Abstract
Objectives:
Breast feeding protects infants from many diseases, including necrotizing enterocolitis, peptic ulceration and infectious diarrhea. Conversely, maternal separation stress and Non-Steroidal Anti-Inflammatory Drugs (NSAID’s) can induce intestinal injury and bleeding. This study aimed to evaluate in suckling rats if maternal separation/formula feeding leads to increased intestinal sensitivity to indomethacin (indo)-induced intestinal injury and to look at potential mechanisms involved.
Methods:
Nine-day-old rats were dam-fed or separated/trained to formula-feed for 6 days prior to indo administration (5 mg/kg/day) or saline (control) for 3 days. Intestinal bleeding and injury were assessed by measuring luminal and Fecal Hemoglobin (Hob) and jejunal histology. Maturation of the intestine was assessed by measuring luminal bile acids, jejunal sucrase, serum corticosterone, and mRNA expression of ileal Apical Sodium-Dependent Bile Acid Transporter (ASBT).
Results:
At 17 days, formula-fed indo-treated pups had a 2-fold increase in luminal Hb compared to formula-fed control pups and had evidence of morphological injury to the small intestinal mucosa as observed at the light microscopic level, whereas indo had no effect on dam-fed littermates. In addition, formula-fed rats had significant increases in luminal bile acid, sucrase specific activity, serum corticosterone, and expression of ASBT mRNA compared to dam-fed rats.
Conclusion:
Maternal separation stress may cause early intestinal maturational changes induced by corticosteroid release, including increased epithelial exposure to bile acids. These maturational changes may have a sensitizing rather than protective effect against indo-induced injury in the new-born.
Keywords: Indomethacin, Intestinal injury, Formula feeding, Intestinal maturation, Premature infants
Introduction
Infants are exposed to Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) when they develop fever, during medical induction of Patent Ductus Arteriosus (PDA) closure, and when they are at risk for thromboembolic events. NSAIDs can induce gastroduodenal and small intestinal injury in both animals and humans via inhibition of prostaglandin synthesis, decrease in mesenteric blood flow and elimination by biliary excretion. The use of indo either prenatally (to delay labor) or postnatally in low birth weight neonates (to induce PDA closure) has been linked to gastrointestinal (GI) bleeding, spontaneous intestinal perforation, and necrotizing enterocolitis (NEC) [1–3]. Bile acid accumulation in the GI lumen may play a role in enhancing intestinal injury by this class of drugs [4] as suggested by prior adult animal studies that demonstrated prevention of NSAID-induced damage in the distal small intestine by bile duct ligation [5]. Maternal separation has been shown to increase intestinal permeability [6] and to induce pro-inflammatory cytokines in the intestine [7]. Cow-milk formula feeding could increase both TH1 (IFNγ) and TH2 (IL4 and IL5)–type cytokines [8] in the intestine of new-born rats. On the protective side, breast feeding protects against NEC [9,10], peptic ulceration [11] and infectious diarrhea [12]. Traditional NEC/acute stress injury has been established in rodent models by separating new-born pups from their dam, feeding with cow-milk formula, and exposure to hypoxia and/or cold stress [13–15]. In this study, we separated the new-born rats from their dams, but instead of hypoxia or cold stress, we trained the new-born rats to self-feed with cow-milk derived formula. This approach allowed us to assess if separation and formula feeding increases the susceptibility of neonatal rats to indomethacin-induced (indo-induced) intestinal injury in the absence of the stress of repeated handling for gavage feeding with insertion of orogastric tube multiple times daily (which would likely have been more stressful for the animals). Additionally, our model also helped to provide insight into the role of NSAID/bile acid interactions in this pathogenic mechanism.
Materials and Methods
Animal model and experimental design
Ethics statement:
Use and handling of rat pups were in accordance with guidelines from the National Institute of Health for humane handling of animals and were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee (Animal approval protocol # HSC-AWC12–040).
Experimental groups:
Three litters of Sprague-Dawley strain rat pups (generally consisted of 6–9 pups per litter) were used for each experiment starting at day of life 9 (DOL). Study groups were designated as followed: Dam-fed control group remained with the nursing dam and received subcutaneous injections of saline (0.1 mL) starting on DOL 14 for 3 days; dam-fed indo group staying with the nursing dam but receiving subcutaneous injections of indo at a dose of 5 mg/kg body weight/day for 3 days; formula-fed control group separated from the dam and receiving saline injections for 3 days; and formula-fed indo group separated from dam and receiving indo injections for 3 days. Dose of indo selected was found by our experience as the lowest dose of indo to be administered to rats without causing significant mortality over a chronic dosing period (4–6 days). Also, in a study from 1999 by Morise et al., various doses of indo were compared with regard to lesions scores and mucosal permeability; the group found that injury occurred in a dose-dependent manner with doses ranging from 5–40 mg/kg and injury was seen at 5 mg/kg dose [16]. Rat pups were observed and body weights were measured daily. Intestinal tissues were collected on DOL 17.
Self-feeding training method and formula composition
Formula was prepared by adding 80 g of Esbilac powder (puppy formula–PetAg Inc., Hampshire, IL) and 45 g of PM 60/40 (infant formula–Abbott Nutrition, Columbus, OH) to 250 mL of water to yield about 300 mL of formula mixture. Similac PM 60/40 plus Esbilac (canine supplement formula) mixture has been used in past NEC studies with the rat pup model [8,10]. The formula mixture simulates rat breast milk with respect to the composition of protein, fat, and calories [17]. Formula-fed animals were hand-fed for the first 2 days after separation, while they were trained to feed from a trough/reservoir set up in their cages. A beaker containing formula was placed on a continuous heat source with constant stirring. Silicone tubing was used to run the formula from this beaker down to the trough/reservoir set up within each cage. A pump attached to a timer was set to run 15 mL of formula from the beaker into the trough every 3 h. Rat pups were observed to be able to drink directly from the trough and formula was visible in their stomach through the abdomen.
Medications
Indomethacin (Sigma Aldrich, St. Louis, MO) was subcutaneously administered at dose of 5 mg/kg body weight or the equivalent volume of saline (0.1 mL) to control rat pups once daily for 3 days. Briefly, indo powder was mixed into phosphate buffer saline (PBS). To dissolve the indo into solution, we used NaOH to increase the pH to 9. Once the indo dissolved, we readjusted the pH back to 7.4 using HCl. The solution was then diluted with PBS to the necessary concentration to get 5 mg/kg dose into a volume of 0.1 mL. The solution was filtered prior to administration.
Tissue collection
At DOL17, blood was collected by cardiac puncture when animals were anesthetized by using 4% isoflurane for hematocrit and corticosterone analysis. The small intestine was resected from the pyloric junction to the cecum and measured. Ice-cold saline was used to flush the resected small bowel, and the fluid was collected and stored at −20°C for subsequent biochemical analyses. The small intestine was then divided into 3 equal sections. The terminal ileum was frozen immediately in liquid nitrogen and stored −80°C for isolation of RNA and to measure mRNA expression of the ASBT. The jejunum was collected, divided equally for RNA and histologic evaluation, respectively. The intact colon was opened longitudinally. The contents from the colon were collected and subsequently extracted to measure Hemoglobin and bile acid levels.
Quantitative Hemoglobin (Hb) assay
Hb concentration was measured in the intestinal flush and colonic contents as described [18]. Briefly, 1 mL of a benzidine solution containing 1% (w/w) of benzidine dihydrochloride in 90% (v/v) of glacial acetic acid was placed in a test tube. After addition of 0.02 mL of the intestinal flush, 1 mL of 1% of hydrogen peroxide was added and mixed. A diluent containing 10% (v/v) glacial acetic acid was added and allowed to stand for 10 min, followed by observation of a color change (~20 min). Absorbance at 515 nm was measured by a spectrophotometer and Hb concentration (mg/mL) was calculated by comparison to a standard curve.
Morphologic analysis
The jejunal tissue was fixed in formalin, embedded in paraffin, and longitudinal sections were stained with hematoxylin and eosin. The villus length (villus tip to the junction at the crypt), crypt depth, and number of mitotic bodies (per 10 aligned crypts) were determined by a “blinded observer,” and the mean and standard deviation were calculated for each animal.
Measurement of marker enzyme activity, protein, mRNA expression and bile acid levels
Sucrase activity in the intestinal tissue lysates was measured as previously described by Dahlquist [19]. Briefly, intestinal homogenate was placed in 37°C water bath for 1 h in the presence of sucrose-buffer solution. Glucose production was measured using glucose oxidase. Sucrase-specific activity was expressed in sucrase activity units/mg of protein. Protein concentration in jejunal tissue homogenate was determined using the Bradford Assay (Bio-Rad).
Apical Sodium-Dependent Bile Acid Transporter (ASBT) mRNA expression by real-time PCR
Expression of the ASBT and the housekeeping gene cyclophilin was measured by real-time PCR as described [20] using primers: rat ASBT (5’-GGTTGCGCTTGTTATTCCTGT-3’; 5’-GGTTCAATGATCCAGGCACTT-3’); rat cyclophilin (5’-ACGTCGTTTTCGGCAAAGT-3’; 5’-CTTGGTGTTCTCCACCTTCC-3’). Values are means of triplicate determinations using RNA from individual animals or pools of RNA samples as indicated, and expression was normalized using cyclophilin.
Measurement of intestinal luminal total bile acid and serum levels of corticosterone
Intestinal luminal total bile acid concentration was measured using a colorimetric total bile acid assay kit from Diazyme Laboratories, USA as described [21].
Serum levels of corticosterone were measured using a Cayman’s Corticosterone EIA kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instructions.
Statistical analysis
Data were represented by mean + SE and visually assessed for normality with histograms. Statistical comparison between groups was performed using one-way Kruskal-Wallis analysis and pairwise test significance verified by Wilcoxon Rank-Sum tests in State SE 13 (College Station, TX: StataCorp LP). Differences were considered statistically significant at two-sided p<0.05.
Results
Measures of growth
Body weight:
The 9 day-old rat pups exhibited similar body weights at the beginning of the study. The growth rate over the following 4 days (day of life 9–12) was approximately 2 g/day in dam-fed pups versus 0.3 g/day in the formula-fed group. After this period for acclimation to formula-feeding leading to a lag in growth as the rats learned to self-feed, the growth rate for the formula-fed groups improved to approximately 2 g/day, compared to the approximately 2.5 g/day observed in the dam-fed groups during this period (days 13–17). Indomethacin treatment had no significant effect on body weight, compared to the control saline-treated group.
Evaluation for indo induced intestinal injury
Hb concentration of intestinal flush and colonic contents:
The luminal Hb concentration was significantly increased in the small intestinal contents from the indo-treated versus saline formula-fed groups (p=0.024) but there was no difference between the indo-treated formula-fed vs. saline-treated dam-fed groups (p=0.102) nor between the indo-treated vs. saline-treated groups among the dam-fed groups (p=0.818) (Figure 1). The Hb concentration measured in colonic contents of the indo-treated vs. saline-treated formula-fed groups did not show a significance difference (p=0.382).
Figure 1:
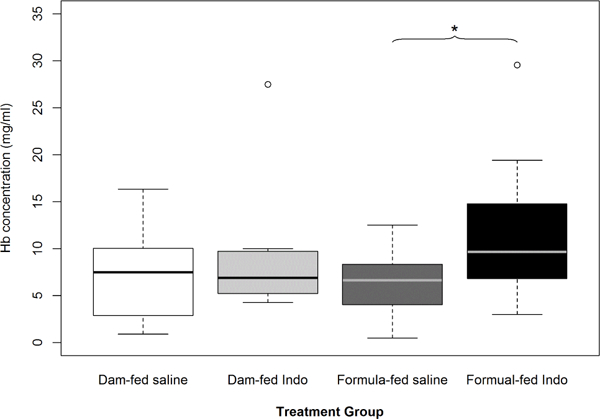
Effects of formula-feeding and indomethacin treatment on luminal hemoglobin (Hb) levels in rat pups. Hb was measured in the small intestinal contents isolated from the indicated groups of rat pups after they have received the 3rd dose of indo. Luminal Hb levels were significantly increased in formula-fed rat pups that received indo versus saline but not in the respective dam-fed groups (p=0.024). Mean values ± SE are shown (n=14 to 18 per group). An asterisk indicates significant differences (*p<0.05) between the indicated groups.
Mortality
No deaths were observed in the dam-fed groups. The formula-fed group that received indo had 2 deaths (one that occurred prior to start of indo on DOL 13 and one on day 3 of indo administration) while the formula-fed group that received saline had 1 death (which occurred prior to start of indo on DOL 13). The deaths could not be directly attributable to any specific cause, as the remains had too greatly deteriorated to determine if intestinal injury was present. The deaths were not included in the data analysis.
Intestinal histology
Histological examination and morphometric measurements of jejunal sections were performed for all 4 groups. Light microscopic histological analysis revealed that the morphology appeared to be altered by formula-feeding and indo treatment. Morphometric measurements revealed a numeric difference in mean villus height between formula-fed versus dam-fed groups but this difference was not statistical significant (Figure 2a).
Figure 2:
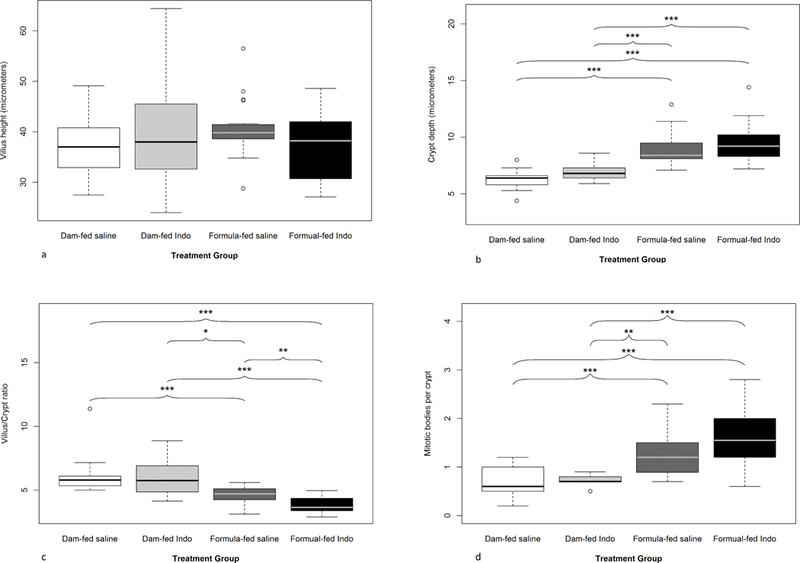
Effects of formula-feeding and indomethacin treatment on intestinal morphology. Quantitative morphometric analysis of the small intestine from the indicated groups was performed. (a) Villus height, (b) crypt depth, (c) villus to crypt ratio, and (d) average number of mitotic bodies per crypt. Mean values ± SE are shown (n=9 to 21 pups per group). An asterisk indicates significant differences (*p<0.05; **p<0.01; ***p<0.001).
Crypt depth comparison showed that formula-fed groups (saline and indo) always had deeper crypts compared to their dam-fed counterparts (Figure 2b), whereas indo treatment induced modest but not statistically significant increases in crypt depth compared to saline controls in each group (dam vs. formula-fed). The villus to crypt ratio (V: C ratio) was calculated. Formula-fed groups had lower V: C ratios as compared to dam-fed groups. In the formula-fed groups, there was a further decrease in V: C ratio in the indo treated group (p=0.003) while no significant difference was observed between the saline vs. indo groups in dam-fed groups (p=0.764) (Figure 2c).
Mitotic bodies in crypts were evaluated as a measure of mucosal injury with ongoing crypt cell proliferation for repair (Figure 2d) [12]. Formula-fed pups treated with indo had the highest average number of mitotic bodies per crypt but did not reach statistical significance (1.6 ± 0.1 vs. 1.2 ± 0.1, p=0.054); in the indo treated vs. saline in formula-fed groups) (Figure 2).
We found that, overall, formula-fed animals had a higher average number of mitotic bodies per crypt (1.4 ± 0.08) when compared to dam-fed animals (0.7 ± 0.06), p=0.0002 (Figures 3a-3d).
Figure 3:
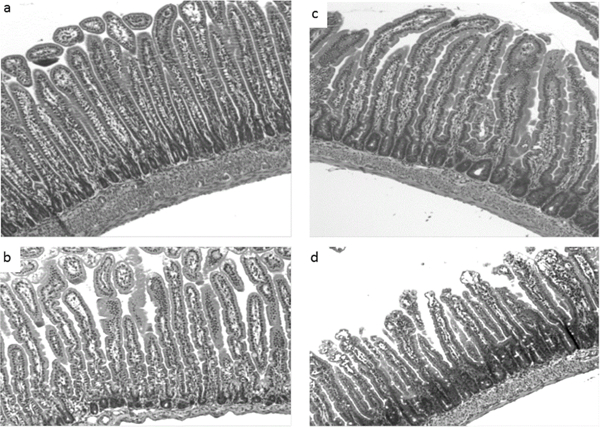
Effects of formula-feeding and indomethacin treatment on intestinal villus and crypt morphology. Representative light micrographs of hematoxylin-eosin-stained transverse sections of jejunum from the indicated treatment groups are shown. (a) Dam-fed saline, (b) Dam-fed indo (c) Formula-fed saline, (d) Formula-fed indo. Formula-fed saline treated pups have significantly deeper crypts (3c) versus the dam-fed saline treated group (3a). The crypts were significantly deeper in the indo-treated formula-fed group (3d) but not dam-fed pups (3b). Images are shown at 10X magnification.
In dam-fed groups, no difference in the number of mitotic bodies was observed between indo versus saline-treated groups (0.7 ± 0.05 vs. 0.7 ± 0.09,) p=0.736; (Figures 4a-4d). These findings demonstrated that the indo-associated microscopic injury at the villous surface (with increased crypt cell proliferation to compensate) occurred only in the formula-fed rat pups, not in the breast-fed pups.
Figure 4:
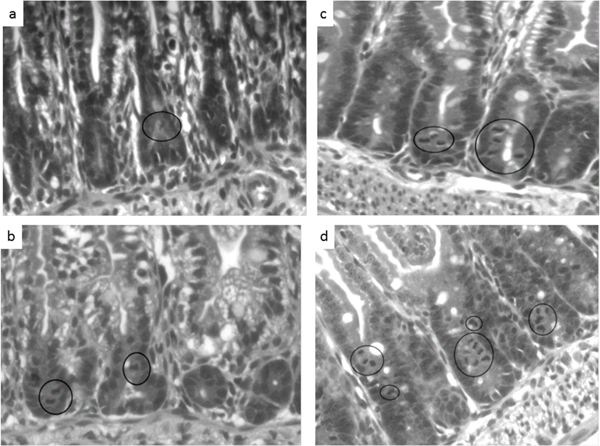
Effects of formula-feeding and indomethacin treatment on intestinal crypt morphology. Representative light micrographs of hematoxylin-eosin-stained transverse sections of jejunum from the indicated treatment groups are shown. (a) Dam-fed saline, (b) Dam-fed indo (c) Formula-fed saline, (d) Formula-fed indo. The mitotic bodies are indicated by the black circles. The formula-fed indo pups (4d) had the highest average number of mitotic bodies per crypt among the treatment groups. No statistically significant different in the number of mitotic bodies was observed in dam-fed saline versus indo treated pups. Images are shown at 10X magnification.
Measures of intestinal development/maturity/stress
Jejunal sucrase activity:
Sucrase activity was measured as a marker of intestinal maturation. The dam-fed groups exhibited a normal low level of sucrase activity typical of immature intestine (0.5 ± 0.2 and 0.3 ± 0.05 units/mg of protein in indo and saline treated groups, respectively). In contrast, sucrase activity was elevated 8–10-fold in the formula-fed rat pups at the same age (3.3 ± 0.4 and 3.5 ± 0.4 units/mg of protein in indo and saline treated groups, respectively), levels that are approximately 50% of those present in adult rats (Figure 5a).
Figure 5:
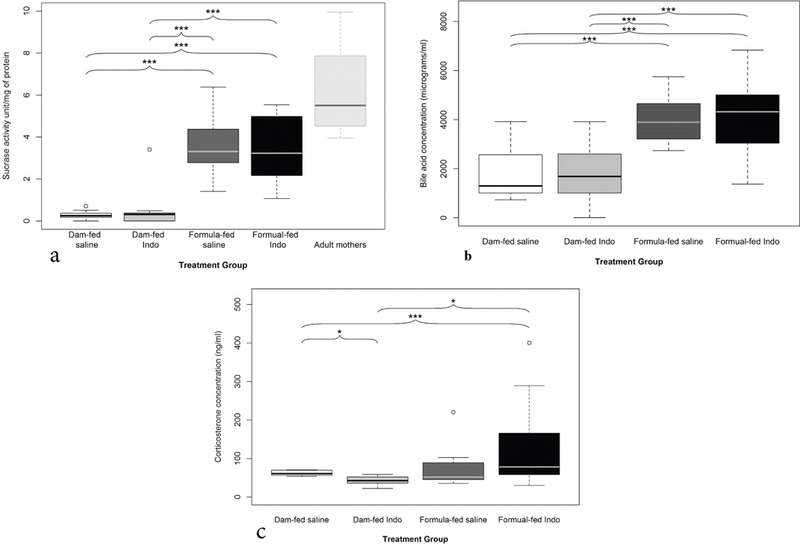
Markers of intestinal maturity. a) Effects of formula-feeding and indomethacin treatment on intestinal sucrase activity. Sucrase activity (as a marker of intestinal maturation) was measured. Mean values ± SE are shown (n=14 to 17 per group). An asterisk indicates significant differences (***p<0.001) between the indicated groups. b) Effects of formula-feeding and indomethacin treatment on luminal bile acid levels. Luminal bile acid concentration from the intestinal flush was measured by enzymatic assay. Mean values ± SE are shown (n=13 to 18 per group). An asterisk indicates significant differences (***p<0.001) between the indicated groups. c) Effects of formula-feeding and indomethacin treatment on corticosterone levels in rat pups: The formula-fed group overall had higher levels of corticosterone than dam-fed groups but did not reach statistical significance (mean 109 ± 19 ng/mL vs. 52 ± 5 ng/mL; p= 0.074, mean data not shown). Indo treated formula-fed group had higher corticosterone level than indo-treated dam-fed group (131 ± 29 ng/mL, n=15 vs. 42 ± 6 ng/mL, n=5, *p<0.05).
Luminal bile acid levels:
Luminal bile acids were measured from the intestinal flush obtained during intestinal collection (Figure 5b). There was a significantly higher concentration of luminal bile acid in formula-fed groups (4,062 ± 344 and 3,974 ± 223 μg/mL in indo and saline treated groups, respectively) compared to dam-fed rat pups (1,933 ± 363 and 1,733 ± 274 in indo and saline treated groups, respectively; p<0.0001. Indo treatment did not affect luminal bile acid levels versus saline (p=0.818 and 0.658 for dam and formula-fed groups, respectively).
Corticosterone level:
Serum corticosterone levels were also measured in the rat pups. Formula-fed pups (saline and indo treated) were found to have higher corticosterone levels (109 ± 19 ng/mL; n=25) than pups in the dam-fed group (52 ± 5 ng/mL, n=10) but did not reach statistical significance (p=0.074). Indo treated groups that were formula-fed had significantly higher circulating corticosterone levels than the dam-fed group that received indo (mean 131 ± 29 ng/mL, n=15 vs. 42 ± 6 ng/mL, n=5; p=0.01) (Figure 5c). When comparing formula-fed groups that received saline vs. indo treatment, corticosterone levels were increased in animals injected with NSAID, but statistical significance was not achieved (mean of 76 ± 18 ng/mL, n=10 vs. 131 ± 29 ng/mL, n=15; p=0.12) (Figure 5).
mRNA expression of Apical Sodium-Dependent Bile Acid Transporter (ASBT):
Developmental mRNA expression the ASBT was measured by real time PCR. Samples of rat ileal tissue were collected on gestational day 21 (G21) and postnatal (p) days 0, 8, 14, and 24. Full weaning occurred on day 21 (Figure 6a). As previously shown in rat pups by Shneider et al. ASBT mRNA expression increases significantly in neonates between the ages of 14 and 24 days of age (wherein negligible expression was recorded at earlier time points) [22,23] (Figure 6b). Ileal ASBT expression was significantly higher (36-fold) in formula-fed groups (323–484 AU) compared to dam-fed groups (9–14 AU) at 16 DOL, with highest expression in the indo treated formula-fed group (Figure 6).
Figure 6:
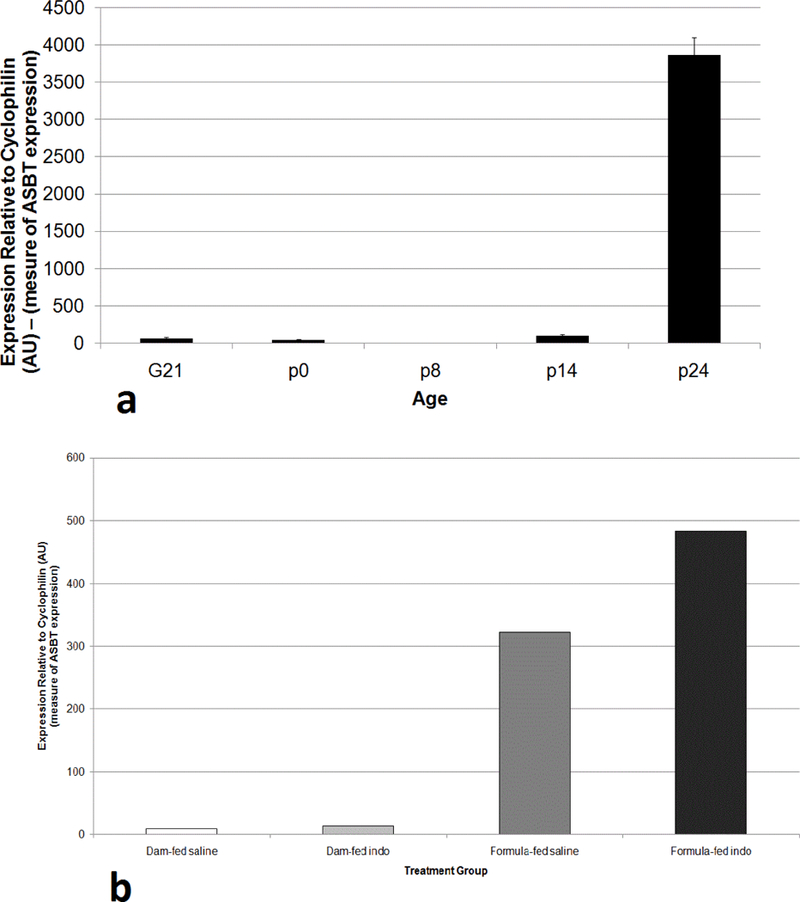
Ileal ASBT mRNA expression in rat pups. Ileal mRNA expression of the ASBT was measured by real time PCR. (a) Samples of rat intestinal tissue were collected on gestational day 21 (G21) and postnatal (p) days 0, 8, 14, and 24. Full weaning occurred on day 21. (b) Ileal ASBT expression was measured at DOL 15–16 in the indicated groups. Values are means of triplicate determinations, and expression was normalized using cyclophilin.
Discussion
We have shown that mild intestinal injury can occur due to stress from formula feeding and maternal separation. These stressors may play a role in accelerating intestinal development, as evidenced by an elevated corticosteroid level in the formula-fed groups that received indo, as they were potentially the most stressed group.
Effect of Maternal Separation (MS) with formula feeding on NSAIDs-induced intestinal injury: NSAIDs such as indo can severely injure the small intestinal mucosa in adult rodents and humans, leading to the development of ulcers, bleeding, and ultimately stricture and/or perforation [24–31]. Low birth weight neonates are sometimes exposed to NSAIDs (indo) either prenatally (to delay labor) or postnatally for the medical induction of PDA closure (with indo or ibuprofen), which can lead to intestinal perforation [32,33]. Based on our results, there appears to be increased indo-induced intestinal injury in rat pups that were formula-fed compared with animals that were fed with maternal milk suggesting that formula feeding/maternal separation may increase a neonate’s susceptibility to NSAID treatment. Similar results were seen in a prior study where rat pups were either breast-fed or formula-fed (similar mixture that was used in our study) but with hypoxia as the stressor [17]. In that study, all formula-fed rat pups exposed to hypoxia developed a NEC-like picture while hypoxic breast-fed rat pups showed no intestinal injury. The formula-fed rat pups that were not challenged with hypoxia did not have observable intestinal injury [17]. In new-born rat pups the GI tract matures gradually during the suckling period and much more rapidly during the weaning period. This process is very different from human infants, in whom the GI tract is more mature at birth and shows a slower gut developmental profile during the first year of post-natal life [34]. Indo has also been shown to block tight-junction repair in an ischemic-injury porcine model by Jacobi et al. There may be a similar mechanism at work in rodents, but that will need further exploration [35].
Histological analysis showed that maternally-separated, formula-fed controls had marginally taller villi than dam-fed controls, which may suggest more advanced intestinal maturity [36,37]. A previous study showed that rat pups fed a formula diet had longer small intestines and taller villi, suggestive of precocious maturation of small intestine as an adaptive mechanism to increase surface area for absorption [36]. The formula-fed rat pups also had deeper intestinal crypts, suggestive of accelerated epithelial turnover [36,37]. Indo treatment did not appear to affect the villus height or crypt depths in the dam-fed group. However, the villus to crypt ratio was significantly reduced in the indo treated versus saline-treated formula-fed pups, which may be a sign of mucosal injury and increased villus regeneration [24,38]. Similar results were obtained when the numbers of mitotic bodies were measured. There was an increase in mitotic bodies in the crypts of formula-fed pups compared to dam-fed. The indo treated formula-fed group had the highest average number of mitotic bodies, suggesting that cell turnover and proliferation is further increased in the setting of intestinal damage [24–39].
In our study, formula-fed rat pups appeared to have comparable growth to dam-fed groups when comparing body weights during the latter stages of the study period. Dam-fed rat pups initially exhibited an expected faster rate of weight gain when the litter size was reduced at DOL 9 in order to transfer some of the rat pups from the dam to formula-feeding groups. A previous study showed rat pups that are at or less than 14 days of life, if given an unlimited supply of milk, will continue to drink until their stomachs are so distended that they can barely walk [40]. Normally, the immaturity of the satiation response is limited by maternal milk supply, which is matched to litter size. If litter size is reduced within 6 h of birth, rat pups exhibit normal weight gain during the nursing period. However, if litter size is reduced later, obesity may ensue [40].
Previous animal studies have noted that when malnutrition is present, small intestinal development may be altered with decreased jejunal mass and delayed epithelial maturation as measured by mucosal enzymes activities [41]. Because the opposite changes (increased intestinal mass and accelerated maturation) were found in our formula-fed animal groups, we conclude that the formula-fed rat pups were not malnourished at the completion of study and that our findings are not secondary to malnutrition. The less intrusive method of feeding also allowed us to avoid trauma associated with traditional means of proving formula via orogastric tube or gastrostomy tube. Self-feeding also avoids gastric irritation that can increase bleeding risk.
Role of corticosteroids in NSAID injury:
Precocious intestinal maturation observed in formula-fed rat pups may, in part, be related to an accelerated progression of intestinal development secondary to stress (from maternal separation and formula feeding). Interestingly, among the rat pups that did not receive indo, formula-fed rat pups did not manifest greater cortisone level than that of the dam-fed rat pups. It is possible that we did not capture the elevation if the response occurred earlier and had resolved by the time the experiment ended or if level was influenced by diurnal variation (corticosteroid levels being normally increased in the evening, while specimens were collected during the afternoon. We hypothesize that environmental stress may induce changes in the intestine by stimulating corticosteroid release, which is a classic adrenal cortical stress signal [42–44]. Previous studies showed early weaning leads to precocious maturation of jejunal disaccharidase activity in intact rats but not in adrenalectomized rats [40–46]. There is a large body of evidence indicating that administration of adrenocortical steroids can induce precocious intestinal maturation in a number of animal species, which is reflected by the development of an adult pattern of disaccharidase enzyme activity, intestinal morphology, and cell proliferation [47–49]. Corticosteroids exacerbate NSAID-induced GI injury, possibly owing to the induction of intestinal transport of luminal bile acid into the enterocyte [50].
Brush border disaccharidases such as sucrase, isomaltase, and α,α-trehalase normally are present at low or undetectable levels in rat pups during first 2 weeks of life and are not expressed until days 15–17 of life, reaching adult levels by day of life 25. Jejunal sucrase is induced by the stress of weaning [40–48]. Other changes in intestinal development secondary to the corticosteroid induction have been documented in mammals, most of which seem to benefit nutrient absorption. These include “closure” of the intestinal barrier, which refers to a decrease in macromolecular permeability [51]. In humans, permeability measured by lactulose/mannitol excretion in the urine following adrenocorticoid decreases with antenatal steroid exposure [52]. Oddly, in rodents, dexamethasone combined with maternal separation increased intestinal permeability [53].
Potential role of epithelial bile acids in NSAIDs injury:
Bile acids may play a role in development of NEC and NSAID-induced injury to the epithelial barrier. For example, Halpern’s lab has shown in a rat necrotizing enterocolitis model that increased luminal bile acids and increased apical ASBT expression is an important part of the pathophysiological mechanism. Strong support for bile acid-contribution to intestinal injury was shown when chemical inhibition or knockout of the ASBT was found to protect from experimental necrotizing enterocolitis [54,55]. Our lab has obtained in vitro and in vivo evidence that the presence of bile acids significantly exacerbate NSAIDs-induced cell/membrane injury, possibly by forming cytotoxic mixed micelles [56,57]. In addition, excretion of indo in bile likely contributed to intestinal injury [57]. In the present study, luminal bile acids were much higher (~2-fold) in formula-fed compared to dam-fed groups. Indo treatment did not affect levels of luminal bile acids. However, formula-feeding-associated stress steroids may have induced maturation of systems responsible for bile acid biosynthesis and enterohepatic cycling, which would increase intestinal bile acid exposure and may have potentiated the indo-induced intestinal injury in the formula-fed group [40,50]. Although we did not measure intracellular bile acid levels, one would predict an increase due to elevated luminal level and apical membrane transporter activity. Investigators previously showed that ileal brush border membrane bile acid transport activity and ileal ASBT mRNA and protein expression normally appears later in the postnatal period, increasing between days 14 and 24 in rat pups [22,23] and this developmental pattern was confirmed in the present study. Moreover, formula-fed animals had markedly higher levels of ASBT mRNA expression when compared to the dam-fed animals at DOL [16–17]. Although the underlying mechanism for the induction of expression was not further explored, previous studies would suggest a connection to the elevated cortisol levels in the formula-fed mice. For example, Hwang and Henning previously demonstrated that administration of dexamethasone during the second postnatal week induced ileal ASBT expression in rat pups [50]. It has also been shown that administration of glucocorticoids induces ileal ASBT expression in rat or mouse models [58,59], and that the ASBT is a direct target glucocorticoid receptor target gene [60].
In summary, dam-feeding appeared to protect against NSAID-induced intestinal injury/bleeding during weeks 2–3 of life. Stress (from maternal separation and formula-feeding) increased intestinal sensitivity to indo-induced injury. Epithelial maturation induced by the systemic adrenocortical burst might have been expected to make the gut more resistant to injury; but we observed that it was instead associated with great level of injury. Our study provides additional insight to factors which may facilitate NSAIDs-induced injury to the infant intestine. Breast milk may play a protean role against indo-induced intestinal injury although the mechanism still requires further exploration. Feeding breast milk to premature infants that require NSAID may provide some protection to one’s lower gut against NSAID-induced intestinal injury.
Acknowledgment and Funding
The authors thank Milton Feingold, MD (Department of Pathology at Baylor College of Medicine) for advice regarding the study, and Jamie Haywood (Department of Internal Medicine, Wake Forest School of Medicine) for assistance with the ileal mRNA expression measurements. This work was supported by grants from DDC (NIDDK P30 056338).
References
- 1.Attridge JT, Clark R, Walker MW, Gordon PV (2006) New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol 26: 93–99. [DOI] [PubMed] [Google Scholar]
- 2.Grosfeld JL, Chaet M, Molinari F, Engle W, Engum SA, et al. (1996) Increased risk of necrotizing enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann Surg 224: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma R, Hudak ML, Tepas JJ, Wludyka PS, Teng RJ, et al. (2010) Prenatal or postnatal indomethacin exposure and neonatal gut injury associated with isolated intestinal perforation and necrotizing enterocolitis. J Perinatol 30: 786–793. [DOI] [PubMed] [Google Scholar]
- 4.Barrios JM, Lichtenberger LM (2000) Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology 118: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 5.Jacob M, Foster R, Sigthorsson G, Simpson R, Bjarnason I (2007). Role of bile in pathogenesis of indomethacin-induced enteropathy. Arch Toxicol 81: 291–298. [DOI] [PubMed] [Google Scholar]
- 6.Oines E, Murison R, Mrdalj J, Gronli J, Milde AM (2012) Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav 105: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy MB, Paik KD, Caraway JD, Schiml PA, Deak T (2011) Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behav Neurosci 125: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, et al. (2009) Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamkin DH (2012) Mother’s milk, feeding strategies, and lactoferrin to prevent necrotizing enterocolitis. JPEN 36: 25–29. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, et al. (2003) Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou PH, Francoual C, Glangeaud MC, Barette A, Dupont C, et al. (2000) Risk factors for severe esophageal and gastric lesions in term neonates: a case-control study. Groupe Francophone d’Hepato-Gastroenterologie et Nutrition Pediatrique. J Pediatr Gastroenterol Nutr 31: 377–380. [DOI] [PubMed] [Google Scholar]
- 12.Morrow AL, Rangel JM (2004. ) Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis 15: 221–228. [DOI] [PubMed] [Google Scholar]
- 13.Caplan MS, Hedlund E, Adler L, Hsueh W (1994) Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 14.Caplan MS, Simon D, Jilling T (2005) The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 145–151. [DOI] [PubMed] [Google Scholar]
- 15.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, et al. (2000) Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77. [DOI] [PubMed] [Google Scholar]
- 16.Morise Z, Granger DN, Fuseler JW, Anderson DC, Grisham MB (1999) Indomethacin induced gastropathy in CD18, intercellular adhesion molecule 1, or P-selectin deficient mice. Gut 45: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, et al. (1974) An experimental study of acute neonatal enterocolitis-the importance of breast milk. J Pediatr Surg 9: 587–595. [DOI] [PubMed] [Google Scholar]
- 18.Crosby WH, Furth FW (1956) A Modification of the Benzidine Method for Measurement of Hemoglobin in Plasma and Urine. Blood 11: 380–383. [PubMed] [Google Scholar]
- 19.Dahlquist A (1984) Assay of intestinal disacchardiases. Scand J Clin Lab Invest 44: 169–172. [DOI] [PubMed] [Google Scholar]
- 20.Lan T, Rao A, Haywood J, Kock ND, Dawson PA (2012) Mouse organic solute transporter alpha deficiency alters FGF15 expression and bile acid metabolism. J Hepatol 57: 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Lan T, Chen Y, Dawson PA (2012) Dietary fish oil increases fat absorption and fecal bile acid content without altering bile acid synthesis in 20-d-old weanling rats following massive ileocecal resection. Pediatr Res 72: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, et al. (1995) Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest 95: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shneider BL, Setchell KD, Crossman MW (1997) Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr Res 42: 189–194. [DOI] [PubMed] [Google Scholar]
- 24.Anthony A, Dhillon AP, Nygard G, Hudson M, Piasecki C, et al. (1993) Early histological features of small intestinal injury induced by indomethacin. Aliment Pharmacol Ther 7: 29–39. [DOI] [PubMed] [Google Scholar]
- 25.Fukumoto A, Tanaka S, Shishido T, Takemura Y, Oka S, et al. (2009) Comparison of detectability of small-bowel lesions between capsule endoscopy and double-balloon endoscopy for patients with suspected small-bowel disease. Gastrointest Endosc 69: 857–865. [DOI] [PubMed] [Google Scholar]
- 26.Iddan G, Meron G, Glukhovsky A, Swain P (2000) Wireless capsule endoscopy. Nature 405: 417. [DOI] [PubMed] [Google Scholar]
- 27.Menozzi A, Pozzoli C, Giovannini E, Solenghi E, Grandi D, et al. (2006) Intestinal effects of nonselective and selective cyclooxygenase inhibitors in the rat. Eur J Pharmacol 552: 143–150. [DOI] [PubMed] [Google Scholar]
- 28.Nygard G, Anthony A, Piasecki C, Trevethick MA, Hudson M, et al. (1994) Acute indomethacin-induced jejunal injury in the rat: early morphological and biochemical changes. Gastroenterology 106: 567–575. [DOI] [PubMed] [Google Scholar]
- 29.Shishido T, Oka S, Tanaka S, Aoyama T, Watari I, et al. (2012) Diagnostic yield of capsule endoscopy vs. double-balloon endoscopy for patients who have undergone total enteroscopy with obscure gastrointestinal bleeding. Hepatogastrol Enterol 59: 955–959. [DOI] [PubMed] [Google Scholar]
- 30.Volterra G, Pisanti N, Meli A (1974) Factors influencing the development of indomethacin-induced intestinal ulcers in the rat. Proc Soc Exp Biol Med 146: 146–152. [DOI] [PubMed] [Google Scholar]
- 31.Whittle BJ (2004) Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol 500: 427–439. [DOI] [PubMed] [Google Scholar]
- 32.Abbasi S, Gerdes JS, Sehdev HM, Samimi SS, Ludmir J (2003) Neonatal outcome after exposure to indomethacin in utero: a retrospective case cohort study. Am J Obstet Gynecol 189: 782–785. [DOI] [PubMed] [Google Scholar]
- 33.Fujii AM, Brown E, Mirochnick M, O’Brien S, Kaufman G (2002) Neonatal necrotizing enterocolitis with intestinal perforation in extremely premature infants receiving early indomethacin treatment for patent ductus arteriosus. J Perinatol 22: 535–540. [DOI] [PubMed] [Google Scholar]
- 34.Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, et al. (2006) Early nutrition and immunity-progress and perspectives. Br J Nutr 96: 774–790. [PubMed] [Google Scholar]
- 35.Jacobi SK, Moeser AJ, Corl BA, Harrell RJ, Blikslager AT, et al. (2012) Dietary long-chain PUFA enhance acute repair of ischemia-injured intestine of suckling pigs. J Nutr 142: 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, et al. (2000) Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr 31: 162–169. [DOI] [PubMed] [Google Scholar]
- 37.Mandir N, FitzGerald AJ, Goodlad RA (2005) Differences in the effects of age on intestinal proliferation, crypt fission and apoptosis on the small intestine and the colon of the rat. Int J Exp Pathol 86: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson RA, Rivera N (1992) Effect of difluoromethylornithine (DFMO) on NSAID-induced intestinal injury in rats. Dig Dis Sci 37: 1833–1839. [DOI] [PubMed] [Google Scholar]
- 39.Ilahi M, Khan J, Inayat Q, Abidi TS (2006) Histological changes in parts of foregut of rat after indomethacin administration. J Ayub Med Coll Abbottabad 18: 29–34. [PubMed] [Google Scholar]
- 40.Henning SJ (1981) Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol 241: 199–214. [DOI] [PubMed] [Google Scholar]
- 41.Butzner JD, Gall DG (1990) Impact of refeeding on intestinal development and function in infant rabbits subjected to protein-energy malnutrition. Pediatr Res 27: 245–251. [DOI] [PubMed] [Google Scholar]
- 42.Economou G, Andronikou S, Challa A, Cholevas V, Lapatsanis PD (1993) Cortisol secretion in stressed babies during the neonatal period. Horm Res 40: 217–221. [DOI] [PubMed] [Google Scholar]
- 43.Grofer B, Bodeker RH, Gortner L, Heckmann M (2010) Maturation of adrenal function determined by urinary glucocorticoid steroid excretion rates in preterm infants of more than 30 weeks of gestational age. Neonatology 98: 200–205. [DOI] [PubMed] [Google Scholar]
- 44.Ng PC (2011) Effect of stress on the hypothalamic-pituitary-adrenal axis in the fetus and newborn. J Pediatr 158: 41–43. [DOI] [PubMed] [Google Scholar]
- 45.Martin GR, Henning SJ (1984) Enzymic development of the small intestine: are glucocorticoids necessary? Am J Physiol 246: 695–699. [DOI] [PubMed] [Google Scholar]
- 46.Miyata T, Minai Y, Haga M (2008) Impaired growth of small intestinal epithelium by adrenalectomy in weaning rats. Acta Histochem Cytochem 41: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galand G (1989) Brush border membrane sucrose-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp Biochem Physiol B 94: 1–11. [DOI] [PubMed] [Google Scholar]
- 48.Henning SJ (1978) Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol 235: 451–456. [DOI] [PubMed] [Google Scholar]
- 49.Yeh K, Yeh M, Holt PR, Alpers DH (1994) Development and hormonal modulation of postnatal expression of intestinal alkaline phosphatase mRNA species and their encoded isoenzymes. Biochem J 301: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang ST, Henning SJ (2001) Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 226: 674–680. [DOI] [PubMed] [Google Scholar]
- 51.Daniels VG, Hardy RN, Malinowska KW, Nathanielsz PW (1973) The influence of exogenous steroids on macromolecule uptake by the small intestine of the new-born rat. J Physiol 229: 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, et al. (1998) Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res 44: 519–523. [DOI] [PubMed] [Google Scholar]
- 53.Moussaoui N, Braniste V, Ait-Belgnaoui A, Gabanou M, Sekkal S, et al. (2014) Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS ONE 9: e88382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, et al. (2006) Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halpern MD, Weitkamp JH, Mount Patrick SK, Dobrenen HJ, Khailova L, et al. (2010) Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dial EJ, Darling RL, Lichtenberger LM (2008) Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol 23: 384–389. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, Dial EJ, Doyen R, Lichtenberger LM (2010) Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol 298: 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowicki MJ, Shneider BL, Paul JM, Heubi JE (1997) Glucocorticoids upregulate taurocholate transport by ileal brush-border membrane. Am J Physiol 273: 197–203. [DOI] [PubMed] [Google Scholar]
- 59.Out C, Dikkers A, Laskewitz A, Boverhof R, van der Ley C, et al. (2014) Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J Hepatol 61: 351–357. [DOI] [PubMed] [Google Scholar]
- 60.Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA (2004) Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut 53: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


