Abstract
Objective
The opioid crisis has had devastating effects on individuals and communities, and it has rapidly increased in severity. However, we still lack nationally representative information on the diversity of comorbidity patterns among DSM-5 prescription opioid use disorder (P-OUD), other substance use disorders (SUDs), and psychopathology. This impedes planning for multiple aspects of intervention, including society-wide allocation of treatment resources, program design at individual treatment centers, and personalized care to individual patients.
Method
To address this critical gap in information, we evaluated clinical profiles of American adults via latent class analysis in a large, recently collected epidemiological dataset that uses structured diagnostic assessment for DSM-5 psychopathology (National Epidemiologic Survey on Alcohol and Related Conditions-III; N=36,309). Variables considered for profiles included lifetime diagnosis for multiple SUDs, a number of externalizing and internalizing conditions, and demographic variables. We then associated clinical profiles with demographic variables and functional impairment.
Results
Comorbid psychopathology and other SUDs were common in latent classes with elevated and very high rates of P-OUD. To illustrate, alcohol use disorder rates were greater than 45% and PTSD rates were greater than 28% in classes with higher P-OUD rates. Higher P-OUD rates were associated with White/non-Hispanic and American Indian/Alaska Native populations. Relationships between P-OUD rates and functional impairment were inconsistent.
Conclusions
Many current treatment delivery systems are not designed to accommodate the heterogeneous profiles associated with high P-OUD rates. We provide specific suggestions for improvements to the mental health service system, individual clinical care programs, and future research approaches.
Keywords: Opioid crisis, personalized medicine, comorbidity
As noted throughout this special issue, the United States (US) is in the midst of an opioid crisis (Kanouse & Compton, 2015; Nelson, Juurlink, & Perrone, 2015). Unfortunately, consequences from prescription and non-prescription opioid misuse have been continuing to climb. Overdoses increased 27.9% from 2015 to 2016, and an estimated 42,249 US residents died from an opioid overdose in 2016, accounting for nearly two-thirds of drug overdose deaths (Seth, Scholl, Rudd, & Bacon, 2018). While recent increases in opioid-related overdose have been driven primarily by heroin and illicitly manufactured fentanyl, over 17,000 overdose deaths in 2016 were prescription opioid-driven (Seth et al., 2018).
A key component of the US federal response to the opioid epidemic is to promote access to prescription opioid use disorder (P-OUD) treatment (Johnson et al., 2018). Such treatment often includes medication-assisted treatment (MAT) with methadone, buprenorphine, or naltrexone, and MAT has considerable evidence of effectiveness in promoting opioid abstinence and reducing associated negative outcomes, such as overdose (Connery, 2015). Psychosocial interventions for P-OUD are generally recommended and less well studied, with more limited evidence of effectiveness (Kampman & Jarvis, 2015). Preliminary evidence suggests that cognitive-behavioral therapy (Moore et al., 2016), 12-step-oriented residential treatment (Schuman-Olivier, Claire Greene, Bergman, & Kelly, 2014), and mindfulness-based treatment (Garland et al., 2014) may promote opioid abstinence.
While efforts have been increasing to offer these treatments, they have a number of problems in application. Early treatment termination is common in MAT, with many trials retaining a roughly 50% or less of those initiating treatment for the entire treatment course (Carroll & Weiss, 2017; Soyka, Zingg, Koller, & Kuefner, 2008). Retention in psychosocial treatment without medication is not well characterized, but given known retention issues for treatment approaches for substance use disorders (SUDs), early psychotherapy termination may also be common (Dutra et al., 2008). This can further impact psychotherapy efficacy for P-OUD.
Efforts to address the opioid crisis also do not occur in isolation, as cross-sectional and longitudinal evidence link prescription opioid misuse and P-OUD with higher levels of other substance use and psychopathology (Kerridge et al., 2015; McCabe, West, Jutkiewicz & Boyd, 2017; Saha et al., 2016; Schepis & Hakes, 2011). This comorbidity can further attenuate MAT efficacy, as poorer MAT outcomes are associated with other substance use as well as depressive, anxiety, and PTSD symptoms (Benningfield et al., 2012; Huhn et al., 2018; King, Brooner, Peirce, Kolodner, & Kidorf, 2014; Leece et al., 2015; Soyka, 2015; Schafer et al., 2010). Research on the effects of comorbid psychopathology (e.g., PTSD, major depression, anxiety disorders) on the MAT process tends to be inconsistent, with some findings that associate comorbidity with early treatment termination or relapse to opioid use/misuse (Benningfield et al., 2012; Dreifuss et al., 2013; Ferri, Finlayson, Wang, & Martin, 2014; Leece et al., 2015; Martin et al., 2017; Saha et al., 2016). However, these studies often-use regional data, are frequently underpowered, and usually do not use the updated DSM-5 criteria. They also often combine participants who are dependent on heroin with those dependent on prescription opioids, potentially clouding an important moderator of outcomes. All of these factors limit the conclusions that can be drawn.
In considering the role of comorbid psychopathology and P-OUD, remarkably little is known about common symptom profiles. Prior work on associations between P-OUD and either psychopathology or SUDs have tended to examine bivariate associations, as opposed to examining multivariate symptom relationships. This lack of information makes it difficult to design treatment programs that are tailored to specific comorbid symptom profiles, and it impedes decision-making regarding allocation of resources to promote access (e.g., additional investment in evidence-based anxiety treatment may be needed for a specific subset of patients with P-OUD). To date, no studies have examined multivariate symptom profiles of patients with P-OUD in large clinical samples.
To address this critical gap in knowledge, we applied latent class analysis (LCA) to data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III), a large nationally representative survey of the US adult noninstitutionalized population that used structured assessment for DSM-5 diagnostic criteria. The present study aims to provide a nationwide characterization of common symptom patterns and highlight how they relate to P-OUD rates, to inform planning for clinic design across a wide range of settings as well as public policy strategy. We hypothesized that a discrete number of classes would best explain comorbidity patterns observed with P-OUD. In particular, we expected that comorbidity patterns would vary depending on different rates of P-OUD, with at least one class containing a high prevalence of individuals with P-OUD. We also hypothesized that classes would be differentially associated with both demographic variables (age, sex, race/ethnicity) and functional impairment.
Method
Participants and Procedure
The study sample included all participants who enrolled in the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III; N=36,309). The NESARC-III is a nationally representative survey which was conducted via in-person interview format from 2012 to 2013. Respondents were selected through multistage probability sampling (for details regarding the sampling design, please see Grant et al., 2014). The NESARC-III epidemiological study was approved by the National Institutes of Health and Westat, Inc. Institutional Review Boards, and all participants provided informed consent (Goldstein et al., 2016). Participants were civilian, non-institutionalized U.S. residents (including those in group quarters, such as college or work dormitories, but not including homeless or incarcerated individuals) over the age of 181 (M=45.63, SD=17.53; 56.3% female). With regards to race and ethnicity, the majority of participants identified as White/non-Hispanic (52.9%) followed by Black/non-Hispanic (21.4%) and Hispanic, any race (19.4%) with the lowest reported race/ethnicities being Asian/Native Hawaiian/Other Pacific Islander, non-Hispanic (5.0%) and American Indian/Alaska Native, non-Hispanic (1.4%). Prevalence of P-OUD for the sample was 2.1%. Regarding diagnostic recency, 43.3% of participants with lifetime P-OUD had also experienced a P-OUD episode in the past year, and 67.7% of participants with lifetime P-OUD reported a symptom episode within the past 10 years.
Measures
The NIAAA Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5; Grant et al., 2015) is a structured diagnostic interview designed to assess DSM-5 diagnostic criteria for alcohol use disorder, nicotine use disorder, and selected drug use disorders and mental disorders. Lifetime diagnosis status was established by responses to questions asking whether psychopathology was experienced in the past year and prior to the past year. For the current study, lifetime diagnosis variables were used for all substance and mental disorder variables. Also consistent with DSM-5 criteria, mood and anxiety diagnoses did not include substance or medically induced occurrences (Grant et al., 2016).
The Short Form Health Survey Version 2 (SF-12; Ware et al., 2002) is a 12-item measure that is commonly used in population surveys to measure functional health or disability. The SF-12 assesses the extent of problems experienced in work or other daily activities over a timeline of the last four weeks. We used the two main summary measures from the SF-12, the Physical Component Summary (PCS) and the Mental Component Summary (MCS), as well as the SF-12 Bodily Pain Scale (BPS). Scores on all SF-12 subscales are standardized with a mean of 50 and a standard deviation of 10, with lower scores reflecting increased functional impairment. Using criteria established by Cohen (1988) regarding interpretation of standardized differences, differences of 2, 5, and 8 units on the SF-12 reflect small, medium, and large differences, respectively.
Analytic Plan
To identify comorbidity profiles relative to lifetime diagnoses of P-OUD, we used latent class analysis (LCA). Latent class analysis evaluates whether participants can be classified into a discrete number of latent classes/groups based on an observed set of variables. It is based on a mixture modeling approach, which uses a categorical latent variable to allow for the possibility of multiple underlying distributions to explain the observed pattern of responses, as opposed to assuming that a variable (or set of variables) is properly represented by a single distribution.
In this investigation, we evaluated whether there were discrete distributions/classes that underlie a set of observed dichotomous diagnosis variables, which included P-OUD, a number of substance use disorders, and a number of externalizing and internalizing disorders (please see Table 1 for a list of all disorders that were used as a part of latent class formation). Latent class models were estimated in Mplus version 8 (Muthén and Muthén, 2017) using full-information maximum likelihood estimation with robust standard errors, while also incorporating complex sample features from the NESARC as a part of model estimation.
Table 1.
Descriptive Characteristics of Observed Latent Classes
| Low-Average P-OUD
Rates |
Elevated P-OUD Rates |
Very High P-OUD Rates |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Qualitative Description | Class 1 Mentally Healthy | Class 2 Depressed | Class 3 Alcohol-Tobacco | Class 4 Alcohol-Tobacco-Cocaine | Class 5 Internalizing | Class 6 Bipolar | Class 7 Polysubstance/Elevated Psychopathology | Class 8 Polysubstance /Very High Psychopathology | Entropya |
| Lifetime Prevalence Rates | |||||||||
| Prescription Opioid Use Disorder | 0.1% | 1.1% | 2.7% | 7.3% | 4.2% | 7.7% | 74.0% | 28.3% | 0.44 |
| Alcohol Use Disorder | 12.0% | 25.1% | 76.0% | 85.0% | 45.9% | 67.1% | 88.0% | 89.9% | 0.50 |
| Tobacco Use Disorder | 11.9% | 25.1% | 69.3% | 81.2% | 50.3% | 59.7% | 86.7% | 85.5% | 0.48 |
| Cannabis Use Disorder | 0.3% | 1.4% | 20.2% | 37.3% | 12.5% | 19.5% | 66.7% | 33.1% | 0.46 |
| Cocaine Use Disorder | 0.0% | 0.0% | 5.9% | 16.4% | 1.4% | 8.0% | 58.2% | 23.3% | 0.44 |
| Heroin Use Disorder | 0.0% | 0.0% | 0.7% | 1.2% | 0.7% | 0.6% | 22.9% | 8.4% | 0.42 |
| Stimulant Use Disorder | 0.0% | 0.3% | 3.3% | 10.7% | 0.3% | 4.5% | 52.1% | 26.6% | 0.43 |
| Sedative Use Disorder | 0.0% | 0.4% | 0.8% | 2.9% | 2.9% | 2.0% | 54.7% | 18.3% | 0.43 |
| Antisocial Personality Disorder | 0.3% | 1.4% | 5.0% | 16.5% | 4.4% | 14.1% | 25.8% | 54.0% | 0.43 |
| Bipolar 1 Disorder | 0.3% | 0.0% | 1.7% | 0.0% | 0.0% | 100.0% | 10.9% | 57.1% | 0.45 |
| Major Depression Disorder | 7.2% | 56.0% | 18.8% | 68.0% | 71.7% | 0.0% | 43.5% | 25.5% | 0.48 |
| Generalized Anxiety Disorder | 0.6% | 23.1% | 2.2% | 34.5% | 50.7% | 36.7% | 16.3% | 82.3% | 0.46 |
| Social Phobia | 0.6% | 6.7% | 1.0% | 12.4% | 45.4% | 16.1% | 6.7% | 79.4% | 0.44 |
| Panic Disorder | 0.7% | 9.8% | 2.7% | 18.0% | 55.9% | 30.2% | 15.7% | 87.2% | 0.45 |
| Agoraphobia | 0.1% | 1.5% | 0.0% | 2.5% | 45.9% | 10.6% | 4.3% | 70.0% | 0.44 |
| Specific Phobia | 2.2% | 13.5% | 4.0% | 17.3% | 47.1% | 20.3% | 11.1% | 59.6% | 0.44 |
| Postraumatic Stress Disorder | 0.4% | 15.0% | 1.9% | 35.6% | 33.8% | 39.3% | 28.1% | 77.8% | 0.45 |
| % with P-OUD Who Have Ever Received Treatmentb | 4.7% | 22.9% | 34.7% | 8.9% | 38.2% | 35.0% | 37.2% | 29.9% | - |
| Model Estimated nc | 22056.11 | 5522.35 | 5456.41 | 1407.92 | 852.24 | 467.32 | 399.91 | 146.71 | - |
| Sample Proportion | 60.75% | 15.21% | 15.03% | 3.88% | 2.35% | 1.29% | 1.10% | 0.40% | - |
Variable-specific entropy
Because number of participants in each class reflect model-based estimates, the results are allowed to take on fractional values
Treatment targeted to P-OUD
To establish the proper number of classes that underlie the observed diagnostic variables (i.e., class enumeration), an iterative process was used. A 1-class model was first fit to the data, and then a 2-class model was fit to the data and its fit was compared the 1-class model. Subsequently, a 3-class model was fit to the data to see if it fit better than the 2-class model, and this sequence continued by comparing each k class model to a respective k-1 class model, up through a 12-class model. The principal criterion used to determine the best final model was the Bayesian Information Criterion (BIC); Kass and Raftery (1995) indicate that a BIC difference of 10 between models suggests “very strong” evidence in favor of the model with lower BIC value. We also decided on the best final model based on the qualitative interpretability of classes as suggested by Masyn (2013), especially in the case of models with highly redundant class structures. Quality of class separation was evaluated via entropy, and quality of contribution of each observed variable to class differentiation was evaluated by variable-specific entropy (i.e., how much did each specific diagnosis contribute to class differentiation; Asparouhov & Muthen, 2018). Values of entropy range from 0 to 1 and values of .40, .60, and .80 have been associated with low, medium, and high degrees of class separation, respectively (Clark & Muthén, 2009).
To use demographic variables to predict class membership, multinomial logistic regression using 3-step model estimation was used following the procedures of Vermunt (2010); each demographic variable was considered as a separate predictor in separate models. To use class membership to predict physical, mental, and pain-related functional impairment on the SF-12, regression-based models using the BCH method of distal outcome evaluation developed by Bakk and Vermunt (2016) were employed. To evaluate how classes related to treatment seeking behavior, manual 3-step estimation (Asparouhov & Muthén, 2014) was used to identify inter-class variation regarding the proportion of patients who had received past treatment specifically for P-OUD. To evaluate equality of means and proportions across classes, Wald tests were used.
Because classes were identified empirically, analyses using class membership as a predictor or outcome reflect a post-hoc approach, and p-values for these analysis were evaluated based on false discovery rate procedures (FDR; Benjamini & Hochberg, 1995). In this case, each single predictor/outcome was considered as a separate family of hypotheses for evaluation (e.g., using age as a predictor of class membership resulted in 7 hypothesis tests, while using race/ethnicity as a predictor of class membership results in 28 hypothesis tests; the FDR procedure corrected for false discoveries based on 7 hypothesis tests for age and 28 hypothesis tests for race/ethnicity).
Results
Based on evaluation of BIC values and qualitative class interpretation, we chose an 8-class model as an optimal fit to the data (please see Supplementary Table S1 for detail on BIC and entropy for all models considered). Two other models showed lower BIC values than the 8-class model, including a 9-class model (ΔBIC=2.83 compared to 8-class model) and 10-class model (ΔBIC=13.87 compared to 9-class model). The difference in BIC for a 9-class model was small compared to the 8-class model, and did not produce an additional class that added meaningful new information. A 10-class model showed a larger difference in BIC (though for comparison, an 8-class model showed a minimum ΔBIC>125 relative to all models with fewer classes). The classes that were added in a 10-class model reflected much overlap with those from the 8-class model, with divisions that appeared artificial (e.g., class 2 from the 8-class model was largely replicated by both classes 3 and 4 in the 10-class model, with the only major difference being that classes 3 and 4 in the 10-class model were split into 2 classes that had 0.00% and 100.00% rates of major depressive disorder). Entropy values were also not substantially different for the 8-class model (entropy=0.75) versus a 9-class (entropy=0.77) or 10-class (entropy=0.77) model.
Class Enumeration Results
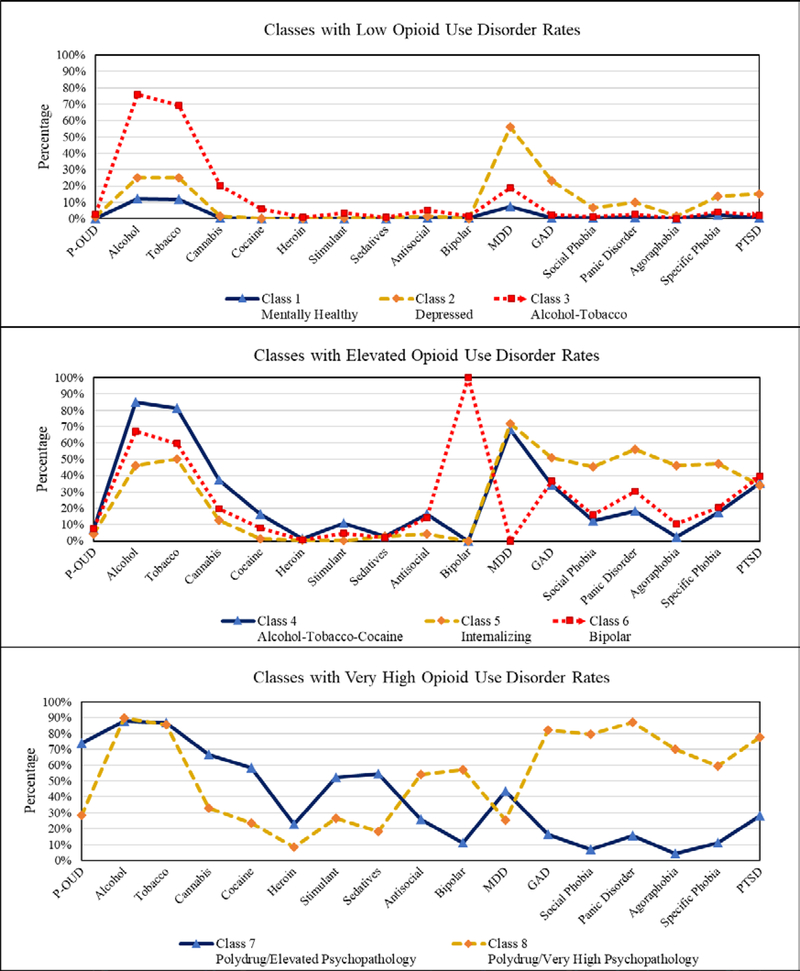
Clinical profiles associated with each class can be found in Table 1 with lower class numbers associated with a higher proportion of the overall sample. Qualitative class descriptions are also provided in Table 1, and a graphical depiction of disorder rates by class can be found in Figure 1. We defined P-OUD rates for each class in terms of the overall NESARC sample, with “elevated” classes reflecting a P-OUD rate that was more than twice the overall sample rate, and “very high” classes reflecting more than 10 times the overall P-OUD rate in the NESARC sample. With regard to the classes that reflected low-average P-OUD rates, class 1 (mentally healthy class, 60.75% of sample, 0.10% P-OUD rate) showed relatively low rates of psychopathology and average overall functioning as measured by the SF-12. Class 2 (depressed class, 15.21% of sample, 1.10% P-OUD rate) had high levels of major depressive disorder (MDD) and generalized anxiety disorder (GAD), relatively average alcohol and tobacco use disorder rates, and relatively low rates of clinical-level illicit drug SUDs. Quality of life was observed to be fairly low in this class, especially with regard to mental well-being (SF-12 Mental Component Summary=38.5). This stands in contrast to class 3 (alcohol-tobacco class, 15.03% of sample, 2.70% P-OUD rate), which had very high alcohol and tobacco use disorder rates, but otherwise relatively average disorder dates for other drug use and psychopathology and relatively higher mental well-being (SF-12 Mental Component Summary=45.6). Overall, these three classes accounted for a majority of participants (90.98% of total sample)
Figure 1.
Profile Plots of Disorder Prevalence Rates for Participants in Each Observed Class Note: P-OUD=Prescription opioid use disorder; alcohol, tobacco, cannabis, cocaine, heroin, stimulant and sedatives all refer to the substance use disorders corresponding to each respective substance; antisocial=antisocial personality disorder ; bipolar=bipolar 1 disorder; MDD=Major depressive disorder; GAD=Generalized anxiety disorder; PTSD=Posttraumatic stress disorder
With regard to classes that reflected elevated P-OUD rates, class 4 (alcohol-tobacco-cocaine class, 3.88% of sample, 7.30% P-OUD rate) reflected high rates of PTSD as well as depression, alcohol, tobacco, cannabis, and other illicit use disorders. It also reflected notable functional impairment via scores on the SF-12 Physical Component Summary (39.28), Mental Component Summary (32.41), and Bodily Pain Scale (34.99). Class 5 (internalizing class, 2.35% of sample, 4.20% P-OUD rate) was high on anxiety and PTSD rates as well as for alcohol and tobacco use disorder, but had low disorder prevalence rates for illicit drug use disorders. Class 6 (bipolar class, 1.29% of sample, 7.70% P-OUD rate) was notable for reflecting high rates of bipolar disorder and alcohol use disorder, as well as elevated rates of cocaine use disorder.
With regard to classes that reflected very high P-OUD rates, class 7 (polysubstance class with elevated psychopathology, 1.1% of sample, 74.00% P-OUD rate) had relatively high rates of psychopathology for nearly all drug classes, but relatively low rates of anxiety disorders. Class 8 (polysubstance class with very high psychopathology rates, 0.40% of sample, 28.30% P-OUD rate) differentiated from class 7 in several ways. These include its lower P-OUD rates, higher prevalence of anxiety disorders and sedative use disorder, and relatively normal SF-12 scores compared to what might be expected for such high rates of psychopathology. Classes 7 and 8 both showed high rates of bipolar disorder and antisocial personality disorder.
Demographic Predictors of Class Membership
Multinomial logistic regression models were used to predict class membership. These models rely on a ratio, where the chance of participant assignment to a specific class is compared to the chance of being assigned to the mentally healthy class. Specifically, these models evaluate if this ratio differs across levels of the predictor, producing an odds ratio (OR) for differences in odds across predictor levels. Predictor reference classes were White/non-Hispanic (for race/ethnicity) and female (for sex), and the outcome reference group was class 1 (mentally healthy class).
Results from analyses using demographic predictors of class membership can be found in Table 2. Increasing age was associated a lower probability of being assigned to classes other than the mentally healthy class (class 1), with the exception of the depressed class (class 2, which showed no significant difference in relationship with age relative to class 1, p=.24). Relative to females, males were less likely to be members of the depressed lass (class 2, OR=0.27) and the internalizing class (class 5, OR=0.36) but more likely to be members of the alcohol-tobacco class (class 3, OR=3.44) and the polysubstance/elevated psychopathology class (class 7, OR=1.65).
Table 2.
Demographic Predictors of Class Membership
| Low-Average OUD Rates |
Elevated OUD Rates |
Very High OUD Rates |
|||||
|---|---|---|---|---|---|---|---|
| Qualitative Description | Class 2 Depressed | Class 3 Alcohol- Tobacco | Class 4 Alcohol- Tobacco- Cocaine | Class 5 Internalizing | Class 6 Bipolar | Class 7 Polysubstance/ Elevated Psychopathology | Class 8 Polysubstance/ Very High Psychopathology |
| Variable | OR | OR | OR | OR | OR | OR | OR |
| American Indian/Alaska Native, non-Hispanic | 0.97 | 1.18 | 4.87* | 2.16 | 3.59* | 0.82 | 6.12* |
| Asian/Native Hawaiian/Other Pacific Islander, non-Hispanic | 0.25* | 0.25* | 0.14* | 0.07* | 0.31* | 0.14* | 0.00* |
| Black, non-Hispanic | 0.48* | 0.62* | 0.61* | 0.44* | 0.85 | 0.21* | 0.41* |
| Hispanic, any race | 0.45* | 0.37* | 0.59* | 0.31* | 0.78 | 0.19* | 0.34* |
| Male | 0.27* | 3.44* | 0.88 | 0.36* | 0.84 | 1.65* | 0.65 |
| Age (−SD)a | 1.04 | 1.52* | 1.47* | 1.28* | 1.52* | 1.75* | 1.63* |
| Age (+1SD)a | 0.97 | 0.66* | 0.68* | 0.78* | 0.66* | 0.57* | 0.61* |
Note: Reference classes for predictors were White/non-Hispanic (for race/ethnicity) and female (for sex). The reference class for outcome was class 1 (mentally healthy class). The * mark reflects significance at the .05 level after adjusting for the false discovery rate. OR=odds ratio
Significance tests for age reflect test of overall mean odds ratio; specific odds ratios were calculated by comparing the estimated odds between those associated with ±1 standard deviation in age (which reflect ages 28.10 and 63.16, respectively) compared to the estimated odds associated with the average sample age (45.63)
With regard to race/ethnicity, most findings reflected that minority race/ethnicity groups were associated with a lower probability of being in class of elevated P-OUD rates. Exceptions to this pattern included the American Indian/Alaska Native group being more likely than the White/non-Hispanic group to be a member of the alcohol-tobacco-cocaine class (class 4 , OR=4.87), the bipolar class (class 6, OR=3.59), and the polysubstance/very high psychopathology class (class 8, OR=6.12). Nonsignificant race/ethnicity differences were also observed in several cases. In particular, members of the American Indian/Alaska Native group did not show significant differences in being a member of the depressed, alcohol-tobacco, internalizing, and polysubstance/elevated psychopathology classes (classes 2, 3, 5, and 7, respectively). Members of the Hispanic group did not show a significant difference with regard to membership in the bipolar class (class 6), and members of the Black/non-Hispanic group also did not show a significant difference with regard to membership in class 6.
Class Membership as a Predictor of Functional Impairment and Treatment Seeking
Relative to the mentally healthy class (class 1), all other classes showed significantly lower SF-12 scores on all SF-12 scales (please see Supplemental Table S2 for detail). Differences at the medium and large levels of magnitude were more frequently observed for the SF-12 Mental Component Summary and Bodily Pain Scale, where the depressed and alcohol-tobacco-cocaine classes (classes 2 and 4, respectively) showed large differences on both scales when compared to the mentally healthy class (class 1). On the Mental Component Summary, large differences were also observed between class 1 and the bipolar and polysubstance/elevated psychopathology classes (classes 6 and 7, respectively), and medium-large differences were observed for the alcohol-tobacco and internalizing classes (classes 3 and 5, respectively). On the SF-12 Bodily Pain Scale, medium-sized differences were also observed for the internalizing, bipolar, and polysubstance/elevated psychopathology classes (classes 5, 6, and 7, respectively). On the SF-12 Physical Component Summary, the alcohol-tobacco-cocaine class (class 4) again showed a large-sized difference, and medium sized differences were observed for the depressed and internalizing classes (classes 2 and 5, respectively). With regard to treatment seeking, a minority of participants with P-OUD had ever received clinical treatment for the problem. Relative to the mentally healthy reference class (class 1), only those in the polysubstance/elevated psychopathology class (class 7) showed a significant difference in likelihood of receiving past treatment (37.2% vs. 4.7%)
Discussion
Multiple population subgroups showed elevated P-OUD prevalence. In particular, 5 of the participant classes had a P-OUD prevalence above 4%. These classes represent 9% of the weighted sample, corresponding to over 25 million Americans. Also, these five classes were clinically heterogeneous, with great differences among them in prevalence of alcohol use disorder (45.9–89.9%), cannabis use disorder (12.5–66.7%), antisocial personality disorder (4.4–54.0%), major depressive disorder (0–71.1%) and anxiety disorders (2.5–87.2%). Overall, these data suggest that the American population presents with a number of discrete clinical presentations that reflect elevated P-OUD rates. Furthermore, these classes of patients are also associated with significant polysubstance use and multiple forms of psychopathology.
In terms of the opioid crisis, the polysubstance classes (classes 7 and 8) are of notable interest. Each was comprised of individuals with a greater than one in four probability of having lifetime P-OUD, with a nearly three in four chance in the polysubstance/elevated psychopathology class (class 7). While these classes had very similar prevalence rates of alcohol and tobacco use disorders, class 7 had the highest heroin use disorder rate, along with higher rates of other drug use disorders. Conversely, the polysubstance/very high psychopathology class (class 8) had higher prevalence rates of every examined form of psychopathology, except for MDD. For the three classes with moderately elevated P-OUD prevalence (classes 4 through 6), similar divergence in the associated substance use disorders and psychopathology was found. Class 4 (alcohol-tobacco-cocaine class) had the highest rates of alcohol, tobacco and cannabis use disorders, and class 5 (internalizing class) had the highest rates of psychopathology with the exceptions of bipolar disorder and PTSD. These were more prevalent in class 6 (bipolar class). Class 7 (polysubstance/elevated psychopathology) was also the only class to have a significantly higher rate of prior substance-related treatment seeking behavior relative to the mentally healthy class. While a minority of patients who stand to benefit from P-OUD treatment actually receive it (Saha et al. 2016), patients with a number of problems may have increased interactions with substance use and mental health treatment, increasing the likelihood of receiving targeted care. It would be beneficial for patients who have less severe symptom presentations but still have elevated P-OUD rates to be identified more frequently as candidates for specialized P-OUD care, such as those in the alcohol-tobacco-cocaine class (class 4).
Taken together, these results suggest that a single treatment approach to reducing P-OUD is unlikely to be successful. Recent models of integrated P-OUD care have emphasized the importance of consulting with or including behavioral health specialists in treatment planning and delivery and note the likelihood of polysubstance use in those presenting for P-OUD treatment (Stoller, Stephens, & Schorr, 2016). The latent classes uncovered here suggest that behavioral health specialists need to play a potentially even larger role in treatment, and that clinicians treating P-OUD will need expertise with the complexities of psychosocial and medication treatment for both P-OUD and other substance use. This can occur in the context of specialty care, as well as through brief interventions provided by psychologists housed in the offices of primary care physicians (an integrative treatment model that has been deployed in a number of Veterans Affairs hospitals). Given past findings that indicate that other substance use and psychopathology can reduce the effectiveness of P-OUD treatment, failure to adequately treat the varying patterns of polysubstance use and psychopathology present in those P-OUD subgroups will only prolong the US opioid epidemic. Results from class differences on SF-12 scores also suggest that treatment will also need to include effective behavioral pain management. For example, class 4 (alcohol-tobacco-cocaine) reported significant bodily pain in addition to polysubstance use.
In designing improved systems for intervention, a long-term approach will be needed to make the changes necessary. A majority of those with P-OUD had symptom onset within the prior 10 years, and other research using NESARC-III data has indicated that P-OUD in both the past year as well as across the lifespan is associated with substantial comorbidity with other substance use disorders and psychopathology (Saha et al., 2016). Thus, a large number of patients will need to be monitored over an extended period, and not all of them will develop symptoms at once. The timeframe for symptom onset that we considered naturally fits into the common 10-year timeframe used by the Congressional Budget Office for resource allocation planning. In this way, current federal program development systems are already designed to allocate resources over the extended period necessary to make the desired impact.
Within classes, higher rates of anxiety disorders co-occurred with relatively lower rates of P-OUD, consistent with data from adolescents suggesting that anxiety symptomatology can protect against substance use (e.g., Scalco et al., 2014). In contrast, higher levels of PTSD covaried with elevated P-OUD prevalence, indicating an acute need for PTSD treatment in those with P-OUD. Briefer interventions may be preferable in treating the common comorbid presentations of individuals with P-OUD, such as a five-session writing-based exposure treatment for PTSD (Sloan, Marx, Lee, & Resick, 2018), but it is unclear whether briefer treatments will be effective in those with significant comorbidity.
With that said, clinicians will likely encounter the challenge of matching treatment options for individuals with P-OUD. Based on the current study, many individuals with P-OUD may have limited or no comorbidity (i.e., those in classes 1 through 3) but those with P-OUD from classes 4 through 8 are also likely to have significant comorbidity and need multidisciplinary, integrated behavioral treatment and MAT. Per American Society of Addiction Medicine (ASAM) Guidelines (2015), MAT is indicated in those with moderate or severe DSM-5 P-OUD, which roughly corresponds to DSM-IV opioid dependence (Compton et al., 2013). Future research, however, is needed to establish optimum treatment for those with moderate or severe P-OUD, whether that is MAT only, MAT with brief interventions for comorbidity or more intensive behavioral treatment for their complex pattern of P-OUD, other SUDs and psychopathology.
Similarly, research is needed to establish the best treatment course for those with mild P-OUD, as pharmacotherapy may not be appropriate for such individuals (Kampman & Jarvis, 2015). Furthermore, clinicians are encouraged to refer to the ASAM practice guidelines for the use of medications for treating OUD and P-OUD including special populations such as adolescents, individuals with pain, pregnant women, criminal justice and homeless populations, and individuals with co-occurring psychiatric disorders (Kampman & Jarvis, 2015). For instance, clinicians should be aware of potential interactions between medications used to treat co-occurring psychiatric conditions and P-OUD. Notably, the ASAM and World Health Organization guidelines (2009) recommend psychosocial treatment in combination of MAT for all individuals with opioid dependence, suggesting that comprehensive treatment may be warranted for all individuals with moderate to severe P-OUD.
Class membership was also associated with a significantly different demographic profile. While American Indian/Alaska Native respondents were more likely to be in three of the five elevated P-OUD prevalence classes than White/non-Hispanic respondents, Asian/Native Hawaiian/Other Pacific Islander, Black/non-Hispanic, and Hispanic respondents were significantly less likely to be in an elevated P-OUD class than White/non-Hispanic individuals. These results are consistent with other research suggesting that White/non-Hispanic and American Indian/Alaska Native individuals have the highest rates of opioid overdose (Case & Deaton, 2015; Venner et al., 2018). Sex was not consistently associated with membership in an elevated P-OUD class, but increasing age was associated with a decreased likelihood of class membership in each of the five highest P-OUD prevalence classes. While older adults have evidenced an increase in prescription opioid misuse prevalence over the past 15 years (Schepis & McCabe, 2016), adults 50 and older remain the lowest prevalence group for prescription opioid misuse (Schepis, McCabe, & Teter, 2018). Finally, the emergence of fentanyl and other synthetic opioids after 2013 indicates the need to account for these products in subsequent OUD investigations (O’Donnell et al., 2017).
Limitations
Several limitations should be noted. First, the data are cross-sectional, which prevents causal inference in terms of the developmental trajectories leading to class membership. Second, self-report measures were used in some instances. At all points the NESARC-III uses assessments that have strong validity data, and while self-report of substance use is a reliable and valid assessment methodology (Grant et al., 2015; Johnston & O’Malley, 1985; O’Malley, Bachman, & Johnston, 1983), it remains possible that individuals can show limited insight into their own functioning. Third, several variables were not collected which could provide further mechanistic insight, including disorder-specific severity, neurobiological correlates, and physical dependence (i.e., opioid tolerance or withdrawal symptoms) as it relates to pain conditions on P-OUD. This information could inform the complex interplay between these concepts and their correspondence to latent class membership. Finally, the exclusion of some institutionalized subpopulations with higher rates of SUDs and mortality, including homeless individuals and incarcerated populations, may have led to underestimation of P-OUD prevalence in the NESARC-III (Keyes, Rutherford, Popham, Martins, & Gray, 2018).
Of additional note is that we used the entire NESARC-III sample in our analysis. We considered limiting the analysis only to participants with P-OUD, which would reduce heterogeneity among participants and could possibly improve clarity in class interpretation. However, looking at classes created using data solely from respondents with P-OUD is less representative of both the general American population as well as the overall clinical population who presents for treatment. Given that a large proportion of Americans have been exposed to opioids at some point, and also that opioids are the most frequently prescribed medications in America (Han et al. 2017; Volkow & McLellan, 2016), limiting analysis to the minority of Americans with P-OUD would lose the connection between exposure and real-world clinical profiles. While there is notable heterogeneity among our classes, utilizing the entire NESARC sample means that results are representative of what clinicians are likely to see in presenting patients. This results in a substantial increase in external validity, has clinical impact in suggesting how non-specialty treatment centers should adapt their capabilities to the opioid crisis, and better informs the design of public policy.
Conclusions
Substantial heterogeneity in comorbid psychopathology and polysubstance use patterns were found in the latent classes with elevated P-OUD prevalence. Thus, when addressing P-OUD, healthcare professionals will likely need to assess and treat multiple conditions simultaneously with multidisciplinary teams. Behavioral health professionals will be key members of these teams, as a combination of medication and psychotherapy will almost certainly be needed for comorbid disorders associated with moderate to severe P-OUD. Public health systems will also need to consider resource allocation for P-OUD treatment in light of these results. While MAT and harm reduction approaches (e.g., naloxone distribution) are essential to reducing P-OUD and its consequences, healthcare and public health systems should expect to allocate significant resources to treat the comorbidities present in those with P-OUD.
At present, empirically-supported interventions for the observed comorbid conditions are often unavailable at treatment sites, which may impede successful P-OUD intervention. While a significant increase in resources has been provided to address the opioid crisis, simply amplifying current treatment initiatives will likely result in inefficient investment, ineffective outcomes, and wasted effort. Based on the diverse profiles in the present study, the likelihood of success can be maximized by tailoring research, clinical intervention strategies, and federal investment to empirical clinical profiles.
Supplementary Material
Public Health Significance Statement.
A number of distinct clinical profiles are associated with elevated rates of prescription opioid use disorder. We highlight specific profiles that can include other substance use disorders, depression, anxiety, PTSD, and externalizing conditions. These profiles can be used to guide public policy, resource allocation, and the design of personalized care strategies for patients in need.
Acknowledgments
The development of this manuscript was supported by research grants from the National Institutes of Health National Institute on Drug Abuse (NIDA) to Dr. McCabe (R01DA031160 and R01DA036541) and Dr. Schepis (R01DA043691 and R01DA042146). This manuscript was prepared using a limited access data set obtained from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The funders had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, and approval of the manuscript, and the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, the National Institutes of Health, or the U.S. Government.
Appendix A. Data Collection and Transparency Statement
The data reported in this manuscript were obtained from publicly available data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). The project website from the NESARC-III can be found at https://www.niaaa.nih.gov/research/nesarc-iii, and a bibliography of work resulting from NESARC-III research can be found at https://www.niaaa.nih.gov/sites/default/files/NESARC/NESARC-III%20publications_Final_8_10_16.pdf Prior NESARC-III papers have focused largely on establishing epidemiological estimates and univariate analyses. In this manuscript, we extend NESARC-III work by employing multivariate inference. The multivariate relationships among variables examined in the present article have not been examined in any previous or current articles, or to the best of our knowledge in any papers that will be under review soon.
Footnotes
Age was measured continuously for all participants except for those age 90 or older (n=187); these participants had their ages fixed to age 90 during the construction of the NESARC dataset
References
- Asparouhov T, & Muthén B (2014). Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Structural Equation Modeling, 21, 329–341. [Google Scholar]
- Asparouhov T, & Muthén B (2018). Variable-specific entropy contribution. Retrieved from: https://www.statmodel.com/download/UnivariateEntropy.pdf
- Bakk Z, & Vermunt JK (2016). Robustness of stepwise latent class modeling with continuous distal outcomes. Structural Equation Modeling, 23, 20–31. doi: 10.1080/10705511.2014.955104 [DOI] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57, 289–300. [Google Scholar]
- Benningfield MM, Dietrich MS, Jones HE, Kaltenbach K, Heil SH, Stine SM, . . . Martin PR (2012). Opioid dependence during pregnancy: Relationships of anxiety and depression symptoms to treatment outcomes. Addiction, 107, 74–82. doi: 10.1111/j.1360-0443.2012.04041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, & Weiss RD (2017). The role of behavioral interventions in buprenorphine maintenance treatment: A review. American Journal of Psychiatry, 174, 738–747. doi: 10.1176/appi.ajp.2016.16070792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2015). Rising morbidity and mortality in midlife among White Non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences, 112, 15078–15083. doi: 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, & Muthén B (2009). Relating latent class analysis results to variables not included in the analysis. Retrieved from: https://www.statmodel.com/download/relatinglca.pdf
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Compton WM, Dawson DA, Goldstein RB, & Grant BF (2013). Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug and Alcohol Dependence, 132, 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23, 63–75. doi: 10.1097/hrp.0000000000000075 [DOI] [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, . . . Weiss RD (2013). Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug and Alcohol Dependence, 131, 112–118. doi: 10.1016/j.drugalcdep.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165, 179–187. doi: 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Ferri M, Finlayson AJ, Wang L, & Martin PR (2014). Predictive factors for relapse in patients on buprenorphine maintenance. American Journal on Addictions, 23, 62–67. doi: 10.1111/j.1521-0391.2013.12074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82, 448–459. doi: 10.1037/a0035798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, . . . Grant BF (2016). The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Social Psychiatry and Psychiatric Epidemiology, 51, 1137–1148. doi: 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Amsbary M, Chu A, Sigman R, Kali J, Sugawana Y, . . . Smith SM (2014). Source and accuracy statement: National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, . . . Hasin DS (2015). The alcohol use disorder and associated disabilities interview schedule-5 (AUDADIS-5): Reliability of substance use and psychiatric disorder modules in a general population sample. Drug and Alcohol Dependence, 148, 27–33. doi: 10.1016/j.drugalcdep.2014.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, . . . Hasin DS (2016). Epidemiology of DSM-5 drug use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry, 73, 39–47. doi: 10.1001/jamapsychiatry.2015.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Dodd DR, Potter JS, Rice LS, Dickinson W, Sparenborg S, & Weiss RD (2014). Baseline characteristics and treatment outcomes in prescription opioid dependent patients with and without co-occurring psychiatric disorder. American Journal of Drug and Alcohol Abuse, 40, 157–162. doi: 10.3109/00952990.2013.842241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, & Jones CM (2017). Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine, 167, 293–301. doi: 10.7326/M17-0865 [DOI] [PubMed] [Google Scholar]
- Huhn AS, Sweeney MM, Brooner RK, Kidorf MS, Tompkins DA, Ayaz H, Dunn KE (2018). Prefrontal cortex response to drug cues, craving, and current depressive symptoms are associated with treatment outcomes in methadone-maintained patients. Neuropsychopharmacology. doi: 10.1038/s41386-018-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Jones C, Compton W, Baldwin G, Fan J, Mermin J, & Bennett J (2018). Federal response to the opioid crisis. Current HIV/AIDS Reports, 15, 293–301. doi: 10.1007/s11904-018-0398-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, & O’Malley PM (1985). Issues of validity and population coverage in student surveys of drug use In Rouse BA, Kozel NJ, & Richard LG (Eds.), Self-report methods of estimating drug use: Meeting current challenges to validity. (National Institute on Drug Abuse Research Monograph No. 57[ADM] 85–1402). Bethesda, MD: National Institute on Drug Abuse. [Google Scholar]
- Kampman K, & Jarvis M (2015). American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. Journal of Addiction Medicine, 9, 358–367. doi: 10.1097/ADM.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouse AB, & Compton P (2015). The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. Journal of Pain & Palliative Care Pharmacotherapy, 29, 102–114. doi: 10.3109/15360288.2015.1037521 [DOI] [PubMed] [Google Scholar]
- Kass RE, & Raftery AE (1995). Bayes factors. Journal of the American Statistical Association, 90, 773–795. doi: 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Keyes KM, Rutherford C, Popham F, Martins SS, & Gray L (2018). How healthy are survey respondents compared with the general population?: Using survey-linked death records to compare mortality outcomes. Epidemiology, 29, 299–307. doi: 10.1097/ede.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge BT, Saha TD, Chou SP, Zhang H, Jung J, Ruan WJ, . . . Hasin DS (2015). Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Drug and Alcohol Dependence, 156, 47–56. doi: 10.1016/j.drugalcdep.2015.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Brooner RK, Peirce J, Kolodner K, & Kidorf M (2014). Challenges and outcomes of parallel care for patients with co-occurring psychiatric disorder in methadone maintenance treatment. Journal of Dual Diagnosis, 10, 60–67. doi: 10.1080/15504263.2014.906132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD, Weiss RD, Anton RF Jr, George TP, Greenfield SF, Kosten TR, . . . Hennessy G (2007). Treatment of patients with substance use disorders. American Journal of Psychiatry, 164, 5–123. [PubMed] [Google Scholar]
- Leece P, Cavacuiti C, Macdonald EM, Gomes T, Kahan M, Srivastava A, . . . Juurlink DN (2015). Predictors of opioid-related death during methadone therapy. Journal of Substance Abuse Treatment, 57, 30–35. doi: 10.1016/j.jsat.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, & Hasin DS (2017). Changes in US lifetime heroin use and heroin use disorder prevalence from the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74, 445–455. doi: 10.1001/jamapsychiatry.2017.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyn K (2013). Latent class analysis and finite mixture modeling In Little TD (Ed.), The Oxford Handbook of Quantitative Methods (Vol. 2, pp. 551–611). New York: Oxford University Press. [Google Scholar]
- McCabe SE, West BT, Jutkiewicz EM, & Boyd CJ (2017). Multiple DSM-5 substance use disorders: A national study of US adults. Human Psychopharmacology: Clinical and Experimental, 32. doi: 10.1002/hup.2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Cutter CJ, Buono FD, Barry DT, Fiellin LE, . . . Schottenfeld RS (2016). Cognitive behavioral therapy improves treatment outcomes for prescription opioid users in primary care buprenorphine treatment. Journal of Substance Abuse Treatment, 71, 54–57. doi: 10.1016/j.jsat.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, & Muthén B (2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Muthén and Muthén [Google Scholar]
- Nelson LS, Juurlink DN, & Perrone J (2015). Addressing the opioid epidemic. JAMA, 314, 1453–1454. doi: 10.1001/jama.2015.12397 [DOI] [PubMed] [Google Scholar]
- O’Donnell JK, Gladden RM, & Seth P (2017). Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region-United States, 2006–2015. Morbidity and Mortality Weekly Report, 66, 897–903. doi: 10.15585/mmwr.mm6634a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley PM, Bachman JG, & Johnston LD (1983). Reliability and consistency in self-reports of drug use. International Journal of Addiction, 18, 805–824. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Brooner RK, King VL, & Kidorf MS (2016). Effect of traumatic event reexposure and PTSD on substance use disorder treatment response. Drug and Alcohol Dependence, 158, 126–131. doi.org/ 10.1016/j.drugalcdep.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, . . . Grant BF (2016). Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. Journal of Clinical Psychiatry, 77, 772–780. doi: 10.4088/JCP.15m10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalco MD, Colder CR, Hawk LW, Read JP, Wieczorek WF, & Lengua LJ (2014). Internalizing and externalizing problem behavior and early adolescent substance use: A test of a latent variable interaction and conditional indirect effects. Psychology of Addictive Behaviors, 28, 828–840. doi: 10.1037/a0035805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer I, Eiroa-Orosa FJ, Verthein U, Dilg C, Haasen C, & Reimer J (2010). Effects of psychiatric comorbidity on treatment outcome in patients undergoing diamorphine or methadone maintenance treatment. Psychopathology, 43, 88–95. doi: 10.1159/000274177 [DOI] [PubMed] [Google Scholar]
- Schepis TS, & Hakes JK (2011). Nonmedical prescription use increases the risk for the onset and recurrence of psychopathology: Results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction, 106, 2146–2155. doi: 10.1111/j.1360-0443.2011.03520.x [DOI] [PubMed] [Google Scholar]
- Schepis TS, & McCabe SE (2016). Trends in older adult nonmedical prescription drug use prevalence: Results from the 2002–2003 and 2012–2013 National Survey on Drug Use and Health. Addictive Behaviors, 60, 219–222. doi: 10.1016/j.addbeh.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, McCabe SE, & Teter CJ (2018). Sources of opioid medication for misuse in older adults: Results from a nationally representative survey. Pain, 159, 1543–1549. doi: 10.1097/j.pain.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman-Olivier Z, Claire Greene M, Bergman BG, & Kelly JF (2014). Is residential treatment effective for opioid use disorders? A longitudinal comparison of treatment outcomes among opioid dependent, opioid misusing, and non-opioid using emerging adults with substance use disorder. Drug and Alcohol Dependence, 144, 178–185. doi: 10.1016/j.drugalcdep.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, & Bacon S (2018). Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. Morbidity and Mortality Weekly Report, 67, 349–358. doi: 10.15585/mmwr.mm6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Marx BP, Lee DJ, & Resick PA (2018). A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: A randomized noninferiority clinical trial. JAMA Psychiatry, 75, 233–239. doi: 10.1001/jamapsychiatry.2017.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M (2015). Alcohol use disorders in opioid maintenance therapy: Prevalence, clinical correlates and treatment. European Addiction Research, 21, 78–87. doi: 10.1159/000363232 [DOI] [PubMed] [Google Scholar]
- Soyka M (2017). Treatment of opioid dependence with buprenorphine: current update. Dialogues in Clinical Neuroscience, 19, 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Kranzler HR, van den Brink W, Krystal J, Möller HJ, Kasper S, & The WFSBP Task Force on Treatment, Guidelines for Substance Use Disorders. (2011). The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of substance use and related disorders (Part 2: Opioid dependence). The World Journal of Biological Psychiatry, 12, 160–187. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, & Kuefner H (2008). Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: Results from a randomized study. International Journal of Neuropsychopharmacology, 11, 641–653. doi: 10.1017/S146114570700836X [DOI] [PubMed] [Google Scholar]
- Stoller KB, Stephens MC, & Schorr A (2016). Integrated service delivery models for opioid treatment programs in an era of increasing opioid addiction, health reform, and parity. Rockville, MD: Substance Abuse and Mental Health Administration [Google Scholar]
- Venner KL, Donovan DM, Campbell ANC, Wendt DC, Rieckmann T, Radin SM, . . . Rosa CL (2018). Future directions for medication assisted treatment for opioid use disorder with American Indian/Alaska Natives. Addictive Behaviors, 86, 111–117. doi: 10.1016/j.addbeh.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK (2010). Latent class modeling with covariates: Two improved three-step approaches. Political Analysis, 18, 450–469. doi: 10.1093/pan/mpq025 [DOI] [Google Scholar]
- Volkow ND, & McLellan AT (2016). Opioid abuse in chronic pain-misconceptions and mitigation strategies. New England Journal of Medicine, 374, 1253–1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., Kosinski M, Turner-Bowker DM, Gandek B (2002) User’s Manual for the SF-12v2 Health Survey. Lincoln, RI: QualityMetric Incorporated. [Google Scholar]
- World Health Organization. (2009). Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva, Switzerland: WHO Press. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.