Abstract
Background
The aetiology of dilated cardiomyopathy (DCM) is highly heterogeneous including genetic and/or acquired (infective, toxic, immune, endocrine, and nutritional) factors. The major part of acquired DCM in developed countries is caused by either viral or autoimmune myocarditis. It is believed that the activation of the T-lymphocyte cell system is the major pathomechanism underlying autoimmune myocarditis and inflammatory DCM (DCMi). However, in the hearts of a subset of patients, a significant number of CD20+ B-lymphocytes can be detected too. Limited information exists on the role of B-cell-dependent mechanisms in the progression of DCMi. Particularly CD20+ B-lymphocytes, which can be targeted by anti-CD20+ B-lymphocytes antibodies or inhibitors, might contribute to the pathogenesis of myocardial damage beyond antibody production.
Case summary
Here, we present a case series of six patients with subacute and chronic endomyocardial biopsy-proven CD20+ B-lymphocyte-associated DCMi, where symptomatic heart failure therapy, with or without combined immunosuppressive therapy with steroid-based treatment regime, was insufficient to improve cardiac function. Five patients improved clinically several weeks after a standard infusion protocol with rituximab, a chimeric monoclonal antibody against the pan-B-cell surface molecule CD20.
Discussion
Our case series shows that CD20+ B-lymphocyte persistence can play a pathophysiologic role in a subset of DCMi patients and highlights the potential of targeting CD20+ B cells in patients with prominent CD20+ B-lymphocyte persistence.
Keywords: Inflammatory dilated cardiomyopathy, CD20+, B-lymphocytes, Rituximab, Case report
Learning points
Measurement of CD20+ B-lymphocytes should be included in the routine endomyocardial biopsies diagnostics.
CD20+ B-lymphocyte persistence can be the reason for steroid-refractory inflammatory dilated cardiomyopathy.
CD20+ B-lymphocyte persistence can be targeted by CD20 inhibitors or antibodies like rituximab.
Introduction
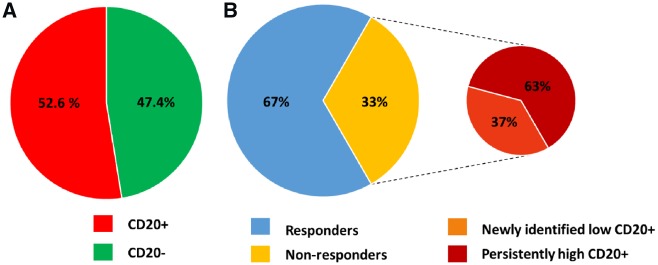
The major part of acquired dilated cardiomyopathy (DCM) in developed countries is caused by either viral or autoimmune myocarditis.1–3 About 30% of myocarditis cases, myocardial inflammation does not resolve but progresses into chronic inflammatory DCM (DCMi).4,5 Particularly in patients with histologically confirmed ongoing inflammation in the absence of viral persistence, an abnormal myocardial immune response with or without autoantibodies are prone to progress to DCMi.4,6 It is believed that the major pathomechanisms in immune myocarditis and DCMi involve the activation of the T-lymphocyte system (like CD4+, CD8+, CD3+ cells) and macrophages (like CD86+ cells), which can be targeted by immunosuppressive interventions including steroid-based treatment regimens.4,7,8 With respect to CD20+ B-lymphocytes, we analysed a panel of 156 endomyocardial biopsies (EMB) from DCMi patients and found that 52.6% displayed in addition to T lymphocytes a presence of more than seven CD20+ cells/mm2 (42% >10 cells/mm2). Immunohistochemical examinations of the EMB were carried out at the Institute of Cardiac Diagnostics and Therapies ‘IKDT’ according to a standard procedure.9 In a subset of DCMi patients, a prominent presence of CD20+ B-lymphocytes was detected (28.5% >20 cells/mm2) (Figure 1A). Importantly, CD20+ staining was independent from the antibody-producing CD138+ plasma cells (Figure 2). Our patient registry shows that prednisolone/azathioprine therapy was ineffective in circa 33% of EMB-proven DCMi patients despite having no underlying viral cause. Looking at CD20+ B-lymphocytes, 63% of the non-responders had persistently high counts (average 20.8 cells/mm2), the other 37% had low counts in the follow-up biopsies only (average 12.50 cells/mm2) (Figure 1B). Limited information exists on the role of B-cell-dependent mechanisms in the progression of DCMi. However, there is accumulating evidence demonstrating that CD20+ B-lymphocytes contribute to the pathogenesis of myocardial damage directly by their own secretome and by aggravating the T-cell system.10–14 Recently, B-lymphocytes were shown to aggravate myocardial inflammation via suppressing the anti-inflammatory M2 macrophages.15 Therefore, we hypothesized that CD20+ B-lymphocytes can contribute independently of the T-cell system to the course of DCMi and may belong to a subclass of DCMi’s, which could benefit from an intervention with rituximab (RTX), a chimeric monoclonal antibody against the pan-B-cell surface molecule CD20.
Figure 1.
Pie graph representations of CD20+ B-lymphocytes-association with inflammatory dilated cardiomyopathy. (A) The pie chart represents 82 patients (red) with endomyocardial biopsies CD20+ B-lymphocytes infiltrates (>7 cells/mm2) out of 156 inflammatory dilated cardiomyopathy patients. (B) The main pie chart represents 24 endomyocardial biopsies-proven inflammatory dilated cardiomyopathy patients who were treated with prednisolone/azathioprine for 6 months, 16 patients (blue) were responders and eight patients (yellow) were non-responders. The pie-of-pie represents the steroid non-responders of which five patients (dark red) showed high-persistent endomyocardial biopsies CD20+ B-lymphocyte infiltrates (average 20.8 cells/mm2), and three patients (orange) with newly identified low-grade CD20+ B-lymphocytes in the follow-up biopsies (average 12.50 cells/mm2).
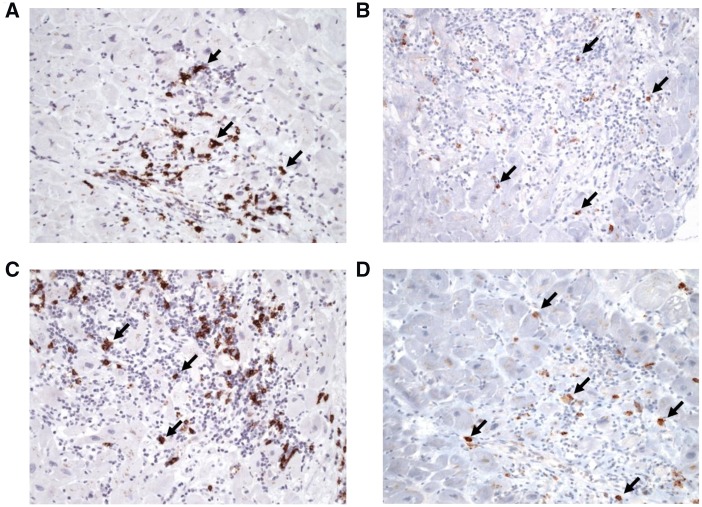
Figure 2.
Representative pictures of CD20+ B-lymphocytes (A, C) and CD138+ plasma cells (B, D) infiltrates in paraffin-embedded endomyocardial biopsies of two patients with CD20+ myocarditis, indicating that the CD20 staining pattern differs from that from CD138 staining. Magnification x 200; CD 138: Anti-CD20: monoclonal mouse antibody, clone 8J662 (Fa. Biomol, Hamburg, Germany); Anti-CD138; clone B-A38 (Roche Diagnostics, Mannheim, Germany).
Case series
Here, we detail our experience with six patients who received RTX in a single patient use approach. Their characteristics are summarized in the Table 1. A standard protocol efficient to deplete B cells in approved indications, consisting of two administrations of 375 mg/m2 RTX (MabThera® Roche Pharma AG) and 150 mg cortisone (to prevent infusion-related reactions) in a 4-week interval, was applied in addition to standard heart failure therapy. No other immunosuppressive agents were administered throughout the RTX treatment period, aiming to exclusively target CD20+ lymphocytes. All administrations were safe and well tolerated in these six patients. No unexpected infusion-related reactions or other treatment-emergent adverse drug reactions including infections occurred.
Table 1.
Baseline and post-treatment characteristics of the patients
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 56 | 68 | 76 | 70 | 73 | 47 |
| Sex | Female | Female | Male | Male | Female | Male |
| NYHA class at diagnosis | IV | III–IV | III | IV | III | II |
| NYHA class 8 weeks after treatment | II | I–II | II | III | II | II |
| LVEF at diagnosis (%) | 29 | 45 | 19 | 14 | 24 | 22 |
| LVEF 4 weeks after treatment (%) | 46 | 53 | 31 | 23 | 37 | 25 |
| LVEDD at diagnosis (mm) | 57 | 55 | 70 | 76 | 72 | 74 |
| LVEDD after treatment (mm) | 47 | 44 | 64 | 65 | 60 | 72 |
| CD20+ count in diagnostic biopsy (cells/mm2) | 20.25 | 633 | 11 | >7 | >7 | 10 |
| CD20+ count in follow-up biopsy (cells/mm2) | ND | ND | 6.52 | ND | ND | 17.5 |
| CD3+ count in diagnostic biopsy (cells/mm2) | 7.4 | 638 | 8.5 | 10.2 | 3.1 | 23 |
| CD3+ count in follow-up biopsy (cells/mm2) | 2.8 | 6.3 | 6.2 | 6 | 6.8 | 82 |
| Mac-1 count in diagnostic biopsy (cells/mm2) | 43 | 230 | 38.4 | 60.7 | 30.8 | 48.57 |
| Mac-1 count in follow-up biopsy (cells/mm2) | 16.3 | 30 | 43.3 | 21.1 | 52 | 120 |
| NT-proBNP at diagnosis (pg/mL) | 2544 | 3316 | 15 318 | 1555 | 7800 | 1329 |
| NT-proBNP after treatment (pg/mL) | 1293 | 362 | 11 814 | 2197 | 4230 | 789 |
LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; ND, not detectable (below technical detection limit); NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Patient 1
Patient 1 was a 56-year-old woman with newly diagnosed heart failure due to DCMi [New York Heart Association (NYHA) IV]. Coronary heart disease had been excluded by coronary angiography. One month later, transthorathic echocardiography (TTE) and angiography showed a mildly dilated left ventricle [left ventricular (LV) end-diastolic diameter (LVEDD) 57 mm] with a significantly reduced systolic function [ejection fraction (EF) 29%]. Cardiac function improved slightly after initiation of symptomatic heart failure therapy including a cardiac resynchronization therapy (CRT) system. Endomyocardial biopsies analyses showed a persistence of a significant number of CD20+ B-lymphocytes infiltrates slightly elevated macrophages and normal CD3+ T-lymphocytes without signs of viral genomes. Upon first and second RTX administration, LV function improved to EF 46% and 52%, respectively. CD3+ T-lymphocytes and macrophages (Mac-1) declined from 7.4 to 2.8 cells/mm2 and 43 to 16.3 cells/mm2, respectively, while CD20+ B-lymphocytes had resolved completely. Eight months after treatment, LVEDD had changed from 57 to 47 mm and a repeated stress TTE showed a further rise in EF from EF 52% (basal) to 63% after dobutamine. All parameters remained stable during the follow-up period of 12 months. NYHA status improved to Class II.
Patient 2
Patient 2 was a 68-year-old woman who had been treated with a Hodgkin-directed chemotherapy 9 years ago. During the last 9 years, cardiac function was documented as normal and stable. Shortly after pneumonia, she presented with a rapid onset of heart failure symptoms and TTE showed impaired systolic function (EF 25%; LVEDD 55 mm; NYHA III–IV). Endomyocardial biopsies were obtained to differentiate a chemotherapy induced-DCM from an inflammatory-driven cardiomyopathy after exclusion of coronary heart disease. Endomyocardial biopsies analyses revealed a massive CD3+ T-lymphocytes (376 cells/mm2) and CD20+ B-lymphocytes (780 cells/mm2) virus-negative acute myocarditis. An immunosuppressive therapy with steroids (prednisolone: initial dose 60 mg/day tapered by 10 mg bi-weekly for 6 months) and azathioprine (150 mg/day) led to an increase of EF up to 45%, whereas N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels decreased from 6621 to 3316 pg/mL. Two months later, the patient decompensated again, despite optimal medical treatment. A follow-up EMB still showed a persistent CD3+ T-lymphocytes- and CD20+ B-lymphocyte-inflammation (CD3: 638 cells/mm2 and CD20: 633 cells/mm2). Upon RTX, the EF improved from 45% to 53%. A control EMB showed normal CD3+ T-lymphocytes (6.3 cells/mm2), no CD20 infiltrates and a significantly reduced NT-proBNP of 362 pg/mL, respectively. The patient stayed stable (EF 57%, NYHA I–II) and NT-proBNP levels were nearly normal (282 pg/mL) 1 year later.
Patient 3
Patient 3 was a 76-year-old male with a 5-year history of DCM. Several EMBs analyses in the past 2 years excluded a significant existence of CD3+ T-lymphocyte infiltrates and macrophages. CD20+ B-lymphocytes had not been evaluated at that time. LV function declined over time from 28% to 19% (Supplementary material online, Video SA). The LV was dilated (LVEDD 70 mm). The next EMB, taken after a second cardiac decompensation despite optimal medical treatment, showed slightly elevated CD3+ T-lymphocytes and significantly elevated CD20+ B-lymphocyte infiltrates (11 cells/mm2), therefore, we initiated RTX therapy. Transthorathic echocardiography, before the second RTX-administration, revealed no changes in LV diastolic diameter but an increase in left ventricular ejection fraction (LVEF) compared with baseline from 19% to 31%. Yet, TTE at 6 and 12 months post the second dose of RTX showed an improved LV function and a smaller left ventricle (EF 36%; LVEDD 64 mm) (Supplementary material online, Video SB).
Patient 4
Patient 4 was a 70-year-old male patient with a 5-year history of EMB-proven DCMi who reported increased dyspnoea at exertion, despite standard heart failure therapy. The EF was initially 14% and a 76 mm LVEDD was recorded. A repeated EMB analysis showed classical CD20+ B-lymphocytes infiltrates. Whereas a 4-week post first dose examination showed no basal echocardiographic changes, an improvement of EF from 15 to 23% accompanied with a change in LVEDD (65 mm) was found at 6 months after the second RTX administration.
Patient 5
Patient 5 was a 73-year-old woman with a 4-year history of DCM. Initially, the EF was about 14%. Neither macrophages, T-lymphocytes, nor a significant persistence of cardiotropic viruses were detected in the first EMB. No specific treatment options could be offered. At that time, no investigation for CD20+ B-lymphocytes had been established. The EF improved up to 24% according to standard heart failure therapy, which included a CRT system. Over the years, she suffered more and more from heart failure symptoms, accompanied by a rise in NT-proBNP levels and a reduction in 6 min walking distance. The EF and LVEDD remained unchanged, but LV contractility reserve started to decline as documented by a stress TTE examination. Re-evaluation of her former taken EMBs showed a significant infiltration of CD20 B-lymphocytes, which were confirmed in a repeat EMB. At that time, RTX was initiated. We found a very rapid improvement of EF already after the first RTX infusion. A follow-up TTE prior to the second RTX administration showed a significantly smaller left ventricle (LVEDD: 72–60 mm) with an increased EF up to 37%, compared to baseline 4 weeks after treatment initiation. After the second dose administration, echocardiographic parameters remained stable. Without changes of the symptomatic heart failure therapy, NT-proBNP values decreased from 7800 to 4230 pg/mL, whereas NYHA functional class improved from III to II and the 6-min-walk test showed an improvement from 350 to 420 m.
Patient 6
Patient 6 was a 47-year-old man who suffered from a mild borderline myocarditis (EF 53%, LVEDD 63 mm) which had resolved spontaneously as documented by two follow-up biopsies 4 and 8 months after baseline EMB. LVEF remained mildly reduced (EF 49%, LVEDD 59 mm). An anti-inflammatory treatment and further biopsies were declined by the patient. Five years later, the patient presented with significant worsening of his cardiac function (EF 22%, LVEDD 72 mm) despite only mild heart failure symptoms (NYHA II). Endomyocardial biopsies revealed a pronounced virus-negative myocardial inflammation with elevated CD3 (107 cells/mm2) and CD20+ B-lymphocytes (9.2 cells/mm2). Upon a 6 months treatment with prednisolone and azathioprine systolic LV function did not improve (EF 22%, LVEDD 74 mm). CD3-positive-lymphocytes decreased moderately (23 cells/mm2), while CD20+ B cells remained unchanged (10 cells/mm2). Since B cells often decline when T cells are reduced spontaneously or upon treatment, we suggested a CD20+ B-lymphocyte-driven myocarditis and started a RTX intervention. The control EMB 4 months after treatment revealed a more focally clustered persistent inflammatory cardiomyopathy with increased T-lymphocytes (82 cells/mm2) and CD20+ B-lymphocytes (17.5 cells/mm2). Myocardial function remained severely restricted (EF 25%, LVEDD 72 mm).
Endomyocardial biopsies material from Patients 1 and 2 further allowed extra experimental insights. RTX treatment led to a reduction in cardiac inflammation as seen by a drop in the EMB mRNA expression of adhesion molecules (VCAM-1 and ICAM-1) as well as of the proinflammatory cytokines TNF-α and IL-18. Furthermore, in Patient 1, RTX led to a reduction in the activation of the innate immunity, which follows from the 3.0-, 6.5-, 5.8-, 5.6-, 6.3-, and 22-fold reduction in the mRNA expression of triggers and components of the NLRP3 inflammasome NOD-2, S100A9, S100A8, NLRP3, caspase 1, and IL1β, respectively. Similar observations were seen in Patient 2.
Discussion
Our case series describe for the first time the existence of a specific CD20+ B-lymphocyte-driven DCMi, which can profit from RTX. Recent experimental data foster the important role of B cells in myocardial remodelling.11,13,14,16 However, although CD20+ and CD138+ cells differed in their staining pattern, the reduction of CD20+ B-lymphocytes may indirectly limit the production of anti-myocardial antibodies and B-cell-mediated T-cell activation in the myocardium.11 Yet, the underlying mechanisms remain to be fully elucidated. It should be emphasized that individual outcomes are not representative, yet the pharmacodynamics of RTX in DCMi patients remains to be shown in a controlled clinical trial.
Patient 1 and 2 experienced the most distinct improvement as seen in TTE accompanied by an improvement in haemodynamic parameters. Patient 1 was the youngest subject who received RTX and had a short medical history of heart failure. Both factors may have contributed to this favourable outcome. However, a spontaneous self-healing mechanism cannot be completely excluded in this case.5 Patient 2, who initially showed massive CD3+ and CD20+ lymphocyte infiltrates and did not respond after immunosuppressive therapy with steroids and azathioprine, healed after RTX. Nevertheless, the cases 3–5 showed a disease onset, years before RTX initiation. A spontaneous healing mechanism can be excluded, especially since we found a very fast long-acting improvement of cardiac function shortly after initiation of RTX intervention. RTX administrations favourably influenced the course of the disease as shown by angiography, TTE, and haemodynamics, except for Patient 6, which we cannot explain and probably just indicate that also under RTX non-responder exists.
In conclusion, patients with prominent CD20+ B-lymphocytes persistence can profit from RTX. In addition, we generally recommend to include the evaluation of CD20+ B-lymphocyte presence into the routine EMB diagnostics.
Lead author biography

Univ. Prof. Dr. med. Carsten Tschöpe is the vise director of the Department of Cardiology, Charité, CVK and chair of the cardiology platform - Berlin Institute of Health Center for Regenerative Therapies. He is a Professor of medicine and cardiology. The main research areas of Prof. Tschöpe include inflammatory cardiomyopathy, immunocardiology and heart failure with preserved ejection fraction.
Supplementary Material
Acknowledgements
We thank Monika Willner, Kerstin Puhl, and Marzena Sosnowski for excellent technical assistance. We are thankful to the members and co-workers of the ‘Institut Kardiale Diagnostik und Therapie’ (IKDT, CEO: Prof Heinz—Peter Schultheiss), who did the biopsy routine analysing.
Funding
This work was supported by the Berlin-Brandenburg Center for Regenerative Therapies – (BCRT) sponsored by the German Federal Ministry of Education and Research (BMBF; 0313911) to C.T.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance. All patients have signed an informed consent according to the local ethics committee (application no. EA2/140/16).
Conflict of interest: An application patent has been filed for a CD20/CD19/CD21-directed therapy in DCMi (Europe: 10713861.2; USA: 13/264.090; registered applicant: Charité). All authors confirm that there is no conflict of interest to be declared.
References
- 1. Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L.. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018;39:1784–1793. [DOI] [PubMed] [Google Scholar]
- 2. Trachtenberg BH, Hare JM.. Inflammatory cardiomyopathic syndromes. Circ Res 2017;121:803–818. [DOI] [PubMed] [Google Scholar]
- 3. Seferovic PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH.. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:553–576. [DOI] [PubMed] [Google Scholar]
- 4. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 5. D'Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Di Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001;85:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Linthout S, Tschöpe C.. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 2017;14:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dominguez F, Kühl U, Pieske B, Garcia-Pavia P, Tschöpe C.. Update on myocarditis and inflammatory cardiomyopathy: reemergence of endomyocardial biopsy. Rev Esp Cardiol (Engl Ed) 2016;69:178–187. [DOI] [PubMed] [Google Scholar]
- 8. Merken J, Hazebroek M, Van Paassen P, Verdonschot J, Van Empel V, Knackstedt C, Abdul Hamid M, Seiler M, Kolb J, Hoermann P, Ensinger C, Brunner-La Rocca H-P, Poelzl G, Heymans S.. Immunosuppressive therapy improves both short- and long-term prognosis in patients with virus-negative nonfulminant inflammatory cardiomyopathy. Circ Heart Fail 2018;11:e004228.. [DOI] [PubMed] [Google Scholar]
- 9. Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL.. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245–274. [DOI] [PubMed] [Google Scholar]
- 10. Cordero-Reyes AM, Youker KA, Torre-Amione G.. The role of B-cells in heart failure. Methodist DeBakey Cardiovasc J 2013;9:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cordero-Reyes AM, Youker KA, Trevino AR, Celis R, Hamilton DJ, Flores-Arredondo JH.. Full expression of cardiomyopathy is partly dependent on B-cells: a pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber MS, Prod'homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CCA, Martin F, Zamvil SS.. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 2010;68:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu M, Wen S, Wang M, Liang W, Li H-H, Long Q, Guo H-P, Liao Y-H, Yuan J.. TNF-alpha-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J Clin Immunol 2013;33:1002–1008. [DOI] [PubMed] [Google Scholar]
- 14. Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre J-S, Mallat Z.. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Huang Y, Wu W, Wei B, Qin L.. B cells increase myocardial inflammation by suppressing M2 macrophage polarization in coxsackie virus B3-induced acute myocarditis. Inflammation 2019;42:953.. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto Y, Park IK, Kohyama K.. B-cell epitope spreading is a critical step for the switch from C-protein-induced myocarditis to dilated cardiomyopathy. Am J Pathol 2007;170:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.