 95.7 ± 5.7** |
| Triglycerides (mg/dL) | 168.8 ± 22.3 | 145.6 ± 18.9 | 152.8 ± 29.9 | 99.5 ± 9.7* |
| Female | ||||
| n | 9 | 10 | 10 | 10 |
| ALP (IU/L) | 57.3 ± 5.6 | 48.0 ± 3.1 | 55.4 ± 8.2 | 78.9 ± 5.7* |
| ALT (IU/L) | 91.7 ± 38.6 | 194.4 ± 116.6 | 112.7 ± 44.5 | 83.6 ± 11.5 |
| TBA (μmol/L) | 2.93 ± 0.45 | 3.93 ± 1.68 | 2.94 ± 0.66 | 2.87 ± 0.46 |
| BUN (mg/dL) | 21.9 ± 0.7 | 20.6 ± 1.0 | 16.4 ± 1.0** | 21.4 ± 1.2 |
| Creatinine (mg/dL) | 0.17 ± 0.02 | 0.18 ± 0.01 | 0.13 ± 0.02 | 0.18 ± 0.02 |
| Glucose (mg/dL) | 165.9 ± 5.1 | 176.0 ± 10.5 | 159.7 ± 4.9 | 160.9 ± 6.4 |
| Albumin (g/dL) | 3.1 ± 0.06 | 3.4 ± 0.06* | 3.2 ± 0.03 | 3.1 ± 0.09 |
| Total protein (g/dL) | 5.3 ± 0.1 | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.2 ± 0.1 |
| Cholesterol (mg/dL) | 113.8 ± 10.1 | 89.2 ± 7.3 | 99.1 ± 7.5 | 91.4 ± 8.7 |
| Triglycerides (mg/dL) | 204.7 ± 21.8 | 206.4 ± 28.7 | 245.3 ± 29.1 | 226.0 ± 25.4 |
Data are given as mean ± standard error; abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, TBA = total bile acids, BUN = blood urea nitrogen.
Significant differences (P ≤0.05) from control using Wilcoxon and Kruskal-Wallis tests.
Significant differences (P ≤0.01) from control using Wilcoxon and Kruskal-Wallis tests.
Hematology
Hematology data are summarized in Table 3. In males, a treatment-related erythron effect occurred in the 300 μg/kg dose as evidenced by decreases in hematocrit, hemoglobin concentration, and erythrocyte count. There were no marked alterations in reticulocyte counts, mean cell volume, or mean cell hemoglobin concentration. Thus, the diminished erythron was normocytic, normochromic, and not responsive. The female animals were unaffected. In the leukon, the leukocyte count was significantly elevated in the 300 μg/kg males. There was a significant dose-related rise in the number of monocytes in the 75 μg/kg males and 300 μg/kg male and female dose groups. In males, lymphocyte counts showed a significant increase in males at the 75 μg/kg and 300 μg/kg dose levels and a significant dose-related trend.
Table 3.
Selected hematology data for mice in the 90-day gavage study of CYNa
| Dose (μg/kg) | 0 | 75 | 150 | 300 |
|---|---|---|---|---|
| Male | ||||
| n | 10 | 10 | 8 | 10 |
| Hematocrit (%) | 51.4 ± 1.5 | 53.4 ± 0.1 | 49.7 ± 0.8 | 47.6 ± 1.3* |
| Hemoglobin (g/dL) | 15.8 ± 0.3 | 16.6 ± 0.3 | 15.4 ± 0.2 | 14.7 ± 0.5* |
| Erythrocyte count (106/μL) | 10.6 ± 0.2 | 10.4 ± 0.2 | 10.1 ± 0.2 | 9.7 ± 0.5* |
| Reticulocyte count (106/μL) | 0.46 ± 0.02 | 0.46 ± 0.02 | 0.42 ± 0.02 | 0.59 ± 0.17 |
| Mean cell volume (fL) | 48.8 ± 0.9 | 51.3 ± 0.6 | 49.4 ± 0.4 | 50.3 ± 2.1 |
| MCHC (g/dL) | 30.8 ± 0.4 | 31.1 ± 0.4 | 31.0 ± 0.3 | 30.7 ± 0.5 |
| Leukocyte count (103/μL) | 8.03 ± 1.15 | 9.68 ± 0.66 | 7.25 ± 0.59 | 10.76 ± 0.67* |
| Neutrophil count (103/μL) | 2.4 ± 0.7 | 2.1 ± 0.3 | 1.5 ± 0.2 | 2.2 ± 0.2 |
| Leukocyte count (103/mL) | 5.2 ± 0.8 | 7.2 ± 0.6* | 5.5 ± 0.6 | 7.9 ± 0.5* |
| Monocyte count (103/μL) | 0.07 ± 0.02 | 0.18 ± 0.04* | 0.17 ± 0.04 | 0.30 ± 0.06* |
| Eosinophil count (103/μL) | 0.21 ± 0.06 | 0.29 ± 0.06 | 0.15 ± 0.05 | 0.31 ± 0.08 |
| Female | ||||
| n | 9 | 10 | 10 | 10 |
| Hematocrit (%) | 49.0 ± 0.6 | 50.2 ± 1.5 | 54.0 ± 0.7 | 50.2 ± 1.0 |
| Hemoglobin (g/dL) | 15.7 ± 0.2 | 15.8 ± 0.4 | 15.9 ± 0.2 | 16.0 ± 0.3 |
| Erythrocyte count (106/μL) | 10.2 ± 0.1 | 10.1 ± 0.3 | 10.3 ± 0.2 | 10.1 ± 0.2 |
| Reticulocyte count (106/μL) | 0.46 ± 0.04 | 0.47 ± 0.04 | 0.37 ± 0.03 | 0.35 ± 0.05 |
| Mean cell volume (fL) | 48.0 ± 0.6 | 50.0 ± 0.7 | 48.6 ± 0.5 | 49.6 ± 0.5 |
| MCHC (g/dL) | 31.9 ± 0.2 | 31.6 ± 0.3 | 31.9 ± 0.2 | 31.9 ± 0.2 |
| Leukocyte count (103/μL) | 7.61 ± 0.56 | 6.04 ± 0.63 | 7.90 ± 0.89 | 8.83 ± 0.69 |
| Neutrophil count (103/μL) | 1.3 ± 0.2 | 1.0 ± 0.2 | 1.8 ± 0.3 | 2.0 ± 0.3 |
| Lymphocyte count (103/μL) | 6.0 ± 0.4 | 4.8 ± 0.5 | 5.8 ± 0.7 | 6.5 ± 0.5 |
| Monocyte count (103/μL) | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.11 ± 0.02 | 0.16 ± 0.03* |
| Eosinophil count (103/μL) | 0.21 ± 0.04 | 0.16 ± 0.03 | 0.23 ± 0.04 | 0.24 ± 0.04 |
Data are given as mean ± standard error; abbreviation: MCHC = mean cell hemoglobin concentration
Significant differences (P ≤0.05) from control using Wilcoxon and Kruskal-Wallis tests.
Histopathology
Histopathological evaluation of the selected tissues demonstrated that liver and kidneys exhibited microscopic lesions related to CYN exposure, and these data are summarized in Tables 4 and 5. In the liver, the predominant lesions included disorganization of hepatic cords, hepatocyte hypertrophy/cytoplasmic alteration, cell death of hepatocytes, variable inflammation, and accumulation of pigment. A distortion of the normal hepatic cord architecture was characterized by disruption of hepatic lobules, the structural units of the liver. These effects are evidenced by altered hexagonal arrangement of hepatocyte plates radiating outward from a central vein (Figure 1(a,c)). In addition, the distortion of the hepatic cord architecture occurred without individualization of hepatocytes or widespread hepatocyte necrosis as noted previously during acute CYN exposure (Chernoff et al. 2010). The disorganization of the hepatic cord architecture was significant in all treated females (Table 4). The hepatocyte hypertrophy/cytoplasmic alterations were characterized by hepatocytes with a panlobular increase of cytoplasmic volume and cytoplasmic granularity and eosinophilia and/or hydropic degeneration (Figure 1(b,d)). The hepatocellular hypertrophy/cytoplasmic changes were significant in all treated females and 300 μg/kg males (Table 4). Hepatocellular cell death was characterized by small, randomly scattered foci or centrilobular foci of hepatocyte degeneration and necrosis. The degenerated/necrotic hepatocytes exhibited increased cytoplasmic eosinophilia and nuclear karyorrhexis and pyknosis; often the foci associated with local aggregates of inflammatory cells (including neutrophils and macrophages). The hepatocellular degeneration/necrosis was significant in the 150 μg/kg male and female dose groups (Table 4). Periportal inflammation, consisting of neutrophils, lymphocytes, and macrophages, was also found with significance detected in the 150 and 300 μg/kg female doses. Golden brown-green pigment, consistent with bile (or a combination of bile, lipofuscin, and hemosiderin), accumulated within hepatocytes, canaliculi, and Kupffer cells, and was significant in all exposed male and females (Table 4). Minimal centrilobular sinusoidal ectasia and hemorrhage were present in 150 and 300 μg/kg female dose groups. While similar liver histopathology appeared in both males and females, generally the changes occurred to a greater extent in female mice.
Table 4.
Incidence and severity of histopathologic liver lesions in mice in the 90-day gavage study of CYNa
| Dose (μg/kg) | 0 | 75 | 150 | 300 |
|---|---|---|---|---|
| Males (n) | 9 | 10 | 9 | 10 |
| Hepatic cord architecture distortion | 4 (1.3) | 3 (1.3) | 4 (1.8) | 7 (1.1) |
| Hepatic sinusoidal ectasia/hemorrhage | 3 (1.0) | 2 (1.5) | 4 (1.3) | 1 (1.0) |
| Hepatocyte hypertrophy with cytoplasmic alteration | 7 (1.7) | 10 (2.1) | 9 (2.0) | 10 (2.5)* |
| Hepatocyte cell death, random | 1 (1.0) | 0 | 5 (1.2) | 6 (1.2)* |
| Hepatocyte cell death, centrilobular with inflammation | 2 (1.0) | 5 (1.0) | 6 (1.3)* | 5 (1.4) |
| Heptic inflammation, periportal | 1 (1.0) | 5 (1.0) | 4 (1.3) | 4 (1.0) |
| Pigmentation, hepatocyte intracytoplasmic/intracanalicular | 0 | 3 (1.0) | 4 (1.3)* | 3 (1.3) |
| Pigmentation, Kupffer cells | 0 | 5 (1.4)* | 5 (1.0)* | 8 (1.3)* |
| Females (n) | 9 | 10 | 10 | 10 |
| Hepatic cord architecture distortion | 0 | 4 (1.5)* | 9 (1.4)* | 9 (1.4)* |
| Hepatic sinusoidal ectasia/hemorrhage | 0 | 3 (1.0) | 7 (1.1)* | 5 (1.4)* |
| Hepatocyte hypertrophy with cytoplasmic alteration | 5 (1.0) | 9 (1.7)* | 10 (1.70)* | 9 (2.1)* |
| Hepatocyte cell death, random | 0 | 2 (1.0) | 7 (1.1)* | 6 (1.3)* |
| Hepatocyte cell death, centrilobular with inflammation | 0 | 0 | 6 (1.2)* | 2 (1.0) |
| Hepatic inflammation, periportal | 2 (1.0) | 4 (1.0) | 8 (1.1)* | 8 (1.4)* |
| Pigmentation, hepatocyte intracytoplasmic/intracanalicular | 0 | 1 (1.0) | 4 (1.3) | 7 (1.4)* |
| Pigmentation, Kupffer cells | 0 | 3 (1.3)* | 7 (1.3)* | 9 (1.6)* |
Data represent the total number of lesions and average severity (in parenthesis) for each lesion by group; lesion severity was based on a 0 – 4 grading scale
Significant differences (P ≤0.05) from control using CMH tests (implicitly including both incidence and severity).
Table 5.
Incidence and severity of histopathologic kidney lesions in mice in the 90-day gavage study of CYNa
| Dose (μg/kg/day) | 0 | 75 | 150 | 300 |
|---|---|---|---|---|
| Male (n) | 9 | 10 | 9 | 10 |
| Cortex | ||||
| Tubule dilation | 2 (1.5) | 10 (1.4)* | 9 (2.0)* | 10 (2.6)* |
| Tubule basophilia | 0 | 5 (1.0)* | 5 (1.6)* | 8 (2.6)* |
| Tubule intraluminal protein | 7 (1.6) | 10 (1.7) | 9 (1.7) | 10 (2.1)* |
| Tubule epithelial cytoplasmic alteration | 1 (1.0) | 7 (1.3)* | 9 (1.8)* | 10 (2.6)* |
| Tubule nuclear crowding | 1 (1.0) | 4 (1.0) | 7 (1.0)* | 9 (1.6)* |
| Outer medulla | ||||
| Thinning of outer stripe | 0 | 4 (1.3) | 8 (1.6)* | 9 (2.2)* |
| Tubule epithelial cytoplasmic alteration of outer stripe | 1 (1.0) | 7 (1.3)* | 9 (1.8)* | 10 (1.9)* |
| Tubule intraluminal protein of inner stripe | 3 (1.0) | 6 (1.3) | 9 (1.4)* | 10 (1.9)* |
| Inner medulla | ||||
| Tubule intraluminal protein | 0 | 6 (1.0)* | 8 (1.3)* | 8 (1.5)* |
| Female (n) | 9 | 10 | 10 | 10 |
| Cortex | ||||
| Tubule dilation | 1 (1.0) | 1 (1.0) | 5 (1.0) | 4 (1.3) |
| Tubule basophilia | 1 (1.0) | 0 | 0 | 2 (1.0) |
| Tubule intraluminal protein | 5 (1.0) | 4 (1.0) | 8 (1.0) | 9 (1.1) |
| Tubule epithelial cytoplasmic alteration | 0 | 1 (2.0) | 3 (1.0) | 3 (1.7) |
| Tubule nuclear crowding | 1 (1) | 0 | 1 (2.0) | 3 (1.3) |
| Outer medulla | ||||
| Thinning of outer stripe | 0 | 0 | 2 (2.0) | 1 (1.0) |
| Tubule epithelial cytoplasmic alteration of outer stripe | 2 (1.0) | 8 (1.5) | 10 (1.8)* | 10 (2.0)* |
| Tubule intraluminal protein of inner stripe | 2 (1.0) | 2 (1.0) | 4 (1.0) | 6 (1.2) |
| Inner medulla | ||||
| Tubule intraluminal protein | 3 (1.0) | 4 (1.0) | 3 (1.0) | 4 (1.3) |
Data represent the total number of lesions and average severity (in parenthesis) for each lesion by group; lesion severity was based on a 0 – 4 grading scale
Significant differences (P ≤0.05) from control using CMH tests (implicitly including both incidence and severity).
Figure 1.
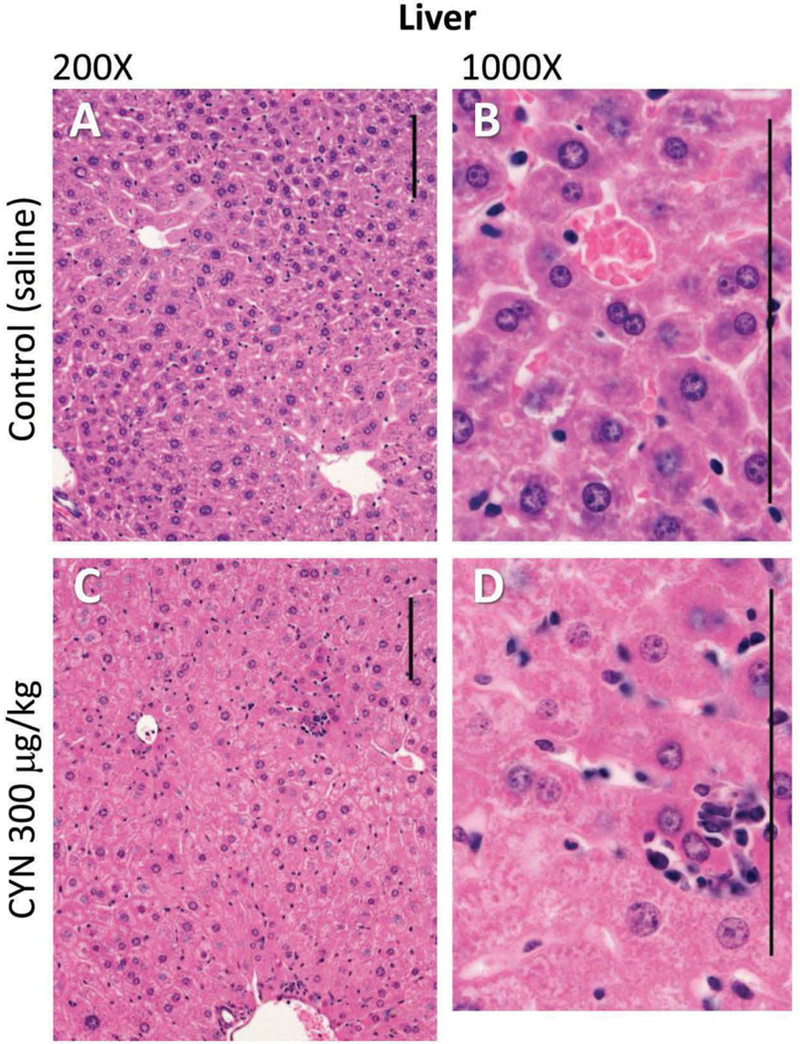
Photomicrographs of the liver tissue of female mice (controls A and B; CYN 300 μg/kg C and D). Liver lesions included distortion of hepatic architecture, hepatocellular hypertrophy, cytoplasmic alteration and degeneration, and/or necrosis of centrilobular hepatocytes often infiltrated by inflammatory cells. Hematoxylin and eosin stain used. Size bars = 100 μm.
In the kidney, the predominant lesions observed involved tubules of the S3 region of the outer medulla, particularly the pars recta of the outer stripe and cortical tubular changes in treated males that were considered morphologically consistent to those of chronic progressive nephropathy (CPN) (Table 5). In the S3 region of the outer medulla, microscopic changes in this region consisted of epithelial cytoplasmic alterations including epithelia swelling or glycogen accumulation, anisokaryosis, and intraluminal protein with thinning or loss of a distinct S3 region (Figure 2(a,c)). The cytoplasmic alterations occurred in 150 and 300 μg/kg females and all male dose groups. However, thinning of the S3 region was detected significantly only in the 150 and 300 μg/kg males. Increased tubule intraluminal protein accumulation was also noted in the inner stripe of the outer medulla and inner medulla of essentially all males. Only in treated male mice were cortical lesions demonstrating features of CPN observed (Figure 2(b,d)). Lesions associated with CPN included dilation and basophilia of tubules, tubule intraluminal protein accumulation, tubule nuclear crowding, and tubule epithelial cytoplasmic alteration. Epithelial cytoplasmic alteration was characterized by epithelial vacuolar change, atrophy, hypertrophy (with increased cytoplasm eosinophilia), and anisokaryosis.
Figure 2.
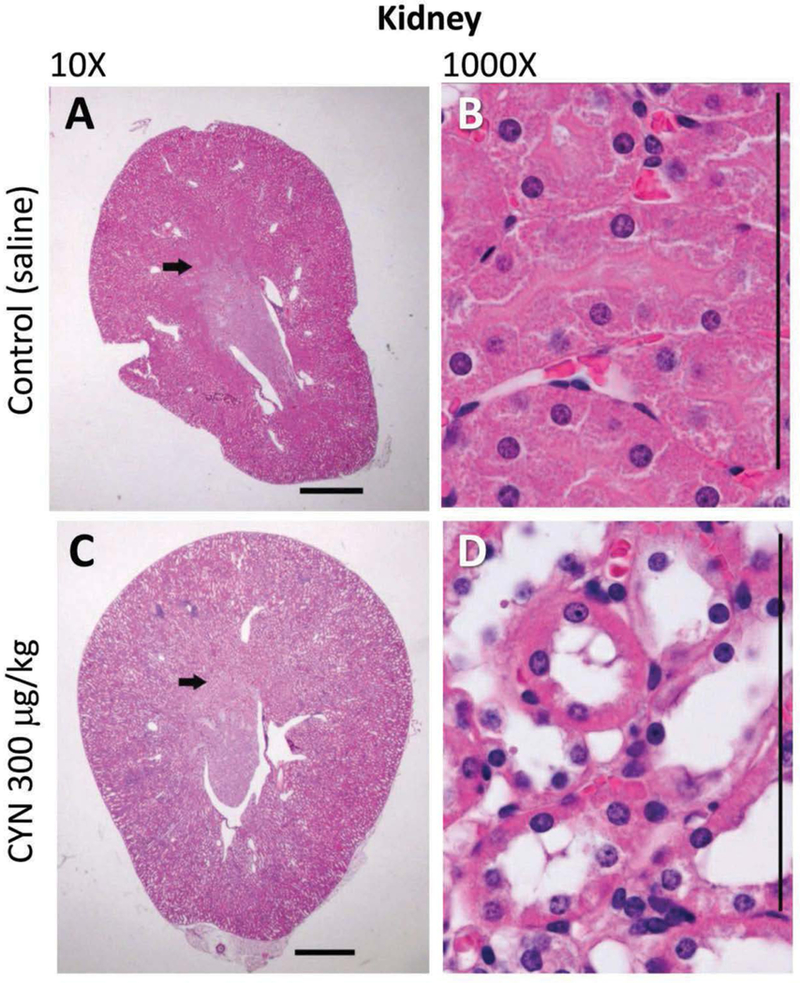
CYN induces a reduction in the outer stripe of the outer medulla of the kidney in male animals (control A; CYN 300 μg/kg C), and in sagittal sections (10x), demarcation of the outer stripe is less pronounced in the CYN-exposed (300 μg/kg) than control kidneys (arrows). Tubular epithelial cells (1000x) in the S3 region (control B; CYN 300 μg/kg D) exhibit epithelial atrophy, loss of luminal microvilli, cytoplasmic swelling or glycogen accumulation (colorless areas), and mild anisokaryosis. Hematoxylin and eosin stain used. Size bars, 1000 μm in 10×; 100 μm in 1000×.
Marked dose-related histological changes were not observed in heart, thymus, spleen, intestine, pancreas, or testes.
Gene expression
Changes in hepatic gene expression were determined for a series of genes known to be affected following i.p. administration of CYN (Chernoff et al. 2010; personal communication). Gene expression data and general functions of genes are summarized in Table 6. There was a significant upregulation of the pro-apoptotic Bcl-2-associated X gene (Bax) at all doses in females and at 150 and 300 μg/kg doses in males which was a dose-related effect in both genders. The fatty acid-binding protein 4 (Fabp4) gene involved with fatty acid uptake and metabolism was significantly downregulated at all dose levels with the exception of high-dose males. Nuclear protein-1, a gene associated with pancreatic disease, exhibited significant upregulation in the female 300 μg/kg group. Ribosomal protein L6 (Rpl6), which is associated with liver regeneration, was significantly upregulated at all dose levels in both genders in a dose-related manner. Both c-Jun that protects against apoptosis and Trp53 that initiates apoptosis were not markedly affected in a consistent pattern. The expression of genes known to be involved in the production of key proteins in the coagulations system was determined. Protein C (Proc) which functions as an anticoagulant by suppressing coagulation factors Va and VIIIa was significantly downregulated in both males and females. Kalikrein (Klkb1) which functions as an activator of plasminogen resulting in the production of plasmin and the dissolution of clots was significantly downregulated at all dose levels in a dose-related manner. Thrombospondin-1 (Thbs-1) and thrombopoietin (Thpo) are genes that regulate platelet formation, aggregation, and clot strengthening, and neither of the genes exhibited biologically consistent altered expressions.
Table 6.
Gene expression
| Dose (μg/kg/day) | 75 | 150 | 300 | |||||
|---|---|---|---|---|---|---|---|---|
| Gene/protein function | Gene Symbol |
Sex | n | Fold Change |
n | Fold Change |
n | Fold Change |
| Bcl2-associated X protein: Involved in the metabolic pathway leading to apoptosis | Bax | M | 8 | −1.13 | 9 | 1.20a | 10 | 1.42b |
| F | 10 | 1.33a | 10 | 1.59b | 10 | 1.62b | ||
| Fatty acid binding protein 4: Transport, uptake, and metabolism of fatty acids | Fabp4 | M | 8 | −2.65b | 9 | −1.46a | 10 | −1.21 |
| F | 10 | −1.53b | 10 | −1.62b | 10 | −1.40a | ||
| Nuclear protein 1: Stress-inducible gene transcription; associated with pancreatitis | Nupr1 | M | 8 | −1.97 | 9 | −1.72 | 10 | −1.44 |
| F | 10 | −1.64 | 10 | 1.17 | 10 | 3.06a | ||
| 60S ribosomal protein L6 is involved in liver regeneration and expressed after liver injury | Rpl6 | M | 8 | 1.36b | 9 | 2.25b | 10 | 2.54b |
| F | 10 | 1.65b | 10 | 2.12b | 10 | 2.62b | ||
| c-Jun and c-Fos form AP-1, early response transcription factor; anti-apoptotic | c-Jun | M | 8 | 1.56 | 9 | −1.64 | 10 | 1.10 |
| F | 8 | 1.52 | 10 | −1.59a | 10 | −1.95b | ||
| Tumor protein p53 is an apoptosis initiator, activating Bax and other BCL2 family genes | Trp53 | M | 8 | 1.58b | 9 | −1.16 | 10 | −1.43b |
| F | 8 | 1.58b | 10 | −1.50b | 10 | −1.07 | ||
| Protein C Inactivates Factors Va and VIIIa, therefore acting as an anti-coagulant | Proc | M | 8 | −1.62b | 9 | −1.52b | 10 | −1.33b |
| F | 10 | −1.55b | 10 | −1.44b | 10 | −1.51b | ||
| Kallikrein protein is an anti-thrombotic factor in the intrinsic coagulation pathway | Klkb1 | M | 8 | −1.26a | 9 | −1.80b | 10 | −1.68b |
| F | 10 | −1.03 | 10 | −1.41b | 10 | −2.10b | ||
| Thrombospondin-1 interacts with plasmin and is involved with platelet aggregation and clot formation | Thbs1 | M | 8 | −1.05 | 9 | −1.10 | 10 | −1.68 |
| F | 8 | 1.22 | 10 | −1.17 | 10 | 1.02 | ||
| Thrombopoietin stimulates production of platelets from megakaryocytes | Thpo | M | 8 | 1.66b | 9 | −1.05 | 10 | −1.15 |
| F | 8 | 1.75b | 10 | −1.16 | 10 | 1.24a |
p<0.05 as compared to the control group
p<0.01 as compared to the control group
Discussion
The oral administration of CYN for 90 consecutive days induced significant alterations observed at the end of the dosing period. The dose-related reduction in weight gain seen in the females is difficult to interpret since this effect was not noted in males. CYN is known to adversely affect both hepatic and renal systems (Bazin et al. 2012; Falconer et al. 1999; Humpage and Falconer 2003; Seawright et al. 1999), and data presented here indicate toxicity in both organs at all dose levels.
In this study, hepatic lesions inmice administered CYN for 90 consecutive days were less severe than those reported in studies of 50 μg/doses administered i.p. for 5 consecutive days that produced widespread disruption of hepatic cords, hepatocellular degeneration and necrosis, and centrilobular inflammation (Chernoff et al. 2010, 2014). Microscopic changes noted in this cohort were more consistent with a subchronic to chronic type, hypertrophic hepatic response to xenobiotic exposure, with elevated hepatocyte volume likely representing increases in smooth endoplasmic reticulum, peroxisomes, mitochondria, or, perhaps, hydropic degeneration (Hardisy and Brix 2014). Many of the detected CYN-induced changes in liver appearance and function were similar to those found in acute or subacute liver disease. Significant increases in absolute and relative liver weights were seen in both genders; however, only males exhibited these effects at the low-dose level, and the degree of change detected in the high-dose group was greater. Centrilobular hepatocytes exhibited features consistent with degeneration, necrosis, and apoptosis. The histological effects noted in the liver included mild-to-moderate centrilobular degeneration, mild hemorrhage, periportal inflammation, and disorganized hepatic cords. Prominent features of the CYN exposure included necrosis and/or apoptosis of hepatocytes possibly resulting from a disruption of the cytoskeleton that was reported in CYN-exposed CHO cells in culture (Fessard and Bernard 2003). Dissociation of hepatic cords, degeneration and necrosis of centrilobular hepatocytes, and indications of inflammation are all consistent with a breakdown of cellular structure that subsequently might be reflective of disrupted synthesis of proteins involved in different aspects of the coagulation process (Chernoff et al. 2014).
Serum markers of the hepatic function such as serum ALP and ALT activity were increased suggestive of hepatic toxicity and consistent with observed histopathology. The ALP activity elevation in conjunction with increased serum TBA concentrations typically indicates cholestasis. However, TBA levels were not markedly altered and although ALP activity rose significantly, data suggest that these effects may not have been related to a cholestatic event. Wiedmeyer (2018) noted that an ALP activity increase without a concomitant change in TBA concentrations may be attributed to a compound-induced hepatocellular induction that is consistent with the observed hypertrophy. In males, ALT activity demonstrated a significant elevation in the 300 μg/kg group, and this effect is usually considered an indicator of hepatocellular injury and associated membrane leakage. TBA concentrations were not markedly altered, suggesting that the ALT increase may not be associated with a hepatocellular injury event. The observed changes in ALT levels may be related to compound induction possibly as an adaptive response (Rosen et al. 1959). Elevated ALT levels in high-dose males were similar to changes seen in studies following i.p. 50 μg/kg exposures to CYN (Chernoff et al. 2010, 2014).
Reduced serum BUN values found in males are another indicator of hepatic dysfunction (Lum and Leal-Khouri 1989). Other indications of general hepatic toxicity included significant reductions in both hematocrit and hemoglobin levels in the high-dose males. Acute intravascular fibrin thrombi or more chronic, organized thrombi were noted in the lumen of hepatic sinusoids of mice, an area where CYN-induced bleeding was previously described by Hawkins et al. (1985).
Although CYN is often described as a hepatic toxin (Zurawell et al. 2005), Falconer and Humpage (2003) found that the kidney was the most sensitive organ with respect to CYN exposure inmalemice. In this investigation, CYN initiated dose-dependent renal toxicity in both cortex and medulla. Lesions in cortical tubules increased with concentration of CYN and occurred exclusively in males. These findings are in agreement with the findings of Falconer and Humpage (2003) and may reflect gender-related differences in response demonstrated in this study. Cortical tubular changes in male mice are morphologically similar to those of CPN, a spontaneous detected condition in mice and rats (Seely and Brix 2014) and may represent a xenobiotic mediated exacerbation of CPN related to CYN administration (Hard and Khan 2004). In general, CPN male animals are more affected than females (Travlos et al. 2011) and that gender bias occurred in this study.
In the medulla, exposure to CYN was associated with tubular lesions. Epithelial cells of the S3 segment of the nephron (proximal straight tubule) were vacuolated or displayed clear spaces, usually in the basal region of the cell, probably representing cell swelling and/or glycogen accumulation. In groups exposed to high concentrations of CYN, there was a reduction in the size of the outer stripe of the outer medulla; a degeneration or loss of S3 segment epithelial cells was suspected. Since the tubular epithelium plays critical roles in homeostasis, damage to tubules is expected to result in impaired renal functions. However, in this study, there was no evidence of tissue dysfunction as noted by serum BUN or creatinine levels. Because of the functional reserve of the kidney, clinical signs or clinical pathology data indicating impaired renal function may not be evident at this point (Hard and Khan 2004). Males appeared to exhibit more severe renal changes than female mice.
The incidence and extent of renal toxicity was related to gender and significant dose-related increases in both kidney absolute and relative weights in males. Although females exhibited significantly elevated relative weight values at the mid and high-dose levels, analysis of covariance indicated a lack of effect of dose group but a significant effect of weight indicating that increased relative weights reflected reduced body weights rather than CYN induced renal effects. A series of pathological changes in the renal cortical and medullary tubules that involved dilation, cellular swelling, glycogen accumulation, intraluminal protein, and cellular loss displayed a higher incidence and greater severity in males. The reason(s) for this gender-related difference in susceptibility is unknown at this time. Ongoing studies are underway to test the possible gender-related expression differences in the Cyp450 gene family that are known to be affected by CYN (Chernoff et al. 2014), and also contain members that exhibit gender-related differences in expression specifically in mouse (Penaloza et al. 2014; Renaud et al. 2011; Waxman and O’Connor 2006).
Reduced serum cholesterol as seen in the 150 and 300 μg/kg treated males may be associated with alterations in lipid metabolism related to hepatic disease, although in humans this is usually indicative of severe levels of toxicity (Ghadir et al. 2010). Increases in plasma cholesterol levels were previously reported in mice exposed to cell-free media containing CYN for periods up to 42 weeks (Sukenik et al. 2006). Significantly, lower serum triglycerides that were noted in high-dose males are also indicative of an impaired hepatic function in humans (Chrostek et al. 2014; Ghadir et al. 2010).
Weight of testes and testes/body weight ratios were increased similar to the effect reported previously (Humpage and Falconer 2003). The underlying cause(s) and potential significance of this effect remains unknown. There have been no apparent studies of potential CYN impacts on the male reproductive system and function, and this data gap needs to be addressed.
In the 300 μg/kg males, a treatment-related fall in the erythron was detected, but there were no marked alterations in reticulocyte counts, mean cell volume, or mean cell hemoglobin concentration. Thus, the decreased erythron was normocytic, normochromic, and not responsive, which is consistent with a treatment-related, suppression type erythron reduction related to CYN administration (Poitout-Belissent and McCartney 2010). In the leukon, there were variable effects related to CYN administration. The leukocyte count was increased in 300 μg/kg treated males with a significant dose-related rise in a number of monocytes in 75 μg/kg males and 300 μg/kg male and females, as well as lymphocyte counts were elevated in males at 75 and 300 μg/kg. While the basis for these leukon changes remains unknown, CYN exposure induced an inflammatory response and increased neutrophil counts in a human population affected by CYN (Byth 1980) as well as in a previous lab study where mice were exposed to 50 μg/kg CYN i.p. (Chernoff et al. 2014). In the current study, elevation was also found in other cells associated with inflammation including eosinophils and monocytes (Ward 1974; Ziegler-Heitbrock 2007).
The significance of gene expression alterations in the absence of data on protein levels is problematic, but efforts were undertaken to identify gene expression profiles in laboratory animals that are indicative of preneoplastic liver disease (Kim and Wang 2003). Various studies included gene expression changes due to nonalcoholic fatty liver disease (NAFLD) (Wang et al. 2016) and acute liver failure after CCl4 exposure (Xu et al. 2013) or hepatectomy (Makino et al. 2011). The utilization of gene expression was suggested as potentially useful in considerations of cyanotoxin NOAEL (Zheng et al. 2011). However, there is little concordance between the pathways leading to human NAFLD and nonalcoholic steatohepatitis, and animal models of the diseases (Teufel et al. 2016). Previously, Chernoff et al. (2010) examined changes in hepatic gene expression in liver after i.p. exposure to CYN where a series of affected genes were identified. Alterations in these genes, while not necessarily being suitable for extrapolation to humans, may indicate some degree of changes that are indicative of organism responses to toxin exposures. Genes known to be affected by CYN within the first day of exposure generate proteins that play roles in critical hepatic functions and/or factors associated with hepatic disease. Bax (Adams and Cory 2007; Elmore 2007) and Rpl6 (Aloni, Peleg, and Meyuhas 1992) remained upregulated at the end of the dosing period in keeping with CYN-induced apoptosis and ongoing cellular repair mechanisms and regeneration. Reduced expression of Fabp4 was associated with decreased triglycerides levels and with drugs that lower cholesterol (Furuhashi et al. 2015; Karpisek et al. 2007), two CYN-induced effects noted in the study. A group of genes involved in the coagulation process were also determined since their expression was known to be affected after exposure with CYN (personal communication). The anticoagulants, Proc (Beckmann et al. 1985) and Klkb1 (Rhaleb, Yang, and Carretero 2011), were downregulated at all doses. Although this effect was linked to procoagulant activity in mice (Kopec and Luyendyk 2014; Lopez et al. 2014), no significant tendency for bleeding was observed. Thbs-1 and Thpo that are involved with platelet production and aggregation and produced in the liver (Hayashi et al. 2012; Jelkmann 2001) were not consistently affected. One difference between responses to i.p. versus oral exposures of CYN is the absence of bleeding after oral exposures. The weaker response of these coagulation-related genes in the current study may reflect the absence of this effect.
CYN-induced effects exhibited basic differences compared to results of studies utilizing the i.p. route. The i.p. route single-dose LD50 is listed as 2100 μg/kg, but this has never been replicated. In mice dosed with 50 μg/kg/d beginning on gestation day 8, CYN produced 14% lethality after four consecutive doses, and 59% lethality after five doses (Chernoff et al. 2014). This indicates that the i.p. route is considerably more toxic than oral dosing as no lethality occurred after 90 consecutive doses as high as 300 μg/kg/d. The most striking difference in response between i.p. and oral routes is the lack of hemorrhage after oral exposures. Hemorrhage was a prominent symptom following CYN exposures in both the Palm Island human poisoning (Byth 1980) and cattle death (Saker, Thomas, and Norton 1999; Thomas et al. 1998). The reason(s) for this difference in response is not known, but it would appear that hemorrhage is indicative of greater toxicity and may also play a significant role in CYN-induced lethality.
All dose levels used in this study induced signs of hepatic and renal injury, withmales more susceptible than females. Liver and kidney/body weight ratios, reduced cholesterol levels, cellular signs of inflammation, and degree and extent of renal histopathological damage all appear to be more pronounced in males, although hepatic cord dissociation, centrilobular hepatocyte changes, and inflammation were more prominent in females. The 75 μg/kg low-dose level was associated with significant increases in liver and kidney/body weight ratios, decreased levels of BUN, signs of hepatic inflammation, and histopathological damage to hepatic and renal tissues, and therefore a no-observed-adverse-effect level could not be determined in this study.
Supplementary Material
Footnotes
Disclaimer
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the US EPA nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
References
- Adams JM, and Cory S. 2007. The Bcl-2-regulated apoptosis switch: Mechanism and therapeutic potential. Current Opinion in Immunology 19:488–96. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Peleg D, and Meyuhas O. 1992. Selective translational control and nonspecific posttranscriptional regulation of ribosomal protein gene expression during development and regeneration of rat liver. Molecular and Cellular Biology 12:2203–12. doi: 10.1128/MCB.12.5.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker R, Carmeli S, Hadas O, Teltsch B, Porat R, and Sukenik A. 1997. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Israel. Journal of Phycology 33:613–16. doi: 10.1111/j.0022-3646.1997.00613.x. [DOI] [Google Scholar]
- Bazin E, Huet S, Jarry G, Le Hégarat L, Munday JS, Humpage AR, and Fessard V. 2012. Cytotoxic and genotoxic effects of cylindrospermopsin in mice treated by gavage or intraperitoneal injection. Environmental Toxicology 27:277–84. doi: 10.1002/tox.20640. [DOI] [PubMed] [Google Scholar]
- Beckmann RJ, Schmidt RJ, Santerre RF, Plutzky J, Crabtree GR, and Long GL. 1985. The structure and evolution of a 46 amino acid human protein C precursor and its messenger RNA, based upon the DNA sequence of cloned human liver cDNAs. Nuclear Acids Researcher 13:5233–547. doi: 10.1093/nar/13.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellem F, Nunes S, and Morais M. 2013. Cyanobacteria toxicity: Potential public health impact in south Portugal populations. Journal of Toxicology and Environmental Health. Part A 76:263–71. doi: 10.1080/15287394.2013.757204. [DOI] [PubMed] [Google Scholar]
- Bernard C, Harvey M, Briand JF, Bire R, Krys S, and Fontaine JJ. 2003. Toxicological comparison of diverse Cylindrospermopsis raciborskii strains: Evidence of liver damage caused by a French C. raciborskii strain. Environmental Toxicology 18:176–86. doi: 10.1002/tox.10112. [DOI] [PubMed] [Google Scholar]
- Bohunická M, Mareš J, Hrouzek P, Urajová P, Lukeš M, Šmarda J, Komárek J, Gaysina LA, and Struneký O. 2015. A combined morphological, ultrastructural, molecular, and biochemical study of the peculiar family Gomontiellaceae (Oscillatoriales) reveals a new cylindrospermopsin-producing clade of cyanobacteria. Journal of Phycology 51:1040–44. doi: 10.1111/jpy.12354. [DOI] [PubMed] [Google Scholar]
- Burns J 2008. Toxic cyanobacteria in Florida waters In Cyanobacterial harmful algal blooms: State of the science and research needs, advances in experimental medicine and biology, ed. Hudnell HK, vol. 619, 127–38. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Byth S 1980. Palm Island mystery disease. The Medical Journal of Australia 2:40–42. [DOI] [PubMed] [Google Scholar]
- Chernoff N, Rogers EH, Zehr RD, Gage MI, Malarkey DE, Bradfield CA, Liu Y, Schmid JE, Jaskot RH, Richards JH, Wood CR, and Rosen MB. 2010. Toxicity and recovery in the pregnant mouse after gestational exposure to the cyanobacterial toxin, cylindrospermopsin. Journal of Applied Toxicology 31:242–54. doi: 10.1002/jat.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff N, Rogers EH, Zehr RD, Gage MI, Travlos GS, Malarkey DE, Brix A, Schmid JE, and Hill D. 2014. The course of toxicity in the pregnant mouse after exposure to the cyanobacterial toxin cylindrospermopsin: Clinical effects, serum chemistries, hematology, and histopathology. Journal of Toxicology and Environmental Health. Part A 77:1040–60. doi: 10.1080/15287394.2014.919838. [DOI] [PubMed] [Google Scholar]
- Chrostek L, Supronowicz L, Panasiuk A, Cylwik B, Gruszewska E, and Flisiak R. 2014. The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clinical and Experimental Medicine 14:417–21. doi: 10.1007/s10238-013-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz AA, Hiskia A, Kaloudis T, Chernoff N, Hill D, Antoniou MG, He X, Loftin K, O’Shea K, Zhao C, Pelaez M, Han C, Lynch TJ, and Dionysiou DD. 2013. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environmental Science. Processes & Impacts 15:1979–2003. doi: 10.1039/c3em00353a. [DOI] [PubMed] [Google Scholar]
- Distressed Watershed Designation Analysis, Grand Lake St. Marys Watershed 2011. Developed by the Ohio Department of Natural Resources. Division of Soil and Water Resources. [Google Scholar]
- Elmore S 2007. Apoptosis: A review of programmed cell death. Toxicologic Pathology 35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer IR, Hardy SJ, Humpage AR, Froscio SM, Tozer GJ, and Hawkins PR. 1999. Hepatic and renal toxicity of the blue-green alga (Cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environmental Toxicology 14:143–50. doi: 10.1002/(ISSN)1522-7278. [DOI] [Google Scholar]
- Falconer IR, and Humpage AR. 2003. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environmental Toxicology 18:94–103. doi: 10.1002/tox.10104. [DOI] [PubMed] [Google Scholar]
- Fastner J, Heinze R, Humpage AR, Mischke U, Eaglesham GK, and Chorus I. 2003. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (Cyanobacteria) isolates. Toxicon 42:313–21. doi: 10.1016/S0041-0101(03)00150-8. [DOI] [PubMed] [Google Scholar]
- Fergusson KM, and Saint CP. 2003. Multiplex PCR assay for Cylindrospermopsis raciborskii and cylindrospermopsin-producing cyanobacteria. Environmental Toxicology 18:120–25. doi: 10.1002/tox.10108. [DOI] [PubMed] [Google Scholar]
- Fessard V, and Bernard C. 2003. Cell alterations but no DNA strand breaks induced in vitro by cylindrospermopsin in CHO K1 cells. Environmental Toxicology 18:353–59. doi: 10.1002/tox.10136. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Saitoh S, Shimamoto K, and Miura T. 2015. Fatty acid-binding protein 4 (FABP4): Pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clinical Medicine Insights: Cardiology 8 (Suppl 3):23–33. doi: 10.4137/CMC.S17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadir MR, Riahin AA, Havaspour A, Nooranipour M, and Habibinejad AA. 2010. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepatitis Monthly 10:285–88. [PMC free article] [PubMed] [Google Scholar]
- Harada KI, Ohtani I, Iwamoto K, Suzuki M, Watanabe MF, Watanabe M, and Terao K. 1994. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 32:73–84. doi: 10.1016/0041-0101(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Hard GC, and Khan KH. 2004. A contemporary overview of chronic progressive nephropathy in the laboratory rat, and its significance for human risk assessment. Toxicologic Pathology 32:171–80. doi: 10.1080/01926230490422574. [DOI] [PubMed] [Google Scholar]
- Hardisy JF, and Brix AE. 2014. Comparative hepatic toxicity: Prechronic/chronic liver toxicity in rodents. Toxicologic Pathology 33:35–40. doi: 10.1080/01926230590522077. [DOI] [PubMed] [Google Scholar]
- Hawkins PR, Chandrasena NR, Jones GJ, Humpage AR, and Falconer IR. 1997. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon 35:341–46. doi: 10.1016/S0041-0101(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Hawkins PR, Runnegar MTC, Jackson ARB, and Falconer IR. 1985. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic supply reservoir. Applied and Environmental Microbiology 50:1292–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Sakai K, Baba H, and Sakai T. 2012. Thrombospodin-1n is a novel negative regulator of liver degeneration after partial hepatectomy via TGF-β1 activation in mice. Hepatology 55:1562–73. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpage AR, and Falconer IR. 2003. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environmental Toxicology 18:94–103. doi: 10.1002/tox.10104. [DOI] [PubMed] [Google Scholar]
- Jelkmann W 2001. The role of the liver in the production of thrombopoietin compared with erythropoietin. European Journal of Gastroenterology & Hepatology 13:791–801. doi: 10.1097/00042737-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Karpisek M, Stejskal D, Kotolova H, Kollar P, Janoutova G, Ochmanova R, Cizek L, Horakova D, Yahia RB, Lichnovska R, and Janout V. 2007. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. European Journal of Clinical Investigation 37:637–42. doi: 10.1111/j.13652362.2007.01835. [DOI] [PubMed] [Google Scholar]
- Kim JW, and Wang XW. 2003. Gene expression profiling of preneoplastic liver disease and liver cancer: A new era for improved early detection and treatment of these deadly diseases? Carcinogenesis 24:363–69. doi: 10.1093/carcin/24.3.363. [DOI] [PubMed] [Google Scholar]
- Kinnear SHW, Duivenvoorden LJ, and Fabbro LD. 2009. Ecotoxicity and bioaccumulation from Cylindrospermopsis raciborskii towards the development of environmental protection guidelines for contaminated water bodies In Lake pollution research progress, ed. Miranda FR and Bernard LM, 81–106. New York, NY: Nova Science. [Google Scholar]
- Kopec AK, and Luyendyk JP. 2014. Coagulation in liver toxicity and disease: Role of hepatocyte tissue factor. Thrombosis Research 133 (Suppl 1):S57–S59. doi: 10.1016/j.thromres.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Carmichael WW, Brittain S, Eaglesham GK, Shaw GR, Liu Y, and Watanabe MM. 2001a. First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Rhaphidiopsis curvata (Cyanobacteria). Journal of Phycology 37:1121–26. doi: 10.1046/j.1529-8817.2001.01075. [DOI] [Google Scholar]
- Li R, Carmichael WW, Brittain S, Eaglesham GK, Shaw GR, Mahakhant A, Noparatnaraporn N, Yongmanitchai W, Kaya K, and Watanabe MM. 2001b. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii Cyanobacteria. Toxicon 39:973–80. doi: 10.1016/S0041-0101(00)00236-1. [DOI] [PubMed] [Google Scholar]
- Loftin KA, Graham JL, Hilborn ED, Lehmann SC, Meyer MT, Dietze JE, and Griffith CB. 2016. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational risks in the EPA National Lakes Assessment 2007. Harmful Algae 56:77–90. doi: 10.1016/j.hal.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Lopez M, Kopec AK, Joshi N, Geddings JE, Cline H, Towery KL, Rockwell CE, Mackman N, and Luyendyk JP. 2014. Fas-induced apoptosis increases hepatocyte tissue factor procoagulant activity in vitro and in vivo. Toxicological Sciences 141:453–434. doi: 10.1093/toxsci/kfu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum G, and Leal-Khouri S. 1989. Significance of low serum urea nitrogen concentrations. Clinical Chemistry 35:639–40. [PubMed] [Google Scholar]
- Makino H, Shimada H, Morioka D, Kunisaki C, Morita T, Matsuyama R, Kubota T, Shimizu D, Ichikawa Y, Tanaka K, Matsuo K, Togo S, Endo I, Nagashima Y, Okazaki Y, and Hayashizaki Y. 2011. Analysis of gene expression profiles in fatal hepatic failure after hepatectomy in mice. The Journal of Surgical Research 169:36–43. doi: 10.1016/j.jss.2009.11.722. [DOI] [PubMed] [Google Scholar]
- Mazmouz R, Chapuis-Hugon F, Mann S, Pichon V, Méjean A, and Ploux O. 2010. Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: Identification of the cyr gene cluster and toxin analysis. Applied and Environmental Microbiology 76:4943–49. doi: 10.1128/AEM.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani I, Moore RE, and Runnegar MTC. 2002. Cylindrospermopsin, a potent hepatotoxin from the bluegreen alga Cylindrospermopsis raciborskii. Journal of the American Chemical Society 114:7941–42. doi: 10.1021/ja00046a067. [DOI] [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). 1998. Test no. 408: Repeated dose 90-day oral toxicity study in rodents. OECD guidelines for testing of chemicals, section 4: Health effects. doi: 10.1787/9789264070707-en. [DOI] [Google Scholar]
- Penaloza CG, Estevez B, Han DM, Norouzi M, Lockshin RA, and Zakeri Z. 2014. Sex-dependent regulation of cytochrome P450 family members Cyp1a1, Cyp2e1, and Cyp7b1 by methylation of DNA. FASEB Journal 28:966–77. doi: 10.1096/fj.13-233320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout-Belissent FM, and McCartney JE. 2010. Interpretation of hematology data in preclinical toxicological studies In Schalm’s veterinary hematology, Sixth edition, ed. Weiss DJ and Wardrop KJ, 78–84. Ames, IA: Wiley-Blackwell. [Google Scholar]
- Poniedziałek B, Rzymski P, and Kokociński M. 2012. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environmental Toxicology and Pharmacology 34:651–60. doi: 10.1016/j.etap.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Preussel K, Stuken A, Wiedner C, Chorus I, and Fastner J. 2006. First report of cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 47:156–62. doi: 10.1016/j.toxicon.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Renaud HJ, Cui JY, Khan M, and Klaassen CD. 2011. Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicological Sciences 124:261–77. doi: 10.1093/toxsci/kfr240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaleb NE, Yang XP, and Carretero OA. 2011. The kallikrein-kinin system as a regulator of cardiovascular and renal function. Comprehensive Physiology 1:971–93. doi: 10.1002/cphy.c100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EH, Zehr RD, Gage MI, Humpage AR, Falconer IR, Marr M, and Chernoff N. 2007. The cyanobacterial toxin, cylindrospermopsin, induces fetal toxicity in the mouse after exposure late in gestation. Toxicon 49:855–64. doi: 10.1016/j.toxicon.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Rosen F, Roberts NR, Budnick LE, and Nichol CA. 1959. Corticosteroids and transaminase activity: The specificity of the glutamic-pyruvic transaminase response. Endocrinology 65:256–64. doi: 10.1210/endo-65-2-256. [DOI] [PubMed] [Google Scholar]
- Saker ML, Nogueira IC, Vasconcelos VM, Neilan BA, Eaglesham GK, and Pereira P. 2003. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicology and Environmental Safety 55:243–50. doi: 10.1016/S0147-6513(02)00043-X. [DOI] [PubMed] [Google Scholar]
- Saker ML, Thomas AD, and Norton JH. 1999. Cattle mortality attributed to the toxic cyanobacterium Cylindrospermopsis raciborskii in an outback region of north Queensland. Environmental Toxicology 14 (1):179–82. doi: 10.1111/j.1751-0813.1998.tb10233.x. [DOI] [Google Scholar]
- SAS/STAT® software v.13.1. Copyright (c) 2013 by SAS Institute Inc., Cary, NC.
- Seawright AA, Nolan CC, Shaw GR, Chiswell RK, Norris RL, Moore MR, and Smith MJ. 1999. The oral toxicity for mice of the tropical cyanobacterium Cylindrospermopsis raciborskii (Woloszynska). Environmental Toxicology 14:135–42. doi:. [DOI] [Google Scholar]
- Seely JC, and Brix A. 2014. Kidney-nephropathy, chronic progressive In National toxicology program nonneoplastic lesion atlas, ed. Cesta MF, Herbert RA, Brix A, Malarkey DE, and Sills RC, 1–4. Research Triangle Park, NC: U.S. Department of Health and Human Services. [Google Scholar]
- Seifert M, McGregor G, Eaglesham G, Wickramasinghe W, and Shaw G. 2007. First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gomont) Speziale and Dyck. Harmful Algae 6:73–80. doi: 10.1016/j.hal.2006.07.001. [DOI] [Google Scholar]
- Shaw GR, Seawright AA, Moore MR, and Lam PK. 2000. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicologic activity. Therapeutic Drug Monitoring 22:88–92. doi: 10.1097/00007691-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Spoof L, Berg KA, Rapala J, Lahti K, Lepistö L, Metcalf JS, Codd GA, and Meriluoto J. 2006. First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environmental Toxicology 21:552–660. doi: 10.1002/tox.20216. [DOI] [PubMed] [Google Scholar]
- Sterling DJ, and Quilliam MA. 2001. First report of the cyanobacterial toxin cylindrospermopsin in New Zealand. Toxicon 39:1219–22. doi: 10.1016/S0041-0101(00)00266-X. [DOI] [PubMed] [Google Scholar]
- Sukenik A, Reisner M, Carmeli S, and Werman M. 2006. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in mice: Long-term exposures to low doses. Environmental Toxicology 21:575–82. doi: 10.1002/tox20220. [DOI] [PubMed] [Google Scholar]
- Teufel A, Itzel T, Erhart W, Brosch M, Wang XY, Kim YO, von Schönfels W, Herrmann AS, Brückner S, Stickel F, Dufour JF, Chavakis T, Hellerbrand C, Spang R, Maass T, Becker T, Schreiber S, Schafmayer C, Schuppan D, and Hampe J. 2016. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology 151:513–25. doi: 10.1053/j.gastro.2016.05.051. [DOI] [PubMed] [Google Scholar]
- Thomas AD, Saker ML, Norton JH, and Olsen RD. 1998. Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. Australian Veterinary Journal 76:592–94. doi: 10.1111/j.1751-0813.1998.tb10233.x. [DOI] [PubMed] [Google Scholar]
- Travlos GS, Hard GC, Betz LJ, and Kissling GE. 2011. Chronic progressive nephropathy in male F344 rats in 90-day toxicity studies: Its occurrence and association with renal tubule tumors in subsequent 2-year bioassays. Toxicologic Pathology 39:381–89. doi: 10.1177/0192623310388432. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (EPA). 2015. Drinking water health advisory for the cyanobacterial toxin cylindrospermopsin EPA-820R15100, Office of Water, Washington, DC. [Google Scholar]
- Wang C, Tao Q, Wang X, Wang X, and Zhang X. 2016. Impact of high-fat diet on liver genes expression profiles in mice model of nonalcoholic fatty liver disease. Environmental Toxicology and Pharmacology 45:52–62. doi: 10.1016/j.etap.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Ward PA 1974. The inflammatory mediators. Annals of the New York Academy of Sciences 221:290–98. doi: 10.1111/j.1749-6632.1974.tb28228.x. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, and O’Connor C. 2006. Growth hormone regulation of sex-dependent liver gene expression. Molecular Endocrinology 20:2613–29. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- White SH, Duivenvoorden LJ, Fabbro LD, and Eaglesham GK. 2006. Influence of intracellular toxin concentrations on cylindrospermopsin bioaccumulation in a fresh water gastropod (Melanoides tuberculata). Toxicon 47:497–509. doi: 10.1016/j.toxicon.2005.12.011. [DOI] [PubMed] [Google Scholar]
- White SH, Duivenvoorden LJ, Fabbro LD, and Eaglesham GK. 2007. Mortality and toxin bioaccumulation in Bufo marinus following exposure to Cylindrospermopsis raciborskii cell extracts and live cultures. Environmental Pollution 147:158–67. doi: 10.1016/j.envpol.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wiedmeyer CE 2018. Evaluation of hepatic function and injury In The clinical chemistry of laboratory animals, ed. Kurtz DM and Travlos GS, 367–406. 3rd ed New York, NY: CRC Press, Taylor & Francis Group. [Google Scholar]
- Xu C, Zhao W, Hao Y, Chang C, and Fan J. 2013. Comparative analysis of gene expression profiles of acute hepatic failure and that of liver regeneration in rat. Gene 528:59–66. doi: 10.1016/j.gene.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Parfett C, Williams A, Yagminas A, Zhou G, Douglas GR, and Yauk CL. 2011. Assessment of subclinical, toxicant-induced hepatic gene expression profiles after low-dose, short-term exposures in mice. Regulatory Toxicology and Pharmacology 60:54–72. doi: 10.1016/j.yrtph.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L 2007. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. Journal of Leukemia Biologic 81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- Zurawell RW, Chen H, Burke JM, and Prepas EE. 2005. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. Journal of Toxicology and Environmental Health. Part B 8:1–37. doi: 10.1080/10937400590889412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


