Abstract
Background
Aortic thrombosis represents a consequence of atherosclerotic disease. In few cases, it can be secondary to large vessel or infective vasculitis. More rarely, aortic thrombosis is the manifestation of a primary malignant neoplasm of the aortic wall. Aortic angiosarcoma is a rare tumour, its clinical presentation is often non-specific and associated signs and symptoms may vary greatly. An early diagnosis is difficult to reach and the presence of metastatic disease is not uncommon at the time of diagnosis. The prognosis is poor overall.
Case summary
We report the case of a female patient who presented to her GP because of fatigue, hyporexia, weight-loss, and anaemia. An ultrasound of the abdomen showed two small pancreatic lesions, confirmed and described as benign cystic pancreatic lesions on computed tomography (CT) imaging; an incidental thrombus in the superior mesenteric artery was also found on CT imaging. The thoracic CT identified a large thrombotic lesion in the descending thoracic aorta with significant narrowing of the aortic lumen and confirmed the presence of an osteolytic bone lesion on the VIII right rib, in the absence of atherosclerotic disease. Signs of increased metabolic activity in the aortic lumen and in the VIII posterior right rib were shown at a subsequent positron emission tomography. A CT-guided biopsy of the bone lesion was performed and at histology the diagnosis of metastatic angiosarcoma of the aortic wall was made.
Discussion
Aortic angiosarcoma is a rare cause of aortic thrombosis, to be taken into consideration in a patient with thrombotic lesions of the aorta in the absence of atherosclerotic disease. The differential diagnosis is difficult because of clinical presentation and radiological features similar to those of inflammatory aortic disease. In our case, the final diagnosis of angiosarcoma was made only by a biopsy of a bone metastatic lesion.
Keywords: Aortic angiosarcoma, Aortic thrombosis, Primary aortic tumour, Case report
Learning points
A mesenteric artery thrombosis can represent the first sign of primary malignant aortic angiosarcoma.
Diagnostic imaging is crucial to characterize a non-atherosclerotic aortic thrombus.
A suspicious of primary aortic angiosarcoma must be confirmed through histology.
The interplay with different specialists is essential in very rare diseases to ensure the best clinical management.
Introduction
Aortic diseases are heterogeneous and include aneurysms, aortic dissection, atherosclerotic ulcers, and less common diseases such as Marfan syndrome, congenital abnormalities, or primary tumours. Aortic diseases may be diagnosed after a long period of subclinical development.1
Aortic angiosarcoma is a malignant neoplasm, and nearly 190 cases of aortic sarcoma have been reported in literature so far.2 In most cases, the lesion involves the intima. The clinical presentation is heterogeneous, and patients most frequently report claudication, abdominal, and/or back pain. The development of hypertension, aneurysm, and pseudoaneurysm are the most frequent findings.
Computed tomography (CT), magnetic resonance imaging, and positron emission tomography (PET) are useful to detect the presence of an aortic angiosarcoma, but the gold standard for the diagnosis of a primary malignant aortic tumour is the histological examination, including immunohistochemical analysis.
In most cases, early diagnosis is challenging because of the non-specific clinical presentation, late onset of symptoms, being the disease rare. The tumour is aggressive and more than 50% of the patients have metastatic disease at the time of diagnosis.
Timeline
| Day 1 | Hospitalization for a recent onset of palpitations, fatigue, epigastric pain, and vomiting. |
| Day 3 | Incidental finding of a mesenteric arterial thrombosis on abdominal computed tomography (CT). |
| Day 5 | Pathological glucose uptake in the descending aorta lumen and at the level of the 8th right rib (positron emission tomography). |
| Day 6 | Obstructive thrombosis and thickening of arterial wall in the descending aorta (thoracic CT). |
| Day 10 | Aortic thrombosis and aortic wall enlargement at magnetic resonance imaging. |
| Day 12 | Computed tomography-guided biopsy of the osteolytic lesion of the rib (metastatic lesion?). |
| Day 20 | Histological diagnosis of metastatic angiosarcoma of the aortic wall. |
| 1 day after diagnosis | Start oral propranolol (anti-proliferative effect); multidisciplinary team excluded surgical procedure. |
| 1 week after diagnosis | Start of palliative radiotherapy on metastatic lesion. |
| 10 days after diagnosis | Start of chemotherapy with Paclitaxel (five cycles completed). |
| 7 months after diagnosis | Patient passed away after rapid progression of the disease. |
Case presentation
A 78-year-old woman was admitted to the internal medicine department because of a recent onset of palpitations, fatigue, epigastric pain, and vomiting. She was on antihypertensive treatment, and she had a diagnosis of hypothyroidism secondary to a previous thyroiditis. An urticarial vasculitis had been diagnosed when she was 75.
At admission, blood pressure (BP) was 140/70 mmHg, heart rate 90 b.p.m., with a regular pulse, temperature 36.2°C, oxygen saturation 96% in room air, and she was not in pain; body weight was 47 kg and height was 152 cm. Thoracic, abdominal, and neurological examinations were normal, heart sounds were normal without murmurs and peripheral pulses were normal, without arterial bruits appreciable. Electrocardiogram (ECG) confirmed sinus rhythm. Laboratory exams displayed increased C-reactive protein and erythrocyte sedimentation rate without clinical evidence of infection or inflammatory disease. Other laboratory test results are shown in Table1.
Table 1.
This showing laboratory parameters measured at admission
| Laboratory parameters | At admission | Normal range |
|---|---|---|
| White blood cells (*103/mm3) | 8.79 | 4–10.8 |
| Red blood cells (*106/mm3) | 4.33 | 4–5.2 |
| Haemoglobin (g/dL) | 10.8 | 12–16 |
| MCV (fL) | 77.9 | 82–99 |
| Platelets (*103/mm3) | 402 | 130–400 |
| Troponin I (ng/mL) | <0.015 | <0.045 |
| Creatine kinase (U/L) | 42 | 43–217 |
| Glucose (mg/dL) | 77 | 70–100 |
| INR | 1.2 | 0.8–1.2 |
| Partial thromboplastin time (pTT) (s) | 30.4 | 24–38 |
| Sodium (mmol/L) | 137 | 138–144 |
| Potassium (mmol/L) | 4.6 | 3.3–4.7 |
| Chloride (mmol/L) | 98 | 100–107 |
| Calcium (mg/dL) | 9.45 | 8.1–9.8 |
| Creatinine (mg/dL) | 1.13 | 0.5–0.95 |
| C-reactive protein (mg/L) | 85.1 | <5 |
| Erythrocyte sedimentation rate (mm) | 71 | 3–46 |
| Total bilirubin (mg/dL) | 0.37 | 0.2–1 |
| LDH (U/L) | 225 | 136–234 |
| Aspartate transaminase (U/L) | 17 | 9–31 |
| Alanine transaminase (U/L) | 16 | 11–29 |
| γ-glutamyl transferase (U/L) | 50 | 10–38 |
| Alkaline phosphatase (U/L) | 70 | 26–98 |
| Urine sediment | Negative |
INR, international normalized ratio; LDH, lactate dehydrogenase; MCV, mean corpuscular volume.
In consideration of the weight loss (4 kg), fatigue and the presence of anaemia, an ultrasound of the abdomen was performed, showing few hypo-echogenic pancreatic lesions. At a subsequent abdomen contrast-enhanced CT the pancreatic lesions were described as uncomplicated cysts, and a thrombus in the superior mesenteric artery with collateral reperfusion was observed (Figure1).
Figure 1.
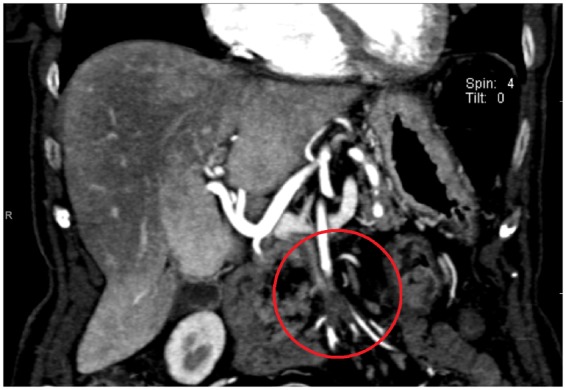
Abdomen contrast-enhanced computed tomography image showing a thrombosis of superior mesenteric artery and collateral reperfusion (circle).
Thromboembolism (for instance embolization in a visceral vessel from a thrombus in aorta or cardio-embolic origin), thrombophilia, or vasculitis may be the cause of a thrombosis of the superior mesenteric artery.
The patient had no previous finding of atherosclerotic diseases and only one cardiovascular risk factor (hypertension), with documented good control of BP values by antihypertensive treatment. An ECG-Holter monitoring showed sinus rhythm throughout the 24 h of recording, in the absence of arrhythmic episodes or ‘silent’ atrial fibrillation. A transthoracic echocardiogram showed normal cardiac chambers dimensions, normal wall thicknesses, and regional contractility, with a 50% ejection fraction, and normal aortic, mitral, and tricuspid valves structure.
Lupus anti-coagulant, anti-phospholipid antibodies (including anti-β2GPI and anti-cardiolipin), and homocysteinaemia normal values excluded thrombophilia. Other autoimmunity tests, including antinuclear antibodies, complement fractions consumption, anti-neutrophil cytoplasmic antibodies, anti-extractable nuclear antigen, and cryoglobulins were all negative.
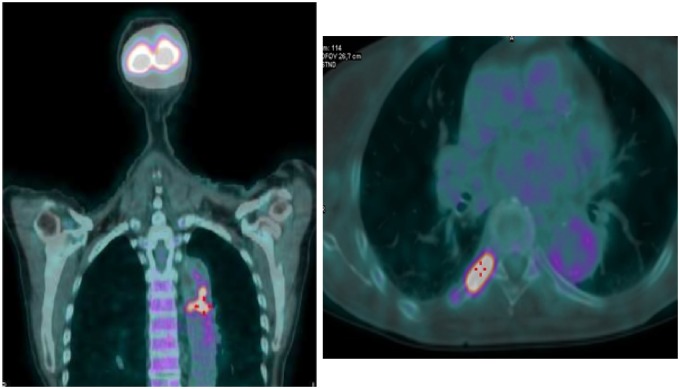
The patient underwent a fludeoxyglucose (18-F) PET in order to investigate the presence of vasculitis, in particular Takayasu’s arteritis, giant cell arteritis, Behcet disease, IgG4 related-disease, or Cogan syndrome. An intense tracer signal was observed in the lumen of the thoracic aorta, suggesting the presence of endovascular thrombus, due to an inflammatory process or a neoplastic disease; in addition, intense metabolic activity was described at the level of the VIII posterior right rib, suggesting a neoplastic process (Figure 2).
Figure 2.
Fludeoxyglucose-positron emission tomography showing intense fixation of the tracer in the thoracic aortic lumen (left side) and fixation of the tracer was at VIII posterior right rib (right side).
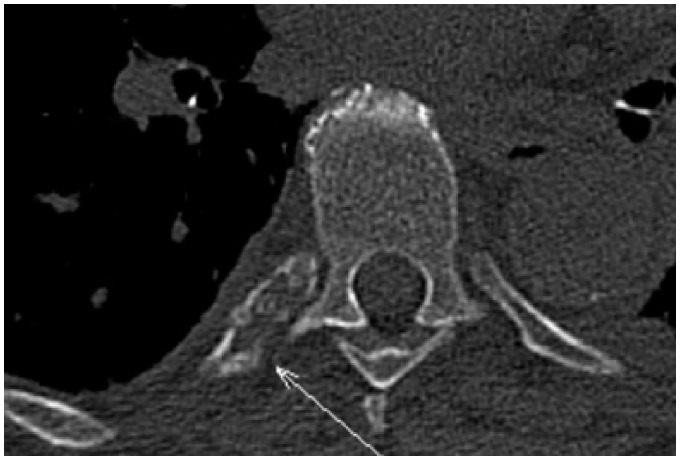
Subsequently, a thorax and abdomen contrast-enhanced CT was performed to evaluate the entire thoracic and abdominal aorta. The CT showed the presence of a large aortic thrombus (7 cm in length) in the descending thoracic tract, causing a focal sub-occlusion (6 mm in diameter), with thickening of the aortic wall, but not atherosclerotic plaques. The rib osteolytic lesion was also confirmed on CT imaging (Figures3 and 4).
Figure 3.
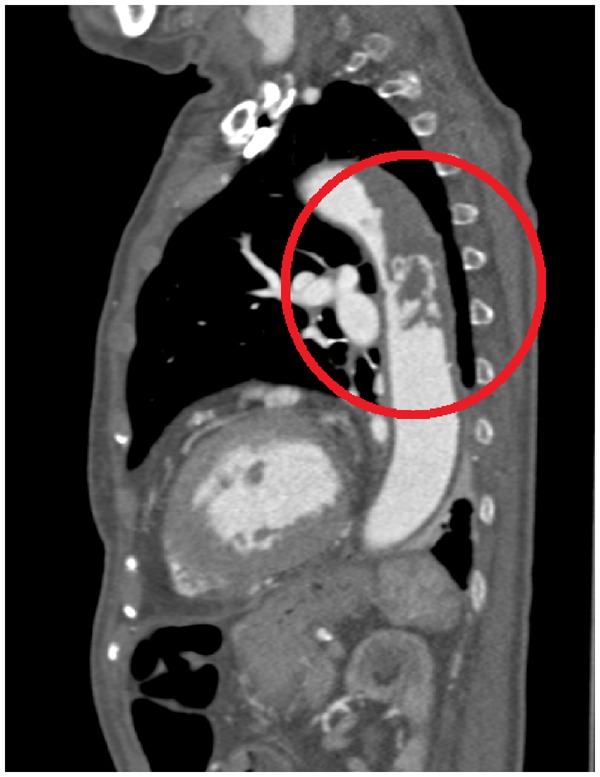
Thoracic contrast-enhanced computed tomography image showing the presence of a large aortic in the descending thoracic tract (circle).
Figure 4.
Skeletal reconstruction computed tomography image showing osteolytic bone lesion at VIII posterior right rib (arrow).
Therefore, two apparently different clinical conditions were to be considered: a large vessel vasculitis and a skeletal neoplastic lesion of unknown origin.
At serum protein electrophoresis a monoclonal gammopathy was shown with a double monoclonal component IgGk and IgGλ at immunofixation, possibly suggesting multiple myeloma. Therefore, a bone marrow biopsy was performed, showing a low percentage of plasma cells and excluding the diagnostic hypothesis of multiple myeloma.
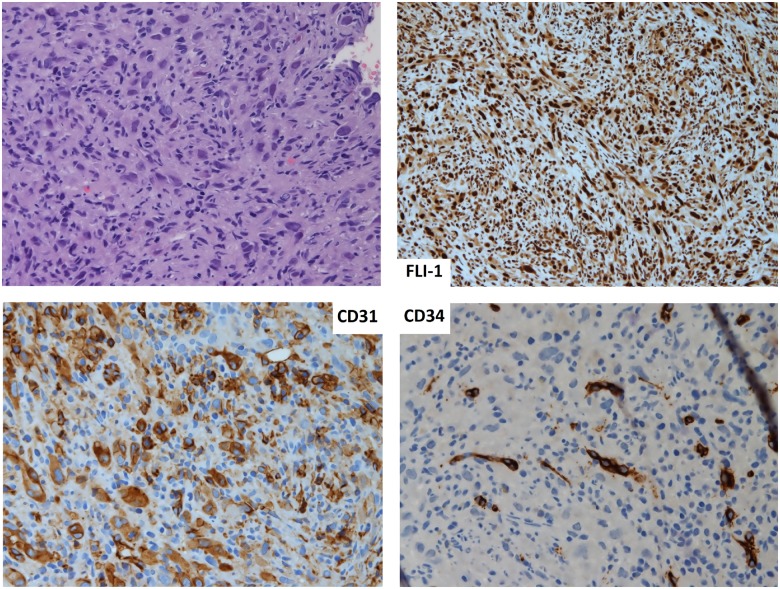
At magnetic resonance, performed in order to more precisely assess the thrombus and the aortic wall structure, the presence of a large size thrombus, with irregular and sub-occluding profile was confirmed, with a clear thickening of the aortic wall and oedema at the site of the thrombus (Figure5). In addition, the patient underwent a CT-guided biopsy of the VIII right rib to evaluate the origin of the metastatic bone lesion; at histology the features of a vascular neoplasm, mostly compatible with an angiosarcoma were described: high cell density including inflammatory cells and atypical cells, with larger size, epithelioid and fusate morphology, pleomorphic and irregular nucleus, hypodense chromatin, and eosinophilic nucleolus. The immunohistochemical analysis showed positivity for endothelial markers (CD-31, WT-1, FLI-1) (Figure 6).
Figure 5.
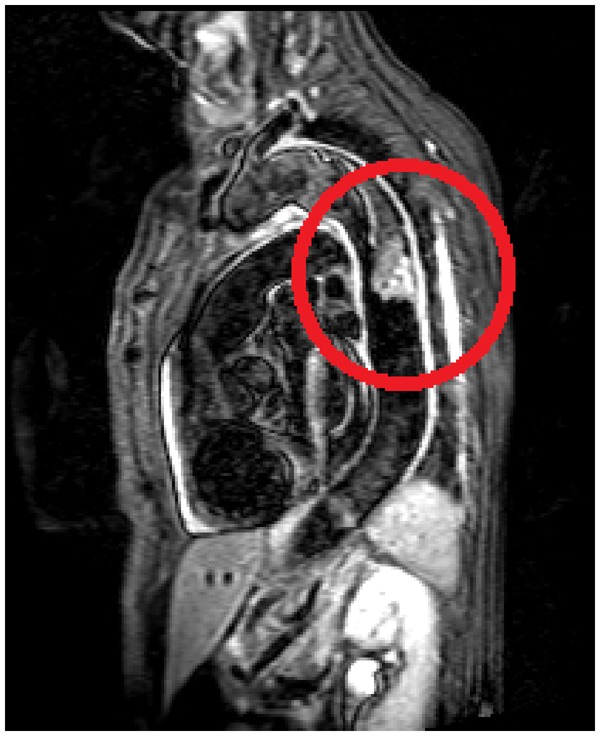
Magnetic resonance imaging image showing aortic wall thickened and frankly oedematous.
Figure 6.
Histological images of bone biopsy. Top left: haematoxylin–eosin staining of sample. Top right: immunohistochemical for FLI-1. Down left: immunohistochemical for CD31. Down right: immunohistochemical for CD34.
After a multidisciplinary discussion, including the vascular surgeon, the oncologist, and the internist, the surgical removal of the neoplastic thrombus was ruled out because of the presence of metastasis and chemotherapy, based on the oral administration of propranolol and paclitaxel, was started. The patient underwent irradiation of the rib and completed five cycles of treatment with paclitaxel.
Despite treatment, the patient died due to the progression of the disease, 7 months after the diagnosis.
Discussion
Aortic sarcoma is a rare tumour; there are only 190 cases reported in literature up to now. The first case was described in 1873 by Brodowski.3
Aortic sarcoma can be classified into intimal angiosarcoma (of endothelial origin), intimal myofibroblastic sarcoma (of mesenchymal origin), and mural sarcoma. The most common site of origin is the intima and the most common location is the descending thoracic aorta, as in our case report. The presence of metastasis is not uncommon at time of diagnosis, and the main sites of metastasis are the bone, the lungs, the liver, the skin, and the adrenal glands.
An early diagnosis is difficult and more than 25% of cases are detected at autopsy.4 Our patient was a 78-year-old woman, while the median age of onset is 62.2 years and the disease is more common in men.5
The clinical presentation is highly variable. In a pooled analysis published in 20144 patients presented with acute arterial embolization in 20.6% of cases, claudication (18.8%), abdominal complaints (12.7%), back pain (9.1%), constitutional symptoms (8.5%), aortic dissection, or rupture (6.1%). An arterial peripheral embolus has been reported in one-third of patients and about 68% of distal embolizations occur at an intra-abdominal site.4
The patient we described complained only of fatigue, loss of appetite, and weight loss, although the occlusion of the superior mesenteric artery did not reduce visceral blood flow, maintained by collateral reperfusion.
The wide spectrum of potential causes of arterial thrombosis requires several instrumental examinations and procedures in order to achieve the correct diagnosis.
Positron emission tomography offers substantial benefits in the diagnostic work-up, since it investigates the potential inflammatory activity due to large vessel vasculitis or neoplastic thrombosis. Contrast-enhanced CT is essential in evaluating aortic and large to medium sized arteries and the presence of atherosclerotic disease features, including thrombosis and plaques. Magnetic resonance imaging provides three-dimensional high-resolution imaging and has been shown to be superior in differentiating intima sarcoma from atherosclerotic disease, based on the features and extent of aortic wall involvement.6,7
Some authors have reported that real-time three-dimensional transoesophageal echocardiography may useful to assess haemodynamic changes in the ascending aorta and aortic arch.8
Despite all these imaging methods offering some specific advantage in the assessment of aortic diseases, none of them represents a specific tool for a definitive diagnosis of angiosarcoma among vasculitis, arteritis, atheroma, and mural thrombus.9
The gold standard for a definitive diagnosis of angiosarcoma is represented by histopathology. In most patients previously described, the histopathological examination was performed on an aortic specimen after surgical resection. Intraortic biopsy is rarely performed for the potential embolic complications.2 In our case, the CT-guided biopsy of the bone metastasis was crucial to reach the correct diagnosis.
Histological features of aortic angiosarcoma are high cell density including inflammatory cells and atypical cells, epithelioid and fusate morphology, with pleomorphic and irregular nucleus, chromatin hypodense,5 and all of them were observed in our patient.
The immunohistochemical analysis of intimal angiosarcoma shows positivity for endothelial markers including CD-31, WT-1, FLI-1, CD-34, and positivity for factor VIII.6 In a review of 13 clinical cases, CD31 was reported as the most common marker, found in more than half of the patients.6 In our patient positivity for CD-31, FLI-1 and factor VIII was reported, while CD-34, S100 and desmin were all negative.
The worst prognostic marker of angiosarcoma is the presence of metastatic disease. In fact, the absence of metastasis at diagnosis is associated to a median survival of 20 months while patients with advanced disease, even after complete resection of the neoplasm, have a median survival of 6 months.4 Overall the prognosis is poor and the estimated 1-, 3-, and 5-year survival rates are 46.7%, 17.1%, and 8.8%, respectively.4
Surgical resection is the treatment of choice for patients with an angiosarcoma confined to the aorta, while only palliative surgery may be considered in the presence of metastatic tumours. Radiation therapy may be indicated for pain control, more often for bone metastasis.
Chemotherapy was shown to be associated with improvement of median survival in some patients’ series, but its role remains controversial.10 More recently, the use of paclitaxel has been proposed.11
The surgical option was definitively ruled-out in our patient because of the presence of metastasis, and the decision was taken during a multidisciplinary discussion among different specialists and vascular surgeons.
The patient received chemotherapy, based on oral administration of propranolol, a non-selective β-adrenergic receptor (β-AR) antagonist, and paclitaxel, in addition to radiation on the bone lesion.12
Propranolol exhibits a selective cytotoxicity and tumour suppressive ability towards angiosarcoma. Several studies have reported a β-AR expression on vascular tumours, including angiosarcoma13; in addition, in vitro studies have shown potential benefits from the inhibition of β2 and β3 receptor on tumour cells by non-selective β blockers.14
Therefore, it has been proposed that the use of relatively safe non-selective β-AR antagonists along with traditional treatment could improve prognosis for patients with advanced disease.
Unfortunately, our patient died because of further progression of the disease, 7 months after the diagnosis was made.
Conclusion
A mesenteric artery thrombosis was the first finding that led us to the diagnosis of a primary malignant aortic tumour. In patients with splanchnic emboli, an aortic sarcoma should be included in the differential diagnosis, in particular in patients with mild or absent underlying atherosclerotic disease. The extremely low incidence and the atypical clinical presentation of aortic angiosarcoma imply great efforts in obtaining the correct diagnosis. A synergistic interplay between different specialists (vascular surgeon, oncologist, and internist) is essential to ensure the best clinical management.
Lead author biography

Maria Lorenza Muiesan, MD, Full Professor of Internal Medicine, Medical and Surgical Sciences Department, University of Brescia and Director of Internal Medicine Unit ASST Spedali Civili Brescia, University of Brescia and Postgraduate Specialization School in Emergency Medicine. She is a member of the nucleus of the working group on aorta and peripheral artery disease of the European Society of Cardiology. Author of more than 250 publications on international peer review scientific journals. Editorial activity: Editorial board of the ‘Journal of Hypertension’ Reviewer for JAMA, Circulation, JACC, Hypertension, ATVB, Eur Heart J, Am J Hypertension, Journal of the American Society of Hypertension, J Clin Hypertension, Annals of Internal Medicine.
Supplementary Material
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 2. Wu Z-Y, Weng L-Q, Chen Z-G, Chen Y-X, Li Y-J.. Primary aortic sarcoma in arch and descending aorta: a case report and literature review. J Thorac Dis 2018;10:E289–E295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodowski, W. Primäres Sarkom der Aorta thoracica mit Verbreitung des Neugebildes in der unteren Körperhälfte. Jahresb Leistung Fortschr ges Med 1873;8:243–246. [Google Scholar]
- 4. Rusthoven CG, Liu AK, Bui MM, Schefter TE, Elias AD, Lu X, Gonzalez RJ.. Sarcomas of the aorta: a systematic review and pooled analysis of published reports. Ann Vasc Surg 2014;28:515–525. [DOI] [PubMed] [Google Scholar]
- 5. Thalheimer A, Fein M, Geissinger E, Franke S.. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg 2004;40:548–553. [DOI] [PubMed] [Google Scholar]
- 6. Fatima J, Duncan AA, Maleszewski JJ, Kalra M, Oderich GS, Gloviczki P, Suri RM, Bower TC.. Primary angiosarcoma of the aorta, great vessels, and the heart. J Vasc Surg 2013;57:756–764. [DOI] [PubMed] [Google Scholar]
- 7. Mohsen NA, Haber M, Urrutia VC, Nunes LW.. Intimal sarcoma of the aorta. AJR Am J Roentgenol 2000;175:1289–1290. [DOI] [PubMed] [Google Scholar]
- 8. Mecklai A, Rosenzweig B, Applebaum R, Axel L, Grossi E, Chan A, Saric M.. Intimal sarcoma in the aortic arch partially obstructing the aorta with metastasis to the brain. Tex Heart Inst J 2014;41:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin S-I, Su M-I, Tsai C-T.. Primary intimal sarcoma of thoracic aorta presenting as hypertensive crisis. Acta Cardiol Sin 2015;31:560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burke AP, Cowan D, Virmani R.. Primary sarcomas of the heart. Cancer 1992;69:387–395. [DOI] [PubMed] [Google Scholar]
- 11. Penel N, Italiano A, Ray-Coquard I, Chaigneau L, Delcambre C, Robin YM, Bui B, Bertucci F, Isambert N, Cupissol D, Bompas E, Bay JO, Duffaud F, Guillemet C, Blay JY.. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol 2012;23:517–523. [DOI] [PubMed] [Google Scholar]
- 12.Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hannu A, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, Van der Graaf W, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv51-iv67. [DOI] [PubMed] [Google Scholar]
- 13. Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE.. β-Adrenergic receptor expression in vascular tumors. Mod Pathol 2012;25:1446–1451. [DOI] [PubMed] [Google Scholar]
- 14. Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, Modiano JF, Kokta V, Boucheron LE, Mitchell DC, Bryan BA.. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One 2013;8:e60021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.