Abstract
Collagen and hyaluronan are the most abundant components of the extracellular matrix (ECM) and their overexpression in tumors is linked to increased tumor growth and metastasis. These ECM components contribute to a protective tumor microenvironment by supporting a high interstitial fluid pressure and creating a tortuous setting for the convection and diffusion of chemotherapeutic small molecules, antibodies and nanoparticles in the tumor interstitial space. This review focuses on the research efforts to deplete extracellular collagen with collagenases to normalize the tumor microenvironment. Although collagen synthesis inhibitors are in clinical development, the use of collagenases is contentious and clinically untested in cancer patients. Pretreatment of murine tumors with collagenases increased drug uptake and diffusion two to ten-fold. This modest improvement resulted in decreased tumor growth but the benefits of collagenase treatment are confounded by risks of toxicity from collagen breakdown in healthy tissues. In this review, we evaluate the published in vitro and in vivo benefits and limitations of collagenase treatment to improve drug delivery.
Keywords: Extracellular Matrix, Collagenase, Hyaluronidase, Interstitial Fluid Pressure, Drug Penetration, Drug diffusion
Graphical Abstract
1. Introduction
The extracellular matrix (ECM) is one of the key elements within a solid tumor that restricts drug penetration into tumor cells. This aspect of tumor biology was recognized for decades and has been extensively reviewed1–3 as have efforts to remove the ECM with enzymes or collagen/ hyaluronan synthesis inhibitors.4–10 Movement of macromolecular oncotherapies, such as antibodies and nanoparticles, are more sensitive to the ECM than low molecular weight drugs so efforts to modify the ECM will be most beneficial to the therapeutic activity of these agents.
Several tumors including breast, pancreatic, colorectal, ovarian, and lung exhibit a dense ECM where higher collagen or hyaluronan content correlates with poor prognosis.11,12 Cancer associated fibroblasts produce large amounts of ECM components which are associated with tumor promoting activities including: angiogenesis13,14, cell proliferation4, and the creation of ‘highways’ for extravasation of metastatic cells or intravasation of pro-tumor immune cells (Figure 1).4,11 Tumor ECM, which is composed primarily of collagen and hyaluronic acid (HA), contributes to several aspects of the tumor microenvironment that obstructs drug delivery, including: 1. Compression of blood vessels, decreasing blood perfusion and restricting adequate vascular access to portions of the tumor;15 2. Limiting drug trafficking through the tumor matrix interstitium preventing drugs from reaching their cellular targets;16,17 and 3. Maintenance of a high interstitial fluid pressure (IFP) generated by plasma leakage from blood, mechanical stress within the tumor and a lack of proper lymphatic drainage.18 The proliferation of tumor cells within the confined microenvironment further contributes to the mechanical stress within the tumor microenvironment.18 All of these restrict the movement of drugs from the blood into tumor cells (Figure 2).15,17
Figure 1: Elevated ECM enables tumor promoting activities.
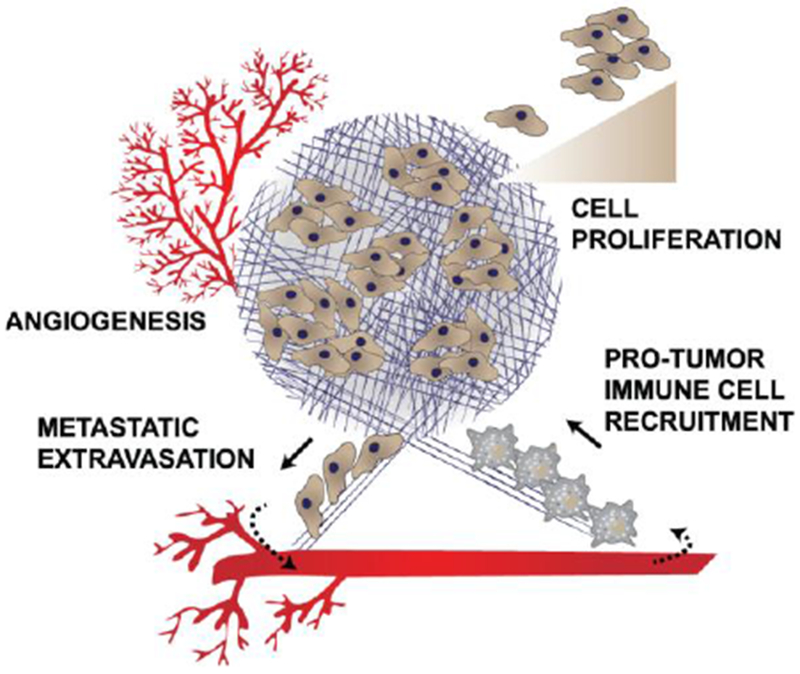
ECM in tumors causes: 1. Increased angiogenesis to supply nutrients for the growing tumor; 2. Growth factor signaling leading to cell proliferation; 3. Recruitment of anti-inflammatory, protumor immune cells; and 4. Creating of a path for metastatic cells to extravasate from the primary tumor.
Figure 2: A dense tumor extracellular matrix limits adequate delivery of drugs.
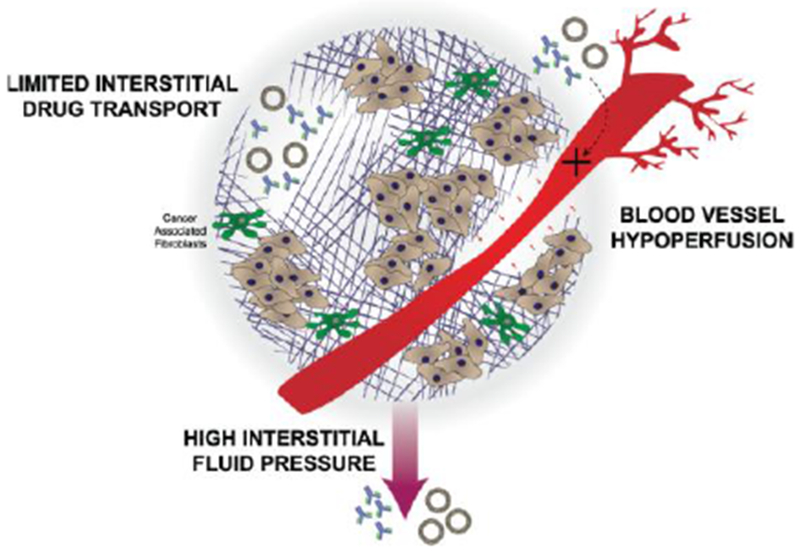
Blood vessels can become compressed preventing drugs from reaching the tumor. Those drugs that are able to enter the tumor microenvironment are faced with a high interstitial fluid pressure and limited interstitial drug transport.
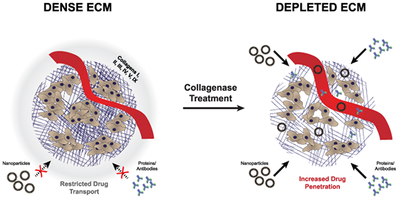
In theory, approaches to decrease the tumor ECM should increase drug penetration and enable higher drug concentrations within tumor cells. There is a subtle aspect to this approach. Complete removal of all biopolymers in the tumor interstitial space might result in collapse of the tumor and a decrease in drug penetration; as such the concept of normalization of the ECM has been suggested to be the goal of such therapies (3,4,10,16,17,18). In this review we highlight key approaches for ECM degradation but focus on the activity of collagenases since these enzymes are less widely used yet can be mechanistically distinguished from the most advanced form of matrix degradation achieved through the reduction of hyaluronic acid (HA).
2. HA Modification
One of the earliest strategies to deplete the tumor ECM is to target hyaluronic acid (HA) either by preventing its production or by digesting what is present in the tumor ECM.19 The utility of HA inhibition has been comprehensively reviewed elsewhere.6,8 Notably, 4-methylumbelliferone (4-MU) has been utilized to prevent HA formation in a variety of tumors by depleting the substrate required for hyaluronan synthesis.20–22 It also downregulates expression of hyaluronan synthase 2 and 3.21 The use of 4-MU illustrates the potential dual effect achieved from inhibiting tumor ECM. It directly decreased tumor growth and metastasis in prostate, liver, breast and skin cancer due to a reduction in tumor HA.6 In addition, treatment of tumored animals with 4-MU enhanced the extravasation of nanoparticles from the blood vessels into the tumor. For example, a combination therapy of a liposome-encapsulated 4-MU prodrug with liposomal doxorubicin decreased tumor volume and increased overall survival compared to liposomal doxorubicin alone in an orthotopic murine tumor.23 The therapeutic enhancement was a result of the improved distribution of liposomal doxorubicin due to a reduction in tumor ECM.23 Oral 4-MU (Cantabiline®), is approved in Europe and Asia as a dietary supplement to increase bile flow. It is a safe drug but has very low oral bioavailability (<3%)8 and its efficacy in oncology remains an open question.
Alternatively, hyaluronidase has been extensively used to enzymatically digest HA.24 Hyaluronidase efficiently degrades large HA polymers comprised of up to 25,000 monosaccharide units down to simple polysaccharides under 10 units.25 Hyaluronidase was explored in oncology clinical trials starting in the 1980s,26 where pretreatment displayed improved outcomes in head and neck, brain, bladder, and gastrointestinal cancer clinical trials.3 Notably, 1 in 3 patients developed immune reactions to the bovine hyaluronidase.26 Detailed assessments of the utility of hyaluronidase for improving interstitial and tumor penetration were previously written. 3,5,7,9,10,27 A pegylated human hyaluronidase (PEGPH20) introduced by Halozyme Therapeutics has advanced to late stage clinical trials. This polymer-modified formulation of hyaluronidase reduces immune recognition and promotes extended circulation, giving the enzyme ample time to accumulate in the tumor ECM.27 PEGPH20 in combination with gemcitabine and nab-paclitaxel (Abraxane®) in a phase II metastatic pancreatic cancer trial showed an increased overall survival in treated patients compared to the control group.28 This is currently the most advanced ECM removal strategy. It will be interesting to learn if the beneficial effects are replicated in the Phase III trial, or in an upcoming gastric cancer trial combining PEGPH20 with Anti-PDL1 immunotherapy.29
3. Collagen Modification
Similar to efforts to remove HA from the tumor matrix, attempts have also been made to limit the synthesis or promote the breakdown of extracellular tumor collagen. Reducing collagen synthesis has been achieved most aggressively through TGF-β inhibition, altering its regulatory role in collagen synthesis.30 The Jain group has spearheaded this through the use of Losartan, an angiotensin II inhibitor, and the use of anti-TGF-β antibodies. Losartan mediates angiotensin II type receptor downregulation of TGF-β activators such as thrombospondin-1 causing a reduction in TGF-β signaling.30 TGF-β is an actively pursued target in oncology so the anti-tumor effects from its inhibition may work in tandem with the benefits of reducing tumor collagen levels. TGF-β inhibition with Losartan led to an increase in drug penetration and subsequent improvement in survival in multiple murine tumors. 30–32 Lorsartan also targets hyaluronan and cancer associated fibroblasts.30,31 Nonetheless, the preclinical success was mirrored in a small phase II clinical trial in pancreatic cancer testing the benefits of Losartan in combination with the FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, oxaliplatin) chemotherapy combination where over 50% of eligible patients identified by radiographic imaging were able to have their tumors resected leaving a margin that had no detectable tumor cells.18,33
Another mode to limit collagen deposition is through inhibition of lysyl oxidase-like-2 (LOXL2) activity. LOXL2 crosslinks collagen to the fibrillar form found in the ECM and an antibody against LOXL2 reduced the number of collagen crosslinks and overall tumor burden in murine xenographs.34 LOXL2 inhibition also caused a decrease in activated fibroblasts and production of growth factors and cytokines involved in cell signaling.34 Thus, as with TGF-β blockade, the anti-tumor effects of LOXL2 inhibition occur in tandem to the benefits of collagen reduction. Despite the preclinical success there is concern that this approach of blocking matrix synthesis may be therapeutically limited to early tumor stages prior to the establishment of the characteristically dense matrix. For instance, a Phase II trial in metastatic pancreatic cancer patients that combined simtuzumab (anti-LOXL2) with gemcitabine failed to improve clinical outcomes likely due to the advanced stage of the cancer.35 Several other antifibrotic drugs have been investigated to reduce tumor ECM including tranilast,36 pirfenidone,37 fasudil,38 and metformin.39 The expanded Losartan trial will be pivotal to understand if inhibition of ECM by repurposing low molecular weight, orally available molecules can translate into the clinic.
The alternative approach to deplete tumor collagen is through the use of collagenases. Collagenases were explored in the 1980s to dissolve excess collagen in patients with severe back pain via collagenase injection into spinal discs.40 The clinical utility of collagenases is validated by the approval of Xiaflex®, a bacterial Clostridium Histolyticum collagenase, for the treatment of Dupuytren’s contracture as an injection against thickening of collagen tissue within the hand. However, the clinical utility of collagenases in improving cancer therapy is much less well established despite collagen being the most abundant tumor ECM component.
In theory, the use of collagenases would be especially attractive for improving drug penetration but unlike hyaluronidase which degrades linear hyaluronan down to short oligosaccharides, collagenases cleave at distinct sites along the collagen triple helix37–40 leaving behind large subunits of approximately 10–95 kDa (Figure 3).41,42 These cleavage products may not effectively separate from collagen fibers resulting in only microscopic local changes to the collagen structure with collagenase therapy.43 Preclinical work to evaluate the use of collagenases in oncology, however, indicated that collagenase treatment improved drug diffusion and penetration in treated tumors (Table 1-Table 6). The challenge for interpreting or comparing results from the studies reviewed in the following sections is that they varied in enzyme exposure (amount and time), route of injection, tumor model, penetration criteria, and type of drug. This makes it difficult to predict the potential clinical utility of injected collagenases in cancer therapy or to evaluate the promise and limitations of the approach.
Figure 3: Cleavage of extracellular collagen and hyaluronan.
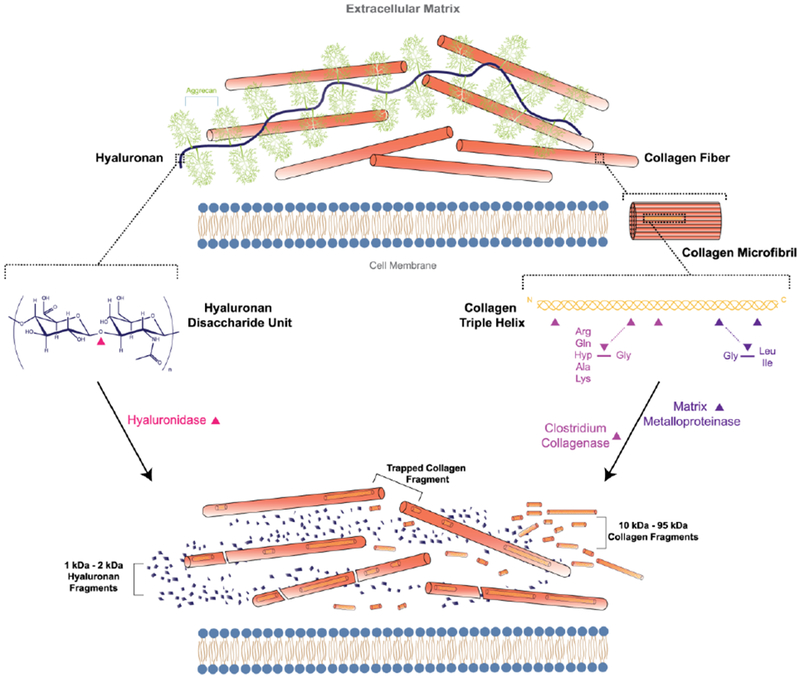
Extracellular hyaluronan and collagen are cleaved by hyaluronidase and collagenase, respectively. Hyaluronidase cleaves at hexosaminidic bonds between β-(1,3)-D-glucuronic acid and β-(1,4)-N-acteyl-D-glycosamine, producing easily cleared oligosaccharide fragments. Bacterial and human collagenases (matrix metalloproteinases-1,3, 8) cleave at defined sites along the collagen triple helix.42 Large collagen cleavage fragments may not effectively be removed from collagen fibers.
Table 1:
In vivo collagenase effect on diffusion
| Particle | Amount of enzyme | Duration of treatment | Route of Injection | Source | Tumor Type | Effect | Change in ECM | Ref |
|---|---|---|---|---|---|---|---|---|
| IgG | 2000 µg | 1 hour | Intratumoral | Bacterial collagenase (Sigma) | Mu89 melonoma (dorsal skinfold chamber) | 1.2X increase in percentage of particles undergoing fast diffusion | N/A | 46 |
| IgG | 30 mg | 24 hour | Intratumoral | Clostridium (biochemical corp) | HSTS sarcoma, U87 (dorsal skinfold chamber) | 2X increase in diffusion coefficient | N/A | 47 |
| 150 kDa Dextran | 100 µg | 24 or 48 hour | Tail vein | Clostridiopeptidase A (Sigma) | OHS osteosarcoma (dorsal skinfold chamber) | 2X increase in diffusion coefficient | N/A | 48 |
| 500 kDa Dextran, Albumin | 2000 µg | 3 hour | Intratumoral | Bacterial collagenase (Sigma) | B16F10 melanoma (shoulder blades) | 2X increase in tumor diffusion coefficient (.5 mm depth). 10X increase in tumor diffusion coefficient (2 mm depth) |
Reduction by western blot | 49 |
Table 6:
MMPs used to improve drug delivery
| Particle | Duration | Source | Tumor Type | Effect | Change in ECM | Ref |
|---|---|---|---|---|---|---|
| HS Vector MGH2 | 7 days | MMP-1 and MMP-8 expressing HSTS26T | HSTS26T Sarcoma | 5–10X increase in viral immunostaining at tumor center 1.3X decrease in tumor growth |
No detectable change in imaged tumor SHG intensity | 101 |
| Adwt300 virus | Up to 50 days | MMP-8 expressing A549 and BxPC-3 cells | A549 alveolar adenocarcinoma BxPC-3 pancreatic cancer |
Increase in viral distribution by viral immunostaining (unquantified) 3X decrease in tumor volume (day 30–50) Complete survival of animals (BxPC-3) |
3X decrease in visible collagen by histology | 102 |
| HSV-eGFP | 21 days | MMP-9 expressing SK-N-AS | SK-N-AS Neuroblastoma | 3X increase in GFP positive cells (Spheroids) Increase tumor vector distribution by viral immunostaining (unquantified) |
N/A | 112 |
4. Diffusion as predictor of drug accessibility in tumors
Diffusion is a key factor that controls delivery of therapeutics into the tumor core. The high IFP and torturous ECM within tumors limits the ability of drugs to freely diffuse.44 Furthermore, the amount of collagen inversely correlates with tumor diffusivity.17 Studies examining the effects of collagenase on drug diffusion focus primarily on protein therapeutics. The findings are especially relevant because protein therapeutics are the most successful class of clinically approved macromolecules in recent years45 and will require improved penetration for the continued clinical success.
Collagenase treatment increased the diffusion of macromolecules in tumors by a modest 2-fold in a majority of studies (Table 1). Alexandrakis et al.46 observed that molecules moving through a Mu89 melanoma tumor undergo both a rapid and slow diffusion. The slower diffusion occurs in parts of the tumor with heightened ECM deposition. Intratumoral (IT) collagenase treatment removed this impediment and caused non-specific IgG antibodies to shift from slow to more rapid diffusion by 1.3-fold within a tumor. Netti et al.47 also investigated the role of ECM components on transport in four different tumors. HST sarcoma and U87 glioblastoma tumors had 2 to 5-fold more collagen relative to tumor mass than the LS174T and MCalV tumors investigated. Higher collagen content in the HST and U87 tumors corresponded with a 2-fold decrease in diffusivity of an IgG antibody. In these tumors, intratumorally injected collagenase doubled the diffusion coefficient for a labeled IgG. Notably, despite the improvement from collagenase treatment, it only restored transport to levels seen in LS174T and MCalV tumors and a larger effect may be required to significantly improve delivery to therapeutically relevant levels.
Eikenes and coworkers48 used an intravenous (IV) injection of collagenase but also obtained a comparable 2-fold increase in diffusion for a 150 kDa FITC-Dextran. Their studies were completed using an osteosarcoma (OHS) tumor model. The change in diffusion following collagenase treatment persisted for at least 48 hours. This is promising because it would allow time for drugs to traffic into tumors and exploit the increased diffusivity to penetrate into the tumor core.
Magzoub et al. is the only study to obtain an increase in diffusion greater that 2-fold.49 They reported a 10-fold increase in diffusion for a 500 kDa dextran at a depth of 2 mm into a tumor. While previous work relied on the more conventional dorsal skinfold window chamber tumor model coupled with fluorescence resonance after photobleaching (FRAP) to determine diffusivity (Table 1), Magzoub designed a fiberoptic probe to intravitally measure diffusion using a more representative melanoma tumor model. Their innovation enabled photobleaching measurements for diffusion at multiple depths within a tumor. Larger macromolecules do not penetrate deeply into dense tissues thus even a minor absolute change in the penetration could lead to a large relative change in penetration, which may be why the magnitude of this scale has not been replicated elsewhere. This is underscored by the fact that at a more superficial depth or with a smaller particle the magnitude is reduced to what was observed in previous studies. At a depth of 0.5 mm, along the tumor periphery, the increase in diffusion for the 500 kDa dextran was only 2-fold. As well, bovine serum albumin whose mass is an order of magnitude less, had an increase in diffusion of only 2-fold at all depths studied. Enhancements in tumor diffusion are overall promising but do not directly address if a 2-fold increase in diffusivity parallels an increase in total drug within tumors.
5. Drug Uptake
5.1. Drug Uptake: Proteins
Interestingly, collagenase treatment increased uptake of protein therapeutics by the roughly the same magnitude as the increase in diffusion. Globally, uptake was enhanced 1.1 to 2-fold in murine tumors (Table 2). Collagenase treatment increased uptake of a radiolabeled, non-specific IgG by 2-fold in Choi et al..50 Choi used an atypical tumor model where they implanted ovarian cancer cells, SKOV-3 or OVCAR-3, in the abdominal wall of rats then affixed a chamber to the peritoneal surface surrounding the exposed tumor. Collagenase solution followed by IgG solution was added to the chamber and allowed to enter the tumor under hydrostatic pressure. Intriguingly, along with boosting total uptake, collagenase treatment also increased the penetration distance of IgG 4-fold beyond the periphery in these ovarian tumors. This added effect points to the ability of collagenases to support delivery of drugs deeper into the tumor core. Still, the mechanism by which this effect occurred is unclear since collagenase failed to significantly lower tumor interstitial pressure or total collagen content.
Table 2:
In vivo collagenase effect on drug uptake
| Particle | Amount of enzyme | Duration of treatment | Route of Injection | Source | Tumor Type | Effect | Change in ECM | Ref |
|---|---|---|---|---|---|---|---|---|
| IgG | 37.5 U/mL | 2 hour | Intraperitoneal Chamber | Bacterial collagenase (Sigma) | SKOV-3, OVCAR-3 ovarian cancer; | 2X increase in IgG concentration in the tumor by radiography 4X increase in penetration distance of IgG beyond the peritoneum |
3X decrease in collagen by OH-Pro (not significant). 30% reduction in IFP (not significant) | 50 |
| IgG (TP-3) | 100 µg | 24 hour | Tail vein | Clostridiopeptidase A (Sigma) | OHS osteosarcoma | 1.10X increase in percent area of imaged tumors exhibiting labeled antibody fluorescence | No detectable change in imaged tumor SHG intensity | 43 |
| IgG (TP-3) | 100 µg | 24 hour | Tail vein | Clostridiopeptidase A (Sigma) | OHS osteosarcoma | 2X increase in antibody fluorescence intensity throughout the tumor | 45% reduction in tumor IFP | 51 |
| Gadopentate-dimeglumine | 10 µg* | 3 hour | Tail vein | Collagenase (Sigma) | NCI-H460 Non Small Cell Lung Cancer | 1.3X Increase in MRI contrast in tumor core | 50% reduction in IFP | 53 |
Total dose determined assuming a 25g mouse
Erikson et al.43 and Eikenes at al.51 used an identical OHS tumor model and enzyme exposure following IV injection but obtained a 1.10-fold and 2-fold increase in labeled TP-3 antibody uptake, respectively. The difference in uptake may be accounted for by changes to TP-3 labeling since the method to attach the fluorophore to the antibody can alter antibody pharmacokinetics.52 Eikenes et al. biotinylated the antibody and quantified it using a fluorescent streptavidin while Erikson directly labeled the TP-3 antibody with a fluorophore. Nonetheless, because TP-3 antibodies are targeted against OHS cells, the combined work confirms that collagenase therapy can enhance uptake of therapeutically relevant antibodies. Furthermore, when looking at changes to the tumor microenvironment, Eikenes measured a 45% reduction in IFP following treatment and a reduction in collagen by histology (not quantified), whereas Erikson and coworkers opted to measure second harmonic generation (SHG) of collagen and found no significant change in total collagen amount following treatment. It is possible that due to the lack of change in the SHG signal, Erikson et al. did not achieve the necessary change in IFP to attain a higher increase in uptake. However, it is uncertain how changes to the tumor microenvironment are related to IFP and which modifications to ECM structure are necessary to improve drug infiltration.
Despite the variety of methods employed to degrade collagen, the degree of the effect is roughly two-fold whether measuring diffusion or uptake. Only two studies reported significant changes in tumor IFP and none demonstrated quantifiable changes to collagen following collagenase treatment so it remains unclear what factors within the tumor microenvironment are good determinants of drug penetration following collagenase treatment (Table 2).
5.2. Drug Uptake: Nanoparticles
Whereas an antibody has a hydrodynamic diameter less than 10 nm, nanoparticles can be upwards of 10-times larger. In drug delivery, nanoparticles can encapsulate drugs often lowering drug toxicity, improving targeting, and extending circulation compared to the free drug.54–56 In cancer, liposomal nanoparticles are the most successful drug carrier but they rely on passive targeting through leaky endothelial fenestrations found in many types of cancer.23,54 This phenomenon known as the enhanced permeability and retention (EPR) effect enables nanoparticles to reach tumor sites. However, nanoparticles are notorious for their entrapment in the tumor periphery and could benefit from removal of the tumor ECM. 57–59
Collagenase treatment modestly increased nanoparticle penetration 1.4 to 2-fold (Table 3). The change in uptake is similar to what was observed with antibodies, however, the large diameter of nanosystems remains a barrier to penetration throughout the tumor. This is highlighted in Erikson et al.43 where uptake of Caelyx™ (liposomal doxorubicin) content in tumors was determined following collagenase treatment. Doxorubicin fluorescence was computed and showed no significant change in payload uptake almost a day following IV injection of collagenase, which can be contrasted with the two-fold increase of the smaller TP-3 antibody under identical conditions. This absence of an increase in liposome uptake from matrix component degradation is also reported by Kolhi and coworkers who inhibited hyaluronan synthase.23
Table 3:
In vivo collagenase effect on nanoparticle uptake
| Particle | Amount of enzyme | Duration of treatment | Route of Injection | Source | Tumor Type | Effect | Change in ECM | Ref |
|---|---|---|---|---|---|---|---|---|
| Caelyx® | 100 µg | 24 hour | Tail vein | Clostridiopeptidase A (Sigma) | OHS osteosarcoma | No change in percent area of imaged tumors exhibiting labeled liposome fluorescence | No detectable change in imaged tumor SHG intensity | 43 |
| Doxil | 25 or 125 µg* | 20 hour | Intratumoral + Intravenous | Collagenase 2 (invitrogen) | SCC-4 tongue sarcoma | 2X increase in radiography intensity of labeled liposomes in extracted tumors at.5% dose (IT) | No detectable change in SHG intensity. 35–40% reduction in IFP (under 2 hours IV) (over 2 hours IT) | 60 |
| Glycol Chitosan Nanoparticles (300 nm) | 11 or 220 U/mg | 72 hour | Intraturmoral | Collagenase Type 1 (Gibco) | A549 alveolar adenocarcinoma | 1.8X increase in fluorescence intensity of labeled nanoparticles in extracted tumors | Visual reduction in tumor collagen by histology (not quantified) | 62 |
| Lipoplexes with CpG plasmid (150 nm) | 300 µg | 4 hour | Intravenous | Clostridium type 1 (Wako Pure Chemical) | LLC lung carcinoma | 1.5X increase in fluorescence intensity of labeled lipoplexes in extracted tumors | 65% reduction in IFP (1 hr after collagenase injection) | 63 |
| Collagenase coated Gold nanoparticles (30 nm) | <300 µg* | 24 hour | Intravenous | Clostridium collagenase (Sigma) | A549 alveolar adenocarcinoma | 1.4X increase in percent injected dose of collagenase labeled gold nanoparticles by ICP-OES quantification | N/A | 66 |
Total dose determined assuming a 25g mouse
Zheng et al.60 also examined uptake of liposomal doxorubicin. Rather than measure fluorescence of free doxorubicin, they encapsulated a radiolabeled probe into their liposomes and measured global scintillation counts. They reported no improvement in uptake following IV injection of collagenase. Interestingly, IT injection of collagenase increased liposomal uptake 2-fold while only using a fraction of the enzymatic dose (scaled based on tumor mass relative to body weight. Although there is no measurement of the amount of enzyme within the tumor, the short serum half-life (6–30 minutes) of collagenases likely resulted in only a small fraction of the enzyme within the tumor 2 hours after IV injection.61 The difference in collagenase concentration between IT and IV injection mirrored the difference in IFP. The IV injection reduced IFP for only 2 hours following collagenase administration, whereas the localized IT injection allowed the reduction in IFP to persist for 24 hours, granting liposomes more time to enter the tumor via the EPR effect.
Lee et al.62 also relied on an IT injection of collagenase and found just under a 2-fold increase in uptake of fluorescently labeled 300 nm glycol chitosan particles. Collagenase treatment amplified nanoparticle intensity in the core of A549 alveolar adenocarcinoma tumors and doubled the signal in individual tumors cells following single-cell isolation. There is no report of changes to tumor IFP, however, these improvements were accompanied by a qualitative reduction in collagen by histology which was not quantified. Kato et al.63 IV injected collagenase prior to labeled 150 nm lipoplexes and found a 1.5-fold enhancement in uptake. They injected lipoplexes 1 hour after collagenase treatment since this window corresponded with a 70% decrease in IFP. It is unclear precisely how long the reduction in IFP persisted – the authors only reported that by 24 hours IFP returned to baseline. Dosing lipoplexes 1 hour after collagenase treatment, when the reduction in IFP was greatest, likely allowed for the significant relative change in uptake compared to studies that dosed nanomaterials 24 hours following IV collagenase treatment (Table 3). The authors did not track pharmacokinetic tumor accumulation of the nanoparticles but this dosing schedule could have mitigated the short serum half-life of lipoplexes (under 1 hour)64 by allowing them to extravasate into the tumor under the reduced IFP.
Another way to exploit transient changes to IFP is to co-deliver the nanoparticle with collagenase. Co-delivery, especially if the enzyme is attached to the drug carrier, may offer improved safety and efficacy through more targeted digestion of collagen at routes of nanoparticle entry.65 Murty et al.66 used this approach and found a 1.4-fold increase in penetration of 30 nm collagenase-coated gold nanoparticles 24 hours after administration. Although, there is no quantification of changes to IFP or tumor collagen, attaching the enzyme to the nanoparticle could extend the circulation time of the enzyme, allowing for a more sustained alteration of the tumor microenvironment. Murty et al. used an A549 alveolar adenocarcinoma model to measure uptake of collagenase-coated gold nanoparticles, the same model used by Lee at al.62 for glycol chitosan nanoparticles (Table 3). Attaching collagenase to the gold nanoparticles promoted increased penetration following IV administration of the combination whereas Lee et al. relied on an IT injection of the enzyme. (Table 3).
In summary, collagenase treatment can modestly improve delivery of nanomedicines and their payload. Tumor IFP appears to be one important criteria for nanoparticle penetration, but its importance compared to other aspects of the tumor microenvironment is still uncertain.
5.3. Drug Uptake: Gene therapy
Gene therapy has long been viewed as a promising tool to combat human cancers.67,68 However, as with other drugs, it is crucial to ensure that nucleic acids get to their intracellular targets especially since many of these products are susceptible to extracellular degradation and rapid elimination. Reduction of tumor collagen can open routes of intracellular delivery for genetic drugs. In mice, improvement in gene delivery with collagenase treatment ranges from 2 to10-fold (Table 4). For instance, Kato et al. used their 150 nm lipoplexes described earlier to deliver luciferase plasmids in a Lewis lung carcinoma tumor model.63 Following intravenous injection of collagenase, lipoplex uptake increased 1.5-fold while luciferase expression was 2-fold higher throughout the tumor. Despite the significant difference in size between the lipid carrier and the plasmid, the similarity in effect suggests that the penetration of the carrier may be critical for the penetration of the contents in gene therapy. The magnitude here also echoes the effect observed for liposomal doxorubicin contents discussed earlier. 43
Table 4:
In vivo collagenase effect on gene delivery
| Particle | Amount of enzyme | Duration of treatment | Route of Injection | Source | Tumor Type | Effect | Change in ECM | Ref |
|---|---|---|---|---|---|---|---|---|
| Lipoplexes with CpG plasmid (150 nm) | 300 µg | 4 hour | Intravenous | Clostridium type 1 (Wako Pure Chemical) | LLC lung carcinoma | 2X increase in luciferase activity in tumor tissue lysate | 65% reduction in IFP | 63 |
| pEGFP-N1, pCMVLuc plasmid | 30 µg | 24 hour | Intratumoral | Collagenase (Roche Diagnostics) | LPB; firbosarcoma | 10X increase in percent of GFP positive areas in imaged tumors (combination treatment, no effect collagenase alone) 10X increase in luciferase activity in tumor lysates |
75% decrease in area density of collagen by histology | 69 |
| Ad-HSV-tk | 10 µg | 24 hour | Intratumoral | Collagenase/dispase (Sigma) | U87, U251 glioblastoma | 6X reduction in tumor volume 50 days post administration of virus with collagenase/dispase | N/A | 70 |
| VP16-GFP HSV | .2 µg | 30 minutes | Intratumoral | Bacterial collagenase (Sigma) | Mu89 melonoma (dorsal skinfold chamber) | 3X increase in viral distribution by fluorescence from injection site | N/A | 72 |
Cemazar et al.69 bypassed the need for a carrier by directly injecting luciferase and green fluorescent protein (GFP) plasmids into various tumors following IT collagenase treatment. They applied electric pulses to aid in gene transfer after administering the plasmid but found no significant changes in gene delivery with collagenase treatment despite a 75% decrease in the area density of collagen. However, treatment with both collagenase and hyaluronidase resulted in a 10-fold increase in percent transfected area of GFP up to 15 days post administration and a 10-fold increase in functional luciferase within the tumor 2 days after administration. The extent of the effect seen here is one of the largest reported following ECM digestion and suggests that administration of two enzymes active against the major extracellular components of the ECM, hyaluronan and collagen, may be needed for large improvements in the gene titers within the tumor.
Kuriyama et al.70 also employed a combination therapy for improved gene therapy in the U-87 glioblastoma mouse model. A collagenase/dispase mixture administered IT improved the delivery of a herpes simplex virus (HSV) coding for thymidine kinase measured by tumor growth. Dispase exhibits extracellular proteolytic activity against both fibronectin and collagen.71 There was no quantification of the change in viral delivery, but collagenase/dispase treatment followed by gene delivery reduced tumor weight and volume by over 80% compared to a PBS control. Even though there is uncertainty around the amount of virus inside the tumor, this study begins to address the downstream question of the anti-tumor efficacy of collagenase pretreatment.
McKee at al.72 computed changes to HSV penetration as well as subsequent effectiveness in limiting tumor growth with collagenase matrix reduction. They found that when a GFP encoding HSV was co-injected IT with collagenase there was a 3-fold increase in viral distribution measured by quantifying by the spread of GFP intensity away from the injection site. In addition, collagenase treated tumors exhibited increased presentation of HSV antigen in tumors two days following treatment and decreased overall tumor growth. There was a 2-fold increase in the time for the tumor to grow ten-times its original size compared to treatment with the oncolytic virus alone. They employed second harmonic generation imaging of collagen to describe the importance of collagen in limiting viral penetration. Viral particle penetration was inversely related to collagen density. The relative change in collagen observed with treatment is, however, not reported.
Notably, these gene therapy studies employed an IT delivery of collagenase and with the exception of the luciferase lipoplexes, the virus or plasmid was also delivered IT (Table 4). It remains unanswered how collagenase treatment would affect gene delivery in an IV setting. Irrespective of the approach taken, further quantification of changes in collagen is needed to understand the potential of matrix removal strategies in solid tumor gene-therapy.
5.4. Drug Uptake: Imaging agent
Beyond augmenting the therapeutic treatment of tumors, collagenases may also aid in tumor diagnosis and evaluation. Hassid et al.53 showed that IV collagenase could increase the concentration of a gadolinium-based MRI contrast agent – GdDTPA in an orthotopic non-small cell murine lung cancer tumor. They measured a 1.33-fold increase in GdDTPA steady state concentration in the tumor core during an IV infusion performed 3 hours following collagenase treatment (Table 2). GdDTPA was also more homogenously distributed throughout the tumor compartment. The authors propose that this increase is likely due to the 65% reduction in IFP observed in treated tumors 5 hours after collagenase therapy. Although the overall effect is modest, using a contrast agent could be a beneficial tool to identify tumors that are most responsive to collagenases, to stratify patients that are more favorable to matrix reduction and to reveal a beneficial window for drug dosing post collagenase therapy.
6. Spheroids
Measuring the effects of collagenase treatment in vivo can be experimentally challenging, timely and costly. The common dorsal skinfold window chamber tumor model, for example, requires complex surgery and specialized equipment to be used effectively.73,74 There is a need for suitable in vitro systems to assess the benefits of collagenases prior to moving in vivo. Traditional 2-D cell culture lacks a suitable architecture and ECM to reliably study the effects of matrix breakdown.75,76 3-D tumor spheroids are the preferred in vitro model for collagenase therapy since they mimic several aspects of the avascular portions of a tumor including epithelial tight junctions, an inhibitory ECM, cellular heterogeneity and a proliferating and quiescent region along with a necrotic core.77–79 The use of tumor spheroids is contentious as a means to evaluate drug delivery systems, however drug penetration studies in tumor spheroids have demonstrated a 2 to 11-fold increase in particle delivery following collagenase treatment which is on the same order as what is observed in vivo (Table 5). However, as with in vivo experiments, spheroid studies seldom report quantifiable changes to tumor collagen with collagenase treatment.
Table 5:
In vitro collagenase effect in tumor spheroids
| Particle | Amount of enzyme | Duration of treatment | Source | Tumor Type | Size | Effect | Ref |
|---|---|---|---|---|---|---|---|
| 2MDa, 150 kDa Dextran | 1–10 mg/mL | 1 hour | Clostridiopeptidase A (Sigma) | OHS osteosarcoma | 150–250 uM | 70% increase in diffusion coefficient 2Mda Dextran. No change for 150 Kda particle (even after 18 hours) | 48 |
| Polystyrene beads | 0.004–1 mg/mL | 5 hour | Clostridium Type I (Sigma) | SiHa cervial carcinoma | 400–500 uM | 12X increase in fluorescence intensity in spheroid core for 40 nm particle; 7X for 20 nm, 3X for 100nm particle; 1.5X for 200 nm; 4X for 100 nm collagenase coated nanoparticles |
80 |
| Collagenase coated albumin nanoparticles | N/A | 96 hour | Clostridium Type I (Sigma) | C8161+ melanoma cocultured and HFF (human foreskin derived fibroblasts) | 300–400 uM | 5% increase in imaged total area of labeled particles in spheroids by 100 nm; 27% for 200 nm | 81 |
| AAVP Bacteriophage vector | 100 µg | 1 hour | Clostridium Type I (Sigma) | 9L Glioma | N/A | 2.6X increase in luciferase expression, 40% decrease in cell viability (67% with combination) | 82 |
Eikenes et al.48 found that despite a 2-fold increase in diffusion of a 150 kDa dextran in vivo, there was no significant change in dextran diffusion in vitro in tumor spheroids. However, Elkenes observed the larger 2 MDa dextran showed an almost 2-fold increase in diffusion following collagenase treatment in the same OHS spheroids. This suggests that spheroids may be useful predictors for the diffusion of larger particles but the diffusion of lower molecular weight drugs may not be sufficiently inhibited by the spheroid ECM to observe the effects of collagenase treatment.
Goodman et al.80 and Cui et al.81 both measured penetration of various nanoparticles as a function of their diameter and observed that collagenase treatment increased penetration in a size-dependent manner. Goodman et al. found a 7, 12, 3, and 1.5-fold increase in fluorescence in the spheroid core for 20, 40, 100 and 200 nm fluorescently labeled polystyrene nanoparticles respectively. The effect of collagenase treatment on particle uptake peaks for 40 nm particles and is much less pronounced for the 100 and 200 nm particles. When collagenase was attached to the surface of the 100 nm particle there was a 4-fold further increase (totaling ~12 fold) in fluorescence signal beyond the spheroid periphery compared to an equivalent particle given with free collagenase.
Cui et al.81 coated albumin nanoparticles with collagenase and found a 6% and 27% increase in penetration for 100 and 200 nm particles respectively. Spheroids were imaged after a 96-hour exposure to collagenase-coated nanoparticles to determine the localization of the nanoparticles. It is important to note that the 200 nm particles exhibited approximately 2-fold greater collagenase activity than the 100 nm particles which could account for the larger percent increase in localization. The authors did not quantify the total fluorescent signal in spheroids which makes it difficult to compare the magnitude of change to what was seen by the nanoparticles in Goodman et al.. However, the authors noted that only the 100 nm collagenase-coated nanoparticles displayed increased signal in the spheroid core (unquantified) which supports the importance of particle size for deep penetration within spheroids. These studies replicate the enhancement in nanoparticle penetration observed in vivo in Murty et al.66 (Table 3) when collagenase is covalently attached to the nanoparticle.
Spheroids were also evaluated as models for determining enhancements in gene therapy following ECM clearance. In 9L rat glioma tumor spheroids, collagenase therapy showed no improvement in delivery of an AAVP tumor-targeting phage carrying a luciferase gene.82 However, the combination of collagenase and hyaluronidase increased luciferase activity almost 3-fold in these tumor spheroids. The magnitude is smaller than what was observed in vivo with luciferase and eGFP plasmids69 but aligns with the finding that digestion of multiple ECM components can provide greater improvement in macromolecular delivery (Table 4).
Although the improvements in drug penetration with spheroids are consistent with those in vivo, their use is not without caveats. The relationship between spheroid collagen content and drug uptake is unclear. Spheroid morphology and response to external stimuli are extremely sensitive to the method used to produce the 3-D cells.78 In addition, spheroid penetration studies are limited to tumor cells which can form spheroids at appropriate sizes to adequately restrict drugs. Spheroids have also not demonstrated the ability to measure changes in penetration of small molecule and protein therapeutics following ECM breakdown. More quantitative studies are needed using standardized methods for growth and viability, and that show that outcomes in spheroids replicate in vivo before spheroids can be considered fully vetted tools for assessing drug penetration.
7.0. Overall outlook; needs and benefits
It is undetermined if the moderate improvements in drug penetration observed in vivo with collagenase treatment in animals will translate to patients. There are major concerns regarding the toxicity of injected collagenase due to the potential for increased tumor metastasis,83–91 degradation of collagen in healthy tissues,62,92 and immune reactions against bacterial collagenases.66 For instance, collagenases that could improve drug delivery when administered at 100–300 µg/mouse resulted in fatalities when injected at greater than 500 µg/mouse.51,63 It is debatable whether the potential efficacy is enough to overcome such a narrow therapeutic window.
7.1. Efficacy: hyaluronidase versus collagenase
Hyaluronidase experiments offer a good benchmark for understanding the translational capacity of collagenases. Several studies compared injected collagenases to injected hyaluronidases to determine which was superior at increasing drug penetration. Despite collagenases only cleaving collagen into large fragments which may become trapped in the ECM (Figure 3), collagenases were generally equal to or better than hyaluronidases. For instance, hyaluronidase treatment reduced diffusion by about 2-fold at all depths of a melanoma tumor for a 2 kDa dextran while collagenase treatment had the opposite effect.49 Collagenase and hyaluronidase both doubled the diffusion coefficient of a larger 150 kDa dextran in an osteosarcoma tumor but the effect did not persist beyond 2 days for hyaluronidase treatment whereas the increased diffusivity remained higher for at least 2 days in collagenase treated tumors.48 For antibodies of similar size, hyaluronidase decreased the portion of labeled IgG antibodies undergoing rapid diffusion within melanoma tumors by 40% whereas collagenase increased it by 20%.46 A similar result revealed that hyaluronidase treatment reduced uptake of a TP-3 antibody by 10% but collagenase treatment enhanced it by 10%.43 Interestingly, hyaluronidase treatment increased uptake of liposomal doxorubicin by 4% despite collagenase treatment having no effect in the same model.43 In another study both collagenase and hyaluronidase increased delivery of glycol chitosan nanoparticles by about 2-fold in an alveolar adenocarcinoma.62 Of note, the authors mentioned that the brightest signal of the labeled particle in tumors came from the collagenase treated group. Additionally, in the tumor microenvironment, hyaluronidase treatment showed no change in IFP and had a minimal effect on IgG transport despite removal of 90% of exposed tumor HA in an ovarian cancer model while collagenase improved uptake 2-fold with only minimal changes to tumor collagen.50 The lack of change in collagen could be due to insufficient clearance of hydrolyzed collagen fragments from fibrillar bundles.
Gene delivery appears to be the one area where collagenase is not clearly superior to hyaluronidase. Collagenase treatment alone was found to not sufficiently improve gene therapy and had to be used in combination with hyaluronidase to enhance uptake.69,82 In multiple in vivo tumors although the combination was superior, hyaluronidase treatment attained a lesser but significant improvement in delivery of a luciferase plasmid in three (SA-1, EAT, B16) of the four (LPB) tumors investigated.69 In 9L glioma tumor spheroids, hyaluronidase alone was no better than collagenase at improving delivery of an AAVP phage.82
Hyaluronidase has been more extensively studied than collagenase and it is generally observed to enhance drug penetration and reduce IFP in tumor models.5 The benefits of hyaluronidase treatment appear to translate clinically, therefore based on the experiments which showed collagenase treatment is a better driver of drug penetration than hyaluronidase treatment, collagenases could have the same if not better clinical efficacy.3,10 Importantly, there are large differences between the ECM turnover of HA and collagen. In parts of the body HA has a rapid half-life of under 2 days while collagen has a significantly slower half-life of several months to upwards of 15 years.9,93 Although tumors can reduce ECM turnover,94 the difference in half-life of collagen and HA could explain why collagen reduction had a more robust effect. Even minor changes in collagen structure could have a pronounced effect on drug penetration since collagen content is unable to recover. In the Phase II PEGPH20 clinical trial hyaluronidase was administered twice per week in a typical 4-week cycle.95 Due to the slower turnover of collagen, long-circulating collagenases could be dosed less frequently reducing the overall medical burden of the therapy. The slow turnover of collagen does, however, increase safety concerns around the effect of removing collagen in healthy tissues.
7.2. Collagenase Safety
Despite a short serum half-life of 6–30 minutes61, intravenous injection of bacterial collagenases at amounts greater than 500 µg (0.5%) is lethal to mice due to abdominal and pulmonary hemorrhaging and necrosis of the lungs.51,63,92 This toxicity is an important concern for injectable collagenase treatments. At a systemic dose below this, the enzyme did not demonstrate lethality, nor drug accumulation in other body compartments while increasing drug penetration in tumors (Table 1- Table 4). Following IT administration of collagenase there was no significant toxicity in any major organ, as well, there was no change in drug biodistribution in tissues outside of the tumor.60,62 The lack of organ toxicity and change in biodistribution also held true after IV collagenase.62 At a dose of 300 µg, there were no observed changes to the delivery of lipoplexes or the luciferase plasmid cargo in any organs outside of the tumor compared to animals without collagenase treatment.63 Collagenases coated nanoparticles reported no injury to the liver or spleen, the major organs for nanoparticle accumulation (as quantified by histology) nor an abnormal elevation in any biochemical blood markers.66
There are concerns that treatment of tumors with collagenase might increase metastases of malignant cells from the parent tumor. This has not been observed. Six weeks following collagenase injection there were no signs of increased metastasis in treated animals.69,72 More data on tolerability in large animals at higher doses (>0.5%) is required to satisfy the safety concerns surrounding collagenase injection. Nevertheless, current data using low collagenase doses shown to be effective, suggests little off-target toxicity and no tumor metastasis.
The immunogenicity of bacterial collagenases is another safety concern. From Xiaflex® human safety trials, a majority of patients exhibited anti-collagenase antibodies by the third injection.61 All patients developed antibodies by the fourth injection. 10–20% of the antibodies were found to be neutralizing which would interfere with subsequent treatments and potentially cross react with human matrix metalloproteinases.61 Perhaps this can be mitigated in the same manner as hyaluronidase by switching to a PEGylated human collagenase.27
8. Ongoing needs to validate matrix reduction therapies to improve cancer treatment
The current literature indicates that collagenases can be both effective and tolerated at low doses in mice but to solidify these findings we must: 1. Better understand the changes to tumor matrix subsequent to collagenase treatment, particularly the clearance of collagen fragments, the rebound of collagen fibers to pre-treatment levels, and the functionality of tumor blood vessels; 2. Use more relevant tumor models which broaden the solid tumor types that are treated with collagenases; and 3. Move beyond unspecific bacterial collagenases. First, few studies quantified changes to tumor collagen content or organization following collagenase treatment. Those that did found little to no effect in collagen that correlates with changes in probe penetration (Table 1–4). Small (12 kDa) degradation fragments of collagen can separate from fibers and be directly measured in plasma whereas larger fragments require additional proteolytic cleavage.93,96 The dose of collagenase used to improve drug infiltration may not allow adequate cleavage of collagen to liberate larger fragments.
For instance, one study validating the use of SHG to measure tumor collagen showed that an IT collagenase dose of 10 mg,97 which is substantially greater than the doses used in a majority of studies measuring drug penetration, was required to observe dramatic changes in tumor SHG intensity. Lower collagenase doses may only exhibit minor changes to tumor collagen. This makes it difficult to know whether a change in total collagen or a change in collagen structure is needed or for how long that change must persist in order to observe increased probe penetration. In addition, there is little understanding of how collagenase treatment reduces tumor mechanical stress and normalizes drug perfusion. Changes in tumor IFP offer a surrogate for the robustness of the effect of collagenase treatment in some instances but a more complete understanding of what happens to collagen, the ECM architecture and tumor pressure after treatment is needed.
Second, the dorsal skinfold window chamber tumor model is the most widely used because it allows for intravital imaging. The problem is that this model is limited to tumors which can grow in that environment and for those which can, they may not exhibit a canonical array of tumor behaviors in that atypical microenvironment.73 As such, traditional orthotropic and spontaneous tumors would serve as better predictors of the benefits of collagenase treatment. In these models, it is essential to demonstrate that an increase in particle penetration causes an increase in overall survival. The few studies that seek to address this show decreases in tumor volume but do not unequivocally show improved in vivo survival. Performing IV over IT injections of collagenase should also be prioritized since the need for defined tumor boundaries for a successful IT injection may limit the tumors that are candidates for matrix reduction therapies.
Third, studies discussed here use bacterial collagenase which exhibits activity on a variety of collagens.98,99 Collagen I is the most abundant in vertebrates but there are 28 different types of collagen identified.11 Several types of collagen including collagen I, II, III, IV, V, IX are implicated in cancer progression.11,12 Therefore, it could be advantageous to tailor the collagenase to the tumor or create a mixture of collagenases that would digest collagens in many different tumor types. In this regard, matrix metalloproteinases (MMP), in particular, should be further explored for ECM degradation because they would limit the immunogenicity from a bacterial collagenase as well as offer a library of proteinases to better match the collagenase to the type of tumor collagen.
8.2. Matrix metalloproteinases
There are 23 different MMPs which target various aspects of the ECM.100 The three collagenases, MMP-1 (human collagenase 1), MMP-8 (human collagenase 2), and MMP-13 (human collagenase 3) and two gelatinases, MMP-2 (gelatinase A), and MMP-9 (gelatinase B) would be the most useful because of their ability to cleave native collagen and its gelatin fragments.100 MMP-1, MMP-8, and MMP-9 were shown to increase delivery of oncolytic viruses when transfected into tumor cells (Table 6). For instance, tumor cells engineered to express MMP-1 or MMP-8 displayed improved penetration of a virus into the tumor core and increased overall viral load 3-fold compared to control tumors in vivo.101 The enhancement in viral delivery was therapeutically efficacious by slowing overall tumor growth. A complimentary study demonstrated complete survival in mice bearing BxPC-3 pancreatic cancer xenographs over 7 weeks when co-injected with an oncolytic virus along with a non-replicating virus carrying an MMP-8 gene. Mice given the oncolytic virus alone exhibited only 20% survival over the same period.102
The primary caveats with MMPs are that they have limited yields when produced recombinantly and certain ones are postulated to be linked to metastasis.83,85–88 Bacterial expression of MMPs requires purification from insoluble inclusion bodies and lengthy refolding protocols.103–105 In addition, bacterial expression is limited to truncated versions of the proteinases, often lacking their collagen binding domain.106,107 Mammalian and insect expression systems can produce full-length MMP protein with a proper glycosylation pattern, but yields generally do not exceed a few milligrams per liter of culture.108 Even with the expression limitations, MMPs may offer a precision in ECM degradation that cannot be achieved with the bacterial collagenases used in previous studies. Production constraints create a bottleneck for advancing the use of MMPs for improved delivery in academic labs but the potential therapeutic upside warrants deriving methods for increased throughput.
MMPs have long been implicated for their role in promoting tumor metastasis by supporting extravasation and subsequent intravasation of tumor cells.109 However, after failure of MMP inhibitors in the clinic,83 there is a greater appreciation for the nuanced roles of MMPs at various stages in tumor progression. For example, MMP-8 has been shown to limit the invasiveness of breast cancer cells.110 In addition, patients with higher MMP-8 expression presented a lower incidence of lymph node metastasis.110 In mice, lung tumors engineered to overexpress MMP-8 showed no evidence of increased metastasis or tumor progression.102 MMP-3, 9, and 12 have also exhibited antitumor activities such as reduced tumor cell migration and invasiveness in murine cancers.111,112 Furthermore, considering that bacterial collagenase experiments showed no tumor metastases despite their broad activity for various collagens, MMP therapies may be much safer than the current wisdom would indicate.60,62,66
8.3. Combination therapies
In addition to broadening the collagenase repertoire, combinations of enzyme digestion treatments should be further studied with the caveat that total depletion of all biopolymers in the ECM may be counterproductive. Collagenase and hyaluronidase combinations have already allowed for improvements in gene therapy.69,82 Additional combinations of ECM degrading enzymes could allow for a more complete clearance of the inhibitory tumor ECM. Synergies could be achieved using collagenases and mammalian gelatinases since the gelatinases can breakdown large collagen fragments produced when collagen is enzymatically cleaved,93 enabling improved clearance of collagen and further opening the ECM to drugs. Selecting the appropriate enzyme or combination may allow for quantifiable changes in total collagen which have alluded researchers thus far. Combinations involving collagenases with collagen30 or hyaluronan synthesis inhibitors22,23,31 should also be attempted to further improve ECM drug penetration into tumors. Intravenous collagenases could first be used to clear the tumor ECM followed by collagen synthesis inhibitors to sustain this effect for an extended period, starving the tumor of the benefits of a dense ECM while allowing more drug to enter.
8.4. Additional Therapeutic Areas
Although the focus of this review is on the benefits of collagenase treatment in cancer, collagenases could be beneficial in other diseases which feature abundant collagen deposition. These areas could offer insights involving pharmacokinetics, pharmacodynamics and biodistribution of collagenases which could inform tumor studies. The benefits of collagenases in disease are reviewed elsewhere.113,114 In short, collagenases could be most beneficial in orthopedics, wound healing, and fibrosis. With early collagenase trials in patients to treat back pain and the approval of Xialfex®, there is a wealth of clinical data available on how collagenases behave near joints and bones.115,116 In homeostatic repair, collagenases are able to clear debris from necrotic tissues and promote dermal cell migration.117 As such collagenases are used clinically to enhance healing in burn injury.118 Collagenases were also explored in fibrosis to clear extracellular deposits. In a rat model of liver cirrhosis, MMP-8 and MMP-13 transfected into hepatic cells showed a significant reduction in liver cirrhosis compared to control.119,120
10. Conclusion
The increased exploration using macromolecules, nanoparticles, and viruses to treat cancer motivates the need for ways to improve penetration of these agents into tumors. The data for injected collagenase treatment suggest it to be relatively safe and modestly efficacious (Table 1–Table 6). However, before that potential can be fully realized, investigators will have to explicate how collagenase treatment affects the amount and structure of tumor collagen and how changes in collagen relate to alterations in the tumor microenvironment. This information could then be used to understand precisely what changes to the ECM are needed to enhance drug delivery as well as enable an appropriate selection of enzyme combinations and matrix metalloproteinases subtypes to maximize drug uptake in tumors. Making these rational improvements could digest a path forward for matrix reduction therapy as a clinical modality to enhance the delivery of drugs, macromolecules, and nanoparticles into solid tumors.
Acknowledgement:
This work was supported by NIH T32 GM007175 and the National Science Foundation Graduate Research Fellowship Program. The authors would like to thank Katherina Chua for helpful discussions.
References
- (1).Khawar IA; Kim JH; Kuh H-J Improving Drug Delivery to Solid Tumors: Priming the Tumor Microenvironment. J. Control. Release 2015, 201, 78–89. [DOI] [PubMed] [Google Scholar]
- (2).Sagnella SM; McCarroll JA; Kavallaris M Drug Delivery: Beyond Active Tumour Targeting. Nanomedicine Nanotechnology, Biol. Med. 2014, 10 (6), 1131–1137. [DOI] [PubMed] [Google Scholar]
- (3).Whatcott CJ; Han H; Posner RG; Hostetter G; Von Hoff DD Targeting the Tumor Microenvironment in Cancer: Why Hyaluronidase Deserves a Second Look. Cancer Discov. 2011, 1 (4), 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bonnans C; Chou J; Werb Z Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15 (12), 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Buhren BA; Schrumpf H; Hoff NP; Bölke E; Hilton S; Gerber PA Hyaluronidase: From Clinical Applications to Molecular and Cellular Mechanisms. Eur. J. Med. Res. 2016, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lokeshwar VB; Mirza S; Jordan A Targeting Hyaluronic Acid Family for Cancer Chemoprevention and Therapy. Adv. Cancer Res. 2014, 123, 35–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).McAtee CO; Barycki JJ; Simpson MA Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. Adv. Cancer Res. 2014, 123 (402), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nagy N; Kuipers HF; Frymoyer AR; Ishak HD; Bollyky JB; Wight TN; Bollyky PL 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015, 6 (March), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Scodeller P Hyaluronidase and Other Extracellular Matrix Degrading Enzymes for Cancer Therapy: New Uses and Nano-Formulations. J. Carcinog. Mutagen. 2014, 5 (4), 2–6. [Google Scholar]
- (10).Shepard HM Breaching the Castle Walls: Hyaluronan Depletion as a Therapeutic Approach to Cancer Therapy. Front. Oncol. 2015, 5 (August), 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Fang M; Yuan J; Peng C; Li Y Collagen as a Double-Edged Sword in Tumor Progression. Tumor Biol. 2014, 35 (4), 2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Karousou E; D’Angelo ML; Kouvidi K; Vigetti D; Viola M; Nikitovic D; De Luca G; Passi A Collagen VI and Hyaluronan: The Common Role in Breast Cancer. Biomed Res. Int. 2014, 2014, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Xiao W; Ruan S; Yu W; Wang R; Hu C; Liu R; Gao H Normalizing Tumor Vessels to Increase the Enzyme-Induced Retention and Targeting of Gold Nanoparticle for Breast Cancer Imaging and Treatment. Mol. Pharm. 2017, 14 (10), 3489–3498. [DOI] [PubMed] [Google Scholar]
- (14).Venning FA; Wullkopf L; Erler JT Targeting ECM Disrupts Cancer Progression. Front. Oncol. 2015, 5 (October), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gkretsi V; Stylianou A; Papageorgis P; Polydorou C; Stylianopoulos T Remodeling Components of the Tumor Microenvironment to Enhance Cancer Therapy. Front. Oncol. 2015, 5 (October). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Jain RK Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. 2013, 31 (17), 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Jain R; Stylianopoulos T Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7 (11), 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Stylianopoulos T; Munn LL; Jain RK Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends in Cancer 2018, 4 (4), 292–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Beckenlehner K; Bannke S; Sprub T; Bernhardt G; Schnenberger H; Schiess W Hyaluronidase Enhances the Activity of Adriamycin in Breast Cancer Models in Vitro and in Vivo. J. Cancer Res. Clin. Oncol. 1992, 118 (8), 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Morohashi H; Kon A; Nakai M; Yamaguchi M; Kakizaki I; Yoshihara S; Sasaki M; Takagaki K Study of Hyaluronan Synthase Inhibitor, 4-Methylumbelliferone Derivatives on Human Pancreatic Cancer Cell (KP1-NL). Biochem. Biophys. Res. Commun. 2006, 345 (4), 1454–1459. [DOI] [PubMed] [Google Scholar]
- (21).Saito T; Tamura D; Nakamura T; Makita Y; Ariyama H; Komiyama K; Oshihara T; Asano R 4-Methylumbelliferone Leads to Growth Arrest and Apoptosis in Canine Mammary Tumor Cells. Oncol. Rep. 2013, 29 (1), 335–342. [DOI] [PubMed] [Google Scholar]
- (22).Nakazawa H; Yoshihara S; Kudo D; Morohashi H; Kakizaki I; Kon A; Takagaki K; Sasaki M 4-Methylumbelliferone, a Hyaluronan Synthase Suppressor, Enhances the Anticancer Activity of Gemcitabine in Human Pancreatic Cancer Cells. Cancer Chemother. Pharmacol. 2006, 57 (2), 165–170. [DOI] [PubMed] [Google Scholar]
- (23).Kohli AG; Kivimäe S; Tiffany MR; Szoka FC Improving the Distribution of Doxil® in the Tumor Matrix by Depletion of Tumor Hyaluronan. J. Control. Release 2014, 191 (1–2), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Plattt VM; Szoka FC Anticancer Therapeutics: Targeting Macromolecules and Nanocarriers to Hyaluronan or CD44, a Hyaluronan Receptor. Mol. Pharm. 2008, 5 (4), 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sugahara K; Yamada S; Sugiura M; Takeda K; Yuen R; Khoo HE; Poh CH Identification of the Reaction Products of the Purified Hyaluronidase from Stonefish (Synanceja Horrida) Venom. Biochem. J. 1992, 283 ( Pt 1, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Baumgartner G; Gomar-Höss C; Sakr L; Ulsperger E; Wogritsch C The Impact of Extracellular Matrix on the Chemoresistance of Solid Tumors – Experimental and Clinical Results of Hyaluronidase as Additive to Cytostatic Chemotherapy. Cancer Lett. 1998, 131 (1), 85–99. [PubMed] [Google Scholar]
- (27).Bookbinder LH; Hofer A; Haller MF; Zepeda ML; Keller GA; Lim JE; Edgington TS; Shepard HM; Patton JS; Frost GI A Recombinant Human Enzyme for Enhanced Interstitial Transport of Therapeutics. J. Control. Release 2006, 114 (2), 230–241. [DOI] [PubMed] [Google Scholar]
- (28).Hingorani S; Bullock A; Seery T; Zheng L; Sigal D; Ritch P; Braiteh F; Zalupski M; Bahary N; Harris W; Pu J; Aldrich C; Khelifa S; Wu W; Baranda J; Jiang P; Hendifar A PEGPH20 Improves Pfs in Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Randomized Phase 2 Study in Combination with Nab-Paclitaxel/gemcitabine. Ann. Oncol. 2017, 28 (suppl_3), mdx262.002-mdx262.002. [Google Scholar]
- (29).Halozyme. Halozyme Initiates Clinical Trial Of PEGPH20 With Anti-PDL1 Immunotherapy In Cholangiocarcinoma And Gallbladder Cancer Patients. Press Release; 2018. [Google Scholar]
- (30).Diop-Frimpong B; Chauhan VP; Krane S; Boucher Y; Jain RK Losartan Inhibits Collagen I Synthesis and Improves the Distribution and Efficacy of Nanotherapeutics in Tumors. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (7), 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chauhan VP; Martin JD; Liu H; Lacorre DA; Jain SR; Kozin SV; Stylianopoulos T; Mousa AS; Han X; Adstamongkonkul P; Popović Z; Huang P; Bawendi MG; Boucher Y; Jain RK Angiotensin Inhibition Enhances Drug Delivery and Potentiates Chemotherapy by Decompressing Tumour Blood Vessels. Nat. Commun. 2013, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Liu J; Liao S; Diop-Frimpong B; Chen W; Goel S; Naxerova K; Ancukiewicz M; Boucher Y; Jain RK; Xu L TGF- Blockade Improves the Distribution and Efficacy of Therapeutics in Breast Carcinoma by Normalizing the Tumor Stroma. Proc. Natl. Acad. Sci. 2012, 109 (41), 16618–16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Murphy JE; Wo JY-L; Ferrone C; Jiang W; Yeap BY; Blaszkowsky LS; Kwak EL; Allen JN; Clark JW; Faris JE; Zhu AX; Goyal L; Mamon HJ; Lillemoe KD; Ryan DP; DeLaney TF; Fernandez-del Castillo C; Boucher Y; Hong TS TGF-B1 Inhibition with Losartan in Combination with FOLFIRINOX (F-NOX) in Locally Advanced Pancreatic Cancer (LAPC): Preliminary Feasibility and R0 Resection Rates from a Prospective Phase II Study. J. Clin. Oncol. 2017, 35 (4_suppl), 386–386. [Google Scholar]
- (34).Barry-hamilton V; Spangler R; Marshall D; Mccauley S; Rodriguez HM; Oyasu M; Mikels A; Vaysberg M; Ghermazien H; Wai C; Garcia CA; Velayo AC; Jorgensen B; Biermann D; Tsai D; Green J; Zaffryar-eilot S; Holzer A; Ogg S; Thai D; Neufeld G; Vlasselaer P Van; Smith, V. Allosteric Inhibition of Lysyl Oxidase – like-2 Impedes the Development of a Pathologic Microenvironment. Nat. Med. 2010, 16 (9), 1005–1013. [DOI] [PubMed] [Google Scholar]
- (35).Benson AB; Wainberg ZA; Hecht JR; Vyushkov D; Dong H; Bendell J; Kudrik F A Phase II Randomized, Double‐Blind, Placebo‐Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First‐Line Treatment of Pancreatic Adenocarcinoma. Oncologist 2017, 22 (3), 241–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Papageorgis P; Polydorou C; Mpekris F; Voutouri C; Agathokleous E; Kapnissi-Christodoulou CP; Stylianopoulos T Tranilast-Induced Stress Alleviation in Solid Tumors Improves the Efficacy of Chemo- and Nanotherapeutics in a Size-Independent Manner. Sci. Rep. 2017, 7 (September 2016), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Polydorou C; Mpekris F; Papageorgis P; Voutouri C; Stylianopoulos T Pirfenidone Normalizes the Tumor Microenvironment to Improve Chemotherapy. Oncotarget 2017, 8 (15), 24506–24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Vennin C; Chin VT; Warren SC; Lucas MC; Herrmann D; Magenau A; Melenec P; Walters SN; Del Monte-Nieto G; Conway JRW; Nobis M; Allam AH; McCloy RA; Currey N; Pinese M; Boulghourjian A; Zaratzian A; Adam AAS; Heu C; Nagrial AM; Chou A; Steinmann A; Drury A; Froio D; Giry-Laterriere M; Harris NLE; Phan T; Jain R; Weninger W; McGhee EJ; Whan R; Johns AL; Samra JS; Chantrill L; Gill AJ; Kohonen-Corish M; Harvey RP; Biankin AV; Evans TRJ; Anderson KI; Grey ST; Ormandy CJ; Gallego-Ortega D; Wang Y; Samuel MS; Sansom OJ; Burgess A; Cox TR; Morton JP; Pajic M; Timpson P Transient Tissue Priming via ROCK Inhibition Uncouples Pancreatic Cancer Progression, Sensitivity to Chemotherapy, and Metastasis. Sci. Transl. Med. 2017, 9 (384). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Incio J; Suboj P; Chin SM; Vardam-Kaur T; Liu H; Hato T; Babykutty S; Chen I; Deshpande V; Jain RK; Fukumura D Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PLoS One 2015, 10 (12), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sussman BJ; Bromley JW; Gomez JC Injection of Collagenase in the Treatment of Herniated Lumbar Disk: Initial Clinical Report. JAMA J. Am. Med. Assoc. 1981, 245 (7), 730–732. [PubMed] [Google Scholar]
- (41).Kafienah W; Buttle DJ; Burnett D; Hollander AP Cleavage of Native Type I Collagen by Human Neutrophil Elastase. Biochem. J. 1998, 330 ( Pt 2, 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).French MF; Bhown A; Van Wart HE Identification of Clostridium Histolyticum Collagenase Hyperreactive Sites in Type I, II, and III Collagens: Lack of Correlation with Local Triple Helical Stability. J. Protein Chem. 1992, 11 (1), 83–97. [DOI] [PubMed] [Google Scholar]
- (43).Erikson A; Tufto I; Bjønnum AB; Bruland ØS; Davies CDL The Impact of Enzymatic Degradation on the Uptake of Differently Sized Therapeutic Molecules. Anticancer Res. 2008, 28 (6 A), 3557–3566. [PubMed] [Google Scholar]
- (44).Baronzio G; Parmar G; Baronzio M Overview of Methods for Overcoming Hindrance to Drug Delivery to Tumors, with Special Attention to Tumor Interstitial Fluid. Front. Oncol. 2015, 5 (July), 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Venditto VJ; Szoka FC Cancer Nanomedicines: So Many Papers and so Few Drugs! Adv. Drug Deliv. Rev. 2013, 65 (1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Alexandrakis G; Brown EB; Tong RT; McKee TD; Campbell RB; Boucher Y; Jain RK Two-Photon Fluorescence Correlation Microscopy Reveals the Two-Phase Nature of Transport in Tumors. Nat. Med. 2004, 10 (2), 203–207. [DOI] [PubMed] [Google Scholar]
- (47).Netti PA; Berk DA; Swartz MA; Grodzinsky AJ; Jain RK Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors. 2000, 60, 2497–2503. [PubMed] [Google Scholar]
- (48).Eikenes L; Tufto I; Schnell E. a.; Bjørkøy A.; Davies CDL. Effect of Collagenase and Hyaluronidase on Free and Anomalous Diffusion in Multicellular Spheroids and Xenografts. Anticancer Res. 2010, 30 (2), 359–368. [PubMed] [Google Scholar]
- (49).Magzoub M; Jin S; Verkman AS Enhanced Macromolecule Diffusion Deep in Tumors after Enzymatic Digestion of Extracellular Matrix Collagen and Its Associated Proteoglycan Decorin. FASEB J. 2007, 22 (1), 276–284. [DOI] [PubMed] [Google Scholar]
- (50).Choi J; Credit K; Henderson K; Deverkadra R; He Z; Wiig H; Vanpelt H; Flessner MF Intraperitoneal Immunotherapy for Metastatic Ovarian Carcinoma: Resistance of Intratumoral Collagen to Antibody Penetration. Clin. Cancer Res. 2006, 12 (6), 1906–1912. [DOI] [PubMed] [Google Scholar]
- (51).Eikenes L; Bruland ØS; Brekken C; De Lange Davies C Collagenase Increases the Transcapillary Pressure Gradient and Improves the Uptake and Distribution of Monoclonal Antibodies in Human Osteosarcoma Xenografts. Cancer Res. 2004, 64 (14), 4768–4773. [DOI] [PubMed] [Google Scholar]
- (52).Weiner GJ Building Better Monoclonal Antibody-Based Therapeutics. Nat. Publ. Gr. 2015, 15 (6), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hassid Y; Eyal E; Margalit R; Furman-Haran E; Degani H Non-Invasive Imaging of Barriers to Drug Delivery in Tumors. Microvasc. Res. 2008, 76 (2), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kohli AG; Kierstead PH; Venditto VJ; Walsh CL; Szoka FC Designer Lipids for Drug Delivery: From Heads to Tails. J. Control. Release 2014, 190, 274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Allen TM; Cullis PR Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65 (1), 36–48. [DOI] [PubMed] [Google Scholar]
- (56).Yan Z; Yang Y; Wei X; Zhong J; Wei D; Liu L; Xie C; Wang F; Zhang L; Lu W; He D Tumor-Penetrating Peptide Mediation: An Effective Strategy for Improving the Transport of Liposomes in Tumor Tissue. Mol. Pharm. 2014, 11 (1), 218–225. [DOI] [PubMed] [Google Scholar]
- (57).Barua S; Mitragotri S Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9 (2), 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Appelbe OK; Zhang Q; Pelizzari CA; Weichselbaum RR; Kron SJ Image-Guided Radiotherapy Targets Macromolecules through Altering the Tumor Microenvironment. Mol. Pharm. 2016, 13 (10), 3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Yu FTH; Chen X; Wang J; Qin B; Villanueva FS Low Intensity Ultrasound Mediated Liposomal Doxorubicin Delivery Using Polymer Microbubbles. Mol. Pharm. 2016, 13 (1), 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zheng X; Goins BA; Cameron IL; Fullerton GD Ultrasound-Guided Intratumoral Administration of Collagenase-2 Improved Liposome Drug Accumulation in Solid Tumor Xenografts. 2011, 173–182. [DOI] [PubMed] [Google Scholar]
- (61).Wharf C; Kingdom U; European Medicines Agency. Assessment Report Xiapex. Report 2011. [Google Scholar]
- (62).Lee S; Han H; Koo H; Na JH; Yoon HY; Lee KE; Lee H; Kim H; Kwon IC; Kim K Extracellular Matrix Remodeling in Vivo for Enhancing Tumor-Targeting Efficiency of Nanoparticle Drug Carriers Using the Pulsed High Intensity Focused Ultrasound. J. Control. Release 2017, 263, 68–78. [DOI] [PubMed] [Google Scholar]
- (63).Kato M; Hattori Y; Kubo M; Maitani Y Collagenase-1 Injection Improved Tumor Distribution and Gene Expression of Cationic Lipoplex. Int. J. Pharm. 2012, 423 (2), 428–434. [DOI] [PubMed] [Google Scholar]
- (64).Uyechi LS; Gagné L; Thurston G; Szoka FC Mechanism of Lipoplex Gene Delivery in Mouse Lung: Binding and Internalization of Fluorescent Lipid and DNA Components. Gene Ther. 2001, 8 (11), 828–836. [DOI] [PubMed] [Google Scholar]
- (65).Villegas MR; Baeza A; Vallet-Regí M Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues. ACS Appl. Mater. Interfaces 2015, 7 (43), 24075–24081. [DOI] [PubMed] [Google Scholar]
- (66).Murty S; Gilliland T; Qiao P; Tabtieng T; Higbee E; Zaki A. Al; Puré E.; Tsourkas A. Nanoparticles Functionalized with Collagenase Exhibit Improved Tumor Accumulation in a Murine Xenograft Model. 2014, 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Dachs GU; Dougherty GJ; Stratford IJ; Chaplin DJ Targeting Gene Therapy to Cancer: A Review. Oncol. Res. 1997, 9 (6–7), 313–325. [PubMed] [Google Scholar]
- (68).Lo HW; Day CP; Hung MC Cancer-Specific Gene Therapy. Adv. Genet. 2005, 54, 233–255. [DOI] [PubMed] [Google Scholar]
- (69).Cemazar M; Golzio M; Sersa G; Escoffre J; Coer A; Vidic S; Teissie J Hyaluronidase and Collagenase Increase the Transfection Efficiency of Gene Electrotransfer in Various Murine Tumors. Hum. Gene Ther. 2012, 23 (1), 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kuriyama N; Kuriyama H; Julin CM; Lamborn KR; Israel M a. Protease Pretreatment Increases the Efficacy of Adenovirus-Mediated Gene Therapy for the Treatment of an Experimental Glioblastoma Model. Cancer Res. 2001, 61 (5), 1805–1809. [PubMed] [Google Scholar]
- (71).Stenn KS; Link R; Moellmann G; Madri J; Kuklinska E Dispase, a Neutral Protease from Bacillus Polymyxa, Is a Powerful Fibronectinase and Type IV Collagenase. J. Invest. Dermatol. 1989, 93 (2), 287–290. [DOI] [PubMed] [Google Scholar]
- (72).McKee TD; Grandi P; Mok W; Alexandrakis G; Insin N; Zimmer JP; Bawendi MG; Boucher Y; Breakefield XO; Jain RK Degradation of Fibrillar Collagen in a Human Melanoma Xenograft Improves the Efficacy of an Oncolytic Herpes Simplex Virus Vector. Cancer Res. 2006, 66 (5), 2509–2513. [DOI] [PubMed] [Google Scholar]
- (73).Maeda A; DaCosta RS Optimization of the Dorsal Skinfold Window Chamber Model and Multi-Parametric Characterization of Tumor-Associated Vasculature. IntraVital 2014, 3 (1), e27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Schreiter Jeannine, Meyer Sophia, Schmidt Christian, Schulz Ronny M., S. L. Dorsal Skinfold Chamber Models in Mice. GMS Interdiscip. Plast. Reconstr. Surg. 2017, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Seo BR; DelNero P; Fischbach C In Vitro Models of Tumor Vessels and Matrix: Engineering Approaches to Investigate Transport Limitations and Drug Delivery in Cancer. Adv. Drug Deliv. Rev. 2014, 69–70 (6), 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Huang K; Boerhan R; Liu C; Jiang G Nanoparticles Penetrate into the Multicellular Spheroid-on-Chip: Effect of Surface Charge, Protein Corona, and Exterior Flow. Mol. Pharm. 2017, 14 (12), 4618–4627. [DOI] [PubMed] [Google Scholar]
- (77).Fracasso G; Colombatti M Effect of Therapeutic Macromolecules in Spheroids. Crit. Rev. Oncol. Hematol. 2000, 36 (2–3), 159–178. [DOI] [PubMed] [Google Scholar]
- (78).Zanoni M; Piccinini F; Arienti C; Zamagni A; Santi S; Polico R; Bevilacqua A; Tesei A 3D Tumor Spheroid Models for in Vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6 (August 2015), 19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Takechi-Haraya Y; Goda Y; Sakai-Kato K Control of Liposomal Penetration into Three-Dimensional Multicellular Tumor Spheroids by Modulating Liposomal Membrane Rigidity. Mol. Pharm. 2017, 14 (6), 2158–2165. [DOI] [PubMed] [Google Scholar]
- (80).Goodman TT; Olive PL; Pun SH Increased Nanoparticle Penetration in Collagenase-Treated Multicellular Spheroids. Int. J. Nanomedicine 2007, 2 (2), 265–274. [PMC free article] [PubMed] [Google Scholar]
- (81).Cui M; Naczynski DJ; Zevon M; Griffith CK; Sheihet L; Poventud-Fuentes I; Chen S; Roth CM; Moghe PV Multifunctional Albumin Nanoparticles As Combination Drug Carriers for Intra-Tumoral Chemotherapy. Adv. Healthc. Mater. 2013, 2 (9), 1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Yata T; Lee ELQ; Suwan K; Syed N; Asavarut P; Hajitou A Modulation of Extracellular Matrix in Cancer Is Associated with Enhanced Tumor Cell Targeting by Bacteriophage Vectors. Mol. Cancer 2015, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Amar S; Fields GB Potential Clinical Implications of Recent Matrix Metalloproteinase Inhibitor Design Strategies. Expert Rev. Proteomics 2015, 12 (5), 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Cao J; Chiarelli C; Kozarekar P; Adler HL Membrane Type 1-Matrix Metalloproteinase Promotes Human Prostate Cancer Invasion and Metastasis. Thromb. Haemost. 2005, 94, 770–778. [DOI] [PubMed] [Google Scholar]
- (85).Foley CJ; Luo C; O’Callaghan K; Hinds PW; Covic L; Kuliopulos A Matrix Metalloprotease-1a Promotes Tumorigenesis and Metastasis. J. Biol. Chem. 2012, 287 (29), 24330–24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kudo Y; Iizuka S; Yoshida M; Tsunematsu T; Kondo T; Subarnbhesaj A; Deraz EM; Siriwardena SBSM; Tahara H; Ishimaru N; Ogawa I; Takata T Matrix Metalloproteinase-13 (MMP-13) Directly and Indirectly Promotes Tumor Angiogenesis. J. Biol. Chem. 2012, 287 (46), 38716–38728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Pulukuri SMK and Rao JS Matrix Metalloproteinase-1 Promotes Prostate Tumor Growth and Metastasis. Int. J. Oncol. 2008, 32 (4), 757–765. [PMC free article] [PubMed] [Google Scholar]
- (88).Sarkar SK; Marmer B; Goldberg G; Neuman KC Single-Molecule Tracking of Collagenase on Native Type I Collagen Fibrils Reveals Degradation Mechanism. Curr. Biol. 2012, 22 (12), 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tester AM; Waltham M; Oh SJ; Bae SN; Bills MM; Walker EC; Kern FG; Stetler-Stevenson WG; Lippman ME; Thompson EW Pro-Matrix Metalloproteinase-2 Transfection Increases Orthotopic Primary Growth and Experimental Metastasis of MDA-MB-231 Human Breast Cancer Cells in Nude Mice. Cancer Res. 2004, 64 (2), 652–658. [DOI] [PubMed] [Google Scholar]
- (90).Yonemura Y; Endo Y; Takino T; Sakamoto K; Bandou E; Kinoshita K; Fushida S; Miwa K; Sugiyama K; Sasaki T Membrane-Type 1 Matrix Metalloproteinase Enhances Lymph Node Metastasis of Gastric Cancer. Clin. Exp. Metastasis 2000, 18 (4), 321–327. [DOI] [PubMed] [Google Scholar]
- (91).Zucker S; Hymowitz M; Rollo EE; Mann R; Conner CE; Cao J; Foda HD; Tompkins DC; Toole BP Tumorigenic Potential of Extracellular Matrix Metalloproteinase Inducer. Am. J. Pathol. 2001, 158 (6), 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Diener B; Carrick L; Berk RS In Vivo Studies with Collagenase from Pseudomonas Aeruginosa. Infect. Immun. 1973, 7 (2), 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).López B; González A; Díez J Circulating Biomarkers of Collagen Metabolism in Cardiac Diseases. Circulation 2010, 121 (14), 1645–1654. [DOI] [PubMed] [Google Scholar]
- (94).Choi I-K; Strauss R; Richter M; Yun C-O; Lieber A Strategies to Increase Drug Penetration in Solid Tumors. Front. Oncol. 2013, 3 (July), 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Hingorani Andrea J. Bullock, Tara Elisabeth Seery Lei Zheng, Sigal Darren, P SR. Randomized Phase II Study of PEGPH20 plus Nab-Paclitaxel/gemcitabine (PAG) vs AG in Patients (Pts) with Untreated, Metastatic Pancreatic Ductal Adenocarcinoma (mPDA). 2018, 13–16. [DOI] [PubMed] [Google Scholar]
- (96).Chalikias GK; Tziakas DN Biomarkers of the Extracellular Matrix and of Collagen Fragments. Clin. Chim. Acta 2015, 443, 39–47. [DOI] [PubMed] [Google Scholar]
- (97).Brown E; McKee T; diTomaso E; Pluen A; Seed B; Boucher Y; Jain RK Dynamic Imaging of Collagen and Its Modulation in Tumors in Vivo Using Second-Harmonic Generation. Nat. Med. 2003, 9 (6), 796–800. [DOI] [PubMed] [Google Scholar]
- (98).Breite AG; McCarthy RC; Dwulet FE Characterization and Functional Assessment of Clostridium Histolyticum Class I (C1) Collagenases and the Synergistic Degradation of Native Collagen in Enzyme Mixtures Containing Class II (C2) Collagenase. Transplant. Proc. 2011, 43 (9), 3171–3175. [DOI] [PubMed] [Google Scholar]
- (99).Watanabe K Collagenolytic Proteases from Bacteria. Appl. Microbiol. Biotechnol. 2004, 63 (5), 520–526. [DOI] [PubMed] [Google Scholar]
- (100).Lu P; Takai K; Weaver VM; Werb Z Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb Perspect Biol 2011, 3 (12), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Mok W; Boucher Y; Jain RK Matrix Metalloproteinases-1 and −8 Improve the Distribution and Efficacy of an Oncolytic Virus. Cancer Res. 2007, 67 (22), 10664–10668. [DOI] [PubMed] [Google Scholar]
- (102).Cheng J; Sauthoff H; Huang Y; Kutler DI; Bajwa S; Rom WN; Hay JG Human Matrix Metalloproteinase-8 Gene Delivery Increases the Oncolytic Activity of a Replicating Adenovirus. Mol. Ther. 2007, 15 (11), 1982–1990. [DOI] [PubMed] [Google Scholar]
- (103).Singh KK; Jain R; Ramanan H; Saini DK Matrix-Assisted Refolding, Purification and Activity Assessment Using a “Form Invariant” Assay for Matrix Metalloproteinase 2 (MMP2). Mol. Biotechnol. 2014, 56 (12), 1121–1132. [DOI] [PubMed] [Google Scholar]
- (104).Nam DH; Ge X Direct Production of Functional Matrix Metalloproteinase−-14 without Refolding or Activation and Its Application for in Vitro Inhibition Assays. Biotechnol. Bioeng. 2016, 113 (4), 717–723. [DOI] [PubMed] [Google Scholar]
- (105).Parkar A. a.; Stow MD.; Smith K.; Panicker A. Guilloteau K, J P.; Jupp R.; Crowe SJ. Large-Scale Expression, Refolding, and Purification of the Catalytic Domain of Human Macrophage Metalloelastase (MMP-12) in Escherichia Coli. Protein Expr. Purif. 2000, 20 (2), 152–161. [DOI] [PubMed] [Google Scholar]
- (106).Pathak N; Hu SI; Koehn J. a. The Expression, Refolding, and Purification of the Catalytic Domain of Human Collagenase-3 (MMP-13). Protein Expr. Purif. 1998, 14 (2), 283–288. [DOI] [PubMed] [Google Scholar]
- (107).Ho TF; Qoronfleh MW; Wahl RC; Pulvino T. a; Vavra KJ.; Falvo J.; Banks TM.; Brake PG.; Ciccarelli RB. Gene Expression, Purification and Characterization of Recombinant Human Neutrophil Collagenase. Gene 1994, 146 (2), 297–301. [DOI] [PubMed] [Google Scholar]
- (108).Rasch MG; Lund IK; Illemann M; Høyer-Hansen G; Gårdsvoll H Purification and Characterization of Recombinant Full-Length and Protease Domain of Murine MMP-9 Expressed in Drosophila S2 Cells. Protein Expr. Purif. 2010, 72 (1), 87–94. [DOI] [PubMed] [Google Scholar]
- (109).Hadler-Olsen E; Fadnes B; Sylte I; Uhlin-Hansen L; Winberg J-O Regulation of Matrix Metalloproteinase Activity in Health and Disease. FEBS J. 2011, 278 (1), 28–45. [DOI] [PubMed] [Google Scholar]
- (110).Gutiérrez-Fernández A; Fueyo A; Folgueras AR; Garabaya C; Pennington CJ; Pilgrim S; Edwards DR; Holliday DL; Jones JL; Span PN; Sweep FCGJ; Puente XS; López-Otín C Matrix Metalloproteinase-8 Functions as a Metastasis Suppressor through Modulation of Tumor Cell Adhesion and Invasion. Cancer Res. 2008, 68 (8), 2755–2763. [DOI] [PubMed] [Google Scholar]
- (111).Eckhard U; Huesgen PF; Schilling O; Bellac CL; Butler GS; Cox JH; Dufour A; Goebeler V; Kappelhoff R; Keller U. auf dem; Klein T.; Lange PF.; Marino G.; Morrison CJ.; Prudova A.; Rodriguez D.; Starr AE.; Wang Y.; Overall CM. Active Site Specificity Profiling of the Matrix Metalloproteinase Family: Proteomic Identification of 4300 Cleavage Sites by Nine MMPs Explored with Structural and Synthetic Peptide Cleavage Analyses. Matrix Biol. 2016, 49 (2016), 37–60. [DOI] [PubMed] [Google Scholar]
- (112).Hong CS; Fellows W; Niranjan A; Alber S; Watkins S; Cohen JB; Glorioso JC; Grandi P Ectopic Matrix Metalloproteinase 9 Expression in Human Brain Tumor Cells Enhances Oncolytic HSV Vector Infection. Gene Ther 2010, 17 (10), 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Warwick D; Arandes-Renú JM; Pajardi G; Witthaut J; Hurst LC Collagenase Clostridium Histolyticum : Emerging Practice Patterns and Treatment Advances. J. Plast. Surg. Hand Surg. 2016, 50 (5), 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Alipour H; Raz A; Zakeri S; Dinparast Djadid N Therapeutic Applications of Collagenase (Metalloproteases): A Review. Asian Pac. J. Trop. Biomed. 2016, 6 (11), 975–981. [Google Scholar]
- (115).Gilpin D; Coleman S; Hall S; Houston A; Karrasch J; Jones N Injectable Collagenase Clostridium Histolyticum: A New Nonsurgical Treatment for Dupuytren’s Disease. J. Hand Surg. Am. 2010, 35 (12), 2027–2038. e1. [DOI] [PubMed] [Google Scholar]
- (116).Yang KK; Bennett N Peyronie’s Disease and Injectable Collagenase Clostridium Histolyticum: Safety, Efficacy, and Improvements in Subjective Symptoms. Urology 2016, 94, 143–147. [DOI] [PubMed] [Google Scholar]
- (117).McCallon SK; Weir D; Lantis JC Optimizing Wound Bed Preparation with Collagenase Enzymatic Debridement. J. Am. Coll. Clin. Wound Spec. 2014, 6 (1–2), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Özcan C; Ergün O; Çelik A; Çördük N; Özok G Enzymatic Debridement of Burn Wound with Collagenase in Children with Partial-Thickness Burns. Burns 2002, 28 (8), 791–794. [DOI] [PubMed] [Google Scholar]
- (119).Abe H; Kamimura K; Kobayashi Y; Ohtsuka M; Miura H; Ohashi R; Yokoo T; Kanefuji T; Suda T; Tsuchida M; Aoyagi Y; Zhang G; Liu D; Terai S Effective Prevention of Liver Fibrosis by Liver-Targeted Hydrodynamic Gene Delivery of Matrix Metalloproteinase-13 in a Rat Liver Fibrosis Model. Mol. Ther. Nucleic Acids 2016, 5 (September 2015), e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Liu J; Cheng X; Guo Z; Wang Z; Li D; Kang F; Li H; Li B; Cao Z; Nassal M; Sun D Truncated Active Human Matrix Metalloproteinase-8 Delivered by a Chimeric Adenovirus-Hepatitis B Virus Vector Ameliorates Rat Liver Cirrhosis. PLoS One 2013, 8 (1), e53392. [DOI] [PMC free article] [PubMed] [Google Scholar]


