Abstract
Hallmark of retinitis pigmentosa (RP) is the primary, genetic degeneration of rods followed by secondary loss of cones, caused by still elusive biologic mechanisms. We previously shown that exposure of rd10 mutant mice, modeling autosomal recessive RP, to environmental enrichment (EE), with enhanced motor, sensorial and social stimuli, results into a sensible delay of retinal degeneration and vision loss. Searching for effectors of EE-mediated retinal protection, we performed transcriptome analysis of the retina of rd10 enriched and control mice and found that gene expression at the peaks of rod and cone degeneration is characterized by a strong inflammatory/immune response, which is however measurably lower in enrichment conditions. Treating rd10 mice with dexamethasone during the period of maximum photoreceptors death lowered retinal inflammation and caused a preservation of cones and cone-mediated vision. Our findings indicate a link between retinal inflammation and bystander cone degeneration, reinforcing the notion that cone vision in RP can be preserved using anti-inflammatory approaches.—Guadagni, V., Biagioni, M., Novelli, E., Aretini, P., Mazzanti, C. M., Strettoi, E. Rescuing cones and daylight vision in retinitis pigmentosa mice.
Keywords: rod, bystander effect, environmental enrichment, microglia, cytokines
The term retinitis pigmentosa (RP) defines a group of clinically alike dystrophies characterized by progressive degeneration of photoreceptors and abnormalities in retinal pigment epithelium. RP has a strong genetic component, with hundreds of mutations in over 70 genes identified so far, displaying both common and rare variants (1). Such elevated genetic heterogeneity corresponds to diversity in disease mechanisms, which might involve any aspect of photoreceptor biology, including development, phototransduction, protein transport, gene expression, etc., but converging on common cell death pathways and phenotypes (2). RP has a prevalence comprised between 1/3000 and 1/5000 (http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=791). Typically, vision loss becomes evident in young adults, and the disease is regarded as intractable as it progresses unrestrainedly toward blindness.
The first sign of RP is night blindness (nyctalopia), which is caused by early damage and subsequent death of rod photoreceptors, with progressive restriction of the visual field (tunnel vision) due to loss of rods from the peripheral retina where these cells predominate. Later, affected individuals experience a progressive decline of visual acuity in the central fields and an impairment of chromatic discrimination caused by the gradual demise of cones (3). Although these cells represent a small fraction (<5%) of all photoreceptors in the retina of most mammals, their role in human vision is crucial and their degeneration might lead to a condition of legal blindness. Thus, the most severe clinical sign of RP (cone-mediated vision loss) is not caused by the primary genetic defect but by the secondary degeneration of cones.
The biologic mechanisms linking the death of rods and the bystander demise of cones remain elusive. Decrease of survival factors, alteration of glucose metabolism, oxidative stress consequent to locally increased oxygen levels are all being investigated as implicated in cone death in RP (4). Because of the cone’s fundamental role in human vision, preservation of even a fraction of these cells would ensure relatively normal lives to patients with RP; moreover, therapeutic approaches targeting generalized processes (i.e., oxidative stress) would act on pathways downstream the causative mutation and probably have efficacy independently of the genetic cause of the disease.
We have previously shown that environmental enrichment (EE)—an experimental paradigm in which laboratory animals are raised in the presence of increased social, sensorial, and motor stimulation—slows down photoreceptor degeneration and preserves vision in the rd10 mouse, a well-known RP model with a mutation in the rod-specific phosphodiesterase gene (5, 6). Analogous studies based on physical exercise alone (one of the components of EE) have shown positive effects on the same and other models of photoreceptor degeneration (7, 8), and the potential beneficial effects of exercise on the progression of human RP are being assessed through a specific pilot study (https://clinicaltrials.gov/ identifier: NCT03381235).
A major effector of the neuroprotective action exerted by EE on the CNS is a measurable increase in the production and release of various neurotrophic factors and in particular of BDNF, a relevant mediator for synaptic plasticity, deemed crucial for the protective effects of physical exercise on the aging and diseased brain (9). Correspondingly, little is known about the main molecular effectors of EE on retinal degeneration, for which systematic studies are still missing. Here, we exploit rd10 mutant mice raised in an enriched environment and control conditions to perform retinal transcriptome analysis at the peak of rod and cone death, respectively. We find that retinal inflammation and immune responses constitute major biologic processes in this model of inherited photoreceptor degeneration and that some of the gene expression associated to these responses is reduced in EE conditions, where photoreceptor survival is confirmed to be higher. We also show that pharmacological administration of commonly used steroids reduces retinal inflammation and is sufficient to preserve a considerable fraction of cones and cone-mediated vision in rd10 mice.
MATERIALS AND METHODS
Animals
Mice were treated in accordance to Italian and European institutional guidelines, following experimental protocols approved by the Italian Ministry of Health (Authorization n° 4/2014-PR, DGSAF10741-A; authorization n° 8/2014-PR, DGSAF10739-AAND protocol 10739.EXT.0) and by the intramural Ethical Committees. Protocols adhere to the Association for Research in Vision and Ophthalmology statement for the use of animals in research.
Homozygous rd10 mice (Pde6brd10/rd10) on a C57Bl6J background were maintained in a local facility with water and food ad libitum and ambient light below 100 Lux. Controls were age-matched C57Bl6J mice [wild type (WT)] maintained in the same conditions. As a rule, 3–6 animals per experimental group were used. For EE studies, a total of 30 mice were used for immunocytochemistry (ICCH) analysis and 54 mice for molecular biology assays. For dexamethasone (Dexa) experiments, a total of 36 mice were used for behavior experiments and morphologic studies; 12 of them were used for Western blots.
Male and female mice were equally represented in all experimental groups.
EE
Three litters of rd10 mice were used to confirm previous experiments on the effects of EE on retinal degeneration. EE conditions were the same as previously described by Barone et al. (6). Briefly, 2 pregnant rd10 females were maintained in large cages containing 3 other helper females, 1 running wheel for voluntary physical exercise, and 5 objects for manipulations and explorations, plus a transparent Plexiglas tunnel and paper material for nest preparation. Manipulation objects were changed every 5 d to stimulate animal curiosity. Enriched litters of rd10 mice were maintained in the same cage (and therefore in a large social group) up to postnatal day (P)45. Then, males and females were separated in 2 large cages, still equipped with a running wheel and the objects previously described. Animals remained in EE until the end of the experiments (from birth to 60 d of age). Control groups were age-matched rd10 mice maintained in standard (ST) laboratory conditions, represented by ST-size cages, with no objects and occupied by small social groups (n = 6 mice/cage). Illumination levels, food and water, as well as manipulation from the animal house personnel were identical for EE and ST animals.
Retinal histology and ICCH
For retinal histologic studies, mice were deeply anesthetized with intraperitoneal injections of 0.1 ml/5 g body weight Avertin (3-bromo-ethanol in 1% tert-amyl alcohol), their eyes enucleated, and the animals humanely killed by cervical dislocation. Eyes were labeled on the dorsal pole, eye cups were obtained, and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 h at room temperature. Eye cups were washed in buffer, cryo-protected in 30% sucrose, frozen in cold isopenthane and stored at −80°C until use. Cryostat sections (12–14 µm thick) were collected on Super frost slides; additional eyes were used to prepare retinal whole mounts in which the retina was separated from the pigment epithelium. ICCH on both retinal sections and whole mounts was performed following the method outlines by Barone et al. (6) and by incubation in: 1) block solution with 0.3% Triton X-100, 5% of the serum of the species in which the secondary antibody was generated, and 0.01 M PBS; incubation time was 2 h for the sections and overnight for whole mounts; 2) primary Ab diluted in PBS, 0.1% Triton X-100, and 1% serum; incubation time was overnight for the sections and 3 d for whole mounts; 3) fluorescent secondary Ab diluted as the primary Ab; incubation time was 2–3 h for the sections and 2 d for whole mounts. Mouse monoclonal primary Abs used for retinal sections were rhodopsin (O4886, diluted 1:1000; MilliporeSigma, Burlington, MA, USA) and synaptotagmin 2 (Znp1; diluted 1:800; Zebrafish International Resource Center, Eugene, OR, USA). Rabbit polyclonal primary Abs were S and M/L cone opsins (AB5405, AB5407; diluted 1:800; MilliporeSigma); cone arrestin (AB15282, diluted 1:5000; MilliporeSigma); ionized calcium-binding adapter molecule 1 (Iba1) (019-19741, diluted 1:800; Wako, Rimini, Italy). Rat mAb was CD11b (M1/70, diluted 1:250; Abcam, Cambridge, MA, USA).
Secondary antibodies were donkey anti-mouse Alexa Fluor 488 (A-21202; Thermo Fisher Scientific, Waltham, MA, USA), donkey anti-rabbit Rhodamine Red X (715296151), and donkey anti-rat Alexa Fluor 488 (712-546-153; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), diluted 1:1000 for retinal sections and for whole-mount preparations. Specimens were rinsed and mounted in Vectashield (H-1000; Vector Laboratories, Burlingame, CA, USA).
Images of retinal preparations were obtained with a Zeiss Imager.Z2 microscope equipped with an Apotome2 device (Carl Zeiss, Oberkochen, Germany) using a Plan Neofluar ×40/1.25 oil objective or with a Leica TCS-SL confocal microscope (Leica-Microsystems, Milan, Italy) equipped with 488 and 543 lasers using a Plan Apochromat ×40/1.40 oil objective. Retinal whole mounts were also imaged at a Zeiss Imager.Z2 microscope with ×10/0.3 M27 and ×20/0.50 M27 objectives; images were tiled with the Tiles and Positions software of the Zen module to reconstruct the entire retinal surface. All original microscopy images were saved as TIFF files; to match computer monitor parameters, brightness and contrast were increased 20% with Adobe Photoshop (Adobe, San Jose, CA, USA) and the corresponding files saved as copies.
Cell counts
Cones and microglia/macrophages were counted in retinal whole-mount preparations double-stained with antibodies against cone arrestin and Iba1. To assess total cell numbers taking into account local anisotropies in retinal degeneration patterns and center-to-periphery changes in cell density, retinas were imaged with a Zeiss Imager.Z2 equipped with a xenon lamp and appropriate fluorescence filter cubes. Cells were counted in 32 fields regularly spaced along the 2 main (horizontal and vertical) retinal meridians (16 fields along the dorso-ventral axis and 16 along the nasotemporal axis, respectively) covering the retina from the far periphery to the proximity of the optic nerve head. Counting areas were 223.8 × 167.6 μm fields within which z stacks of consecutive images spaced 1 μm apart were simultaneously obtained along the red and green channels using an alternate mode of acquisition. Stacks used for cone counts encompassed the outer segments and the cell bodies throughout the outer nuclear layer. Stacks for microglial cells encompassed the photoreceptor and outer nuclear layers extending on average across 20 μm. Stacks for microglia/macrophage counts in Dexa and control specimens extended from the subretinal space to the OPL. Microglia/macrophages counts were always done focusing selectively in the outer retina; however, observations on cell morphology were conducted throughout the entire retinal thickness also examining the inner microglial plexus.
Series of single plane optical sections were used to count cells with Metamorph software and using the manual counting tool. Each cell was counted in a single focal plane and tracked across the z stack. The mean number of cells/field was obtained, converted in cells/square mm, and multiplied to the total retina area (measured on low power images obtained with a bright field microscope using a ×1.25 objective). The total number of cells/retina (or the mean density) were finally calculated.
Statistics
Comparisons of experimental groups (each of them consisting of a minimum of 4–5 age and sex-matched mice) was executed in SigmaPlot applying 1-way ANOVA test with specific post hoc methods. Histologic data (i.e., cell counts) obtained from rd10-EE mice were compared with those of rd10 ST and to WT groups; similarly, data from rd10 mice treated with Dexa were compared to those obtained from matched controls treated with vehicle alone. A value of P ≤ 0.05 was considered significant.
Molecular biology
RNA samples obtained from retinas of rd10 ST, rd10 EE, and WT mice aged 24 and 45 d (n = 9 mice for experimental group, 54 mice total) were used for both whole transcriptome analysis and quantitative real-time-PCR (q real-time PCR).
Retinal RNA were extracted from the 2 retinas of each mouse using the RNeasy mini kit (74104; Qiagen, Germantown, MD, USA) following the manufacturer’s protocol for fresh tissue. An optional On-Column, DNase Digestion with the RNase-Free DNase Set (79254; Qiagen) was performed. RNA concentration and purity were determined with a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific).
The RNA samples from groups of 3 animals of the same age and experimental group were pooled (400 ng from each sample) to obtain a total of 1200 ng/group. Of these, 900 ng were used for next generation sequencing (NGS) and 300 ng for q real-time PCR.
Whole transcriptome analysis of mouse retinas with NGS
Library preparation
RNA-seq was performed using Ion Proton Sequencer for NGS by using products produced by Thermo Fisher Scientific. Starting material (900 ng of pooled RNA) was treated with the Low Input RiboMinus Eukaryote Kit v2. RNA was fragmented with RNAse III for 3 min following the Ion Total RNA-seq Kit protocol. For cDNA amplification, the barcode sequence was added to the 5′ end to distinguish between 2 libraries during Ion Proton sequencing. The yield and size distribution of each library were assessed loading 1 µl of the library on the Agilent 2100 Bioanalyzer and using the Agilent High Sensitivity DNA Kit (Agilent Technologies, Santa Clara, CA, USA).
Template preparation
Amplification, ion sphere particles (ISPs) loading, and enrichment was performed following the manufacturer’s protocol with Ion OneTouch 2 Instrument (Thermo Fisher Scientific).
Sequencing with the Ion Proton
Samples were sequenced using an Ion PI Sequencing 200 Kit and an Ion Proton Sequencer (Thermo Fisher Scientific) initialized prior to the protocol. Enriched retinal samples (template-positive ISPs) were prepared for sequencing adding the ion PI Control Ion ISPs and annealing the sequencing primer. The Ion PI Chip was prepared, calibrated, and loaded with samples and subsequently placed in the Sequencer A Torrent Suite software, which was programmed for a running routine.
Computational methods
Data collected from 9 animals from each experimental group (WT, rd10 ST, and rd10 EE at P24 and P45) were pooled. Various preparations steps were assessed by ad hoc programs. Specifically, the FastQC quality control tool was employed to perform quality assessment. Raw data were examined, and contamination from different organisms (bacteria, fungi, virus) was eliminated (if necessary) by applying FastqScreen. RNA-Seq reads were aligned to the mouse genome (mm10; University of California–Santa Cruz, Santa Cruz, CA, USA) with STAR aligner 2.5.1 (https://github.com/alexdobin/STAR). Cufflinks (http://cole-trapnell-lab.github.io/cufflinks/) was used to quantify gene expression levels. The gene differential expression was performed by using Cuffdiff, a software included in the Cufflinks package that employs transcript-based detection methods. Correlation of gene expression within each experimental group was assessed globally and for photoreceptor-specific groups of genes by Spearman correlation analysis. Matrices including different experimental groups were built and analyzed. Elaborated sets of data were visualized with Gene Ontology (http://geneontology.org/), Vennt, Excel, CummeRbund (an R tool), and Database for Annotation, Visualization, and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) analysis (10). We set a stringent P value threshold of 0.05 for identifying differentially expressed genes.
Analysis of gene expression with RT2 profiler PCR arrays
Reverse transcription
Starting from 300 ng of pooled RNA mix, cDNA synthesis was performed with RT2 First Strand Kit (330401; Qiagen) following the manufacturer’s protocol, which comprises a further step of genomic DNA elimination. After reverse transcription, the cDNA amount and purity were assessed again with the NanoDrop 2000 as described for RNA.
Real-time quantitative PCR
Each cataloged RT2 Profiler PCR Array incorporates laboratory-verified assays for 84 pathway-focused genes (5 of which are housekeeping genes) and controls for sample and reaction quality (PAMM-150Z; Qiagen). Each q real-time PCR reaction was performed adding equal amounts of cDNA to a 2× SYBR Green Mix and nuclease-free water, portioned into aliquots for a 96-well plate, and run on Step One Plus Machine (Thermo Fisher Scientific) following the manufacturer’s protocol.
Data analysis
Data analysis was performed with an online dedicated software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php) based on the ΔΔCt method. The first step of data analysis consisted in checking the Ct values of controls for both genomic contamination and positive and reverse transcription controls to guarantee proper comparison of different arrays. Levels of gene expression were compared statistically and referred to housekeeping genes with ΔΔCt method. Gene expression quantification for each sample was assessed with Cufflinks, which was also used for quantification among the groups with Cuffdiff. Data were graphically represented as histograms, clustergrams with dendrograms, and scatter plots (11). Statistical significance was assessed by 1-way ANOVA on genes with fold change >2 or < −2 with respect to WT expression.
Drug delivery
rd10 mice aged 23 d were divided into 2 groups (12 animals/group) and received subcutaneous injection of 4 mg/kg body weight Dexa (Soldesam Forte 4 mg/ml; Laboratorio Torino Medica, Turin, Italy) or vehicle (an identical amount of distilled water) once a day (between 8 am and 9 am) from P23 to P45. Two additional groups received the treatment from P23 to P60. The dose of Dexa was chosen based on previous rodent studies (12, 13).
Body weight was monitored every 2 d. Blood sugar level was measured on the first and last day of treatment using a drop of blood obtained from the tail vein and test strips for the glucometer. Ocular pressure was assessed at the beginning and at the end of the treatment period with a TonoLab air tonometer to rule out the possibility of secondary glaucoma development. Treated and control mice were harvested at P45 or at P60. The eyes were rapidly removed and processed as described for immunohistochemistry or for Western blot analysis.
Western blot
Retinal homogenates were obtained from 3 Dexa-treated and 3 water-treated rd10 mice aged P45 and P60, using both retinas of each animal for each preparation as described in Barone et al. (6). Proteins (ranging from 20 to 60 µg) from each retinal sample were electrophoresed on a 12% Bis-Tris Criterion XT Precast gel (Bio-Rad, Hercules, CA, USA), then blotted on 0.2 nitrocellulose membrane using a semidry transfer system (Trans-Blot Turbo; Bio-Rad). The protein blot was blocked by exposure to Odyssey Blocking Buffer-PBS Tween 0.2% 1:1 solution at room temperature for 90’. Nitrocellulose filters were then incubated overnight at 4°C with the following primary antibodies: anti-cone arrestin (Ab15282, at 1:2000 dilution; MilliporeSigma), anti–monocyte chemoattractant protein 1 [chemokine chemoattractant (CCL) 2] (PAA087Mu01 at 1:1000; Cloud-Clone, Katy, TX, USA) and anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ab8245; Abcam), which served as an internal ST for protein quantification. Secondary antibodies conjugated with infrared-emitting dyes (anti-mouse IRDye 680LT at 1:20,000 and anti-rabbit 800CW at 1:20,000; Li-Cor Biosciences, Lincoln, NE, USA) were used. Reacted filters were scanned using an Odyssey IR scanner (Li-Cor Biosciences); densitometry was performed with Image Studio software v.5.2 (Li-Cor Biosciences). Cone arrestin and the 13 KDa band of CCL2 blots were measured and normalized to corresponding values of GAPDH used as loading controls. The entire GAPDH band was used for measurements.
Visual behavior
Visual acuity of Dexa-treated and control mice was assessed at 45 and 60 d of age under photopic conditions using a Prusky water maze as described in detail in Barone et al. (6).
Data sharing
NGS original data of this study are visible in the Mendely data repository (https://data.mendeley.com/datasets/jvtzrpc26s/draft?a=4515f216-bcd9-4c37-b988-66ac3fb1cb55).
RESULTS
Enriched environment is confirmed to support cone survival
As observed in other rodent models and in patients with RP, cone degeneration in the rd10 mouse follows major rod death (which reaches a maximum between P18 and P24), with a peak around P45 and a similar center-to-periphery topographical gradient (14, 15). Around P60, only cones devoid of outer segments and aberrant morphologies persist in the retina and cone-mediated light responses detectable with an electroretinogram become extinct (15). Scattered clusters of heavily remodeled cones survive for many months in this and other mutants (16).
To confirm previous studies showing beneficial effects on retinal survival and function in rd10 mice exposed to a habitat rich in sensory, motor, and social stimulation, we studied retinal morphology by quantitative immunohistochemistry in rd10 mice born and raised in EE (rd10, EE mice) compared with animals raised in ST conditions (rd10 ST mice) and to age-matched WT of the same genetic backgrounds (C57Bl6J WT mice). We focused on retinal cones. Results are summarized in Fig. 1, which shows how exposure to EE supports morphologic preservation and survival of cones in rd10 mutant mice. No sex-related differences were detected in the effects of EE in this study.
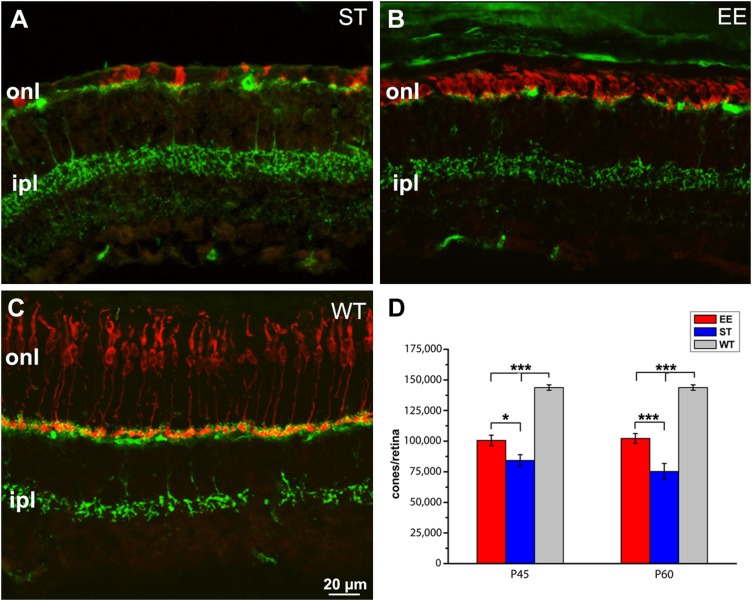
Figure 1.
Enhanced cone survival upon exposure to enriched environment. A, B) Vertical sections of the retina of rd10 mice born and maintained in ST conditions (A) or in an enriched environment (B). C) retina of a WT control mouse. Age is 60 d (P60). Red staining: cone arrestin; green staining: synaptotagmin-2, a marker of cone bipolar cells highlighting retinal laminar organization (A–C). Cones (labeled in red) are scant, devoid of outer segments and with aberrant morphologies in A, whereas they still form a continuous row in B. The WT (C) retina shows regularly arranged, elongated cones. Green labeling of dendrites and axonal endings of cone bipolar cells provides references for the outer and inner plexiform layers, respectively. D) Cumulative cone counts from retinas of EE and ST rd10 mice at 2 time points, showing 30% higher survival in EE at P60. The retina of C57Bl6J WT mice contains ∼150,000 cones. Ipl, inner plexiform layer; onl, outer nuclear layer. *P ≤ 0.05, ***P < 0.005 (1-way ANOVA).
Mice kept in EE conditions maintain a higher number of cones than ST counterparts at both the time points examined; surviving cones reach 70% of the WT number at P60, are shorter than normal (their length being only 25% of the WT size) but retain outer segments that are particularly appreciable in the peripheral retina. Surviving cones are less numerous (50% of the WT; 1-way ANOVA, P < 0.001) in the rd10 ST counterparts, where they form a scattered row in the outer retina and exhibit remodeled morphologies with ovoidal bodies and virtually absent outer segments. Hence, the present data reproduce entirely previous results from our laboratory in which exposure to EE returned higher photoreceptor preservation and survival in rd10 mutant mice (5, 6).
Transcriptome analysis shows a major inflammatory component of retinal degeneration at the peaks of rod and cone death
To search for molecular effectors responsible of the beneficial effects of EE on photoreceptor survival and to identify gene expression and biologic processes specifically associated to the active phases of photoreceptor degeneration, we used RNA deep sequencing and compared transcriptional changes occurring in the retinas of 3 experimental groups (rd10 EE, rd10 ST, and WT mice), each group represented at 2 ages (P24 and P45), respectively corresponding to the times near the peaks of rod and cone degeneration in the rd10 strain. RNA-seq and trascriptome analysis were performed on these 6 groups of mice, each composed of 9 animals, for a total of 54 mice and 108 retinal samples. Spearman correlation analysis was performed to assess sample quality and homogeneity (Supplemental Fig. S1). The complete list of genes was loaded onto the Vennt program, which allowed isolating gene lists as a function of statistical significance and gene expression levels. We chose as significant P values of 0.05 and lower and fold-changes of 2. Results were further subjected to gene ontology (GO) analysis by DAVID (10), and biologic process pathways were visualized as block diagrams; main results were also validated by quantitative PCR analysis on the same retinal samples.
Gene expression original data are available at: https://data.mendeley.com/datasets/jvtzrpc26s/draft?a=4515f216-bcd9-4c37-b988-66ac3fb1cb55.
At P24, we found a total of 341 genes up-regulated in the retina of rd10 ST mice and 187 genes up-regulated in the retina of rd10 EE mice compared to WT controls. Intersecting the list of up-regulated genes with Vennt software, we identified a core of 159 genes up-regulated in both ST and EE retinas at P24 with respect to age-matched WT samples.
Similarly, at P45, we found 507 genes differentially expressed in rd10 ST mice compared to WT, and 368 in EE compared to WT; we also found 246 genes up-regulated in both rd10 ST and in rd10 EE mice with respect to WT. Intersecting the lists with Vennt software, we found a core of 93 genes commonly up-regulated in the rd10 retinas, both ST and EE at P45, with respect to age-matched WT retinas.
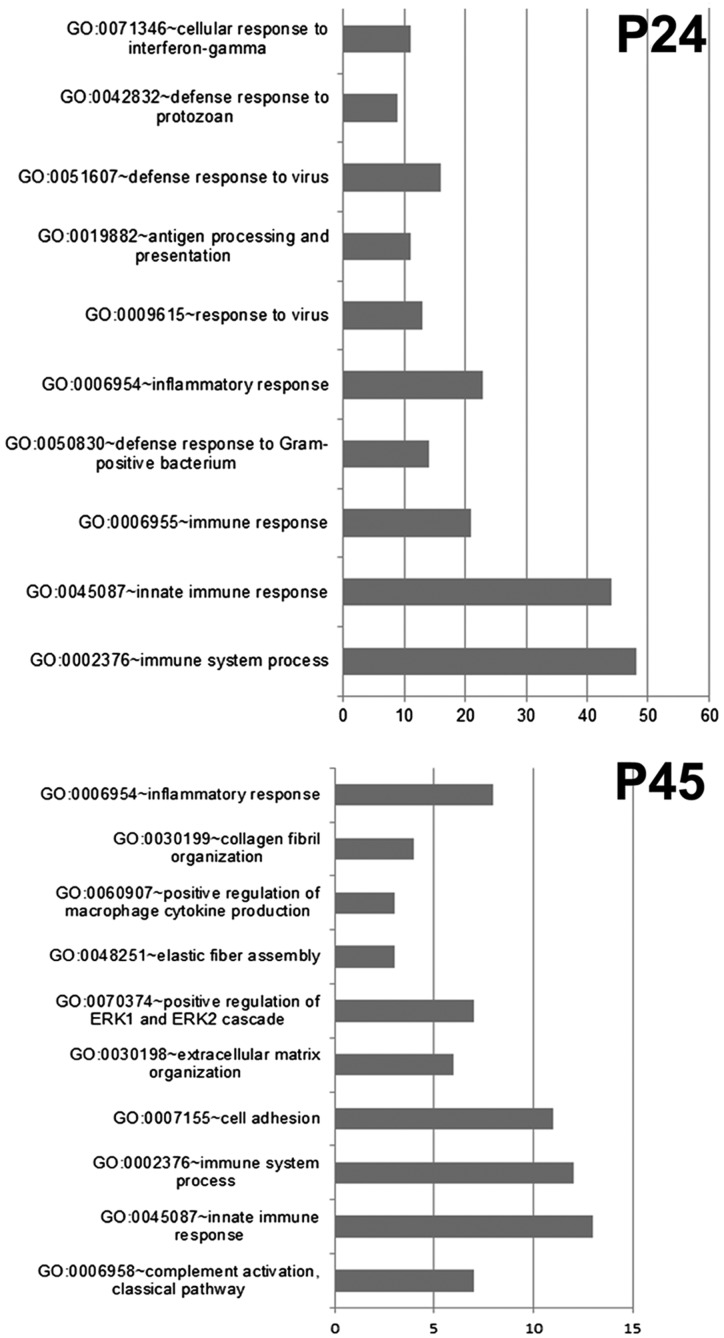
Pathway analysis using unbiased Gene Ontology on Web-based Gene Set Analysis Toolkit (WEBGESTALT) disclosed a major role of the inflammatory/immune response, both innate and adaptive, at P24 as well as at P45. DAVID representation clearly demonstrates that at P24, the majority of up-regulated genes in the rd10 retina (65 out of the 159 genes) belong to the inflammatory pathway (Fig. 2); similarly, at P45, 30 of the 93 up-regulated genes in the rd10 retina fall into the inflammatory/immune response pathway (Fig. 2). This and similar analyses indicate a predominant impact of inflammation on a classic paradigm of inherited retinal degeneration (Supplemental Fig. S2). Other predictable biologic pathways known to occur in this disease model (such as oxidative stress) are revealed by GO interrogation only when gene expression levels are set at lower values (i.e., fold change lower than 1), suggesting a main influence of inflammation on gene expression at the ages analyzed here.
Figure 2.
DAVID plots of Gene Ontology analysis at 24 and 45 d of life. At both ages (proximal to the peaks of rod and cone degeneration, respectively), inflammation/immune response emerges as a major biologic process underlying retinal degeneration. The signatures of both innate and immune reaction are revealed by this data grouping strategy. Bars represent gene count. The GO terms plotted show FDR values well below the threshold of 0.05.
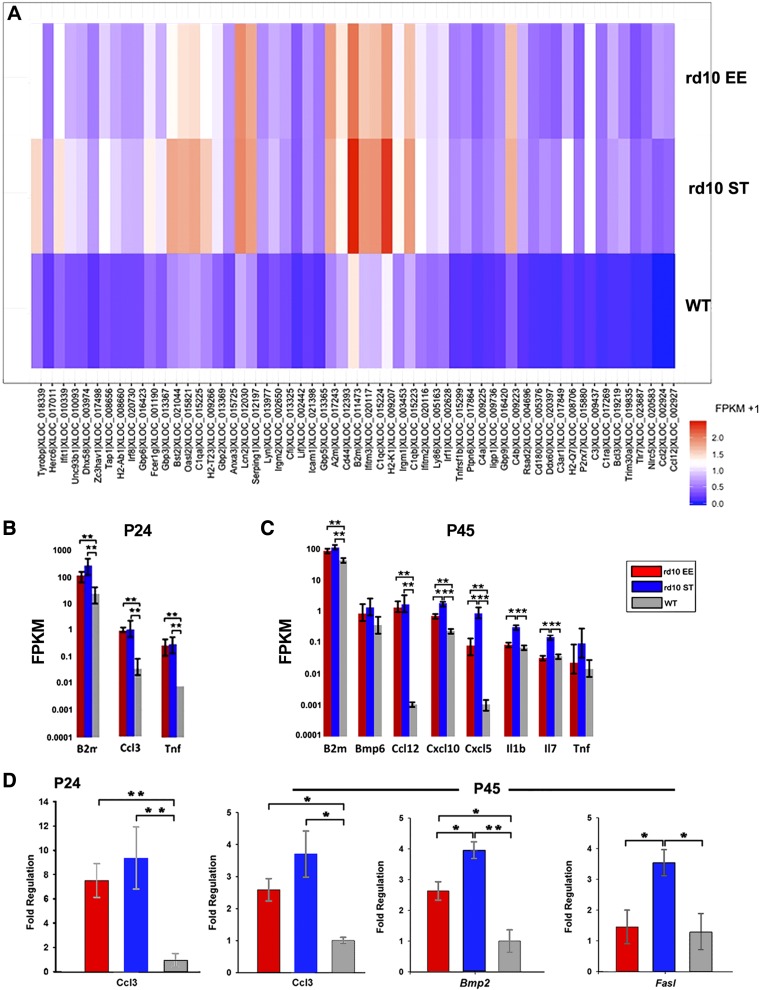
Transcriptome profiling reveals a statistically significant lower expression of various proinflammatory molecular species in EE compared to matched ST retinal samples. Both at P24 and P45, the number of up-regulated genes is lower in the EE rd10 sample group compared to matched ST samples. This is best appreciated in paired heat maps of the 3 experimental groups studied here (Fig. 3A), clearly showing a reduction of the intensity of the hottest bands of the inflammatory gene clusters in EE compared to ST cases. Three noticeable examples are Il1b, Il7, and Tnfa (Fig. 3B, C).
Figure 3.
Retinal inflammation in rd10 mice. A) Expression of genes of the immune response pathway chosen from GO databases selecting terms showing the highest differences in expression levels in rd10 EE, rd10 ST, and WT retinas, shown here as paired heat maps. P24 data, highly indicative, are shown. The hottest profiles are visible in the retinas of rd10 ST mice. Groups of genes show visibly lower levels of expression in EE samples. B, C) Plots from transcriptome analysis of retinas from rd10 EE, rd10 ST, and WT mice at 24 and 45 d of age showing representative samples of up-regulated genes involved in the inflammatory response (i.e., Ccl3, Il1b). These genes demonstrate a typically higher pattern of expression in rd10 ST samples, lower in healthy, WT mice and intermediate in rd10 EE animals. D) q real-time PCR at P24 and P45 detects high levels of inflammatory chemokines in all rd10 samples compared to WT but lower expression in rd10 EE samples compared to ST. FPKM, fragments per kilobase of exon per million fragments mapped. *0:01 ≤ P < 0:05, **0:001 ≤ P < 0:01, ***P < 0:001.
A selective cytokine-chemokine panel of 84 genes used for q real-time PCR array confirms the up-regulation of genes typically associated with inflammation (including cytokines as Il1b, Tnf, Il6, and chemokines such as Ccl3 and Ccl5) and of glial regulatory pathways (Fig. 3D). Quantitative real-time PCR analysis also confirms a clear shift toward a lowered inflammatory molecular profile in retinas from EE cases compared to ST controls at both the age points examined (Figs. 3D and 4 and Supplemental Fig. S3). Although their inflammation markers are obviously higher than in the WT, retinas from rd10 EE mice show lower expression levels of proinflammatory genes and higher expression levels of anti-inflammatory genes compared to the rd10 and ST counterparts (Fig. 4). This supports the conclusion that exposure to EE partially reverts the main inflammatory profile triggered by photoreceptor degeneration.
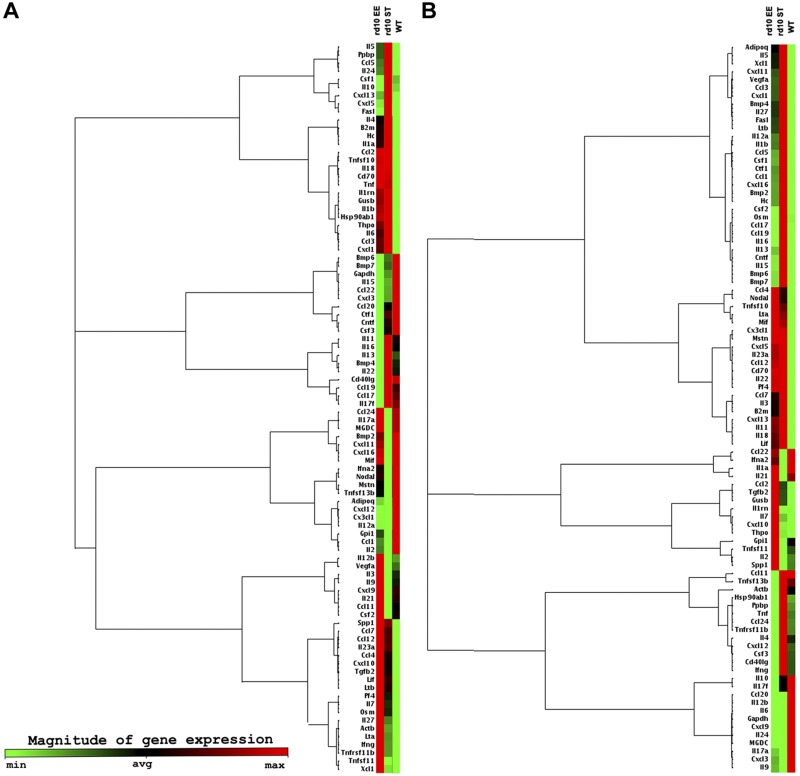
Figure 4.
q real-time PCR analysis for 84 cytokines-chemokines bound to inflammatory pathways was used to confirm transcriptome data in the 3 experimental groups. Shown here are clustergrams with relative gene expression and dendrograms (tree diagrams) indicating coregulated genes across groups at P24 (A) and P45 (B). Expression levels of proinflammatory genes are high in the ST rd10 retina, lower in the WT, and intermediate in EE cases.
Altogether, transcriptomic profiling associated to q real-time PCR array provided insight on the general consequences of rod and cone degeneration and on the effects of EE on these processes: specifically, we found that a pattern of exacerbated retinal inflammation constitutes a major molecular signature of inherited photoreceptor degeneration and that exposure to an enriched environment partially reverts this pattern.
Hallmarks of retinal degeneration transcriptome
The main hallmark of retinal degeneration is the above described predominance of inflammation/immune response at both P24 and P45. Yet, inflammatory signatures at P24 and at P45 are not identical. Major differences are constituted by the higher expression levels of Bmp2 and 4, as well as those of other proinflammatory species (Cxcl 5 and 10, Il1b, Il7), which become evidently more expressed approaching the peak of cone death (Fig. 3C, D). Conversely, Tnfa, an early mediator of the inflammatory response, decreases from P24 to P45 (Fig. 3C, D). In general, inflammatory genes show lower expression in EE than in ST samples; an exception is represented by Cxcl10 (a C-X-C motif chemokine strongly chemotactic for lymphocytes and with an important role in angiogenesis), showing higher levels of expression in retinas of EE rd10 mice at P45 by q real-time PCR analysis (Fig. 3).
EE retinas also show higher levels of expression of genes for glycolysis enzymes (Hk1, Hk2, Ldha, Aldoa) in comparison to ST counterparts (Supplemental Fig. S4). Although this can easily be explained by the higher number of photoreceptors surviving in EE retinas, a concomitant increased in glucose metabolism in the retina of enriched animals (which have constant access to a running wheel) is expected on the base of previous demonstration of enhanced metabolism and glycolysis in the brain of mice exposed to physical exercise (17). In addition, various genes of the neurotrophin family (such as Igf1 and Bdnf) are found to be expressed at higher levels in EE retinas, confirming previous data from our group and other laboratories (see transcriptome data).
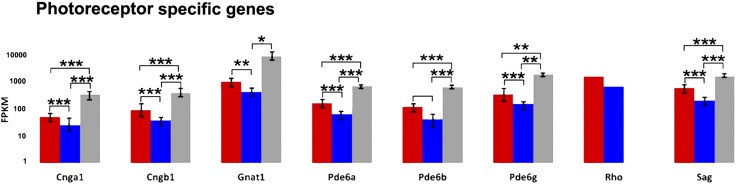
Profiling of rd10 mutant retinas brings to light additional patterns of gene expression consistent with the pathology under study. In particular, a number of photoreceptor-specific genes (such as Cgna1, Gnat1, Pde, etc.) show evidently lower levels of expression in the rd10 samples as an obvious consequence of massive photoreceptor death occurring in the time window examined. Once more, samples from EE retinas demonstrate higher expression levels of the same genes compared to ST counterparts, confirming the higher survival rate of photoreceptors in retinas mice exposed to EE. Differences between the 2 groups can be well appreciated at P45 (Fig. 5).
Figure 5.
Higher survival of photoreceptors in enriched mice is translated in higher expression of genes selectively expressed in these cells. Red columns: rd10, EE mice; blue columns: rd10, ST mice; gray columns: WT mice. Age is P45. Cnga, cyclic nucleotide-gated channel; Gnat, transducin; Pde, phosphodiesterase; Rho, rhodopsin; Sag, arrestin; FPKM, fragments per kilobase of exon per million fragments mapped. Values of Rho are not indicative because the gene expression in the WT is saturating. *0:01 ≤ P < 0:05, **0:001 ≤ P < 0:01, ***P < 0:001.
Retinal microglia/macrophage activation and recruitment closely mirror photoreceptor degeneration
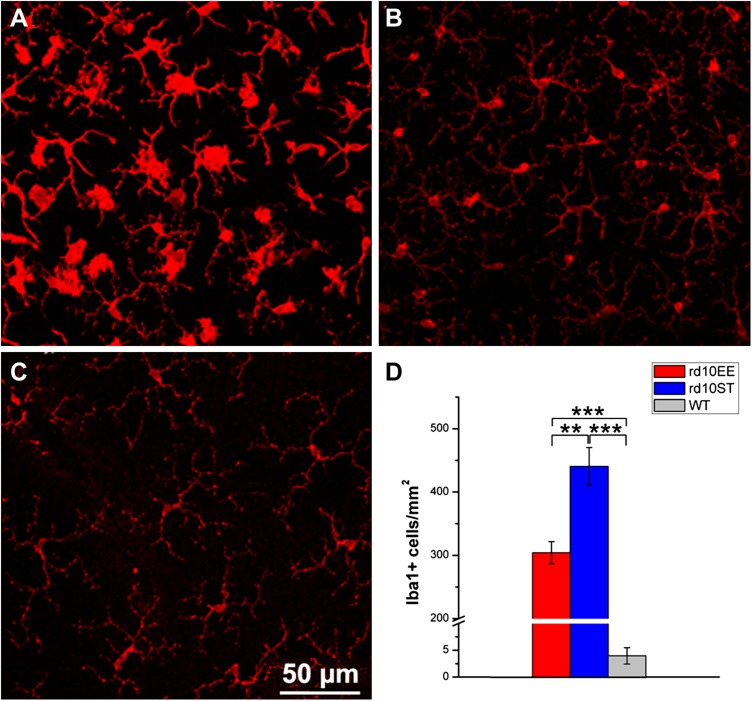
As elsewhere in the CNS, early inflammatory responses in the retina are mediated by macro and microglial cells, becoming rapidly activated upon signaling in the outer retina, which precede and accompany photoreceptor degeneration. Activated microglia and infiltrating macrophages are the main source of highly reactive, signaling molecules (cytokines and chemokines), primarily influencing T-cell adhesion and recruiting circulating monocytes and lymphocytes. Glial secreted factor mRNAs are detected by transcriptome analysis and quantitative real-time PCR and are known to mediate inflammation. Morphologic changes of microglia and monocyte-derived macrophages in the degenerating retina can be monitored with antibodies against Iba1, a microglia/macrophage-specific calcium-binding protein, with actin-bundling activity and participating in membrane ruffling and phagocytosis. Iba1 staining of rd10 retinas in whole mount shows transition from highly ramified, quiescent morphology to amoeboid, activated shape (Fig. 6).
Figure 6.
Effects of EE on retinal microglia. A–C) Immunostaining of retinal microglia with Iba1 antibodies at P45. Whole-mount visualization of the outer microglial plexus. rd10, ST retina. Microglial/macrophagic cells have large, ovoid cells bodies and display high reactivity to the immunostaining (A). Processes are short and transition to active status evident. The same preparation from rd10, EE retina shows a high number of Iba1-positive cells with higher ramified morphology and lower activation status (B). In the WT retina, microglial cells and macrophages retain a quiescent morphology and are less numerous than in the diseased retina (C). D) Histograms of Iba-1 positive cells show lower number of microglial cells in EE rd10 retinas compared to ST. The number of these cells is much lower in the healthy, WT retina. **P ≤ 0.01, ***P ≤ 0.001 (ANOVA, followed by Holm-Sidak test).
Activation of local microglia mainly (but not exclusively) involves cells residing in the outer retinal plexus and progresses according to a center-to-periphery gradient that mirrors faithfully the spatial progression of photoreceptor loss, progressively invading the outer retina and the subretinal space, normally devoid of microglia. Iba1-stained cellular profiles, also positive for CCL2, are observed on the choroidal side (Supplemental Fig. S5).
Whole-mount retinal samples show visibly decreased number of activated microglial/macrophages (Fig. 6A–C) in rd10 EE samples compared to rd10 ST preparations, as confirmed by quantification of of Iba1-positive cells (Fig. 6D). This goes in line with lower expression of inflammation-related genes detected by transcriptome analysis in rd10 EE as compared to rd10 retinal samples and indicates proportionally lower levels of inflammation in retinas of enriched animals.
Steroid treatment lowers retinal inflammation and protects cones
A lower level of activation of microglia/macrophages in retinas from EE mice could be attributable 1) to a direct neuroprotective effect of the enriched environment on photoreceptor survival, in turn driving a lower degree of microglia and monocyte-derived macrophages recruitment and activation; 2) to a primary anti-inflammatory effect of EE per se; or 3) to a combination of both effects. The possibility to reproduce EE beneficial action with environmental-mimetic drugs relies strictly on the identification of EE mechanistic effectors. Based on the present findings and on existing studies of inflammation in RP, we postulated that the initial loss of rods creates a local environment rich in highly reactive, proinflammatory molecules, particular detrimental for the survival of cones, the cells located in close proximity to rods, and known to depend on them for survival factors. We tested the hypothesis that some of the beneficial effects of EE could be mimicked by targeting retinal inflammation alone, treating rd10 mice with Dexa, a synthetic steroid with powerful anti-inflammatory properties but with more limited action compared to EE, a very complex experimental paradigm. Primary effect of Dexa is a depression of inflammatory/immune response by inhibition of microglial recruitment and migration (18). The choice of this anti-inflammatory drug is further justified by its wide employment in human ophthalmology, in particular to treat cystoid macular edema, a common complication of RP (19, 20). We administered subcutaneous Dexa to rd10 mice from P23 (anticipating the peak of rod death) to P60, again encompassing most of the time window of rod and cone degeneration in this mutant; then, we studied the effects of drug administration on the survival of cones and on the maintenance of daylight vision.
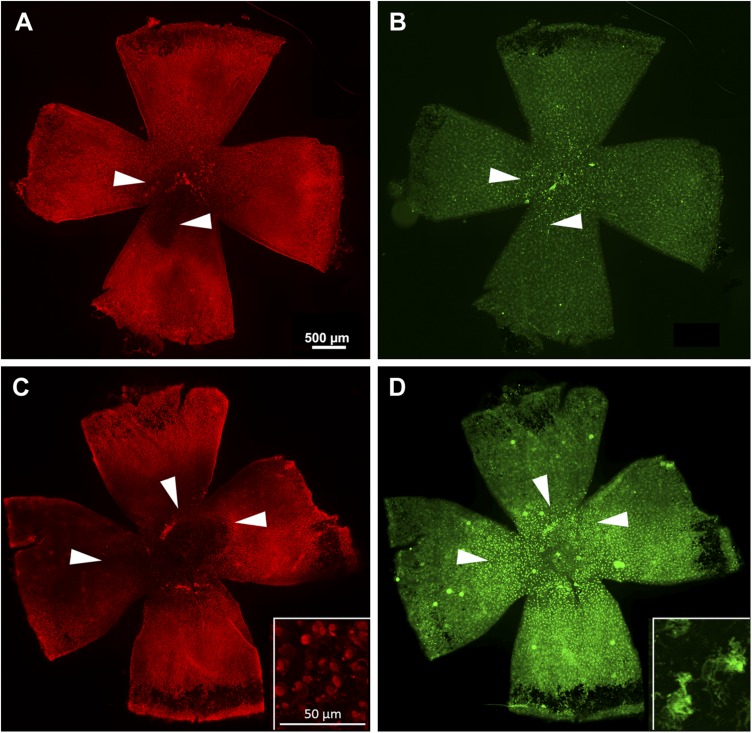
As anticipated from the known anti-inflammatory properties of Dexa, we found that rd10 mice treated with this drug show a lower degree of retinal microglia/macrophages activation, both at P45 and at P60, compared to mice treated with vehicle alone. This is readily visible in retinal whole mounts stained for CD11b integrin α M—a microglia/macrophages marker—and examined at low magnification. Activated mononuclear phagocytes (i.e., microglial cells and invading macrophages) form hot spots that overlap and are contiguous with retinal areas of active cone degeneration and already virtually devoid of most photoreceptors (Fig. 7B, D). The same images highlight higher cone survival in Dexa-treated retinas (Fig. 7A, C). Image analysis and cell counts in retinal whole mounts confirm that Dexa-treated retinas have a larger surface in which cones are still present and a higher total number of these cells (Fig. 7A) as well as a statistically significative lower number of activated microglia/macrophages in the outer retinal plexus (Fig. 7B).
Figure 7.
Effects of Dexa administration on retinal microglia and cone survival. A, B) Whole-mount retina from rd10 mouse treated with Dexa. C, D) Similar preparations from a rd10 control mouse treated with vehicle. Age is 60 d. Cone arrestin staining (red signal) (A, C). Same retinas shown in A and C labeled for CD11b, a marker of microglia/macrophages (green signal) (B, D). Note the bright staining of numerous cells in D compared to B. The extension of a dark, cone-devoid area is visibly larger in C compared to A. Retinal zones of maximum cone degeneration overlap with areas of high microglia/macrophages reactivity (arrowheads). High magnification insets in C and D show details of cone and microglial cell morphologies, respectively.
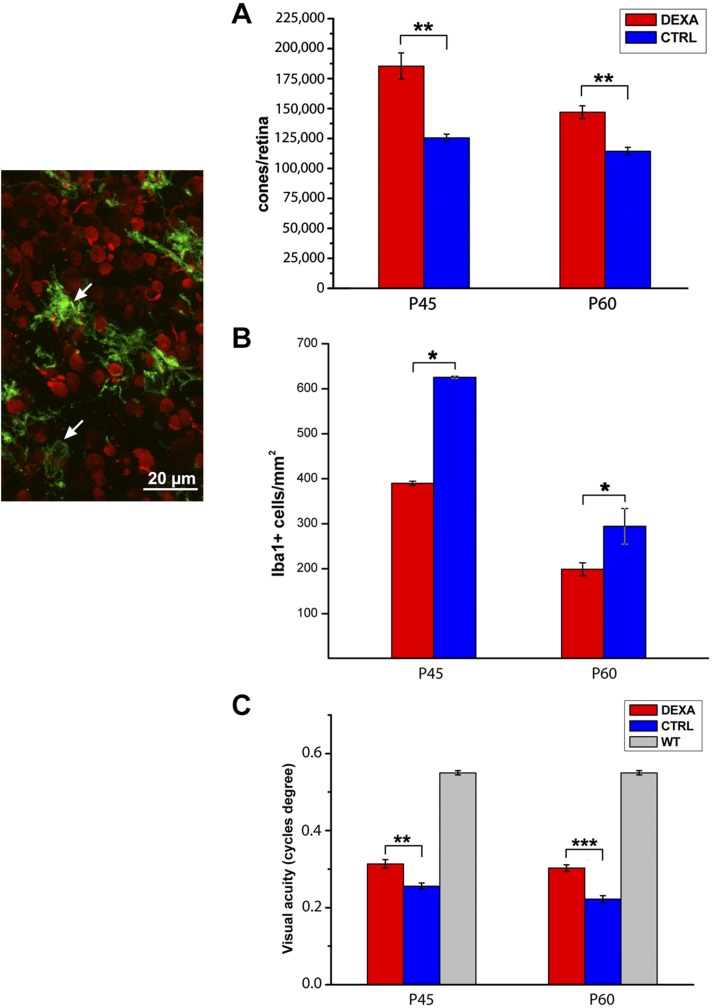
Increased cone survival in Dexa-treated mice translates into improvement of photopic vision compared to untreated rd10 mice. Behavioral testing with Prusky water maze, specifically assessing visual performance in photopic conditions, demonstrates that rd10 mice administered with Dexa (although showing impaired vision than WT, healthy animals) have a higher visual acuity than rd10 untreated animals, indicating that surviving cones are viable (Fig. 8C).
Figure 8.
Dexa effects on rd10 mice. The micrograph shows a detail of a whole-mount retina with a focal plane on cones (stained red by cone arrestin antibodies) and the outer plexus of microglial cells (stained green by Cd11b; arrows). A) Cone counts shows preservation of 30% cones in the retinas of rd10 mice treated with Dexa (red columns) compared to control mice (blue columns). B) The same retinal samples show a lower number of activated microglia/macrophages in Dexa-administered mice. C) Photopic visual acuity of Dexa-treated rd10 mice is higher than in age-matched controls (although sensibly lower than in WT animals). Ctrl, control. For all experimental groups, n = 3/4. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Student’s t test).
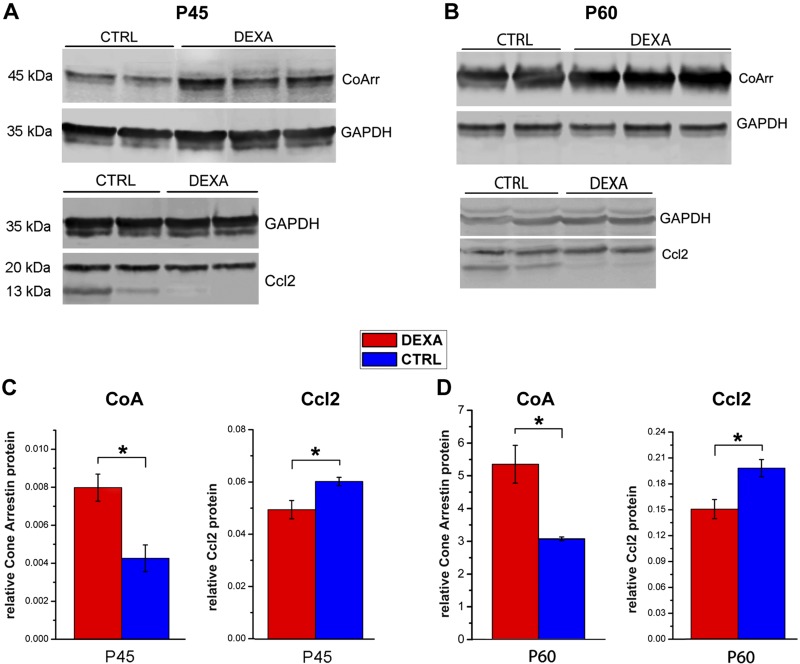
Western blot analysis confirms that cone arrestin, a photoreceptor-specific, soluble protein that blocks photopigment-G protein interaction, is detected in higher amounts in the retinas of Dexa-treated mice at P45, confirming survival of a higher number of cones (Fig. 9A, C). The same retinal specimens show decreased presence of CCL2, a common proinflammatory chemokine primarily involved in the recruitment of blood-derived monocytes, in the retina of rd10 mice treated with Dexa (Fig. 9A, C). Similar results are obtained at P60 (Fig. 9B, D). Western blot for CCl2 bands returns 2 bands (of ∼13 and ∼ 20 kDa), representing the monomeric native form of the protein and the product of its dimerization and glycosylation, respectively (21). Only the lower one, corresponding to the monomeric form necessary to mediate signaling events (22) was used for quantification. Statistics were conducted using samples from retinas of 3 different mice per experimental group.
Figure 9.
Western blot analysis of retinal extracts at P45 (A, C) and P60 (B, D) detects lower levels of CCL2 and higher levels of cone arrestin in Dexa-treated compared to vehicle-treated preparations. Loading of up to 60 µg of protein was necessary to reveal CCL2, a cytokine with low expression level. Ctrl, control. *0:01 ≤ P < 0:05.
Altogether, Dexa treatment decreases retinal glial activation, lowers reactive cytokine concentration in the retina, supports cone structural and functional preservation, and largely mimics the entity and type of beneficial effects produced by EE in rd10 mutant mice.
DISCUSSION
Beside perceptive impairment, vision loss brings in high risks of traumatic injuries, depression, cognitive decline, and shortened life expectancy (23). Therapies are becoming increasingly available to restore vision or delay progressive visual decline in inherited retinal degenerations. Approaches to treatment comprise gene, cellular or ribozyme therapies, RNAi, dietary supplementation, implant of retinal prostheses, pharmacological use of neurotrophic factors, and antiapoptotic agents (24).
Pharmacological treatments delaying vision loss and preserving cone-mediated vision in human RP are virtually missing. Yet, drug-based therapies could be of general use, especially if targeting widespread processes associated to the disease (i.e., oxidative stress), likely independent of the underlying, causative mutation. Besides prolonging useful vision, such pharmacological approaches would preserve photoreceptor viability in view of a true therapeutic option.
Here, we provide experimental evidence supporting the occurrence of major inflammatory responses in the retina of RP mice at the peaks of rod and cone loss. Our NGS study confirms and expands previous transcriptome data for the same mutant (25), which were not obtained from age-matched rd10 and control samples and did not comprise enriched mice. We deemed important to focus specifically on 2 biologically critical time points, near the peaks of rod and cone degeneration in rd10 mice. We compared rd10 raised in ST and EE conditions using age- and sex-matched controls, thus minimizing effects due to differences in inflammatory and environmental responses due to disease stage and sex (26). We also expanded our previous findings showing that exposure to an enriched environment from birth delays the secondary loss of cones and preserves cone-mediated vision, concomitantly reducing retinal inflammation, in line with anti-inflammatory effects of EE recently described in other disease models (27). Finally, we demonstrated that targeting the sole inflammation process with general administration of commonly used steroids is sufficient to delay cone degeneration and to preserve a population of these cells large enough to allow behaviorally tests based on photopic visual acuity.
Despite the known existence of an eye immune privilege, ocular inflammation has been implicated in the pathogenesis of a wide variety of eye diseases, comprising noninfectious uveitis, macular edema, diabetic retinopathy, and age-related macular degeneration (28), for which anti-inflammatory therapies are in use to ameliorate the symptoms (29–31). Different studies in humans indicate that inflammatory processes occur during RP as well. As early as 1985, antiretinal autoimmune activity was detected in the blood of patients with inherited retinal degenerations (32, 33) and various lymphocyte subsets along with macrophages were found in vitreous samples of RP individuals (34). More recently, the specific role of inflammation in RP pathogenesis has been investigated, at first demonstrating that death of rods attracts resident microglia in the outer retina, where they are observed to actively engulf rods in regions of ongoing cell death (35). Later, a clinical study (36) reported elevated levels of various proinflammatory cytokines and chemokines (including CCL2) in the aqueous and vitreous humors of humans affected by RP, as well as the presence of numerous inflammatory cells in their vitreous; noticeably, the number of inflammatory cells correlated with the entity of decreased visual function. In view of previous findings of the critical role of CCL2 in photoreceptor apoptosis via microglia/macrophages activation in retinal detachment (37), these studies demonstrate that up-regulation of CCL2 may concur per se to the degenerative process of RP (38). Indeed, in this study, we find that rd10 retinas show high levels of CCL2, a proinflammatory marker known to activate microglia and to recruit monocytes, memory T cells, and dendritic cells (36). Additional clinical findings in patients with RP include posterior subcapsular cataract and macular edema and a long-known presence of aqueous flare (39). Approximately 10–37% of patients with RP may have circulating antiretinal antibodies and have higher chances to develop macular edema (40). Yet, it is unclear if these antibodies precede or are a consequence of retinal degeneration.
Animal models of RP confirm the occurrence of retinal inflammation. Cytokines, including IL1b, are elevated in the T17 M rhodopsin mouse model (41), whereas genetic ablation of MyD88, a proinflammatory signaling protein slows down retinal degeneration in a knockout mouse model of RP (42). Dysregulation of the inflammatory response is supposed to further propagate photoreceptor damage, among others through generation of ROS, and secretion of IL1b (43, 44). Distressed rods express an eat-me signal (i.e., externalization of phosphatidylserine), up-regulating phagocytosis in microglia; in turn, phagocytosis of photoreceptor proteins increases the activation of microglia and macrophages, or both, via TLR4, creating a self-maintained circle, which can be interrupted by pharmacologic blockade of proinflammatory cytokines, such as IL1b (45). In addition, the CX3CR1-CX3CL1 axis, an immune-modulating pathway of crosstalk between neurons and microglia, has been implicated in the degeneration of photoreceptors, as demonstrated by the finding that increasing CX3CL1-CX3CR1 signaling effectively improves structure and function of these cells in a mouse model of RP (43).
Altogether, existing experimental data and clinical observations indicate that although inflammation in RP arises secondarily to the primary, genetic cause of photoreceptor loss, the sustained immune response developing thereafter may exacerbate the retinal deconstructive processes occurring in this disease.
Recent experimental studies have clarified the early and massive involvement of retinal microglia in removing dying rods, elucidating signaling regulating the phagocytic clearance of dead photoreceptors as well of those at risk (43, 46) but also contributing to photoreceptor loss (47, 48). Hence, microglial ablation, or inhibition of microglial activation (49), delay photoreceptor death in RP and slow the rate of disease progression.
If sustained inflammation arising from clearance of dying cells is expected in a chronic disease and also explains anticipated removal of mutated cells doomed to death, it does not justify tout court the secondary death of cones in RP because these cells do not express mutations and yet are affected by the death of nearby rods.
This study concentrates specifically on the concomitant occurrence of sustained inflammation and cone death in mice with a mutation in a photoreceptor-specific Pde6b gene, which is also causative of human RP (50). The unbiased results obtained by interrogation of the data by Gene Ontology, together with the choice of the time points for NGS analysis, show a net correlation of expression level changes in inflammatory genes and extent of photoreceptor death. Retinal transcriptome signature at P24 is similar to that observed at P45, with a main role of inflammation/immune response, although levels of gene expression are higher at P24. At both ages, markers of this biologic response are lower in samples from age-matched rd10 mice born and raised in an enriched environment. These samples also show higher expression of photoreceptor-specific genes, supporting the observation of increased survival of these cells in EE conditions. Noticeably, overexpression of Tnfa is higher at the peak of rod death, as expected from this early marker of inflammatory response (51).
Molecular indicators of glycolytic activity are also higher in the retina of EE samples. This can be explained by the higher number of photoreceptors surviving in these samples as well as to a raise in glucose retinal metabolism found for other areas of the CNS in response to physical exercise (52), a crucial component of EE, and of the underlying BDNF increased production typical of this experimental manipulation (53). Indeed, a raise in glucose mobilization is known to be beneficial for cones, which exhibit a very high glucose metabolism and have been shown to undergo autophagy as a consequence of rod death. Hence, part of the protective action of EE on the retina can be attributed to enhanced glucose use, with an action similar to that of rod-derived, cone viability factor, known to promote aerobic glycolysis, thereby supporting survival of cones (54). Additional beneficial effects of EE can be ascribed to increased expression of retinal neurotrophic factors, as demonstrated by our laboratory (6) and other groups (8).
Besides these (expected) effects of EE, our data reveal a concomitant relation between enrichment exposure and retinal immune response that goes in line with recent studies: environmental stimulation promotes increased expression of antioxidant genes and reduces the expression of inflammatory genes in a mouse model of accelerated senescence (55), preventing oxidative stress, and inflammation. Similarly, EE attenuates expression of proinflammatory and pro-oxidative mediators in a mouse model of Alzheimer disease (56).
To test the hypothesis that chronic inflammation concurs to the secondary degeneration of cones, we treated rd10 mice with Dexa, using this widely employed steroid to inhibit glial responses directly. However, this pharmacological treatment was chosen to attempt an environmental-mimetic protocol, taking advantage of a direct action on the immune system (typical of steroids), without directly interfering with photoreceptor survival. Dexa-general treatment of rd10 mice mimicked nature and entity of positive effects of EE exposure, insofar as morphology and function of retinal cones were partially preserved and retinal inflammation down-regulated. The effect was mostly visible on cones because at P45 rods had largely degenerated as in untreated animals, suggesting that reduction of chronic inflammation generated a benefit for cones in particular. This goes in line with experiments showing rescue of cones in RP and macular degeneration models upon selective targeting of infiltrating monocytes (57), also inhibited by steroid treatments. This also supports beneficial effects in patients with RP treated with a number of pharmaceuticals exhibiting (albeit not exclusively) anti-inflammatory attributes, such as vitamin E and the calcium blocker Diltiazem (38).
A direct effect of Dexa on photoreceptors cannot be ruled out completely considering the ubiquitous distribution of the glucocorticoid receptor (GR) throughout the CNS. However, a considerable variety in GR gene expression levels has been demonstrated in different cell types, whereas the presence of GR on photoreceptors, despite being postulated, has never been detected directly (58). Moreover, both steroidal and nonsteroidal medications have been demonstrated to improve photoreceptor survival after laser retinal photocoagulation, concomitantly reducing inflammation in the retina (59). This evidence suggests that the effects of steroid on photoreceptors survival act through down-regulation of inflammation rather than through a direct, neuroprotective effect.
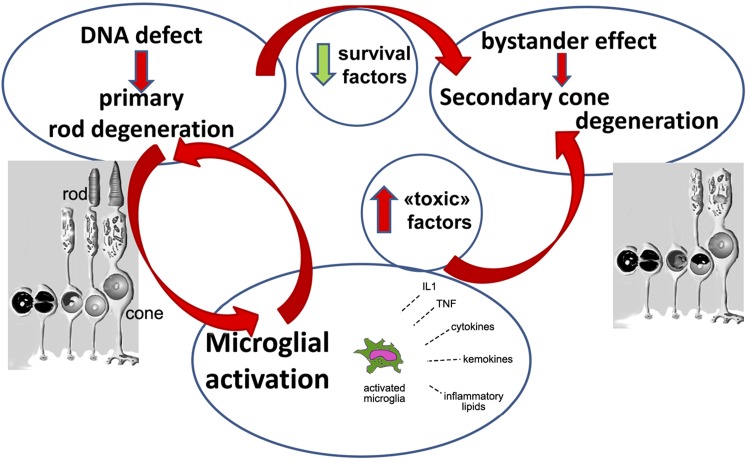
Our studies support the notion that the primary mutation responsible for RP triggers a vicious circle by which photoreceptor degeneration self-reinforces, supplying the production of reactive molecules from nearby glial cells, at the same time depleting the reservoir of prosurvival factors (Fig. 10). Both EE and anti-inflammatory treatment have the ability to attenuate this loop, thus prolonging the lifespan of cones.
Figure 10.
Inhibiting inflammation breaks a vicious circle in retinal degeneration. In RP, a genetic defect leads to the primary degeneration of rods. This creates simultaneously: 1) a decrement in the production of survival factors, which in turn contributes to the bystander death of cones; and 2) a local inflammatory response, mediated among others by microglial cells and invading macrophages. This implicates chronic release of toxic molecules worsening the microenvironment where cones reside, further contributing to the death of these cells. Anti-inflammatory treatments help breaking down this vicious circle.
Compared to genetic ablation of retinal immune response, previously shown to ameliorate the retinal degeneration phenotype (45), the employment of a widely used drug to attenuate inflammation used here can be translated to humans relatively more easily and these data lay the ground for immediate testing of general or ocular steroids to delay cone and daylight vision loss in patients with RP: Dexa itself, as well as other drugs of the same family (i.e., Triamcinolone), are used routinely in ophthalmology and chronically administered to individuals with autoimmune diseases (60). Although side effects are numerous, long-term treatments are generally well tolerated. A straightforward clinical trial for RP could be started upon simple repurposing the use of Dexa for RP and initiate a study with patients already intended for steroid administration to treat macular edema. Treatment and observation time should be extended to appreciate a decrement in the rate of cone vision loss given the slow progression of RP.
More studies will be necessary to unravel the molecular cascades mediating the retinal inflammatory response in inherited photoreceptor degeneration and to develop methods of targeting selected molecular species and population of microglia/macrophages mostly contributing to the adverse retinal response have to be identified. Disclosure of the predominance of a strong inflammatory component in this family of genetic disorders and demonstration that attenuation (rather than complete suppression) of inflammation is capable of rescuing retinal cones constitute useful achievements.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Francesca Lessi and Sara Franceschi (Pisa Science Foundation) for valuable support with next generation sequencing experiments; Renzo Di Renzo (Institute of Neuroscience, Consiglio Nazionale delle Ricerche, Pisa, Italy) for technical assistance; Tommaso Pizzorusso (University of Florence, Italy) for the generous use of quantitative real-time PCR apparatus. Macula Vision Research Foundation (Conshohocken, PA, USA). Funded by Fondazione Roma (Rome, Italy), retinitis pigmentosa call; and by Velux Foundation Stiftung (Zurich, Switzerland). ES was beneficiary of a EU Horizon 2020 research and innovation program - Marie Sklodowska-Curie grant agreement No 674901. The sponsors had no role in the study design, in the collection, analysis, and interpretation of data, in writing the report, or in the decision to submit the paper for publication. The authors declare no conflicts of interest.
Glossary
- CCL
chemokine chemoattractant
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- Dexa
dexamethasone
- EE
environmental enrichment
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GO
gene ontology
- GR
glucocorticoid receptor
- Iba1
ionized calcium-binding adapter molecule 1
- ICCH
immunocytochemistry
- ISP
ion sphere particle
- NGS
next generation sequencing
- OPL
Outer plexiform layer
- PDE
Phosphodiesterase
- q real-time PCR
quantitative real-time PCR
- RP
retinitis pigmentosa
- ST
standard
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
V. Guadagni and M. Biagioni equally contributed to the study design, data collection, data analysis, data interpretation, and writing of the manuscript; E. Novelli contributed to the study design, data collection and interpretation, and provided technical assistance; P. Aretini and C. M. Mazzanti contributed to the study design, data analysis and interpretation, and manuscript writing. E. Strettoi supervised the study, contributed to the study design and to data analysis and interpretation, and wrote the manuscript; and all authors read, edited, and approved the final manuscript.
REFERENCES
- 1.Daiger S. P., Bowne S. J., Sullivan L. S. (2007) Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 125, 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright A. F., Chakarova C. F., Abd El-Aziz M. M., Bhattacharya S. S. (2010) Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 11, 273–284 [DOI] [PubMed] [Google Scholar]
- 3.Zobor D., Zrenner E. (2012) Retinitis pigmentosa - a review. Pathogenesis, guidelines for diagnostics and perspectives [in German]. Ophthalmologe 109, 501–514; quiz 515 [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro P. A., Mir T. A. (2018) The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 62, 24–37 [DOI] [PubMed] [Google Scholar]
- 5.Barone I., Novelli E., Strettoi E. (2014) Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol. Vis. 20, 1545–1556 [PMC free article] [PubMed] [Google Scholar]
- 6.Barone I., Novelli E., Piano I., Gargini C., Strettoi E. (2012) Environmental enrichment extends photoreceptor survival and visual function in a mouse model of retinitis pigmentosa. PLoS One 7, e50726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardue M. T., Chrenek M. A., Schmidt R. H., Nickerson J. M., Boatright J. H. (2015) Potential role of exercise in retinal health. Prog. Mol. Biol. Transl. Sci. 134, 491–502 [DOI] [PubMed] [Google Scholar]
- 8.Lawson E. C., Han M. K., Sellers J. T., Chrenek M. A., Hanif A., Gogniat M. A., Boatright J. H., Pardue M. T. (2014) Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J. Neurosci. 34, 2406–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sale A., Berardi N., Maffei L. (2014) Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol. Rev. 94, 189–234 [DOI] [PubMed] [Google Scholar]
- 10.Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 11.Ozkan A., Biçer A., Avşar T., Seker A., Toktaş Z. O., Bozkurt S. U., Başak A. N., Kılıç T. (2012) Temporal expression analysis of angiogenesis-related genes in brain development. Vasc. Cell 4, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wróbel A., Serefko A., Wlaź P., Poleszak E. (2014) The depressogenic-like effect of acute and chronic treatment with dexamethasone and its influence on the activity of antidepressant drugs in the forced swim test in adult mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 243–248 [DOI] [PubMed] [Google Scholar]
- 13.Wróbel A., Zebrowska-Łupina I., Wielosz M. (2005) Dexamethasone reduces locomotor stimulation induced by dopamine agonists in mice. Pharmacol. Rep. 57, 451–457 [PubMed] [Google Scholar]
- 14.Arango-Gonzalez B., Trifunović D., Sahaboglu A., Kranz K., Michalakis S., Farinelli P., Koch S., Koch F., Cottet S., Janssen-Bienhold U., Dedek K., Biel M., Zrenner E., Euler T., Ekström P., Ueffing M., Paquet-Durand F. (2014) Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One 9, e112142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargini C., Terzibasi E., Mazzoni F., Strettoi E. (2007) Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J. Comp. Neurol. 500, 222–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin B., Masland R. H., Strettoi E. (2009) Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res 88, 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dornbos D., III, Zwagerman N., Guo M., Ding J. Y., Peng C., Esmail F., Sikharam C., Geng X., Guthikonda M., Ding Y. (2013) Preischemic exercise reduces brain damage by ameliorating metabolic disorder in ischemia/reperfusion injury. J. Neurosci. Res. 91, 818–827 [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y., Ling E. A., Dheen S. T. (2007) Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J. Neurochem. 102, 667–678 [DOI] [PubMed] [Google Scholar]
- 19.Stewart M. W. (2012) Corticosteroid use for diabetic macular edema: old fad or new trend? Curr. Diab. Rep. 12, 364–375 [DOI] [PubMed] [Google Scholar]
- 20.Ahn S. J., Kim K. E., Woo S. J., Park K. H. (2014) The effect of an intravitreal dexamethasone implant for cystoid macular edema in retinitis pigmentosa: a case report and literature review. Ophthalmic Surg. Lasers Imaging Retina 45, 160–164 [DOI] [PubMed] [Google Scholar]
- 21.Deshmane S. L., Kremlev S., Amini S., Sawaya B. E. (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y., Tsirka S. E. (2014) Mouse monocyte chemoattractant protein 1 (MCP1) functions as a monomer. Int. J. Biochem. Cell Biol. 55, 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chia E. M., Wang J. J., Rochtchina E., Smith W., Cumming R. R., Mitchell P. (2004) Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Invest. Ophthalmol. Vis. Sci. 45, 71–76 [DOI] [PubMed] [Google Scholar]
- 24.Strettoi E., Gargini C., Novelli E., Sala G., Piano I., Gasco P., Ghidoni R. (2010) Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 107, 18706–18711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uren P. J., Lee J. T., Doroudchi M. M., Smith A. D., Horsager A. (2014) A profile of transcriptomic changes in the rd10 mouse model of retinitis pigmentosa. Mol. Vis. 20, 1612–1628 [PMC free article] [PubMed] [Google Scholar]
- 26.Bessinis D. P., Dalla C., Kokras N., Pitychoutis P. M., Papadopoulou-Daifoti Z. (2013) Sex-dependent neurochemical effects of environmental enrichment in the visual system. Neuroscience 254, 130–140 [DOI] [PubMed] [Google Scholar]
- 27.Xu H., Rajsombath M. M., Weikop P., Selkoe D. J. (2018) Enriched environment enhances β-adrenergic signaling to prevent microglia inflammation by amyloid-β. EMBO Mol. Med. 10, e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez V. L., Caspi R. R. (2015) Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 36, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao J. H., Mulvahill M., Zhang L., Joondeph B. C., Dacey M. S. (2014) Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology 121, 1871–1876 [DOI] [PubMed] [Google Scholar]
- 30.Cheung N., Mitchell P., Wong T. Y. (2010) Diabetic retinopathy. Lancet 376, 124–136 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell P., Liew G., Gopinath B., Wong T. Y. (2018) Age-related macular degeneration. Lancet 392, 1147–1159 [DOI] [PubMed] [Google Scholar]
- 32.Chant S. M., Heckenlively J., Meyers-Elliott R. H. (1985) Autoimmunity in hereditary retinal degeneration. I. Basic studies. Br. J. Ophthalmol. 69, 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heckenlively J. R., Solish A. M., Chant S. M., Meyers-Elliott R. H. (1985) Autoimmunity in hereditary retinal degenerations. II. Clinical studies: antiretinal antibodies and fluorescein angiogram findings. Br. J. Ophthalmol. 69, 758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newsome D. A., Michels R. G. (1988) Detection of lymphocytes in the vitreous gel of patients with retinitis pigmentosa. Am. J. Ophthalmol. 105, 596–602 [DOI] [PubMed] [Google Scholar]
- 35.Gupta N., Brown K. E., Milam A. H. (2003) Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 76, 463–471 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida N., Ikeda Y., Notomi S., Ishikawa K., Murakami Y., Hisatomi T., Enaida H., Ishibashi T. (2013) Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 120, 100–105 [DOI] [PubMed] [Google Scholar]
- 37.Nakazawa T., Hisatomi T., Nakazawa C., Noda K., Maruyama K., She H., Matsubara A., Miyahara S., Nakao S., Yin Y., Benowitz L., Hafezi-Moghadam A., Miller J. W. (2007) Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc. Natl. Acad. Sci. USA 104, 2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMurtrey J. J., Tso M. O. M. (2018) A review of the immunologic findings observed in retinitis pigmentosa. Surv. Ophthalmol. 63, 769–781 [DOI] [PubMed] [Google Scholar]
- 39.Nagasaka Y., Ito Y., Ueno S., Terasaki H. (2016) Increased aqueous flare is associated with thickening of inner retinal layers in eyes with retinitis pigmentosa. Sci. Rep. 6, 33921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heckenlively J. R., Aptsiauri N., Nusinowitz S., Peng C., Hargrave P. A. (1996) Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Trans. Am. Ophthalmol. Soc. 94, 179–200; discussion 200–206 [PMC free article] [PubMed] [Google Scholar]
- 41.Rana I., Badoer E., Alahmadi E., Leo C. H., Woodman O. L., Stebbing M. J. (2014) Microglia are selectively activated in endocrine and cardiovascular control centres in streptozotocin-induced diabetic rats. J. Neuroendocrinol. 26, 413–425 [DOI] [PubMed] [Google Scholar]
- 42.Syeda S., Patel A. K., Lee T., Hackam A. S. (2015) Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88. Exp. Neurol. 267, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabel M. K., Zhao L., Zhang Y., Gonzalez S. R., Ma W., Wang X., Fariss R. N., Wong W. T. (2016) Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 64, 1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng H., Ding M., Chen X. X., Lu Q. (2014) Microglial NADPH oxidase activation mediates rod cell death in the retinal degeneration in rd mice. Neuroscience 275, 54–61 [DOI] [PubMed] [Google Scholar]
- 45.Zhao L., Zabel M. K., Wang X., Ma W., Shah P., Fariss R. N., Qian H., Parkhurst C. N., Gan W. B., Wong W. T. (2015) Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 7, 1179–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang K. J., Lee J. E., Wang Y. D., Ma W., Fontainhas A. M., Fariss R. N., Wong W. T. (2009) Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest. Ophthalmol. Vis. Sci. 50, 4444–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida N., Ikeda Y., Notomi S., Ishikawa K., Murakami Y., Hisatomi T., Enaida H., Ishibashi T. (2013) Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 120, e5–e12 [DOI] [PubMed] [Google Scholar]
- 48.Zhao L., Ma W., Fariss R. N., Wong W. T. (2011) Minocycline attenuates photoreceptor degeneration in a mouse model of subretinal hemorrhage microglial: inhibition as a potential therapeutic strategy. Am. J. Pathol. 179, 1265–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng B., Xiao J., Wang K., So K. F., Tipoe G. L., Lin B. (2014) Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J. Neurosci. 34, 8139–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLaughlin M. E., Sandberg M. A., Berson E. L., Dryja T. P. (1993) Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat. Genet. 4, 130–134 [DOI] [PubMed] [Google Scholar]
- 51.Owens T. (2002) Identification of new therapeutic targets for prevention of CNS inflammation. Expert Opin. Ther. Targets 6, 203–215 [DOI] [PubMed] [Google Scholar]
- 52.Dienel G. A. (2019) Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045 [DOI] [PubMed] [Google Scholar]
- 53.Van Praag H., Fleshner M., Schwartz M. W., Mattson M. P. (2014) Exercise, energy intake, glucose homeostasis, and the brain. J. Neurosci. 34, 15139–15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aït-Ali N., Fridlich R., Millet-Puel G., Clérin E., Delalande F., Jaillard C., Blond F., Perrocheau L., Reichman S., Byrne L. C., Olivier-Bandini A., Bellalou J., Moyse E., Bouillaud F., Nicol X., Dalkara D., van Dorsselaer A., Sahel J. A., Léveillard T. (2015) Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161, 817–832 [DOI] [PubMed] [Google Scholar]
- 55.Griñan-Ferré C., Puigoriol-Illamola D., Palomera-Ávalos V., Pérez-Cáceres D., Companys-Alemany J., Camins A., Ortuño-Sahagún D., Rodrigo M. T., Pallàs M. (2016) Environmental enrichment modified epigenetic mechanisms in SAMP8 mouse hippocampus by reducing oxidative stress and inflammaging and achieving neuroprotection. Front. Aging Neurosci. 8, 241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herring A., Blome M., Ambrée O., Sachser N., Paulus W., Keyvani K. (2010) Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol. 20, 166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eandi C. M., Charles Messance H., Augustin S., Dominguez E., Lavalette S., Forster V., Hu S. J., Siquieros L., Craft C. M., Sahel J. A., Tadayoni R., Paques M., Guillonneau X., Sennlaub F. (2016) Subretinal mononuclear phagocytes induce cone segment loss via IL-1β. eLife 5, e16490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wenzel A., Grimm C., Seeliger M. W., Jaissle G., Hafezi F., Kretschmer R., Zrenner E., Remé C. E. (2001) Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest. Ophthalmol. Vis. Sci. 42, 1653–1659 [PubMed] [Google Scholar]
- 59.Brown J., Jr., Hacker H., Schuschereba S. T., Zwick H., Lund D. J., Stuck B. E. (2007) Steroidal and nonsteroidal antiinflammatory medications can improve photoreceptor survival after laser retinal photocoagulation. Ophthalmology 114, 1876–1883 [DOI] [PubMed] [Google Scholar]
- 60.Rodríguez Villanueva J., Rodríguez Villanueva L., Guzmán Navarro M. (2017) Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 516, 342–351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.