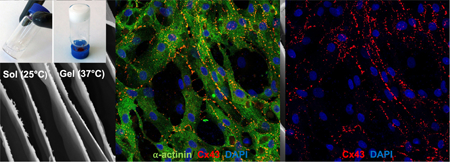
Abstract
Utilizing polymers in cardiac tissue engineering holds promise for restoring function to the heart following myocardial infarction, which is associated with grave morbidity and mortality. To properly mimic native cardiac tissue, materials must not only support cardiac cell growth but also have inherent conductive properties. Here, we present an injectable reverse thermal gel (RTG)-based cardiac cell scaffold system that is both biocompatible and conductive. Following the synthesis of a highly functionalizable, biomimetic RTG backbone, gold nanoparticles (AuNPs) were chemically conjugated to the backbone to enhance the system’s conductivity. The resulting RTG-AuNP hydrogel supported targeted survival of neonatal rat ventricular myocytes (NRVMs) for up to 21 days when cocultured with cardiac fibroblasts, leading to an increase in connexin 43 (Cx43) relative to control cultures (NRVMs cultured on traditional gelatin-coated dishes and RTG hydrogel without AuNPs). This biomimetic and conductive RTG-AuNP hydrogel holds promise for future cardiac tissue engineering applications.
Keywords: cardiac tissue engineering, reverse thermal gel, gold nanoparticles, injectable polymer, tissue engineering
Graphical Abstract
INTRODUCTION
Post-myocardial infarction (MI) cardiomyocyte (CM) loss triggers matrix degradation and fibrosis that drives the progression to heart failure (HF).1–3 HF leads to a poor quality of life and an increased risk of mortality.4 Heart transplantation remains the gold standard treatment for those with end-stage HF, but availability of donor hearts is a major limitation.5–7 Because of the limited supply of donor hearts and the limited regenerative ability of the myocardium, there is an urgent need for novel treatment approaches following MI. Investigators have focused on the development of biomaterials aimed at supporting the infarct site and overall restoring cardiac function.8–12
Injectable hydrogels provide a particularly attractive approach for MI treatment because of their potential to be delivered in a minimally invasive manner, thus minimizing mechanical stress on the cells during injection and at the infarcted area, while providing structural support for the infarct.13–15 In addition, because of the three dimensional (3D) nature of these platforms, they better mimic the in vivo microenvironment than two-dimensional (2D) platforms.16 Compared to other injectable hydrogels, reverse thermal gel (RTG) systems in particular are advantageous in that they undergo a reversible solution-to-gelation (sol-to-gel) transition solely through temperature stimuli,17 a less harmful process for encapsulated cells and tissue than systems that require UV radiation for cross-linking, which can cause oxidative damage to DNA.18
Because native cardiac tissue has unique electrophysiological behavior that is critical for the transfer of electrical signals and function of CMs,19 an ideal hydrogel system would be conductive, supporting electrical signaling between cells. Whereas injectable hydrogels hold great potential for use in cardiac tissue engineering, the majority of them are electrically insulated.20 To improve the electrical properties of injectable hydrogels, investigators have modified these hydrogels through chemical conjugation or mixing with conductive nanoparticles, such as gold nanoparticles (AuNPs).21–27 AuNPs are highly conductive biocompatible biostructures25 that confer conductive properties to otherwise inert cells.
Previously, we developed an injectable and highly biocompatible RTG designed to promote long-term CM survival.28 This RTG consists of poly(serinol hexamethylene urea)-co-poly(N-isopropylacrylamide) functionalized with lysine, termed “RTG-lysine”. The RTG-lysine hydrogel contains several free amine groups for functionalization, which are useful for a wide variety of bio-conjugations.29–33 Here, we used the free amine groups of the RTG-lysine system to chemically conjugate AuNPs functionalized with carboxylic acid (COOH) groups, creating a novel RTG-AuNP hydrogel. We found that CMs cocultured with cardiac fibroblasts (CFs) had improved long-term viability, cardiac marker expression, and increased Cx43 area when cultured in the 3D RTG-AuNP hydrogel compared to standard 2D culture systems and the unmodified RTG (hydrogel without AuNPs).
MATERIALS AND METHODS
Materials.
Materials were purchased and prepared as previously described,34 except for the following materials: Auric chloride (HAuCl4), trisodium citrate, sodium nitrate, sodium chloride, and 4-mercaptobutryic acid were purchased from Sigma-Aldrich and used as received. Water was purified using a Barnstead MicroPure system (Thermo Fisher).
Equipment.
AuNP size was estimated via both UV/vis spectroscopy and transmission electron microscopy (TEM). UV/vis readings were collected using a Nanodrop 2000 spectrophotometer (Thermo-Fisher). TEM analyses were performed by dispersing AuNPs onto formvar-coated copper grids followed by interrogation using a JEOL JSM-1400plus TEM operated at 120 kV. Thermogravimetric analyses (TGAs) were carried out as previously described,34 except that the decreased nitrogen flow used here was 25 mL/min. Resistance, viscosity, and mechanical (storage and loss moduli) analyses were conducted as previously described,34 with the following exceptions. For resistance and viscosity measurements, a 3% (w/w) polymer solution was used. Mechanical properties were determined using a constant angular frequency of 0.5 rad/s at 1.0% stain. Frequency sweep analysis was performed using an 8 mm parallel plate geometry with frequency sweeps ranging from 0.1 to 10 rad/s (20 points per decade collected). The dynamic decay of C, N, O, and Au was performed using a SPECS SAGE HR 100 system spectrometer (Sage). A Mg Kα (1253.6 eV) X-ray source at 12.5 kV and 10 mA was used for the analysis with a take-off angle of 90° and operating pressure of 8 × 10−8 mbar. Quantitative spectral analysis was performed with the Casa X-ray photoelectron spectroscopy (XPS) 2.3 software. Morphological characterization of a 3% (w/w) RTG-AuNP solution was performed using a JSM-6010LA scanning electron microscope (JEOL, Tokyo, Japan), as previously described,34 using cryogenic horizontal and vertical cuts with an approximate width of 2 mm. 3D and 2D cell cultures were imaged using a Zeiss-LSM780 confocal microscope.
Preparation of Citrate-Stabilized AuNPs.
Glassware used for the synthesis of AuNPs was cleaned using aqua regia (3:1 HCl/ HNO3), rinsed with Barnstead water, and allowed to air dry. AuNPs were prepared by mixing 5 mL of 0.01 M HAuCl4, 20 mL of 0.01 M sodium citrate, and 25 mL of Barnstead water. The solution was refluxed until a deep red color developed and then was allowed to cool to room temperature. The resulting AuNPs were concentrated to approximately 10 nM in pH 11 water. AuNP functionalization was carried out by adding 1 mL of 10 nM 4-mercaptobutyric acid per 10 mL aliquot of AuNPs. Excess acid was removed by aging the solution in salt up to a concentration of 3.6 mM NaCl. AuNPs were resuspended to a final concentration of 10 nM in pH 11 water.
Polymer Synthesis.
AuNPs were conjugated to RTG-lysine, which was synthesized as previously described,28,34 by dissolving 10 nM of AuNPs-COOH in 15 mL of phosphate-buffered saline (PBS). Five molar excess of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride/N-hydroxysuccinimide was added to activate the COOH groups (15 min at room temperature). Then, 5 mL of RTG-lysine in solution (0.1 g/mL) prepared in PBS was added drop-wise and the reaction carried out for 48 h at RT. The RTG-AuNP polymer was then dialyzed, lyophilized, and underwent L-lysine addition as previously described.34 The final polymer was dialyzed, sterile-filtered through a 2 μm filter, and lyophilized, as previously described.34
Neonatal Rat Ventricular Myocyte Culture.
Primary neonatal rat ventricular myocytes (NRVMs) were prepared from 1 to 3-day-old, Sprague Dawley rat pups (Charles River), as previously described34–36 according to the University of Colorado Denver Animal Care and Use Committee guidelines. The resultant NRMVs were cultured in 2D gelatin-coated plates (2D gelatin controls) or in the 3D hydrogels.
3D in Vitro Cell Culture.
3D in vitro culture experiments were performed as previously reported.34 Briefly, NRVMs (9 × 104) were pelleted, media-aspirated, and the cell pellet mixed with room temperature polymeric solution (150 μL of 3% [w/w]) dissolved in complete media. The cell-polymer solution was deposited into a glass bottom dish and incubated at 37 °C for 15 min at 5% CO2, which allows gel formation. Following incubation, warm media (200 μL) was deposited on top of the solidified gel with encapsulated cells. Scheme 1 shows the schematic representation of the 3D cell culture.
Scheme 1.
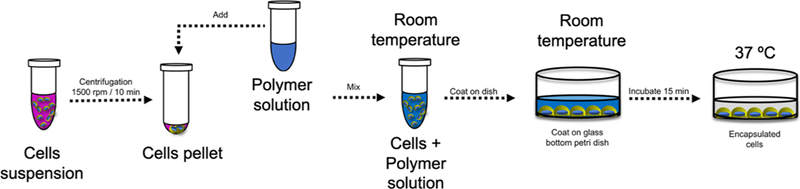
Representation of the 3D Cell Culture Method Using the RTG Hydrogels
Immunocytochemistry.
Immunocytochemistry was performed as previously described,34 using cells cultured for 21 days34 and primary antibodies against alpha actinin (Abcam ab9465, at 1:100), Cx43 (SIGMA c6219, at 1:100), and vimentin (Abcam ab24525, at 1; 100). Goat antimouse conjugated to Alexa Fluor 488 (Invitrogen, at 1:200), goat antirabbit conjugated to TRITC (Sigma, at 1:200), and goat antichicken Cy5 (Abcam, at 1:200) were used as secondary antibodies. Cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI; 1:2000).
Statistical Analysis.
Data were collected in triplicate from ≥3 independent experiments. Statistical significance was calculated and determined using ANOVA. P-values <0.05 were considered statistically significant.
RESULTS
RTG-AuNP Synthesis.
Using our previously established RTG-lysine as a platform,28 here we developed a novel 3D RTG-AuNP hydrogel. The objective of this system was to provide (1) a conductive, low viscous injectable hydrogel that (2) supports long-term survival of cocultured CMs and CFs. First, we synthetized AuNPs with COOH functional groups (Figure 1A), which are easily dispersed in water (Figure S1A). Next, the size and morphological characterization of the AuNPs-COOH was analyzed by both UV/vis spectroscopy and TEM (Figure 1B,C). UV/vis readings showed a surface plasmon absorbance peak at 528 nm, indicating an AuNPs-COOH size of approximately 35 nm diameter (Figure 1B). Using TEM analysis, the AuNPs-COOH were shown to have a rounded morphology with an approximate average diameter of 32 ± 8 nm (Figure 1C).
Figure 1.
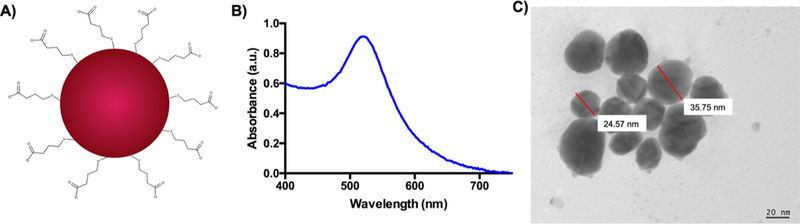
AuNP characterization. (A) AuNPs-COOH chemical structure. (B) UV/vis reading showed an absorbance peak around 528 nm. (C) AuNPs-COOH present a rounded morphology with a diameter of ~32 nm, as shown via TEM analysis.
The RTG-lysine then had its free primary amine groups covalently conjugated to the AuNPs-COOH to obtain RTG-AuNPs, as shown in Scheme 2.
Scheme 2.
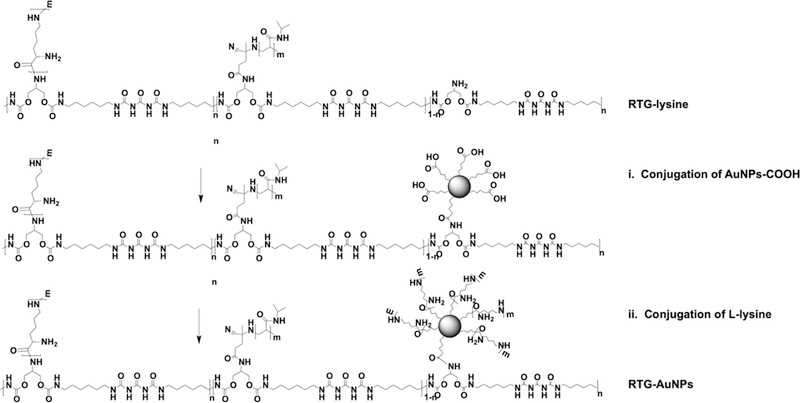
Representation of the RTG-AuNP Synthesis
To confirm the chemical linkage of the AuNPs within the RTG-lysine, TGA analysis was performed (Figure 2A). No component separation was observed during the material decomposition in both the RTG-lysine and RTG-AuNP hydrogels, meaning no residuals of unreacted compounds.12 The single weight loss in the RTG-AuNPs confirms the chemical conjugation of the AuNPs to the RTG-lysine backbone. Both polymer systems presented similar decomposition kinetics; initial decomposition of the RTG-lysine was observed at 435 °C with a mass of 97%, whereas the RTG-AuNPs began to decompose at 425 °C with a mass loss of 95%. Both polymers were completely decomposed by 700 °C. As expected, resistance of the RTG-AuNP polymer was found to be significantly lower than that of the RTG-lysine (hydrogel without conjugated AuNPs), as measured at 37 °C (140.1 kΩ ± 34.9 and 333.653 kΩ ± 50.46, respectively), validating the conductive properties of the RTG-AuNPs (Figure 2B).
Figure 2.
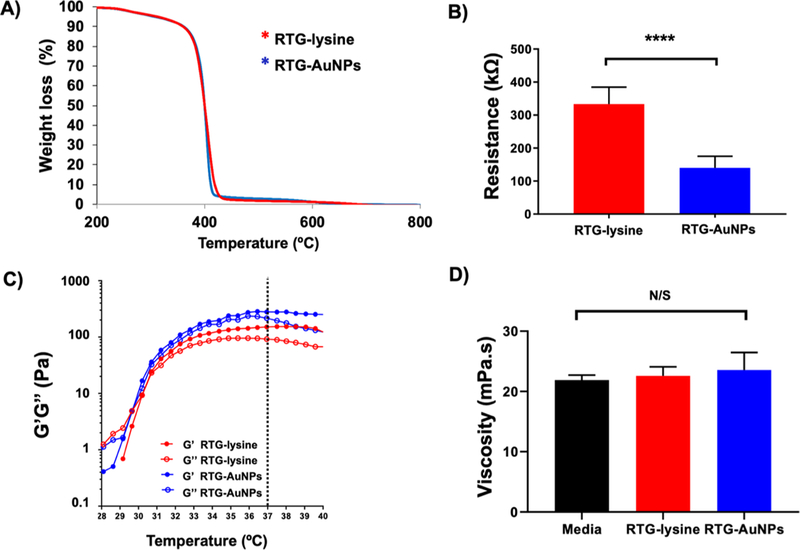
Characterization of the RTG-lysine and RTG−AuNP hydrogels. (A) A single weight loss monitored by TGA analysis demonstrates the chemical conjugation of the AuNPs to the RTG-lysine. (B) Resistance measurements demonstrating that the RTG−AuNP system is more conductive than the RTG-lysine system. p value: ****<0.0001. Data are presented as mean ± S.D. (C) The RTG−AuNP system presents significantly higher mechanical properties to those of the RTG-lysine system. Data are presented as mean ± S.D. (D) The viscosities of both the RTG−AuNP and RTG-lysine systems at 3% (w/w) concentration are similar to that of the NRVM cell culture media (N/S: non-significant). Data presented as mean ± S.D.
The mechanical properties of both hydrogels were determined using oscillatory shear rheology (Figure 2C). Both hydrogels were found to have a sol-to-gel phase transition of approximately 35 °C, making them ideal for biomedical applications as this is close to body temperature. Viscoelastic properties of both hydrogels were present at 37 °C; however, the RTG-AuNPs presented a significantly higher G’ moduli (G’ = 255.3 ± 45.2 Pa; n = 6) than the RTG-lysine (G’ = 181.7 ± 53.06 Pa; n = 6) (Figure S1B). A frequency sweep analysis was also performed. Figure S1C shows that below 1 rad/s the hydrogels present viscoelastic properties that increase with the angular frequency. At high frequencies, above 1 rad/s, G” values dominate in both hydrogels.
The viscosities of both hydrogels were also analyzed. NRVM culture media was used for comparison. Both RTG-lysine and RTG-AuNP solutions (at 3% [w/w]) possess viscosities similar to that of cell culture media (Figure 2D) (media: 21.9 ± 0.7 mPa-s; RTG-lysine: 22.58 ± 1.3 mPa-s; RTG-AuNPs: 23.56 ± 2.5 mPa·s).
XPS analysis was performed to further confirm the presence of the AuNPs in the RTG-AuNPs. Figure 3A shows XPS survey spectra of the RTG-AuNP and the RTG-lysine hydrogels. As expected, the results demonstrate the presence of Au 4f in the RTG-AuNP hydrogel. Elemental analysis revealed that Au comprises around 0.3% of the hydrogel composition (Figure 3B).
Figure 3.
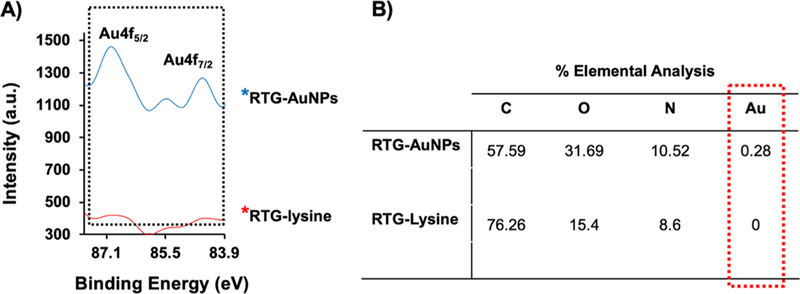
High-resolution XPS spectra relevant to Au 4f regions of RTG−AuNP and RTG-lysine hydrogels, respectively. (A) Characteristic peaks of Au4f were observed at 87 and 84.5 eV, which confirm the presence of AuNPs in the RTG−AuNPs. (B) Elemental analysis of both hydrogels further indicates the AuNPs within the RTG−AuNPs.
3D morphological characterization of the RTG-AuNP system was also performed. Side and cross sections (generated via vertical and horizontal cuts, respectively, as shown in Figure 4A) revealed the 3D structure of the RTG-AuNP system. Specifically, side sections demonstrated that the RTG-AuNPs assemble into a laminar sheet-like conformation upon gelling, providing a structure ideal for supporting cell orientation (Figure 4B). Cross sections revealed a highly interconnected and porous network (Figure 4C).
Figure 4.
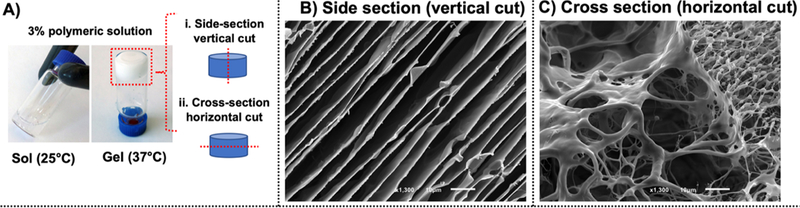
(A) Morphological characterization of the RTG−AuNPs was analyzed in vertical and horizontal cuts. (B) Vertical cuts of the hydrogel demonstrate a laminar sheet-like configuration. (C) Horizontal cuts of the hydrogel showed a highly interconnected porosity.
In Vitro Long-Term Survival of NRVM and CF Coculture.
To determine whether the RTG-AuNP hydrogel would be supportive of a long-term 3D culture of cells similar to those found in vivo, we cocultured NRVMs and CFs within this hydrogel system over a period of 21 days. CFs normally comprise approximately 10% of the total cell population derived from our standard NRVM preparation protocol.36,37 We had previously analyzed the cell population of the non-CM cells within our cell culture isolation protocol and we were not able to detect non-CM cells but CFs only.28,34 The RTG-lysine hydrogel and traditional 2D, gelatin-coated dishes were both used as controls; such gelatin-coated dishes are typically recommended and used to successfully culture NRVM.35 Both 2D and 3D culture systems received the same growth media, using a similar media change schedule protocol. Following 21 days of culture in the 3D or 2D systems, immunocytochemistry was performed using antibodies against α-actinin, a CM-specific marker, and vimentin, a CF-specific marker (Figure 5A), to determine how the percentages of subpopulations varied in these culture systems. Confocal microscopy revealed that a significantly greater percentage of cells expressed α-actinin (i.e., were NRVMs) in the RTG-AuNP system compared to the 2D gelatin control system (56 ± 10% vs 43.3 ± 7.7%, respectively). The percentage of α-actinin-positive cells was significantly higher in the RTG-lysine system (73.5 ± 0.97%) compared to both the 2D gelatin control and RTG-AuNP system (Figure 5B).
Figure 5.
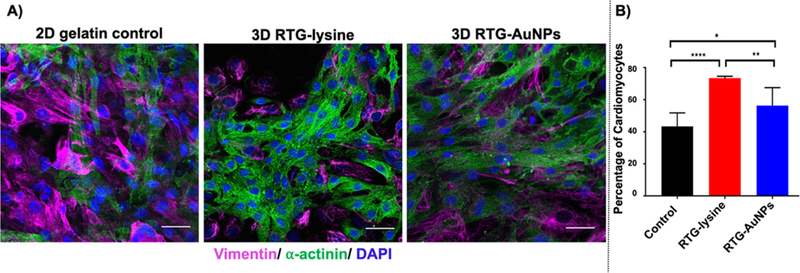
Immunocytochemistry labeling of NRVMs and CFs cultured in 2D and 3D systems for 21 days. (A) Antibody staining against α-actinin (green) and vimentin (pink) label NRVMs and CFs, respectively, with nuclei labeled using DAPI (blue). (B) Quantification of immunocytochemistry staining against α-actinin indicates the percentage of cells likely to be NRVMs, showing both 3D systems to contain a greater percentage of NRVMs than the 2D gelatin control. Scale bar 40 μm. p values: *<0.023, **<0.0017, and ****<0.0001. Data are presented as mean ± S.D.
Following 21 days of culture in the 3D or 2D systems, immunocytochemistry was performed using an antibody against connexin 43 (Cx43), a gap junction protein, to determine the degree and localization of expression of this protein in the culture systems (Figure 6A). Confocal microscopy revealed that cells cultured in the RTG-AuNP hydrogel have greater Cx43 staining area (3.2 ± 1.96 μm2) compared to cells in the 2D gelatin control system (0.26 ± 0.1 μm2) and the RTG-lysine system (0.89 ± 0.13 μm2) (Figure 6B).
Figure 6.
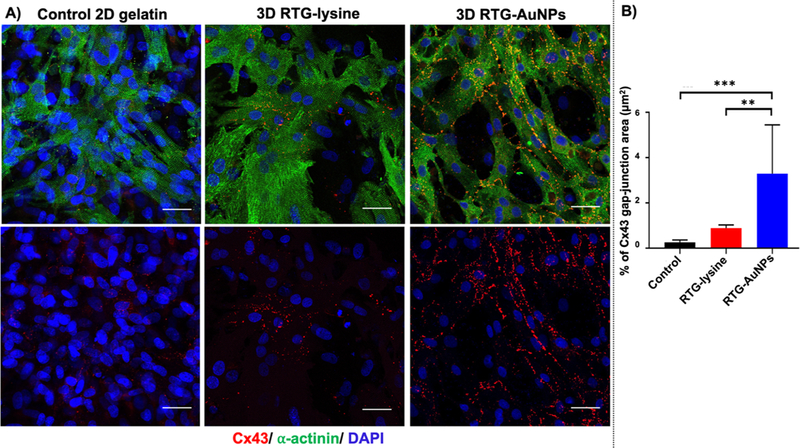
Immunocytochemistry labeling of gap junctions in NRVMs cultured in 2D and 3D systems for 21 days. (A) Antibody staining against connexin 43 (Cx43) (red) and α-actinin (green), with nuclei labeled using DAPI (blue). (B) Quantification of immunocytochemistry staining against Cx43 to indicate the surface area of NRVMs positive for this gap junction protein, showing the RTG-AuNP system to contain the largest Cx43-positive area. Scale bar: 40 μm. p values: **<0.0021 and *** <0.0002. Data are presented as mean ± S.D.
DISCUSSION
Previously, we demonstrated that our RTG-lysine hydrogel system supports long-term CM survival for up to 21 days. For the current work, our aim was to develop a conductive, injectable RTG functionalized with AuNPs and to assess its ability to function as a 3D scaffold supportive of a coculture of CMs and CFs but emphasizing long-term CMs’ survival. AuNPs were selected as an ideal conductive material to incorporate because of their high biocompatibility and inert properties.27,38 In addition, several recent studies have shown that polymeric materials functionalized with AuNPs can improve electrical communication between conductive cells such as CMs and neurons.39 For example, Nair et al. found that a decellularized porcine matrix conjugated with AuNPs promoted growth and proliferation of rat myoblasts (H9C2 cells).40 Similarly, Shevach et al. found that fibrous decellularized omental matrices with deposited AuNPs improved elongation, alignment, and cardiac function of NRVMs (compared to matrices devoid of AuNPs).22 Baranes et al. similarly demonstrated that AuNPs embedded within the surface of electrospun nanofibers encouraged longer neurite outgrowth of primary neurons.23
In this work, we were able to chemically incorporate AuNPs-COOH within our highly biocompatible RTG-lysine hydrogel. AuNPs-COOH were characterized with TEM and UV/vis spectroscopy. It has been reported that spherical AuNP diameter can be calculated using multipole scattering theory from UV/vis spectroscopy data.41,42 Using this method, we determined the diameter of the AuNPs-COOH’ which was in the range of 35 nm. Similar values were obtained using TEM (32 ± 8 nm), demonstrating the precision of this approach. AuNPs-COOH were well dispersed in water as shown in Figure S1A. This property simplified their conjugation within the RTG-lysine hydrogel and avoided the use of further polymer purification methods. The chemical incorporation of AuNPs-COOH was demonstrated by both TGA and XPS. TGA results show a single weight loss event in the RTG—AuNP hydrogel, meaning no residuals of unreacted compounds.34 These results confirm the conjugation of the AuNPs to the RTG-lysine backbone. The presence of gold and its % composition within the polymer was evaluated by XPS. Figure 3 shows a high-resolution XPS spectra of the RTG-lysine and the RTG—AuNPs in which two doublets of Au 4f, Au 4f 5/7, and Au 4f 7/2, located at 87.1 and 84.5 eV are present in the RTG—AuNP hydrogel. The positions of these doublets are similar to those reported by other authors,43–45 thus demonstrating that the AuNPs were well conjugated into the RTG-lysine hydrogel.
The injectable RTG—AuNP hydrogel system presented here holds several advantages over bioengineered patch systems. The first advantage of the RTG—AuNP system is that it possesses a highly porous structure that is able to host embedded cells within its 3D matrix. Thanks to its highly interconnected porosity, encapsulated cells can easily elongate and interconnect with each other, without forced rearrangement of their surrounding microenvironment. It has previously been reported that the porosity and pore size of 3D scaffolds unsurprisingly have an important effect on encapsulated cells, as open and interconnected porous networks are essential for proper cell nutrition, proliferation, and migration.46,47 As previously mentioned, side sections of the RTG—AuNPs observed via SEM show a laminar sheet-like configuration, which likely aids in proper NRVM orientation and elongation. Cell alignment is particularly important for immature CMs as it promotes mechanical integrity, electrical conduction, contractile efficiency, and ultimately leads to cell maturation.48–51 A laminar sheet-like configuration has been also observed in our other RTG systems as well30 and it has favored other cell types. We have previously reported that this laminar assembly depends on the concentration of the polymer solution.30 Hydrogels with polymer concentrations below 2.5% (w/w %) assemble into heterogeneous structures.30
A second advantage of the RTG—AuNP system is that it possesses decreased resistance, and thus increased conductivity, compared to the RTG-lysine system. Although the resistance of metallic bulk gold is approximately 2.4 10−11 kΩ,52,53 a reflection of it being a highly conductive material, the number of free amine groups in the RTG-lysine system limits the number of AuNPs-COOH conjugation sites and thus, the amount of Au within the RTG—AuNPs was only 0.3% as estimated by XPS elemental analysis. However, despite the small amount of AuNPs conjugated with the RTG-lysine hydrogel, the RTG—AuNP hydrogel still has a higher resistance than the plain RTG-lysine and that can be appreciated in the increased amount of Cx43 observed within the CMs cultured in the RTG—AuNP hydrogel.
Another advantage of the RTG—AuNP hydrogel is its low viscosity, which is similar to that of cell culture media. This property allows the RTG-lysine and the RTG-AuNP hydrogels to be easily injected through a small gauge needle, as demonstrated in the Supporting Information Videos S1 and S2. It has been previously reported that hydrogels with low viscosity are easier to inject than hydrogels with a high viscosity.54 From an application point of view, minimally invasive therapies are very appealing for tissue engineering approaches12,55 and therefore the injectable property of RTG—AuNPs is a major advantage for both in vivo studies and future translational therapeutic applications.
In the current work, we aimed to first develop and characterize the injectable biocompatible conductive hydrogel. We were able to show that both the mechanical properties, storage and loss modulus of the RTG—AuNP hydrogel are low, which is characteristic of physical gels at a low polymer concentration.28,34 Moreover, we found that at a high angular frequency (above 1 rad/s) both hydrogels behave like viscoelastic liquids. These results further confirm that both RTG hydrogels are weak gels because of their weak physical gelation properties.56 Although we have reported previously that increasing the concentration of the polymer in solution leads to an increase in the mechanical properties of the type of RTG hydrogels tested for this investigation,30 several investigations have reported the successful use of hydrogels with low mechanical properties in both in vitro28,34,57—60 and in in vivo61,62 cardiac tissue engineering studies. We have previously demonstrated that NRVMs cultured in our soft 3D RTG-lysine hydrogel present long-term viability for up to 21 days with increased Cx43 area when compared with traditional 2D gelatin controls.28 We also demonstrate the functional improvement of NRVMs cultured in 3D RTG-CNT soft gels and their increased Cx43 area and more homogeneous calcium transients for up to 21 days when cultured in these soft hydrogel systems.34 Geuss et al. demonstrated that HL-1 CMs cultured in PEGylated Fibrin hydrogels, with storage moduli of 123.5 ± 29.6 Pa, spread better than PEGylated Fibrin hydrogels, with storage moduli of 966.8 + 85.5 Pa.59 Hao et al. tested fullerene/alginate hydrogels, with storage moduli in the range of 600 Pa, as a delivery system of brown adipose-derived stem cells in a rat MI model. They demonstrated that the hydrogels improve the retention and survival of the implanted cells and induced angiogenesis, which promoted cardiac function recovery.61 Singelyn et al. proved that hydrogels derived from decellularized pig ventricular extracellular matrix, with low mechanical properties, were able to maintain cardiac function in a rat MI model without inducing arrhythmias.63 Wassennaar et al. have shown that porcine myocardial hydrogel with low mechanical properties alters several remodeling pathways after MI promoting a pro-regenerative environment in the injured tissue.64 Moreover, Rane et al. have demonstrated that structural reinforcement of injured cardiac tissue, because of the mechanical properties of hydrogels, is insufficient to prevent cardiac remodeling: they found that bioactivity and cell infiltration within injectable materials have a key role in improving cardiac function in the injured tissue.65
Material degradation is another important property for tissue engineering applications. Whereas degradable hydrogels may be desirable, inert, nondegradable materials can also be used for cardiac regeneration.11 We have previously reported that modifying the chemistry composition of the PNIPAAm copolymer used in the synthesis of our RTG systems can lead to fast or slow degradation rates of our hydrogels.66
Finally, we evaluated the ability of our RTG—AuNP system to support NRVMs and CF in a coculture. This analysis was crucial to determine the feasibility of this material for use as a scaffold in cardiac tissue applications. As discussed above, we encapsulated a coculture of NRVMs and CFs within the RTG—AuNP system. Because the RTG—AuNPs is in a solution at room temperature, encapsulation of cardiac cells was easily achieved through simple mixing of the RTG—AuNPs with the cell suspension at room temperature. Whereas the electroactive RTG—AuNPs maintained the growth of NRVMs in coculture with CFs for at least 21 days, the percentage of NRVMs was surprisingly lower in the 3D RTG— AuNP system compared with the RTG-lysine system. However, this may be beneficial for the NRVMs as CFs provide mechanotransductive cues that can improve CM function.67 Finally, we demonstrated that when cultured in the 3D RTG—AuNPs, the NRVMs had a significantly greater area of Cx43-positive cells when compared to 2D gelatin controls and the RTG-lysine hydrogel. This may be due to the electrical cues of the RTG—AuNPs promoting a more organized, and hence better defined, area of Cx43 expression. It has been reported by several authors that correct organization of Cx43 plays an important role in normal cardiac function. Unorganized Cx43 can lead to cardiac arrhythmias.68—70 Here, we have demonstrated that RTG—AuNP hydrogel induces a more organized and increased Cx43 localization, which is beneficial for CM function.36 Overall, our results suggest that the RTG—AuNP system supports long-term cardiac cell survival (for at least 21 days) in coculture with CFs with increased and more organized Cx43 expression, making the RTG—AuNP polymer promising for use in in vivo applications (on-going study). Although this study was performed with cardiac cells, this system should not be limited to cardiac tissue engineering applications as it can be beneficial for other conductive cells such as neurons.
CONCLUSIONS
Here, we demonstrate that our injectable, conductive, and low-viscosity RTG—AuNP hydrogel, which conveniently transitions to a 3D matrix by temperature stimuli, can provide both topographical and electrophysiological cues supportive of culturing both NRVMs and CFs. This system specifically promotes long-term CM survival with an increased surface area positive for Cx43. Finally, we believe that our RTG—AuNP system holds tremendous potential for both minimally invasive approaches to repairing damaged heart tissues as well as use for 3D in vitro cardiac modeling investigations.71
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Walther R. Olivas for helping with the injection videos. The authors would like also to thank the University of Colorado Denver Advanced Microscopy Core for their facilities and support. The authors also thank Eric P. Wartchow and the Electron Microscopy Lab at Children’s Hospital Colorado for facilities and support in the SEM and TEM analysis. Dr. Pena would like to specially thank Dr. Peter Buttrick for all his help, support, and advise during this investigation.
Funding
This study was supported by generous grants of the John Patrick Albright (L.M., M.R.G.T., and B.P.) and Foreman Casali (L.M.) Foundations, NIH/NHLBI RO1 HL116905 (L.M.), NIH (HL116848 and HL127240) (T.A.M), American Heart Association (16SFRN31400013) (T.A.M), HL147558 (T.A.M) and DK119594 (T.A.M), PDS HL116906 (B.P.), AHA SFRN Heart Failure Fellow (16SFRN31400013) (b.P.), 1R01HL109209–01A1 (M.R.G.T.), NIH RO1 HL114753 (R.S.), NIH K24 HL081506 (R.S.), NIH F32 (1 F32 HL137256–01) (B.A.A.) and the Burroughs Welcome Fund Postdoctoral Enrichment Program (B.A.A.). This work was supported in part by a Trans-Atlantic Network of Excellence grant from the Leducq Foundation (14 CVD 03) (L.M., M.R.G.T., and B.P.). Part of this work was supported by AXA research funds (M.P.), the Spanish Ministry of Economy and Competitiveness MINECO (project CTQ2016–76721-R) (M.P.), the University of Trieste and the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency—grant no. MDM-2017–0720 (M.P.). This investigation was also supported in part by the AHA (17GRNT33661024) (D.P.) and the R21 HL124100–01 (D.P.).
ABBREVIATIONS
- RTG
reverse thermal gel
- AuNPs
gold nanoparticles
- 3D
three-dimensional
- NRVMs
neonatal rat ventricular myocytes
- TGA
thermogravimetric analysis
- SEM
scanning electron microscopy
- XPS
X-ray photoelectron spectroscopy
- CMs
cardiomyocytes
- CFs
cardiac fibroblasts
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.9b00666.
AuNPs-COOH are well-dispersed in water; the RTG— AuNP system presents significantly higher G’ to the RTH-lysine system (PDF)
RTG-lysine hydrogel injection at 37 °C using a 31-gauge needle. The injection is made in water. Polymer concentration: 3% (w/w) (AVI)
RTG—AuNP hydrogel injection at 37 °C using a 31-gauge needle. The injection is made in water. Polymer concentration: 3% (w/w) (AVI)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Konstam MA; Kramer DG; Patel AR; Maron MS; Udelson JE Left Ventricular Remodeling in Heart Failure. JACC Cardiovasc. Imaging 2011, 4, 98–108. [DOI] [PubMed] [Google Scholar]
- (2).Inamdar A; Inamdar A Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cho E; Kim M; Ko YS; Lee HY; Song M; Kim MG; Kim H-K; Cho W-Y; Jo S-K Role of Inflammation in the Pathogenesis of Cardiorenal Syndrome in a Rat Myocardial Infarction Model. Nephrol. Dial. Transplant. 2013, 28, 2766–2778. [DOI] [PubMed] [Google Scholar]
- (4).Braunwald E Heart Failure. JACC Hear. Fail. 2013, 1, 1–20. [DOI] [PubMed] [Google Scholar]
- (5).Li S; Loganathan S; Korkmaz S; Radovits T; Hegedűs P; Zhou Y; Karck M; Szabó G Transplantation of Donor Hearts after Circulatory or Brain Death in a Rat Model. J. Surg. Res. 2015, 195, 315–324. [DOI] [PubMed] [Google Scholar]
- (6).Barr ML; Taylor DO Changes in Donor Heart Allocation in the United States without Fundamental Changes in the System: Rearranging Deck Chairs and Elephants in the Room. Am. J. Transplant. 2015, 15, 7–9. [DOI] [PubMed] [Google Scholar]
- (7).Chin C; Miller J; Robbins R; Reitz B; Bernstein D The Use of Advanced-Age Donor Hearts Adversely Affects Survival in Pediatric Heart Transplantation. Pediatr Transpl 1999, 3, 309–314. [DOI] [PubMed] [Google Scholar]
- (8).Nelson DM; Ma Z; Fujimoto KL; Hashizume R; Wagner WR Intra-Myocardial Biomaterial Injection Therapy in the Treatment of Heart Failure: Materials, Outcomes and Challenges. Acta Biomater. 2011, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Johnson TD; Braden RL; Christman KL Injectable ECM Scaffolds for Cardiac Repair. Cardiac Tiss. Eng. 2014, 1181, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Tous E; Purcell B; Ifkovits JL; Burdick JA Injectable Acellular Hydrogels for Cardiac Repair. J. Cardiovasc. Transl. Res. 2011, 4, 528–542. [DOI] [PubMed] [Google Scholar]
- (11).Radisic M; Christman KL Materials Science and Tissue Engineering: Repairing the Heart. Mayo Clin. Proc. 2013, 88, 884–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Peña B; Laughter M; Jett S; Rowland TJ; Taylor MRG; Mestroni L; Park D Injectable Hydrogels for Cardiac Tissue Engineering. Macromol Biosci. 2018, 18, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hasan A; Khattab A; Islam MA; Hweij KA; Zeitouny J; Waters R; Sayegh M; Hossain MM; Paul A Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Janani A; Sridhar Skylab R Injectable Hydrogel for Cardiac Tissue Engineering. Int. J. ChemTech Res. 2014, 6, 2233–2236. [Google Scholar]
- (15).Aguado BA; Mulyasasmita W; Su J; Lampe KJ; Heilshorn SC; Ph D; Heilshorn SC; Ph D Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Eng. Part A 2012, 18, 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hirt MN; Hansen A; Eschenhagen T Cardiac Tissue Engineering. Circ. Res. 2014, 114, 354–367. [DOI] [PubMed] [Google Scholar]
- (17).Park MH; Joo MK; Choi BG; Jeong B Biodegradable Thermogels. Acc. Chem. Res. 2012, 45, 424–433. [DOI] [PubMed] [Google Scholar]
- (18).Rastogi RP; Richa; Kumar A; Tyagi MB; Sinha RP Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Tandon N; Cannizzaro C; Chao P-HG; Maidhof R; Marsano A; Au HTH; Radisic M; Vunjak-Novakovic G Electrical Stimulation Systems for Cardiac Tissue Engineering. Nat. Protoc. 2009, 4, 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Shin SR; Jung SM; Zalabany M; Kim K; Zorlutuna P; Kim SB; Nikkhah M; Khabiry M; Azize M; Kong J; Wan K.-t.; Palacios T; Dokmeci MR; Bae H; Tang X; Khademhosseini A Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li Y; Shi X; Tian L; Sun H; Wu Y; Li X; Li J; Wei Y; Han X; Zhang J; Jia X; Bai R; Jing L; Ding P; Liu H; Han D AuNP-Collagen Matrix with Localized Stiffness for Cardiac-Tissue Engineering: Enhancing the Assembly of Intercalated Discs by β1-Integrin-Mediated Signaling. Adv. Mater. 2016, 28, 10230–10235. [DOI] [PubMed] [Google Scholar]
- (22).Shevach M; Fleischer S; Shapira A; Dvir T Gold Nanoparticle-Decellularized Matrix Hybrids for Cardiac Tissue Engineering. Nano Lett. 2014, 14, 5792–5796. [DOI] [PubMed] [Google Scholar]
- (23).Baranes K; Shevach M; Shefi O; Dvir T Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [DOI] [PubMed] [Google Scholar]
- (24).McCaffrey R; Long H; Jin Y; Sanders A; Park W; Zhang W Template Synthesis of Gold Nanoparticles with an Organic Molecular Cage. J. Am. Chem. Soc. 2014, 136, 1782–1785. [DOI] [PubMed] [Google Scholar]
- (25).Wu Y; Li Y; Liu P; Gardner S; Ong BS Studies of Gold Nanoparticles as Precursors to Printed Conductive Features for Thin-Film Transistors. Chem. Mater. 2006, 18, 4627–4632. [Google Scholar]
- (26).He L; Dragavon J; Cho S; Mao C; Yildirim A; Ma K; Chattaraj R; Goodwin AP; Park W; Cha JN Self-Assembled Gold Nanostar-NaYF4:Yb/Er Clusters for Multimodal Imaging, Photothermal and Photodynamic Therapy. J. Mater. Chem. B 2016, 4, 4455–4461. [DOI] [PubMed] [Google Scholar]
- (27).Merino S; Martín C; Prato M; Vazquez E; Kostarelos K Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [DOI] [PubMed] [Google Scholar]
- (28).Pena B; Martinelli V; Jeong M; Bosi S; Lapasin R; Taylor MRG; Long CS; Shandas R; Park D; Mestroni L Biomimetic Polymers for Cardiac Tissue Engineering. Biomacromolecules 2016, 17, 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Peña B; Shandas R; Park D A Heparin-Mimicking Reverse Thermal Gel for Controlled Delivery of Positively Charged Proteins. J. Biomed. Mater. Res. - Part A 2015, 103, 2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Laughter MR; Ammar DA; Bardill JR; Pena B; Kahook MY; Lee DJ; Park D A Self-Assembling Injectable Biomimetic Microenvironment Encourages Retinal Ganglion Cell Axon Extension in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 20540–20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Yun D; Famili A; Lee YM; Jenkins PM; Freed CR; Park D Biomimetic Poly(Serinol Hexamethylene Urea) for Promotion of Neurite Outgrowth and Guidance. J. Biomater. Sci. Polym. Ed. 2014, 25, 354–369. [DOI] [PubMed] [Google Scholar]
- (32).Yun D; Laughter MR; Park D A Biomimetic Reverse Thermal Gel for 3-Dimensional Neural Tissue Engineering. Austin J. Biomed. Eng. 2014, 1, 1019. [Google Scholar]
- (33).Jenkins PM; Laughter MR; Lee DJ; Lee YM; Freed CR; Park D A Nerve Guidance Conduit with Topographical and Biochemical Cues: Potential Application Using Human Neural Stem Cells. Nanoscale Res. Lett. 2015, 10, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Peña B; Bosi S; Aguado BA; Borin D; Farnsworth NL; Dobrinskikh E; Rowland TJ; Martinelli V; Jeong M; Taylor MRG; Long CS; Shandas R; Sbaizero O; Prato M; Anseth KS; Park D; Mestroni L Injectable Carbon Nanotube-Functionalized Reverse Thermal Gel Promotes Cardiomyocytes Survival and Maturation. ACS Appl. Mater. Interfaces 2017, 9, 31645–31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Martinelli V; Cellot G; Toma FM; Long CS; Caldwell JH; Zentilin L; Giacca M; Turco A; Prato M; Ballerini L; Mestroni L Carbon Nanotubes Promote Growth and Spontaneous Electrical Activity in Cultured Cardiac Myocytes. Nano Lett. 2012, 12, 1831–1838. [DOI] [PubMed] [Google Scholar]
- (36).Martinelli V; Cellot G; Toma FM; Long CS; Caldwell JH; Zentilin L; Giacca M; Turco A; Prato M; Ballerini L; Mestroni L Carbon Nanotubes Instruct Physiological Growth and Functionally Mature Syncytia: Nongenetic Engineering of Cardiac Myocytes. ACS Nano 2013, 7, 5746–5756. [DOI] [PubMed] [Google Scholar]
- (37).Martinelli V; Cellot G; Fabbro A; Bosi S; Mestroni L; Ballerini L Improving Cardiac Myocytes Performance by Carbon Nanotubes Platforms. Front. Physiol. 2013, 4, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Amezcua R; Shirolkar A; Fraze C; Stout D Nanomaterials for Cardiac Myocyte Tissue Engineering. Nanomaterials 2016, 6, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Vial S; Reis RL; Oliveira JM Recent Advances Using Gold Nanoparticles as a Promising Multimodal Tool for Tissue Engineering and Regenerative Medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar]
- (40).Nair RS; Ameer JM; Alison MR; Anilkumar TV A Gold Nanoparticle Coated Porcine Cholecyst-Derived Bioscaffold for Cardiac Tissue Engineering. Colloids Surf. B 2017, 157, 130–137. [DOI] [PubMed] [Google Scholar]
- (41).Haiss W; Thanh NTK; Aveyard J Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [DOI] [PubMed] [Google Scholar]
- (42).Amendola V; Meneghetti M Size Evaluation of Gold Nanoparticles by UV-vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar]
- (43).Božanić DK; Luyt AS; Trandafilović LV; Djoković V Glycogen and Gold Nanoparticle Bioconjugates: Controlled Plasmon Resonance via Glycogen-Induced Nanoparticle Aggregation. RSC Adv. 2013, 3, 8705–8713. [Google Scholar]
- (44).Castillo C; Buono-Core G; Manzur C; Yutronic N; Sierpe R; Cabello G; Chornik B Molybdenum Trioxide Thin Films Doped with Gold Nanoparticles Grown by a Sequential Methodology: Photochemical Metal-Organic Deposition (PMOD) and DC-Magnetron Sputtering. J. Chit Chem. Soc. 2016, 61, 2816–2820. [Google Scholar]
- (45).Ke X; Zhang X; Zhao J; Sarina S; Barry J; Zhu H Selective Reductions Using Visible Light Photocatalysts of Supported Gold Nanoparticles. Green Chem. 2013, 15, 236–244. [Google Scholar]
- (46).Annabi N; Nichol JW; Zhong X; Ji C; Koshy S; Khademhosseini A; Dehghani F Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Loh QL; Choong C Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pasqualini FS; Sheehy SP; Agarwal A; Aratyn-Schaus Y; Parker KK Structural Phenotyping of Stem Cell-Derived Cardiomyocytes. Stem Cell Rep. 2015, 4, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ye F; Keller BB; Nakane T; Dwenger M; Yuan F; Masumoto H; Tinney JP; Kowalski WJ Quantification of Cardiomyocyte Alignment from Three-Dimensional (3D) Confocal Microscopy of Engineered Tissue. Microsc. Microanal. 2017, 23, 826–842. [DOI] [PubMed] [Google Scholar]
- (50).Weeke-Klimp A; Bax NAM; Bellu AR; Winter EM; Vrolijk J; Plantinga J; Maas S; Brinker M; Mahtab EAF; Gittenberger-de Groot AC; van Luyn MJA; Harmsen MC; Lie-Venema H Epicardium-Derived Cells Enhance Proliferation, Cellular Maturation and Alignment of Cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 49, 606–616. [DOI] [PubMed] [Google Scholar]
- (51).Li J; Minami I; Shiozaki M; Yu L; Yajima S; Miyagawa S; Shiba Y; Morone N; Fukushima S; Yoshioka M; Li S; Qiao J; Li X; Wang L; Kotera H; Nakatsuji N; Sawa Y; Chen Y; Liu L Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium. Stem Cell Rep. 2017, 9, 1546–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lloyd David H Physics, 2nd ed.; Saunders college Publishing: Florida, 1998. [Google Scholar]
- (53).Cutnell JD; Johnson KW Physics, 4th ed.; Wiley: New York, 1998. [Google Scholar]
- (54).Chen MH; Wang LL; Chung JJ; Kim Y-H; Atluri P; Burdick JA Methods to Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 2, 3146–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Kondiah P; Choonara YE; Kondiah PPD; Marimuthu T; Kumar P; Du Toit LC; Pillay V A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Jyoti BVS; Baek SW Rheological Characterization of Ethanolamine Gel Propellants. J. Energ. Mater. 2016, 34, 260–278. [Google Scholar]
- (57).Jeffords ME; Wu J; Shah M; Hong Y; Zhang G Tailoring Material Properties of Cardiac Matrix Hydrogels to Induce Endothelial Differentiation of Human Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 11053–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ungerleider JL; Johnson TD; Rao N; Christman KL Fabrication and Characterization of Injectable Hydrogels Derived from Decellularized Skeletal and Cardiac Muscle. Methods 2015, 84, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Geuss LR; Allen ACB; Ramamoorthy D; Suggs LJ Maintenance of HL-1 Cardiomyocyte Functional Activity in PEGylated Fibrin Gels. Biotechnol Bioeng. 2015, 112, 1446–1456. [DOI] [PubMed] [Google Scholar]
- (60).Navaei A; Truong D; Heffernan J; Cutts J; Brafman D; Sirianni RW; Vernon B; Nikkhah M PNIPAAm-Based Biohybrid Injectable Hydrogel for Cardiac Tissue Engineering. Acta Biomater. 2016, 32, 10–23. [DOI] [PubMed] [Google Scholar]
- (61).Hao T; Li J; Yao F; Dong D; Wang Y; Yang B; Wang C Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [DOI] [PubMed] [Google Scholar]
- (62).Wassenaar JW; Braden RL; Osborn KG; Christman KL Modulating in Vivo Degradation Rate of Injectable Extracellular Matrix Hydrogels. J. Mater. Chem. B 2016, 4, 2794–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Singelyn JM; Sundaramurthy P; Johnson TD; Schup-Magoffin PJ; Hu DP; Faulk DM; Wang J; Mayle KM; Bartels K; Salvatore M; Kinsey AM; Demaria AN; Dib N; Christman KL Catheter-Deliverable Hydrogel Derived from Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Wassenaar JW; Gaetani R; Garcia JJ; Braden RL; Luo CG; Huang D; Demaria AN; Omens JH; Christman KL Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Rane AA; Chuang JS; Shah A; Hu DP; Dalton ND; Gu Y; Peterson KL; Omens JH; Christman KL Increased Infarct Wall Thickness by a Bio-Inert Material Is Insufficient to Prevent Negative Left Ventricular Remodeling after Myocardial Infarction. PLoS One 2011, 6, No. e21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Famili A; Kahook MY; Park D A Combined Micelle and Poly(Serinol Hexamethylene Urea)-Co-Poly(N-Isopropylacrylamide) Reverse Thermal Gel as an Injectable Ocular Drug Delivery System. Macromol. Biosci. 2014, 14, 1719–1729. [DOI] [PubMed] [Google Scholar]
- (67).Vunjak-Novakovic G; Tandon N; Godier A; Maidhof R; Marsano A; Martens TP; Radisic M Challenges in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 2009, 16, 169–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Severs N; Coppen SR; Dupont E; Yeh HI; Ko YS; Matsushita T Gap Junction Alterations in Human Cardiac Disease. Cardiovasc. Res. 2004, 62, 368–377. [DOI] [PubMed] [Google Scholar]
- (69).Severs NJ; Bruce AF; Dupont E; Rothery S Remodelling of Gap Junctions and Connexin Expression in Diseased Myocardium. Cardiovasc. Res. 2008, 80, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kostin S; Dammer S; Hein S; Klovekorn WP; Bauer EP; Schaper J Connexin 43 Expression and Distribution in Compensated and Decompensated Cardiac Hypertrophy in Patients with Aortic Stenosis. Cardiovasc. Res. 2004, 62, 426–436. [DOI] [PubMed] [Google Scholar]
- (71).Aguado BA; Grim JC; Rosales AM; Watson-Capps JJ; Anseth KS Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10, eaam8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


