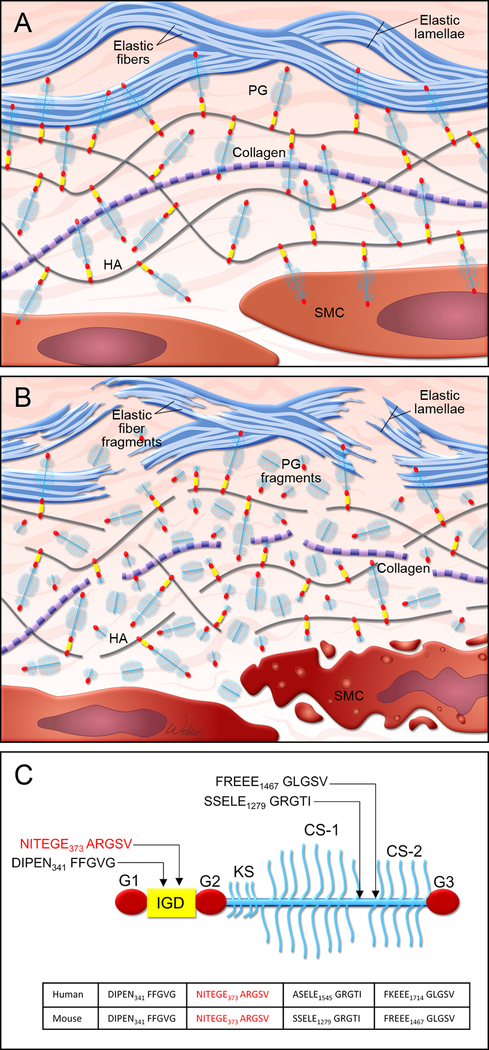
Proteoglycans are important components of the extracellular matrix in vascular tissue and exert critical roles in maintaining aortic structure and function.1 In the normal vasculature (Figure 1A), proteoglycans such as versican interact with hyaluronan to form large aggregates that enhance water retention and create reversible, compressive structures that are necessary for smoothing out pressure waves and abrogating the deformation of blood vessels.2–5 Normal proteoglycan aggregates also pressurize the intralamellar spaces and promote the connection between smooth muscle cells (SMCs) and elastic laminae in the arterial wall, thereby facilitating mechanosensing.5 In addition, proteoglycans maintain aortic hemostasis by regulating elastic fiber assembly6,7 and SMC proliferation.3,8 However, the abnormal accumulation of proteoglycans (Figure 2B) can be destructive to aortic structure and function. Recent studies using computational simulations have suggested that an abnormal increase in proteoglycan aggregate mass in the aorta can increase intralamellar swelling pressure, disrupt cell-matrix interactions, and delaminate the microstructure of the aortic wall, leading to compromised aortic mechanosensing and structural integrity.5,9,10 Thus, an imbalance between proteoglycan production and turnover may disturb the integrity of aortic structure and function.1,5,9,10
Figure. Proteoglycan metabolism in aortic wall.
A, Illustration of normal aortic wall with intact proteoglycans (PG), such as aggrecans, attached to hyaluronan (HA) and healthy smooth muscle cells (SMC). B, Illustration of degenerative aortic wall with accumulated proteoglycan (eg, aggrecan) fragments, elastic fiber fragmentation, and SMC death. C, Schematic structure of an aggrecan core protein that contains globular domains (G1, G2, and G3) and an interglobular domain (IGD). Glycosaminoglycan keratan sulfate (KS) and chondroitin sulfate (CS1 and CS2) chains are attached to the core protein. The location of cleavage sites along the core protein is indicated.
In normal vascular tissue, the abundance of proteoglycans is low, but it is increased significantly in vascular disease.1 Abnormal proteoglycan accumulation has been detected frequently in the degenerative aortic media of thoracic aortic aneurysms and dissections (TAAD), as described in mucoid extracellular matrix accumulation11 and the outdated concept of “cystic medial necrosis.”12 The acidic glycosaminoglycans in proteoglycans give alcian blue staining its characteristic bluish-green color. Accumulation of glycosaminoglycans is observed often in areas with severe elastic fiber fragmentation and SMC loss. Recently, using liquid chromatography–tandem mass spectrometry analysis, Cikach et al13 and Yin et al14 have identified different proteoglycans and glycopeptides in ascending aortic tissues from TAAD patients, including those with Marfan syndrome. Large aggregates of aggrecan and versican were detected in ascending aortas from TAAD patients and mice with Marfan syndrome,13,14 particularly in dissected and ruptured aortas.13 These findings have suggested that the accumulation of aggregated proteoglycans, particularly aggrecan and versican, in the aortic media contributes to aortic degeneration in TAAD. Thus, it is critically important to understand the regulation of proteoglycan production, degradation, and turnover and the interaction between proteoglycan metabolism and other components of the aortic wall.
Proteoglycans are cleaved by matrix metalloproteases and a disintegrin-like and metalloprotease domain with thrombospondin-type motifs (ADAMTSs). In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Dupuis et al15 describe the importance of ADAMTS5-mediated aggrecan cleavage for normal aortic wall development. The authors showed that Adamts5-deficient (Adamts5−/−) mice displayed anomalies in the ascending aorta, such as increased aortic thickness, aortic stenosis, altered outflow tract rotation and aortic geometry, SMC loss, and blood cells in the aortic wall. These aortic abnormalities were associated with aggrecan accumulation. The authors further identified the site of ADAMTS5-mediated aggrecan cleavage that was important for aggrecan turnover and aortic development. Using antibodies that recognized neo-epitopes of aggrecan generated by ADAMTS-mediated cleavage (Figure 2C), they observed that cleaved aggrecan fragments with FFGVG, SSELE, FREEE, and GLGS neo-epitopes accumulated in aortas of Adamts5−/− mice. In contrast, they observed a reduced abundance of cleaved aggrecan fragments with the TEGE neo-epitope in the interglobular domain at the N-terminus core. These findings indicate that ADAMTS5 may be involved in aggrecan cleavage at the TEGE373↓374ALGSV site. Furthermore, the authors demonstrated that mice carrying a non-cleavable mutation at this site (TEGE373↓374NVYS) displayed mild aortic abnormalities, suggesting that cleavage at this site may be important for aggrecan turnover and normal aortic wall development. These findings are in line with those of several recent studies that support an emerging role of ADAMTS5 as a key enzyme involved in proteoglycan turnover and aortic protection in mice.16,17 Indeed, mice lacking the catalytic domain of ADAMTS5 (Adamts5Δcat) showed aggrecan accumulation and thoracic aortic dilatation,16 as well as exacerbated ascending aortic enlargement in mice with angiotensin II–induced aortic dilatation.17 Further analyses revealed that the accumulation of an ADAMTS-specific versican cleavage product (versikine) was decreased and that the abundance of versican was increased in Adamts5Δcat mice.17 These findings suggest that the ADAMTS5-mediated cleavage and turnover of aggrecan and versican are critical for aortic morphogenesis during development and the maintenance of aortic integrity throughout adulthood. Others have reported the increased production of proteoglycans (particularly aggrecan and versican) in the aortic wall of TAAD tissues.13 Together, these findings have raised the possibility that aggrecan and versican accumulation in ascending TAAD may result from increased production and reduced turnover of these proteoglycans.13–17
The elegant study by Dupuis et al15 leads to several additional questions. First, it is not clear whether induction of proteoglycan production results from aortic inflammation or the adaptive response to aortic injury. Further work is needed to determine the factors and pathways that stimulate proteoglycan production and to characterize the dynamic roles of proteoglycan metabolism in aortic inflammation, injury, repair, and remodeling. Second, the comprehensive regulation of proteoglycan cleavage is not well understood. Proteoglycans are important for the homeostasis of aortic structure and function. Unwanted proteoglycan cleavage not only causes the disruption of normal proteoglycan function but also generates cleaved products that can further promote an inflammatory response. In contrast, insufficient proteoglycan cleavage and turnover cause abnormal proteoglycan accumulation. Therefore, the precise control of proteoglycan cleavage is critical. It is not clear whether site-specific cleavage is specifically responsible for degrading functional proteoglycans or clearing dysfunctional proteoglycan fragments. The possibility also remains that cleavage at the same site, through more complex regulation, has diverse effects on proteoglycan processing, degradation, and clearance. Finally, it is likely that different ADAMTS family members such as ADAMTS1,18 ADAMTS5,15–17 and ADAMTS419 are differentially involved in proteoglycan metabolism. Their specific proteoglycan substrates and their regulation of the specific cleavage sites on each proteoglycan remain to be determined. The upstream stressors and pathways that regulate ADAMTS expression and activity and subsequent proteoglycan dysfunction and/or turnover also remain to be identified. With the increasing use of advanced technologies such as proteomics (glycoproteomics, proteoglycanomics) and conditional knockout mice, our understanding of the dynamic regulation of proteoglycan metabolism will continue to unfold.
Acknowledgment
We gratefully acknowledge Nicole Stancel, PhD, ELS(D), of Scientific Publications at the Texas Heart Institute, for editorial support, and Scott Weldon, MA, FAMI, from the Division of Cardiothoracic Surgery, Baylor College of Medicine, for creating the illustrations.
Sources of Funding
Our research activities are supported by grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (HL131980, HL143359, HL127111, HL133723, HL139748) and from the American Heart Association Vascular Diseases Strategically Focused Research Networks (SFRN) (AHA18SFRN33960114). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA. Dr. LeMaire’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine.
Abbreviations
- ADAMTS
disintegrin-like and metalloprotease domain with thrombospondin-type motif
- SMC
smooth muscle cell
- TAAD
thoracic aortic aneurysms and dissections
Footnotes
Disclosures
The authors have no potential conflicts of interest to disclose.
Contributor Information
Ying H. Shen, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX Department of Cardiovascular Surgery, Texas Heart Institute, Houston, TX.
Hong S. Lu, Saha Cardiovascular Research Center, University of Kentucky, Lexington, KY Department of Physiology, University of Kentucky, Lexington, KY.
Scott A. LeMaire, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX Department of Cardiovascular Surgery, Texas Heart Institute, Houston, TX.
Alan Daugherty, Saha Cardiovascular Research Center, University of Kentucky, Lexington, KY; Department of Physiology, University of Kentucky, Lexington, KY.
REFERENCES
- 1.Wight TN. A role for proteoglycans in vascular disease. Matrix Biol. 2018:71–72: 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992:267:10003–10010. [PubMed] [Google Scholar]
- 3.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999:19:1004–1013. [DOI] [PubMed] [Google Scholar]
- 4.Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008:294:H1197–1205. [DOI] [PubMed] [Google Scholar]
- 5.Roccabianca S, Bellini C, Humphrey JD. Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface. 2014:11:20140397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002:90:481–487. [DOI] [PubMed] [Google Scholar]
- 7.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006:98:370–377. [DOI] [PubMed] [Google Scholar]
- 8.Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002:190:38–45. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey JD. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-beta. J Vasc Res. 2013:50:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014:13:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halushka MK, Angelini A, Bartoloni G, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc Pathol. 2016:25:247–257. [DOI] [PubMed] [Google Scholar]
- 12.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977:39:13–20. [DOI] [PubMed] [Google Scholar]
- 13.Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X, Wanga S, Fellows A, et al. Glycoproteomic Analysis of the Aortic Extracellular Matrix in Marfan Patients. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA118312175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupuis LE, Nelson EL, Hozik B, Porto SC, Rogers-DeCotes A, Fosang A, Kern CB. Adamts5(−/−) mice exhibit altered acan (aggrecan) proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119313077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suna G, Wojakowski W, Lynch M, et al. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation. 2018:137:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fava M, Barallobre-Barreiro J, Mayr U, Lu R, Didangelos A, Baig F, Lynch M, Catibog N, Joshi A, Barwari T, Yin X, Jahangiri M, Mayr M. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2018:38:1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oller J, Mendez-Barbero N, Ruiz EJ, et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017:23:200–212. [DOI] [PubMed] [Google Scholar]
- 19.Ren P, Hughes M, Krishnamoorthy S, Zou S, Zhang L, Wu D, Zhang C, Curci JA, Coselli JS, Milewicz DM, LeMaire SA, Shen YH. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci Rep. 2017:7:12351. [DOI] [PMC free article] [PubMed] [Google Scholar]