Abstract
Study Objective:
We compared the efficacy and safety of intravenous lidocaine to that of hydromorphone for the treatment of acute abdominal pain in the emergency department.
Methods:
This was a randomized, double blind clinical trial conducted in two EDs in the Bronx, NY. Adults weighing 60–120 kg were randomized to receive 120 mg of IV lidocaine or 1 mg of IV hydromorphone. 30 minutes after administration of the first dose of study drug, participants were asked if they needed a second dose of the investigational medication to which they were randomized. Patients were also stratified based on clinical suspicion of nephrolithiasis. The primary outcome was improvement in 0–10 pain scores between baseline and 90 minutes. An important secondary outcome was need for “off-protocol” parenteral analgesics, including opioids or non-steroidal anti-inflammatory drugs.
Results:
We enrolled 154 patients of whom 77 received lidocaine and 77 received hydromorphone. By 90 minutes, patients randomized to lidocaine improved by a mean of 3.8 points on the 0–10 scale, while those randomized to hydromorphone improved by a mean of 5.0 points (mean difference 1.2, 95% CI: 0.3, 2.2). Need for off-protocol “rescue” analgesics occurred in 39/77 (51%) of lidocaine patients and 20/77 (26%) hydromorphone patients (95%CI for difference of 25%: 10, 40%). Adverse events were comparable between groups. Among the subset of 22 patients with nephrolithiasis, lidocaine patients reported a mean improvement of 3.4 points on the pain scale, while hydromorphone patients reported a mean improvement of 6.4 points (mean difference 3.0, 95% CI: 0.5 to 5.5).
Conclusion:
IV hydromorphone was superior to IV lidocaine, both for general abdominal pain and a subset with nephrolithiasis. A majority of patients randomly allocated to lidocaine required additional analgesia.
Introduction
Background
Nearly 12 million patient visits to US emergency departments (EDs) annually are due to abdominal pain.(1) While ED providers have a variety of pharmacological agents at their disposal to treat this pain, severe undifferentiated abdominal pain often requires intravenous (IV) opioids to achieve adequate analgesia. When used in the ED for acute pain, parenteral opioids are generally effective, safe and well-tolerated, although they may cause side effects such as nausea, vomiting, drowsiness, dizziness, pruritis, and uncommonly, respiratory depression.(2–6)
IV lidocaine has emerged as a possible therapeutic alternative for management of acute severe pain in the ED.(7) IV lidocaine has been used for acute pain for more than half of a century.(8) Accumulated data indicate that IV lidocaine is superior to placebo and as effective as morphine for neuropathic pain.(9) Perioperative IV lidocaine may also improve post-surgical pain outcomes.(10, 11) In the ED, published data suggest that IV lidocaine may be efficacious for nephrolithiasis,(12) acute limb ischemia,(13) long bone fractures,(14) and undifferentiated severe pain.(15)
Importance
Acute pain is a very common chief complaint in the ED. There is a continuing need for medications that relieve pain rapidly, effectively, and durably with minimal side effects. It is still unclear whether lidocaine can replace opioid regimens as primary parenteral treatment of severe pain in the ED.
Goals of this Investigation
In this study, we tested the hypothesis that among ED patients with acute abdominal pain, IV lidocaine would provide superior analgesia when compared to IV hydromorphone as determined by improvement on a 0–10 pain scale between baseline and 90 minutes later.
Methods
Study design and setting
This was a randomized, double blind, comparative effectiveness trial conducted in two EDs of Montefiore Medical Center, an urban teaching institution in the Bronx, NY, with an annual visit volume exceeding 170,000 visits. Bilingual (Spanish and English), salaried, research associates collected data 24 hours per day, seven days per week. We assessed outcomes throughout the ED stay and by telephone 7 days later. The Montefiore Medical Center IRB reviewed and approved this protocol. We registered the trial online at http://www.clinicaltrials.gov (NCT03300674).
Selection of participants
Eligible patients were between 18 and 64 years old, weighed between 60 and 120 kg, and presented to one of our EDs for treatment of acute, severe abdominal pain. Acute was defined as pain for no more than seven days. Severe was defined as warranting the use of intravenous opioids, as determined by the attending physician. Patients were excluded from participation for cardiac conduction system impairment, known renal or liver disease, hemodynamic instability (as determined by the attending physician), pregnancy, breastfeeding, or for allergy to either medication. Patients were also excluded if they self-reported use of prescription or illicit opioids within the previous week, or if they had a chronic pain disorder, defined as use of any analgesic medication on more days than not during the month preceding the acute episode of pain. Patients who received off-protocol medication in the ED prior to enrollment were eligible for enrollment if ≥ 1 hour had elapsed since off-protocol medication administration and the patient still met inclusion criteria (pain warranting intravenous opioids).
Interventions
Patients were randomized in a 1:1 ratio to 120 mg of IV lidocaine or 1 mg of IV hydromorphone. Each of these medications was administered as an intravenous drip over 10 minutes. If patients reported insufficient relief of pain when specifically queried at 30 minutes, they could receive a second dose of the same medication to which they were randomized. Patients who required additional medication beyond 90 minutes were administered parenteral analgesia at the discretion of the ED attending physician.
Hydromorphone, dosed using a 1 mg titration strategy every 30–60 minutes, has been shown to be a safe and effective analgesic for management of acute severe pain in the ED.(2, 5) The optimal dose of IV lidocaine for acute pain is unknown. For this study, we chose a dose that was most likely to be efficacious while minimizing potential for adverse events. In the peri-operative setting, IV lidocaine boluses ranged from 1–3 mg/kg.(10) ED-based studies have used lower doses of 1–2 mg/kg (Appendix Table). Using weight boundaries of 60 and 120 kg as criteria for study entry, all participants assigned to the lidocaine arm received at least 1 mg/kg of IV lidocaine and, if they opted for a second dose of medication, up to 4 mg/kg of lidocaine over one hour.
Randomization occurred in blocks of four based on a random number generator. Allocation was concealed. Research subjects, clinicians, and research personnel were blinded. The research pharmacist presented research personnel with identical vials containing a clear solution of either lidocaine or hydromorphone, labeled as an investigational medication. The clinical nurse removed the solution from the vial, inserted it into a 50 or 100 cc bag containing normal saline, and administered the medication as a ten-minute intravenous drip. The same mechanism was used for the optional second dose of investigational medication. Subjects were stratified by study site and diagnosis (presumptive diagnosis of nephrolithiasis versus other causes of pain). The rationale for stratification based on presumptive diagnosis of kidney stones was two-fold: 1) kidney stones represent a large subset of abdominal pain diagnoses; 2) kidney stones may be more likely to respond to IV lidocaine than other causes of abdominal pain.(12)
Measurements
Baseline variables of interest included age, sex, weight, pain severity and duration. Diagnosis was determined by querying the treating attending physician at the time of ED discharge. Pain intensity was measured using a verbal numerical scale on which 0 represented no pain and 10 represented the worst pain imaginable. Satisfaction with a specific medication is a highly patient-centered outcome, in which individuals determine for themselves the benefit of a particular drug versus the adverse effects experienced. We included in this study a measure that has been used in multiple ED-based pain trials—”The next time you come to the ER for treatment of pain, do you want to receive the same medication again?”(16) Patients were asked to choose among the following responses: “Yes,” “No,” or “Not sure”.
We determined the presence of medication-induced side effects by asking the following question: “Did you have any new symptoms that began only after you got the study medication?” An affirmative response was followed by an open-ended question eliciting details. Seven days after the ED visit, we called all discharged patients to determine whether they revisited an ED after the initial ED discharge.
Outcomes
The primary efficacy outcome for this study was improvement in 0–10 pain scores between medication administration (time 0 minutes) and 90 minutes later. We chose 90 minutes because we believed that would be sufficient time for patients to receive two doses of the investigational medication if a second dose was requested. An important secondary outcome was need for additional “off-protocol” analgesia, defined as parenteral opioids or non-steroidal anti-inflammatory drugs administered subsequently during their ED stay. Exploratory outcomes included patient satisfaction with the medication to which they were randomly allocated. We also recorded pain scores at 15, 30, 45, 60, 90,120, and 180 minutes after investigational medication administration and report these as well as the frequency with which patients experienced a ≥50% improvement in pain between baseline and 90 minutes. Safety endpoints included development of any new symptom after administration of the investigational medication, need for naloxone, change in the disposition of the patient attributable to investigational medication, and unplanned return to any ED within 7 days of the index visit. During each patient’s ED course, we assessed for the development of side effects every hour for three hours.
Analysis
Baseline characteristics are reported as mean (SD), median (IQR), or n/N (%), as appropriate. The primary outcome is reported as the between-group difference in the mean improvement on 0–10 pain score at 90 minutes. Results are considered statistically significantly different if the 95% CI does not include the null point of 0. All dichotomous values are reported as percentages with 95% confidence intervals (CI).
We performed an intention to treat analysis. Once the investigational medication was initiated, the patient was included in the analysis, regardless of whether or not the patient completed the medication infusion and regardless of whether or not the patient received additional analgesic medication. We also repeated the primary outcome analysis for an a priori pre-determined subgroup of patients with the clinical diagnosis of nephrolithiasis.
When 0–10 pain score data were missing at an intermediary time-point, we averaged the adjacent pain scores. When missing data occurred at final measurements, we carried forward the last available pain score.
We based our sample size calculation on previous work. We anticipated a mean improvement in 0–10 pain score of 4.9 and a standard deviation of 2.8. Using a between-group difference of 1.3 as a minimum clinically significant difference, an alpha = 0.05 and a beta =0.20, we determined the need for 73 patients in each group. We enrolled 105% of this N in anticipation of protocol violations and missing data, thus leaving us with a sample size of 154 patients, 77 in each group.
Results
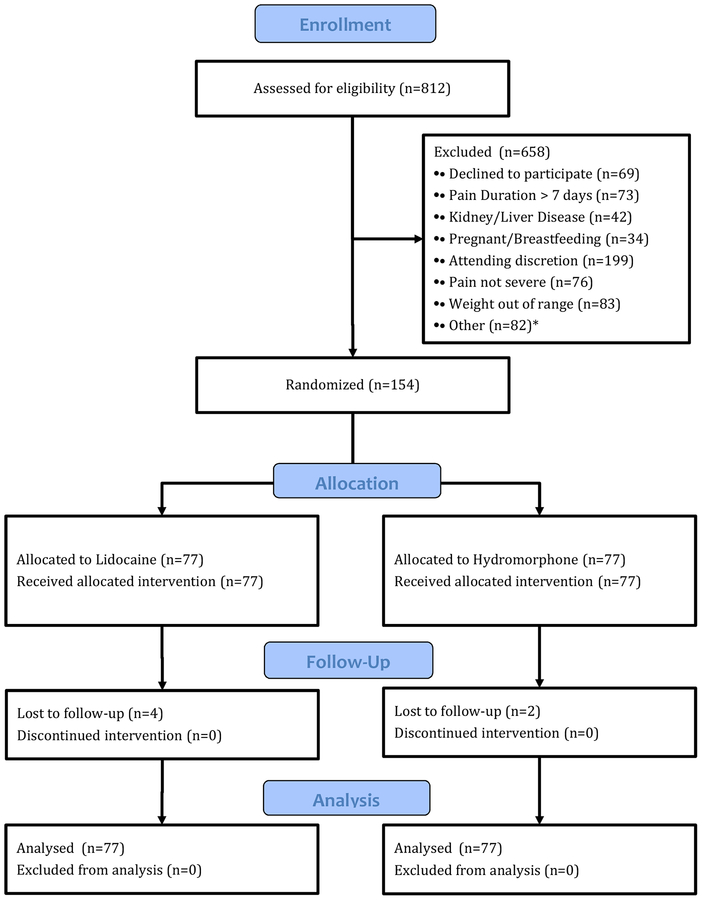
Enrollment commenced in January 2018 and concluded seven months later. During this time, we screened 812 patients for eligibility, 154 of whom met criteria and consented to participate (see CONSORT flow chart, Figure 1). 77 patients were randomized to each group.
Figure 1.
CONSORT flow diagram.
* Other: Use of opioids prior to ED presentation (26), lacked capacity to consent (20), chronic pain syndrome (9), hemodynamically unstable (8), not predominantly abdominal pain (7), abnormal EKG (7), allergic to investigational medication (5)
Baseline characteristics were similar in the two study arms (Table 1), though the lidocaine group was randomly allocated more women than men. Non-specific abdominal pain was the single most common diagnosis. Twenty-two patients (14%), 11 in each group, were diagnosed with nephrolithiasis. The mean initial dose of lidocaine received by the 77 patients who received IV lidocaine was 1.5 mg/kg (SD: 0.3); the mean total dose received was 2.1mg/k (SD 0.8).
Table 1.
Baseline characteristics
| Variable | Lidocaine (n=77) | Hydromorphone (n=77) |
|---|---|---|
| Age in years, mean (SD) | 42 (12) | 40(13) |
| Sex | ||
| Female | 54 (70%) | 44 (57%) |
| Male | 23 (30%) | 33 (43%) |
| Weight in pounds, mean (SD) | 177 (32) | 180 (33) |
| Pain duration in days, median (IQR) | 2 (1,4) | 2 (1,3) |
| Clinical diagnosis* | ||
| Non-specific abdominal pain | 27 (35%) | 22 (29%) |
| Nephrolithiasis | 11 (14%) | 11 (14%) |
| Colitis/Diverticular disease | 12 (16%) | 8 (10%) |
| Biliary pathology | 6 (8%) | 12 (16%) |
| Esophageal/gastric/duodenal pathology | 5 (6%) | 8 (10%) |
| Pelvic pain | 7 (9%) | 5 (6%) |
| Appendicitis | 4 (5%) | 4 (5%) |
| Small bowel obstruction/ileus/hernia | 2 (3%) | 2 (3%) |
| Urinary tract infection | 2 (3%) | 2 (3%) |
| Pancreatitis | 1 (1%) | 1 (1%) |
| Other | 0 (0%) | 2 (3%) |
Based on attending physician’s clinical impression at discharge
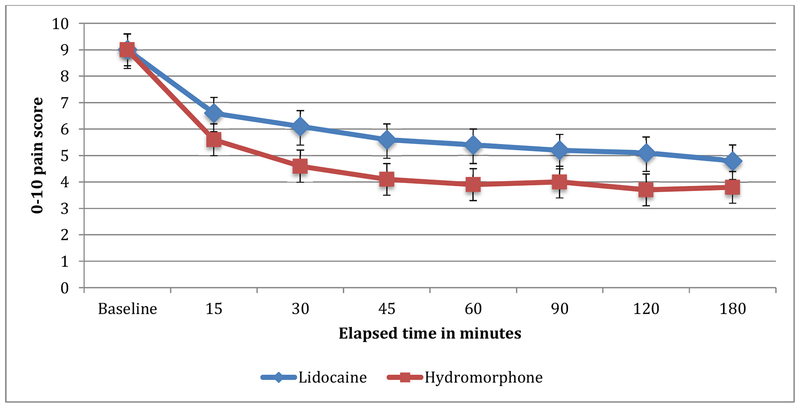
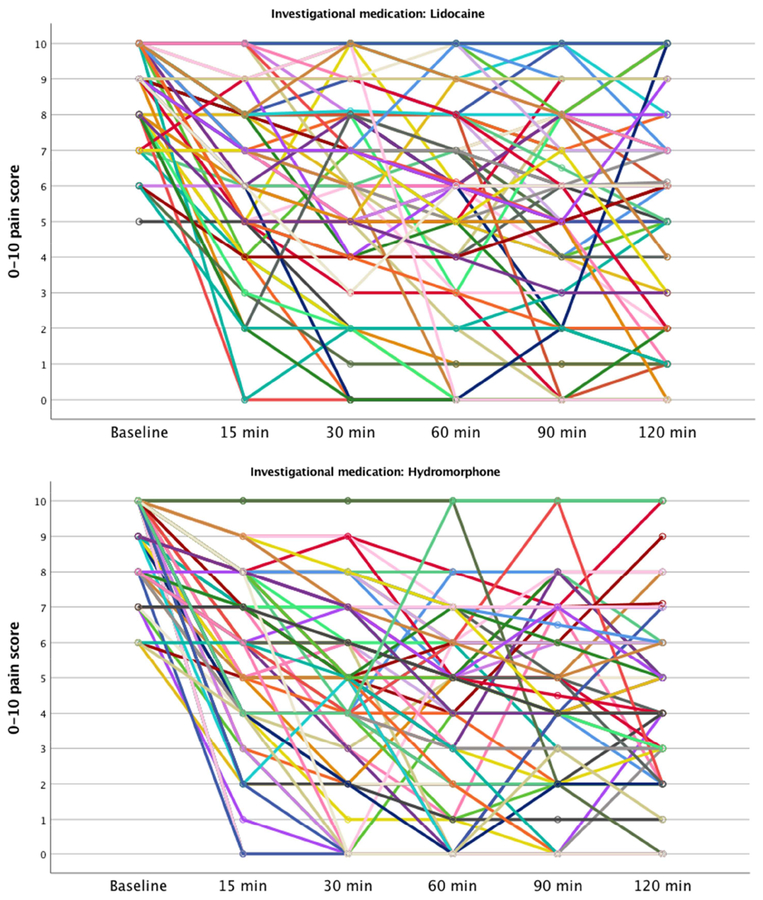
At the 90-minute assessment, patients randomized to lidocaine improved by an average of 3.8 points on the 0–10 pain scale, while those randomized to hydromorphone improved by an average of 5.0 points (mean difference 1.2, 95% CI 0.3 to 2.2) (Appendix data and Appendix Figure). Pain scores were lower in the hydromorphone arm than the lidocaine arm at all time points except baseline (Table 2 and Figure 2). At 90 minutes, more hydromorphone patients (47/77, 61%) than lidocaine patients (30/77, 39%) reported a ≥50% improvement in their pain (difference 22%, 95% CI: 7% to 37%).
Table 2.
0–10 pain scores throughout the study period, reported as mean (SD)
| Time point | Lidocaine (n=77) | Hydromorphone (n=77) | Difference (95% CI) |
|---|---|---|---|
| Baseline | 9.0 (1.3) | 9.0 (1.2) | 0.0 (-0.4, 0.4) |
| 15 minutes | 6.6 (2.4) | 5.6 (2.6) | 1.0 (0.2, 1.8) |
| 30 minutes | 6.1 (2.8) | 4.6 (2.8) | 1.5 (0.6, 2.4) |
| 45 minutes | 5.6 (3.0) | 4.1 (2.7) | 1.5 (0.6, 2.4) |
| 60 minutes | 5.4 (3.0) | 3.9 (2.8) | 1.5 (0.5, 2.4) |
| 90 minutes | 5.2 (3.1) | 4.0 (2.9) | 1.2 (0.3, 2.2) |
| 120 minutes | 5.1 (3.2) | 3.7 (2.8) | 1.4 (0.4, 2.3) |
| 180 minutes | 4.8 (2.8) | 3.8 (2.9) | 1.0 (0.1, 2.0) |
Figure 2.
0–10 pain scores. Error bars depict 95% CI
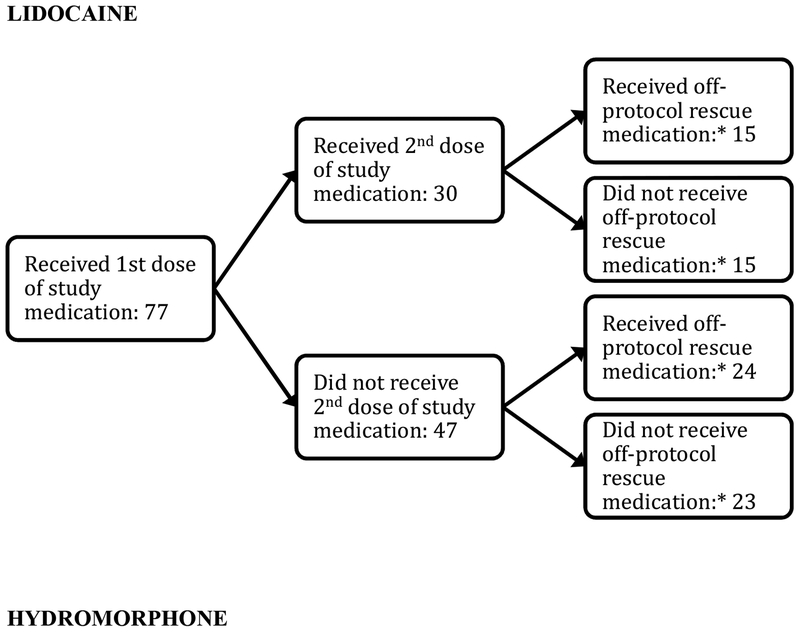
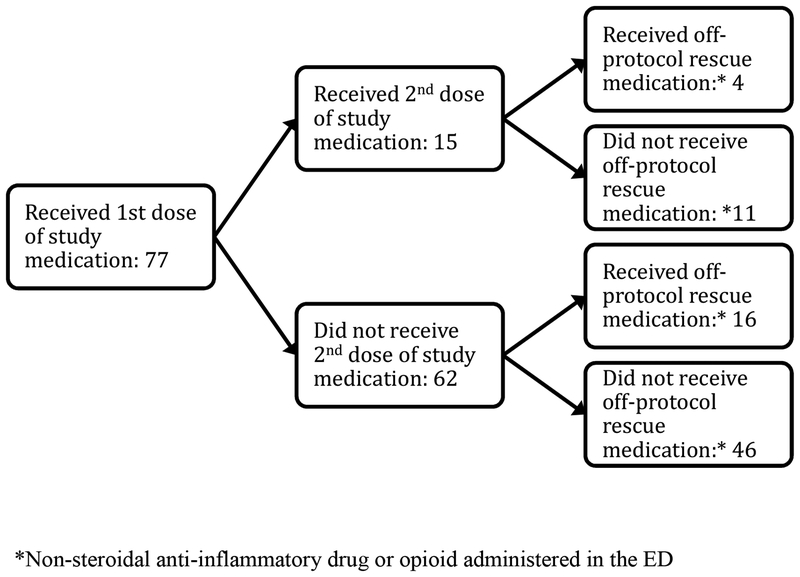
Need for off-protocol “rescue” analgesics occurred in 39/77 (51%) of lidocaine patients and 20/77 (26%) hydromorphone patients (95%CI for difference of 25%: 10, 40%) (Table 3 and Figure 3). Similarly, more hydromorphone patients (64/71, 90%) than lidocaine patients (47/73, 64%) would want to receive the study medication again (difference 26%, 95% CI: 13% to 39%).
Table 3.
Exploratory outcomes
| Outcome | Lidocaine (n=77) | Hydromorphone (n=77) | Difference (95% CI) |
|---|---|---|---|
| Requested additional dose of investigational medication | |||
| Yes | 30 (39%) | 15 (19%) | 20% (5%, 34%) |
| No | 47 (61%) | 62 (81%) | |
| Would want the same medication again | |||
| Yes | 47 (64%) | 64 (90%) | 26% (13%, 39%) |
| No | 19 (26%) | 5 (7%) | |
| Not sure | 7 (10%) | 2 (3%) | |
| Missing data | 4 | 6 |
Figure 3.
Flowchart of medication administered to study participants
Medication-associated symptomatology was comparable between the two study arms (Table 4). The most commonly reported symptoms were dizziness, drowsiness, headache, nausea, and pruritis. No other symptom was reported by more than one patient. There were no serious adverse events in the study. No patient required administration of naloxone.
Table 4.
Adverse events
| Adverse event | Lidocaine | Hydromorphone | Difference (95% CI) |
|---|---|---|---|
| Any patient reported symptom | |||
| Yes | 23 (30%) | 28 (36%) | 6% (−8, 21%) |
| No | 54 (70%) | 49 (64%) | |
| Specific symptoms reported by patients | |||
| Dizziness | 4 (5%) | 14 (18%) | |
| Drowsiness | 6 (8%) | 4 (5%) | |
| Headache | 6 (8%) | 3 (4%) | |
| Nausea | 9 (12%) | 13 (17%) | |
| Pruritis | 1 (1%) | 2 (3%) | |
| Change in management due to investigational medication | |||
| Yes | 0 (0%) | 0 (0%) | |
| No | 77 (100%) | 77 (100%) | 0% |
| Unplanned return visit to ED within 1 week | |||
| Yes | 2 (3%) | 0 (0%) | 3% (−1, 6%) |
| No | 71 (97%) | 75 (100%) | |
| Missing | 4 | 2 | |
| Required naloxone | |||
| Yes | 0 (0%) | 0 (0%) | |
| No | 77 (100%) | 77 (100%) | 0% |
Among the 22 patients diagnosed with clinical nephrolithiasis (based on the attending physician’s discharge impression), those who received lidocaine reported an improvement of 3.4 points on the 0–10 pain scale, while those who received hydromorphone reported an improvement of 6.4 points (mean difference 3.0, 95% CI: 0.5 to 5.5).
We performed a post-hoc analysis in which we compared weight-based dose of lidocaine with the primary outcome. There was a clinically important inverse association between patient weight and improvement in pain score. This is detailed in Appendix 1. Patients who weighed 73kg or less reported a mean improvement in pain scores that was substantially better than patients who weighed 85kg or more (mean difference =1.9, 95%CI: 0.5, 3.4). Patients who weighed no more 73 kg reported mean improvement in 0–10 pain scores of 5.0 (95% CI: 3.8, 6.2).
Limitations
The primary limitation of this trial was that we may have under-dosed the initial bolus of IV lidocaine. Post-hoc data presented in the Appendix 1 demonstrate that initial doses of IV lidocaine approaching 2 mg/kg were more effective than lower weight-based doses and as effective as IV hydromorphone. When designing this study, we did not identify dose-finding studies of IV lidocaine for acute pain (Appendix Table). We anticipated that our one-or-two-dose titration scheme would deliver an appropriate dose to each patient, maximizing efficacy while minimizing adverse events. This strategy may have been less effective due to delays, sometimes substantial, in administering the second dose of investigational medication. We did not use weight-based dosing in this study because we did not have research pharmacy resources available 24 hours of the day.
Second, due to constraints inherent in conducting clinical research in an active ED, we had some difficulty delivering the second dose of study medication in a timely manner to all patients. As demonstrated in Appendix 2, the elapsed time to the second dose of medication, in some cases, was so long that the efficacy was not captured in our primary outcome at 90 minutes. However, as demonstrated in Table 2, our conclusions would not have changed, even if our primary outcome was delayed until 120 minutes.
Third, our mechanism of blinding may have been inadequate. Patients administered hydromorphone were more likely to suspect that they received hydromorphone, while research associates were more likely to guess correctly that lidocaine patients received lidocaine (Appendix). The reason for this and its significance is not clear. If patients suspected that they received hydromorphone, this may have caused them to overstate the efficacy of hydromorphone. Alternatively, if it was the pain relief itself that allowed patients to surmise which medication they received, then the impact on the stated pain scores may have been minimal.
Fourth, we did not use a pain score cutoff for medication re-dosing. We merely asked patients if they would want another dose of the medication. Therefore, a between-group difference in pain tolerance may have impacted total dose of medication received, and thereby, the primary outcome.
Finally, this study took place in two urban academic emergency departments in the Bronx, NY, caring for an under-served inner-city population. It is uncertain whether these data can be generalized to other settings.
Discussion
In this ED-based, randomized comparative effectiveness trial, IV hydromorphone was substantially more efficacious than IV lidocaine for acute abdominal pain. Hydromorphone was also superior to lidocaine in a subset of patients with nephrolithiasis. While generally well-tolerated, IV lidocaine, on all measures, was substantially less efficacious for acute pain.
Hydromorphone, a standard of care for treatment of acute, new-onset, severe pain in the ED, has been shown repeatedly to be safe, effective, and well-tolerated.(2–6) Easily titratable and reversible, with a widely available antidote, there are no compelling, evidence-based reasons not to administer IV hydromorphone for severe abdominal pain in a monitored setting such as the ED. It was a highly effective analgesic in this study too, though approximately 1/3 of hydromorphone patients reported medication-related side-effects, and approximately 1/3 did not achieve a 50% improvement in pain by 90 minutes. These results are generally in keeping with published data, which have demonstrated that 1 mg doses of IV hydromorphone, administered repeatedly every 30–60 minutes over the first two hours of an ED course, are an effective way to achieve high levels of patient satisfaction with analgesic treatment.(5)
Our data do not support the use of a 120mg dose of IV lidocaine as a first-line analgesic for severe abdominal pain. Pain scores among those who received lidocaine were higher throughout the study period, need for additional analgesia occurred much more frequently among patients randomized to lidocaine versus hydromorphone, and on average, patients who received lidocaine reported less than a 50% reduction in pain. Our data do not provide any compelling reasons to choose IV lidocaine as a first-line analgesic over hydromorphone for abdominal pain. Placebo-controlled studies are needed to determine whether IV lidocaine should play any role in the management of ED patients with pain.
In other randomized, ED-based studies of visceral pain, pain scores after administration of IV lidocaine improved 45% by 60 minutes in a general pain study,(15) and 88% by 30 minutes in a study of kidney stones.(12) While the former results are generally consistent with the 40% improvement our lidocaine patients experienced at 60 minutes, the latter are substantially different, and may be related to dosing of the medication or differences in the patient populations.
IV lidocaine can cause serious adverse events such as hypotension, cardiac dysrhythmias, and seizures, although these are generally not seen at doses up to 5 mg/kg.(7) At doses less than 2 mg/kg, side effects are generally minor and transient (see Appendix Table). As demonstrated in our exploratory analysis in Appendix 1 and in the Appendix Table, initial doses of 2 mg/kg may have more efficacy than lower doses of IV lidocaine. The 2 mg/kg dose thus may be a reasonable starting point in future investigations of IV lidocaine and for patients who have contra-indications to IV opioids.
In conclusion, IV hydromorphone was more efficacious than 120mg doses of IV lidocaine, both for general abdominal pain and a subset of nephrolithiasis patients. Based on these data, lidocaine should not replace hydromorphone as a first-line analgesic.
Acknowledgments
This publication was supported in part by the Harold and Muriel Block Institute for Clinical and Translational Research at Einstein and Montefiore grant support (UL1TR001073)
Appendix 1. Optimizing the dose of IV lidocaine
Among patients who received IV lidocaine, there was an inverse association between weight and improvement in 0–10 pain score between baseline and 90 minutes: r2 = 0.10
To understand the clinical significance of this association, we divided our dataset into thirds based on weight and compared mean improvement in 0–10 pain score between baseline and 90 minutes among the different groups of lidocaine patients.
60–73 kg: 5.0 (95% CI: 3.8, 6.2)
73–85 kg: 3.0 (95% CI: 1.7, 4.3)
85–120 kg: 3.1 (95% CI: 2.1, 4.0)
These data suggest to us that, if used for acute pain, the initial dose of IV lidocaine should be 2 mg/kg. Patients in the middle tertile (approximately 1.5mg/kg) and the heaviest tertile (< 1.5mg/kg) did not benefit as much.
IV lidocaine can cause serious adverse events such as hypotension, cardiac dysrhythmias, and seizures, although these are generally not seen at doses up to 5 mg/kg.(7)
Appendix 2. Elapsed time to initiation of second dose of medication. (Time 0 was initiation of the first dose of investigational medication).
Hydromorphone arm. 15 patients requested a second dose of investigational medication.
Median= 51 minutes, IQR= 48, 65 minutes
Number of patients who received the second dose of medication beyond 70 minutes: 1 (85 minutes)
Lidocaine arm. 30 patients requested a second dose of investigational medication.
Median =55 minutes, IQR= 50, 78 minutes
Number of patients who received the second dose of medication beyond 70 minutes: 8 (78 minutes × 2, 79 minutes, 80 minutes × 2, 82 minutes, 102 minutes, 148 minutes)
Appendix 3. Imputed primary outcome (90 minute) data
| Study ID | Actual 45 minute data | Actual 60 minute data | Actual 120 minute data | Actual 180 minute data | Imputed 90 minute value |
|---|---|---|---|---|---|
| 94 | 8.0 | 8.0 | 5.0 | 5.0 | 6.5 |
| 122 | 6.0 | 7.0 | 6.0 | 5.0 | 6.5 |
| 125 | 6.0 | 5.0 | 4.0 | 4.0 | 4.5 |
Appendix 4. What happens to the primary outcome when nephrolithiasis patients are removed?
Improvement in Baseline −90 minute pain scores, hydromorphone arm: 4.8 (SD=2.9)
Improvement in Baseline – 90 minute pain scores, lidocaine arm: 3.8 (SD=3.0)
Rounded difference: 0.9 (95%CI: −0.1, 1.9)
Appendix 5. An assessment of the adequacy of blinding
Prior to discharge, patients were asked to guess which medication they received
| Patient’s guess: | Lidocaine (n=77) | Hydromrophone (n=77) |
|---|---|---|
| I think I got lidocaine | 37 (54%) | 27 (38%) |
| I think I got hydromorphone | 32 (46%) | 45 (63%) |
| Missing | 8 | 5 |
The research associates were also asked to guess which medication the patient received.
| Research associate’s guess: | Lidocaine (n=77) | Hydromrophone (n=77) |
|---|---|---|
| I think s/he got lidocaine | 51 (70%) | 30 (42%) |
| I think s/he got hydromorphone | 22 (30%) | 42 (58%) |
| Missing | 4 | 5 |
Appendix Figure.
0–10 pain scores at various time points
Appendix Table.
ED-based randomized studies of IV lidocaine for acute pain
| Author/year | Etiology of pain | Dose of IV lidocaine | Comparator | Primary outcome | Secondary outcome | Adverse effects of lidocaine |
|---|---|---|---|---|---|---|
| Clattenburg, 2018(15) | Severe pain | <50 kg = 75 mg, 50–100 kg =100 mg, >100 kg = 150 mg, over 10 minutes, followed by drip of same dose over 50 minutes | Morphine, provider directed dose | Pain at 60 minutes: No difference | Need for rescue medication: No difference | 2/16 with transient side effects |
| Forouzan, 2017(14) | Long bone fracture | 1.5 mg/kg over 2 minutes | Morphine 0.1 mg/kg | 3 point improvement in pain score at 30 minutes: Lidocaine superior | None | Serious adverse events: None |
| Firouzian, 2016(17) | Renal colic | 1.5 mg/kg + morphine 0.1 mg/kg | Morphine 0.1 mg/kg | VAS at intervals up to 120 minutes: No difference | None | None |
| Tanen, 2014(18) | Acute radicular low back pain | 100 mg over 2 minutes | Ketorolac 30 mg | Pain at 60 minutes: No difference | Rescue medication: No difference | Not reported |
| Vahidi, 2014(13) | Critical limb ischemia | 2 mg/kg over 5 minutes | Morphine 0.1 mg/kg | VAS at 30 minutes: Lidocaine superior | None | None |
| Soleimanpour, 2012(12) | Renal colic | 1.5 mg/kg + metoclopramide 0.15 mg/kg | Morphine 0.1 mg/kg + Metoclopramide 0.15 mg/kg | Pain score < 3: Lidocaine superior | None | Perioral numbness (3/120); Transient dizziness (10/120); Transient dysarthria (2/120) |
| Reutens, 1991(19) | Migraine | 1mg/kg over 2 minutes | Normal saline | VAS at 10 and 20 minutes: No difference | Rescue medication: No difference | None |
| Bell, 1990(20) | Migraine | 50 mg initially. Could receive two additional doses of 50 mg at 20 minute intervals |
1) Dihydroergotamine 1 mg IV, re-dose
available at 30 minutes 2) Chloropromazine 12.5 mg, allowed 2 re-doses |
Pain at one hour: Lidocaine comparable to dihydroergotamine; inferior to chlorpromazine | Would use again: Lidocaine Comparable to dihydroergotamine; Inferior to chlorpromazine, | 5/17 reported minor adverse events |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to report.
References
- 1.Rui P, Kang K; Pages https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf on September 22, 2018 2018.
- 2.Chang AK, Bijur PE, Campbell CM, Murphy MK, Gallagher EJ. Safety and efficacy of rapid titration using 1mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: the “1+1” protocol. Ann Emerg Med. 2009;54(2):221–5. [DOI] [PubMed] [Google Scholar]
- 3.Chang AK, Bijur PE, Davitt M, Gallagher EJ. Randomized clinical trial comparing a patient-driven titration protocol of intravenous hydromorphone with traditional physician-driven management of emergency department patients with acute severe pain. Ann Emerg Med. 2009;54(4):561–7 e2. [DOI] [PubMed] [Google Scholar]
- 4.Chang AK, Bijur PE, Gallagher EJ. Randomized clinical trial comparing the safety and efficacy of a hydromorphone titration protocol to usual care in the management of adult emergency department patients with acute severe pain. Ann Emerg Med. 2011;58(4):352–9. [DOI] [PubMed] [Google Scholar]
- 5.Chang AK, Bijur PE, Holden L, Gallagher EJ. Efficacy of an Acute Pain Titration Protocol Driven by Patient Response to a Simple Query: Do You Want More Pain Medication? Ann Emerg Med. 2016;67(5):565–72. [DOI] [PubMed] [Google Scholar]
- 6.Chang AK, Bijur PE, Napolitano A, Lupow J, Gallagher EJ. Two milligrams i.v. hydromorphone is efficacious for treating pain but is associated with oxygen desaturation. J Opioid Manag. 2009;5(2):75–80. [DOI] [PubMed] [Google Scholar]
- 7.LOJ ES, Scherber K, Cabrera D, Motov S, Erwin PJ, West CP, et al. Safety and Efficacy of Intravenous Lidocaine for Pain Management in the Emergency Department: A Systematic Review. Ann Emerg Med. 2018;72(2):135–44 e3. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert CR, Hanson IR, Brown AB, Hingson RA. Intravenous use of xylocaine. Curr Res Anesth Analg. 1951;30(6):301–13. [PubMed] [Google Scholar]
- 9.Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg. 2005;101(6):1738–49. [DOI] [PubMed] [Google Scholar]
- 10.Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventham NT, Kennedy ED, Brady RR, Paterson HM, Speake D, Foo I, et al. Efficacy of Intravenous Lidocaine for Postoperative Analgesia Following Laparoscopic Surgery: A Meta-Analysis. World J Surg. 2015;39(9):2220–34. [DOI] [PubMed] [Google Scholar]
- 12.Soleimanpour H, Hassanzadeh K, Vaezi H, Golzari SE, Esfanjani RM, Soleimanpour M. Effectiveness of intravenous lidocaine versus intravenous morphine for patients with renal colic in the emergency department. BMC Urol. 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahidi E, Shakoor D, Aghaie Meybodi M, Saeedi M. Comparison of intravenous lidocaine versus morphine in alleviating pain in patients with critical limb ischaemia. Emerg Med J. 2015;32(7):516–9. [DOI] [PubMed] [Google Scholar]
- 14.Forouzan A, Barzegari H, Motamed H, Khavanin A, Shiri H. Intravenous Lidocaine versus Morphine Sulfate in Pain Management for Extremity Fractures; a Clinical Trial. Emerg (Tehran). 2017;5(1):e68. [PMC free article] [PubMed] [Google Scholar]
- 15.Clattenburg EJ, Nguyen A, Yoo T, Flores S, Hailozian C, Louie D, et al. Intravenous Lidocaine Provides Similar Analgesia to Intravenous Morphine for Undifferentiated Severe Pain in the Emergency Department: A Pilot, Unblinded Randomized Controlled Trial. Pain Med. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Green SM, Krauss BS. The Numeric Scoring of Pain: This Practice Rates a Zero Out of Ten. Ann Emerg Med. 2016;67(5):573–5. [DOI] [PubMed] [Google Scholar]
- 17.Firouzian A, Alipour A, Rashidian Dezfouli H, Zamani Kiasari A, Gholipour Baradari A, Emami Zeydi A, et al. Does lidocaine as an adjuvant to morphine improve pain relief in patients presenting to the ED with acute renal colic? A double-blind, randomized controlled trial. Am J Emerg Med. 2016;34(3):443–8. [DOI] [PubMed] [Google Scholar]
- 18.Tanen DA, Shimada M, Danish DC, Dos Santos F, Makela M, Riffenburgh RH. Intravenous lidocaine for the emergency department treatment of acute radicular low back pain, a randomized controlled trial. J Emerg Med. 2014;47(1):119–24. [DOI] [PubMed] [Google Scholar]
- 19.Reutens DC, Fatovich DM, Stewart-Wynne EG, Prentice DA. Is intravenous lidocaine clinically effective in acute migraine? Cephalalgia. 1991;11(6):245–7. [DOI] [PubMed] [Google Scholar]
- 20.Bell R, Montoya D, Shuaib A, Lee MA. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079–82. [DOI] [PubMed] [Google Scholar]