Abstract
Background
Exercise training (ET) has beneficial effects on multiple sclerosis and its animal model experimental autoimmune encephalomyelitis (EAE). However, the intensity‐dependent effects of ET on the systemic immune system in EAE remain undefined.
Objective
(1) To compare the systemic immune modulatory effects of moderate versus high‐intensity ET protocols in protecting against development of EAE; (2) To investigate whether ET affects autoimmunity selectively, or causes general immunosuppression.
Methods
Healthy mice performed moderate or high‐intensity treadmill running programs. Proteolipid protein (PLP)‐induced transfer EAE was utilized to examine ET effects specifically on the systemic immune system. Lymph node (LN)‐T cells from trained versus sedentary donor mice were transferred to naïve recipients and EAE severity was assessed, by clinical assessment and histopathological analysis. LN‐T cells derived from donor trained versus sedentary PLP‐immunized mice were analyzed in vitro for proliferation assays by flow cytometry analysis and cytokine and chemokine receptor gene expression using real‐time PCR. T cell‐dependent immune responses of trained versus sedentary mice to the nonautoantigen ovalbumin and susceptibility to Escherichia coli‐induced acute peritonitis were examined.
Results
High‐intensity training in healthy donor mice induced significantly greater inhibition than moderate‐intensity training on proliferation and generation of encephalitogenic T cells in response to PLP‐immunization, and on EAE severity upon their transfer into recipient mice. High‐intensity training also inhibited LN‐T cell proliferation in response to ovalbumin immunization. E. coli bacterial counts and dissemination were not affected by training.
Interpretation
High‐intensity training induces superior effects in preventing autoimmunity in EAE, but does not alter immune responses to E. coli infection.
Introduction
Exercise training (ET) attenuates symptoms and delays the progression of disability in multiple sclerosis (MS) patients,1, 2, 3, 4 while low levels of physical fitness have been suggested as a risk factor for developing MS.5 Accordingly, ET has been shown to modulate other autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and others.6 Beneficial effects of ET in experimental autoimmune encephalomyelitis "EAE", the animal model of MS,7, 8, 9, 10, 11, 12 have been established. Notably, moderate‐intensity ET reduced the encephalitogenicity of autoreactive T cells to attenuate transfer EAE, but did not induce a direct protective effect on the CNS from encephalitogenic T cells.12
Several studies demonstrated an anti‐inflammatory effect of ET in various mouse models of systemic inflammation.13, 14, 15 Other studies suggested complex effects of ET on the systemic immune system, including activation of both pro‐ and anti‐inflammatory processes.2, 16, 17 This discrepancy may derive from the use of protocols of varying exercise intensity that can differentially affect the balance of T cells and pro‐ versus anti‐inflammatory cytokines in mice.13, 14, 15, 18, 19, 20 Moreover, while moderate ET can ameliorate chronic neuroinflammation and its related pathologies and enhance antigen‐specific immune response, intense ET may impair immune function and lead to transient increases in susceptibility to infection.12, 19, 21, 22, 23, 24 Thus, a major unanswered question is whether ET modulates autoimmunity selectively, or causes general effects (positive or negative) on the immune system. Uncertainty is highlighted by the observation that intense exercise may cause transient immune suppression.25, 26, 27
In this study, we aimed: (1) To compare moderate versus high‐intensity training on immunomodulation and the development of EAE; (2) To investigate whether the more potent ET protocol affects autoimmunity selectively, or causes general immunosuppression. We employed a unique experimental paradigm using the chronic‐relapsing transfer EAE model that enabled identification of selective effects of ET on the systemic immune system in EAE.
Materials and Methods
Experimental animals
Female SJL/JCrHsd mice (6–7 weeks of age) were purchased from Envigo Inc, Israel. Animal experimentation was approved by the Institutional Animal Care and Use Committee. The studies were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
Experimental design
The transfer EAE experimental setup enabled comparison of the effects of moderate‐intensity continuous training (MICT) and high‐intensity continuous training (HICT) on systemic autoimmunity, as indicated by induction of lymph node (LN)‐derived T cell encephalitogenicity (Fig. 1). To assess the modulatory effects of the two exercise protocols on systemic autoimmunity, we examined in vivo and in vitro the amount, potency and encephalitogenicity of LN‐derived T cells from donor mice that underwent MICT or HICT programs or control sedentary (SED) mice prior to PLP immunization. To that end, healthy mice were subjected to defined treadmill running programs, followed by their immunization with a PLP peptide. Then, inguinal LN‐T cells were isolated, stimulated in culture with PLP peptide and injected into naïve recipient mice, which developed EAE. Recipient mice that were injected with PLP‐reactive LN‐T cells from SED mice served as controls. Encephalitogenicity was evaluated (1) in vivo by clinical and pathological severity of EAE following transfer of LN‐T cells from MICT or HICT versus SED donor mice into recipient naïve mice; (2) in vitro following secondary activation of lymph node cells (LNCs) by the PLP autoantigen.
Figure 1.
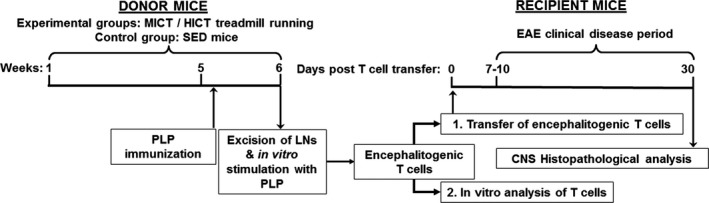
Experimental protocol to investigate effects of exercise training on the systemic immune system in experimental autoimmune encephalomyelitis (EAE). Transfer EAE model in mice was used to isolate the effects of exercise training (ET) on systemic immune system. Healthy mice were subjected to a 6 week moderate (MICT) or high‐intensity treadmill‐running program (HICT). At the end of the 5th week of training, MICT, HICT,and sedentary (SED) mice were immunized with a PLP peptide and at the end of the 6th week their lymph node (LN) was removed and stimulated in culture with proteolipid (PLP) peptide. Donor MICT‐, HICT‐ or SED‐derived encephalitogenic T cells were injected to naïve recipient mice, which developed EAE and were scored daily for neurological symptoms up to 30 days post transfer. PLP stimulated LN‐T cells from MICT, HICT and SED mice were also analyzed in vitro for activation, proliferation and gene expression characteristics. Mice were sacrificed for central nervous system (CNS) histopathology analyses 30 days post EAE induction.
Treadmill exercise training (ET)
Six‐ to seven‐week‐old female SJL healthy mice underwent 6‐week treadmill running programs, including pre‐ and posttraining performance tests on a 5‐lane treadmill designed for mice (Panlab Harvard Apparatus, USA), as previously described.12 MICT and distance‐matched HICT protocols (Table 1) were based on the initial exhaustion speed performance tests. We defined MICT as 55–60% of exhaustion speed, according to widely used training protocols, and HICT as 70–75% of exhaustion speed, as mice did not tolerate higher speeds to complete the designated distance (Table 2). Lack of facilities to measure maximal oxygen consumption (VO2max) led us to use the aforementioned protocols, rather than intensities expressed as % of VO2max.
Table 1.
Moderate and high‐intensity training protocols.
| 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | ||
|---|---|---|---|---|---|---|---|
| MICT | Duration per session | 10 min | 20 min | 30 min | |||
| Speed per session | 23 cm/sec | ||||||
| Sessions per week | 5 d/w | ||||||
| HICT | Duration per session | 10 min | 20 min | 30 min | 23 min | ||
| Speed per session | 23 cm/sec | 28 cm/sec | 30 cm/sec | ||||
| Sessions per week | 5 d/w | 3 d/w | |||||
MICT, Moderate intensity continuous training; HICT, High intensity continuous training; Min, minutes; cm/sec, centimeters per second; d/w, days per week.
Table 2.
Moderate and high‐intensity training improve performance.
| Test type | SED (n = 10) | MICT (n = 10) 1 | HICT (n = 10) | |||
|---|---|---|---|---|---|---|
| Pre‐SED period | Post‐SED period | Pre‐ET | Post‐ET | Pre‐ET | Post‐ET | |
| Exhaustion Speed (cm/sec) | 42 ± 1 | 43 ± 1 | 40 ± 1 | 45 ± 1* | 44 ± 1 | 48 ± 1*** |
| Exercise Tolerance (min:sec) | 14:03 ± 0:50 | 18:24 ± 1:44** | 14:18 ± 0:32 | 22:33 ± 1:29*** | 15:15 ± 0:26 | 29:47 ± 2:50*** |
Data are represented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, as compared to pretraining period. SED, sedentary; MICT, moderate intensity continuous training; HICT, high‐intensity continuous training.
Taken from.12
Transfer experimental autoimmune encephalomyelitis (EAE)
Proteolipid protein (PLP) 139–151 transfer EAE model in 6‐ to 7‐week‐old female SJL/JCrHsd mice was utilized as previously described.12, 28 EAE was developed in recipient mice, induced by transfer of LN‐T cells obtained from MICT, HICT or SED donor mice (Fig. 1). Recipient EAE mice were scored daily for neurological symptoms up to 30 days after EAE induction as previously described.12, 28
Histopathology analyses
Thirty days after LN‐T cell transfer, SED‐ and HICT EAE animals were sacrificed for histopathological analysis as previously described.12, 28 Serial paraffin‐embedded transverse sections were obtained from mid‐cervical, mid‐thoracic, and mid‐lumbar levels of the spinal cords. Sections were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB)/nuclear fast red to assess inflammation and demyelination, respectively. Immunohistochemistry was performed in adjacent serial sections for macrophages (rat anti‐mouse Mac3, 553322, 1:800, BD Pharmingen), T cells (monoclonal rabbit anti–CD3, RM‐9107‐SO; 1:800, Thermo‐Scientific) and nonphosphorylated neurofilament H (anti‐mouse SMI32, NE1023, 1:2000, Calbiochem). For each staining, the whole white matter of three sections per mouse was quantified, one section per each spinal cord level. The number of immune cells in perivascular infiltrates was counted in H&E stained sections, and reported as total average number per square millimeter. Mac3+ and CD3+ cells were counted both in the perivascular infiltrates and parenchyma, and reported as total average number of each cell type per square millimeter. Demyelination and axonal damage were assessed by calculating the area of LFB loss and SMI32 protein expression, respectively. All pathology measurements were performed by using the Image J software analysis (ver. 1.51H, NIH, USA).
In vitro proliferation assays of LN‐T cells
Lymph nodes were excised from MICT, HICT, or SED mice at 10 days after PLP immunization (n = 12–15/group). Stimulation index (SI) was calculated as LNC number in the experimental group divided by LNCs number in naïve, nonimmunized mice. LNCs were cultured as single cell suspensions with 10 μg/mL PLP peptide or 2.5 μg/mL concanavalin A (ConA) or no stimulation, as previously described.12, 28 The proliferation of T cells was evaluated by flow cytometry analysis for bromodeoxyuridine (BrdU) incorporation as previously described.12, 28 SI was calculated as fraction of CD3+, BrdU+ cells (relative to total) in the experimental group divided by the fraction in naïve, nonimmunized mice with no secondary activation (n = 3). All samples were analyzed in a Cytomics FC 500 apparatus (Beckman Coulter, Life Science) using the CXP analysis software (ver. 2.1; Informer Technologies, Inc).
Cytokines and chemokine receptor genes determination of PLP‐reactive LNCs
Total RNA was prepared using the RNeasy Plus Mini Kit (QIAGEN) from LNCs that were excised from mice 10 days after PLP immunization following their activation in vitro with PLP peptide (n = 12–15/group). cDNA was prepared from 300 ng total RNA using qScript cDNA Synthesis Kit (Quanta Biosciences), according to manufacturer's instructions. Semiquantitative real‐time PCR was performed using the PerfeCTa SYBR Green FastMix, ROX (Quanta Biosciences).
Ovalbumin immunization and in vitro proliferation assays of LN‐T cells
To evaluate T cell‐dependent immune responses to a nonautoantigen stimulus, HICT, and SED mice (n = 10/group) were immunized with 250 μg ovalbumin (OVA; Tamar laboratories Supplies Ltd.) in 100 μL saline and an equal volume of complete Freund's adjuvant (CFA; Fig. 6A). Other groups of HICT and SED mice (n = 5/group) were injected intraperitoneally with 100 μL of CFA alone. Ten days after immunization, LNCs were harvested and cultured in vitro for 72 h in the presence of 200 μg/mL OVA. LNCs counts and the proliferation of LN‐T cells were evaluated as described.
Figure 6.
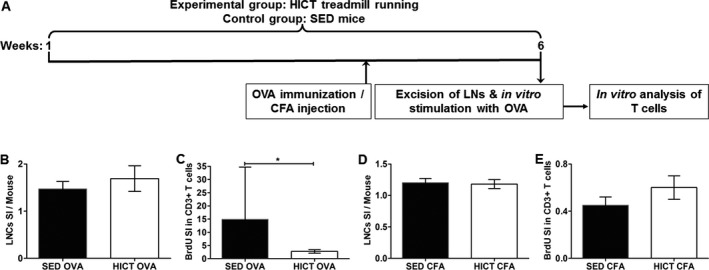
High‐intensity training inhibits T cell‐dependent responses to ovalbumin (OVA) immunization. (A) Experimental outline. Healthy mice were subjected to a 6 week high‐intensity treadmill running program (HICT, n = 15). Sedentary mice served as controls (SED, n = 15). At the end of the 5th week of training, HICT and control SED mice were immunized with ovalbumin (SED OVA, HICT OVA; n = 10/group) or injected with complete Freund′s adjuvant alone (SED CFA, HICT CFA; n = 5/group). At the end of the 6 weeks, lymph node (LN) was removed, stimulated in vitro for 72 h in culture with OVA and analyzed for their proliferation activity. Number of lymph node cells (LNCs) per mouse at day of LN excision from OVA‐immunized HICT and SED mice (B) or CFA‐injected HICT and SED mice (D) represented by stimulation index (SI): The number of LNCs in the experimental group divided by the number of LNCs in naïve, nonimmunized mice (n = 3). Flow cytometry analysis at 72 h in culture for bromodeoxyuridine (BrdU) incorporation into CD3+ T cells derived from OVA‐immunized HICT and SED mice (C) or CFA‐injected HICT and SED mice (E), represented by SI: The fraction of CD3+ BrdU+ T cells in the experimental group divided by the fraction of CD3+ BrdU+ T cells from naïve, mice (n = 3). In OVA‐immunized mice, HICT did not affect the number of LNCs (B), but significantly reduced T‐cell proliferation (C). HICT did not affect the number of LNCs (D) nor T‐cell proliferation (E) in CFA‐injected mice. Data are mean ± SE.*P < 0.05.
Escherichia coli (E. coli)‐induced acute peritonitis‐sepsis model in PLP‐immunized HICT and SED mice
To evaluate innate immune responses to bacterial infection, PLP‐immunized–HICT and SED mice (n = 9/group) were injected intraperitoneally with an extraintestinal pathogenic E. coli strain 76 (a previously characterized clinical bloodstream isolate,29 a logarithmic culture of 6 × 105 CFU per mouse) 4 days after immunization, at the end of the training program (Fig. 7A). The infecting inoculum did not cause animal mortality but was proven to cause morbidity, bacterial propagation, and systemic infection involving innate immune responses. Twenty‐four hours postinfection mice were sacrificed and peritoneal fluids (as a measure of local proliferation) and spleens (as a measure of systemic infection) were collected aseptically from all animals. Bacterial counts were determined by serial dilutions of peritoneal fluids or spleen homogenates and by colony counting on LB agar plates after an ON incubation.
Figure 7.
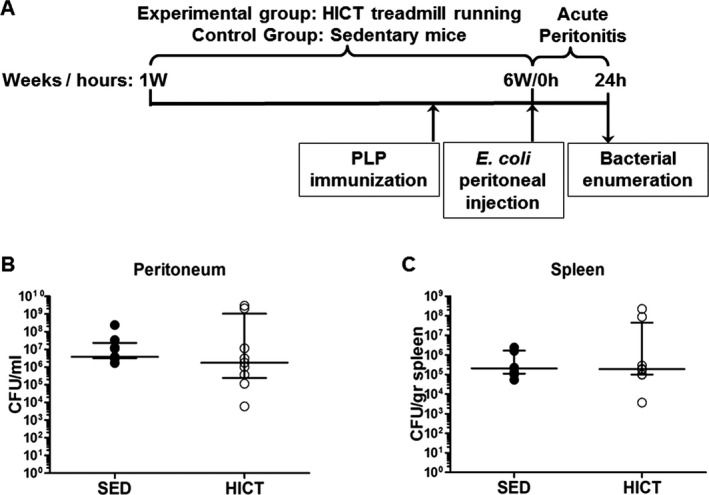
High‐intensity training sustains immune responses to acute peritoneal Escherichia coli bacterial infection. (A) Experimental outline. Healthy mice were subjected to a 6 week‐ high‐intensity treadmill running program (HICT, n = 9). Sedentary (SED, n = 9) mice served as controls. At the 6th week of the training program, HICT and SED mice were immunized with the autoantigen proteolipid (PLP) peptide. At the end of the 6th week, 4 days following PLP immunization, HICT and SED mice were intraperitoneally injected with E.‐coli bacteria to induce acute peritonitis. Twenty‐four hours after E. coli infection mice were sacrificed and bacterial enumeration was performed in their peritoneal fluid and spleens. (B and C) Bacterial propagation in mice in the acute‐peritonitis‐sepsis model. Bacterial counts assessed (n = 9) at 24 h in peritoneal fluid (B) and in spleens indicating systemic infection (C). Data are represented as median ± interquartile range.
Statistical analyses
All data are presented as mean ± standard error of mean (SE). For performance tests, the values before and after training for each experimental group were compared using Student’s paired t test. For differences in physical performance following the training program, in EAE clinical parameters and in LNCs and T cells characteristics, the experimental groups were compared using two‐way analysis of variance (ANOVA) followed by Newman–Keuls multiple comparison tests. For pathology parameters, OVA‐immunized or CFA‐injected mice‐derived LNCs and T cell analyses and for bacterial counts the experimental groups were compared using two tailed Mann–Whitney test. Data were analyzed in GraphPad Prism software v.5. Differences were considered statistically significant at P < 0.05.
Results
Exercise training improves physical performance
In SED mice, there was a statistically significant but limited (see below) increase in exercise tolerance, but not in maximal speed (Table 2). Improvement in exercise tolerance may be due to age‐dependent maturation of mice. MICT and HICT protocols improved significantly both maximal speed and exercise tolerance, compared to baseline performance (Table 2). MICT induced >10% increase in maximal speed and ~60% increase in exercise tolerance. HICT increased maximal speed similarly (~9%) and markedly increased exercise tolerance by >90% (Table 2). ANOVA test confirmed that HICT improved exercise tolerance significantly more than MICT (P < 0.001) and SED (P < 0.001).
Exercise training induces systemic immune modulation in donor mice to attenuate the clinical course of transfer EAE
The transfer of LN‐T cells derived from PLP‐immunized MICT and HICT mice induced a significantly milder clinical course of EAE in recipient mice, as compared to the clinical course in control EAE group that received LN‐T cells derived from PLP‐immunized SED mice (Fig. 2A). LN‐T cells from PLP‐immunized HICT mice induced the least severe overall clinical course of disease along the 30‐day observation (Fig. 2A). The day of onset was significantly delayed in the two experimental groups, compared to the control group (Fig. 2B). The average maximal clinical score of disease was similar in SED‐ and MICT‐treated groups, but was significantly decreased in the HICT‐treated group (Fig. 2B). Finally, the burden of disease in mice that received PLP‐immunized, MICT‐derived LN‐T cells was ~30% lower than in mice receiving PLP‐immunized, SED‐derived LN‐T cells. Remarkably, mice that received PLP‐immunized, HICT‐derived LN‐T cells exhibited over 70% reduction in the burden of disease (Fig. 2B). ANOVA test indicated that LN‐T cells derived from PLP‐immunized HICT mice induced a significant reduction in the maximal clinical score (P < 0.01) and a trend toward delayed onset and reduction in the burden of disease, as compared to LN‐T cells from MICT mice.
Figure 2.
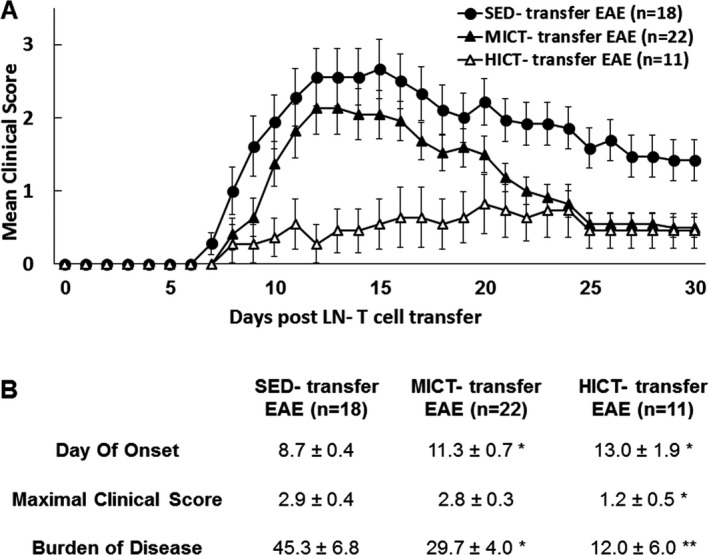
Superior inhibitory effect of high‐intensity training on the encephalitogenicity of lymph node (LN) T cell‐derived from proteolipid (PLP)‐immunized mice in transfer model of experimental autoimmune encephalomyelitis (EAE). Clinical course (A) and clinical parameters (B) of transfer EAE in mice that received PLP‐reactive LN‐T cells from moderate‐intensity continuous trained (MICT‐transfer EAE, n = 22), high‐intensity continuous trained (HICT‐transfer EAE, n = 11) or sedentary (SED‐transfer EAE, n = 18) mice. The severity of EAE was scored on a 0–6 scale. Transfer of LN‐T cells derived from HICT, PLP‐immunized mice to naïve recipients induced the most significant attenuation of EAE development. Data are mean ± SE. *P < 0.05, **P < 0.01, as compared to SED‐transfer EAE.
PLP‐reactive LN‐T cells from HICT mice induce less tissue damage and milder inflammation in recipient EAE mice
Next, we examined whether the milder disease induced by LN‐T cells derived from HICT mice was associated with reduced tissue injury. In HICT‐transfer EAE mice, there was a 65% reduction in the area of demyelination (Fig. 3A–C) and ~10% decrease in nonphosphorylated‐neurofilament SMI32‐expressing injured axons (Fig. 3D–F), as compared to control SED‐transfer EAE mice. While SED‐transfer EAE mice exhibited an aggressive and extensively distributed pattern of demyelination and axonal injury, in HICT‐transfer EAE mice, the tissue damage was milder and scattered.
Figure 3.
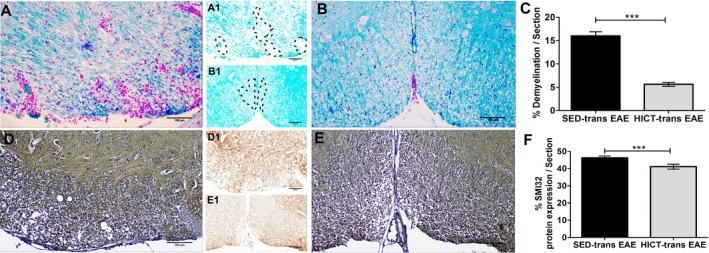
Attenuation of pathology parameters in the spinal cords of experimental autoimmune encephalomyelitis (EAE) mice injected with proteolipid (PLP)‐reactive lymph node (LN)‐T cells from high‐intensity trained mice. Evaluation of demyelination (A–C) and axonal damage (D–F) was performed on cross sections of the spinal cords in EAE mice that were injected with LN‐T cells from control sedentary (SED‐transfer [trans] EAE; A, A1, D, D1; n = 6) or from high‐intensity trained mice (HICT‐trans EAE; B, B1, E, E1; n = 5) mice, at 30 days post EAE induction. C, F – quantification of tissue pathology in spinal cord white matter. A, B – luxol fast blue (LFB) histochemistry with periodic acid schiff (PAS) counterstaining, A1, B1 – LFB histochemistry without PAS counterstaining, dashed lines: represent areas of demyelination shown in A, B, respectively; D, E – SMI32 immunohistochemistry with hematoxylin counterstaining, D1, E1 – SMI32 immunohistochemistry without hematoxylin counterstaining, in brown SMI32+ injured axons. LFB staining showed reduction in the area of demyelination in HICT‐transfer EAE (B, B1), as compared to SED‐transfer EAE mice (A, A1, C). SMI32 immunostainnings showed less axonal damage (F) in HICT‐transfer EAE (E, E1) than in control SED‐transfer EAE mice (D, D1). Scale bars = 100 μm. Data are mean ± SE. **P < 0.01, ***P < 0.001.
There was also a marked reduction in the inflammatory process in HICT‐transfer EAE (Fig. 4A, D and G), as compared to SED‐transfer EAE controls (Fig. 4B, E and H). This was indicated by ~80% decrease in perivascular immune cell infiltrations (Fig. 4C), 60% decrease in CD3+ T‐cell counts (Fig. 4F) and 90% decrease in Mac3+ macrophage counts (Fig. 4I) in HICT‐transfer EAE spinal cords.
Figure 4.
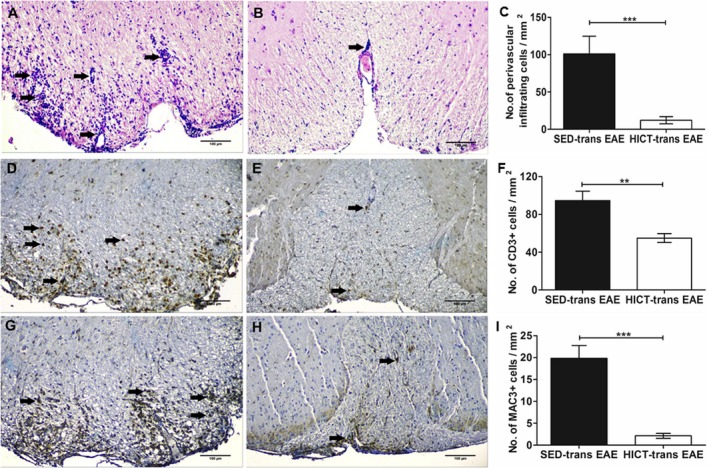
Attenuation of inflammatory parameters in the spinal cords of experimental autoimmune encephalomyelitis (EAE) mice injected with proteolipid (PLP)‐reactive lymph node (LN)‐T cells from high‐intensity trained mice. Evaluation of inflammation was performed on cross sections of the spinal cords in EAE mice that were injected with LN‐T cells from control sedentary (SED‐trans EAE; A, D, G; n = 6) or from high‐intensity trained HICT‐trans EAE; B, E, H; n = 5) mice, at 30 days post EAE induction. C, F, I – counts of inflammatory cell types in spinal cord white matter. In HICT trans‐EAE mice, there was a significant reduction in total perivascular immune cell infiltrations (B), in CD3+ T cells (E) and in Mac3+ macrophages (H), as compared to SED tans‐EAE mice (A, D, G, respectively). A, B: arrows – indicate perivascular infiltrates, D, E: arrows – indicate perivascular CD3+ T cells, G, H: arrows – indicate Mac3+ macrophages. Scale bars = 100 μm. Data are mean ± SE. **P < 0.01, ***P < 0.001.
High‐intensity training modulates T cell reactivity to the autoantigen
Since there was a reduction in encephalitogenicity of LN‐T cells derived from PLP‐immunized MICT and HICT mice to induce brain inflammation in vivo, we investigated the training effects on LNCs and T cells activation, proliferation, and pro‐inflammatory gene expression in response to PLP stimulation in vitro. Training decreased the stimulation index of LNCs by 40–50% versus control with no significant difference between training intensities (Fig. 5A). MICT inhibited PLP‐stimulated T‐cell proliferation by 55% and HICT inhibited T‐cell proliferation in response to PLP by ~80%, versus control (Fig. 5B). HICT inhibited PLP‐induced T cell proliferation by >50%, as compared to MICT. Both MICT and HICT decreased TNFα and TGFβ mRNA levels in stimulated T cells, as compared to PLP‐stimulated SED T cells (Fig. 5C). HICT markedly inhibited mRNA levels of IFNγ IL‐17 and IL‐10.
Figure 5.
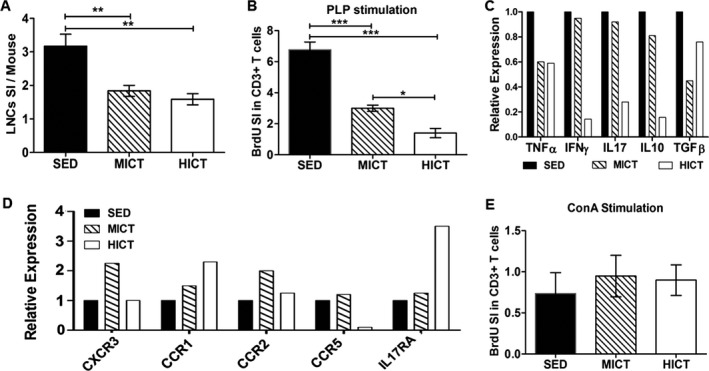
Superior inhibitory effect of high‐intensity training on lymph node cells (LNCs) and T cells derived from proteolipid (PLP) immunized donor mice. LNCs were excised from moderate‐intensity continuous trained (MICT), high‐intensity continuous trained (HICT) and sedentary (SED) mice at 10 days post PLP immunization and were stimulated in vitro for 72 h with PLP peptide (A–D, n = 12–15/group) or concanavalinA (ConA, E). (A) Number of LNCs per mouse at day of LN excision represented by stimulation index (SI): The number of LNCs in the experimental group divided by the number of LNCs in naïve, nonimmunized mice (n = 3). Flow cytometry analysis for bromodeoxyuridine (BrdU) incorporation into CD3+ T cells at 72 h after PLP (B) or ConA (E) stimulation in vitro, represented by SI: The fraction of CD3+ BrdU+ T cells in the experimental group divided by the fraction of CD3+ BrdU+ T cells in naïve, nonimmunized mice. Real‐time PCR analysis at 72 h after PLP stimulation in vitro for cytokine mRNA levels of tumor necrosis factor (TNF)‐α, interferon (IFN)‐γ, interleukin (IL)‐10 and transforming growth factor (TGF)‐β (C) and chemokine receptors mRNA levels of CXCR3, CCR1, CCR2, CCR5 and interleukin‐10 receptor α (IL17RA) (D). HICT in PLP‐immunized mice induced the most prominent reduction in the number of LNCs (A), T cell proliferation (B) and mRNA levels of cytokines (C) in response to the autoantigen PLP in vitro. This was accompanied by an overall increase in mRNA levels of chemokine receptors (D). Training did not affect T cell proliferation in response ConA nonspecific stimulation (E). (A, B, E) Summary of three independent experiments. Data are mean ± SE. (C, D) Representatives of one of three independent experiments. Relative expression to SED group = 1. *P < 0.05, **P < 0.01, ***P < 0.001.
While ET induced a reduction in cytokine mRNA levels, this was not accompanied by suppression of other immune‐related genes in T cells. MICT and HICT had differential effects on mRNA levels of several chemokine receptors from T cells ranging from marked increases to mark decreases (Fig. 5D). Interestingly, there was a 10‐fold decrease in CCR5 mRNA levels in PLP‐immunized HICT derived T cells, compared to that of PLP‐immunized SED or MICT T cells.
To examine the overall ability of T cells to respond to stimuli, we examined the proliferation of T cell derived from MICT, HICT or SED, PLP‐immunized mice in response to the nonspecific mitogen ConA (Fig. 5E). Flow cytometry analysis for CD3+, BrdU+ T cells showed that neither training protocol affected the proliferative response of T cells to 72 h ConA stimulation in vitro, as compared to the SED group (Fig. 5E).
High‐intensity training inhibits T cell response to ovalbumin (OVA)
Since HICT induced superior immune modulation to PLP autoimmunity, we further investigated the effects of HICT on immune responses to other immunogenic challenges. We first addressed the question whether the inhibitory effect of HICT was selective to the autoantigen or was a general suppressive on T cell‐dependent immunity. Immunization with OVA induced a mild 1.5‐fold increase in total LNCs counts in SED mice that were not affected by HICT (Fig. 6B). However, HICT markedly inhibited the proliferation of OVA‐stimulated T cells by ~75%, compared to OVA‐stimulated T cell proliferation from SED mice (Fig. 6C). To exclude the possibility that the inhibitory effects of ET on PLP and OVA immunizations were related to differences in response to the adjuvant, similar experiments were performed with CFA. CFA had no noteworthy effects (Fig. 6D and E).
High‐intensity training does not affect innate immune system response to E. coli infection
Finally, the effects of HICT on innate immune system response, as studied by susceptibility of mice to bacterial infection, were examined (Fig. 7A). Proliferation of bacteria in the peritoneum (Fig. 7B) and systemic bacterial dissemination (Fig. 7C) at 24 h post‐infection were similar in HICT and SED mice groups.
Discussion
The major findings of the present study are that preventive intervention of high‐intensity training: (1) modulates the systemic autoimmune system more effectively than moderate‐intensity training; (2) inhibits the potency of encephalitogenic T cells to induce EAE; (3) induces inhibition of T cell‐dependent response to a nonautoantigen; and (4) does not increase susceptibility to acute bacterial infection, suggesting preserved innate immune system response.
Previous studies on the effects of ET on EAE yielded variable results. Clinical outcomes ranged from worsening of symptoms,30 no effect on clinical severity,10, 31, 32 to disease attenuation, as indicated by delayed onset and peak of disease33, 34 and even overall attenuation of the clinical course.11, 12 The inconsistencies in these studies likely derived from variations in training modes (running, swimming) and protocols (intensity, speed, volume, duration) that were employed. To provide standardization of the association between ET intensity and its effects on EAE, we employed two controlled treadmill running ET protocols. These protocols were distance matched, but differed in their intensity level, and therefore were comparable in terms of training volume (i.e. work output). Both training protocols improved physical performance, but the high‐intensity‐training program achieved higher improvement in exercise tolerance.
Positive effects of high‐intensity, but not moderate‐intensity, swimming were recently reported in myelin oligodendrocyte glycoprotein‐induced EAE.35 In contrast, we found that both training intensities attenuated autoimmunity in an intensity‐dependent manner. Noteworthy is that swimming is not the natural mode of physical activity for mice. Importantly, our experimental design using the transfer EAE model enables investigation of the effect of ET specifically on the peripheral immune system. Further, we utilized the transfer EAE model to examine effect of ET on the systemic response to other immunologic challenges.
Our results indicate that ET induces a dose‐dependent decrease in the proliferative response of PLP‐reactive T cells. However, no significant changes in cytokine and chemokine receptors gene expression, nor trends that could be attributable to the level of training intensity were notable. We therefore suggest that the modulatory effect of training is mediated by a reduction in the amount of encephalitogenic T cells, rather than by their cytokine/chemokine profile.
While studies in different experimental setups suggested that ET may improve the ability of the immune system to respond to deleterious stimuli,17, 36, 37 others demonstrated that intense ET may cause transient immune suppression.25, 26, 27 Several lines of evidence in our study demonstrate that ET modulates PLP‐autoimmunity rather than inducing general immune suppression. First, T cells from MICT, HICT and SED, PLP‐immunized mice proliferated similarly following ConA stimulation in vitro, indicating that ET does not reduce the maximal capacity of T cell response to a nonspecific mitogen. Second, no differences were measured in the number of LNCs obtained from OVA‐immunized HICT and SED mice. However, HICT induced significant inhibition of T cell proliferation in response to OVA immunization. These findings indicate that the inhibitory effect of high‐intensity ET is not selective to an autoantigen, but is also observed in T cell responses to a nonautoantigen. Thus, one potential consequence of HICT may be a limitation in effective immunization to deleterious immune challenges. The observation that HICT does not increase susceptibility of mice to E. coli‐induced acute peritonitis suggests, however, that the innate immune response of trained mice is not compromised.
In the current study, we investigated selected immunogenic stimuli to test the effects of high‐intensity training on the systemic immune system in the EAE model. Therefore, we cannot provide generalized conclusions on global adaptive and innate immune responses. Further studies are required to examine the mechanisms whereby high‐intensity training induces inhibition of CNS autoimmunity.
In conclusion, our findings demonstrate intensity‐dependent effects of ET on modulating the systemic autoimmune system to attenuate EAE development. Defining the optimal training protocol to attenuate EAE will further our understanding of the cellular and molecular mechanisms underlying the beneficial immune modulatory effects of ET on EAE. The current findings provide a basic biological rationale that can be further translated to clinical trials in MS patients. Notably, while the experimental design here was of EAE prevention by ET, our findings may be relevant for relapsing‐remitting MS patients, in whom intense physical training during remissions may have protective effects against development of further relapses. Translation of these training programs from rodents to human patients may be challenging, yet possible. Importantly, to maintain the therapeutic effect of ET, it may be necessary to constantly adjust the training program to the improvement in physical fitness, by gradually increasing its intensity.
Conflict of Interest
The authors declare that no competing interests exist.
Acknowledgments
This work was supported by The Judy and Sidney Swartz Fund for research in Multiple Sclerosis and by the Chief Scientist Office of the Israeli Ministry of Health.
Funding Information
This work was supported by The Judy and Sidney Swartz Fund for research in Multiple Sclerosis and by the Chief Scientist Office of the Israeli Ministry of Health.
Funding Statement
This work was funded by The Judy and Sidney Swartz Fund for research in multiple sclerosis grant ; Chief Scientist Office of the Israeli Ministry of Health grant .
References
- 1. Heine M, Wens I, Langeskov‐Christensen M, et al. Cardiopulmonary fitness is related to disease severity in multiple sclerosis. Mult Scler 2016;22:231–238. [DOI] [PubMed] [Google Scholar]
- 2. Mokhtarzade M, Ranjbar R, Majdinasab N, et al. Effect of aerobic interval training on serum IL‐10, TNFalpha, and adipokines levels in women with multiple sclerosis: possible relations with fatigue and quality of life. Endocrine 2017;57:262–271. [DOI] [PubMed] [Google Scholar]
- 3. Pilutti LA, Platta ME, Motl RW, Latimer‐Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci 2014;343:3–7. [DOI] [PubMed] [Google Scholar]
- 4. Sandroff BM, Motl RW, Scudder MR, DeLuca J. Systematic, Evidence‐based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev 2016;26:271–294. [DOI] [PubMed] [Google Scholar]
- 5. Cortese M, Riise T, Bjornevik K, et al. Body size and physical exercise, and the risk of multiple sclerosis. Mult Scler 2018;24:270–278. [DOI] [PubMed] [Google Scholar]
- 6. Sharif K, Watad A, Bragazzi NL, et al. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev 2018;17:53–72. [DOI] [PubMed] [Google Scholar]
- 7. Benson C, Paylor JW, Tenorio G, et al. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE). Exp Neurol 2015;271:279–290. [DOI] [PubMed] [Google Scholar]
- 8. Bernardes D, de Oliveira ALR. Regular exercise modifies histopathological outcomes of pharmacological treatment in experimental autoimmune encephalomyelitis. Front Neurol 2018;9:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernardes D, Oliveira‐Lima OC, Silva TV, et al. Differential brain and spinal cord cytokine and BDNF levels in experimental autoimmune encephalomyelitis are modulated by prior and regular exercise. J Neuroimmunol 2013;264:24–34. [DOI] [PubMed] [Google Scholar]
- 10. Klaren RE, Stasula U, Steelman AJ, et al. Effects of exercise in a relapsing‐remitting model of experimental autoimmune encephalomyelitis. J Neurosci Res 2016;94:907–914. [DOI] [PubMed] [Google Scholar]
- 11. Souza PS, Goncalves ED, Pedroso GS, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood‐brain barrier disruption. Mol Neurobiol 2016;54:4723–4737. [DOI] [PubMed] [Google Scholar]
- 12. Einstein O, Fainstein N, Touloumi O, et al. Exercise training attenuates experimental autoimmune encephalomyelitis by peripheral immunomodulation rather than direct neuroprotection. Exp Neurol 2018;299(Pt A):56–64. [DOI] [PubMed] [Google Scholar]
- 13. Kruger K, Alack K, Ringseis R, et al. Apoptosis of T‐cell subsets after acute high‐intensity interval exercise. Med Sci Sports Exerc 2016;48:2021–2029. [DOI] [PubMed] [Google Scholar]
- 14. Kruger K, Mooren FC, Pilat C. The immunomodulatory effects of physical activity. Curr Pharm Des 2016;22:3730–3748. [DOI] [PubMed] [Google Scholar]
- 15. Mardare C, Kruger K, Liebisch G, et al. Endurance and resistance training affect high fat diet‐induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J Diabetes Res 2016;2016:4536470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deckx N, Wens I, Nuyts AH, et al. 12 weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediators Inflamm 2016;2016:6789276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gleeson M, Bishop NC, Stensel DJ, et al. The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–615. [DOI] [PubMed] [Google Scholar]
- 18. McAlees JW, Smith LT, Erbe RS, et al. Epigenetic regulation of beta2‐adrenergic receptor expression in T(H)1 and T(H)2 cells. Brain Behav Immun 2011;25:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Song H, Tang X, et al. Effect of exercise training intensity on murine T‐regulatory cells and vaccination response. Scand J Med Sci Sports 2012;22:643–652. [DOI] [PubMed] [Google Scholar]
- 20. Zhao G, Zhou S, Davie A, Su Q. Effects of moderate and high intensity exercise on T1/T2 balance. Exerc Immunol Rev 2012;18:98–114. [PubMed] [Google Scholar]
- 21. Florindo M. Inflammatory cytokines and physical activity in multiple sclerosis. ISRN Neurol 2014;2014:151572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Handzlik MK, Shaw AJ, Dungey M, et al. The influence of exercise training status on antigen‐stimulated IL‐10 production in whole blood culture and numbers of circulating regulatory T cells. Eur J Appl Physiol 2013;113:1839–1848. [DOI] [PubMed] [Google Scholar]
- 23. Simpson RJ, Kunz H, Agha N, Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci 2015;135:355–380. [DOI] [PubMed] [Google Scholar]
- 24. Simpson RJ, Lowder TW, Spielmann G, et al. Exercise and the aging immune system. Ageing Res Rev 2012;11:404–420. [DOI] [PubMed] [Google Scholar]
- 25. Gleeson M, Pyne DB. Respiratory inflammation and infections in high‐performance athletes. Immunol Cell Biol 2016;94:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lakier Smith L. Overtraining, excessive exercise, and altered immunity: is this a T helper‐1 versus T helper‐2 lymphocyte response? Sports Med 2003;33:347–364. [DOI] [PubMed] [Google Scholar]
- 27. Moreira A, Delgado L, Moreira P, Haahtela T. Does exercise increase the risk of upper respiratory tract infections? Br Med Bull 2009;90:111–131. [DOI] [PubMed] [Google Scholar]
- 28. Einstein O, Fainstein N, Vaknin I, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol 2007;61:209–218. [DOI] [PubMed] [Google Scholar]
- 29. Kondratyeva K, Wollman A, Gerlitz G, Navon‐Venezia S. Adhesion and invasion to epithelial cells and motility of extended‐spectrum beta‐lactamase‐producing Escherichia coli reveal ST131 superiority: a comparative in vitro study of extraintestinal pathogenic E. coli lineages. J Med Microbiol 2017;66:1350–1357. [DOI] [PubMed] [Google Scholar]
- 30. Patel DI, White LJ, Lira VA, Criswell DS. Forced exercise increases muscle mass in EAE despite early onset of disability. Physiol Res 2016;65:1013–1017. [DOI] [PubMed] [Google Scholar]
- 31. Keytsman C, Blancquaert L, Wens I, et al. Muscle carnosine in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Relat Disord 2018;21:24–29. [DOI] [PubMed] [Google Scholar]
- 32. Patel DI, White LJ. Effect of 10‐day forced treadmill training on neurotrophic factors in experimental autoimmune encephalomyelitis. Appl Physiol Nutr Metab 2013;38:194–199. [DOI] [PubMed] [Google Scholar]
- 33. Le Page C, Bourdoulous S, Beraud E, et al. Effect of physical exercise on adoptive experimental auto‐immune encephalomyelitis in rats. Eur J Appl Physiol Occup Physiol 1996;73:130–135. [DOI] [PubMed] [Google Scholar]
- 34. Wens I, Dalgas U, Verboven K, et al. Impact of high intensity exercise on muscle morphology in EAE rats. Physiol Res 2015;64:907–923. [DOI] [PubMed] [Google Scholar]
- 35. Xie Y, Li Z, Wang Y, et al. Effects of moderate‐ versus high‐ intensity swimming training on inflammatory and CD4(+) T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol 2018;18:60–67. [DOI] [PubMed] [Google Scholar]
- 36. Cao Dinh H, Beyer I, Mets T, et al. Effects of physical exercise on markers of cellular immunosenescence: a systematic review. Calcif Tissue Int 2017;100:193–215. [DOI] [PubMed] [Google Scholar]
- 37. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev 2011;17:6–63. [PubMed] [Google Scholar]


