Abstract
Background:
Childhood neuroblastoma describes a heterogeneous group of extracranial solid tumors, that are treated per risk profile. We sought to describe treatment patterns and survival using population-based data from throughout the United States.
Materials and Methods:
Using the National Cancer Institute (NCI)’s Patterns of Care data, we analyzed treatment provided to newly diagnosed, histologically confirmed neuroblastoma patients in 2010 and 2011, registered to one of 14 Surveillance, Epidemiology, and End Results (SEER) cancer registries. Data were re-abstracted from hospital records and treating physicians contacted for verification. Application of the Children’s Oncology Group (COG)’s 3-level (low, intermediate and high) neuroblastoma risk classification system for therapeutic decision-making provided insight to community-based treatment patterns. Kaplan-Meier survival analyses, based on 5-years of follow-up, were also performed.
Results:
76% of the 250 patients were enrolled on an open/active clinical trial. All low-risk patients received surgery. Most intermediate-risk patients (81%) received a chemotherapy regimen that included carboplatin, etoposide, cyclophosphamide and doxorubicin. High-risk patients received extensive, multimodal treatment consisting of chemotherapy, surgery, myeloablative chemotherapy with stem cell rescue (transplant), radiation, immunotherapy (dinutuximab), and isotretinoin therapy. 21% patients had died at the end of the maximum 60-month follow-up period. The 5-year estimated survival rates were lower for patients diagnosed with stage 4 disease, unfavorable DNA ploidy, MYCN gene amplification or classified as high-risk.
Conclusion:
Most neuroblastoma patients are registered on a risk-based open/active clinical trial. Variation in modality, systemic agents and sequence of treatment reflects the heterogeneity of therapy received by these patients.
Keywords: Neuroblastoma, Therapeutics, Immunotherapy, Survival
Introduction
Neuroblastoma is a rare, but common childhood, solid tumor cancer. [1] Approximately, 700–800 new cases are diagnosed annually in the United States. [2] [3] Clinical heterogeneity is a hallmark of this cancer: some patients, mainly infants, have tumors that undergo spontaneous regression without any intervention or require only surgery, while others require multimodal therapy. [4] The purpose of this study was to describe patient characteristics, variation in treatment and survival of neuroblastoma patients treated in communities throughout the US.
The Children’s Oncology Group (COG) established a classification system that used age at diagnosis, MYCN gene amplification status, DNA ploidy, histology, and the International Neuroblastoma Staging System (INSS) to assign low, intermediate or high-risk status for therapeutic decision-making. [5] This treatment classification system was used in many COG clinical trial studies, though recently superseded by the International Neuroblastoma Risk Group (INRG) system. [6]
In low-risk patients, treatment aims to deliver the minimum therapy while maintaining excellent patient survival. [7] Intermediate-risk treatments depends on histological/biological characteristics of the tumor and consists of chemotherapy with or without surgery and RT. [6] The current standard of care strategy for high-risk neuroblastoma consists of three treatment blocks – induction, consolidation and post-consolidation/maintenance. [8] Induction chemotherapy seeks to reduce tumor burden by shrinking the primary tumor and reducing metastases using a combination chemotherapy regimen, followed by delayed surgery to remove the primary tumor. Consolidation involves myeloablative chemotherapy supported by autologous hematopoietic stem-cell rescue that attempts to eradicate minimal residue disease and repopulate the bone marrow. [6] Maintenance treatment follows for minimal residual disease with anti-GD2 monoclonal antibody and cytokine immunotherapy in addition to differentiating therapy with isotretinoin. [8] Treatment options for recurrent neuroblastoma are dependent upon previous initial risk classification with several phase 2 clinical trials being conducted in this cohort of patients. [9]
Using population-based data collected by the NCI Patterns of Care study, our objective was to describe the treatment and survival outcomes of newly diagnosed neuroblastoma patients throughout the United States in 2010–2011 with follow up through 2014.
Materials and Methods
NCI Patterns of Care Data:
The NCI’s Surveillance, Epidemiology and End Results (SEER) program collects information on all cancer diagnosed in defined geographic regions. Currently, SEER covers about 30% of the US population. [10] Information for each patient in SEER is obtained primarily from hospital and pathology records and includes tumor characteristics, treatment, and select demographic characteristics. [11] Because much of the adjunct therapy is provided in an outpatient setting and SEER data collection is primarily hospital-based, the NCI annually conducts a more comprehensive data collection on a sample of patients diagnosed with specific cancers under the Patterns of Care/Quality of Care studies. [12] More detailed information about this study is available at the NCI website. [13]
Each SEER registry obtained approval, as necessary, from their Institutional Review Board before study initiation. After a central training, abstractors from the 14 participating SEER registries (the urban metropolitan areas of San Francisco/Oakland/San Jose/Monterey, Detroit, Seattle, Atlanta, and Los Angeles County; the states of Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah; and the remainder of California) re-abstracted the hospital records of patients to verify tumor characteristics, demographic and treatment information. Each patient’s physician was asked to verify the patient’s risk profile and details of treatment (radiation, chemotherapy, and/or immunotherapy, including the use of novel agents), as well as whether the patient was registered on an open/active clinical trial. In the case of facilities such as Health Maintenance Organizations (HMOs) or hospitals with consolidated inpatient and outpatient records, this information was utilized for treatment verification. For data quality control, 5% of patients’ records were also re-abstracted by a second cancer registrar.
Patient Sample:
Using data collected from the NCI’s Patterns of Care study, all patients less than 20 years of age, registered in the SEER region with newly diagnosed, histologically confirmed neuroblastoma in 2010 and 2011 were eligible. There were 268 histologically confirmed neuroblastoma patients in SEER. Patients (n=5) without histological confirmation were ineligible. Patterns of care data were available for 252 patients. Because olfactory neuroblastoma (esthesioneuroblastoma) is treated differently, these patients (n=2) were excluded, leaving the treatment patterns of 250 childhood neuroblastoma patients for analyses.
Patient, Hospital and Treatment Measures:
After stratifying by the COG risk classification system, bivariate analyses of patient sociodemographic characteristics included age at diagnosis (<1 year, 1–2 years, 2–3 years, 3–4 years and 4+years), sex, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic or other), insurance (any private or public-based) were performed. Bivariate analyses of clinical characteristics included year of diagnosis (2010 or 2011), tumor primary site, INSS stage (1, 2A/2B, 3, 4 or 4S), and hospital/physician characteristics: hospital bed size (<200, 200–499, or ≥500), ownership (government, not-for-profit), subspecialty consulted (pediatric hematology/oncology, oncology/hematology, radiation oncology, surgery, internal medicine, or other/unknown) and number of physician subspecialties consulted.
Modes and sequence of treatment included cancer-directed surgery and/or radiation to the primary site, stem-cell rescue transplantation and systemic therapies including chemotherapy. Systemic agents were coded with date the agent was first administered and whether therapy was completed or terminated early. Survival following diagnosis was measured through December 31, 2014, which provided a 36 months to 60 months (5-years) follow-up period. For patients who died during this period, date and cause of death was recorded.
Statistical analysis:
All descriptive information, including use of treatment modalities is presented as observed counts and weighted percentages for the study population. These weights were used to calculate percentages that reflected the SEER populations from which the data were obtained. Kaplan-Meier models stratifying by risk profiles, stage and biological characteristics calculated the probability of all-cause mortality during the 60-month follow-up time. SAS version 9.3 (SAS Institute, Cary, NC), Stata version 13.0 (StataCorp LP, College Station, TX) and SUDAAN version 11.0.1 (Research Triangle Institute, Raleigh, NC) were used to conduct all analyses.
Results
In this study’s cohort, high-risk patients (n=100) predominated; with 57 patients classified as intermediate-risk and 36 patients as low-risk patients (Table 1). The remaining 57 patients had an unknown risk status profile due to missing physician verification information. As expected, the clear majority of low (89%) and intermediate-risk (97%) patients were under the age of two. Overall, there were more non-Hispanic white neuroblastoma patients (N=130) in this cohort. Neuroblastoma emanating from the adrenal gland as the primary site was also associated with high-risk disease. Most patients were covered by private health insurance, treated in larger (>200 bed) hospitals, that were non-government, not-for-profit institutions and were seen primarily by a pediatric oncologist/hematologist in combination with another subspecialist.
Table 1:
Sociodemographic, clinical and hospital characteristics of 250 childhood neuroblastoma patients newly diagnosed in 2010–2011
| Variables | Stratifying by COG Risk Group Unweighted numbers Weighted % | |||||
|---|---|---|---|---|---|---|
| Frequency (N) | Low (N=36) | Intermediate (N=57) | High (N=100) | Unknown (N=57) | Overall (N=250) | |
| Sociodemographic characteristics | ||||||
| Age at diagnosis, years | ||||||
| <1 | 82 | 47.2 | 67.9 | 8.1 | 32.1 | 32.8 |
| 1 to 2 | 66 | 41.6 | 28.7 | 23.7 | 19.4 | 26.4 |
| 2 to 3 | 29 | 8.5 | 1.8 | 19.7 | 10.4 | 11.9 |
| 3 to 4 | 27 | 2.7 | 1.6 | 15.9 | 15.4 | 10.6 |
| 4+ | 46 | 0 | 0 | 32.6 | 22.8 | 18.3 |
| Sex | ||||||
| Female | 118 | 33.3 | 51.2 | 46.6 | 52.5 | 47.2 |
| Male | 132 | 66.7 | 48.8 | 53.4 | 47.5 | 52.8 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 130 | 46.9 | 48.1 | 58.2 | 50.9 | 52.6 |
| Non-Hispanic Black | 28 | 8.4 | 5.0 | 14.3 | 12.3 | 10.9 |
| Hispanic | 70 | 33.6 | 36.5 | 19.8 | 30.1 | 27.9 |
| Other (e.g., Native Indian, Asian) | 22 | 11.1 | 10.3 | 7.8 | 6.8 | 8.6 |
| Health insurance coverage | ||||||
| Any private | 151 | 69.0 | 53.7 | 63.6 | 51.6 | 59.4 |
| Public | 99 | 31.0 | 46.3 | 36.4 | 48.4 | 40.6 |
| Clinical characteristics | ||||||
| Primary site (ICD-O-3 code) | ||||||
| Adrenal glands, NOS (749) | 117 | 30.2 | 26.4 | 67.1 | 42.0 | 46.9 |
| Retroperitoneum (480) | 28 | 11.0 | 15.2 | 7.9 | 12.2 | 11.0 |
| Posterior mediastinum (382) | 17 | 5.6 | 14.3 | 2.3 | 9.3 | 7.1 |
| Others (<10 cases) ^ | 88 | 53.1 | 44.1 | 22.6 | 36.6 | 35.0 |
| Tumor stage (INSS Stage) | ||||||
| 1 | 28 | 45.3 | 0 | 0 | 21.9 | 11.3 |
| 2A/2B | 24 | 30.2 | 10.9 | 1.0 | 10.6 | 9.5 |
| 3 | 31 | 0 | 32.1 | 8.8 | 7.0 | 12.5 |
| 4 | 104 | 2.7 | 33.1 | 74.0 | 19.3 | 42.2 |
| 4S | 8 | 0 | 5.2 | 0 | 8.7 | 3.2 |
| Missing/unknown | 55 | 21.7 | 18.8 | 16.2 | 32.5 | 21.3 |
| Open/active clinical trial registration | ||||||
| Registered | 189 | 39.1 | 88.9 | 89.2 | 61.6 | 75.9 |
| Not registered/unknown | 61 | 60.9 | 11.1 | 10.8 | 38.4 | 24.1 |
| Year of diagnosis | ||||||
| 2010 | 127 | 44.3 | 58.4 | 51.1 | 46.8 | 50.9 |
| 2011 | 123 | 55.7 | 41.6 | 48.9 | 53.2 | 49.1 |
| Hospital/physician characteristics | ||||||
| Hospital bed size* | ||||||
| <200 bed | 24 | 16.7 | 13.9 | 7.0 | 5.5 | 9.6 |
| 200–499 bed | 139 | 61.2 | 62.8 | 51.6 | 49.9 | 55.2 |
| 500+ bed | 86 | 22.1 | 23.3 | 41.3 | 44.7 | 35.2 |
| Hospital Ownership* | ||||||
| Government | 31 | 8.1 | 8.2 | 9.2 | 26.4 | 12.6 |
| Non-government, not-for-profit | 216 | 91.9 | 91.8 | 90.8 | 73.6 | 87.4 |
| Subspecialist consulted | ||||||
| Pediatric hematology/oncology | 227 | 78.0 | 91.6 | 96.2 | 89.6 | 91.1 |
| Oncology and/or hematology | 26 | 19.3 | 11.9 | 7.9 | 6.8 | 10.2 |
| Radiation oncology | 81 | 0 | 5.2 | 61.6 | 27.6 | 32.3 |
| Surgery (general/orthopedic) | 105 | 55.0 | 29.0 | 42.8 | 42.3 | 41.2 |
| Internal medicine | 21 | 13.6 | 3.3 | 11.8 | 3.6 | 8.2 |
| Other or unknown specialist | 123 | 24.8 | 61.2 | 46.3 | 61.6 | 50.2 |
| Number of physician subspecialties consulted | ||||||
| 1 | 42 | 33.7 | 23.7 | 7.0 | 15.7 | 16.5 |
| 2 | 86 | 39.0 | 39.9 | 23.3 | 43.6 | 33.9 |
| 3 | 59 | 19.1 | 15.5 | 34.3 | 15.3 | 23.6 |
| 4 | 63 | 8.2 | 20.9 | 35.5 | 25.4 | 26.0 |
| Deceased (12/31/2014) | 52 | 0 | 5.2 | 40.7 | 15.2 | 21.0 |
Abbreviations: COG: Children’s Oncology Group INSS=International Neuroblastoma Staging System, NOS= Not Otherwise Specified
For respondents, hospital characteristics coded as unknown are not placed in any category.
27 other primary sites were identified. Percentages may not add to 100% because of rounding.
Most patients (76%) were registered on an open/active clinical trial. Stratifying by clinical trial registration, these patients did not differ by age, race/ethnicity, risk group, insurance status, or hospital ownership (data not shown). However, significantly more females and high-risk patients were on clinical trials. Clinical trials patients consulted significantly more physician specialties. The predominant trial (n=95) in this SEER-based cohort was a phase III randomized trial of single versus tandem myeloablative consolidation therapy for high-risk neuroblastoma (ANBL0532 - ClinicalTrials.gov identifier: ). This was followed by a phase III study (n=53) Response- and Biology-Based Therapy for Intermediate-risk Neuroblastoma (ANBL0531- ClinicalTrials.gov identifier: ).
In this study’s cohort, the variation in treating this disease is evident. (Table 2) Stratified by risk profile, all 36 patients classified as low-risk patients received surgery, 30% of whom also received chemotherapy. The clear majority (97%) of intermediate-risk patients received chemotherapy with 63% also having received surgery. Aggressive multimodal treatment is standard for high-risk patients. Of the high-risk patients, 86% had surgery, 84% had stem cell rescue transplant and 78% received radiation (not mutually exclusive). Moreover, chemotherapy (99%) was the most often used modality (Table 2) with 32% of patients also receiving dinutuximab immunotherapy, and 30% receiving retinoid therapy (data not shown). Dinutuximab (previously known as ch14.18 monoclonal antibody), was still under investigation at the time data were being collected for this study.
Table 2:
Treatment modality, average time from diagnosis to treatment and sequence of treatment stratified by risk status (weighted %)
| Treatment Modality | Stratifying by COG Risk Group | |||
|---|---|---|---|---|
| Low (N=36) | Intermediate (N=57) | High (N=100) | Unknown (N=57) | |
| Treatment modality (weighted % by risk group: not mutually exclusive) | ||||
| Surgery (n=207) | 100.0 | 63.2 | 86.0 | 86.2 |
| Chemotherapy (n=203) | 30.4 | 96.7 | 99.1 | 66.7 |
| Radiation (n=105) | 0 | 3.4 | 78.4 | 41.3 |
| Stem Cell Rescue Transplant (n= 104) | 0 | 0 | 83.9 | 34.7 |
| Dinutuximab (n=42) | 0 | 0 | 32.0 | 17.2 |
| Retinoid therapy (n=43) | 0 | 9.4 | 29.9 | 13.9 |
| Mean days from date of diagnosis to: | ||||
| Surgery | 4 | 108 | 117 | 69 |
| Chemotherapy | 99 | 10 | 16 | 40 |
| Radiation | NA | NA | 251 | 250 |
| Stem Cell Rescue Transplant | NA | NA | 180 | 213 |
| Dinutuximab | NA | NA | 300 | 298 |
| Retinoid therapy | NA | 371 | 326 | 321 |
| Sequence of treatment (weighted % by risk group) | ||||
| Systemic therapy before surgery | 2.7 | 31.2 | 16.1 | 11.9 |
| Systemic therapy after surgery | 27.5 | 16.0 | 5.9 | 11.3 |
| Systemic therapy both before & after surgery | 2.9 | 14.4 | 64.0 | 33.1 |
| Radiation after surgery | 0 | 1.6 | 72.6 | 34.5 |
| Radiation after systemic therapy | 0 | 1.6 | 42.4 | 20.5 |
| Radiation after stem cell rescue transplant | 0 | 0 | 62.1 | 25.9 |
| Systemic therapy before & after radiation | 0 | 0 | 27.5 | 17.3 |
The mean time-to-initiation of treatment modalities revealed that low-risk patients received their initial therapy, surgery, within a mean of 4 days (min 0 days, max 25 days) from date of diagnosis (Table 2). Intermediate- and high-risk patients were much more likely to receive chemotherapy as initial regimen therapy with mean time-to-initiation of 10 days and 16 days respectively. The median times to initiation of therapy are similar to the mean values, except for chemotherapy use in low-risk patients. A bi-modal distribution emerges with chemotherapy use in low-risk patients giving rise to 2 groups, those who receive chemotherapy within 12 days of diagnosis and those who received chemotherapy after 5 months, increasing the mean to 99 days from diagnosis to initiation.
The sequence of treatment indicated that among histologically confirmed low-risk patients who received additional systemic cancer treatment, most was given post-surgery. (Table 2) The clear majority of intermediate-risk patients received chemotherapy first, whereas high-risk patients received chemotherapy before and after surgery. Many high-risk patients then received stem cell rescue transplant, radiation, as well as other systemic therapies. The mean time between date of diagnosis and transplant was 180 days (min 35 days, max 276 days) in the high-risk group. Only two patients were recorded as having an allogenic rather than autologous transplant (data not shown).
Systemic Therapies
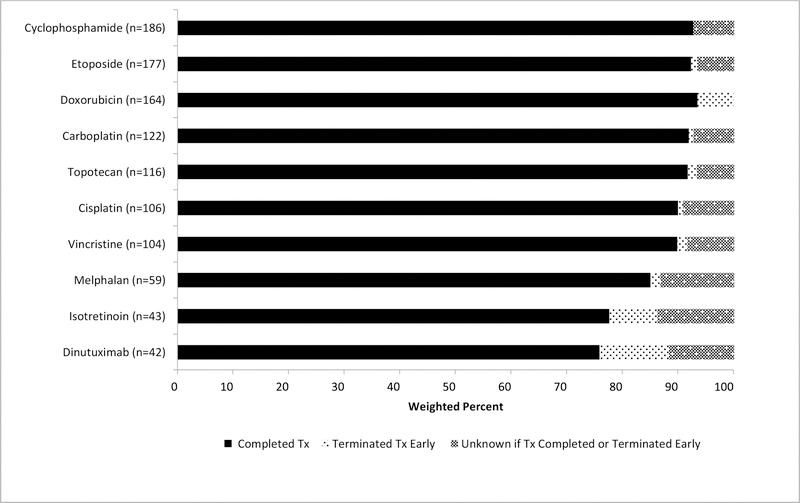
Given the nature of the cancer and multimodal treatment options available, various systemic therapies were administered. In this study, cyclophosphamide was the chemotherapeutic agent most frequently administered (n=186). (Figure 1) Patients were most likely to have completed doxorubicin (94%), with dinutuximab most likely to be terminated early (13%) when administered.
Figure 1:
Percentage completion and termination of top 10 systemic agents in Neuroblastoma diagnosed in 2010–11 (weighed)
Among low-risk patients, approximately 11% received a regimen consisting of carboplatin and etoposide followed by cyclophosphamide and doxorubicin; an additional 17% received only a single agent, cyclophosphamide, carboplatin or cisplatin (data not shown). Among intermediate-risk patients 81% received a regimen consisting of carboplatin, etoposide, cyclophosphamide and doxorubicin. Of the high-risk patients on clinical trials, 81% received multiple permutations of chemotherapies (data not shown). Approximately, two out of three patients (65%) received five to eight different chemotherapy agents and one in five (21%) received nine or more. (Table 3)
Table 3:
Percentage of chemotherapy agents in neuroblastoma patients diagnosed in 2010–11 by risk status (weighed)
| Chemotherapy* | Stratifying by COG Risk Group | |||
|---|---|---|---|---|
| Low (N=36) | Intermediate (N=57) | High (N=100) | Unknown (N=57) | |
| (weighted % by risk group) | ||||
| No chemotherapy | 69.6 | 3.3 | 0.9 | 33.3 |
| 1–4 Chemotherapy agents | 30.4 | 76.6 | 12.5 | 32.1 |
| 5–8 Chemotherapy agents | 0 | 20.1 | 65.1 | 34.6 |
| 9–11 Chemotherapy agents | 0 | 0 | 21.4 | 0 |
Chemotherapy agents from: Cyclophosphamide, etoposide, doxorubicin, carboplatin, topotecan, cisplatin, vincristine, melphalan, busulfan, ifosfamide, temozolomide, thiotepa, daunorubicin, vinblastine, fludarabine, dactinomycin and irinotecan.
At the time of data abstraction (2013 in 96% of cases), the clear majority of high-risk patients (85%), had received chemotherapy and surgery. Of the patients who received dinutuximab, 96% had a stem cell transplant and 72% were recorded to be part of the COG study (ANBL0532). In 57% of patients given dinutuximab, granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-2 (IL-2) were also administered (data not shown). Retinoid therapy, initiated close to a year (mean = 318 days, min 67 days, max 505 days) after diagnosis, was administered to about a third (30%) of all high-risk patients (data not shown).
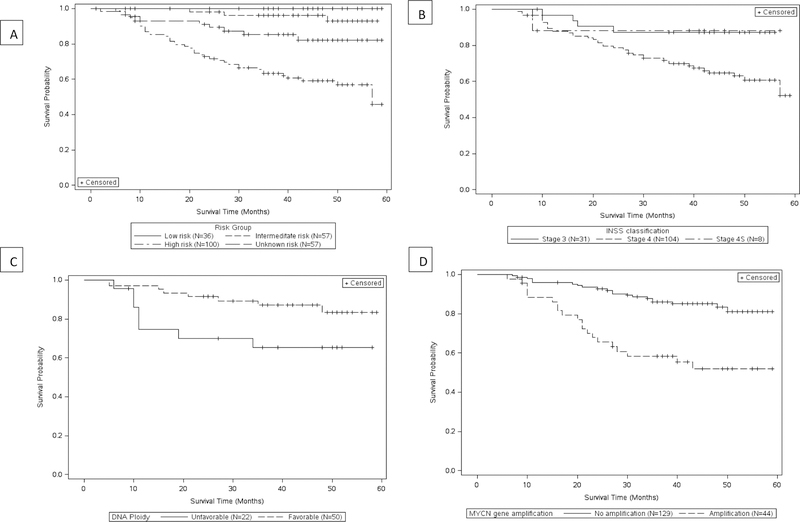
Overall, 52 patients (21%) had died, with 41% of high-risk patients in the cohort dying (Table 1). The time between initiation of the final therapy and death was within 3 months for 36%, between 4 and 11 months for 25%, and 12 months or more for 39% of patients that had died. The cause of death was coded as being related to the ‘endocrine system’ in 90% of the decedent patients (data not shown). The estimated 5-year Kaplan-Meier survival curve for high-risk is approximately 46% compared to 93% for intermediate-risk patients (Figure 2a). Stratifying by stage (INSS), MYCN gene amplification and DNA ploidy provides additional information on prognostic value of such variables. Within this cohort, patients with stage 4 diagnosis, unfavorable DNA ploidy and MYCN gene amplification had poorer survival rates (Figures 2b–d).
Figure 2:
Kaplan-Meier all-cause survival curve for childhood neuroblastoma patients by A) risk profile B) INSS staging C) DNA ploidy D) MYCN gene amplification
Discussion
Findings from this population-based study of patients with pathologically confirmed childhood neuroblastoma highlight the clinical heterogeneity of patients and the treatments they received. This observational study captures information across health care settings within the US. Approximately 76% of patients, received all or part of their treatment on a clinical trial. International evidence shows that survival rates are better within the strict framework of clinical trials. [14] The 24% of patients not on protocol were significantly more likely to be males and were seen by significantly fewer physician specialties. These children were significantly more likely to be low-risk patients than patients on clinical trials. It is probable that there were more open clinical trials for higher risk patients.
All patients in this study received some form of treatment. Only 5 patients in 2010 and 2011 registered in the SEER database had no histologic confirmation of neuroblastoma. It is possible that patients without histologic confirmation were not reported to the SEER program, therefore these data may under-represent the true prevalence of the low-risk neuroblastoma patients.
In this cohort, low- and intermediate-risk patients were younger in age, received less intensive treatment and had better survival outcomes, which is consistent with the literature. [15] A phase 3 nonrandomized trial of intermediate-risk patients highlighted the need for more refined risk stratification to support further appropriate reduction in chemotherapy use. [16] However, most published research in treating neuroblastoma focuses on improving the poor survival rates of high-risk patients. In this cohort, 41% of high-risk patients died. Overall 39% of the neuroblastoma patients in our study died 12 months or more after the initiation of the last treatment modality, which suggests that their death was due to either disease progression or recurrence.
In this analysis of neuroblastoma patients most were on clinical trials. Dinutuximab was not approved by the Food and Drug Administration (FDA) at the time these patients were diagnosed. Of patients receiving this immunotherapy, 42% received it without both interleukin-2 and GM-CSF, which was not per protocol. Most patients in the dataset who were administered dinutuximab (93%) were on a registered clinical trial. For many high-risk patients, the standard approach includes isotretinoin. We observed that only 30% of high-risk patients in our cohort received isotretinoin. Some died prior to reaching that stage of treatment, others may have had disease progression while still others were simply not being treated with standard approaches.
In comparing our findings with other studies, our data provided a detailed snapshot of treatment administered to patients diagnosed in 2010 and 2011. In terms of treatment with specific chemotherapeutic agents, a 2009 report based on 823 long-term childhood neuroblastoma survivors from the Childhood Cancer Survivor Study (CCCS) diagnosed between 1970 and 1986, found a total of over 20 different chemotherapeutic agents were used. [17] Similar to our findings, cyclophosphamide (43%), vincristine (37%) and doxorubicin (27%) were the three most frequently used agents. [17] In contrast, decarbazine (20%), teniposide (10%) and mechlorethamine/nitrogen mustard (4%) were used in the CCCS study patients but not recorded in the more contemporary Patterns of Care dataset.
The emerging immunotherapy, dinutuximab, approved by the FDA in March 2015, is one of the newer treatments for neuroblastoma patients. [18] The results of the phase III randomized study of dinutuximab in high-risk neuroblastoma following myeloablative therapy and autologous stem cell rescue () published in 2010 showed a superior outcome for the treatment-arm patients (n=113) who received this immunotherapy-based treatment compared to standard therapy. [19] It is anticipated that immune-based therapies will be part of combinatorial regimens likely to emerge during the next decade. [20] The main immune therapies relevant to neuroblastoma are cytokine, vaccine, antibody and cellular therapies. [21] It appears the future of pharmacotherapy for neuroblastoma lies in capturing the unique, multifaceted molecular signature of a particular neuroblastoma and differentiating it from normal tissue. [22]
Stratifying by treatment risk category, our survival analysis also found that low- and intermediate-risk patients had excellent survival, similar to the findings of prominent clinical trials. [16] [23] In this cohort with a maximum of 60 months of follow-up, the estimated 5-year survival was 45% in high-risk patients. This was near the 50% 5-year survival reported for patients diagnosed between 2005 and 2010; an improvement on the 29% for patients diagnosed between 1990 and 1994. [8] This increase in overall survival has been attributed to the introduction of myeloablative therapy and immunotherapy. [7] However, a report from the CCCS on late mortality and long-term outcomes in survivors of neuroblastoma show that 6% of patients (N=954) died more than 5-years after their diagnosis with the most common causes of death being disease recurrence and second malignant neoplasms. [24]
Limitations
Despite the strengths of utilizing a large population-based sample to analyze treatment information among patients with histologically confirmed neuroblastoma, our study has limitations. Study numbers limited sub-type analysis (i.e., <10 patients were stage 4S). A substantial number of patients had no known risk profile due to lack of verification by their physician. Data from these patients were included in our analyses but we are unable to draw any substantial conclusions from those with unknown risk profiles. For a small number of patients, date of death is after the date of treatment information abstraction and it is possible that these patients received additional treatment not captured in this dataset. The data were observational and unmeasured patient characteristics may have influenced both treatment selection and survival following diagnosis. Parent/guardian preferences or physician recommendations were not captured. In addition, the registries’ follow-up includes only vital status and does not include information on progression, recurrence of relapse for any patient.
Future Research
This cohort of childhood neuroblastoma patients can be prospectively followed to give further long-term survival data. Furthermore, the NCI’s Patterns of Care studies may again collect treatment information on childhood neuroblastoma in the future, which will enable a comparison on how the newer therapies have diffused into practice and whether survival metrics have improved. Of interest, will be the shift in reducing treatment in low- and intermediate-risk patients and the promise of novel treatments for high-risk patients. This comparison will likely be based on the refinement in risk classification that has emerged from INRG to define 19 pretreatment groups stratified by prognostic markers with 4 risk groups (very-low, low, intermediate and high) for treatment. [7]
Conclusion
Analysis of this population-level dataset found that risk characterization was the most important variable in predicting treatment and survival for neuroblastoma patients. Variation exists in the use of treatment modalities, but being on open/active clinical trial was common for these patients. Evaluation of the diffusion of newer targeted agents and dissemination of improved protocols for treating high-risk neuroblastoma in community practice will be important for future research.
Acknowledgements
This study was made possible through the efforts of the Principal Investigators and the cancer registry personnel at the participating Surveillance, Epidemiology and End Results registries.
Disclosure: This work was supported by National Cancer Institute contracts: HHSN261201000024C; HHSN261201000025C, HHSN261201000032C, HHSN261201000027C, HHSN261201000026C, HHSN261201000140C, HHSN261201000037C, HHSN261201000033C, HHSN261201000034C, HHSN261201000035C, HHSN261201000029C, HHSN261201000031C, HHSN261201000028C, and HHSN261201000030C.
Footnotes
Conflict of interest statement: The authors report no conflicts of interest.
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. This article was produced by employees of the US government as part of their official duties and, as such, is in the public domain in the United States of America. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- [1].Jiang M, Stanke J, Lahti JM. The Connections Between Neural Crest Development and Neuroblastoma. Curr Top Dev Biol 2011;94:77–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003;3:203–16. [DOI] [PubMed] [Google Scholar]
- [3].St. Jude’s Children Research Hospital Website. Neuroblastoma. https://www.stjude.org/disease/neuroblastoma.html (Accessed June 14, 2017).
- [4].Nuchtern JG, London WB, Barnewolt CE, Naranjo A, McGrady PW, Geiger JD, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg 2012;256:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am 2008; 55:97–120. [DOI] [PubMed] [Google Scholar]
- [6].PDQ® Pediatric Treatment Editorial Board. PDQ Neuroblastoma Treatment. Bethesda, MD: National Cancer Institute; Updated 04/14/2017 Available at: https://www.cancer.gov/types/neuroblastoma/patient/neuroblastoma-treatment-pdq Accessed 06/15/2017. [Google Scholar]
- [7].Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. Neuroblastoma. Nat Rev Dis Primer 2016; 2:16078. [DOI] [PubMed] [Google Scholar]
- [8].Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol 2015;33:3008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KK, et al. Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013;60:985–93. [DOI] [PubMed] [Google Scholar]
- [10].Surveillance Research Program. Number of Persons by Race and Hispanic Ethnicity for SEER Participants (2010 Census Data). Bethesda, MD: http://seer.cancer.gov/registries/data.html Accessed 01/06/2017. [Google Scholar]
- [11].Banegas MP, Harlan LC, Mann B, Yabroff KR. Renal cell cancer: a shift in approaches for treatment of advanced disease in the United States. J Natl Compr Cancer Netw 2014;12:1271–9. [DOI] [PubMed] [Google Scholar]
- [12].Harlan LC, Eisenstein J, Russell MC, Stevens JL, Cardona K. Gastrointestinal stromal tumors: treatment patterns of a population-based sample. J Surg Oncol 2015;111:702–7. [DOI] [PubMed] [Google Scholar]
- [13].Patterns of Care/Quality of Care Studies. Bethesda, MD: National Cancer Institute; Updated 02/24/2017 Available at: https://healthcaredelivery.cancer.gov/poc/ Accessed 06/12/2017. [Google Scholar]
- [14].Di Cataldo A, Agodi A, Balaguer J, Garaventa A, Barchitta M, Segura V, et al. Metastatic neuroblastoma in infants: are survival rates excellent only within the stringent framework of clinical trials? Clin Transl Oncol 2017; 19(1): 76–83. [DOI] [PubMed] [Google Scholar]
- [15].Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther 2017;17:369–86. [DOI] [PubMed] [Google Scholar]
- [16].Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 2010;363:1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229–39. [DOI] [PubMed] [Google Scholar]
- [18].FDA Press Announcements - FDA approves first therapy for high-risk neuroblastoma. Silver Spring, MD: Food and Drug Administration; Updated 03/10/2015 Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm437460.htm Accessed 04/20/2017. [Google Scholar]
- [19].Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mackall CL, Merchant MS, Fry TJ. Immune-based therapies for childhood cancer. Nat Rev Clin Oncol 2014;11:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Navid F, Armstrong M, Barfield RC. Immune Therapies for Neuroblastoma. Cancer Biol Ther 2009;8:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ganeshan VR, Schor NF. Pharmacologic management of high-risk neuroblastoma in children. Paediatr Drugs 2011;13:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Strother DR, London WB, Schmidt ML, Brodeur GM, Shimada H, Thorner P, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. J Clin Oncol 2012;30:1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laverdière C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2009;101:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]